Abstract
Objective. IFN α Kinoid (IFN-K) is a therapeutic vaccine composed of IFNα2b coupled to a carrier protein. In a phase I/II placebo-controlled trial, we observed that IFN-K significantly decreases the IFN gene signature in whole blood RNA samples from SLE patients. Here, we analysed extended follow-up data from IFN-K-treated patients, in order to evaluate persistence of neutralizing anti-IFNα Abs antibodies (Abs), and gene expression profiling.
Methods. Serum and whole blood RNA samples were obtained in IFN-K-treated patients included in the follow-up study, in order to determine binding and neutralizing anti-IFNα Ab titres, and perform high-throughput transcriptomic studies.
Results. Neutralization studies of 13 IFNα subtypes demonstrated the polyclonal nature of the Ab response induced by IFN-K. Follow-up analyses in six patients confirmed a significant correlation between neutralizing anti-IFNα Ab titres and decrease in IFN scores compared to baseline. These analyses also revealed an inhibitory effect of IFNα blockade on the expression of B cell associated transcripts.
Conclusions. IFN-K induces a polyclonal anti-IFNα response that decreases IFN- and B cell-associated transcripts.
Trial registration: ClinicalTrials.gov, clinicaltrials.gov, NCT01058343
Keywords: systemic lupus erythematosus, interferon alpha, interferon alpha kinoid, interferon signature, B cells
Rheumatology key messages
IFNα kinoid induces a polyclonal anti-IFNα antibody response with a broad neutralizing capacity of IFNα subtypes.
IFNα neutralization in SLE patients decreases the expression of genes involved in B cell activation.
Introduction
IFN α-kinoid (IFN-K) is a therapeutic vaccine composed of inactivated IFNα2b coupled to a carrier protein. When administered to human IFNα transgenic mice, IFN-K induced a polyclonal antibody (Ab) response that neutralized all 13 human IFNα subtypes, but not IFNβ or IFNγ [1]. In lupus-prone NZB/W mice, mouse IFN-K prevented disease manifestations (death, renal disease) triggered by exogenous IFNα [2].
IFN-K was tested in a placebo-controlled phase I/II study including 28 SLE patients. We previously reported our observations up to day 168 after the first IFN-K injection. IFN-K induced 10× higher anti-IFNα binding Abs in IFN gene signature–positive (two/three of the patients) compared with IFN gene signature–negative individuals. In these IFN-signature–positive patients, IFN-K administration resulted in a significant decrease in the expression of IFN-induced genes, and the amplitude of the inhibition correlated with binding anti-IFNα Ab titres [3, 4].
Here, we report on the characterization of the neutralizing anti-IFNα Ab response induced by IFN-K in the phase I/II-included patients. We also analysed extended follow-up data obtained in six IFN-K–treated patients in order to obtain transcriptomic and biological insights into the long-term effects of the drug.
Patients and methods
Twenty-eight patients with SLE (aged 18–50 years), according to the ACR criteria for SLE [5], were recruited in a multicentre, randomized, double-blind placebo-controlled, phase I/II staggered dose-escalation trial of IFN-K (ClinicalTrials.gov registry number NCT01058343). Patients were randomized to receive three or four injections of placebo (n = 7) or 30 μg (n = 3), 60 μg (n = 6), 120 μg (n = 6) or 240 μg (n = 6) IFN-K. Clinical and safety data, induction of anti-IFNα Ab and gene expression profiling studies for up to day 168 after the first IFN-K injection were reported previously [3]. Institutional ethics committee approval for the present follow-up study was obtained for all participating centres. All subjects provided written informed consent.
Follow-up sera were obtained every 3 months after the last study visit in a majority of patients, up to day 336 in placebo-treated, and up to day 1558 in IFN-K treated patients. In six patients, whole blood samples were collected in PAXgene Blood RNA tubes (Qiagen, Courtaboeuf, France) every 6 months during follow-up, together with biological (binding and neutralizing anti-IFNα Ab titres, C3 concentrations and anti-ds DNA Ab titres) data (see supplementary Table S1 and supplementary materials and methods, available at Rheumatology Online). RNA was extracted from these samples, and was also re-extracted from baseline (month 0) and day 168 (month 6) PAXgene tubes stored at −80° from the same patients, and from 10 healthy volunteers (described in [3]). RNA extraction and hybridization of HGU133 Plus2.0 arrays (Affymetrix, High Wycombe, UK) are described in the supplementary material and methods, available at Rheumatology Online. The Affymetrix.CEL files were deposited in the Gene Expression Omnibus of the National Center for Biotechnology Information, and are accessible through Gene Expression Omnibus accession number GSE72754.
Analysis of the gene expression data was performed on GeneSpring (Agilent, Santa Clara, CA) after normalization by robust multi-array analysis [6]. In order to mine our microarray data, we looked at correlations between serum neutralizing anti-IFNα Ab titres and differences in gene expression compared with baseline. We used several time points from the same patients, in view of the strong changes in anti-IFNα Ab titres over time, to increase the sensitivity of our analyses. Pathway analyses were done using DAVID [7, 8]. Calculation of the IFN [9] and B cell scores are described in the supplementary materials and methods, available at Rheumatology Online. Statistical analyses were performed on Prism v5.0 software. Correlations with serum neutralizing anti-IFNα Ab titres were evaluated using non-parametric tests (Spearman ρ). Between-group differences in B cell score evolution over time was evaluated using a Kruskal–Wallis test. Additional gene set enrichment analyses were performed using all samples from the initial IFN-K trial (GSE39088), as well as samples from 10 SLE patients with nephritis, before administration of immunosuppressive therapy (GSE72747), IFNα-stimulated control whole blood cells (GSE39088) and CpG-stimulated purified B cells from healthy individuals (GSE45113). [10] A description of these samples is provided in supplementary Table S2, available at Rheumatology Online.
Results
As described previously, 10 out of 21 patients who received IFN-K (mainly in the 120 and 240 μg groups) developed neutralizing anti-IFNα Abs, which were still detectable in 6 of them at last follow-up visit (range of persistence: 168–1558 days, see supplementary Fig. S1, available at Rheumatology Online). Neutralization studies on 13 different IFNα subtypes were performed using sera from 2 IFN-K–treated patients, and compared with the neutralization pattern of 9F3, an anti-IFNα2b mAb. The results displayed in supplementary Table S3, available at Rheumatology Online, confirmed the polyclonal nature of the neutralizing Ab response induced by IFN-K.
Extended follow-up data were collected in 6 out of the 21 IFN-K–treated patients. One of them (in the 240 μg group) did not have a positive IFN gene signature at baseline. In the five other patients, normalization of the IFN signature was observed in two of them who had developed moderate or high titres neutralizing anti-IFNα antibodies (Fig. 1A). Accordingly, there was a significant correlation between serum neutralizing anti-IFNα Ab titres and decreased expression of IFN-induced genes (Fig. 1B). There was also a moderate correlation between increase in serum C3 and neutralizing anti-IFNα Ab titres, but the P-value was not significant (r = 0.32, P = 0.054) (Fig. 1C). Serum anti dsDNA Ab titres increased in one patient who did not develop neutralizing anti-IFNα Abs, and were stable in all other patients (Fig. 1D).
Fig. 1.
Effects of neutralizing anti-IFNα Abs on the expression of IFN-induced genes
(A) Mean-centred, log2-transformed normalized expression of 21 IFN-induced probe sets (green square = −2.5; red square = +2.5) used to calculate IFN scores (probe set identifications are displayed in supplementary Table S7), IFN scores and serum anti-IFNα Ab titres (U/ml) in 10 controls and 6 IFN-K−treated SLE patients are displayed at the indicated time points, at which transcriptomic, biological and serological data were available. (B) Correlation between log10-transformed serum neutralizing anti-IFNα Abs and changes in IFN scores compared with baseline or (C) changes in serum C3 compared with baseline. (D) Evolution of serum dsDNA Ab titres at the same time points. Spearman ρ correlation coefficients are displayed on the graphs. Patients’ study numbers: filled square = 030501; open circle = 030402; filled circle = 060102; filled rhombus = 010101; filled inverted triangle = 030401; filled triangle = 060101.
In these 6 patients, serum anti-IFNα neutralizing antibody titres displayed a strong (−0.9 <r <−0.7) negative correlation with decreased expression of 156 transcripts (supplementary Table S4, available at Rheumatology Online). Pathway analyses showed that these transcripts were significantly enriched in immunoglobulin genes (supplementary Table S5, available at Rheumatology Online). Gene set enrichment analyses showed that the majority of them were overexpressed in SLE patients compared with in controls. These transcripts were also induced by IFNα in control cells, thereby confirming that they are IFNα-dependent. Finally, many of them were induced in proliferating CD27high B cells stimulated with a TLR9 agonist, which confirmed their potential link with B cell activation processes (Fig. 2A).
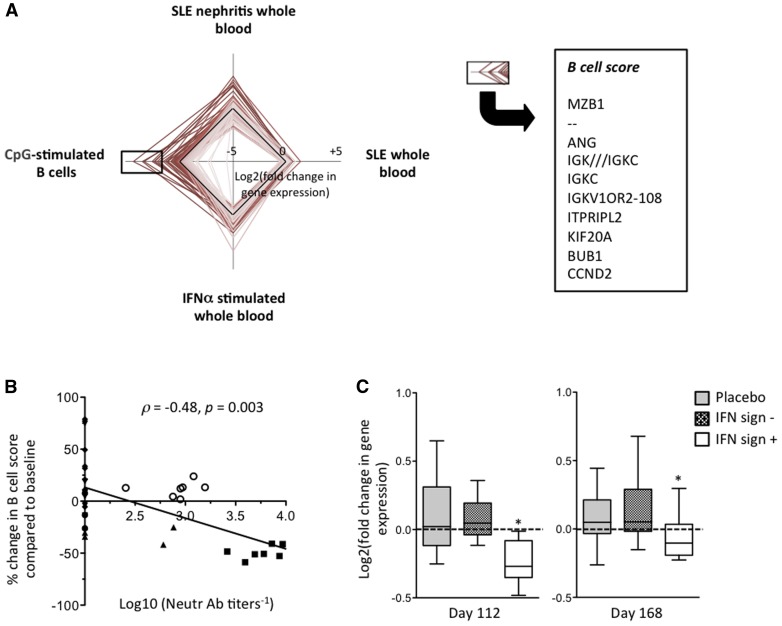
Fig. 2.
Genes downregulated by IFN-K are significantly enriched in B cell–associated transcripts
Serum neutralizing anti-IFNα Ab titres display a strong (−0.9 < r < −0.7) correlation with decreased expression of 156 transcripts (compared with baseline) in 6 IFN-K−treated SLE patients observed during the extended follow-up period. (A) Radar plot showing the relative expression of these 156 transcripts in the indicated experimental conditions. The B cell score is calculated using the 10 most differentially expressed genes in proliferating CpG-stimulated B cells. (B) Correlation between log10-transformed serum neutralizing anti-IFNα Abs and changes in B cell scores compared with baseline. Patients’ study numbers: filled square = 030501; open circle = 30402; filled circle = 060102; filled rhombus = 010101; filled inverted triangle = 030401; filled triangle = 060101. (C) Box plots (median, interquartile range, 10th and 90th percentiles) showing the changes from day 0 in the expression of the 10 probe sets used in the B cell score in all patients included in the phase I/II trial. The differences in the average log2 expression of each of these probe sets between the indicated day and day 0 were calculated for patients treated with placebo (n = 7), IFN signature−negative (n = 7) and IFN signature−positive (n = 12) patients treated with IFN-K. *P < 0.05 using the Kruskal−Wallis tests.
We designed a score using the 10 (out of 156 listed above) transcripts that were most overexpressed in C27high CpG-stimulated B cells (Fig. 2A, supplementary Table S6, available at Rheumatology Online). Not surprisingly, decrease in the CpG-stimulated B cell score correlated significantly with serum anti-IFNα neutralizing Ab titres in the extended follow-up group of patients (Fig. 2B). Because the observed correlation was driven by two patients with high neutralizing Ab titres, we returned to the original phase I/II trial data on all patients in order to increase sample size. We found that IFN-K significantly decreased the expression of these transcripts at day 112 and at day 168 in patients with a positive IFN signature at baseline, compared with IFN signature–negative or placebo-treated patients (Fig. 2C).
Discussion
Extended follow-up data from SLE patients included in the phase I/II IFN-K trial demonstrate that IFN-K induces not only binding, but also neutralizing anti-IFNα Abs, mainly in the higher dose groups. The neutralizing response is polyclonal and extends to numerous IFNα subtypes. Inhibition of the IFN signature correlates with serum neutralizing anti-IFNα Abs. In addition, in vivo IFNα neutralization is associated with decreased expression of transcripts associated with B cell activation in peripheral blood, an observation that we could extend from the small subset of patients included in the extended follow-up, to the whole population of IFN-K–treated patients.
More efficient target neutralization by a polyclonal, rather than a monoclonal antibody response is a well-established concept, and experimental evidence confirms the synergistic blocking effects of a mix, compared with single mAbs [11]. In the case of IFNα, the ability of such monoclonal Abs to inhibit each individual subtype is variable, and it is possible that some in vivo IFNα activity remains even under therapy. The observation that rontalizumab induces clinical improvement in patients with little or no in vivo IFNα bioactivity, but not in patients with higher levels, is further indirect support in favour of this hypothesis [12]. In contrast, the polyclonal anti-IFNα response induced by IFN-K enhances the probability of neutralization of all IFNα subtypes. In addition, production of higher titres of anti-IFNα Abs in IFN signature–positive patients results in IFN-blocking activity, even in patients with elevated IFNα bioactivity [3].
Further data mining indicated that IFN-K–induced anti-IFNα Abs decreased the expression of immunoglobulin and other B cell–associated transcripts. This observation is in line with the known direct and indirect effects of IFNα on B cell activation and differentiation [13]. Whether this effect of IFN-K on circulating B cells will affect activity and size of the autoAb-producing pool of long-lived plasma cells in lupus will be key in evaluating the disease-modifying effect of IFNα neutralization in SLE.
In conclusion, these observations confirmed the broad IFNα-neutralizing ability of IFN-K, and indicate that further evaluation of the therapeutic effects of the drug in a large placebo-controlled trial is warranted.
Supplementary Material
Acknowledgements
J.D. is funded by the “UCB/UCL Chaire sur les rhumatismes inflammatoires et systémiques.”
Funding: This work was funded by an unrestricted grant from Neovacs, and from the Fonds de la Recherche Scientifique.
Disclosure statement: G.G.-V., M.L., T.C., C.R. and F.C. are employees of Neovacs. P.V. was an employee of Neovacs at the time of the study, owns Neovacs stock and is currently on the Neovacs scientific advisory board. All other authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology Online.
References
- 1.Mathian A, Amoura Z, Adam E. et al. Active immunization of human interferon α transgenic mice with a human interferon α Kinoid induces antibodies that neutralize interferon α in sera from patients with systemic lupus erythematosus. Ann Rheum Dis 2011;70:1138–43. [DOI] [PubMed] [Google Scholar]
- 2.Zagury D, Le Buanec H, Mathian A. et al. IFNalpha kinoid vaccine–induced neutralizing antibodies prevent clinical manifestations in a lupus flare murine model. Proc Natl Acad Sci U S A 2009;106:5294–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lauwerys BR, Hachulla E, Spertini F. et al. Down-regulation of interferon signature in systemic lupus erythematosus patients by active immunization with interferon α-kinoid. Arthritis Rheum 2013;65:447–56. [DOI] [PubMed] [Google Scholar]
- 4.Lauwerys BR, Ducreux J, Houssiau FA. Type I interferon blockade in systemic lupus erythematosus: where do we stand? Rheumatology 2014;53:1369–76. [DOI] [PubMed] [Google Scholar]
- 5.Hochberg MC, for the Diagnostic and Therapeutic Criteria Committee of the American College of Rheumatology. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus [letter]. Arthritis Rheum 1997;40:1725. [DOI] [PubMed] [Google Scholar]
- 6.Irizarry RA, Hobbs B, Collin F. et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 2003;4:249–64. [PMC][10.1093/biostatistics/4.2.249] [12925520] [DOI] [PubMed] [Google Scholar]
- 7.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 2009;4:44–57. [DOI] [PubMed] [Google Scholar]
- 8.Huang da W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 2009;37:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao Y, Higgs BW, Morehouse C. et al. Development of potential pharmacodynamic and diagnostic markers for anti-IFN-α monoclonal antibody trials in systemic lupus erythematosus. Hum Genomics Proteomics 2009;17:374312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henn AD, Laski M, Yang H. et al. Functionally distinct subpopulations of CpG-activated memory B cells. Sci Rep 2012;2:345.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kontsek P, Borecky L, Novak M. et al. Enhancement of neutralizing efficacy by combining three monoclonal antibodies to human interferon-alpha. Immunology 1991;73:8–11. [PMC free article] [PubMed] [Google Scholar]
- 12.Kalunian K, Merrill JT, Maciuca R. et al. Efficacy and safety of rontalizumab (anti-interferon alpha) in SLE subjects with restricted immunosuppressant use: results of a randomized, double-blind, placebo-controlled phase 2 study. Arthritis Rheum 2012;64 (Suppl 10):2622. [abstract]. [Google Scholar]
- 13.Kiefer K, Oropallo MA, Cancro MP. et al. Role of type I interferons in the activation of autoreactive B cells. Immunol Cell Biol 2012;90:498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.