Abstract
Background
A prophylactic HIV-1 vaccine is a global health priority.
Objective
To assess a novel vaccine platform as a prophylactic HIV-1 vaccine regimen.
Design/Setting
This randomized, double-blind, placebo-controlled trial assessed two candidate HIV-1 vaccines (Ad26.EnvA and Ad35-Env both at 5×1010 vp) in homologous and heterologous combinations in three geographic regions (US, East and South Africa). Both subjects and study personnel were blinded to treatment allocation. (NCT 01215149).
Patients
Healthy HIV uninfected adults.
Measurements
Safety and immunogenicity were assessed and the impact of baseline vector immunity was analyzed.
Results
217 subjects received at least 1 vaccination and 210 (>96%) completed follow-up, No vaccine-associated serious adverse events occurred. All regimens were generally well tolerated though more vaccine recipients had transient moderate or severe systemic reactions (36.5%) compared to placebo recipients (20.5%). All regimens elicited humoral and cellular immune responses in nearly all volunteers. There was no impact of pre-existing Ad26 or Ad35 neutralizing antibody titers on vaccine safety and little on immunogenicity. In both homologous and heterologous regimens the second vaccination significantly increased EnvA antibody titers (~20 fold from median ELISA titers of 30–300 to 3000). The heterologous regimen Ad26-Ad35 elicited significantly higher EnvA antibody titers than Ad35-Ad26. T cell responses were modest and lower in East Africa than in South Africa and the United States.
Conclusions
Both vaccines elicited significant immune responses in all populations. Baseline vector immunity did not have a significant impact on immune responses. Second vaccinations in all regimens significantly boosted EnvA titers though vaccine order in the heterologous regimen had a modest effect on the immune response.
Primary Funding
IAVI, NIAID/NIH, and the Ragon Institute in collaboration with Crucell Holland BV.
Introduction
The development of a vaccine to prevent HIV infection is a global health priority. To date four concepts have been assessed for possible efficacy, and only one has shown modest and short-lived efficacy(1–5). A significant challenge is how to elicit robust and durable anti-HIV-1 immune responses. A variety of approaches are being investigated to augment anti-HIV-1 immune responses including: repeated vaccine administration, increased vaccine dose, cytokine co-administration, vectored delivery systems and heterologous prime-boost strategies(6–8). Here we describe a study of different prime-boost regimens employing two human adenovirus vectors (heterologous vectors) to which most people have little or no immunity and which differ in their biological characteristics from adenovirus serotype 5 such as utilization of a different primary cellular receptor and elicitation of innate cytokine responses. The vaccines each carried an HIV clade A Env gene, however, the sequences were not matched.
Certain vectored vaccine strategies may be limited by immunity to the delivery vector, either pre-existing or induced by the first immunization(s): vaccine safety and tolerability may be affected by prior experience with the vaccine vector(9–12) or immune responses to the vector may impair responses to the vaccine insert. One strategy to avoid or minimize preexisting immunity is to utilize adenovirus delivery systems based on less common serotypes(13,14) in heterologous vectored vaccine regimens. Adenovirus vectored HIV-1 vaccines currently in development include adenovirus serotype 26(15,16) and 35(17), both of which have been shown to protect in the NHP model and to be safe and immunogenic in initial Phase I human testing. These platforms are also being developed as vaccine candidates for other pathogens (18–22).
We report on the first assessment of an Ad26 and Ad35 heterologous vaccine regimen, with HIV clade A envelope gene inserts, in a randomized, double-blind, placebo-controlled, multicenter, international clinical trial in the US, Kenya, Rwanda and South Africa.
METHODS
Design Overview and Setting and Participants
This trial was a multi-center, randomized, double-blind (with respect to vaccine or placebo as well as homologous or heterologous treatment groups but not to schedule), placebo-controlled trial to evaluate the safety and immunogenicity of two different HIV adenovirus-vectored vaccines, Ad26.ENVA.01 (Ad26) and Ad35-ENV (Ad35), both at 5 × 1010 vp (viral particles) in homologous and heterologous regimens and at two dose schedules (0, 3 months or 0, 6 months). Both subjects and study personel (clinical and laboratory) were blinded to treatment allocation. Volunteers were healthy HIV-negative adults aged 18–50 years who reported low risk for acquiring HIV; eligibility was not affected by preexisting natural immunity to Ad26 or Ad35. The different groups allowed a comparison of homologous and heterologous regimens at the 0, 3-month interval between different African regions and the two dose schedules at the US clinical research center. The study was conducted at six clinical research centers in four countries and was approved by all relevant local and governmental ethics and regulatory bodies for each clinical research center. Written informed consent was obtained from each volunteer. The trial was registered at ClinicalTrials.gov (NCT01215149).
The modular trial schema is presented in Table 1. Homologous regimens (Ad26-Ad26 and Ad35-Ad35) had previously been assessed in the US thus comparison of these regimens was replaced with a comparison of 0, 3 vs 0, 6 month schedule at the US site(15–17). All vaccines were given by intramuscular (IM) injection in the deltoid muscle. For full study details see supplemental appendix.
Table 1.
Study Schema
| Region | Group | Vaccines Regimens* |
N | M0 | M3 | M6 | Abbreviation† |
|---|---|---|---|---|---|---|---|
| US | A | Heterologous | 11 | Ad26 | - | Ad35 | Ad26-Ad35 |
| B | 10 | Ad35 | - | Ad26 | Ad35-Ad26 | ||
| C | 10 | Ad26 | Ad35 | - | Ad26-Ad35 | ||
| D | 9 | Ad35 | Ad26 | - | Ad35-Ad26 | ||
|
East Africa |
E | Heterologous | 17 | Ad26 | Ad35 | - | Ad26-Ad35 |
| F | 17 | Ad35 | Ad26 | - | Ad35-Ad26 | ||
| G | Homologous | 18 | Ad26 | Ad26 | - | Ad26-Ad26 | |
| H | 16 | Ad35 | Ad35 | - | Ad35-Ad35 | ||
|
South Africa |
I | Heterologous | 17 | Ad26 | Ad35 | - | Ad26-Ad35 |
| J | 16 | Ad35 | Ad26 | - | Ad35-Ad26 | ||
| K | Homologous | 16 | Ad26 | Ad26 | - | Ad26-Ad26 | |
| L | 16 | Ad35 | Ad35 | - | Ad35-Ad35 | ||
| Total | 173 Active 44 Placebo‡ Total 217 |
All vaccine doses were 5 × 1010 viral particles (vp).
Abbreviation used in text, tables and figures. M= month.
Placebo recipients were evenly divided between all groups with 11 in US, 17 in East Africa, and 16 in South Africa
Interventions: Vaccines
The Ad26.ENVA.01 vaccine manufactured by Crucell Holland BV (The Netherlands) was a replication-deficient adenovirus type 26 constructed to contain an HIV-1 Clade A Env gene encoding a modified envelope gp140 protein (RW020 GenBank # U08794). The Ad35-ENV vaccine manufactured by Transgene (France) was a recombinant replication-incompetent adenovirus type 35 constructed to contain an HIV-1 subtype A Env gene encoding a modified gp140 protein (01TZA173 GenBank # AY253305). These 2 candidate HIV-1 vaccines had been developed by independent programs but given the substantial homology between the Env A inserts this prime-boost study was designed. For aligned regions of the Ad26.EnvA and Ad35-Env there is a 72.7% amino acid sequence identity. The placebo was the final formulation buffer for the Ad35 vaccine.
Outcomes: Safety and Immunogenicity Assessments
The primary objective of this study was to evaluate the safety and tolerability of these vaccines and regimens. Secondary analyses included immunogenicity of the heterologous regimens at 3 vs 6 months and the heterologous vs homologous regimens at 3 month interval, and the impact of anti-vector immunity on the immune responses elicited by the vaccines and regimens. Systematic safety assessments were conducted. Details of trial schema and safety assessments are presented in the supplemental material. Reactogenicity and adverse events were assessed using an adapted version of the Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events, Version 1.0, December 2004; clarification August 2009. Serum and peripheral blood mononuclear cell (PBMC) samples were collected at baseline and in the 0, 6-month groups at weeks 2, 4, 8, 24, 26, 28, 32 and 52 and in the 0, 3-month groups at weeks 2, 4, 8, 12, 14, 16, 20, 36 and 52 as described previously(15–17). Direct enzyme-linked immunosorbent assays (ELISAs) were performed to assess EnvA-specific serum binding antibodies against the vaccine immunogens with a titer of ≥100 defined as positive for either of the proteins(15–17). T cell responses were assessed by IFN-γ ELISpot and intracellular cytokine assay (ICS) as described previously(15–17). Criteria for positive Ad35 and Ad26 neutralizing antibody (Nab) responses were titer >16. Full details of the immunology methods are presented in the supplemental material. Immunology assays were performed at three centralized laboratories; the IAVI Human Immunology Laboratory (HIL) at Imperial College, London; Beth Israel Deaconess Medical Center (BIDMC), Boston and the HIV Vaccine Trials Network (HVTN) laboratory at Fred Hutchinson Cancer Research Center (FHCRC), Seattle. All assays were performed blinded to treatment allocation(23).
Statistical Analysis
All analyses are based on the as-treated population (2 volunteers were randomized to placebo but received 2 vaccine administrations instead. One received Ad26-Ad35, the other Ad35-Ad35). Analyses of all safety and immunology data (except for Intracellular Cytokine staining [ICS]) were performed by the EMMES Corporation (Rockville, MD, USA) using SAS software version 9.3 (SAS Institute, Cary, NC). ICS data were analyzed by the FHCRC. Comparisons of the proportions of subjects with events (safety and immunology) were made using the Chi-Square or Fisher’s exact 2-tailed test, as appropriate. Simple comparison of the magnitude of ELISpot and ELISA values was by the Wilcoxon rank-sum test for 2 classification levels and by the Kruskal-Wallis test otherwise. To investigate the simultaneous effects of age, gender, BMI, region and regimen on the magnitude of ELISpot and ELISA values at specific visits, multivariable regression models of the log10 response were used. There were no significant pair-wise interactions so final models included only main effects. No imputation was done for missing data, which were treated as missing completely at random. A two-sided p value of less than 0.05 was considered to indicate statistical significance. For more details, see the Supplementary Document on Statistical Methods.
Role of the Funding Source
The investigators designed, conducted, and analysed the data and made all decisions regarding the manuscript and the submission for publication.
RESULTS
Demographics and Disposition of Volunteers
The trial was conducted between October 2010 and November 2012. Of the 218 volunteers randomized 217 received at least one study vaccination (Supplemental Figure SF1). Fifty-two volunteers (23.9%) were enrolled in Boston, 45 volunteers (20.6%) in Rwanda, 40 volunteers (18.3%) in Kenya and 81 volunteers (37%) in South Africa, (27 volunteers [12.4%] at each of the three clinical research centers). Of the 217 vaccinated volunteers, 107 (49.3%) were female and average age was 27.0 years (range: 18–50). Across all sites the proportion of black race was 78.3%; in the US 66.7% (34/51) volunteers identified as white, followed by Asian (9.8%, 5/51) and black (7.8%, 4/51) (Supplemental Table ST1A/B). A total of 96.8% (210/217) of volunteers completed follow-up and 208 (95.8%) volunteers received both study vaccinations (165/173 active; 43/44 placebo).
Safety and Tolerability
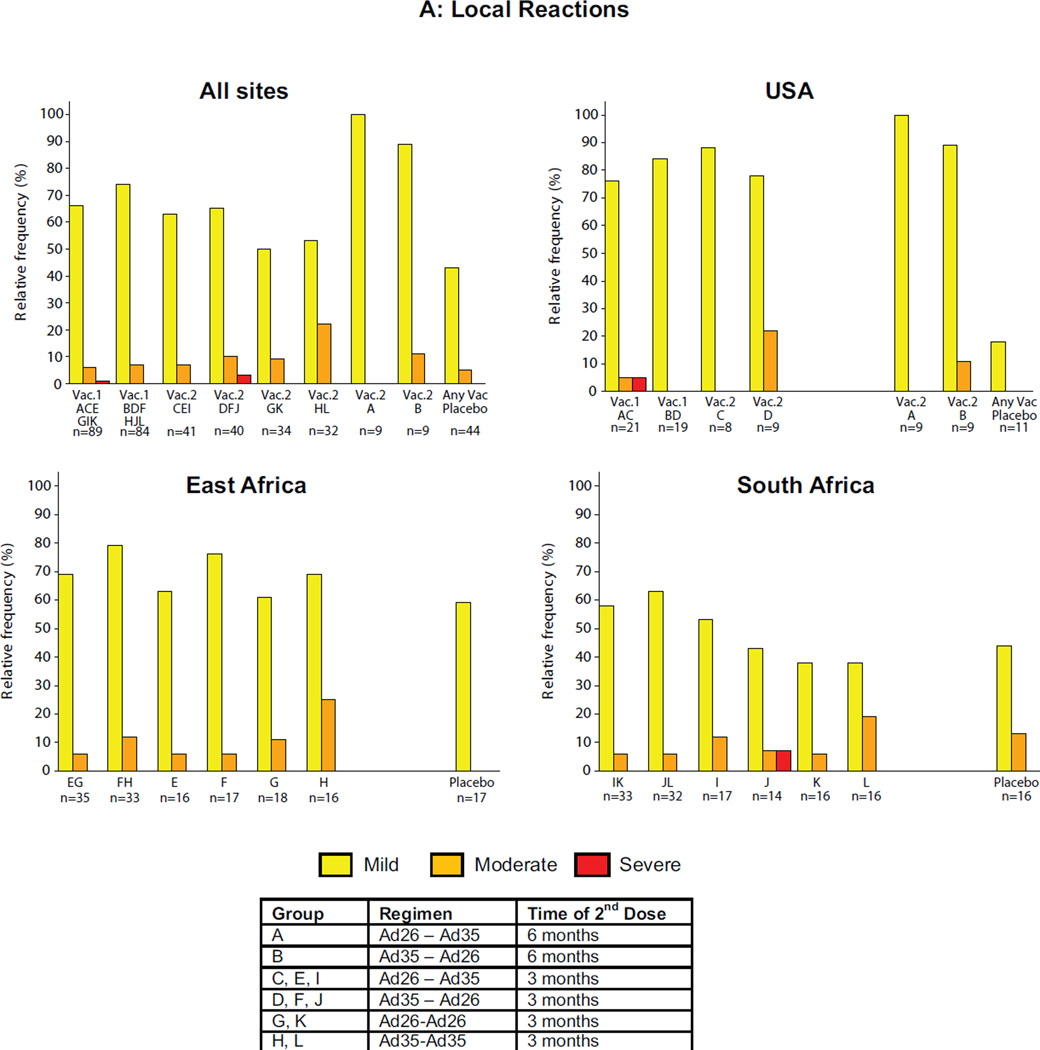
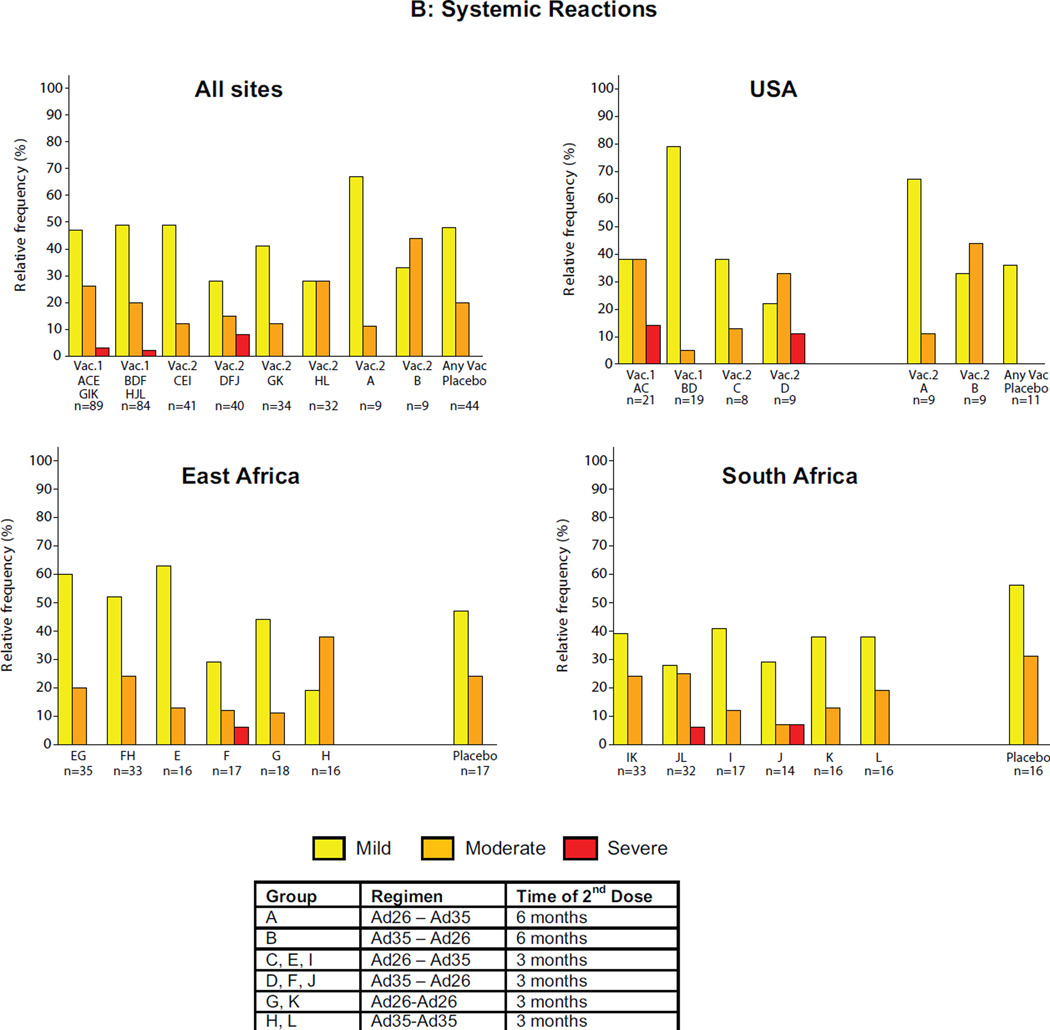
The majority of local reactions were graded as mild or moderate with the overall frequency of any local reaction 86.1% (95% CI: 80.1–90.9) in the vaccine groups and 47.7% (95% CI: 32.5–63.3%) among placebo recipients (Figure 1A/B, ST2). The overall proportion of volunteers with local reactions graded as moderate or severe was 27/173 (15.6%; 95% CI: 10.5–21.9%) in the vaccine groups compared with 2/44 (4.5%; 95% CI: 0.6–15.5%) in the placebo group (p=0.054, chi-square). The difference between individual regimens in proportion of volunteers with moderate or severe local reactions was not statistically significant (p=0.181, Fisher’s exact 2-tail test).
Figure 1. Maximum local and systemic reactions.
Data from all first vaccinations were pooled based on vaccine received and then respective homologous and heterologous regimens were examined. The Y-axis represents the percentage of volunteers experiencing reactogenicity events and the X-axis the study group(s). Panel A for local reactions and panel B for systemic reactions through Day 7 after each vaccination by study group all sites combined and by region. Volunteers self-assessed reactogenicity with a memory aid on Day 0 (evening of vaccine/placebo administration) and daily through Day 7. Safety data for placebos by study group for all sites combined and by region is shown in the far right column of each quadrant. The figure shows the maximum severity assessment grade recorded. The severity grade of the reactogenicity events is indicated by color codes (none: white; mild: yellow; moderate: orange; severe: red).
The majority of systemic reactions were mild or moderate with the overall frequency of any systemic reaction 80.9% (95% CI: 74.3–86.5%) in the vaccine groups and 68.2% (95% CI: 52.4–81.4%) among placebo recipients. There was a statistically significant difference (p=0.045, chi-square) in moderate or severe systemic reactions between vaccine (63/173, 36.4%; 95% CI: 29.2–44.1%) and placebo recipients (9/44, 20.5%; 95% CI: 9.8–35.3% but not between the individual treatment regimens, including placebo (p=0.23, chi-square). Approximately 5% (95% CI; 2.0–8.9%) of vaccine recipients (8 volunteers) reported severe systemic reactions; six after Ad26 and two after Ad35 administration, all in volunteers who were seronegative at baseline to the respective adenovirus vector. No severe reactogenicity was noted in placebo recipients. The difference between vaccine and placebo groups in the proportion of volunteers with moderate or greater nonsolicited adverse events (AE) was not statistically significant. No deaths or vaccine-related serious adverse events were reported and there was no apparent pattern in clinical or laboratory adverse events. Further details of safety assessments are presented in the supplemental material.
IMMUNOGENICITY
HIV-1 Env-Specific Antibody Responses
Samples from 215 volunteers (excluding 2 volunteers who became HIV infected) from all groups were analyzed at BIDMC using two HIV clade A envelope proteins: UG37 and RW020 proteins. Similar results were seen in analyses with UG37 at the HIL, therefore the data from BIDMC are reported here. No volunteers had baseline UG37 antibodies, two volunteers (<1%) had baseline RW020 antibodies and no placebo recipients had responses to either Env at any time point.
Env ELISA titer and response rates across groups
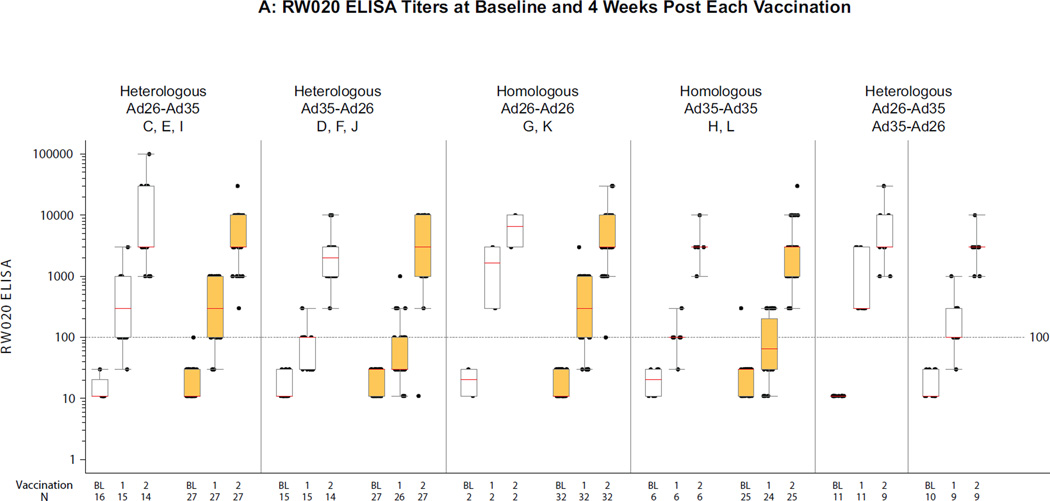
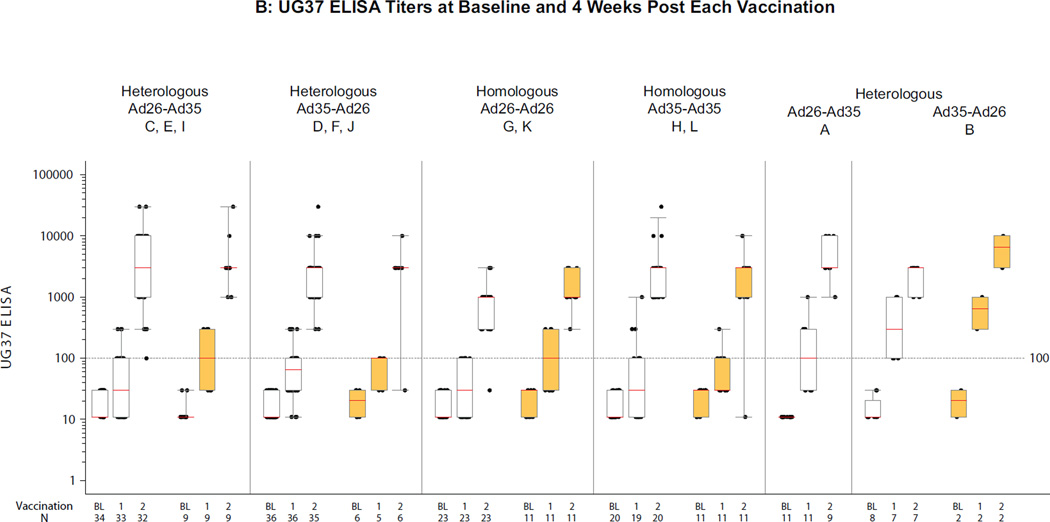
Figure 2A/B (ST3, ST4A/B) show a summary of RW020 and UG37 ELISA titers across groups, stratified by heterologous or homologous regimens and the presence or absence of Ad26 and Ad35 neutralizing antibody (NAb) titers at baseline. At 4 weeks post first vaccination, median ELISA titers to Env in different groups ranged from 30–300 for RW020 and UG37; response rates were 49–100% for RW020 and 38–100% for UG37. After the second vaccination titers increased ~20 fold to a median across different groups of 3000 for RW020 and to 1000–3000 for UG37 with response rates >97% across the vaccination regimens.
Figure 2. Distribution of ELISA titers 4 weeks after each vaccination per regimen.
A) RW020 ELISA Titers (RW020 Env is homologous to the Ad26 Env insert); B) UG37 ELISA Titers (UG37 Env is 81.2% homologous to the Ad35 Env insert). Second vaccinations were at month 6 in Groups A and B, and month 3 otherwise. Boxes represent the 1st and 3rd quartiles (IQR). Red lines indicate median titer. Whiskers extend to the 5th and 95th percentiles. White/orange boxes represent negative/positive baseline Ad26 NAb (A) and Ad35 NAb (B) values. On the X-axis, B, 1 & 2 represent baseline and 4 weeks post first and second vaccinations respectively.
Multivariable Analyses
All vaccine regimens elicited significantly higher anti-HIV Env titers than placebo (all p<0.0001). No statistically clear advantage for any of the four short-interval regimens was observed by regimen or region. At 4 weeks after the second vaccination, a regimen with an Ad35 vaccination elicited higher UG37 responses than one without an Ad35 vaccination: Ad26-Ad26 (least squares mean (LSM) titer 840 (95% CI: 583–1211)) versus Ad26-Ad35 (LSM 2954 (95% CI: 2218–3934), p<0.001), versus Ad35-Ad26 (LSM 2073 (95% CI: 1561–2753), p=0.002), and versus Ad35-Ad35 (LSM 2148 (95% CI: 1468–3143), p=0.003). Of note, in the heterologous regimens Ad26-Ad35 elicited greater RW020 responses than Ad35-Ad26 in South African subjects (p=0.007); (SF2A–D). There were no differences in ELISA response rates and titers across regimen stratified by schedule (see supplement).
Preexisting immunity did not impact ELISA responses
No clear impact of preexisting NAbs to Ad26 or Ad35 on Env ELISA responses, either response rate or magnitude, was identified after the first vaccine administration. Responses elicited for UG37 4 weeks after the first vaccination with Ad35 vaccine (SF3A) were identified in 33/62 (53%; 95% CI: 40–66%) of baseline Ad35 seronegative vs. 10/18 (56%; 95% CI: 31–78%) of seropositive volunteers (p=0.86, chi-square). Responses elicited for RW020 4 weeks after the first vaccination with Ad26 vaccine (SF3B) were identified in 27/28 (96%; 95% CI: 82–100%) of baseline Ad26 seronegative vs. 52/59 (88%; 95% CI: 77–95%) seropositive volunteers (p=0.43, Fisher’s exact 2-tail test). Four weeks after the second administration (homologous or heterologous) in all groups, the immune response was 100% in Ad26- and Ad35-seronegative volunteers and 97% to 99% in Ad35- or Ad26-seropositive volunteers by both ELISA assays (SF3C/D). A single dose of Ad26 elicited higher Env seroconversion rates compared to Ad35 (96% vs 53% [seronegative] p<0.001; and 88% vs 56% [seropositive] p=0.005).
Homologous vs heterologous adenovirus vector regimens
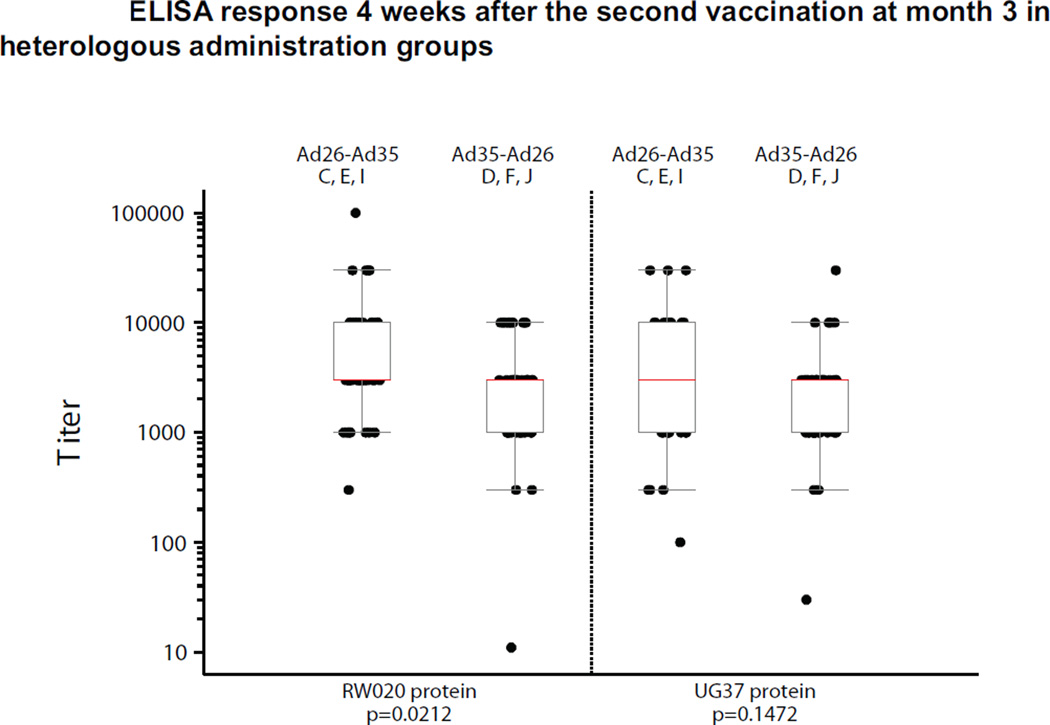
The median increase of 2700 (95% CI: 2700–2970) in the Env ELISA titer from first to second vaccination with Ad26 in the Ad26-Ad26 regimen was statistically significant (p<0.001) against the matched EnvA protein, of which 32 out of the 34 paired samples were from the Ad26 baseline seropositive group (ST4A). Similar results were observed with the UG37 titers. The second vaccination with Ad35 in the Ad35-Ad35 regimen resulted in a median increase of 2800 (95% CI: 970–2970, p<0.001) in ELISA titer (Figure 2B) against the UG37 Env protein and a significant median increase was observed in both baseline Ad35 seropositive (n=11, 2700 (95% CI: 970–9900), p=0.002) and Ad35 baseline seronegative (n=19, 2900 (95% CI: 989–2970), p<0.001) volunteers (Figure 2B, ST3, ST4B). Similar results were observed for RW020 titers. For early boost heterologous regimens, higher titers were observed with the Ad26-Ad35 regimen compared to the Ad35-Ad26; this was statistically significant for the RW020 Env ELISA (matched to Ad26) (GMT 4696 (95% CI: 3213–6865) vs 2274 (95% CI: 1507–3431); p=0.021) but not for the UG37 ELISA using Env matched to the Ad35 vaccine (GMT 2819 (95% CI: 1888–4208) vs. 1959 (95% CI: 1365–2811); p=0.15) (Figure 3, ST3). Thus, the regimen with Ad26 prime and Ad35 boost induced more Env-binding antibodies than the reverse order. Further studies are underway to characterize the function of the antibodies elicited.
Figure 3. Distribution of ELISA titers 4 weeks after the second vaccination at month three in heterologous administration groups.
The two left boxes are for RW020 ELISA Titers (RW020 Env is homologous to the Ad26 Env insert) and the two right boxes are for UG37 ELISA Titers (UG37 Env is 81.2% homologous to the Ad35 Env insert). Boxes represent the 1st and 3rd quartiles (IQR). Red lines indicate median titer. Whiskers extend to the 5th and 95th percentiles.
HIV-1 Env-Specific Cellular Immune Responses
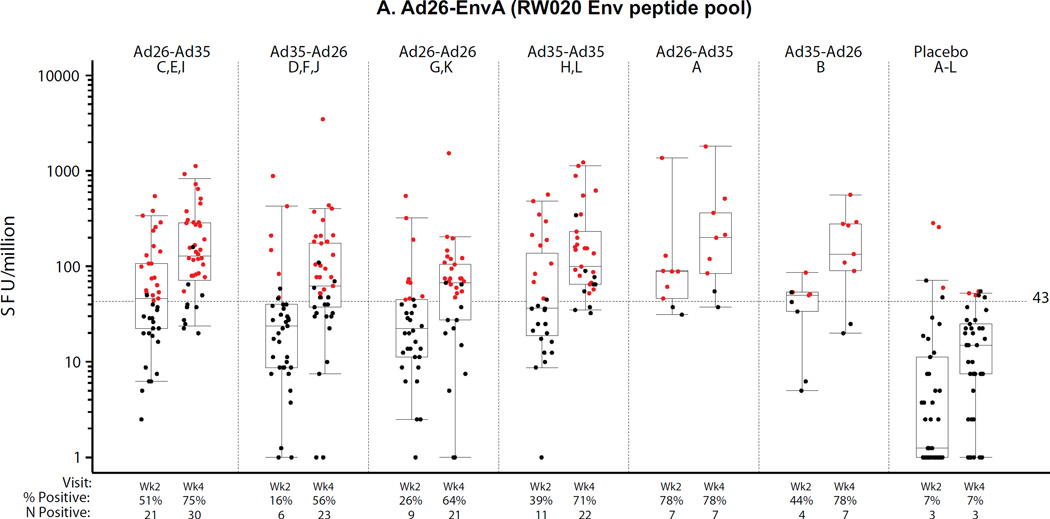
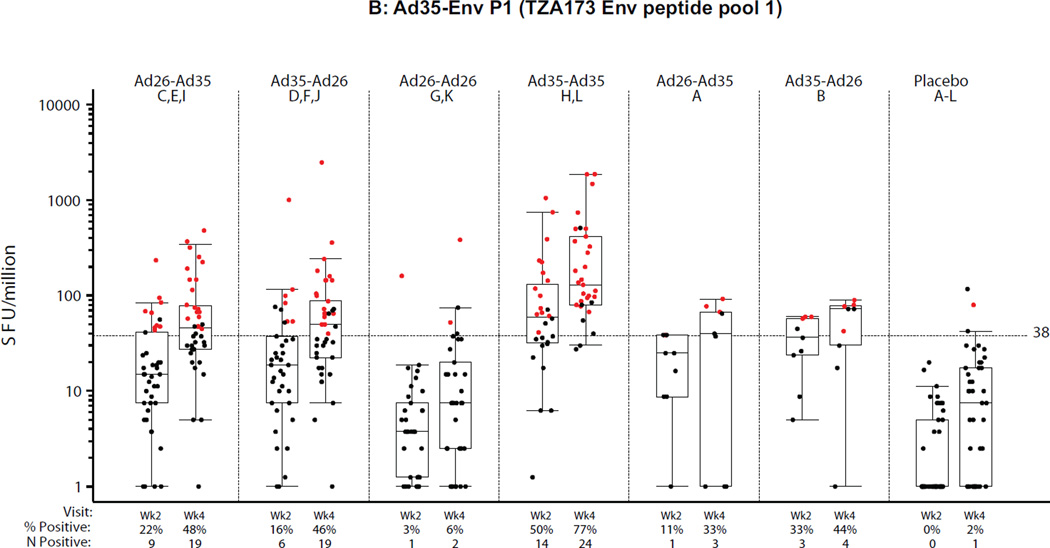
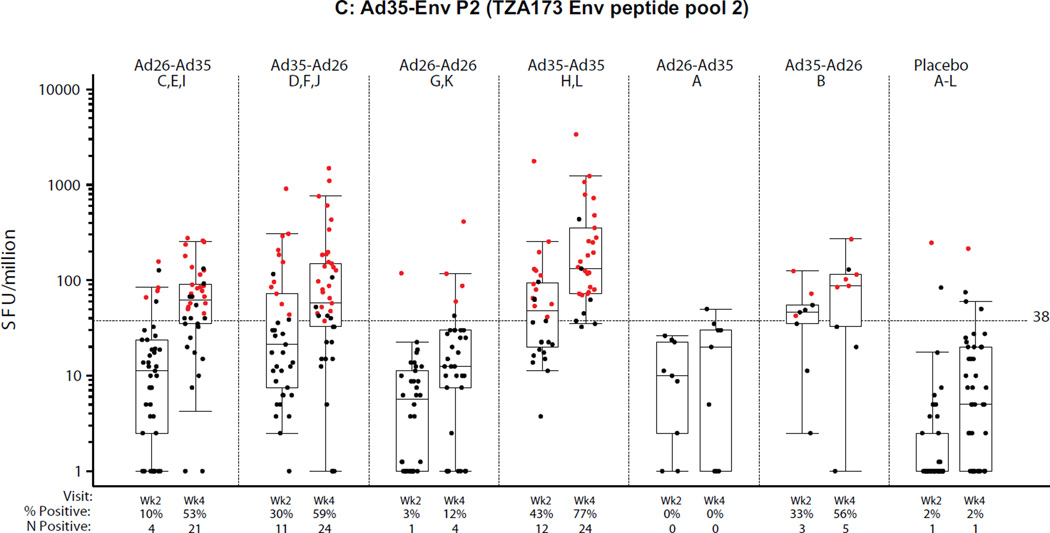
PBMC samples from all groups were analyzed by ELISpot at the HIL (n=201, 2 weeks post second vaccination) and at BIDMC (n=204, 4 weeks post second vaccination). The results are presented in Figures 4A–C for each of the three peptide pools assessed, two pools matched to the Ad35-Env (Ad35-EnvP1, Ad35-EnvP2; TZA173; SF4) and one pool matched to the Ad26-Env (Ad26-Env; RW020; SF4). Overall the ELISpot magnitude and response rates were higher at 4 weeks compared to 2 weeks after the second vaccination regardless of homologous or heterologous vaccine regimens or order of the Ad vectors. For the purposes of comparing across regimens, the week 4 time point is described below, but the results were similar at 2 weeks post second vaccination. Further analysis of ELISpot and ICS reponses are described in the supplement (ST5 and SF6).
Figure 4. Distribution of IFNγ ELISpot responses 2 and 4 weeks after the second vaccination.
A) Ad26.EnvA (RW020 Env peptide pool); B) Ad35-Env P1 (TZA173 Env peptide pool 1); C) Ad35-Env P2 (TZA173 Env peptide pool 2). X-axis shows study group (treatment), percent (%) and frequency (n) of positive responses. Week 2 and 4 samples were analyzed at HIL and BIDMC respectively. Second vaccinations were at month 6 in Groups A and B, and month 3 otherwise. All responses are background-subtracted. Mean responses <1 were set to 1. Boxes show median and IQR. Whiskers extend to the 5th and 95th percentiles. Black and Red dots represent negative and positive responses, respectively.
Multivariable Analyses
At 4 weeks post-boost, all regimens elicited significantly higher ELISpot responses compared to placebo by all 3 peptide pools (Ad26 EnvA, Ad35 EnvA P1, and Ad35 EnvA P2) (p<0.001) except for the Ad26-Ad26 regimen by the Ad35 pools. Regimens with an Ad35 vaccination compared to the Ad26-Ad26 vaccination had stronger responses detected with the Ad35 peptide pools and this was stronger when two doses of Ad35 were given than as one dose of Ad35 in a heterologous regimen. Lower responses were observed in East African subjects by all 3 peptide pools (SF5A–C). No significant differences were observed between the 3- and 6-month regimens.
Neutralizing Antibody Responses to the Adenovirus Vectors
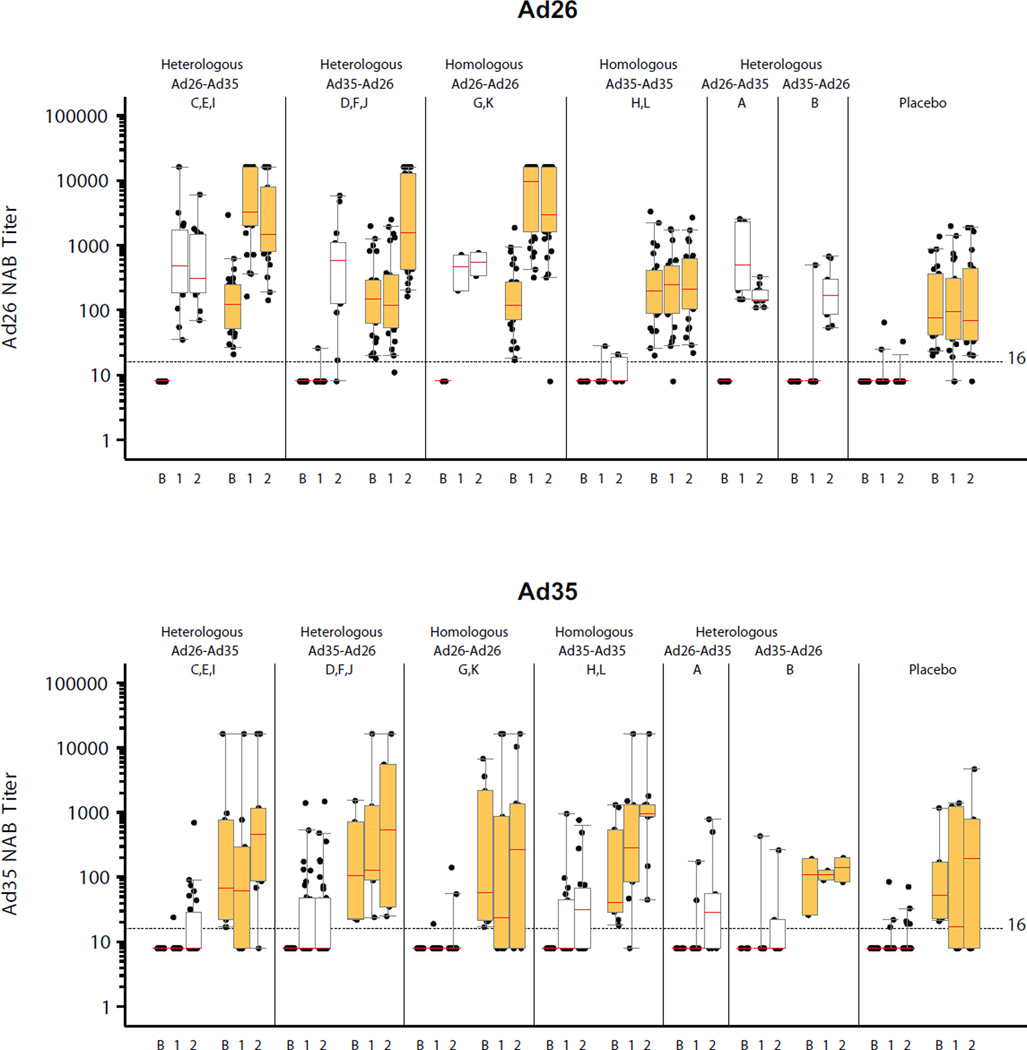
Neutralization of adenovirus was used to assess baseline and vaccine-induced responses to the vector. Of the 215 samples analyzed, 134 (62%) volunteers had positive Ad26 titers (GMT 144; 95% CI 117–177) and 46 volunteers (21%) had positive Ad35 titers (GMT 115; 95% CI 67–199) at baseline (Figure 5, ST6). At 4 weeks after first vaccination, Ad26 NAb titers were detected in 100% and 64% of samples to Ad26 and Ad35, respectively (p<0.001). At 4 weeks post second vaccination there were no statistically significant differences observed in the proportions with detectable titers, with 90% or more being positive in all active vaccine groups/regimens (Figure 5). After a single vaccination, the magnitude of Ad26 NAb titers was higher than Ad35 (GMT 2270 and 184, respectively; p<0.001). After the second vaccination in African volunteers, two Ad26.EnvA vaccinations induced a higher Ad26 NAb titer (GMT=3815) than a single Ad26 vaccination whether it was the first (GMT=1320) or second (GMT=1210) vaccination, (p=0.003; Kruskal-Wallis). Four weeks after first Ad35-Env vaccination Ad35 NAb titers were detected in 46% of Ad35 naïve volunteers (GMT = 181) (Figure 5). Four weeks post second vaccination the highest proportion of positive Ad35 NAb titers was in the homologous Ad35 group (71% after second vaccination), which was significantly greater (p=0.01) than the proportion in the groups with homologous Ad26 or heterologous regimens (the difference between these 3 groups is not statistically significant).
Figure 5. Ad26 and Ad35 neutralizing titers.
Individual Ad26 and Ad35 neutralizing antibody responses from volunteers at baseline and 4 weeks post first and second vaccination are shown. Second vaccinations were at month 6 in Groups A and B, and month 3 otherwise. White/orange boxes represent negative/positive baseline Ad26 (top panel) and Ad35 (bottom panel) neutralizing antibodies. Boxes represent median, IQR and the 5th + 95th percentiles of the titer distribution. On the x-axis, B, 1 & 2 represent baseline and 4 weeks post first and second vaccinations respectively. Placebo responses represent the proportion of volunteers with a positive titer at any time.
Conclusion/Discussion
This is the first heterologous adenovirus vectored HIV-1 vaccine study conducted in the US and sub-Saharan Africa. All vaccine regimens were found to be well tolerated and to elicit both humoral and cellular immune responses. Both heterologous and homologous regimens significantly increased humoral Env responses. Presence of baseline anti-vector (Ad26 or Ad35) NAbs did not significantly impact the anti-Env immune responses elicited. Safety was similar across the three geographic regions studied.
The 5×1010 vp dose of both the Ad26.EnvA and Ad35-Env vectored vaccines given individually and in combination was generally well tolerated, with a slight increase in reactions reported in the vaccine groups compared to placebo but with no evidence for serious adverse events, increased AEs or laboratory abnormalities related to vaccine. Severe reactions were observed in eight volunteers and all occurred in volunteers who were seronegative at baseline for the respective adenovirus vaccine vector serotype and was brief and self-limited. These findings are consistent with prior adenovirus based vaccines where some severe but transient systemic reactions have been seen, primarily at doses of 1011 vp(3,10,12,15,17).
The 3-month regimen appears to have similar humoral and cellular immunogenicity as the 6-month regimen. The potential to utilize a shorter vaccination schedule could have substantial benefits clinically and in vaccine development. Thus, further evaluation of a shorter vaccination schedule should be studied, including evaluating the durability of immune responses.
Both homologous and heterologous vector boosting of humoral immune responses was observed (~20 fold) which was not significantly impacted by presence of baseline homologous serotype adenoviral vector immunity. Interestingly though, increased Env antibody titers were elicited in the Ad26-Ad35 compared with Ad35-Ad26 heterologous regimens. This impact of vector order on antibody responses is similar to what has been observed in pre-clinical models and raises important considerations in defining the optimal regimen for candidate vaccines in human trials.
T cell response magnitudes by ELISpot and flow cytometry were modest with some differences noted across regimens and dependent on whether matched or mismatched peptides were used. This was most apparent for the Ad35 homologous groups where both higher ELISpot magnitude and response rates were found. This can partly be explained because Ad26.EnvA in a homologous DNA prime-boost regimen has been shown to induce T-cell responses to an average of only one Env epitope per volunteer, whereas an average of two Env epitopes per volunteer were recognized after Ad35-Env homologous boosting. Epitope mapping from samples would be required to confirm whether there is boosting of responses or whether new epitopes are recognized after the second vaccination in the different homologous and heterologous regimens. It is possible that the differences between the two EnvA inserts used in the heterologous vaccine regimens amounted to two primes rather than a prime-boost. Lower ELISpot responses were observed in East African volunteers, which requires further study, and highlights the importance of assessing vaccine candidates in target populations.
Both vectored vaccines consistently elicited EnvA humoral and cellular immune responses after a single vaccination, which was not significantly impaired by preexisting anti-vector NAbs. In addition, a homologous boost significantly increased humoral responses despite eliciting significant vector specific NAbs after the first vaccination. The Ad26 vectored vaccine more often elicited detectable ELISA responses in both Ad26 baseline seropositive and seronegative volunteers than the Ad35 vectored vaccine. The lack of detectable impact of baseline Ad26 and Ad35 NAbs on the elicitation of EnvA responses is different than what has been reported for Ad5 vectored vaccines and the reasons for this difference are unclear; it may be due to the biologic differences between these adenovirus serotypes or the lower NAb titers to these less common adenovirus serotypes (Ad26/Ad35). Taken together, neither preexisting nor vaccine elicited anti-vector immune response appeared to blunt elicitation of insert-specific responses.
These data demonstrate that humoral HIV-1 responses following an adenovirus-based vaccine can be boosted with a homologous (vector and insert) or heterologous (vector and insert) adenovirus vaccine; pre-existing vector immunity does not appear to impact safety or immunogenicity; and vaccine order can significantly impact immune responses. T cell response rates and magnitudes by ELISpot and flow cytometry were modest and similar across regimens, with some differences depending on whether matched or mis-matched Env peptides were used and by region. Both heterologous and homologous adenovirus vaccine regimens are promising vaccination strategies.
Supplementary Material
Acknowledgments
Thanks to all the dedicated volunteers across the trial sites, the staff at the clinical research centers and immunology support laboratories. Thanks to EMMES and SCHARP for data analysis and clinical trial database support. Thanks to the United States Agency for International Development, to the National Institutes of Health and to the Ragon Institute of MGH for their support. Special thanks to Nicholas Mangeya and Llewellyn Fleurs from the Desmund Tutu HIV Centre Institute of Infectious Disease and Molecular Medicine Faculty of Health Sciences (South Africa) for clinical support and from IAVI to Michele Fong-Lim and Donna Cordasco for quality assurance support and Claudia Schmidt and Kristen Syvertsen for clinical trial support. Thanks to Lorna Clark, Laura Sharp and technical staff at IAVI-Human Immunology Laboratory and Kaitlin Smith, Chelsea Bleckwehl, Anna McNally, Ann Cheung, and Mark Justin Iampietro from the BIDMC Laboratory.
FUNDING
Funding for this study was provided by the Ragon Institute of MGH, MIT, and Harvard; National Institutes of Health grants AI066305, (D.H.B.); UM1AI068618, UMAI068635, UMAI068614; and the International AIDS Vaccine Initiative (IAVI). IAVI’s work is made possible by generous support from many donors including: the Bill & Melinda Gates Foundation; the Ministry of Foreign Affairs of Denmark; Irish Aid; the Ministry of Finance of Japan in partnership with the World Bank; the Ministry of Foreign Affairs of the Netherlands; the Norwegian Agency for Development Cooperation (NORAD); the United Kingdom Department for International Development (DFID), and the United States Agency for International Development (USAID). The full list of IAVI donors is available at www.iavi.org. This study is made possible by the generous support of the American people through USAID. The contents are the responsibility of the IAVI and do not necessarily reflect the views of USAID or the United States Government.
Funding and Potential Conflicts of Interest
The work was funded by the International AIDS Vaccine Initiative (IAVI), NIAID/NIH, and the Ragon Institute of MGH, MIT, and Harvard in collaboration with Crucell Holland BV (now JNJ). Patents for the Ad26 vector are held in part by Crucell (JNJ) and BIDMC; and Ad35 vector by IAVI.
References
- 1.Buchbinder SP, Mehrotra DV, Duerr A, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flynn NM, Forthal DN, Harro CD, Judson FN, Mayer KH, Para MF. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis. 2005;191:654–665. doi: 10.1086/428404. [DOI] [PubMed] [Google Scholar]
- 3.Hammer SM, Sobieszczyk ME, Janes H, et al. Efficacy Trial of a DNA/rAd5 HIV-1 Preventive Vaccine. New Engl J Med. 2013 doi: 10.1056/NEJMoa1310566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McElrath MJ, De Rosa SC, Moodie Z, et al. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet. 2008;372:1894–1905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 6.Baden LR, Blattner WA, Morgan C, et al. Timing of plasmid cytokine (IL-2/Ig) administration affects HIV-1 vaccine immunogenicity in HIV-seronegative. J Infect Dis. 2011;204:1541–1549. doi: 10.1093/infdis/jir615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bart PA, Goodall R, Barber T, et al. EV01: a phase I trial in healthy HIV negative volunteers to evaluate a clade C HIV vaccine, NYVAC-C undertaken by the EuroVacc Consortium. Vaccine. 2008;26:3153–3161. doi: 10.1016/j.vaccine.2008.03.083. [DOI] [PubMed] [Google Scholar]
- 8.McCormack S, Stohr W, Barber T, et al. EV02: a Phase I trial to compare the safety and immunogenicity of HIV DNA-C prime-NYVAC-C boost to NYVAC-C alone. Vaccine. 2008;26:3162–3174. doi: 10.1016/j.vaccine.2008.02.072. [DOI] [PubMed] [Google Scholar]
- 9.Nicholson O, Dicandilo F, Kublin J, et al. Safety and Immunogenicity of the MRKAd5 gag HIV Type-1 Vaccine in a Worldwide Phase 1 Study of Healthy Adults. AIDS Res Hum Retroviruses. 2010 doi: 10.1089/aid.2010.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Catanzaro AT, Koup RA, Roederer M, et al. Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 candidate vaccine delivered by a replication-defective recombinant adenovirus vector. J Infect Dis. 2006;194:1638–1649. doi: 10.1086/509258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harro C, Sun X, Stek JE, et al. Safety and immunogenicity of the Merck adenovirus serotype 5 (MRKAd5) and MRKAd6 human immunodeficiency virus type 1 trigene vaccines alone and in combination in healthy adults. Clinical and vaccine immunology : CVI. 2009;16:1285–1292. doi: 10.1128/CVI.00144-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Priddy FH, Brown D, Kublin J, et al. Safety and immunogenicity of a replication-incompetent adenovirus type 5 HIV-1 clade B gag/pol/nef vaccine in healthy adults. Clin Infect Dis. 2008;46:1769–1781. doi: 10.1086/587993. [DOI] [PubMed] [Google Scholar]
- 13.Barouch DH, Kik SV, Weverling GJ, et al. International seroepidemiology of adenovirus serotypes 5, 26, 35, and 48 in pediatric and adult populations. Vaccine. 2011;29:5203–5209. doi: 10.1016/j.vaccine.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mast TC, Kierstead L, Gupta SB, et al. International epidemiology of human pre-existing adenovirus (Ad) type-5, type-6, type-26 and type-36 neutralizing antibodies: correlates of high Ad5 titers and implications for potential HIV vaccine trials. Vaccine. 2010;28:950–957. doi: 10.1016/j.vaccine.2009.10.145. [DOI] [PubMed] [Google Scholar]
- 15.Baden LR, Walsh SR, Seaman MS, et al. First-in-human evaluation of the safety and immunogenicity of a recombinant adenovirus serotype 26 HIV-1 Env vaccine (IPCAVD 001) J Infect Dis. 2013;207:240–247. doi: 10.1093/infdis/jis670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barouch DH, Liu J, Peter L, et al. Characterization of humoral and cellular immune responses elicited by a recombinant adenovirus serotype 26 HIV-1 Env vaccine in healthy adults (IPCAVD 001) J Infect Dis. 2013;207:248–256. doi: 10.1093/infdis/jis671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keefer MC, Gilmour J, Hayes P, et al. A phase I double blind, placebo-controlled, randomized study of a multigenic HIV-1 adenovirus subtype 35 vector vaccine in healthy uninfected adults. PloS One. 2012;7:e41936. doi: 10.1371/journal.pone.0041936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geisbert TW, Bailey M, Hensley L, et al. Recombinant adenovirus serotype 26 (Ad26) and Ad35 vaccine vectors bypass immunity to Ad5 and protect nonhuman primates against ebolavirus challenge. J Virol. 2011;85:4222–4233. doi: 10.1128/JVI.02407-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Radosevic K, Rodriguez A, Lemckert AA, et al. The Th1 immune response to Plasmodium falciparum circumsporozoite protein is boosted by adenovirus vectors 35 and 26 with a homologous insert. Clinical and vaccine immunology: CVI. 2010;17:1687–1694. doi: 10.1128/CVI.00311-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ouedraogo A, Tiono AB, Kargougou D, et al. A phase 1b randomized, controlled, double-blinded dosage-escalation trial to evaluate the safety, reactogenicity and immunogenicity of an adenovirus type 35 based circumsporozoite malaria vaccine in burkinabe healthy adults 18 to 45 years of age. PloS One. 2013;8:e78679. doi: 10.1371/journal.pone.0078679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radosevic K, Wieland CW, Rodriguez A, et al. Protective immune responses to a recombinant adenovirus type 35 tuberculosis vaccine in two mouse strains: CD4 and CD8 T-cell epitope mapping and role of gamma interferon. Infect Immun. 2007;75:4105–4115. doi: 10.1128/IAI.00004-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Creech CB, Dekker CL, Ho D, et al. Randomized, placebo-controlled trial to assess the safety and immunogenicity of an adenovirus type 35-based circumsporozoite malaria vaccine in healthy adults. Human vaccines & immunotherapeutics. 2013;9 doi: 10.4161/hv.26038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarzotti-Kelsoe M, Cox J, Cleland N, et al. Evaluation and recommendations on good clinical laboratory practice guidelines for phase I-III clinical trials. PLoS Medicine. 2009;6:e1000067. doi: 10.1371/journal.pmed.1000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horton H, Thomas EP, Stucky JA, et al. Optimization and validation of an 8-color intracellular cytokine staining (ICS) assay to quantify antigen-specific T cells induced by vaccination. J Immunol Methods. 2007;323:39–54. doi: 10.1016/j.jim.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sprangers MC, Lakhai W, Koudstaal W, et al. Quantifying adenovirus-neutralizing antibodies by luciferase transgene detection: addressing preexisting immunity to vaccine and gene therapy vectors. J Clin Microbiol. 2003;41:5046–5052. doi: 10.1128/JCM.41.11.5046-5052.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.