Abstract
The yellow peach moth, Conogethes punctiferalis, is an extremely important polyphagous insect in Asia. The chemosensory systems of moth play an important role in detecting food, oviposition sites and mate attraction. Several antennal chemosensory receptors are involved in odor detection. Our study aims to identify chemosensory receptor genes for potential applications in behavioral responses of yellow peach moth. By transcriptomic analysis of male and female antennae, 83 candidate chemosensory receptors, including 62 odorant receptors, 11 ionotropic receptors and 10 gustatory receptors were identified. Through Blast and sequence alignment, the highly conserved co-receptor Orco was annotated, eight unigenes clustered into pheromone receptors, and two clustered as sugar receptor. Among the IRs, one unigenes was similar with co-receptors IR25a. Expression levels of 50 odorant receptors were further evaluated by quantitative real-time PCR in antennae. All the ORs tested were detected in antennae and some of which were associated with sex-biased expression. The chemosensory receptors identified in C. punctiferalis provide a foundational resource for further analysis on olfaction for behavior. The expression profiles of ORs in antennae indicated variant functions in olfactory recognition, and our results provided the possibility for the potential application of semiochemical to control this pest moth.
Insect olfactory system enables insects to detect and discriminate different odor molecules from their environment. The odorant and pheromone perception in insects are a complex series of process that transfer external stimulus from the environment to behavioral response. Diverse proteins consisting in this signal transduction pathway include odorant binding proteins (OBPs), chemosensory proteins (CSPs), chemosensory receptor (CRs), odorant degrading enzymes (ODEs) and sensory neuron membrane proteins (SNMPs)1,2,3,4,5,6,7.
The gene family encoding CRs in insects mainly divides into three groups: olfactory receptors (ORs), gustatory receptors (GRs) and ionotropic receptors (IRs)8,9,10,11,12. The insect ORs and the GRs were firstly identified in the genomic analysis of Drosophila melanogaster13. Previous studies suggested that the OR and GR families belong to a superfamily based on the transmembrane domain13,14. Both ORs and GRs are seven transmembrane-domain receptors with an inverted transmembrane topology in contrasts to the OR of mammalian14,15,16. ORs are housed targeted in the dendritic membrane of olfactory sensory neurons (OSNs). Most OSNs express a single OR gene, along with odorant receptor co-receptor (Orco)17. Insect OR genes are highly diverse, and their numbers among species are various13,18,19,20,21,22. ORs have been shown to form a voltage-gated ion channel following heterodimerization with Orco, which is an ortholog of the D. melanogaster OR, DmOr83b23,24. ORs in Lepidopteran pheromone system are classified into two major groups, pheromone receptors (PRs) and general odorant receptors according to perception of chemicals25. PRs are thought to be associated with olfactory signal transduction of pheromone compounds in peripheral olfactory reception, and general odorant receptors are considered to detect plant volatiles. PRs were reported in several species20,26,27,28. Also pheromone-pheromone receptor relationships also have been identified. In Bombyx mori, general odorant receptor BmOR19 responds to linalool, furthermore, BmOR45 and BmOR47 respond to benzoic acid, 2-phenylethanol, and benzaldehyde respectively29. While PRs (BmOR1 and BmOR3) respond specifically to bombykol (E10Z12-16: OH) and bombykal (E10Z12-16: Ald) respectively, which are major and minor sex pheromone components in B. mori.30,31. Although we classified receptors on the basis of their responses to chemicals, their functions are still unknown. PRs are more conserved compared with other ORs32,33. Orco is the only highly conserved OR protein among divergent insect species, which suggests the crucial role in chemical recognition34,35. The GRs are generally expressed in gustatory receptor neurons (GRNs) within gustatory organs. Differently, several GRs are usually expressed in one neuron. Most GRs sense non-volatile compounds, including sugar and bitter compounds36. Interestingly, Some GRs expressed in antennae cannot identify volatile odorants, but respond to carbon dioxide37. IRs belongs to a variant subfamily of ionotropic glutamate receptors (iGluRs), involving in detection of chemical signals38,39. Based on the expression location, two subfamily of IRs are identified: conserved antennal IRs and divergent IRs, indicating the function of IRs in olfactory and taste39. In antennal IRs, IR8a and IR25a appear to act as co-receptors and form heteromeric complexes with odor- specific receptors40,41. In contrast with ORs, many IR-expressing neurons expressed two or three IR genes17.
The yellow peach moth Conogethes punctiferalis (Guenée) (Lepidoptera: Crambidae), is a serious polyphagous insect of crop (sunflower, maize, sorghum, cotton, etc.) and fruit (peach, plum, durian, etc.). It’s hard to control by traditional method, due to larvae bored the inside fruit or stem42,43. Few olfactory receptor genes have been reported in this moth44, however more receptor genes investigated allows a better understanding of the molecular basis of olfaction. Identification of the chemosensory genes can lead to comprehensive understanding of how to recognize and locate host for this moth. In the present study, the antennal transcriptome of C. punctiferalis were sequenced on the Illumina HiSeq 4000 platform. In total 83 chemoreceptor genes including 62 ORs, 11 IRs and 10 GRs were identified. The expression levels of ORs, which may play important roles in chemoreception of C. punctiferalis were analyzed by using of quantitative real-time PCR (qRT-PCR). The results assist with the identification of genes involved in C. punctiferalis olfactory, help better understand the gene expression in sex, and provide the molecular basis for further study of pheromone and host volatile recognition.
Results
Illumina sequencing
To identify the chemosensory protein genes from C. punctiferalis, the cDNA from male and female antennae were sequenced using the Illumina HiSeqTM 4000 sequencing platform. A total of 59,375,582 and 57,353,054 raw reads were obtained from female and male antennae, respectively. After removing adaptor sequences, low quality sequences and containing N sequences, 58,053,354 and 55,592280 clean reads were generated from the antennae of female and male raw data, respectively. After assembled, the two datasets result in 112,933 contigs with a mean length of 902 bp and an N50 of 1750 bp. From these datasets, 76,486 unigenes were obtained with a mean length of 727 bp and an N50 of 1499 bp (Table 1). The raw reads of the C. punctiferalis are available from the SRA database (accession number: (SRX 1604701 and SRX 1604705).
Table 1. Summary of assembled contigs and unigenes.
| Contigs | Unigenes | |
|---|---|---|
| Total length (bp) | 101,865,459 | 55,582,691 |
| Minimum length (bp) | 201 | 201 |
| Mean length (bp) | 902 | 727 |
| Median length (bp) | 411 | 320 |
| Maximum length (bp) | 29,150 | 29,150 |
| N50 (bp) | 1,782 | 1,499 |
| N90 (bp) | 306 | 254 |
Functional annotation of assembled unigenes
The proportion of unigenes annotated in at least one database of Nr, Nt, KO, SwissProt, PFAM, GO, KOG reach to 40%. Only 4.75% unigenes were annotated in all databases (Table 2). Using BlastX database, 26,114 (34.14%) unigenes were matched to known proteins. For species distribution, the results showed 33.43%, 24.85%, and 1.91% of protein similar with the sequences from B. mori, Danaus plexippus and Papilio xuthus, respectively (Fig. 1). GO annotation was used to classify the function of transcripts according to the GO terms (Fig. 2A). In the biological process terms, cellular, metabolic and single-organism were the highest classified. In the cellular component terms, cell, cell part and organelle were the most abundant. In the molecular function category, the genes expressed in the antennae were mostly related to binding, catalytic activity and transporter activity. KOG annotation was based on the relationship between orthologous genes from different species (Fig. 2B). Using KOG functional annotation, “General function prediction only”, “Signal transduction”, and “Posttranslational modification, protein turnover, chaperones” were three largest groups assigned to. For KEGG annotation, 10,298 unigenes were classified into five groups, Cellular processes, environmental information processing, genetic information processing, metabolism and organismal systems (Fig. 2C). Transport and catabolism, signal transduction, translation, carbohydrate metabolism, endocrine systems were the main pathways in each group, respectively.
Table 2. Summary of annotations of unigenes.
| Number of Unigenes | Percentage (%) | |
|---|---|---|
| Annotated in NR | 26,114 | 34.14 |
| Annotated in NT | 11,354 | 14.84 |
| Annotated in KO | 10,298 | 13.46 |
| Annotated in SwissProt | 18,229 | 23.83 |
| Annotated in PFAM | 18,142 | 23.71 |
| Annotated in GO | 18,434 | 24.1 |
| Annotated in KOG | 11,475 | 15 |
| Annotated in all Databases | 3,640 | 4.75 |
| Annotated in at least one Database | 30,596 | 40 |
| Total Unigenes | 76,486 | 100 |
Figure 1. Best hits of the BLASTx results.

Figure 2.
(A) Gene Ontology (GO) analysis for the transcriptomic sequences. (B) euKaryotic Ortholog Groups (KOG) annotation for the unigenes.(C) KEGG pathway annotation of the transcriptome. (A) Cellular processes. (B) Environmental information processing. (C) Genetic information processing. (D) Metabolism. (E) Organismal systems.
Putative chemoreceptor genes
All the unigenes were searched by BLAST to identified chemoreceptor genes. Total 62 OR genes, 11 IR genes and 10 GR genes were identified through bioinformatics analysis. All the chemosensory receptor genes of C. punctiferalis were submitted to the GenBank (accession numbers: KX084452-KX084521 and KX096203-KX096215). Of these genes, 45 unigenes (39 ORs and 6 IRs) showed sequence identity to the previously identified in C. punctiferalis which are already submitted in GenBank. Among the identified ORs, 38 were found to represent full-length open reading frames (ORF) with 4–7 transmembrane domains (Table 3). Half of those sequences were annotated against homologs of Ostrinia furnacalis. As expected, Orco/OR2 was identified with high identity compared with the conserved insect OR2/Orco. The IRs and GRs sequences in C. punctiferalis antennal transcriptome were identified according to the similarity with known insect IRs and GRs. Seven of eleven putative IRs were similar with IRs of O. furnacalis. Six unigenes with full-length ORF, contained 3~4 transmembrane domains. Of GR genes, 3 putative GR genes contained full-length ORF with 6 transmembrane regions, while 7 sequences were partial (Table 4).
Table 3. Odorant receptors in Conogethes punctiferalis antennae.
| contigs | Gene name | Accession number | residues | Full length | Top blastx hit | Score | E-value | % ID | TM | FPKM |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| Female | Male | ||||||||||
| c45607_g1 | OR1 | KX084452 | 449 | Yes | BAG71417.1 | olfactory receptor-1 [Diaphania indica] | 739 | 0.00E + 00 | 81% | 4 | 0.68 | 9.61 |
| c50176_g1 | OR2 /Orco | KX084453 | 474 | Yes | AGF29886.1 | odorant co-receptor [Conogethes punctiferalis] | 951 | 0.00E + 00 | 99% | 7 | 534.09 | 523.08 |
| c45303_g1 | OR3 | KX084454 | 329 | No | AFK30395.1 | odorant receptor 3 [Ostrinia furnacalis] | 280 | 3.00E−87 | 42% | 4 | 2.33 | 283.65 |
| c35425_g1 | OR4 | KX084455 | 418 | No | BAJ22891.1 | odorant receptor [Ostrinia furnacalis] | 283 | 5.00E−87 | 39% | 3 | 11.5 | 0 |
| c43578_g2 | OR5 | KX084456 | 421 | Yes | NP_001296037.1 | odorant receptor 13a-like [Plutella xylostella] | 318 | 1.00E−100 | 45% | 4 | 3.86 | 91.95 |
| c43248_g1 | OR6 | KX084457 | 419 | No | AFK30403.1 | odorant receptor 6 [Ostrinia furnacalis] | 316 | 1.00E−99 | 43% | 4 | 0.17 | 170.9 |
| c42117_g1 | OR7 | KX084458 | 378 | Yes | AIZ94614.1 | putative pheromone receptor OR6 [Ostrinia nubilalis] | 175 | 2.00E−47 | 41% | 7 | 0.08 | 24.79 |
| c40235_g2 | OR8 | KX084459 | 428 | Yes | BAG71424.1 | olfactory receptor [Diaphania indica] | 449 | 1.00E−151 | 50% | 6 | 43.43 | 9.77 |
| c39383_g1 | OR9 | KX084460 | 426 | Yes | AFC91723.1 | putative odorant receptor OR15 [Cydia pomonella] | 211 | 3.00E−61 | 45% | 6 | 5.81 | 1.27 |
| c50460_g1 | OR10 | KX084461 | 394 | Yes | BAR43452.1 | putative olfactory receptor 10 [Ostrinia furnacalis] | 475 | 1.00E−162 | 59% | 6 | 5.04 | 2.8 |
| c40543_g1 | OR11 | KX084462 | 389 | Yes | BAR43453.1 | putative olfactory receptor 11 [Ostrinia furnacalis] | 493 | 4.00E−170 | 68% | 6 | 5.17 | 4.22 |
| c44553_g2 | OR12 | KX084463 | 353 | No | BAR43454.1 | putative olfactory receptor 12 [Ostrinia furnacalis] | 505 | 3.00E−175 | 65% | 4 | 31.85 | 12.76 |
| c46066_g1 | OR13 | KX084464 | 402 | Yes | BAR43455.1 | putative olfactory receptor 13 [Ostrinia furnacalis] | 645 | 0.00E + 00 | 81% | 5 | 32.09 | 14.17 |
| c46413_g1 | OR14 | KX084465 | 416 | Yes | BAR43456.1 | putative olfactory receptor 14 [Ostrinia furnacalis] | 546 | 0.00E + 00 | 64% | 6 | 19.59 | 8.43 |
| c50375_g1 | OR15 | KX084466 | 388 | Yes | NP_001166603.1 | olfactory receptor 13 [Bombyx mori] | 442 | 3.00E−150 | 61% | 6 | 22.17 | 9.13 |
| c46668_g4 | OR16 | KX084467 | 420 | Yes | BAR43458.1 | putative olfactory receptor 16 [Ostrinia furnacalis] | 523 | 1.00E−180 | 60% | 5 | 10.49 | 4.84 |
| c47537_g1 | OR17 | KX084468 | 418 | No | EHJ78030.1 | olfactory receptor 29 [Danaus plexippus] | 574 | 0.00E + 00 | 73% | 0 | 2.79 | 2.99 |
| c45438_g1 | OR18 | KX084469 | 424 | No | BAR43460.1 | putative olfactory receptor 18 [Ostrinia furnacalis] | 593 | 0.00E + 00 | 74% | 6 | 5.9 | 3.44 |
| c45263_g1 | OR19 | KX084470 | 415 | Yes | BAR43461.1 | putative olfactory receptor 19 [Ostrinia furnacalis] | 290 | 6.00E−90 | 40% | 7 | 13.51 | 7.2 |
| c42557_g3 | OR20 | KX084471 | 399 | Yes | BAR43462.1 | putative olfactory receptor 20 [Ostrinia furnacalis] | 437 | 1.00E−147 | 59% | 5 | 24.84 | 8.24 |
| c42436_g1 | OR21 | KX084472 | 395 | Yes | AGK90020.1 | olfactory receptor 17 [Helicoverpa assulta] | 461 | 2.00E−157 | 64% | 5 | 25.06 | 19.27 |
| c14092_g1 | OR22 | KX096208 | 150 | No | XP_013191807.1 | PREDICTED: gustatory and odorant receptor 22 [Amyelois transitella] | 305 | 2.00E−99 | 94% | 3 | 0.91 | 0.22 |
| c45775_g1 | OR23 | KX084473 | 401 | Yes | BAR43467.1 | putative olfactory receptor 25 [Ostrinia furnacalis] | 423 | 5.00E−142 | 52% | 6 | 14.52 | 7.73 |
| c46864_g1 | OR24 | KX084474 | 450 | Yes | BAR43466.1 | putative olfactory receptor 24 [Ostrinia furnacalis] | 627 | 0.00E + 00 | 80% | 6 | 16.34 | 14.37 |
| c49436_g1 | OR25 | KX084475 | 432 | Yes | BAR43467.1 | putative olfactory receptor 25 [Ostrinia furnacalis] | 671 | 0.00E + 00 | 78% | 6 | 10.79 | 10.78 |
| c44068_g1 | OR26 | KX084476 | 396 | Yes | NP_001166611.1 | olfactory receptor 59 [Bombyx mori] | 363 | 1.00E−118 | 47% | 5 | 50.52 | 26.05 |
| c42831_g1 | OR27 | KX084477 | 401 | Yes | BAR43469.1 | putative olfactory receptor 27 [Ostrinia furnacalis] | 647 | 0.00E + 00 | 86% | 6 | 16.3 | 6.65 |
| c43774_g1 | OR28 | KX084478 | 326 | No | BAR43470.1 | putative olfactory receptor 28 [Ostrinia furnacalis] | 309 | 2.00E−98 | 47% | 6 | 22.87 | 14.79 |
| c38000_g1 | OR29 | KX084479 | 413 | Yes | AJF23799.1 | olfactory receptor OR32 [Planotortrix octo] | 365 | 8.00E−119 | 48% | 5 | 7.71 | 2.3 |
| c47502_g1 | OR30 | KX084480 | 458 | Yes | AIG51850.1 | odorant receptor [Helicoverpa armigera] | 177 | 5.00E−50 | 72% | 6 | 21.24 | 0.9 |
| c45926_g1 | OR31 | KX084481 | 388 | Yes | CUQ99414.1 | Olfactory receptor 34 [Manduca sexta] | 399 | 4.00E−133 | 55% | 7 | 12.74 | 7.83 |
| c45783_g1 | OR32 | KX084482 | 390 | Yes | AII01045.1 | odorant receptor [Dendrolimus houi] | 354 | 2.00E−115 | 44% | 6 | 4.86 | 1.1 |
| c46810_g1 | OR33 | KX084483 | 404 | No | BAR43475.1 | putative olfactory receptor 33 [Ostrinia furnacalis] | 646 | 0.00E + 00 | 81% | 7 | 4.97 | 1.95 |
| c47522_g3 | OR34 | KX084484 | 306 | No | BAR43476.1 | putative olfactory receptor 34 [Ostrinia furnacalis] | 320 | 6.00E−103 | 56% | 4 | 0.69 | 14.2 |
| c41738_g1 | OR35 | KX084485 | 430 | Yes | BAR43477.1 | putative olfactory receptor 35 [Ostrinia furnacalis] | 809 | 0.00E + 00 | 89% | 6 | 3.52 | 1.89 |
| c42979_g1 | OR36 | KX084486 | 328 | No | BAR43478.1 | putative olfactory receptor 36 [Ostrinia furnacalis] | 370 | 1.00E−122 | 60% | 6 | 13.92 | 5.12 |
| c49487_g1 | OR37 | KX084487 | 389 | Yes | BAR43479.1 | putative olfactory receptor 37 [Ostrinia furnacalis] | 579 | 0.00E + 00 | 77% | 6 | 11.94 | 5.43 |
| c50585_g1 | OR38 | KX084488 | 398 | Yes | BAR43480.1 | putative olfactory receptor 38 [Ostrinia furnacalis] | 411 | 2.00E−137 | 49% | 6 | 10.58 | 7.68 |
| c42903_g1 | OR39 | KX084489 | 405 | Yes | BAR43481.1 | putative olfactory receptor 39 [Ostrinia furnacalis] | 249 | 2.00E−74 | 35% | 5 | 13.98 | 0 |
| c46193_g1 | OR40 | KX084490 | 414 | No | BAR43461.1 | putative olfactory receptor 19 [Ostrinia furnacalis] | 296 | 6.00E−92 | 44% | 5 | 20.14 | 6.25 |
| c45009_g1 | OR41 | KX084491 | 356 | Yes | BAR43483.1 | putative olfactory receptor 41 [Ostrinia furnacalis] | 429 | 5.00E−145 | 61% | 6 | 4.75 | 1.93 |
| c40483_g1 | OR42 | KX084492 | 370 | No | BAR43484.1 | putative olfactory receptor 42 [Ostrinia furnacalis] | 452 | 6.00E−155 | 61% | 6 | 4.26 | 1.55 |
| c44512_g1 | OR43 | KX084493 | 326 | No | NP_001091818.1 | olfactory receptor 42 [Bombyx mori] | 305 | 4.00E−97 | 54% | 6 | 10.81 | 6.51 |
| c45800_g1 | OR44 | KX084494 | 433 | Yes | BAR43486.1 | putative olfactory receptor 44 [Ostrinia furnacalis] | 555 | 0.00E + 00 | 69% | 5 | 28.41 | 14.73 |
| c44781_g1 | OR45 | KX084495 | 401 | Yes | NP_001157210.1 | olfactory receptor 17 [Bombyx mori] | 333 | 5.00E−107 | 41% | 7 | 13.46 | 8.28 |
| c45498_g3 | OR46 | KX084496 | 215 | No | BAR43488.1 | putative olfactory receptor 46 [Ostrinia furnacalis] | 377 | 6.00E−127 | 83% | 2 | 9.53 | 5.48 |
| c39174_g1 | OR47 | KX084497 | 203 | No | XP_013195066.1 | PREDICTED: putative odorant receptor 92a [Amyelois transitella] | 272 | 2.00E−86 | 63% | 2 | 1.94 | 1.09 |
| c28776_g1 | OR48 | KX084498 | 277 | No | BAR43481.1 | putative olfactory receptor 39 [Ostrinia furnacalis] | 199 | 3.00E−57 | 39% | 4 | 1.45 | 0 |
| c42096_g2 | OR49 | KX084499 | 431 | Yes | BAR43491.1 | putative olfactory receptor 49 [Ostrinia furnacalis] | 523 | 0.00E + 00 | 66% | 6 | 14.61 | 7.74 |
| c43755_g1 | OR50 | KX084500 | 410 | Yes | KOB74670.1 | Odorant receptor 50 [Operophtera brumata] | 477 | 6.00E−163 | 53% | 7 | 6.07 | 1.65 |
| c47178_g1 | OR51 | KX084501 | 330 | No | AII01110.1 | odorant receptor [Dendrolimus kikuchii] | 393 | 2.00E−131 | 53% | 4 | 9.2 | 4.48 |
| c30858_g1 | OR52 | KX084502 | 408 | Yes | BAR43494.1 | putative olfactory receptor 52 [Ostrinia furnacalis] | 491 | 1.00E−168 | 56% | 6 | 7.22 | 1.26 |
| c40944_g1 | OR53 | KX084503 | 402 | Yes | BAR43495.1 | putative olfactory receptor 53 [Ostrinia furnacalis] | 496 | 1.00E−170 | 66% | 5 | 15.15 | 0 |
| c48055_g1 | OR54 | KX084504 | 410 | Yes | AFC91736.1 | putative odorant receptor OR28 [Cydia pomonella] | 457 | 4.00E−155 | 52% | 5 | 14.89 | 5.16 |
| c49083_g1 | OR55 | KX084505 | 415 | Yes | BAR43458.1 | putative olfactory receptor 16 [Ostrinia furnacalis] | 538 | 0.00E + 00 | 63% | 5 | 32.58 | 30.96 |
| c48693_g1 | OR56 | KX084506 | 393 | Yes | BAR43452.1 | putative olfactory receptor 10 [Ostrinia furnacalis] | 425 | 4.00E−143 | 56% | 6 | 20.86 | 8.35 |
| c45498_g2 | OR57 | KX084507 | 215 | No | BAR43488.1|putative olfactory receptor 46 [Ostrinia furnacalis] | 340 | 2.00E−112 | 75% | 3 | 10.91 | 4.52 |
| c16587_g1 | OR58 | KX096203 | 195 | No | BAR43458.1 | putative olfactory receptor 16 [Ostrinia furnacalis] | 234 | 3.00E−71 | 61% | 2 | 0.69 | 0.78 |
| c38597_g3 | OR59 | KX096204 | 125 | No | ACF32962.1 | olfactory receptor 4 [Helicoverpa armigera] | 229 | 1.00E−70 | 87% | 1 | 1.62 | 1.12 |
| c10513_g1 | OR60 | KX096205 | 107 | No | BAR43458.1 | putative olfactory receptor 16 [Ostrinia furnacalis] | 156 | 4.00E−43 | 70% | 2 | 0.4 | 1.46 |
| c84526_g1 | OR61 | KX096206 | 107 | No | AIT69908.1 | olfactory receptor 66 [Ctenopseustis herana] | 122 | 1.00E−30 | 51% | 1 | 1.65 | 0.98 |
| c47522_g1 | OR62 | KX096207 | 135 | No | XP_013165286.1 | PREDICTED: odorant receptor 85b-like [Papilio xuthus] | 102 | 7.00E−23 | 35% | 0 | 0.69 | 14.2 |
Table 4. Ionotropic and gustatory receptors in Conogethes punctiferalis antennae.
| Contigs | Gene name | Accession number | Residues | Full length | Top blastx hit | Score | E-value | % ID | TM |
|---|---|---|---|---|---|---|---|---|---|
| c45400_g1 | IR25a | KX084508 | 937 | No | BAR64798.1|ionotropic receptor [Ostrinia furnacalis] | 1709 | 0.00E + 00 | 97% | 3 |
| c46878_g3 | IR1 | KX084509 | 652 | Yes | BAR64810.1|ionotropic receptor [Ostrinia furnacalis] | 1144 | 0.00E + 00 | 87% | 3 |
| c47407_g1 | IR2 | KX084510 | 657 | Yes | KOB72397.1|Ionotropic receptor [Operophtera brumata] | 662 | 0.00E + 00 | 53% | 4 |
| c43961_g1 | IR3 | KX084511 | 659 | NO | XP_013194002.1|PREDICTED: glutamate receptor ionotropic, NMDA 2A [Amyelois transitella] | 1098 | 0.00E + 00 | 78% | 4 |
| c47528_g1 | IR4 | KX084512 | 548 | Yes | BAR64809.1|ionotropic receptor [Ostrinia furnacalis] | 875 | 0.00E + 00 | 77% | 3 |
| c51268_g2 | IR5 | KX084513 | 488 | No | BAR64796.1|ionotropic receptor [Ostrinia furnacalis] | 756 | 0.00E + 00 | 74% | 0 |
| c45899_g2 | IR6 | KX084514 | 499 | Yes | BAR64797.1|ionotropic receptor [Ostrinia furnacalis] | 781 | 0.00E + 00 | 85% | 4 |
| c46846_g2 | IR7 | KX084515 | 578 | Yes | BAR64800.1|ionotropic receptor [Ostrinia furnacalis] | 805 | 0.00E + 00 | 71% | 3 |
| c49721_g1 | IR8 | KX084516 | 371 | Yes | BAR64803.1|ionotropic receptor [Ostrinia furnacalis] | 451 | 3.00E−150 | 60% | 2 |
| c12165_g1 | IR9 | KX096209 | 162 | No | KPJ10740.1|Glutamate [NMDA] receptor subunit 1 [Papilio machaon] | 258 | 6.00E−82 | 98% | 1 |
| c43148_g1 | IR10 | KX096210 | 133 | No | ADR64688.1|putative chemosensory ionotropic receptor IR1 [Spodoptera littoralis] | 153 | 8.00E−41 | 62% | 1 |
| c37515_g1 | GR1 | KX084517 | 372 | No | NP_001233217.1|gustatory receptor 68 [Bombyx mori] | 194 | 6.00E−54 | 37% | 8 |
| c42471_g1 | GR2 | KX084518 | 333 | Yes | NP_001233217.1|gustatory receptor 68 [Bombyx mori] | 148 | 4.00E−35 | 34% | 6 |
| c38597_g1 | GR3 | KX084519 | 221 | No | AGA04648.1|gustatory receptor [Helicoverpa armigera] | 224 | 2.00E−66 | 74% | 4 |
| c40152_g1 | GR4 | KX084520 | 454 | Yes | AGK90023.1|gustatory receptor 1 [Helicoverpa assulta] | 622 | 0.00E + 00 | 72% | 6 |
| c45098_g1 | GR5 | KX084521 | 427 | Yes | AGK90012.1|gustatory receptor 5 [Helicoverpa armigera] | 444 | 2.00E−149 | 53% | 6 |
| c30207_g1 | GR6 | KX096211 | 156 | No | XP_013189983.1|PREDICTED: gustatory receptor for sugar taste 64f-like [Amyelois transitella] | 214 | 8.00E−64 | 73% | 3 |
| c16511_g1 | GR7 | KX096212 | 170 | No | KOB74472.1|Gustatory receptor 53 [Operophtera brumata] | 230 | 2.00E−70 | 71% | 3 |
| c127_g1 | GR8 | KX096213 | 147 | No | DAA06380.1|TPA: gustatory receptor 17 [Bombyx mori] | 113 | 8.00E−27 | 38% | 3 |
| c21792_g1 | GR9 | KX096214 | 122 | No | XP_013189983.1|PREDICTED: gustatory receptor for sugar taste 64f-like [Amyelois transitella] | 122 | 5.00E−30 | 54% | 3 |
| c15265_g1 | GR10 | KX096215 | 117 | No | XP_013189983.1|PREDICTED: gustatory receptor for sugar taste 64f-like [Amyelois transitella] | 153 | 2.00E−41 | 48% | 3 |
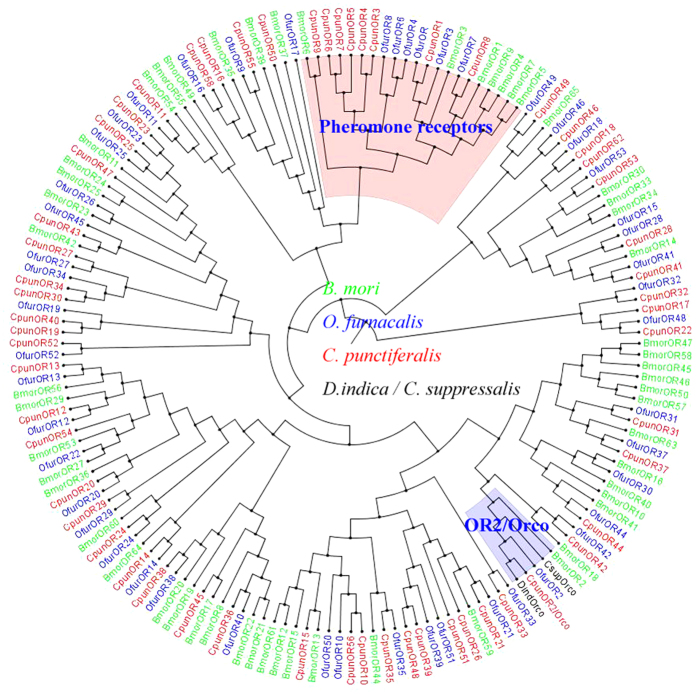
Phylogenetic tree
Phylogenetic tree of ORs was constructed using the sequences of 162 ORs from B. mori, O. furnacalis and C. punctiferalis (Fig. 3). The hits with a length of less than 200 amino acids were ignored. The OR sequences were clustered into PRs, Orco and other divergent ORs. The 8 amino acid sequences, OR1 and OR3-9 clustered into a clade with pheromone receptors of B. mori and O. furnacalis. Orco/OR2 was highly conserved and clustered with Orco/OR2 family. In the neighbor-joining tree of IRs (Fig. 4), the IRs were clustered into ionotropic glutamate receptors (iGluRs), IR25/IR8a and other divergent IRs. Among the nine IRs in C. punctiferalis, CpunIR25a grouped with the highly conserved IR25a/IR8a. Alignment analysis revealed that the similarity of IR25a was higher than 76.3% in D. melanogaster, B. mori, Tribolium castaneum and C. punctiferalis (Fig. 5). CpunIR4, CpunIR6, CpunIR3, CpunIR7 shared identity with IR76b, IR21a, IR68a, IR41a from D. melanogaster, B. mori and T. castaneum, respectively. Unfortunately, we cannot obtain the sequences belong to iGluRs. In phylogenetic tree of the GRs, GRs from B. mori, Manduca sexta and C. punctiferalis were analyzed (Fig. 6), CpunGR5 and CpunGR4 were respectively classified into BmorGr64a and MexGR6 subgroup, which were known as sugar receptors. CpunGR3 was cluster into a clade of fructose receptors.
Figure 3. Phylogenetic relationship of putative olfactory receptors from Conogethes punctiferalis and other insects.
The tree was constructed by MEGA 5.2 program using the neighbor-joining method with the Bootstrapping model by 1000 replication.
Figure 4. Phylogenetic relationship of putative ionotropic receptors from Conogethes punctiferalis and other insects.
The tree was constructed by MEGA 5.2 program using the neighbor-joining method with the Bootstrapping model by 1000 replication.
Figure 5. Amino acid alignment of the putative IR25a with other insects.
Blue shadings indicate the same sequence among insects. Pink shading indicated amino acids which show 75% identity between sequences. Green shading ink shading indicated amino acids which show 50% identity between sequences.
Figure 6. Phylogenetic relationship of putative gustatory receptors from Conogethes punctiferalis and other insect.
The tree was constructed by MEGA 5.2 program using the neighbor-joining method with the Bootstrapping model by 1000 replication.
Expression level of OR genes in female and male antennae
Quantitative real-time PCR (qPCR) was used to analyze the expression levels of 50 OR genes in the antennae of male and female moths. The results indicated that all the tested genes were detected in antennae. Among the OR genes, OR8, OR39, OR48, OR4 and OR30 were highly enriched in female, with 182.6, 93.9, 84.4, 15.6, and 13.5 times to male, respectively. PR subfamily members, OR1, OR3, OR5 and OR7 were highly expressed in male antennae, as well as the odorant receptor OR62 (Fig. 7). Meanwhile, Orco/OR2, OR26, and OR8 had high expression level in female with an FPKM value of 534.09, 50.52, and 43.43, respectively. Orco/OR2, OR3 and OR6 had high expression level in male with an FPKM value of 523.08, 283.65, 170.90, respectively (Table 3). As co-receptor, Orco/OR2 was most abundant in female and male antennae. Because OR4, OR39, OR48 and OR53 were only detected in female antenna, so we cannot obtain the FPKM value (Table 3).
Figure 7. Relative expression levels of putative ORs in the female and male moth antennae.
FA: female antennae; MA: female antennae. The expression levels were estimated using delta delta CT method. Standard error for each sample is represented by error bar.
Discussion
With the rapidly development of next generation sequencing technology, a large number of unigenes were identified. RNA-seq effectively increased the depth of sequencing compared to the previous sequence data from cDNA library46,47,48. From the transcriptome of C. punctiferalis, we identified 83 chemoreceptors including 62 ORs, 11 IRs and 10 GRs. In Crambidae family insect, totally 56 ORs were identified in O. furnacalis20, and in other moths, Cao et al. found 47 ORs and 20 IRs in Chilo suppressalis49, suggested that the olfactory related genes we identified were equivalent to other moths. Total number of identified OR genes varied in different orders or species due to sequencing methods and depth, and/or sample preparations. In Lepidopteran, B. mori, totally 66 ORs were reported in genomic data50. In Dipteran, there were 79 candidate OR genes from Anopheles gambiae and in Hemipteran, 170 OR genes from Apis mellifera were annotated18,21. Especially in Nasonia vitripennis, total number of ORs was up to 30151. However, identified OR genes were only obtained from the antennae, ORs expressed in other tissues might be difficult to identify and the physiological states perhaps influence the amount of ORs in the antennae22. It seemed that more OR genes in Hymenopteran insect, especially the parasitoid wasp, might be due to need more receptors were needed to identify and locate the host.
In Lepidopteran insects, the PRs are more conserved and clustered in a subgroup within the OR family9. In our study, OR1 and OR3-9 of C. punctiferalis were clustered with pheromone receptors of B. mori and O. furnacalis, and we inferred that some or all of them might be putative pheromone receptors. But the expression profiles of these sequences showed that not all of them were male-specific. Koenig considered that at least one of the putative PR should exhibit male-specific expression associated with trichoid sensilla9. Recent studies showed that some PR genes expressed in both sexes. The PR1, 2 and 4 identified from the antennae of C. suppressalis have been detected in both male and female antennae49. From the antennal transcriptome of Spodoptera littoralis, four unigenes were enriched in male antennae, only two of which were annotated as putiative PRs and found to be expressed in antennae of both sexes48. In B. mori, Bmor19, Bmor45 and Bmor47 were also reported as female-specific or highly female-biased receptors, which responded to linalool, benzoic acid and benzaldehyde, suggesting the potential roles in detecting oviposition site and male-produced sex pheromone29. In our study, five unigenes were enriched in the female antennae than in the male, which may have provide important function during the host plant seeking.
IRs are derived from ionotropic glutamate receptors and comprise a novel family of chemosensory receptors. In D. melanogaster, 15 of total 66 IR genes were specifically expressed in antennae38 that was similar with our study that only 11 IRs were identified from the antennae transcriptomes of C. punctiferalis. Phylogenetic analysis showed that IRs from C. punctiferalis were not closely related to the iGluRs. Animal iGluRs are highly conserved family of ligand-gated ion channels39, while IRs are extremely divergent exclusive of IR8a and IR25a38. IR25a and IR8a are most similar primary sequence to iGluRs, suggesting that they are the IR genes most similar to the ancestral IRs9,39,52. In our study, CpunIR25a clustered with the highly conserved IR25a from B. mori, D. melanogaster and T. castenum, while in N. vitripennis and Microplitis mediator, two putative IR25a orthologues were identified22,39. Therefore, the congruent function of IR25a orthologues is still not clear. In Lepidoptera insect, IR8a were identified from the antennal transcriptome of Helicoverpa armigera, H. assulta and C. suppressalis26,49. Surprisingly, IR8a was not found in our antennal transcriptome. We speculated that it maybe need more deeply sequencing and increasing the samples, suggesting that IR8a maybe not expressed in our present samples.
In the previous studies, 56 and 77 candidate GR genes were respectively identified from the Drosophila and Acyrthosiphon pisum genome14,53. In B. mori, 65 GR genes were annotated from the silkworm genome54. However, only 10 GRs were identified from the antennal transcriptome of C. punctiferalis. It might be that GRs have a low expression level in the antennae and mainly expressed in gustatory organs (proboscis, legs, wings and genitalis)55,56. GRs have been divided into four classes: non-fructose sugar, bitter, CO2, and fructose receptors9,54. In phylogenetic tree of GRs, the gustatory receptor genes, GR3-5 were clustered into sugar and fructose receptors, suggesting the potential sugar detection function in the antennae of this moth. However, the recent report showed a wide range of non-gustatory sensory functions of GRs57, indicating that GRs may have more divergent functions in insect antennae.
In conclusion, the main purpose of this study was to identify the chemosensory receptor genes involved in the chemoreception. Based on the transcriptomic analysis, 83 CRs were identified from the antennae of C. punctiferalis. Our method was successful in identifying CR genes with low-expressing levels and the results made it possible for further studies on the molecular level and behaviors, providing the possibility to applicate potential target genes for controlling this pest.
Materials and Methods
Insects rearing and antennae collection
C. punctiferalis larvae were collected from the infested sunflower at Langfang Experimental Station of Chinese Academy of Agricultural Sciences, Hebei Province, China. In laboratory, the larvae were raised on fresh corn ear under conditions 27 ± 1 °C, with 70–80% relative humidity (RH) and a photo period of 16:8 h light: dark (L:D). After eclosion, adults were fed with 10% honey solution. The antennae (70 pairs of each sex) from adults within three days after eclosion were separately collected and frozen immediately in liquid nitrogen.
RNA extraction
Total RNAs were separately extracted and purified using Trizol Reagent (Invitrogen, Carlsbad, CA, USA) following the product manuals. The integrity of total RNA was assessed with Agilent 2100 (Agilent Technologies Inc., CA, USA) and the purity was detected on a NanoDrop 2000 spectrophotometer (NanoDrop products, Wilmington, DE, USA). The concentration was accurately measured by Qubit. Illumina sequencing was performed at Novogen Co., Ltd. Beijing, China, The cDNA libraries were sequenced using Illumina HiSeq 4000 system under effective concentration. In order to obtain the high-quality transcriptome sequence data, the raw reads were filtered to eliminate adaptor sequences and low quality reads. Reads with uncertain nucleotides larger than 10% of the fragment sequence were removed. Trinity de novo program with a default k-mer was used to assemble the clean reads45. Redundant sequences were removed to obtain unigenes sequences by means of selecting longest transcript contigs.
Unigenes annotation and classification
To obtain comprehensive information of gene functions, assembled unigenes were searched against Nr (NCBI non-redundant protein sequences), Nt (NCBI nucleotide), Pfam (Protein family), KOG (euKaryotic Ortholog Groups), SwissProt, KEGG (Kyoto Encyclopedia of Genes and Genomes), GO (Gene Ontology) database using BlASTX with an E-value < 10−5. CDS (coding sequence) were predicted in two-step process, the unigenes were firstly aligned using NR and SwissProt protein database to obtain ORF. If the sequences mismatch with the two databases, estscan (version 3.0.3) software was used to predict the ORF to obtain the nucleic acid and amino acid sequence. FPKM (fragments per kilobase pairs per million mapped reads) was used for gene expression analysis.
Identification of chemosensory receptor genes
Homology searches of OR, GR and IR sequence were performed using BLAST (http://blast.ncbi.nlm.nih.gov/blast.cgi), and the ORFs were predicted using ORF finder (http://www.ncbi.nlm.nih.gov/gorf/gorf.html). Transmembrane domain was predicted using TMHMM (http://www.cbs.dtu.dk/services/TMHMM/). Phylogenetic tree was constructed by MEGA 5.2 software using the neighbor-joining method with the Bootstrapping model by 1000 replication. The evolutionary distances were computed by using the Poisson correction method. The phylogenetic tree image was further created by Figtree software.
Quantitative real-time PCR
The expression levels of ORs were examined in male and female antennae using qRT-PCR. Total RNA was extracted as described above. First-strand cDNAs were synthetize followed One-Step gDNA removal and cDNA Synthesis kit (TransGen Biotech, China) with oligo dT-primer following the kit manual. The primers for qPCR were designed using Primer premier5.0 program (Table S1). qRT-PCR were performed on ABI 7500 fast real-time PCR system (Applied Biosysterm, USA) in a reaction volume of 20 μL using SYBR Premix Ex Taq II (Tli RNaseH Plus) master mix (Takara-Bio, Shiga, Japan), according to the manufacturers’ instructions. Two-step program were performed as follows, 95 °C for 30 s, followed by 40 cycles of 95 °C for 3 s and 60 °C for 30 s. For each gene, three biological replications were performed with each biological replication measured in three technique replications. Relative quantification was analyzed using the comparative 2−ΔΔCT method, with the housekeeping genes β-actin (accession number JX119014) as the reference gene.
Additional Information
How to cite this article: Ge, X. et al. Identification of putative chemosensory receptor genes from yellow peach moth Conogethes punctiferalis (Guenée) antennae transcriptome. Sci. Rep. 6, 32636; doi: 10.1038/srep32636 (2016).
Supplementary Material
Acknowledgments
This work was supported by Special Fund for Agro-scientific Research in the Public Interest (201303026) and China Agriculture Research System (CARS-02).
Footnotes
Author Contributions X.G., T.Z. and Z.W. conceived and designed the experimental plan. X.G. preformed the experiments. X.G., T.Z., K.H., S.B. and Z.W. analyzed the sequence data. T.Z. and Z.W. revised the manuscript. All authors read and approved the final manuscript.
References
- Vogt R. G. & Riddiford L. M. Pheromone binding and inactivation by moth antennae. Nature 293, 161–163 (1981). [DOI] [PubMed] [Google Scholar]
- Rybczynski R., Reagan J. & Lerner M. R. A pheromone-degrading aldehyde oxidase in the antennae of the moth Manduca sexta. J Neurosci 9, 1341–1353 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J., Francis F., Liu Y., Chen J. L. & Cheng D. F. An overview of odorant-binding protein functions in insect peripheral olfactory reception. Genet Mol Res 10, 3056–3069 (2011). [DOI] [PubMed] [Google Scholar]
- Leal W. S. Odorant reception in insects: roles of receptors, binding proteins, and degrading enzymes. Annu Rev Entomol 58, 373–391 (2013). [DOI] [PubMed] [Google Scholar]
- Gu S. H. et al. Functional characterizations of chemosensory proteins of the alfalfa plant bug Adelphocoris lineolatus indicate their involvement in host recognition. Plos One 7, e42871 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybczynski R., Vogt R. G. & Lerner M. R. Antennal-specific pheromone-degrading aldehyde oxidases from the moths Antheraea polyphemus and Bombyx mori. J Biol Chem 265, 19712–19715 (1990). [PubMed] [Google Scholar]
- Forstner M. et al. Differential expression of SNMP-1 and SNMP-2 proteins in pheromone-sensitive hairs of moths. Chem Senses 33, 291–299 (2008). [DOI] [PubMed] [Google Scholar]
- Hansson B. S. & Stensmyr M. C. Evolution of insect olfaction. Neuron 72, 698–711 (2011). [DOI] [PubMed] [Google Scholar]
- Koenig C. et al. A reference gene set for chemosensory receptor genes of Manduca sexta. Insect Biochem Mol Biol 66, 51–63 (2015). [DOI] [PubMed] [Google Scholar]
- Cande J., Prud’homme B. & Gompel N. Smells like evolution: the role of chemoreceptor evolution in behavioral change. Curr Opin Neurobiol 23, 152–158 (2013). [DOI] [PubMed] [Google Scholar]
- Nei M., Niimura Y. & Nozawa M. The evolution of animal chemosensory receptor gene repertoires: roles of chance and necessity. Nat Rev Genet 9, 951–963 (2008). [DOI] [PubMed] [Google Scholar]
- Missbach C. et al. Evolution of insect olfactory receptors. Elife 3, e02115 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson H. M., Warr C. G. & Carlson J. R. Molecular evolution of the insect chemoreceptor gene superfamily in Drosophila melanogaster. P Natl Acad Sci USA 100, 14537–14542 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott K. et al. A chemosensory gene family encoding candidate gustatory and olfactory receptors in Drosophila. Cell 104, 661–673 (2001). [DOI] [PubMed] [Google Scholar]
- Benton R., Sachse S., Michnick S. W. & Vosshall L. B. Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLoS Biol 4, e20 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P. Seven-transmembrane proteins as odorant and chemosensory receptors. Science 286, 707–711 (1999). [DOI] [PubMed] [Google Scholar]
- Zhou C. X., Min S. F., Yan-Long T. & Wang M. Q. Analysis of antennal transcriptome and odorant binding protein expression profiles of the recently identified parasitoid wasp. Sclerodermus sp. Comp Biochem Physiol Part D Genomics Proteomics 16, 10–19 (2015). [DOI] [PubMed] [Google Scholar]
- Hill C. A. et al. G protein coupled receptors in Anopheles gambiae. Science 298, 176–178 (2002). [DOI] [PubMed] [Google Scholar]
- Wanner K. W. et al. Female-biased expression of odourant receptor genes in the adult antennae of the silkworm. Bombyx mori. Insect Mol Biol 16, 107–119 (2007). [DOI] [PubMed] [Google Scholar]
- Zhang T. T. et al. Male- and female-biased gene expression of olfactory-related genes in the antennae of Asian corn borer, Ostrinia furnacalis (Guenée) (Lepidoptera: Crambidae). Plos One 10, e0128550 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson H. M. & Wanner K. W. The chemoreceptor superfamily in the honey bee, Apis mellifera: Expansion of the odorant, but not gustatory, receptor family. Genome Research 16, 1395–1403 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. N. et al. Identification and expression analysis of putative chemosensory receptor genes in Microplitis mediator by antennal transcriptome screening. Int J Biol Sci 11, 737–751 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha T. S. & Smith D. P. Insect odorant receptors: Channeling scent. Cell 133, 761–763 (2008). [DOI] [PubMed] [Google Scholar]
- Sato K., Pellegrino M., Nakagawa T., Vosshall L. B. & Touhara K. Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature 452, 1002–1006 (2008). [DOI] [PubMed] [Google Scholar]
- Yang B., Ozaki K., Ishikawa Y. & Matsuo T. Identification of candidate odorant receptors in Asian corn borer Ostrinia furnacalis. Plos One 10, e0121261, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. et al. Antennal transcriptome analysis and comparison of chemosensory gene families in two closely related noctuidae moths, Helicoverpa armigera and H. assulta. Plos One 10, e0117054 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse-Wilde E., Gohl T., Bouche E., Breer H. & Krieger J. Candidate pheromone receptors provide the basis for the response of distinct antennal neurons to pheromonal compounds. Eur J Neurosci 25, 2364–2373 (2007). [DOI] [PubMed] [Google Scholar]
- Montagne N. et al. Functional characterization of a sex pheromone receptor in the pest moth Spodoptera littoralis by heterologous expression in Drosophila. Eur J Neurosci 36, 2588–2596 (2012). [DOI] [PubMed] [Google Scholar]
- Anderson A. R. et al. Molecular basis of female-specific odorant responses in Bombyx mori. Insect Biochem Molec 39, 189–197 (2009). [DOI] [PubMed] [Google Scholar]
- Sakurai T. et al. Identification and functional characterization of a sex pheromone receptor in the silkmoth Bombyx mori. Proc Natl Acad Sci USA 101, 16653–16658 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T., Sakurai T., Nishioka T. & Touhara K. Insect sex-pheromone signals mediated by specific combinations of olfactory receptors. Science 307, 1638–1642 (2005). [DOI] [PubMed] [Google Scholar]
- Engsontia P., Sangket U., Chotigeat W. & Satasook C. Molecular evolution of the odorant and gustatory receptor genes in lepidopteran insects: implications for their adaptation and speciation. J Mol Evol 79, 21–39 (2014). [DOI] [PubMed] [Google Scholar]
- Krieger J. et al. Genes encoding candidate pheromone receptors in a moth (Heliothis virescens). Proc Natl Acad Sci USA 101, 11845–11850 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi X., Zhao H., Wang P., Hu M. & Zhong G. BdorOrco is important for oviposition-deterring behavior induced by both the volatile and non-volatile repellents in Bactrocera dorsalis (Diptera: Tephritidae). J Insect Physiol 65C, 51–56 (2014). [DOI] [PubMed] [Google Scholar]
- Zhou Y. L. et al. Silencing in Apolygus lucorum of the olfactory coreceptor Orco gene by RNA interference induces EAG response declining to two putative semiochemicals. J Insect Physiol 60, 31–39 (2014). [DOI] [PubMed] [Google Scholar]
- Thorne N., Chromey C., Bray S. & Amrein H. Taste perception and coding in Drosophila. Curr Biol 14, 1065–1079 (2004). [DOI] [PubMed] [Google Scholar]
- Jones W. D., Cayirlioglu P., Kadow I. G. & Vosshall L. B. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature 445, 86–90 (2007). [DOI] [PubMed] [Google Scholar]
- Benton R., Vannice K. S., Gomez-Diaz C. & Vosshall L. B. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell 136, 149–162 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croset V. et al. Ancient protostome origin of chemosensory ionotropic glutamate receptors and the evolution of insect taste and olfaction. Plos Genetics 6, e1001064 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai M. et al. Ionotropic glutamate receptors IR64a and IR8a form a functional odorant receptor complex in vivo in Drosophila. J Neurosci 33, 10741–10749 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abuin L. et al. Functional architecture of olfactory ionotropic glutamate receptors. Neuron 69, 44–60 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephrajkumar A., Chakrabarty R. & Thomas G. Midgut proteases of the cardamom shoot and capsule borer Conogethes punctiferalis (Lepidoptera: Pyralidae) and their interaction with aprotinin. B Entomol Res 96, 91–98 (2006). [DOI] [PubMed] [Google Scholar]
- Luo Z. & Honda H. Olfactory and biophysical assessment of the oviposition stimulating potential of host and non-host plants for the yellow peach moth, Conogethes punctiferalis (Lepidoptera: Crambidae). Appl Entomol Zool 50, 183–189 (2015). [Google Scholar]
- Ge X., Zhang T-tao, H. K.-l., Wang Q-ying, Li Y.-L. & Wang Z-ying. Cloning and tissue expression profiling of the olfactory receptor coreceptor gene in adults of Conogethes punctiferalis (Lepidoptera: Crambidae). Acta Entomologica Sinica 56, 243–250 (2013).
- Grabherr M. G. et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol 29, 644–652 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W., Liu L., Qin W. Q., Li C. X. & Peng Z. Q. Transcriptomic identification of chemoreceptor genes in the red palm weevil Rhynchophorus ferrugineus. Genet Mol Res 14, 7469–7480 (2015). [DOI] [PubMed] [Google Scholar]
- Zhang T., Gu S., Wu K., Zhang Y. & Guo Y. Construction and analysis of cDNA libraries from the antennae of male and female cotton bollworms Helicoverpa armigera (Hübner) and expression analysis of putative odorant-binding protein genes. Biochem Bioph Res Co 407, 393–399 (2011). [DOI] [PubMed] [Google Scholar]
- Legeai F. et al. An expressed sequence tag collection from the male antennae of the noctuid moth Spodoptera littoralis: a resource for olfactory and pheromone detection research. BMC Genomics 12, 86 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao D. et al. Identification of candidate olfactory genes in Chilo suppressalis by antennal transcriptome analysis. Int J Biol Sci 10, 846–860 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K. et al. Highly selective tuning of a silkworm olfactory receptor to a key mulberry leaf volatile. Curr Biol 19, 881–890 (2009). [DOI] [PubMed] [Google Scholar]
- Robertson H. M., Gadau J. & Wanner K. W. The insect chemoreceptor superfamily of the parasitoid jewel wasp Nasonia vitripennis. Insect Mol Biol 19 Suppl 1, 121–136 (2010). [DOI] [PubMed] [Google Scholar]
- He M., Zhang Y. N. & He P. Molecular characterization and differential expression of an olfactory receptor gene family in the white-backed planthopper Sogatella furcifera based on transcriptome analysis. Plos One 10, e0140605 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smadja C., Shi P., Butlin R. K. & Robertson H. M. Large gene family expansions and adaptive evolution for odorant and gustatory receptors in the pea aphid, Acyrthosiphon pisum. Mol Biol Evol 26, 2073–2086 (2009). [DOI] [PubMed] [Google Scholar]
- Wanner K. W. & Robertson H. M. The gustatory receptor family in the silkworm moth Bombyx mori is characterized by a large expansion of a single lineage of putative bitter receptors. Insect Mol Biol 17, 621–629 (2008). [DOI] [PubMed] [Google Scholar]
- Clyne P. J., Warr C. G. & Carlson J. R. Candidate taste receptors in Drosophila. Science 287, 1830–1834 (2000). [DOI] [PubMed] [Google Scholar]
- Silbering A. F. & Benton R. Ionotropic and metabotropic mechanisms in chemoreception: ‘chance or design’? EMBO Rep 11, 173–179 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C. Gustatory receptors: not just for good taste. Curr Biol 23, R929–R932, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.