Abstract
Regional brain sizes of very-preterm infants at term-equivalent age differ from those of term-born peers, which have been linked with later cognitive impairments. However, dependence of regional brain volume loss on gestational age has not been studied in detail. To investigate the spatial pattern of brain growth in neonates without destructive brain lesions, head MRI of 189 neonates with a wide range of gestational age (24–42 weeks gestation) was assessed using simple metrics measurements. Dependence of MRI findings on gestational age at birth (Agebirth) and the corrected age at MRI scan (AgeMRI) were assessed. The head circumference was positively correlated with AgeMRI, but not Agebirth. The bi-parietal width, deep grey matter area and the trans-cerebellar diameter were positively correlated with both Agebirth and AgeMRI. The callosal thickness (positive), atrial width of lateral ventricle (negative) and the inter-hemispheric distance (negative) were exclusively correlated with Agebirth. The callosal thickness and cerebral/cerebellar transverse diameters showed predominant dependence on Agebirth over AgeMRI, suggesting that brain growth after preterm-birth was considerably restricted or even became negligible compared with that in utero. Such growth restriction after preterm birth may extensively affect relatively more matured infants, considering the linear relationships observed between brain sizes and Agebirth.
During the last decade, magnetic resonance imaging (MRI) scans at term-equivalent age have been established as a reliable prognostic biomarker of motor, verbal and cognitive outcomes in preterm infants1,2,3. In addition to the detection of overt destructive brain injury, evaluation of subtle brain injury, represented by diffuse-excessive high signal intensity and mild brain atrophy, has been established using a composite assessment scale for both white matter and grey matter2. For more objective assessment of regional brain sizes, quantitative analysis of brain MRI has been developed. Three-dimensional volumetric analysis gives direct measures of regional brain volume4,5, where reduced brain volume in preterm infants is indicative of adverse neurodevelopmental outcomes up to 2 years of age6,7. A more recent study using the same technique demonstrated that low brain volumes observed in very-preterm infants are associated with long-term functional outcomes of up to 7 years old8. Even without specialised software and expertise, reliable regional brain sizes can be obtained using simple biometric analysis9. Although a relatively greater inter-observer variability was noted for the measurement of fluid spaces9, Kidokoro and colleagues reported that the predictive value of cognitive development may be improved by incorporating one-dimensional measurements of regional brain sizes into the aforementioned composite MRI scoring system10.
Brain sizes obtained using simple metric analysis in the parietal and frontal lobes, and cerebellum show consistent dependences on the corrected age of preterm infants at the MRI scan (dependence on gestational age at birth not assessed)9. This finding suggests that brain growth after preterm birth at least mimics that within the uterus. However, previous comparative studies showed a significant reduction of regional brain volume in very-preterm infants compared with their full-term peers when assessed at term-equivalent age, suggesting a difference between intra- and extra-uterine patterns of brain growth11,12,13.
Important questions are raised whether regional brain volume loss is specific to very-preterm infants or is extensively observed in a gestational-age dependent manner, and whether smaller regional brain sizes at term represent the consequence of permanent brain injury or a temporary delay in growth that can eventually catch up. However, the spatial patterns of altered regional brain growth and their mechanism have not been fully elucidated especially in moderately- and late-preterm infants.
To investigate spatial growth patterns of the brain in preterm infants, we performed an MRI study using simple metrics measurement. Instead of comparing MRI findings between several groups of preterm and term infants, we assessed the dependences of regional brain sizes on the gestational age at birth (Agebirth) and corrected age at the MRI scan (AgeMRI) in a single cohort of newborn infants spanning a wide range of gestational ages. This was based on an assumption that, in specific brain regions, where brain growth following preterm birth is substantially restricted, regional brain sizes may depend on Agebirth rather than AgeMRI.
Results
Clinical characteristics
Five newborn infants were diagnosed with congenital cerebral anomalies (congenital hydrocephaly, n = 1; major chromosomal abnormality, n = 2; and congenital cytomegalovirus infection, n = 2), and 11 newborn infants showed severe, destructive cerebral lesions (intra-ventricular haemorrhage ≥ grade 3, n = 5; cerebral venous or arterial infarction, n = 3; and cystic encephalomalacia due to severe neonatal encephalopathy, n = 3). Of these 16 newborn infants, moderate to severe brain injury in white matter, cortical grey matter, deep grey matter, cerebellum and the whole brain was observed in 10 (62.5%), 2 (12.5%), 7 (43.8%), 7 (43.8%) and 10 (62.5%) newborn infants, respectively. These subjects were excluded from further analysis.
Subsequently, MRI findings were assessed for 189 preterm and term infants, whose Agebirth and AgeMRI were 31.8 ± 4.1 (range, 22.6–42.0) weeks and 38.9 ± 1.6 (range, 36.3–44.3) weeks, respectively (Table 1). AgeMRI was positively correlated with Agebirth (p = 0.021). Antenatal and/or postnatal glucocorticoids were used in 43.3% and 15.3% of the population, respectively; 66.1% of the newborn infants were born via caesarean section; mechanical ventilation was required in 49.7%.
Table 1. Clinical characteristics of the study population.
| Variables | Mean ± SD, or number (%) |
|---|---|
| Gestational age at birth (week) | 31.8 ± 4.1 |
| <28 | 29 (15.3) |
| 28 ≤<32 | 58 (30.7) |
| 32 ≤<36 | 73 (38.6) |
| 37≤ | 29 (15.3) |
| Birth weight (g) | 1537 ± 709 |
| Male sex | 91 (48.1) |
| Antenatal glucocorticoid | 82 (43.3) |
| Multiple pregnancy | 50 (26.5) |
| Caesarean delivery | 125 (66.1) |
| Intrauterine growth restriction | 71 (37.6) |
| Apgar score <7 | |
| 1 min. | 90 (47.6) |
| 5 min. | 29 (15.3) |
| Duration of mechanical ventilation (day) | 9.8 ± 17.5 |
| Chronic lung disease | 45 (23.8) |
| Symptomatic patent ductus arteriosus | |
| Indomethacin | 49 (25.9) |
| ligation | 10 (5.3) |
| Enteral feeding >100 ml/kg (day) | 7.6 ± 5.5 |
| Postnatal glucocorticoid | 29 (15.3) |
| Corrected age at MRI scan (week) | 38.9 ± 1.6 |
| Head circumference at MRI scan (cm) | 34.2 ± 1.6 |
Abbreviation: SD, standard deviation.
MRI findings
Moderate to severe brain injury in white matter, cortical grey matter, deep grey matter, cerebellum and the whole brain was observed in 15 (7.9%), 1 (0.5%), 0 (0.0%), 8 (4.3%), and 4 (2.1%) newborn infants, respectively (Supplemental Table 1). Moderate to severe injury in the whole brain was exclusively observed in very-preterm infants <28 weeks gestation. Measures from simple metric assessments are presented in Table 2.
Table 2. Relationships between simple brain metrics and age.
| Variables | Mean | SD | Agebirth |
AgeMRI |
||||
|---|---|---|---|---|---|---|---|---|
| r | (95% CI) | p | r | (95% CI) | p | |||
| Head circumference (cm) | 34.2 | 1.6 | 0.120 | (−0.023, 0.258) | 0.100 | 0.447 | (0.325, 0.554) | <0.001 |
| Cerebral hemisphere | ||||||||
| Bi-parietal width (mm) | 75.4 | 6.5 | 0.611 | (0.513, 0.693) | <0.001 | 0.348 | (0.216, 0.468) | <0.001 |
| Fronto-occipital diameter (mm) | 100.3 | 5.3 | −0.004 | (−0.147, 0.147) | 0.957 | 0.067 | (−0.076, 0.208) | 0.358 |
| Corpus callosum (thickness in [mm]) | ||||||||
| Genu | 4.4 | 0.8 | 0.332 | (0.199, 0.453) | <0.001 | 0.139 | (−0.004, 0.276) | 0.056 |
| Body | 2.5 | 0.5 | 0.328 | (0.194, 0.450) | <0.001 | 0.078 | (−0.065, 0.218) | 0.287 |
| Splenium | 3.5 | 0.7 | 0.515 | (0.402, 0.613) | <0.001 | −0.058 | (−0.199, 0.085) | 0.426 |
| Deep grey matter | ||||||||
| Deep-grey-matter area (cm2) | 10.9 | 0.8 | 0.252 | (0.113, 0.381) | <0.001 | 0.364 | (0.233, 0.482) | <0.001 |
| Cerebellum | ||||||||
| Trans-cerebellar diameter (mm) | 50.2 | 2.8 | 0.561 | (0.455, 0.652) | <0.001 | 0.483 | (0.365, 0.585) | <0.001 |
| Antero-posterior cerebellar diameter (mm) | 16.4 | 1.4 | 0.105 | (−0.038, 0.244) | 0.151 | 0.367 | (0.237, 0.484) | <0.001 |
| Fluid measures | ||||||||
| Atrial width of Lateral ventricle (mm) | ||||||||
| Right | 5.1 | 1.6 | ||||||
| Left | 6.4 | 2.1 | ||||||
| Mean | 5.8 | 1.7 | −0.232 | (−0.363, −0.092) | 0.001 | 0.061 | (−0.082, 0.202) | 0.404 |
| Thalamo-occipital distance (mm) | 25.5 | 4.3 | −0.165 | (−0.301, −0.023) | 0.024 | −0.043 | (−0.185, 0.100) | 0.552 |
| Inter-hemispheric distance (mm) | 1.9 | 1.1 | −0.628 | (−0.707, −0.533) | <0.001 | 0.006 | (−0.137, 0.149) | 0.936 |
Abbreviations: SD, standard deviation; Agebirth, gestational age at birth; AgeMRI, corrected age at MRI scan. P-values are from the Pearson’s correlation coefficient, using the Fisher Transformation to calculate the 95% confidence intervals.
Dependence of MRI findings on gestational and corrected age
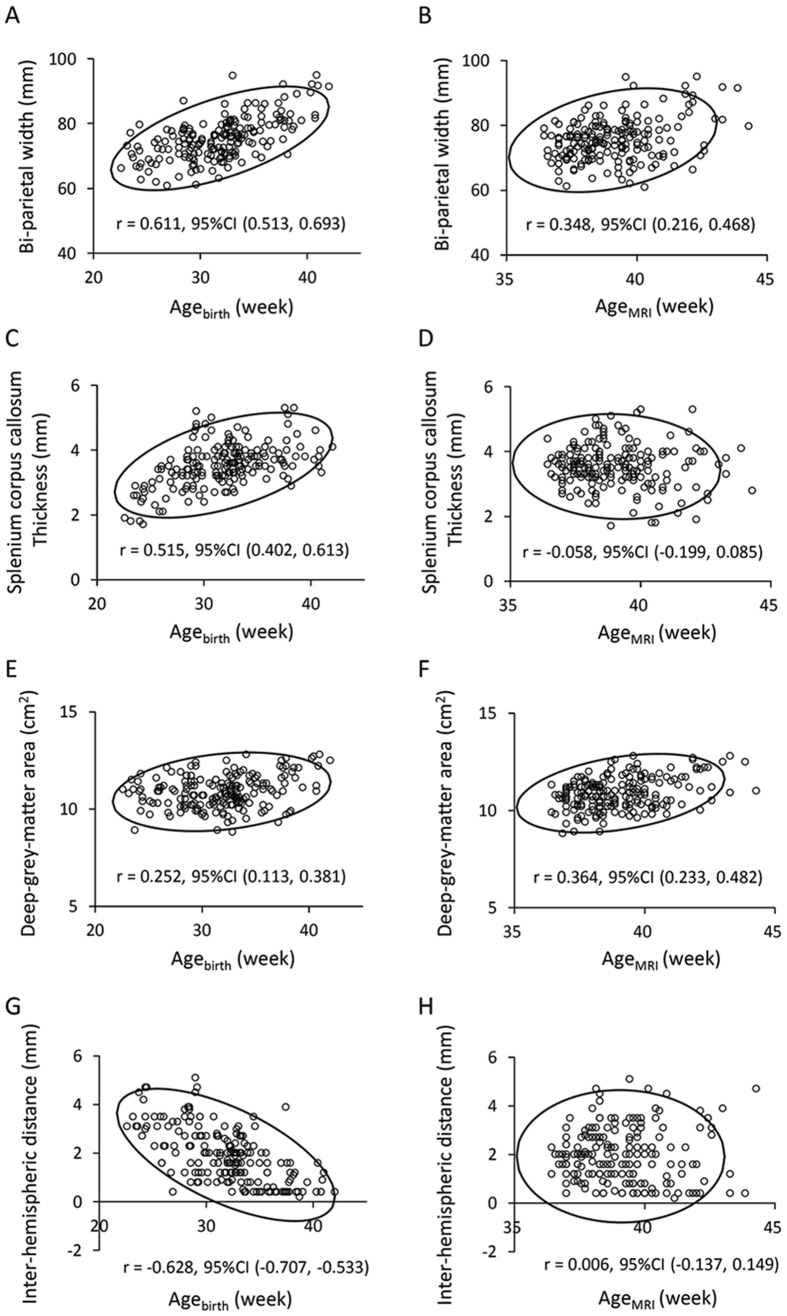
Of the qualitative evaluation items within the composite MRI scoring system, the status of myelination was dependent on AgeMRI (p < 0.001), but not Agebirth (Supplemental Table 2). The severity of brain injury for the white matter (p < 0.001), cortical grey matter (p = 0.002), cerebellum (p < 0.001) and the whole brain (p < 0.001) was linearly associated with Agebirth, but not AgeMRI. The bi-parietal width, deep grey matter area and the trans-cerebellar diameter showed positive linear correlations with both AgeMRI and Agebirth (all p < 0.001), which were most prominent between the bi-parietal width and Agebirth (Table 2, Fig. 1 and Supplemental Fig. 1; see Supplemental Table 3 for findings from exploratory analysis, which assessed the dependence of regional brain sizes on other clinical variables). The callosal thickness for all three regions (positive) (all p < 0.001), atrial width of lateral ventricle (negative) (p = 0.001) and the inter-hemispheric distance (negative) (p < 0.001) were linearly correlated with Agebirth, but not with AgeMRI. The head circumference was positively correlated with AgeMRI (p < 0.001), but not with Agebirth.
Figure 1. Relationships between regional brain sizes and age.
Regional brain sizes are plotted against gestational age at birth (Agebirth) (A,C,E,G) and corrected age at MRI scan (AgeMRI) (B,D,F,H) with 95% confidence ellipse. The bi-parietal width (A,B) and deep-grey-matter area (E,F) were positively correlated with both Agebirth and AgeMRI. The thickness of the splenium of the corpus callosum and inter-hemispheric distance were correlated with Agebirth, but not AgeMRI (C,D,G,H).
Relationship between head circumference and brain metrics
The bi-parietal width was not correlated with the fronto-occipital diameter, whereas a positive relationship was observed between the trans-cerebellar diameter and the antero-posterior cerebellar diameter (p < 0.001) (Table 3). The fronto-occipital diameter and the bi-parietal width were linearly correlated with the antero-posterior cerebellar diameter and the trans-cerebellar diameter, respectively (both p < 0.001). The deep-grey-matter area was correlated with the bi-parietal width, fronto-occipital diameter, trans-cerebellar diameter, antero-posterior cerebellar diameter (all p < 0.001) and the splenial thickness of the corpus callosum (p = 0.001). The atrial width and the thalamo-occipital distance of the lateral ventricle were positively correlated with the fronto-occipital diameter (both p < 0.001). The atrial width of the lateral ventricle was correlated with the thalamo-occipital distance of the lateral ventricle (p < 0.001), but not with the inter-hemispheric distance. The head circumference was correlated with the bi-parietal width, fronto-occipital diameter, deep-grey-matter area, trans-cerebellar diameter, antero-posterior cerebellar diameter and the thalamo-occipital distance of the lateral ventricle (all p < 0.001), where the most robust relationship was seen with the fronto-occipital diameter.
Table 3. Correlation coefficient and 95% confidence interval between regional brain sizes.
| BPW | FOD | gCC | bCC | sCC | DGMA | TCD | APCD | AWLV | TOD | IHD | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Head circumference (HC) | 0.418 | 0.713 | 0.025 | 0.154 | 0.279 | 0.574 | 0.585 | 0.560 | 0.183 | 0.397 | 0.029 |
| (0.293, 0.529) | (0.635, 0.777) | (−0.118, 0.167) | (0.012, 0.290) | (0.142, 0.406) | (0.470, 0.662) | (0.483, 0.672) | (0.454, 0.651) | (0.041, 0.317) | (0.270, 0.511) | (−0.114, 0.171) | |
| Bi-parietal width (BPW) | −0.043 | 0.176 | 0.201 | 0.336 | 0.355 | 0.674 | 0.193 | −0.231 | −0.173 | −0.207 | |
| (−0.185, 0.100) | (0.034, 0.311) | (0.060, 0.334) | (0.203, 0.457) | (0.224, 0.474) | (0.588, 0.745) | (0.052, 0.327) | (−0.362, −0.091) | (−0.308, −0.031) | (−0.340, −0.066) | ||
| Fronto-occipital diameter (FOD) | −0.001 | 0.061 | 0.190 | 0.338 | 0.246 | 0.479 | 0.294 | 0.480 | 0.049 | ||
| (−0.144, 0.142) | (−0.082, 0.202) | (0.049, 0.324) | (0.205, 0.459) | (0.107, 0.376) | (0.361, 0.582) | (0.158, 0.419) | (0.362, 0.583) | (−0.094, 0.190) | |||
| Genu of the corpus callosum (gCC) | 0.448 | 0.329 | 0.172 | 0.232 | 0.018 | −0.049 | −0.010 | −0.174 | |||
| (0.326, 0.555) | (0.195, 0.451) | (0.030, 0.307) | (0.092, 0.363) | (−0.125, 0.160) | (−0.190, 0.094) | (−0.153, 0.133) | (−0.309, −0.032) | ||||
| Body of the corpus callosum (bCC) | 0.453 | 0.173 | 0.253 | 0.084 | −0.065 | −0.043 | −0.272 | ||||
| (0.332, 0.560) | (0.031, 0.308) | (0.114, 0.382) | (−0.059, 0.224) | (−0.206, 0.078) | (−0.185, 0.100) | (−0.399, -.134) | |||||
| Splenium of the corpus callosum (sCC) | 0.255 | 0.363 | 0.153 | −0.084 | 0.057 | −0.384 | |||||
| (0.117, 0.384) | (0.232, 0.481) | (0.010, 0.289) | (−0.224, 0.059) | (−0.086, 0.198) | (−0.499, -.255) | ||||||
| Deep-grey-matter area (DGMA) | 0.506 | 0.352 | 0.178 | 0.237 | −0.048 | ||||||
| (0.392, 0.605) | (0.220, 0.471) | (0.036, 0.313) | (0.098, 0.367) | (−0.189, 0.095) | |||||||
| Trans-cerebellar diameter (TCD) | 0.356 | −0.034 | 0.064 | −0.164 | |||||||
| (0.225, 0.475) | (−0.176, 0.109) | (−0.079, 0.205) | (−0.300, −0.022) | ||||||||
| Antero-posterior cerebellar diameter (APCD) | 0.065 | 0.143 | 0.011 | ||||||||
| (−0.078, 0.206) | (0.000, 0.280) | (−0.132, 0.153) | |||||||||
| Atrial width of Lateral ventricle (AWLV) | 0.533 | 0.145 | |||||||||
| (0.422, 0.628) | (0.002, 0.282) | ||||||||||
| Thalamo-occipital distance (TOD) | 0.103 | ||||||||||
| (−0.040, 0.242) |
Associations between regional brain sizes were assessed with Pearson correlation, using the Fisher Transformation to calculate the 95% confidence intervals. Abbreviation, IHD, inter-hemispheric distance.
Discussion
Previous MRI studies have highlighted the poor regional brain growth of preterm infants compared with their term-born peers9,11,12. In the current study, instead of comparing brain sizes between preterm- and term-born cohorts, we assessed the dependence of brain size on Agebirth and AgeMRI in a single cohort of newborn infants with a spectrum of maturation stages. This was based on an assumption that, in regions where postnatal brain growth mimics that in utero, regional brain sizes primarily depend on AgeMRI, but not Agebirth. However, we found that measures such as deep-grey-matter area and transverse diameters of the cerebrum and cerebellum depended on both Agebirth and AgeMRI, whereas the thickness of the corpus callosum depended exclusively on Agebirth. This suggests that brain growth in these regions after preterm-birth is significantly restricted or is even negligible compared with that in utero. Considering that abnormal size of the corpus callosum and lateral ventricles are good indicators of cognitive impairment in children and adolescents born prematurely14,15,16,17,18, the region-specific growth patterns of the immature brain observed in our study may represent the irreversible consequence of adverse extra-uterine conditions for the brain of preterm infants. In addition, given the linear relationships observed between brain sizes and Agebirth in some regions, growth restriction of the brain is a continuum, which is not specific to extremely- and very-preterm infants, but may affect even more matured newborn infants.
Despite the established relationship between callosal size and neurodevelopmental outcomes following preterm birth14,15,16,17,18, the mechanism of altered callosal growth remain largely unknown. Volume loss in the corpus callosum of preterm-born children is most evident in the posterior region14,15,17,19. The rudimentary corpus callosum originates in the anterior body at the end of the first trimester, and expands towards remaining parts during development20. Similarly, the genu and the body show a consistent increase in thickness during pregnancy21,22, whereas the splenium shows the maximum growth in thickness between 18 and 26 weeks gestation, which slows down after 28 weeks gestation22. Preterm infants, even with their favourable clinical course, experience dramatic environmental changes at birth, which are followed by a prolonged period with stressful procedures, malnutrition, and separation from the mother. The burden of such adverse events, experienced just when the splenium is supposed to grow most rapidly, might be responsible for altered callosal growth after preterm birth.
In preterm infants, increased cerebrospinal fluid space is persistently observed even in adolescence17,23. Ventriculomegaly without preceding severe intra-ventricular haemorrhage is known as an independent predictive marker for adverse neurodevelopmental outcomes in preterm infants18,24,25. Interestingly, the thalamo-occipital distance of the lateral ventricle measured shortly after birth showed little or no dependence on gestational age26,27, suggesting that intra-uterine brain growth is accompanied by the reduction of the ventricular size relative to the whole brain. In the current study, the sizes of ventricular and subarachnoid spaces were negatively correlated with Agebirth, indicating that the growth rate of brain tissue surrounding the ventricle is significantly restricted after preterm birth. In addition, consistent with previous studies24,28, we observed no direct relationship between the sizes of ventricular and subarachnoid spaces. The primary cause of the increase in cerebrospinal fluid-space volume likely differs between newborn infants, because increase in ventricular and subarachnoid spaces can be caused by the volume reduction of the adjacent brain tissue as well as the increase in cerebrospinal fluid itself.
In the current study, the bi-parietal width and the fronto-occipital diameter were not correlated with each other. Unlike the bi-parietal width, the fronto-occipital diameter showed no correlation with Agebirth and AgeMRI. However, the fronto-occipital diameter was tightly correlated with both the head circumference and the thalamo-occipital distance of the lateral ventricle, suggesting that the brain size along the longitudinal axis might be determined by both physiological postnatal growth and pathological dilatation of ventricles. This characteristic balance of cerebral growth between the longitudinal and transverse axes may explain the scaphocephalic head shape commonly observed in preterm infants. It is widely accepted that scaphocephaly in healthy preterm infants does not influence deep brain structures29. However, the current findings suggest that excessive scaphocephaly might be indicative of altered brain volume and structure subsequent to preceding brain injury. In addition, given that approximately 51% of the head circumference can be explained by the fronto-occipital diameter (based on the observed r2 = 0.51 between these variables), careful interpretation is required to assess the brain growth using the head circumference in preterm infants.
Although no correlation was observed between the bi-parietal width and the fronto-occipital diameter, the longitudinal and transverse diameters of the cerebrum were tightly correlated with those of the cerebellum. The transverse diameters of the cerebrum and the cerebellum both showed predominant correlations with Agebirth despite significant differences in the environment between the supra- and infra-tentorial spaces. This suggests the presence of a common mechanism causing the growth restriction of the cerebrum and cerebellum along the transverse axis in preterm infants. Considering that the sideways head position is generally preferred for preterm infants during intensive care, gravity may predominantly affect brain growth towards the transverse axis of the brain after birth. This may influence the growth pattern of the immature brain, as studies in developing rat demonstrated that exposure to hyper-gravity environment causes various types of cerebellar injury, including Purkinje cell loss and subsequent reduction in cerebellar volume30.
In the current study, the severity of brain injury assessed using the composite MRI scores depended on Agebirth, where AgeMRI is already incorporated within the scoring system10. Given that we did not include newborn infants with major cerebral lesions in the current cohort, preterm birth itself was likely to be the primary independent variable of non-destructive brain injury at term. Recently, the incidence of moderate to severe neurodevelopmental impairments in preterm infants has decreased, in part due to the reduced incidence of destructive brain lesions such as intra-ventricular haemorrhage and periventricular leukomalacia31,32. Hence, early diagnosis of non-destructive brain lesions would be important for the prevention of subsequent cognitive impairment. Comprehensive assessment of brain growth and maturation at term using qualitative measures, regional brain size and other quantitative markers may allow more precise detection of injury.
We excluded newborn infants with apparent destructive brain lesions. This led to uncertainty regarding typical brain growth in extremely-preterm infants, who often develop severe cerebral lesions. We did not use volumetric analysis in our current study. While volumetric data are easy to translate, their use in clinical practice remains limited because of additional requirements for software and expertise for data processing. The present study and others suggest the benefit of a simple metric approach, which is reliable, reproducible and readily available in clinical practice9. Future studies should incorporate other quantitative magnetic resonance biomarkers including apparent diffusion coefficients, fractional anisotropy and T2-relaxation time.
We found that the size of specific brain regions, including the thickness of the corpus callosum and the transverse diameter of the cerebrum, depended on Agebirth, but not AgeMRI, suggesting a difference between intra- and extra-uterine brain growth after preterm birth. The linear relationships observed between brain sizes and Agebirth in these regions suggested that regional brain growth restriction is not specific to very-preterm infants, but is likely to affect even more matured infants in a gestational-age dependent manner. Further studies are required to elucidate the mechanism and direct consequence of brain growth patterns specific to preterm infants. Serial cranial ultrasound sonography might help delineate the temporal process of region-specific growth pattern in these infants.
Methods
This study was conducted in compliance with the Declaration of Helsinki under the approval of the Ethics Committee of Kurume University School of Medicine. Informed parental consent was obtained for each participating newborn infant before enrolment into this study.
Study population
Two hundred and five newborn infants, who were admitted to a tertiary neonatal intensive care unit of Kurume University Hospital (Kurume, Fukuoka, Japan) immediately after birth and underwent MRI scans between 36 and 44 weeks corrected age during the period from September 2007 to March 2012, were enrolled into the study. In this unit, head MRI is routinely obtained (i) for preterm infants <34 weeks gestation, and (ii) for near term- and term-born infants with either neurological abnormality, clinical details suggestive of perinatal hypoxia–ischaemia, respiratory failure, major congenital anomaly, chromosome aberration, or metabolic disease, after the clinical condition is stabilised.
MRI study
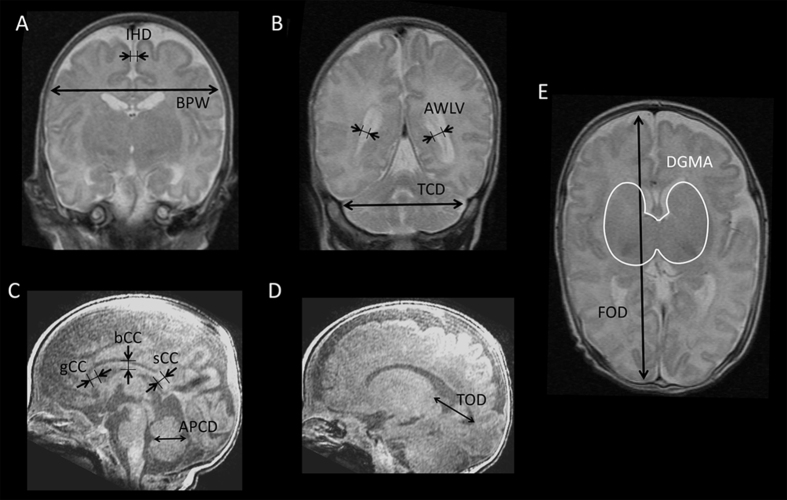
A 3-Tesla Signa HDxt scanner (GE Medical Systems, Milwaukee, WI, USA) was used to obtain head MRI with a three-dimensional brain volume imaging (BRAVO) for T1-weighted images (TR 11 ms; TE 5 ms; slice thickness 1 mm; matrix 384 × 224, interpolated 512 × 512; field of view 200 × 200 mm; both coronal and sagittal sections reconstructed from axial slices) and a fast spin echo imaging for T2-weighted images (TR 5000 ms; TE 87 ms; slice thickness 4 mm; matrix 384 × 224, reconstruction matrix 512 × 512; field of view 200 × 200 mm). Diffusion-tensor imaging was also obtained, information of which was not used in the current study. MRI was visually inspected for its quality, and was assessed using an established MRI scoring system for brain maturation, growth, and injury10. This system gives 0–4 stepwise scores over 13 items, according to the qualitative and quantitative findings of the brain on T1- and T2-weighted images, so that composite scores are calculated for the white matter (range, 0–17), cortical grey matter (0–9), deep grey matter (0–7) and cerebellum (0–7). The MRI was categorised as having no, mild, moderate, or severe injury according to the regional and global composite scores (Supplemental Fig. 2). Simple metric measures were obtained for T2-weighted coronal images (bi-parietal width, trans-cerebellar diameter, interhemispheric distance, and atrial width of the lateral ventricle), T1-weighted sagittal images (thalamo-occipital distance of the lateral ventricle, thickness of the corpus callosum at the genu, mid-portion of the body, and splenium, and antero-posterior cerebellar diameter) and T2-weighted axial images (fronto-occipital diameter and deep-grey-matter area) sections9 (Fig. 2).
Figure 2. Representative images showing the measurement of regional brain sizes.
A coronal section at the level of the cochlea and basilar truncus apparent (A) and the lateral ventricular atrium (B) are measured for the bi-parietal width (BPW) and interhemispheric distance (IHD) on A and for the atrial width of each lateral ventricle (AWLV) and trans-cerebellar diameter (TCD) on B. A midline sagittal section (C) was used for the measurement of callosal thickness at the genu (gCC), mid-portion of the body (bCC), and splenium (sCC), and antero-posterior cerebellar diameter (APCD). A sagittal section, with which a plain from the thalamus to the occipital horn is maximally visible (D), was used for the measurement of the thalamo-occipital distance of the lateral ventricle (TOD). An axial section, with which the caudate, lentiform nuclei and the thalamus are observed with their maximal sizes (E), was used for the measurement of the fronto-occipital diameter (FOD) and the deep-grey-matter area (DGMA).
Clinical information
Clinical information for the newborn infants was obtained including intrauterine growth restriction, gestational age and body weight at birth, delivery mode, use of antenatal and postnatal glucocorticoid, duration of mechanical ventilation, postnatal age when enteral feeding exceeded 100 mL/kg, symptomatic patent ductus arteriosus requiring treatments with intravenous indomethacin (excluding prophylactic administration within 72 h of life) or surgical ligation, chronic lung disease (oxygen dependence on Day 28 and/or 36 weeks corrected age), and the body weight and head circumference on the day of the MRI scan.
Data analysis
To further understand the extra-uterine brain growth of newborn infants without major anomalies and destructive brain injury, newborn infants with congenital cerebral anomalies or destructive brain lesions (intra-ventricular haemorrhage ≥ grade 3, cerebral infarction and cystic encephalomalacia due to severe neonatal encephalopathy) were excluded from the analysis. Correlations between brain metrics, head circumference on the day of the MRI scan, severity of MRI findings on composite scores, Agebirth and AgeMRI were assessed using either Pearson’s correlation coefficient or Spearman’s correlation coefficient when applicable. For multiple comparisons over 11 simple brain metrics and 18 items of the composite scoring system, statistical significance was assumed for p < 0.0045 and 0.0028, respectively (Bonferroni correction).
Additional Information
How to cite this article: Iwata, S. et al. Region-specific growth restriction of brain following preterm birth. Sci. Rep. 6, 33995; doi: 10.1038/srep33995 (2016).
Supplementary Material
Acknowledgments
We thank the patients who participated in the study and their parents for their cooperation; the nurses of the Neonatal Intensive Care Unit and the radiologists of Kurume University Hospital for their enthusiastic support; Profs Yasuki Maeno and Yusuke Uchiyama for their consistent support and encouragement. This work was supported by the Japan Society for the Promotion of Science, The Ministry of Education, Culture, Sports, Science, and Technology (Grant-in-Aid for Scientific Research C24591533 and C15K09733) and the Japan Science and Technology Agency and the Ministry of Education, Culture, Sports, Science and Technology (Grant-in-Aid for Scientific Research B01-24119004, Constructive Developmental Science, Innovative Areas, and H27-001, Special research in perinatal medicine).
Footnotes
Author Contributions S.I., T.A. and O.I. designed the study protocol. S.I., R.K., M.K., M.S. and O.I. recruited study subjects and performed the MRI assessment. Y.A., S.I. and O.I. performed the statistical analyses. S.I., S.T. and O.I. contributed to interpretation of findings. S.I. drafted the initial manuscript. Y.A., R.K. and T.A. drafted the technical part of the manuscript related with MRI, and Y.A., M.K., M.S. and O.I. revised the manuscript. All authors have seen and approved the final version of this manuscript.
References
- Kwon S. H., Vasung L., Ment L. R. & Huppi P. S. The role of neuroimaging in predicting neurodevelopmental outcomes of preterm neonates. Clinics in perinatology 41, 257–283, 10.1016/j.clp.2013.10.003 (2014). [DOI] [PubMed] [Google Scholar]
- Woodward L. J., Anderson P. J., Austin N. C., Howard K. & Inder T. E. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N.Engl.J.Med. 355, 685–694 (2006). [DOI] [PubMed] [Google Scholar]
- Iwata S. et al. Qualitative brain MRI at term and cognitive outcomes at 9 years after very preterm birth. Pediatrics 129, e1138–e1147, 10.1542/peds.2011-1735 (2012). [DOI] [PubMed] [Google Scholar]
- Huppi P. S. et al. Quantitative magnetic resonance imaging of brain development in premature and mature newborns. Annals of neurology 43, 224–235, 10.1002/ana.410430213 (1998). [DOI] [PubMed] [Google Scholar]
- Boardman J. P. et al. Early growth in brain volume is preserved in the majority of preterm infants. Annals of neurology 62, 185–192, 10.1002/ana.21171 (2007). [DOI] [PubMed] [Google Scholar]
- Rathbone R. et al. Perinatal cortical growth and childhood neurocognitive abilities. Neurology 77, 1510–1517, 10.1212/WNL.0b013e318233b215 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward L. J., Edgin J. O., Thompson D. & Inder T. E. Object working memory deficits predicted by early brain injury and development in the preterm infant. Brain 128, 2578–2587 (2005). [DOI] [PubMed] [Google Scholar]
- Monson B. B. et al. Examination of the Pattern of Growth of Cerebral Tissue Volumes From Hospital Discharge to Early Childhood in Very Preterm Infants. JAMA pediatrics 170, 772–779, 10.1001/jamapediatrics.2016.0781 (2016). [DOI] [PubMed] [Google Scholar]
- Nguyen The Tich S. et al. A novel quantitative simple brain metric using MR imaging for preterm infants. AJNR Am J Neuroradiol 30, 125–131, 10.3174/ajnr.A1309 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidokoro H. et al. Brain injury and altered brain growth in preterm infants: predictors and prognosis. Pediatrics 134, e444–e453, 10.1542/peds.2013-2336 (2014). [DOI] [PubMed] [Google Scholar]
- Mewes A. U. et al. Regional brain development in serial magnetic resonance imaging of low-risk preterm infants. Pediatrics 118, 23–33, 10.1542/peds.2005-2675 (2006). [DOI] [PubMed] [Google Scholar]
- Koyama T., Osada H., Tsujii H. & Kurita H. Utility of the Kyoto Scale of Psychological Development in cognitive assessment of children with pervasive developmental disorders. Psychiatry Clin Neurosci 63, 241–243, doi: PCN1931 [pii] 10.1111/j.1440-1819.2009.01931.x (2009). [DOI] [PubMed] [Google Scholar]
- Thompson D. K. et al. Perinatal risk factors altering regional brain structure in the preterm infant. Brain 130, 667–677, 10.1093/brain/awl277 (2007). [DOI] [PubMed] [Google Scholar]
- Nosarti C. et al. Corpus callosum size and very preterm birth: relationship to neuropsychological outcome. Brain 127, 2080–2089, 10.1093/brain/awh230 (2004). [DOI] [PubMed] [Google Scholar]
- Rademaker K. J. et al. Larger corpus callosum size with better motor performance in prematurely born children. Seminars in perinatology 28, 279–287 (2004). [DOI] [PubMed] [Google Scholar]
- Narberhaus A. et al. Gestational age at preterm birth in relation to corpus callosum and general cognitive outcome in adolescents. Journal of child neurology 22, 761–765, 10.1177/0883073807304006 (2007). [DOI] [PubMed] [Google Scholar]
- Peterson B. S. et al. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA 284, 1939–1947, doi: joc00361 [pii] (2000). [DOI] [PubMed] [Google Scholar]
- Northam G. B., Liegeois F., Chong W. K., Wyatt J. S. & Baldeweg T. Total brain white matter is a major determinant of IQ in adolescents born preterm. Annals of neurology 69, 702–711, 10.1002/ana.22263 (2011). [DOI] [PubMed] [Google Scholar]
- Thompson D. K. et al. Characterization of the corpus callosum in very preterm and full-term infants utilizing MRI. NeuroImage 55, 479–490, 10.1016/j.neuroimage.2010.12.025 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kier E. L. & Truwit C. L. The normal and abnormal genu of the corpus callosum: an evolutionary, embryologic, anatomic, and MR analysis. AJNR Am J Neuroradiol 17, 1631–1641 (1996). [PMC free article] [PubMed] [Google Scholar]
- Achiron R. & Achiron A. Development of the human fetal corpus callosum: a high-resolution, cross-sectional sonographic study. Ultrasound in obstetrics & gynecology: the official journal of the International Society of Ultrasound in Obstetrics and Gynecology 18, 343–347, 10.1046/j.0960-7692.2001.00512.x (2001). [DOI] [PubMed] [Google Scholar]
- Malinger G. & Zakut H. The corpus callosum: normal fetal development as shown by transvaginal sonography. AJR. American journal of roentgenology 161, 1041–1043, 10.2214/ajr.161.5.8273605 (1993). [DOI] [PubMed] [Google Scholar]
- Nosarti C. et al. Adolescents who were born very preterm have decreased brain volumes. Brain 125, 1616–1623 (2002). [DOI] [PubMed] [Google Scholar]
- Brouwer M. J. et al. Sequential cranial ultrasound and cerebellar diffusion weighted imaging contribute to the early prognosis of neurodevelopmental outcome in preterm infants. PloS one 9, e109556, 10.1371/journal.pone.0109556 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox L. M. et al. The relationship between ventricular size at 1 month and outcome at 2 years in infants less than 30 weeks’ gestation. Arch Dis Child Fetal Neonatal Ed 99, F209–F214, 10.1136/archdischild-2013-304374 (2014). [DOI] [PubMed] [Google Scholar]
- Davies M. W., Swaminathan M., Chuang S. L. & Betheras F. R. Reference ranges for the linear dimensions of the intracranial ventricles in preterm neonates. Arch Dis Child Fetal Neonatal Ed 82, F218–F223 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer M. J. et al. New reference values for the neonatal cerebral ventricles. Radiology 262, 224–233, 10.1148/radiol.11110334 (2012). [DOI] [PubMed] [Google Scholar]
- Horsch S., Muentjes C., Franz A. & Roll C. Ultrasound diagnosis of brain atrophy is related to neurodevelopmental outcome in preterm infants. Acta Paediatr 94, 1815–1821, 10.1080/08035250500297745 (2005). [DOI] [PubMed] [Google Scholar]
- Mewes A. U. et al. Displacement of brain regions in preterm infants with non-synostotic dolichocephaly investigated by MRI. NeuroImage 36, 1074–1085, 10.1016/j.neuroimage.2007.04.011 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajdel-Sulkowska E. M. Brain development, environment and sex: what can we learn from studying graviperception, gravitransduction and the gravireaction of the developing CNS to altered gravity? Cerebellum 7, 223–239, 10.1007/s12311-008-0001-8 (2008). [DOI] [PubMed] [Google Scholar]
- Kusuda S., Fujimura M., Uchiyama A., Totsu S. & Matsunami K. Trends in morbidity and mortality among very-low-birth-weight infants from 2003 to 2008 in Japan. Pediatr Res 72, 531–538, 10.1038/pr.2012.114 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruegger C., Hegglin M., Adams M. & Bucher H. U. Population based trends in mortality, morbidity and treatment for very preterm- and very low birth weight infants over 12 years. BMC pediatrics 12, 17, 10.1186/1471-2431-12-17 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.