Abstract
The ability to spatially control cell signaling can help resolve fundamental biological questions. Optogenetic and chemical dimerization techniques along with fluorescent biosensors to report cell signaling activities have enabled researchers to both visualize and perturb biochemistry in living cells. A number of approaches based on mechanical actuation using force-field gradients have emerged as complementary technologies to manipulate cell signaling in real time. This review covers several technologies, including optical, magnetic, and acoustic control of cell signaling and behavior and highlights some studies that have led to novel insights. I will also discuss some future direction on repurposing mechanosensitive channel for mechanical actuation of spatial cell signaling.
Main Text
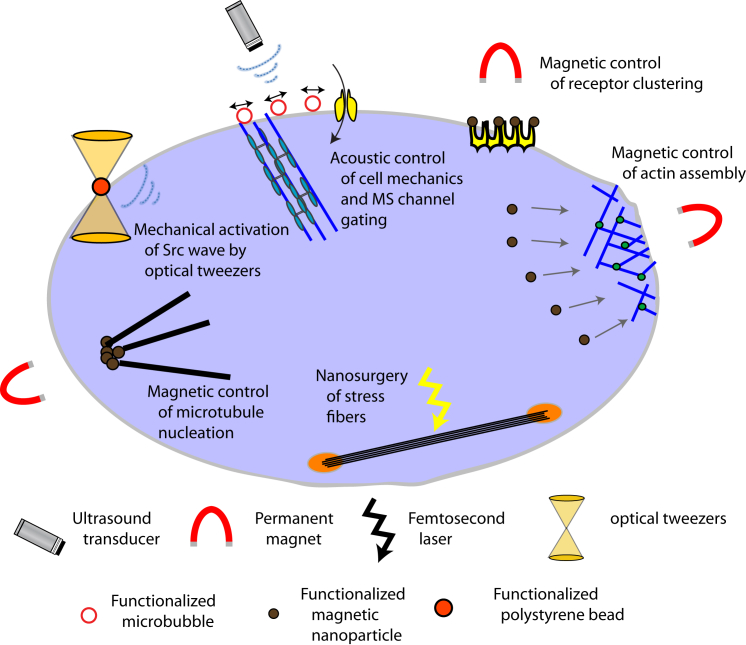
The ability to sense and respond physiologically to environmental cues is a hallmark of all cellular life. The field of signal transduction emerges from the seminal work of Martin Rodbell in the 1970s, where he examined the effects of glucagon on membrane receptors from rat liver cells (1). This led to the 1994 Nobel Prize in Physiology or Medicine that he shared with Alfred Gilman. The explosive growth of signal transduction studies since then has revealed the complexity of signaling pathways. Classically, signal transduction involves the binding of extracellular signaling molecules and ligands to cell-surface receptors that triggers events inside the cell leading to cellular responses. This is complimented by the concept originated in the 1980s that cellular responses to soluble hormones may be regulated mechanically through changes in extracellular matrix structure and mechanics (2), which is now referred to as mechanotransduction. Today, it is well recognized that mechanical stimuli can influence a myriad of cellular processes, including differentiation, proliferation, and cell migration via altered signaling. This is in part due to the development and availability of biophysical tools that have enabled mechanical actuation of single cells with subcellular resolution. This review focuses on the application of biophysical tools, in particular ones based on using a force field (i.e., optical, magnetic, and acoustic), which allow for the interrogation of subcellular mechanotransduction and manipulation of cell signaling (Fig. 1).
Figure 1.
Mechanical actuation of cell signaling and cytoskeleton remodeling by field gradient approaches including optical tweezers, magnetic tweezers, acoustic tweezing cytometry, and nanosurgery. Cartoon depicts some example studies described in this article. To see this figure in color, go online.
Active modes of mechanotransduction
Ricca et al. (3) recently proposed a framework for classifying mechanotransduction in terms of their inputs and outputs, which is a helpful starting point to parse out a growing body of mechanobiology literature. Passive mechanical inputs include physical properties such as substrate stiffness, ECM alignment, and adhesive affinity….etc. The static physical property of the microenvironment can have a profound impact on cell physiology, producing active biological outputs in cytoskeleton remodeling, gene expression, changes in membrane trafficking, altered signaling, and stem cell differentiation (4, 5, 6, 7, 8, 9). Active inputs, on the other hand, encompass externally applied forces, such as cell stretching and fluid shear stress. These dynamic active inputs also can generate active biological outputs by altering cytoskeleton and signaling (10, 11), and have a response timescale ranging from seconds (signaling and contractile responses) to weeks (proliferation and differentiation). At the single cell level, forces at the level of tens of piconewtons to nanonewtons necessitate the utility of specialized approaches to mechanically actuate biochemical signaling.
Feel the force: overview of field gradient methods
The easiest way to mechanically perturb something is through physical contact with a probe. For decades, glass microneedles have been used as force probes to investigate biological systems from single actin filament mechanics to muscle physiology (12, 13). With the development of atomic force microscopy, more sophisticated cantilever force probes with sensitive force resolution have provided measurements of forces generated by single cells and in vitro cytoskeleton networks (14, 15). Because of the requirement of a physical probe as a force transducer, force application is limited locally to the surface contact to a cell. As a significant number of probe-based studies focus on studying cells’ passive material properties, I will instead focus on force-field methods for manipulating cellular mechanical environments as an approach to study active cellular outputs in organizing cell signaling and cytoskeleton remodeling.
Common force-field methods include the use of electric, optical, magnetic, and hydrodynamic fields to generate traps (in some cases), and offer a contact-free approach for force transduction. Forces can be applied globally over the entire cell or locally via the use of a force handle (not to be confused with force probes). These traps can be understood as potential energy wells where forces on a cell or a force handle are balanced. For microscale cell manipulation with electric forces, high-gradient electric field is used in dielectrophoresis where a neutral particle experiences a net force up or down the electric field, depending on the polarizability of the particle compared to the medium (16, 17). Optical tweezers work by the conservation of momentum on spherical particles (for particles greater than wavelength of light) where a change of momentum from photons that strike the particle imparts an equal and opposite momentum change on the particle, thus drawing the particle to the most intensive part of a beam (18). With a similar concept to optical tweezers, magnetic tweezers use a pair of permanent magnets to generate a constant magnetic force to move or rotate a paramagnetic particle due to the large characteristic length of the magnetic field gradient (19). Detailed working principle for these force spectroscopy techniques and their applications to single molecule studies where they have made the most impact have been well described in other reviews (20). In combination with microfluidics, one area where field gradient methods have contributed in recent years is in cell separation applications, where cells can be handled as objects to be moved around (21, 22, 23, 24). Besides whole cell manipulation, these techniques have been harnessed for subcellular mechanical actuation to enable more precise control of cytoskeleton remodeling and signaling pathways. In the sections below, I will discuss various force application approaches in the context of remote control of subcellular activities.
Mechanical activation of signaling by optical tweezers
Optical tweezers function by tightly focusing a laser beam to a diffraction-limited spot using a high numerical aperture objective where the gradient force exerts an attractive force (typically between 1 and 500 pN) toward the focus of the beam. By combining mechanical actuation of fibronectin-coated polystyrene bead using optical tweezers and a genetically encoded Src biosensor based on fluorescence resonance energy transfer, Wang et al. (25) observed directional Src wave propagation along the plasma membrane and a rapid distal Src activation. In addition, this complex spatial signal transduction pattern induced by local mechanical stimulation depended on an intact actin cytoskeleton.
One of the most complex signaling systems is in the immune system where a T cell needs to differentiate between large numbers of antigens before eliciting a proper immune response. Using antigenic peptide bound to major histocompatibility complex-coated bead on T cells to simulate the interactions between T cell receptor complex and major histocompatibility complex ligation, an optical tweezers assay revealed that an external tangential force, but not a normal force, is stimulatory in inducing a calcium flux (26). This study highlights the T cell receptor as an evolved mechanosensor that is activated by direction-specific physical force.
The use of glass or polystyrene beads as force handles is usually required for optical tweezers experiments, but cell-cell interfaces can be directly manipulated by optical tweezers, and thus not limited to manipulating single molecules. Applying precise optical forces in epithelial cell junctions in Drosophila embryo using optical tweezers in a pull-and-release fashion, Bambardekar et al. (27) reported time-dependent cell junctional mechanics properties consistent with a viscoelastic model. By comparison, manipulating single molecules in living cells by optical tweezers is extremely challenging and it has been restricted to force-measurement studies (28), and the application of optical tweezers for manipulating subcellular signaling has not been realized.
Optical ablation of intracellular structures
While optical tweezers can trap and manipulate individual gold nanorods as slender as 8 nm in diameter (29), heating and the creation of free radicals can have detrimental effects upon cell physiology (30, 31). Endogenous particles such as lipid granules can be used as intracellular force handles (32), but the specific biological context of lipid granules and the aforementioned limitations with metallic nanostructures make it challenging to adopt optical tweezers for intracellular manipulation.
Using the ablation property of a focused laser, it is possible to perform nanosurgery in living cells with diffraction-limited resolution. Using near-infrared low-energy, low-repetition-rate femtosecond laser pulses, the optical ablation technique is a noninvasive one, and subcellular ablation does not compromise cell viability nor affect neighboring structures (33). Nanosurgery by optical ablation has been used to ablate cellular structures including mitochondria, Golgi, centrosomes, spindle microtubules, and actin stress fibers (33, 34, 35, 36, 37). Notably, Kumar et al. (34) observed viscoelastic retraction of single actin stress fiber in living cells using nanosurgery and demonstrated that tensional prestress exists in stress fiber bundles. Leveraging the precision of subcellular control of nanosurgery, the spatial tensional variation was mapped by measuring the viscoelastic recoil of contractile stress fibers (38). A similar study has been performed in Caenorhabditis elegans zygotes in which actomyosin meshwork was ablated and the initial outward velocity of adjacent cortex was proportional to cortical tension (39). In separate studies, we know cytoskeleton tension is tightly connected to the Hippo-Yes-associated protein pathway that controls cell proliferation and organ growth (40, 41, 42). With the advance in genetically encoded, ratiometric biosensors for cell signaling proteins (43), there is some exciting potential in investigating how the distribution of tensile load by optical ablation could modulate spatial signaling.
Magnetic control of intracellular signaling
Compared with optical tweezers, magnetic tweezers have spawned numerous studies in spatial intracellular perturbations based on mechanical actuation of magnetic nanoparticles. Although there are no organelles (at least not normally) that behave as permanent or inducible magnets inside a living cell, superparamagnetic nanoparticles can either be microinjected or endocytosed into cells and used as force handles. A key advantage of magnetic tweezers over optical tweezers for live cell studies is the noninvasive nature of magnetic fields, and that the force application can be highly parallelized (44). Magnetic nanoparticles come in a variety of sizes and coatings in which conjugation of ligand and antibodies can be relatively straightforward because numerous commercially available kits are available. Thus, force-transducing handles targeted to different molecules of interest can be readily made to realize magnetic-controlled, remote mechanical actuation of a variety of signaling pathways and cellular organization (45).
Spatial perturbation using magnetic mechanical actuation works by displacing protein-functionalized nanoparticles, and this can occur in two scenarios: (1) ion channels or receptors at the cell membrane or (2) intracellular distribution of signaling proteins. Attaching magnetic nanoparticles to mechanosensitive (MS) ion channel TREK-1 permitted highly localized force to be applied and subsequently activated the channels (46). Similarly, functionalized magnetic nanoparticles attached to the surface of a single hair cell bundle from a North American bullfrog enabled ultrafast mechanical stimulation of hair cells for studying mechanotransduction of sensory systems (47). In other cases, the mechanical forces are applied to the force handles to spatially organize bound proteins where the mechanics of proteins do not play a direct role. Akin to multivalent ligand for receptor clustering and activation, oligomerization of individual IgE/FcεRI receptor complexes in mast cells by a magnetic switch can recapitulate calcium signaling (48). Because intracellular signaling mostly originates at the cell plasma membrane, spatially targeting ion channels and receptors represents a fitting strategy for remote control of cell signaling.
Because magnetic nanoparticles can be delivered to inside a cell, direct remote control of intracellular signaling without activating cell surface channels or receptors is conceivable. This approach has inspired several unique studies on magnetic control of intracellular signaling.
While endocytosis are typically thought to downregulate incoming signals, signaling endosomes provide physical platforms for cross talk between different signaling pathways (49), and endocytosed magnetic nanoparticles can be manipulated as signaling endosomes. In the context of axonal regeneration after injury, neurotrophin-activated tropomyosin-related kinase B receptors are endocytosed and functions as signaling endosomes. In one study, magnetic nanoparticles functionalized with tropomyosin-related kinase B agonist antibodies were allowed to be endocytosed in primary neurons. In the absence of magnetic field, nanoparticle-activated signaling endosomes were trafficked into nascent neurites and promoted their growth. However, by retarding the trafficking of signaling endosomes using a highly localized gradient of magnetic field, growth cone motility was significantly hampered (50).
Rho-GTPases play a central role in organizing the actin cytoskeleton and their precise spatiotemporal control underlies dynamic morphological changes during cell migration. Five-hundred-nanometer-size magnetic particles conjugated to Rac GTP exchange factor TIAM-1 were microinjected into fibroblasts and used as nanoscale templates to probe the activity of molecular complexes inside living cells. Mechanical actuation to displace a magnetic particle to the cell’s leading edge stimulated protrusive activity and an actin comet tail off the particle (51). Five-hundred-nanometer particles are large enough to clog micropipettes such that low particle concentrations need to be used and their motions are frequently constrained by intracellular obstacles. By using smaller functionalized magnetic nanoparticles down to 15 nm, intracellular diffusive motion of nanoparticles can be used to generate an intracellular protein gradient controlled by magnetic forces with a temporal resolution of a few tens of seconds (52).
Introduction of magnetic nanoparticles by microinjection and endocytosis both have their pros and cons and specific biological contexts to which they are best applied. Another scenario where magnetic remote control of cell signaling is used is in a membrane-free system where direct delivery of magnetic nanoparticles to cell-free extract negates the need for endocytosis and microinjection. A cell extract droplet has been used as a model system for investigating microtubule nucleation and assembly by magnetic control. Gradient of GTPase RanGTP, driven by the GTP exchange factor RCC1, has been shown to stabilize and promote microtubule assembly in extracts (53). Magnetic nanoparticles conjugated to Ran can be added to the extract and encapsulated in single emulsion droplets. Accumulation of Ran-nanoparticles induced microtubule nucleation under a magnetic field (54). Dynein motors can organize the polarized microtubule arrays with their orientation controlled by the direction of magnetic forces. Collectively, these studies highlight the versatility and power of remote control of signaling by magnetic-based mechanical actuation.
Acoustic control of intracellular signaling
Acoustic tweezers present a relatively new technique, one that joins the myriad of single-cell and subcellular manipulation tools. Sound waves carry momentum, and the acoustic radiation force can be used to move particles or cells in almost any medium, thus having advantages over magnetic tweezers that require magnetic particles. Similar to how magnetic tweezers use a magnet to produce a magnetic field, the sound wave is produced by a transducer made of a piezo-electric material that converts electrical energy to mechanical energy to create sound waves when high-frequency AC voltage is applied (55). Like optical and magnetic tweezers, acoustic tweezers have been exploited for cell or droplet separation applications (56, 57).
Recently, a technique termed “acoustic tweezing cytometry” was developed based on mechanical actuation of micron-sized lipid coated gaseous microbubbles (58). Microbubbles can be functionalized to bind to cell surface receptors, and Fan et al. (58) targeted microbubbles to adhesion receptor integrins. Ultrasonic excitation exerts a rapid acoustic radiation force that is counteracted by intracellular cytoskeleton contractility. Local mechanical actuation by acoustic tweezing cytometry did not affect cell viability and led to a transient calcium flux. Using acoustic tweezing cytometry to generate subcellular stress in epithelial cells expressing a bacterial mechanosensitive (MS) channel MscL, Heureaux et al. (59) found that MscL, which is activated by increased membrane tension, can be gated with mechanical actuation that targets the integrin-focal adhesion-cytoskeleton connection. In this context, MscL activation is dependent on an intact cytoskeleton and the study revealed that local cytoskeleton stress can induce an increase in membrane tension to activate MscL. When two microbubbles are bound to a cell, the scattered acoustic fields from one microbubble generate a secondary acoustic radiation force on the neighboring microbubble. This can have a dramatic effect on microbubble behaviors in which microbubbles in an ultrasound field pulsating in phase are attracted to one another, regardless of the direction of the ultrasound wave (60). This type of mechanical actuation is quite unique and suitable for studying force-dependent adhesion-mediated signaling.
While acoustic tweezing cytometry provides a simple yet efficient force application using ultrasound that does not have known negative effects on cell physiology, current applications are limited to force application on the cell surface. This is due to the fact submicron microbubbles are not commercially available and the current sizes cannot be microinjected or endocytosed. Acoustic radiation force is a function of microbubble size and it remains to be tested whether smaller microbubbles could exert biologically relevant forces. Another challenge is that microbubbles are gas-filled and have lower density than cellular fluids and will thus float unless attached to cellular components. Delivery of microbubbles to inside a cell might be a challenge; nonetheless, synthesis of smaller microbubbles may provide potentially exciting opportunities for intracellular mechanical actuation using acoustic tweezing cytometry.
Future outlook
I have presented a brief survey of biophysical approaches that provide complementary strategies to chemical and optogenetic tools for producing a spatial gradient of cell signaling. Chemically inducible dimerization, in particular, has been widely adopted for manipulating spatial signaling by targeting a protein of interest to a specific location inside a cell (61). Because the use of dimerization domains requires using genetically encoded fusion proteins, the organellar membrane targets are predetermined. Optogenetic systems use proteins that change conformation in response to light for controlling spatiotemporal dynamics of signaling proteins, and it has inherently better spatial control than chemically inducible dimerization (62). Optogenetics is an alternative strategy to manipulating localization of signaling proteins with magnetic tweezers, but it also requires introduction of genetically encoded fusion proteins. While very powerful for subcellular control of signaling, optogenetics systems require more engineering work to be done toward optimizing the affinity, photoactivation rates, and wavelengths of photoswitching of photosensitive proteins.
In comparison to chemically inducible dimerization, mechanical actuation can have more precise spatiotemporal control. Even though spatial signaling can be activated both from the cell surface and intracellularly, direct mechanical actuation of intracellular signaling is still relatively challenging, with magnetic control of functionalized nanoparticles currently having the most success. Depending on the application, one key question is whether it is the mechanical force that triggers spatial signaling or the organization of the functionalized force handles. Moving forward, I expect the combination of chemical, optogenetic, and mechanical approaches to manipulate spatial signaling will be a fertile ground for new investigations. Because these approaches are entirely orthogonal, their combinations could enable some unique studies looking at cross talk (competition or synergy) between different signaling pathways.
A second area where innovation abounds is the repurposing of the MS channel for mechanotransduction. The MS channel provides an evolutionarily conserved mechanism for sensing mechanical force at the membrane and it actuates by opening a pore to allow molecular transport (63). Mechanical force is sufficient to trigger a calcium wave in Drosophila oocytes from extracellular calcium entering through MS channels (64). The bacterial MS channel MscL has a nonselective pore opening of ∼2.5 nm and can be expressed and functionally reconstituted by activation with osmotic pressure or microfluidic micropipette aspiration in mammalian cells (59, 65). Furthermore, localized stress on a cell surface can increase membrane tension and induce MS channel opening (59). By combining with mechanical probes such as microfluidic micropipette aspiration (66), tension of the lipid bilayer can be harnessed to control gating of a MS channel and introduce a new dimension of mechanosensitivity that can be exploited for spatial signaling.
Finally, cell signaling spans a wide range of timescale and responds to both sustained and transient input. Extending mechanical actuation to beyond short-term signaling can have profound effect on cell physiology (67). Further development and implementation of mechanical actuation of spatial cell signaling covering these key features will further our basic understanding of cellular signaling and provide opportunity for creating cellular network devices using living cells with defined input-output relationships. Manipulating subcellular signaling will continue to be of great interest to cell and developmental biologists, biophysicists, chemists, and engineers embarking on endeavors to take full control of cells.
Acknowledgments
I thank Sapun Parekh and Jianping Fu for providing helpful feedback on the article.
A.P.L. was partially supported by the National Institutes of Health (DP2 HL117748-01 and R21CA198404-01).
Editor: Ewa Paluch.
References
- 1.Rodbell M. The role of hormone receptors and GTP-regulatory proteins in membrane transduction. Nature. 1980;284:17–22. doi: 10.1038/284017a0. [DOI] [PubMed] [Google Scholar]
- 2.Ingber D.E., Jamieson J.D. Cells as tensegrity structures: architectural regulation of histodifferentiation by physical forces transduced over basement membrane. In: Andersson L.C., Gahmberg C.G., Eklom P., editors. Gene Expression During Normal and Malignant Differentiation. Academic Press; Orlando, FL: 1985. [Google Scholar]
- 3.Ricca B.L., Venugopalan G., Fletcher D.A. To pull or be pulled: parsing the multiple modes of mechanotransduction. Curr. Opin. Cell Biol. 2013;25:558–564. doi: 10.1016/j.ceb.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan X., Heureaux J., Liu A.P. Cell spreading area regulates clathrin-coated pit dynamics on micropatterned substrate. Integr. Biol. (Camb.) 2015;7:1033–1043. doi: 10.1039/c5ib00111k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen J.L., Cooke M.E., Alliston T. ECM stiffness primes the TGFβ pathway to promote chondrocyte differentiation. Mol. Biol. Cell. 2012;23:3731–3742. doi: 10.1091/mbc.E12-03-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang N., Tytell J.D., Ingber D.E. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat. Rev. Mol. Cell Biol. 2009;10:75–82. doi: 10.1038/nrm2594. [DOI] [PubMed] [Google Scholar]
- 7.Liu A.P., Loerke D., Danuser G. Global and local regulation of clathrin-coated pit dynamics detected on patterned substrates. Biophys. J. 2009;97:1038–1047. doi: 10.1016/j.bpj.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engler A.J., Sen S., Discher D.E. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 9.Raucher D., Sheetz M.P. Membrane expansion increases endocytosis rate during mitosis. J. Cell Biol. 1999;144:497–506. doi: 10.1083/jcb.144.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu B., Lu S., Wang Y. RhoA and membrane fluidity mediates the spatially polarized Src/FAK activation in response to shear stress. Sci. Rep. 2014;4:7008. doi: 10.1038/srep07008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shao Y., Tan X., Liu A.P. Uniaxial cell stretching device for live-cell imaging of mechanosensitive cellular functions. Rev. Sci. Instrum. 2013;84:114304. doi: 10.1063/1.4832977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kishino A., Yanagida T. Force measurements by micromanipulation of a single actin filament by glass needles. Nature. 1988;334:74–76. doi: 10.1038/334074a0. [DOI] [PubMed] [Google Scholar]
- 13.Minozzo F.C., Baroni B.M., Rassier D.E. Force produced after stretch in sarcomeres and half-sarcomeres isolated from skeletal muscles. Sci. Rep. 2013;3:2320. doi: 10.1038/srep02320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parekh S.H., Chaudhuri O., Fletcher D.A. Loading history determines the velocity of actin-network growth. Nat. Cell Biol. 2005;7:1219–1223. doi: 10.1038/ncb1336. [DOI] [PubMed] [Google Scholar]
- 15.Prass M., Jacobson K., Radmacher M. Direct measurement of the lamellipodial protrusive force in a migrating cell. J. Cell Biol. 2006;174:767–772. doi: 10.1083/jcb.200601159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Voldman J. Electrical forces for microscale cell manipulation. Annu. Rev. Biomed. Eng. 2006;8:425–454. doi: 10.1146/annurev.bioeng.8.061505.095739. [DOI] [PubMed] [Google Scholar]
- 17.Graham D.M., Messerli M.A., Pethig R. Spatial manipulation of cells and organelles using single electrode dielectrophoresis. Biotechniques. 2012;52:39–43. doi: 10.2144/000113802. [DOI] [PubMed] [Google Scholar]
- 18.Zhang H., Liu K.K. Optical tweezers for single cells. J. R. Soc. Interface. 2008;5:671–690. doi: 10.1098/rsif.2008.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Vlaminck I., Dekker C. Recent advances in magnetic tweezers. Annu. Rev. Biophys. 2012;41:453–472. doi: 10.1146/annurev-biophys-122311-100544. [DOI] [PubMed] [Google Scholar]
- 20.Neuman K.C., Nagy A. Single-molecule force spectroscopy: optical tweezers, magnetic tweezers and atomic force microscopy. Nat. Methods. 2008;5:491–505. doi: 10.1038/nmeth.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang M.M., Tu E., Butler W.F. Microfluidic sorting of mammalian cells by optical force switching. Nat. Biotechnol. 2005;23:83–87. doi: 10.1038/nbt1050. [DOI] [PubMed] [Google Scholar]
- 22.Gao J., Riahi R., Wong P.K. Electrokinetic focusing and separation of mammalian cells in conductive biological fluids. Analyst (Lond.) 2012;137:5215–5221. doi: 10.1039/c2an35707k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tasoglu S., Khoory J.A., Demirci U. Levitational image cytometry with temporal resolution. Adv. Mater. 2015;27:3901–3908. doi: 10.1002/adma.201405660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhagat A.A., Hou H.W., Han J. Pinched flow coupled shear-modulated inertial microfluidics for high-throughput rare blood cell separation. Lab Chip. 2011;11:1870–1878. doi: 10.1039/c0lc00633e. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y., Botvinick E.L., Chien S. Visualizing the mechanical activation of Src. Nature. 2005;434:1040–1045. doi: 10.1038/nature03469. [DOI] [PubMed] [Google Scholar]
- 26.Kim S.T., Takeuchi K., Reinherz E.L. The αβ T cell receptor is an anisotropic mechanosensor. J. Biol. Chem. 2009;284:31028–31037. doi: 10.1074/jbc.M109.052712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bambardekar K., Clément R., Lenne P.F. Direct laser manipulation reveals the mechanics of cell contacts in vivo. Proc. Natl. Acad. Sci. USA. 2015;112:1416–1421. doi: 10.1073/pnas.1418732112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Norregaard K., Jauffred L., Oddershede L.B. Optical manipulation of single molecules in the living cell. Phys. Chem. Chem. Phys. 2014;16:12614–12624. doi: 10.1039/c4cp00208c. [DOI] [PubMed] [Google Scholar]
- 29.Selhuber-Unkel C., Zins I., Oddershede L.B. Quantitative optical trapping of single gold nanorods. Nano Lett. 2008;8:2998–3003. doi: 10.1021/nl802053h. [DOI] [PubMed] [Google Scholar]
- 30.Peterman E.J., Gittes F., Schmidt C.F. Laser-induced heating in optical traps. Biophys. J. 2003;84:1308–1316. doi: 10.1016/S0006-3495(03)74946-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neuman K.C., Chadd E.H., Block S.M. Characterization of photodamage to Escherichia coli in optical traps. Biophys. J. 1999;77:2856–2863. doi: 10.1016/S0006-3495(99)77117-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sims P.A., Xie X.S. Probing dynein and kinesin stepping with mechanical manipulation in a living cell. ChemPhysChem. 2009;10:1511–1516. doi: 10.1002/cphc.200900113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shen N., Datta D., Mazur E. Ablation of cytoskeletal filaments and mitochondria in live cells using a femtosecond laser nanoscissor. Mech. Chem. Biosyst. 2005;2:17–25. [PubMed] [Google Scholar]
- 34.Kumar S., Maxwell I.Z., Ingber D.E. Viscoelastic retraction of single living stress fibers and its impact on cell shape, cytoskeletal organization, and extracellular matrix mechanics. Biophys. J. 2006;90:3762–3773. doi: 10.1529/biophysj.105.071506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tängemo C., Ronchi P., Reynaud E.G. A novel laser nanosurgery approach supports de novo Golgi biogenesis in mammalian cells. J. Cell Sci. 2011;124:978–987. doi: 10.1242/jcs.079640. [DOI] [PubMed] [Google Scholar]
- 36.Khodjakov A., Cole R.W., Rieder C.L. Centrosome-independent mitotic spindle formation in vertebrates. Curr. Biol. 2000;10:59–67. doi: 10.1016/s0960-9822(99)00276-6. [DOI] [PubMed] [Google Scholar]
- 37.Grill S.W., Gönczy P., Hyman A.A. Polarity controls forces governing asymmetric spindle positioning in the Caenorhabditis elegans embryo. Nature. 2001;409:630–633. doi: 10.1038/35054572. [DOI] [PubMed] [Google Scholar]
- 38.Tanner K., Boudreau A., Kumar S. Dissecting regional variations in stress fiber mechanics in living cells with laser nanosurgery. Biophys. J. 2010;99:2775–2783. doi: 10.1016/j.bpj.2010.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mayer M., Depken M., Grill S.W. Anisotropies in cortical tension reveal the physical basis of polarizing cortical flows. Nature. 2010;467:617–621. doi: 10.1038/nature09376. [DOI] [PubMed] [Google Scholar]
- 40.Rauskolb C., Sun S., Irvine K.D. Cytoskeletal tension inhibits Hippo signaling through an Ajuba-Warts complex. Cell. 2014;158:143–156. doi: 10.1016/j.cell.2014.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dupont S., Morsut L., Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 42.Sun Y., Yong K.M., Fu J. Hippo/YAP-mediated rigidity-dependent motor neuron differentiation of human pluripotent stem cells. Nat. Mater. 2014;13:599–604. doi: 10.1038/nmat3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fritz R.D., Letzelter M., Pertz O. A versatile toolkit to produce sensitive FRET biosensors to visualize signaling in time and space. Sci. Signal. 2013;6:rs12. doi: 10.1126/scisignal.2004135. [DOI] [PubMed] [Google Scholar]
- 44.Tseng P., Judy J.W., Di Carlo D. Magnetic nanoparticle-mediated massively parallel mechanical modulation of single-cell behavior. Nat. Methods. 2012;9:1113–1119. doi: 10.1038/nmeth.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bonnemay L., Hoffmann C., Gueroui Z. Remote control of signaling pathways using magnetic nanoparticles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015;7:342–354. doi: 10.1002/wnan.1313. [DOI] [PubMed] [Google Scholar]
- 46.Hughes S., McBain S., El Haj A.J. Selective activation of mechanosensitive ion channels using magnetic particles. J. R. Soc. Interface. 2008;5:855–863. doi: 10.1098/rsif.2007.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee J.H., Kim J.W., Cheon J. Magnetic nanoparticles for ultrafast mechanical control of inner ear hair cells. ACS Nano. 2014;8:6590–6598. doi: 10.1021/nn5020616. [DOI] [PubMed] [Google Scholar]
- 48.Mannix R.J., Kumar S., Ingber D.E. Nanomagnetic actuation of receptor-mediated signal transduction. Nat. Nanotechnol. 2008;3:36–40. doi: 10.1038/nnano.2007.418. [DOI] [PubMed] [Google Scholar]
- 49.Pálfy M., Reményi A., Korcsmáros T. Endosomal crosstalk: meeting points for signaling pathways. Trends Cell Biol. 2012;22:447–456. doi: 10.1016/j.tcb.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steketee M.B., Moysidis S.N., Goldberg J.L. Nanoparticle-mediated signaling endosome localization regulates growth cone motility and neurite growth. Proc. Natl. Acad. Sci. USA. 2011;108:19042–19047. doi: 10.1073/pnas.1019624108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Etoc F., Lisse D., Dahan M. Subcellular control of Rac-GTPase signalling by magnetogenetic manipulation inside living cells. Nat. Nanotechnol. 2013;8:193–198. doi: 10.1038/nnano.2013.23. [DOI] [PubMed] [Google Scholar]
- 52.Etoc F., Vicario C., Dahan M. Magnetogenetic control of protein gradients inside living cells with high spatial and temporal resolution. Nano Lett. 2015;15:3487–3494. doi: 10.1021/acs.nanolett.5b00851. [DOI] [PubMed] [Google Scholar]
- 53.Kaláb P., Pralle A., Weis K. Analysis of a RanGTP-regulated gradient in mitotic somatic cells. Nature. 2006;440:697–701. doi: 10.1038/nature04589. [DOI] [PubMed] [Google Scholar]
- 54.Hoffmann C., Mazari E., Gueroui Z. Spatiotemporal control of microtubule nucleation and assembly using magnetic nanoparticles. Nat. Nanotechnol. 2013;8:199–205. doi: 10.1038/nnano.2012.246. [DOI] [PubMed] [Google Scholar]
- 55.Marx V. Biophysics: using sound to move cells. Nat. Methods. 2015;12:41–44. doi: 10.1038/nmeth.3218. [DOI] [PubMed] [Google Scholar]
- 56.Ding X., Peng Z., Huang T.J. Cell separation using tilted-angle standing surface acoustic waves. Proc. Natl. Acad. Sci. USA. 2014;111:12992–12997. doi: 10.1073/pnas.1413325111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee C., Lee J., Shung K.K. Microfluidic droplet sorting with a high frequency ultrasound beam. Lab Chip. 2012;12:2736–2742. doi: 10.1039/c2lc21123h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fan Z., Sun Y., Fu J. Acoustic tweezing cytometry for live-cell subcellular modulation of intracellular cytoskeleton contractility. Sci. Rep. 2013;3:2176. doi: 10.1038/srep02176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heureaux J., Chen D., Liu A.P. Activation of a bacterial mechanosensitive channel in mammalian cells by cytoskeletal stress. Cell. Mol. Bioeng. 2014;7:307–319. doi: 10.1007/s12195-014-0337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen D., Sun Y., Deng C.X. Two-bubble acoustic tweezing cytometry for biomechanical probing and stimulation of cells. Biophys. J. 2015;108:32–42. doi: 10.1016/j.bpj.2014.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.DeRose R., Miyamoto T., Inoue T. Manipulating signaling at will: chemically-inducible dimerization (CID) techniques resolve problems in cell biology. Pflugers Arch. 2013;465:409–417. doi: 10.1007/s00424-012-1208-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tischer D., Weiner O.D. Illuminating cell signalling with optogenetic tools. Nat. Rev. Mol. Cell Biol. 2014;15:551–558. doi: 10.1038/nrm3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Anishkin A., Loukin S.H., Kung C. Feeling the hidden mechanical forces in lipid bilayer is an original sense. Proc. Natl. Acad. Sci. USA. 2014;111:7898–7905. doi: 10.1073/pnas.1313364111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kaneuchi T., Sartain C.V., Wolfner M.F. Calcium waves occur as Drosophila oocytes activate. Proc. Natl. Acad. Sci. USA. 2015;112:791–796. doi: 10.1073/pnas.1420589112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee L.M., Liu A.P. A microfluidic pipette array for mechanophenotyping of cancer cells and mechanical gating of mechanosensitive channels. Lab Chip. 2015;15:264–273. doi: 10.1039/c4lc01218f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee L.M., Liu A.P. The application of micropipette aspiration in molecular mechanics of single cells. J. Nanotechnol. Eng. Med. 2014;5:0408011–0408016. doi: 10.1115/1.4029936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen D., Sun Y., Fu J. Improving survival of disassociated human embryonic stem cells by mechanical stimulation using acoustic tweezing cytometry. Biophys. J. 2015;108:1315–1317. doi: 10.1016/j.bpj.2015.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]