Abstract
Cytoplasmic dynein and kinesin are both microtubule-based molecular motors but are structurally and evolutionarily unrelated. Under standard conditions, both move with comparable unloaded velocities toward either the microtubule minus (dynein) or plus (most kinesins) end. This similarity is important because it is often implicitly incorporated into models that examine the balance of cargo fluxes in cells and into models of the bidirectional motility of individual cargos. We examined whether this similarity is a robust feature, and specifically whether it persists across the biologically relevant temperature range. The velocity of mammalian cytoplasmic dynein, but not of mammalian kinesin-1, exhibited a break from simple Arrhenius behavior below 15°C—just above the restrictive temperature of mammalian fast axonal transport. In contrast, the velocity of yeast cytoplasmic dynein showed a break from Arrhenius behavior at a lower temperature (∼8°C). Our studies implicate cytoplasmic dynein as a more thermally tunable motor and therefore a potential thermal regulator of microtubule-based transport. Our theoretical analysis further suggests that motor velocity changes can lead to qualitative changes in individual cargo motion and hence net intracellular cargo fluxes. We propose that temperature can potentially be used as a noninvasive probe of intracellular transport.
Introduction
Intracellular transport along microtubules (MTs) facilitates and maintains cellular order, and makes possible the existence of spatially extended cells such as neurons. In most cells, cargo transport operates in a variety of environmental conditions, including a wide range of temperatures. Even for mammals, this range can be as wide as 5–45°C for hibernating species (1). The temperature dependence of in vivo transport velocities has often been reported to be Arrhenius-like (2), but non-Arrhenius deviations, including cold block of transport below ∼12°C, are also well established (2). The origin of this rich set of in vivo phenotypes is unclear.
Intracellular transport is often driven by small ensembles (n = ∼2–6) of molecular motors (3, 4). When opposite polarity motors are present in the same ensemble, the sign and magnitude of net cargo velocity reflect the balance of competitive or alternating mechanochemical activity of various motors (5, 6, 7). Thus, it is impossible to gain a quantitative understanding of temperature-dependent in vivo phenotypes without knowing the temperature dependence of the enzymatic activity of individual kinesin and dynein motors that drive transport. However, this aspect of motor function is poorly understood. Previous variable-temperature in vitro motility assays with kinesins (8, 9, 10) have been limited to a relatively narrow temperature window that roughly covers the survival range of most mammals. Broader ranges have been explored via biochemical methods (11, 12), but not biophysical ones. Although published data on kinesin is somewhat limited, scant variable temperature data are available for cytoplasmic dynein. In this work, we aimed to address this gap in understanding by examining kinesin and cytoplasmic dynein motilities under controlled in vitro conditions, with identical environmental factors affecting motor activity.
We first used bead assays to examine how the velocities and force-production abilities of kinesin-1 and mammalian cytoplasmic dynein motors change with temperature. Measurements of velocity were prioritized over measurements of the motor enzymatic rate because mechanochemical coupling efficiency remains controversial for many motors (especially cytoplasmic dynein) even at the single-molecule level. We were surprised to find significant differences between kinesin and dynein motilities as a function of temperature. We use theoretical modeling to examine how our findings relate to motor ensemble performance, and further discuss the implications of our data for biological phenotypes and cell biology experiments.
Materials and Methods
Motor purification
Mammalian cytoplasmic dynein was purified from rat brain as previously described (13). Full-length KIF5A heavy-chain dimers were expressed and purified as previously described (14). The minimal, GST-dimerized Saccharomyces cerevisiae dynein construct (15) (purified protein) was a generous gift from the lab of Dr. Ronald Vale.
In vitro motility assay
In vitro motility assays involving attachment of motor proteins to 1-μm-diameter polystyrene beads were performed as previously described (14). Motility and force production were determined using a 980 nm laser trap in accordance with previously described protocols (16) except that bead positions were recorded using a high-speed video camera (MQ003MG-CM; Ximea, Golden, CO) at 4000 fps and subsequently tracked using custom software (MATLAB, The MathWorks, Natick, MA). This approach was used to avoid condenser contact with the sample and thereby ensure better temperature uniformity and control. Note that the pKa shift of our PIPES-based buffers was negligible over the relevant temperature range.
For motility measurements, the optical trap was shut off as soon as bead binding to MTs was observed. Processivity was measured for bead binding fractions of 0.3 or below.
Temperature control
The full details of our setup differ from existing variable-temperature optical trapping setups (10, 17, 18, 19) and will be described in detail elsewhere. Briefly, a customized Peltier thermoelectric stage (PE120; Linkam, Tadworth, UK) was used to control the slide temperature. A sapphire cover glass was used to maximize heat conductivity between the temperature control plate and the assay. We ensured good temperature contact by using a thin layer of water between the sapphire cover glass and the thermal stage. We also minimized heat sinking by eliminating condenser contact with the sapphire cover glass. To do so, we avoided using back focal plane interferometry to record and track bead positions, and instead used a high-speed camera (recording rate: 4000 fps) and custom video tracking software for this purpose. We calibrated the assay sample temperature with ±0.5°C precision using several independent noncontact methods, including measuring bead diffusion (20, 21, 22), performing fixed-point calibration, and measuring the thermal shift of the peak of emission spectra for Cd-Se and Cd-Te quantum dot fluorescence (23). Further details of the temperature control and calibration will be published elsewhere.
Simulations
We used the stochastic model developed by Kunwar et al. (24, 25) to simulate the temperature dependence of bidirectional cargo transport by multiple molecular motors of opposite types. The details of our model are further described in Supporting Materials and Methods in the Supporting Material.
Results
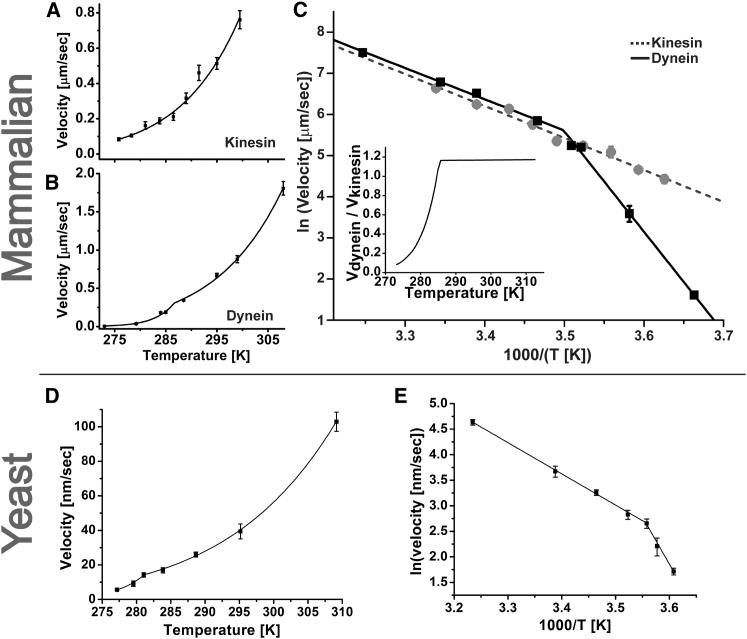
We first examined the temperature dependence of the velocity of mammalian cytoplasmic dynein and mammalian kinesin-1 (Fig. 1). The velocities of beads driven by the kinesin-1 family member KIF5A and mammalian cytoplasmic dynein (in the absence of any regulatory cofactors; Fig. S1) were similar between ∼15°C and ∼27°C (Fig. 1 A) and followed a simple Arrhenius temperature dependence in this range. The activation energy extracted from the Arrhenius fit to the velocity data was ∼65 kJ/mol, which is slightly higher than that previously reported for another kinesin-1 isoform, KIF5B (9). This trend persisted above 27°C when velocity was measured within ∼10 min of temperature change. However, beads were increasingly immotile on MTs afterward, consistent with previous reports of kinesin denaturation at elevated temperatures (8, 10). The activation energy for mammalian cytoplasmic dynein above ∼15°C was just slightly below that of KIF5A: ∼59 kJ/mol and dynein showed no signs of motor degradation up to 37°C.
Figure 1.
Temperature impacts the velocities of kinesin and mammalian cytoplasmic dynein differently. (A) Kinesin velocity and Arrhenius fit (solid line) down to 8°C. (B) Dynein velocity and fit to a piecewise Arrhenius trend (solid line) with a crossover at ∼15°C. (C) Direct comparison of the trends in (A) (dashed gray line) and (B) (solid black line) on the logarithmic Arrhenius plot. Inset: ratio of the fit curves obtained in (A) and (B) plotted on a linear scale. (D) Yeast cytoplasmic dynein velocity and fit to a piecewise Arrhenius trend (solid line) with a crossover at ∼8°C (activation energy 50.5 kJ/mol and 151.5 kJ/mol above and below 8°C, respectively). (E) Arrhenius plot of (D). Error bars, mean ± SE.
In contrast to kinesin-1, the velocity of mammalian cytoplasmic dynein (but not kinesin-1) crossed over to a distinct Arrhenius trend below 15°C (Fig. 1, B and C) with an effective activation energy of ∼154 kJ/mol. The net effect was that the dynein velocity declined far more quickly than the kinesin velocity (Fig. 1 C). To test whether the rapid decline in velocity at low temperatures is a universal feature of cytoplasmic dyneins, we measured the temperature dependence of velocity for a minimal recombinant S. cerevisiae cytoplasmic dynein construct whose movement has biophysical characteristics similar to those of native yeast dynein (15). We found that this dynein also showed a break from simple Arrhenius behavior, but at a much lower temperature: the Arrhenius trend at high temperatures had an activation energy of 50.5 kJ/mol above 8°C and 151.5 kJ/mol below that point (Fig. 1, D and E).
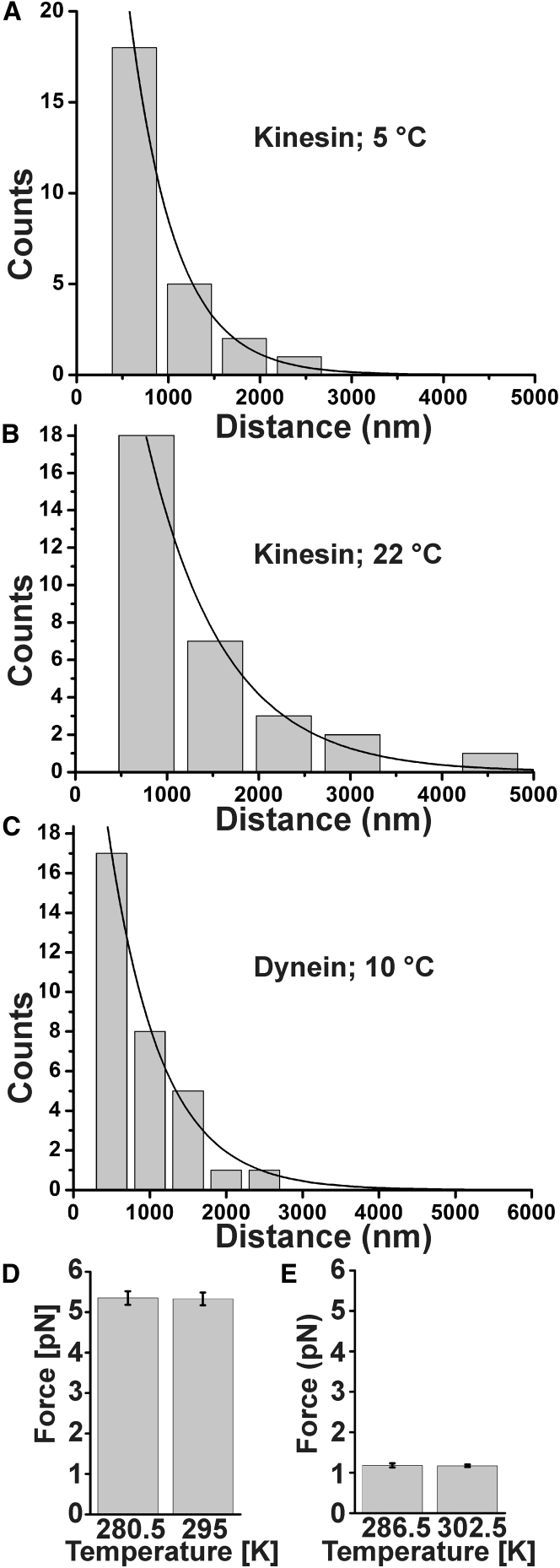
The striking divergence between dynein and kinesin velocities at low temperatures prompted us to examine whether other key motility parameters showed a comparable change at low temperatures (Fig. 2). We found that cytoplasmic dynein’s processivity was 0.69 ± 0.09 μm/s at 10°C. This value is within error bars of the processivity measured for identically purified cytoplasmic dynein at room temperature (0.74 ± 0.08 μm/s (13)). We observed a statistically significant decline in processivity for kinesin-1, from 841.5 ± 0.2 μm/s at room temperature to 497.7 ± 0.09 μm/s at 5°C, confirming a previous qualitative observation of a decline in kinesin’s processivity with temperature (10). We also measured kinesin (Fig. 2 D) and dynein (Figs. 2 E and S2) force production at the lowest temperatures where motility was fast enough to allow for reliable force measurements, and observed no statistically significant changes relative to room temperature. In the case of KIF5A kinesin-1, this parallels and slightly extends a previous report for KIF5B (10).
Figure 2.
Temperature dependence of kinesin and mammalian cytoplasmic dynein processivity and stall force. (A and B) Kinesin processivity at 5°C (A) is 497.7 ± 0.09 μm/s, which is significantly lower than the 841.5 ± 0.2 μm/s obtained at room temperature (B). (C) Dynein processivity is 0.69 ± 0.09 μm/s at 10°C. Error bars, mean ± SE. (D and E) Force production by single kinesin (D) and mammalian cytoplasmic dynein (E) motors (kinesin: 5.3 ± 0.2 pN at 295 K vs. 5.2 ± 0.2 pN at 280.5 K; dynein: 1.2 ± 0.1 pN at 286.5 K vs. 1.2 ± 0.1 pN at 302.5 K; error bars: mean ± SE).
Among the above results, the divergence of kinesin-1 and cytoplasmic dynein velocities at low temperatures stands out as the major qualitative effect because dynein-based motility essentially shuts down below 15°C. By comparison, the decline of kinesin’s processivity is significant, but is not sufficient to effectively abrogate transport. This contrast suggests that the net velocities of cargos driven by heterogeneous ensembles of cytoskeletal motors should show an unusual temperature dependence. Without intracellular regulation, kinesin (but not dynein) could effectively move along MTs at low temperatures. Therefore, we expect that cargos that show net retrograde motion at room temperature will move in the anterograde direction at low temperatures. As dynein activity becomes negligible with decreasing temperature, its main contribution to cargo transport will increasingly consist of just binding-unbinding dynamics. In cases where the force production of kinesins and dyneins on the same cargo is balanced (so that four to six dyneins are available for transport versus each kinesin (6, 7)), we expect that kinesin activity will almost always be opposed by a team of bound dynein motors, and therefore its role will be to bias the cargo position toward the MT plus end so that rebinding events for dynein motors will be biased in that direction. As a result, this transport has the essential character of a Brownian ratchet whereby the random attachment-detachment kinetics is rectified by an enzymatically active agent.
The effect predicted above is essentially qualitative. Consider motor ensembles in which dynein motors dominate transport at room temperature. If the dyneins shut down at low temperature, then, absent additional regulation, the kinesins would be expected to win by default (using sports terminology). We therefore expect that the prediction would be broadly applicable for biological transport, even though, e.g., dynein’s processivity can be modulated substantially by its cofactors (26, 27). However, it is also clear that the predicted effect should be amenable to regulation, including regulation of motor parameters other than velocity. For example, kinesin-based cargo motility can effectively shut down at low temperature if, e.g., the motor processivity drops to near zero. We therefore decided to explore quantitatively the range of conditions in which our predicted effect could be observable. To do this, we performed bidirectional transport simulations for a variable number of kinesin and dynein motors under a variety of realistic motor parameters. In this report, we focus primarily on the case of one kinesin versus four dyneins; however, we also considered a case in which kinesin and dynein forces were balanced, and an example of such a simulation is shown in Fig. S3.
Our simulations indeed demonstrate the change in transport directionality at low temperatures (Figs. 3 and 4; see Supporting Materials and Methods for simulation details). Furthermore, they predict this effect not only for realistic motor parameters (Figs. S4 and S5) but also for parameter values that were recently obtained in the presence of dynein cofactors:
Processivity. Our simulations revealed reversals in transport directionality even when dynein processivity was changed by as much as 10-fold (Figs. 4 B and S6) (26, 27). In addition, we also expect the effect to be present regardless of whether kinesin’s processivity is temperature dependent (Fig. S4) or independent (Fig. S5).
Motor force production. Reversals in transport directionality also occurred in simulations in which dynein and kinesin motor stall forces were balanced. The stall force of 2.5 pN was chosen to model the case in Ref. (28) (Fig. S3); however, a different stall force choice would not alter the conclusions qualitatively.
Motor persistence. The detachment rate of kinesin and dynein motors is known to strongly affect the character of bidirectional transport (24). Therefore, we simulated ensemble motor motility using detachment kinetics measured previously by Kunwar et al. (24). The reversal of directional bias with temperature was again a prominent feature of the simulated cargo tracks (Fig. 4, A–C). Simulations in which kinesin and dynein persistence under superstall load was varied also showed a directionality reversal with temperature.
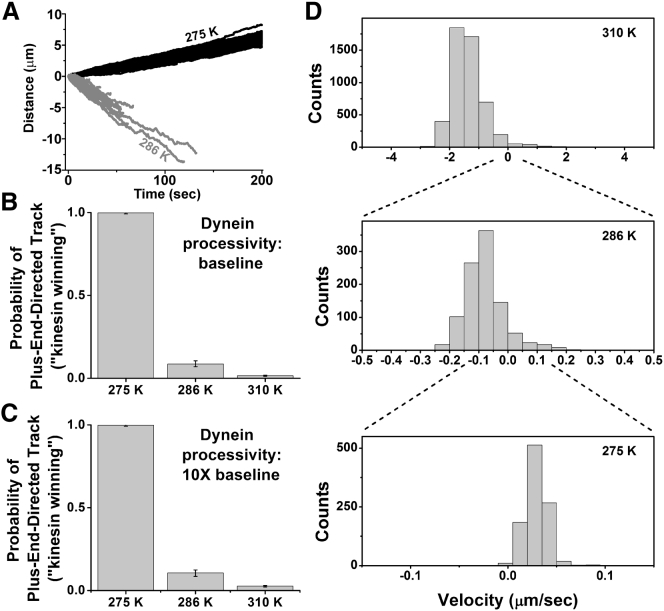
Figure 3.
Simulations of cargo transported by a team of one kinesin and four dyneins (5 pN and 1.25 pN stall force, respectively). Motors were simulated using the anisotropic force-detachment relationship (Fig. S4 and Supporting Materials and Methods). (A) Independent traces of simulated bead motion at 275 K (black) and 286 K (dark gray) are shown superimposed (100 traces for each temperature). (B and C) Probability that a simulated trace will have a positive final location (i.e., the probability of kinesin winning) for (B) baseline and (C) 10× higher dynein processivity values at high, intermediate, and low temperatures (310 K, 286 K, 275 K). (D) Transport velocity histograms illustrate that the directional preference reverses sign as a function of temperature. Note that the velocity undergoes large changes with temperature, necessitating the rescaling of the x axis.
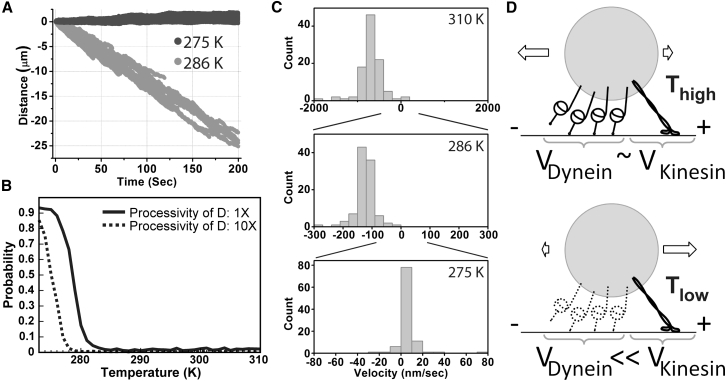
Figure 4.
Simulations of cargo transported by a team of one kinesin and four dyneins (5 pN and 1.25 pN stall force, respectively). Motors were simulated using the isotropic force-detachment relationship (model B, Fig. S5). (A) Independent traces of simulated bead motion at 275 K (dark gray) and 286 K (light gray) are shown superimposed (100 traces for each temperature). (B) Probability that a simulated trace will have a positive final location (i.e., the probability of kinesin winning) for baseline (1×: solid line) and 10× (dashed line) higher dynein processivity values. (C) Transport velocity histograms reveal that the directional preference reverses sign as a function of temperature. Note that the velocity undergoes large changes with temperature, necessitating the rescaling of the x axis. (D) Model: when all motors are active at high temperatures (top), some ensembles of kinesin and dynein motors will exhibit motility with an overall bias in the minus-end direction on MTs. However, at low temperatures (bottom), dynein steps dramatically more slowly than kinesin, leading to an overall transport bias in the plus-end direction, as well as other potentially observable effects (Movies S1, S2, and S3).
Discussion
We have observed that mammalian kinesin-1 and cytoplasmic dynein show divergent mechanochemical activity trends. The extremely high activation energy for dynein at low temperatures means that within just a few degrees below 15°C, dynein transport essentially shuts down relative to kinesin motility. This observation naturally lends itself to understanding the mechanistic origins behind the long-standing mystery of cold block, a phenomenon in which fast axonal transport (FAT) in mammals ceases below ∼12°C, even in animals capable of hibernating (2, 29). It has long been established that cold block cannot be explained by MT depolymerization at low temperatures (29). On the other hand, the strong temperature-dependent decline of dynein-based motility would likely be sufficient to cause a shutdown of dynein-based and kinesin-based FAT at low temperatures in vivo: a halt of dynein motility by means other than temperature typically results in a gradual stoppage of kinesin-based transport as well, due to intracellular motor regulation (30).
If our hypothesis is correct, one may expect the dynein activation energy to evolve smoothly through 15°C in organisms that are not prone to cold block. Indeed, although we observe a similar piecewise Arrhenius trend in yeast cytoplasmic dynein, the trend break occurs at 8°C. This trend break likely carries no physiological implications: cytoplasmic dynein is nonessential in yeast (31), and in addition, MT stability (and hence MT-associated activity) is compromised at such low temperatures (32).
Many biological enzymes show a simple Arrhenius trend for enzymatic activity near room or physiological temperature but break from this trend at low temperatures, i.e., they have a limited thermal dynamic range (33). We propose that the architecture of cytoplasmic dynein allows for tuning of this dynamic range between species. In principle, the observed cytoplasmic dynein velocity trend break at low temperatures could be due to either a change in the enzymatic rate of the motor or a change in mechanochemical coupling between the two motor domains of dynein. In this context, it is very suggestive that the activation energies above and below the break temperature are extremely close for mammalian and yeast dyneins, and in particular are very high at low temperatures. Thus, we speculate that the thermal dynamic range of cytoplasmic dyneins may be tunable via the same mechanism. If so, we propose that regulation of dynein’s thermal dynamic range could occur via its enzymatic domain(s). The AAA ring of dynein certainly has ample complexity (34) to potentially allow for such a mechanism. Coordination between dynein heavy chains is less likely to be the culprit because the velocity of cytoplasmic dyneins is not strongly dependent on the amount of coordination between motor domains (35, 36, 37). Furthermore, if such coordination were important under some conditions, one might naively expect the effect to be different for an artificial dimer (the yeast construct we examined) versus a full cytoplasmic dynein complex.
It is also worthwhile to contrast our observations with recent work (36) that identified the C-terminus domain of the dynein heavy chain (present in mammalian but not yeast motors) as a regulator of dynein’s top force production, which likely accounts for the observed differences between yeast and mammalian motor stall forces. It is conceivable that this effect is due to an interaction between the C-terminus domain and the AAA1 domain (36). However, the unchanged activation energy for motor velocity between yeast and mammalian dynein hints that perhaps the force production is not exclusively set within the enzymatically active part of the motor. For instance, the possibility of the C-terminus domain interacting with dynein’s linker domain (directly or indirectly) has been put forth before (37), and an allosteric effect is structurally feasible (38). One can therefore envision scenarios in which the C-terminus domain modulates linker docking. This would be conceptually (though not structurally) similar to what is seen in kinesin, where the cover-neck bundle affects the neck-linker affinity for the motor domain during the powerstroke, thereby directly affecting motor force production (39, 40).
Our simulations suggest that the reversal of the overall transport direction as a function of temperature is likely to be a very robust effect. This is because the effect arises with very few extremely relaxed starting assumptions. First, bidirectional transport at room temperature needs to be dominated by dynein—a condition that is known to occur for many intracellular cargos. The specific properties of individual dynein motors (processivity, force production, persistence under load, etc.) are not critically important here. All that critically matters is that the dynein ensemble on average wins over the kinesin ensemble at some temperature above the dynein crossover point. The other thing that is needed for directionality reversal is for dynein to effectively cease motility at low temperatures so that the dynamics of the dynein ensemble would be mostly reduced to simple on/off kinetics. We make explicit assumptions in our simulations about the temperature dependence of the on/off rates of dynein as a function of temperature. However, all we really need is for these rates to not differ by many orders of magnitude from the rates at high temperatures. This is a reasonable assumption in light of our experimental data, and would be expected a priori from general biochemistry considerations. Finally, we need the kinesin ensemble to still be active at low temperatures. With these conditions in place, the net cargo motility at low temperatures arises from random binding and unbinding of dynein motors. Indeed, the average number of engaged dyneins remains nearly identical at low temperatures, but the difference grows appreciably at high temperatures as dynein’s processivity increases 10-fold (Fig. S7). This remains true regardless of the force-dissociation model we use for kinesin and dynein motors. The fluctuations are minimally biased by any remnant dynein motility, but they are appreciably biased by the kinesin activity. Our simulations show that regardless of the force-dissociation model we use for kinesin and dynein motors, and for both 1× and 10× choices of dynein processivity, the fraction of time in which kinesin is pulling the cargo with a force equal to or exceeding its stall force grows very gradually as the temperature declines and is above 60% at the lowest temperatures (Fig. S8). Any unbinding dynein motor will diffuse around its anchor point on the cargo, which is displaced however slightly by kinesin motor(s) toward the MT plus end. This dynein is then likely to rebind with an average displacement toward the MT plus end (relative to its previous bound position).
From a biophysics perspective, kinesin activity drives the system far from equilibrium and rectifies the on/off fluctuations of the dynein ensemble. In other words, the multiple motor ensemble at low temperatures behaves mostly as a Brownian ratchet (41), whereas the displacement at high temperatures has a significant or even dominant contribution from the powerstroke mechanism. This transition is at the core of why the effect we propose is robust and likely to be quite insensitive to individual motor properties. What is particularly remarkable about this system is that the transition can happen over a very small temperature window relative to the absolute temperature scale.
Keeping in mind that the directionality reversal is likely to be a robust effect, we propose that temperature regulation may present an opportunity to develop a new approach for probing intracellular transport. The qualitative directionality reversal effect should be observable in cell culture or even in vivo after an acute cold shock to ≤10°C. The shock would need to occur much faster than any possible cold-shock protein expression (42) to provide a temporal window during which a plus- versus minus-end-directed motility imbalance would be observable and quantifiable. In addition, cold shock can induce cell permeabilization with resultant changes in, e.g., metal ion concentrations and cellular pH (42)—all factors that need to be controlled for. Despite such immediate experimental challenges, we envision that the above approach will become increasingly feasible when the response of cell culture to cold shock is generally better understood.
Also of note, activation energies are not identical (12) across the many kinesin families. Therefore, even though the velocities of cytoplasmic dynein and KIF5A measured here (Fig. 1) are fairly well matched above 15°C, this situation cannot hold for all kinesins. We thus speculate that in other systems (e.g., motor ensembles including kinesin-3), bidirectional transport likely requires additional regulation to maintain even qualitative homeostasis across the mammalian survival range. Of course, such temperature-based feedback could be used in cells to sense temperature.
The temperature dependence of fast MT-based transport can be far more complex in lower species than in mammals (2). This suggests one of two possibilities. First, it is possible that cytoplasmic dynein, like its axonemal counterpart (43), is thermally adaptable to match the thermal range of its species. A tantalizing alternative is that an as yet unidentified cofactor(s) can regulate the temperature dependence of dynein motility. This is hinted at by the observation that cold acclimation in poikilotherms can lead to increased rates of FAT at low temperatures (44). Either way, the thermal properties of motor-driven transport and its regulation clearly warrant further study.
Author Contributions
M.V. initiated and guided the project. W.H. and O.O. performed experiments. A.K. directed and guided the theoretical modeling. A.T. implemented the theoretical modeling and contributed to conceptual model refinement. All authors co-wrote the manuscript.
Acknowledgments
We thank the members of Dr. Richard Vallee’s lab for purifying and supplying the mammalian cytoplasmic dynein motors, Dr. R. McKenney and Dr. R. Vale for the generous gift of purified yeast dynein, and Dr. S. Gross and J. Bergman for helpful discussions.
A.K. is supported in part by the Industrial Research and Consultancy Centre at IIT Bombay and by an Innovative Young Biotechnologist Award from the Department of Biotechnology (grant number BT/06/IYBA/2012). This work was supported by National Science Foundation grant number ENG-1563280 to M.V.
Editor: Jennifer Ross.
Footnotes
Supporting Materials and Methods, eight figures, and three movies are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(16)30664-6.
Supporting Material
Data for one representative simulation are shown in movie form (red, dynein; green, kinesin; black, cargo). Motors that are not attached to the MT are not shown, so the number of motors visualized may change from frame to frame.
Data for one representative simulation are shown in movie form (red, dynein; green, kinesin; black, cargo). Motors that are not attached to the MT are not shown, so the number of motors visualized may change from frame to frame.
Data for one representative simulation are shown in movie form (red, dynein; green, kinesin; black, cargo). Motors that are not attached to the MT are not shown, so the number of motors visualized may change from frame to frame.
References
- 1.Carey H.V., Andrews M.T., Martin S.L. Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol. Rev. 2003;83:1153–1181. doi: 10.1152/physrev.00008.2003. [DOI] [PubMed] [Google Scholar]
- 2.Cancalon P. Influence of temperature on various mechanisms associated with neuronal growth and nerve regeneration. Prog. Neurobiol. 1985;25:27–92. doi: 10.1016/0301-0082(85)90022-x. [DOI] [PubMed] [Google Scholar]
- 3.Mallik R., Rai A.K., Kunwar A. Teamwork in microtubule motors. Trends Cell Biol. 2013;23:575–582. doi: 10.1016/j.tcb.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Gross S.P., Vershinin M., Shubeita G.T. Cargo transport: two motors are sometimes better than one. Curr. Biol. 2007;17:R478–R486. doi: 10.1016/j.cub.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 5.Müller M.J.I., Klumpp S., Lipowsky R. Bidirectional transport by molecular motors: enhanced processivity and response to external forces. Biophys. J. 2010;98:2610–2618. doi: 10.1016/j.bpj.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soppina V., Rai A.K., Mallik R. Tug-of-war between dissimilar teams of microtubule motors regulates transport and fission of endosomes. Proc. Natl. Acad. Sci. USA. 2009;106:19381–19386. doi: 10.1073/pnas.0906524106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hendricks A.G., Perlson E., Holzbaur E.L.F. Motor coordination via a tug-of-war mechanism drives bidirectional vesicle transport. Curr. Biol. 2010;20:697–702. doi: 10.1016/j.cub.2010.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Böhm K.J., Stracke R., Unger E. Effect of temperature on kinesin-driven microtubule gliding and kinesin ATPase activity. FEBS Lett. 2000;466:59–62. doi: 10.1016/s0014-5793(99)01757-3. [DOI] [PubMed] [Google Scholar]
- 9.Kawaguchi K., Ishiwata S. Temperature dependence of force, velocity, and processivity of single kinesin molecules. Biochem. Biophys. Res. Commun. 2000;272:895–899. doi: 10.1006/bbrc.2000.2856. [DOI] [PubMed] [Google Scholar]
- 10.Kawaguchi K., Ishiwata S. Thermal activation of single kinesin molecules with temperature pulse microscopy. Cell Motil. Cytoskeleton. 2001;49:41–47. doi: 10.1002/cm.1019. [DOI] [PubMed] [Google Scholar]
- 11.Zhao Y.C., Kull F.J., Cochran J.C. Modulation of the kinesin ATPase cycle by neck linker docking and microtubule binding. J. Biol. Chem. 2010;285:25213–25220. doi: 10.1074/jbc.M110.123067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adio S., Woehlke G. Properties of the kinesin-3 NcKin3 motor domain and implications for neck function. FEBS J. 2009;276:3641–3655. doi: 10.1111/j.1742-4658.2009.07083.x. [DOI] [PubMed] [Google Scholar]
- 13.McKenney R.J., Vershinin M., Gross S.P. LIS1 and NudE induce a persistent dynein force-producing state. Cell. 2010;141:304–314. doi: 10.1016/j.cell.2010.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith T.E., Hong W., Vershinin M. Single-molecule inhibition of human kinesin by adociasulfate-13 and -14 from the sponge Cladocroce aculeata. Proc. Natl. Acad. Sci. USA. 2013;110:18880–18885. doi: 10.1073/pnas.1314132110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reck-Peterson S.L., Yildiz A., Vale R.D. Single-molecule analysis of dynein processivity and stepping behavior. Cell. 2006;126:335–348. doi: 10.1016/j.cell.2006.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vershinin M., Carter B.C., Gross S.P. Multiple-motor based transport and its regulation by Tau. Proc. Natl. Acad. Sci. USA. 2007;104:87–92. doi: 10.1073/pnas.0607919104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Svoboda K., Schmidt C.F., Block S.M. Conformation and elasticity of the isolated red blood cell membrane skeleton. Biophys. J. 1992;63:784–793. doi: 10.1016/S0006-3495(92)81644-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mao H., Arias-Gonzalez J.R., Bustamante C. Temperature control methods in a laser tweezers system. Biophys. J. 2005;89:1308–1316. doi: 10.1529/biophysj.104.054536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bennet M.A., Richardson P.R., Jones A.C. Optically trapped microsensors for microfluidic temperature measurement by fluorescence lifetime imaging microscopy. Lab Chip. 2011;11:3821–3828. doi: 10.1039/c1lc20391f. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez A.C., Phillies G.D.J. Temperature dependence of the diffusion coefficient of polystyrene latex spheres. Biopolymers. 1983;22:593–595. [Google Scholar]
- 21.Müller C.B., Richtering W. Sealed and temperature-controlled sample cell for inverted and confocal microscopes and fluorescence correlation spectroscopy. Colloid Polym. Sci. 2008;286:1215–1222. [Google Scholar]
- 22.Chung K., Cho J.K., Lu H. Three-dimensional in situ temperature measurement in microsystems using Brownian motion of nanoparticles. Anal. Chem. 2009;81:991–999. doi: 10.1021/ac802031j. [DOI] [PubMed] [Google Scholar]
- 23.Li S., Zhang K., Yang H. Single quantum dots as local temperature markers. Nano Lett. 2007;7:3102–3105. doi: 10.1021/nl071606p. [DOI] [PubMed] [Google Scholar]
- 24.Kunwar A., Tripathy S.K., Gross S.P. Mechanical stochastic tug-of-war models cannot explain bidirectional lipid-droplet transport. Proc. Natl. Acad. Sci. USA. 2011;108:18960–18965. doi: 10.1073/pnas.1107841108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kunwar A., Vershinin M., Gross S.P. Stepping, strain gating, and an unexpected force-velocity curve for multiple-motor-based transport. Curr. Biol. 2008;18:1173–1183. doi: 10.1016/j.cub.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schlager M.A., Hoang H.T., Carter A.P. In vitro reconstitution of a highly processive recombinant human dynein complex. EMBO J. 2014;33:1855–1868. doi: 10.15252/embj.201488792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKenney R.J., Huynh W., Vale R.D. Activation of cytoplasmic dynein motility by dynactin-cargo adapter complexes. Science. 2014;345:337–341. doi: 10.1126/science.1254198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shubeita G.T., Tran S.L., Gross S.P. Consequences of motor copy number on the intracellular transport of kinesin-1-driven lipid droplets. Cell. 2008;135:1098–1107. doi: 10.1016/j.cell.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brimijoin S., Olsen J., Rosenson R. Comparison of the temperature-dependence of rapid axonal transport and microtubules in nerves of the rabbit and bullfrog. J. Physiol. 1979;287:303–314. doi: 10.1113/jphysiol.1979.sp012660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yi J.Y., Ori-McKenney K.M., Vallee R.B. High-resolution imaging reveals indirect coordination of opposite motors and a role for LIS1 in high-load axonal transport. J. Cell Biol. 2011;195:193–201. doi: 10.1083/jcb.201104076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eshel D., Urrestarazu L.A., Gibbons I.R. Cytoplasmic dynein is required for normal nuclear segregation in yeast. Proc. Natl. Acad. Sci. USA. 1993;90:11172–11176. doi: 10.1073/pnas.90.23.11172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bellocq C., Andrey-Tornare I., Edelstein S.J. Purification of assembly-competent tubulin from Saccharomyces cerevisiae. Eur. J. Biochem. 1992;210:343–349. doi: 10.1111/j.1432-1033.1992.tb17427.x. [DOI] [PubMed] [Google Scholar]
- 33.Fink A.L., Cartwright S.J. Cryoenzymology. CRC Crit. Rev. Biochem. 1981;11:145–207. [PubMed] [Google Scholar]
- 34.Qiu W., Derr N.D., Reck-Peterson S.L. Dynein achieves processive motion using both stochastic and coordinated stepping. Nat. Struct. Mol. Biol. 2012;19:193–200. doi: 10.1038/nsmb.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeWitt M.A., Chang A.Y., Yildiz A. Cytoplasmic dynein moves through uncoordinated stepping of the AAA+ ring domains. Science. 2012;335:221–225. doi: 10.1126/science.1215804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nicholas M.P., Höök P., Gennerich A. Control of cytoplasmic dynein force production and processivity by its C-terminal domain. Nat. Commun. 2015;6:6206. doi: 10.1038/ncomms7206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Höök P., Mikami A., Vallee R.B. Long range allosteric control of cytoplasmic dynein ATPase activity by the stalk and C-terminal domains. J. Biol. Chem. 2005;280:33045–33054. doi: 10.1074/jbc.M504693200. [DOI] [PubMed] [Google Scholar]
- 38.Kon T., Oyama T., Kurisu G. The 2.8 Å crystal structure of the dynein motor domain. Nature. 2012;484:345–350. doi: 10.1038/nature10955. [DOI] [PubMed] [Google Scholar]
- 39.Hwang W., Lang M.J., Karplus M. Force generation in kinesin hinges on cover-neck bundle formation. Structure. 2008;16:62–71. doi: 10.1016/j.str.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 40.Khalil A.S., Appleyard D.C., Lang M.J. Kinesin’s cover-neck bundle folds forward to generate force. Proc. Natl. Acad. Sci. USA. 2008;105:19247–19252. doi: 10.1073/pnas.0805147105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cubero D., Renzoni F. 1st ed. Cambridge University Press; Cambridge, UK: 2016. Brownian Ratchets: From Statistical Physics to Bio and Nano-motors. [Google Scholar]
- 42.Underhill M.F., Smales C.M. The cold-shock response in mammalian cells: investigating the HeLa cell cold-shock proteome. Cytotechnology. 2007;53:47–53. doi: 10.1007/s10616-007-9048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.King S.M., Marchese-Ragona S.P., Detrich H.W., 3rd Inner and outer arm axonemal dyneins from the Antarctic rockcod Notothenia coriiceps. Biochemistry. 1997;36:1306–1314. doi: 10.1021/bi962229q. [DOI] [PubMed] [Google Scholar]
- 44.Edström A., Hanson M. Temperature effects on fast axonal transport of proteins in vitro in frog sciatic nerves. Brain Res. 1973;58:345–354. doi: 10.1016/0006-8993(73)90006-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data for one representative simulation are shown in movie form (red, dynein; green, kinesin; black, cargo). Motors that are not attached to the MT are not shown, so the number of motors visualized may change from frame to frame.
Data for one representative simulation are shown in movie form (red, dynein; green, kinesin; black, cargo). Motors that are not attached to the MT are not shown, so the number of motors visualized may change from frame to frame.
Data for one representative simulation are shown in movie form (red, dynein; green, kinesin; black, cargo). Motors that are not attached to the MT are not shown, so the number of motors visualized may change from frame to frame.