Abstract
Relapse is the rule rather than the exception among smokers attempting to quit, and compared to men, women may have higher relapse rates. The current study was a randomized clinical trial testing a palmtop computer-delivered treatment for smoking relapse prevention among women. The intervention was individualized based on key theoretical constructs that were measured using ecological momentary assessment (EMA). All participants (N=302) received standard smoking cessation treatment consisting of nicotine replacement therapy and group counseling, and completed EMA procedures for one week after quitting. At the completion of the group counseling sessions and EMA procedures, participants were randomized to either computer-delivered treatment (CDT) or no further computer-delivered treatment or assessment (EMA-Only). CDT participants received a palmtop computer-delivered relapse prevention treatment for one additional month. CDT did not improve abstinence rates relative to EMA-Only. Process analyses suggested that heavier smokers were more likely to use CDT and that greater use among CDT participants may be associated with more positive outcomes. The rapid pace of technological advances in mobile computer technology and the ubiquity of such devices provide a novel platform for developing new and potentially innovative treatments. However, the current study did not demonstrate the efficacy of such technology in improving treatment outcomes.
Keywords: smoking cessation, relapse prevention, women, computer-delivered treatment
Introduction
Although smoking cessation can dramatically reduce the risk of tobacco-related disease (USDHHS, 2001), relapse is the rule rather than the exception among smokers attempting to quit with rates as high as 70–90%, even among smokers who have been abstinent for a week or longer (Brandon, Tiffany, Obremski, & Baker, 1990; Garvey, Bliss, Hitchcock, Heinold, & Rosner, 1992). Although there is not a consensus, there is evidence suggesting that women have higher relapse rates than do men (Wetter et al., 1999) and a recent meta-analysis found that women had higher relapse rates than men across all conventional forms of treatment (Scharf & Shiffman, 2004). Thus, the development of effective relapse prevention treatments has been identified as a priority in reducing smoking among women (USDHHS, 2001).
Social cognitive theory (Bandura, 1986) posits that key variables influencing relapse include affective state, self-efficacy, drug and coping outcome expectancies, and coping behaviors (Marlatt & Donovan, 2005). However, relapse prevention interventions based on social cognitive theory have not yielded consistently superior results relative to other treatment approaches (Carroll, 1996; Hajek, Stead, West, Jarvis, & Lancaster, 2009). One possible strategy for improving the efficacy of relapse prevention interventions is to combat relapse precipitants in real-time during acute episodes of high risk (Carter, Day, Cinciripini, & Wetter, 2007; Shiffman, 2006), congruent with an “episodic” model of relapse (Shiffman, 1989). Real-time treatment might also strengthen adaptive behaviors through repeated exposure and repetition of coping strategies, which could contribute to better acquisition, retention, and use of such skills over time. One strategy for delivering a behavioral relapse prevention intervention in real-time is with the use of palmtop personal computers (PPCs) such as smartphones or personal digital assistants (Carter, Day, Cinciripini, & Wetter, 2007).
PPCs have been used extensively for ecological momentary assessment (EMA). EMA provides a means to collect data in real time in the real world, and EMA research has supported the basic propositions of social cognitive theory, as well as highlighted the dynamic nature of the relapse process (Gwaltney, Shiffman, Balabani & Paty, 2005; Shiffman et al., 2000). Because retrospective recall of cognitions and behaviors surrounding temptations and lapses can be poor and biased (Shiffman, 2005; Stone & Shiffman, 1994), EMA may provide more accurate data for individualization of treatment than data based on traditional self-report approaches (e.g., a PPC-delivered intervention could be individualized based on key theoretical constructs assessed during actual high risk situations, as well as allow for real-time modifications in the treatment program as individuals move through the process of quitting).
A critical advantage of a PPC-delivered intervention is unparalleled access to context-specific quitting strategies in real-time (Carter, Day, Cinciripini, & Wetter, 2007). Moreover, A PPC-delivered intervention might lead to improved abstinence rates for other reasons as well. For example, negative affect interferes with the performance of coping behaviors (Drobes, Meier, & Tiffany, 1994), perhaps by occupying cognitive workspace (Drobes, Elibero, & Evans, 2006), and is a powerful predictor of relapse (Wetter et al., 1999). A real-time intervention may be able to counteract the effects of negative affect on coping behavior and relapse by directly recommending relevant and appropriate context-specific coping strategies, and by eliminating the need for a search of memory (with perhaps the exception of remembering to use the PPC). Given the prominence that negative affect has often been assigned in explaining gender differences in relapse (Walitzer & Dearing, 2006), interventions designed to increase coping during the experience of negative affect could be particularly important for women.
The current study tested a real-time, theoretically-based, PPC-delivered treatment for smoking relapse prevention among women. The intervention was individualized for each woman based on key theoretical constructs derived from social cognitive theory and assessed using EMA. A recent review concluded that incorporating EMA into health behavior interventions using mobile technology should be a major focus of future research (Heron & Smyth, 2010).
Method
Study Design
WIN (Women’s INtervention) was a randomized clinical trial evaluating the efficacy of a PPC-delivered relapse prevention intervention for female smokers (N=302). All participants received standard smoking cessation treatment consisting of group counseling and six weeks of nicotine replacement therapy (NRT; nicotine patches). All participants completed EMA procedures during the week immediately following the quit date. All group counseling sessions and EMA procedures ended on postcessation Day 7. Following completion of the group counseling and EMA procedures on Day 7, participants were randomized to either computer-delivered treatment (CDT; n = 151) or standard treatment (EMA-Only; n = 151). CDT participants then utilized the PPC to receive an individualized relapse prevention intervention for one additional month (from Day 7 until Day 35 postcessation). All participants were followed for one year postcessation. This research was approved by the Institutional Review Boards at both the University of Texas M.D. Anderson Cancer Center and Group Health Research Institute.
Participants
Participants were recruited from the Seattle metropolitan area from 1999 to 2002 using advertisements (local newspapers, radio, bus), public service announcements, and fliers in local clinics. Follow-up was completed in 2003. Individuals were screened for eligibility criteria over the telephone, and eligible respondents attended an orientation during which a detailed study overview was provided, informed consent was obtained, and eligibility criteria were finalized. Inclusion criteria were: female; aged 18 to 70 years; current smoking of at least 10 cigarettes per day for at least the past year; expired breath carbon monoxide (CO) level of 10 parts per million (ppm) or greater; and the ability to speak, read, and write in English. Exclusion criteria were: regular use of tobacco other than cigarettes; active substance abuse disorder, major depression, anxiety disorder, or eating disorder; current use of bupropion; and contraindications for NRT. A modified version of the Primary Care Evaluation of Mental Disorders (PRIME-MD; Spitzer et al., 1995) was used to screen for psychiatric disorders. Ineligible respondents were referred to other smoking cessation programs. As shown in Figure 1, 739 individuals made inquiries about the study, and 302 were eligible, consented to participate, and randomized to treatment.
Figure 1.
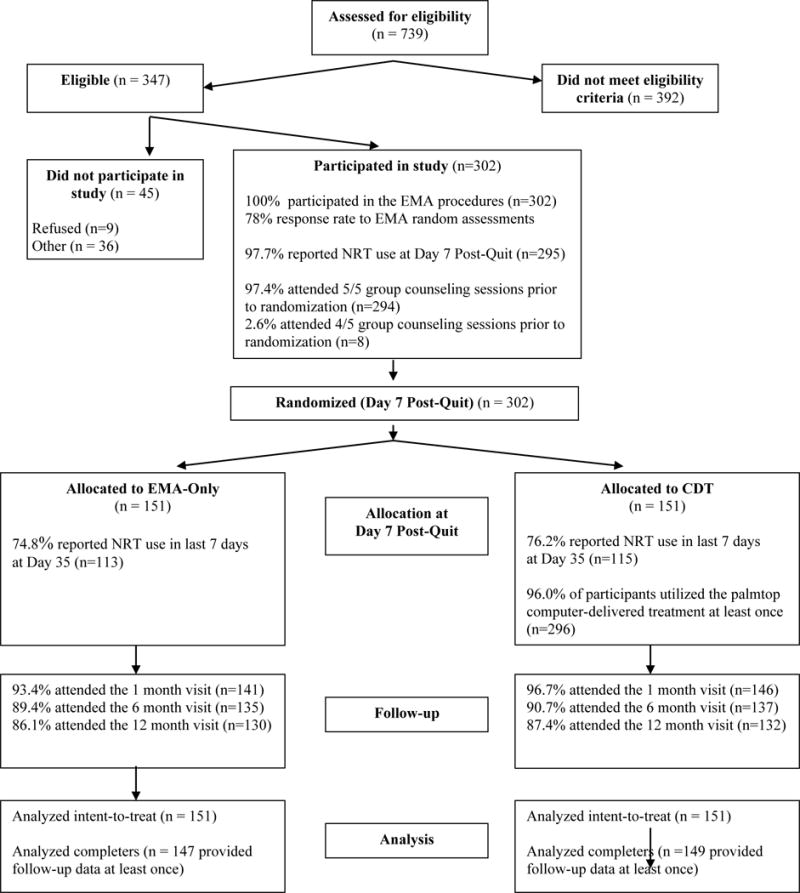
Flow Diagram of Participants from Eligibility Assessment to Follow-up
Procedure
At orientation, clinic visits were scheduled three days before the quit date (Pre-Quit Day -3), Pre-Quit Day -1, three days after the quit date (Day 3), Day 5, Day 7, and Day 35; and follow-up visits 6 and 12 months post-quit day. During each visit, participants completed questionnaires and provided a breath sample to measure CO level. At Pre-Quit Day -1, all participants received a PPC and were asked to complete EMA assessments for one week (until Day 7). EMA procedures were identical for all participants and were completed prior to randomization. After completion of all counseling sessions and EMA procedures at Day 7, participants were randomly assigned to CDT or EMA-Only after stratification for race, cigarettes smoked per day, and depression history. Data collected using EMA were used to individualize the PPC-delivered treatment for each woman in CDT. The study biostatistician generated the randomization sequence.
EMA Procedures and Measures
The Casio E-10 PPC was used, with custom software developed specifically for the study. All participants received the PPC at Pre-Quit Day -1, were trained in its use, and completed PPC-based assessments for one week in response to random prompts (four per day), as well as each time they experienced an urge to smoke (temptation assessments). Participants received gift certificates based on their completion rate for random assessments: $10 for 50–69% completion, $25 for 70–89% completion, and $50 for 90% or more completion.
During the course of the first 7 postcessation days, the EMA program delivered 7,381 random prompts and 5,746 were completed (78% compliance). Participants completed 5,062 temptation assessments, an average of 17.10 temptation assessments per person for the week (SD = 11.20) and 2.44 temptation assessments per person per day (SD = 1.60). Four participants did not provide any temptation assessments. There were no significant differences in compliance with EMA between the participants who were later randomized to EMA-Only or CDT.
Situation Types
EMA items for the random prompts and temptation episodes assessed three critical dimensions of high-risk situations: 1) presence/absence of negative affect, 2) availability/unavailability of cigarettes, and 3) presence/absence of alcohol use or a plan to use alcohol. Dimensions were selected based on both the empirical relation of the dimension to relapse and the ability to delineate specific coping strategies tailored to the dimension. These dimensions defined eight possible “types” of high-risk situations (e.g., presence/absence of negative mood × availability/unavailability of cigarettes × presence/absence of alcohol use).
Coping Strategies
For each type of situation, the PPC displayed 10 situation-specific coping strategies. Strategies for each of the eight situations were generated by a pool of five smoking cessation experts. Strategies were then rated by the experts with respect to their efficacy in each of the eight situations and the top ten strategies for each situation were retained. Coping strategies were common cognitive and behavioral strategies for abstaining from smoking. Cognitive strategies included distraction, self-efficacy enhancement, and reminders of smoking consequences. Behavioral strategies included problem-solving, direct action, escaping the situation, assertiveness, soliciting social support, substituting behavior, and relaxation. For example, if a participant reported that she was experiencing negative affect, but cigarettes were not available and alcohol was not used, coping strategies such as relaxation and soliciting social support were presented. If the participant’s report reflected drinking alcohol and other people smoking, but no negative affect, coping strategies such as escaping the situation and moderating alcohol use were presented. The final pool of coping strategies included 63, rather than 80, strategies as some coping techniques were applicable to several types of high risk situations.
Efficacy and Outcome Expectations
For each of the ten situation-specific coping strategies, efficacy (participant’s confidence that she could perform the strategy) and outcome (whether the strategy would help her maintain abstinence) expectations were assessed.
EMA Tailoring Algorithm
Based on the EMA data collected during the first week postcessation, an individualized hierarchy of coping strategies for each of the eight high-risk situations was generated for each woman in CDT. Strategies within each situation type were ordered based on the mean efficacy and outcome expectation ratings for that strategy using a specific algorithm. The algorithm is available from the authors upon request.
Treatment
All Participants
All participants (both EMA-Only and CDT) received two group counseling sessions prior to quitting and three after quitting (i.e., Pre-Quit Day -3 through Day 7), and six weeks of the 21-mg nicotine patch (Nicoderm CQ; GlaxoSmithKline).
Computer-Delivered Treatment (CDT)
CDT participants received PPC-delivered treatment from Day 7 to Day 35 following the completion of the counseling sessions and EMA procedures (EMA-Only no longer had access to the PPC). CDT consisted of three major modules: 1) Managing My Urge, 2) Treatment Information, and 3) Motivational Messages.
Managing My Urge included the questions defining the type of high-risk situation, the answers to which generated the 10 individualized, context-specific coping strategies for that situation (see EMA Tailoring Algorithm). If desired, the woman could re-order the strategies at any time based on new answers to efficacy and outcome expectation questions, and maintain the reordered sequence for the future. Thus, as the process of quitting progressed, each woman could modify her PPC-delivered treatment to better optimize the coping strategies presented for each type of high risk situation if desired.
Treatment Information consisted of: 1) General Information, and 2) Quitting Strategies. General Information provided guidance on topics such as the risks of smoking, benefits of quitting, understanding nicotine dependence and withdrawal, using the nicotine patch, and weight management. Quitting Strategies included specific tips on preparing to quit, coping with urges and withdrawal, managing negative emotions, identifying high-risk situations, using deep breathing, and what to do if a lapse occurred.
Motivational Messages provided various messages of encouragement and support, such as: “Keep up the good work. Quitting is a process that takes time…” As many or as few messages could be viewed as the participant wished. The women could also program the PPC to automatically deliver a motivational message at self-selected times during the day.
Measures
Point-prevalence abstinence was defined as a self-report of no smoking during the previous 7 days and a CO level of <10 ppm. Abstinence was assessed at every visit. The following questionnaires were administered to all participants prior to quitting. The Mood History Questionnaire (Spitzer, Kroenke, & Williams, 1999) assessed history of depression. The Demographic Information Questionnaire included age, gender, marital status, ethnicity, education, and occupation. The Fagerstrom Test for Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991) measured nicotine dependence. The Smoking Cessation History Questionnaire assessed previous quit attempts and nicotine use history.
Data Analysis
The efficacy of CDT versus EMA-Only was evaluated using longitudinal generalized linear mixed models regression (GLMM; McCulloch & Searle, 2001), specifically the GLIMMIX procedure implemented in SAS/STAT 9.1, across the Day 35, Month 6, and Month 12 follow-ups for both completers only and intent-to-treat. Completers only analyses included all women with data for at least one of the three follow-ups. Only six of 302 participants did not provide data for at least one follow-up. Intent-to-treat analyses assumed that women with missing abstinence data for that follow-up had smoked (see Figure 1). All analyses included time as a covariate. Both unadjusted analyses, and adjusted analyses that controlled for age, race/ethnicity, education, partner status, cigarettes smoked per day, depression history, and abstinence at Day 7 (i.e., abstinence at the time of randomization), were conducted. The interaction of each of the pre-quit variables with treatment on abstinence was also examined. Process analyses within CDT participants examined the relationship between abstinence and: 1) usage of each of the three main modules (times accessed), and 2) total program usage (times accessed).
Results
Participant flow through the study is shown in Figure 1, and participant characteristics by group assignment are shown in Table 1. Overall, 97.4% (n=294) of the women attended all five counseling sessions and 2.6% (n=8) attended 4 of the 5 counseling sessions.
Table 1.
Participant Characteristics by Treatment Group
| Variable | EMA-Only (n = 151) |
CDT (n = 151) |
p |
|---|---|---|---|
| Demographics | |||
| Age | 41.8 (10.4) | 44.0 (11.2) | 0.07 |
| Race | |||
| % Caucasian | 82.7 | 82.0 | |
| % Hispanic | 2.0 | 0 | |
| % African American | 8.0 | 6.7 | |
| % Asian | 3.3 | 2.7 | |
| % Other | 4.0 | 8.7 | 0.50 |
| % > high school education | 78.1 | 74.2 | 0.25 |
| % professional occupation | 51.6 | 51.3 | 0.53 |
| % with partner | 43.6 | 35.8 | 0.17 |
| Tobacco | |||
| % abstinent at day 7 | 72.2 | 77.5 | 0.29 |
| Years smoked | 22.6 (10.0) | 24.6 (11.3) | 0.10 |
| Cigarettes per day | 20.6 (7.6) | 20.5 (8.0) | 0.94 |
| Fagerstrom Test for Nicotine Dependence | 5.1 (1.9) | 5.2 (2.0) | 0.61 |
| % ≤5 5 min to 1st cig | 34.4 | 37.3 | 0.34 |
| Baseline carbon monoxide level | 22.7 (10.3) | 23.7 (11.6) | 0.43 |
| % partner smokes | 51.8 | 41.1 | 0.12 |
| Affect | |||
| % with Depression History | 30.7 | 31.3 | 0.50 |
Note: EMA-Only = EMA only treatment; CDT = computer-delivered treatment. Chi-square tests were used for categorical variables and two-tailed t-tests for continuous variables.
Abstinence rates by treatment group for both completers only and intent-to-treat across Day 35, Month 6, and Month 12 are presented in Figure 2. GLMM analyses of biochemically verified 7 day point prevalence abstinence revealed no significant differences between CDT and EMA-Only for unadjusted completers only (OR= 0.89 (95% CI: 0.56–1.40); p = .60), unadjusted intent-to-treat (OR = 0.94 (95% CI: 0.60–1.46); p = .77), adjusted completers only (OR = 0.77 (95% CI: 0.48–1.26) p = .32), or adjusted intent-to-treat analyses (OR = 0.82 (95% CI: 0.51–1.32); p = .25). Among all participants, 74% reported no puff since quit date on Day 7. However, the treatment effect was not significant when controlling for abstinence status at Day 7, or among both complete abstainers at Day 7 or those individuals who smoked at least a puff by Day 7. The interaction between treatment and any smoking by Day 7 was examined, but it was not significant. There were no significant interactions between treatment and any of the pre-quit characteristics.
Figure 2.
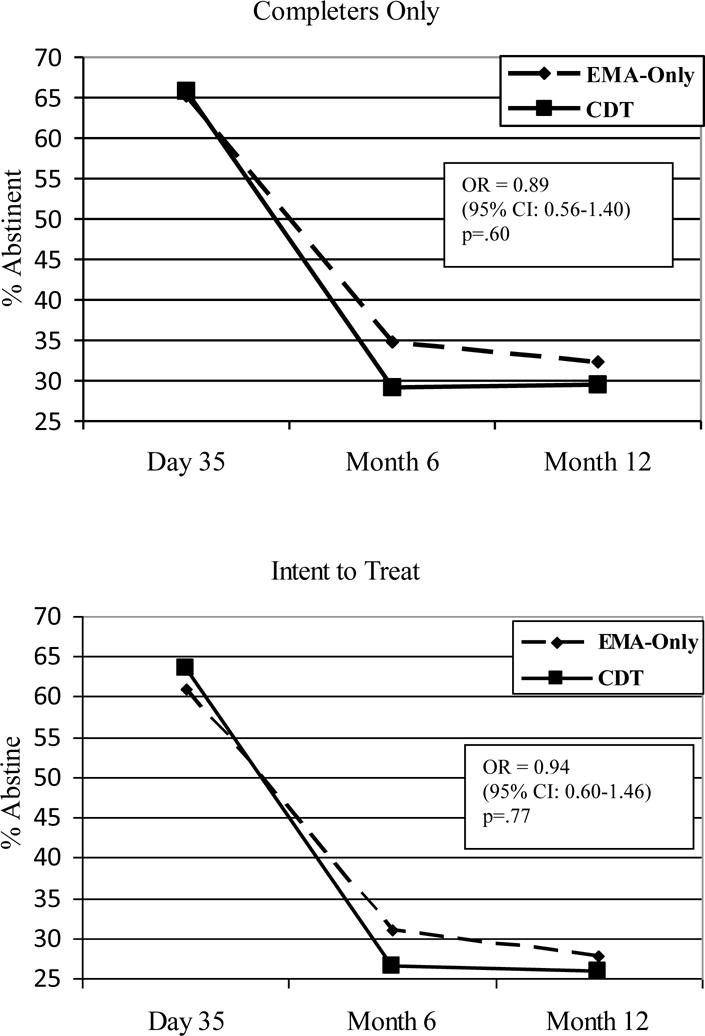
Biochemically Confirmed 7 Day Point Prevalence Abstinence By Treatment Group
Note: EMA-Only = EMA-Only Treatment; CDT = Computer Delivered Treatment
Process analyses investigated the relationship between the number of times a CDT participant accessed the overall program or a particular module and abstinence. Of the 151 women randomized to CDT, all but six (96%) utilized the PPC during the treatment period. Table 2 shows wide variation in treatment usage. The relationship between Managing My Urge and abstinence approached significance for both completers only (OR = 1.02 (95% CI: 0.99–1.03); p = .07) and intent to treat analyses (OR = 1.02 (95%CI: 0.99–1.03); p = .06). Additionally, the relationship between total PPC use and abstinence for completers only (OR = 1.01 (95% CI: 0.99–1.01); p = .09) approached significance. It is important to note that the change in odds is per CDT use. Women who had greater use of either the Managing My Urge module or of the total PPC program, tended to manifest higher rates of abstinence.
Table 2.
CDT Usage and its Relationship to Abstinence among CDT Participants (n=151)
| Module | % of participants who accessed module | Mean number of times accessed (SD; range) |
7 day point prevalence (completers) OR (CI) p |
7 day point prevalence (intent-to-treat) OR (CI) p |
|---|---|---|---|---|
| Managing My Urge | 94.0 | 14.3 (17.9; 0–121) | 1.02 (0.99–1.03) 0.07 |
1.02 (0.99–1.03) 0.06 |
| Motivational Messages | 90.1 | 23.1 (31.1; 0–148) | 1.01 (0.99–1.02) 0.24 |
1.01 (0.99–1.01) 0.31 |
| Treatment Information | 86.8 | 6.7 (7.9; 0–51) | 1.02 (0.98–1.07) 0.25 |
1.02 (0.98–1.06) 0.27 |
| CDT Total Access | 96.0 | 44.2 (46.3; 0–279) | 1.01 (0.99–1.01) 0.09 |
1.01 (0.99–1.01) 0.11 |
Note: CDT = computer-delivered treatment.
Additional analyses investigated whether any of the characteristics in Table 1 were associated with greater utilization of CDT. Greater tobacco consumption (cigarettes per day, CO level) was significantly associated with greater use of CDT. Specifically, greater cigarettes per day was correlated with greater usage of motivational messages (r=.22, p=.01), treatment information (r=.26, p=.003), and overall CDT use (r=.21, p=.01). Higher baseline CO values were correlated with greater usage of managing my urge (r=.25, p=.002), motivational messages (r=.28, p=.001), treatment information (r=.18, p=.04), and overall CDT use (r=.31, p<.001).
Discussion
The current study evaluated a PPC-delivered, theoretically-based, smoking relapse prevention intervention among women. Women are, arguably, at higher risk for smoking relapse and may particularly benefit from intervention efforts designed to prevent relapse. Treatment was tailored based on state of the science behavioral assessment methodology and provided real-time access to individualized, context-specific coping strategies, motivational messages, and general quitting and relapse prevention information via PPCs. Nevertheless, the PPC-delivered intervention did not improve abstinence rates relative to a standard treatment control group. There was at least a suggestion that among CDT participants, heavier smokers were more likely to utilize PPC-delivered treatment and that greater usage may be associated with more positive outcomes.
There are a number of factors that could have contributed to the nonsignificant findings. First, PPCs may simply be an ineffective methodology for delivering smoking relapse prevention interventions. However, a growing body of literature indicates that computer-based interventions can improve smoking prevention and cessation outcomes (Brendryen, Drozd & Kraft, 2008; Norman, Maley, Li, & Skinner, 2008; Prokhorov et al., 2008; Rodgers et al., 2005; Strecher et al., 2008b), including interventions delivered using mobile technology (Heron & Smyth, 2010). In addition, process analyses suggested that there may be an association between greater use of the PPC-delivered treatment, and of the Managing My Urge module in particular, and a decreased likelihood of relapse. However, these results only approached significance. Greater use of the treatment program was significantly correlated with greater tobacco consumption, and this association would tend to reduce the relationship between greater treatment usage and better outcomes. Unfortunately, we cannot determine the direction or meaningfulness of these results currently. For example, greater treatment usage could simply be an artifact of abstinence rather than a determinant of cessation success, or greater motivation to quit could increase both abstinence rates and treatment usage, creating a spurious relationship. Nevertheless, while the current study does not provide evidence for the efficacy of a PPC-delivered treatment, process analyses are at least suggestive of the need for more research on the development and evaluation of PPC-delivered treatments.
Second, the use of the PPC to collect EMA data from all of the women prior to randomization could have turned the PPC into a conditioned cue for craving, leading to either an aversion to using CDT during high risk situations, or to reduced efficacy by activating craving processes through use of the PPC. In addition, EMA is burdensome and engaging in the EMA procedures for one week prior to receiving CDT may have created an aversion to using the PPC.
Third, the intensive EMA procedures, which included an average of 36 separate presentations of 10 context-specific coping strategies for one week postcessation, may have functioned as a coping-skills training intervention and diluted the experimental manipulation. In another study (Rowan et al., 2007), the identical EMA procedures alone did not increase abstinence relative to a group with no EMA, but that study was not adequately powered to detect differences in cessation. Nonetheless, future research would be well-served by study designs that provide for better control of the various dimensions of PPC-delivered interventions.
Fourth, the PPC-delivered intervention may have lacked appeal or have been too complicated in its current format as women used the program an average of less than 2 times per day. Recent results from an online tailored cessation program found that program engagement is important for improving outcomes (Strecher et al., 2008a). Participants may also have been unwilling or unable to effectively use the PPC when under duress, the time when intervention may be most needed.
Fifth, because all participants received intensive behavioral treatment and pharmacotherapy, abstinence rates were very high across both treatment groups at both Day 35 postcessation (61–64% for intent to treat) and at 12 months postcessation (25% for intent-to-treat). Therefore, the provision of a powerful multi-component treatment may have contributed to a ceiling effect. Given that PPC-delivered interventions are likely to be utilized in isolation in the real-world (e.g., smokers might download a program onto their smartphone), future research should evaluate PPC-delivered interventions used in isolation or as adjuvants to less intensive interventions.
Finally, behavioral interventions based on social cognitive theory have not outperformed other treatment approaches (Carroll, 1996; Hajek, Stead, West, & Jarvis, 2005; Hajek, Stead, West, Jarvis, & Lancaster, 2009; Lancaster, Hajek, Stead, West, & Jarvis, 2006), nor has the track record for tailored interventions been universally positive (Lennox et al. 2001). The findings from the current study are consistent with those conclusions. Thus, it may be important to rethink how relapse prevention is addressed in future intervention research.
There are a number of limitations to the current research. The sample was largely White and consisted of women only. In addition, because both intervention content and delivery modality differed between CDT and ST, it was impossible to discern whether the null findings were attributable to content or modality. Further, there was intensive adjuvant treatment. Thus, the results should not be generalized to other populations or to other study approaches. To the best of our knowledge, this is one of the first studies to evaluate a theoretically grounded, PPC-delivered intervention in a well-controlled, randomized clinical trial. In particular, we are unaware of any other smoking cessation/relapse prevention studies that have utilized EMA data to individually tailor treatment. Although there are numerous theoretical and technical issues still to address in future research (e.g., assessment burden vs. tailoring specificity, increasing appeal and usage), the rapid pace of technological advances in mobile computer technology and the ubiquity of such devices provide a novel platform for the design and evaluation of new treatment approaches. Efficacy remains to be demonstrated however.
Acknowledgments
The authors are very appreciative of the efforts of Christine Mahoney, M.A., for her stellar efforts in guiding the project, and GlaxoSmithKline for providing nicotine replacement therapy for all participants.
Funding
This manuscript was supported by grants from the National Cancer Institute (R01CA74517, R01CA74517 S1, K07CA84603) and the Centers for Disease Control and Prevention (K01DP001120).
Appendix: CONSORT checklist
| PAPER SECTION And topic | Item | Description | Reported on Page # |
|---|---|---|---|
| TITLE & ABSTRACT | 1 | How participants were allocated to interventions (e.g., “random allocation”, “randomized”, or “randomly assigned”). | 8 |
| INTRODUCTION Background | 2 | Scientific background and explanation of rationale. | 3–7 |
| METHODS Participants | 3 | Eligibility criteria for participants and the settings and locations where the data were collected. | 8–9 |
| Interventions | 4 | Precise details of the interventions intended for each group and how and when they were actually administered. | 13–14 |
| Objectives | 5 | Specific objectives and hypotheses. | 7 |
| Outcomes | 6 | Clearly defined primary and secondary outcome measures and, when applicable, any methods used to enhance the quality of measurements (e.g., multiple observations, training of assessors). | 15 |
| Sample size | 7 | How sample size was determined and, when applicable, explanation of any interim analyses and stopping rules. | 9, 15 |
| Randomization – Sequence generation | 8 | Method used to generate the random allocation sequence, including details of any restrictions (e.g., blocking, stratification) | 9 |
| Randomization – Allocation concealment | 9 | Method used to implement the random allocation sequence (e.g., numbered containers or central telephone), clarifying whether the sequence was concealed until interventions were assigned. | 9 |
| Randomization – Implementation | 10 | Who generated the allocation sequence, who enrolled participants, and who assigned participants to their groups. | 9 |
| Blinding (masking) | 11 | Whether or not participants, those administering the interventions, and those assessing the outcomes were blinded to group assignment. When relevant, how the success of blinding was evaluated. | 9 |
| Statistical methods | 12 | Statistical methods used to compare groups for primary outcome(s); Methods for additional analyses, such as subgroup analyses and adjusted analyses. | 15–16 |
| RESULTS Participant flow | 13 | Flow of participants through each stage (a diagram is strongly recommended). Specifically, for each group report the numbers of participants randomly assigned, receiving intended treatment, completing the study protocol, and analyzed for the primary outcome. Describe protocol deviations from study as planned, together with reasons. | 16, 34, Figure 1 |
| Recruitment | 14 | Dates defining the periods of recruitment and follow-up. | 8 |
| Baseline data | 15 | Baseline demographic and clinical characteristics of each group. | 16, 32, Table 1 |
| Numbers analyzed | 16 | Number of participants (denominator) in each group included in each analysis and whether the analysis was by “intention-to-treat”. State the results in absolute numbers when feasible (e.g., 10/20, not 50%). | 17–18, 34, Figure 1 |
| Outcomes and estimation | 17 | For each primary and secondary outcome, a summary of results for each group, and the estimated effect size and its precision (e.g., 95% confidence interval). | 17–18, 35, Figure 2 |
| Ancillary analyses | 18 | Address multiplicity by reporting any other analyses performed, including subgroup analyses and adjusted analyses, indicating those pre-specified and those exploratory. | 16–19 |
| Adverse events | 19 | All important adverse events or side effects in each intervention group. | NA |
| DISCUSSION Interpretation | 20 | Interpretation of the results, taking into account study hypotheses, sources of potential bias or imprecision and the dangers associated with multiplicity of analyses and outcomes. | 19–23 |
| Generalizability | 21 | Generalizability (external validity) of the trial findings. | 23 |
| Overall evidence | 22 | General interpretation of the results in the context of current evidence. | 19–23 |
References
- Bandura A. Social Foundations of Thought and Action. New Jersey: Prentice Hall; 1986. [Google Scholar]
- Brandon TH, Tiffany ST, Obremski KM, Baker TB. Postcessation cigarette use: the process of relapse. Addictive Behaviors. 1990;15(2):105–114. doi: 10.1016/0306-4603(90)90013-n. [DOI] [PubMed] [Google Scholar]
- Brendryen H, Drozd F, Kraft P. A digital smoking cessation program delivered through internet and cell phone without nicotine replacement (happy ending): randomized controlled trial. Journal of Medical Internet Research. 2008;10(5):e51. doi: 10.2196/jmir.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll K. Relapse prevention as a psychosocial treatment: A review of controlled clinical trials. Experimental and Clinical Psychopharmacology. 1996;4(1):46–54. [Google Scholar]
- Carter B, Day S, Cinciripini P, Wetter DW. Where are we and where are we going? The Science of Real-Time Data Capture: Self-Reports in Health Research. New York: Oxford University; 2007. Momentary health interventions; pp. 289–307. [Google Scholar]
- Drobes DJ, Elibero A, Evans DE. Attentional bias for smoking and affective stimuli: a Stroop task study. Psychology of Addictive Behaviors. 2006;20(4):490–495. doi: 10.1037/0893-164X.20.4.490. [DOI] [PubMed] [Google Scholar]
- Drobes DJ, Meier EA, Tiffany ST. Assessment of the effects of urges and negative affect on smokers’ coping skills. Behaviour Research and Therapy. 1994;32(1):165–174. doi: 10.1016/0005-7967(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Garvey AJ, Bliss RE, Hitchcock JL, Heinold JW, Rosner B. Predictors of smoking relapse among self-quitters: a report from the Normative Aging Study. Addictive Behaviors. 1992;17(4):367–377. doi: 10.1016/0306-4603(92)90042-t. [DOI] [PubMed] [Google Scholar]
- Gwaltney CJ, Shiffman S, Balabanis MH, Paty JA. Dynamic self-efficacy and outcome expectancies: prediction of smoking lapse and relapse. Journal of Abnormal Psychology. 2005;114(4):661–675. doi: 10.1037/0021-843X.114.4.661. [DOI] [PubMed] [Google Scholar]
- Hajek P, Stead LF, West R, Jarvis MJ. (Cochrane Database System Review: CD003999).Relapse prevention interventions for smoking cessation. 2005 doi: 10.1002/14651858.CD003999.pub2. [DOI] [PubMed] [Google Scholar]
- Hajek P, Stead LF, West R, Jarvis M, Lancaster T. (Cochrane Database System Review: CD003999).Relapse prevention interventions for smoking cessation. 2009 doi: 10.1002/14651858.CD003999.pub3. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heron KE, Smyth JM. Ecological momentary interventions: Incorporating mobile technology into psychosocial and health behaviour treatments. British Journal of Health Psychology. 2010;15:1–39. doi: 10.1348/135910709X466063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster T, Hajek P, Stead LF, West R, Jarvis MJ. Prevention of relapse after quitting smoking: a systematic review of trials. Archives of Internal Medicine. 2006;166(8):828–835. doi: 10.1001/archinte.166.8.828. [DOI] [PubMed] [Google Scholar]
- Lennox AS, Osman LM, Reiter E, Robertson R, Friend J, McCann I, Skatun D, Donnan PT. Cost effectiveness of computer tailored and non-tailored smoking cessation letters in general practice. Randomised controlled trial. British Medical Journal. 2001;322:1–7. doi: 10.1136/bmj.322.7299.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlatt G, Donovan D, editors. Relapse prevention: Maintenance strategies in the treatment of addictive behaviors. 2nd. New York: Guilford Press; 2005. [Google Scholar]
- McCulloch C, Searle S. Generalized, Linear, and Mixed Models. New York: John Wiley & Sons, Inc; 2001. [Google Scholar]
- Norman CD, Maley O, Li X, Skinner HA. Using the internet to assist smoking prevention and cessation in schools: a randomized, controlled trial. Health Psychology. 2008;27(6):799–810. doi: 10.1037/a0013105. [DOI] [PubMed] [Google Scholar]
- Prokhorov AV, Kelder SH, Shegog R, Murray N, Peters R, Jr, Agurcia-Parker C, et al. Impact of A Smoking Prevention Interactive Experience (ASPIRE), an interactive, multimedia smoking prevention and cessation curriculum for culturally diverse high-school students. Nicotine & Tobacco Research. 2008;10(9):1477–1485. doi: 10.1080/14622200802323183. [DOI] [PubMed] [Google Scholar]
- Rodgers A, Corbett T, Bramley D, Riddell T, Wills M, Lin RB, et al. Do u smoke after txt? Results of a randomised trial of smoking cessation using mobile phone text messaging. Tobacco Control. 2005;14(4):255–261. doi: 10.1136/tc.2005.011577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan PJ, Cofta-Woerpel L, Mazas CA, Vidrine JI, Reitzel LR, Cinciripini PM, Wetter DW. Evaluating reactivity to ecological momentary assessment during smoking cessation. Experimental and Clinical Psychopharmacology. 2007;15(4):382–389. doi: 10.1037/1064-1297.15.4.382. [DOI] [PubMed] [Google Scholar]
- Scharf D, Shiffman S. Are there gender differences in smoking cessation, with and without bupropion? Pooled- and meta-analyses of clinical trials of bupropion SR. Addiction. 2004;99(11):1462–1469. doi: 10.1111/j.1360-0443.2004.00845.x. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Conceptual issues in the study of relapse. In: Gossop M, editor. Relapse and Addictive Behaviour. Kent, England: Croom Helm, Ltd; 1989. pp. 149–179. [Google Scholar]
- Shiffman S. Dynamic influences on smoking relapse process. Journal of Personality. 2005;73(6):1715–1748. doi: 10.1111/j.0022-3506.2005.00364.x. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Reflections on smoking relapse research. Drug and Alcohol Review. 2006;25(1):15–20. doi: 10.1080/09595230500459479. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Balabanis MH, Paty JA, Engberg J, Gwaltney CJ, Liu KS, et al. Dynamic effects of self-efficacy on smoking lapse and relapse. Health Psychology. 2000;19(4):315–323. doi: 10.1037//0278-6133.19.4.315. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. Journal of the American Medical Association. 1999;282(18):1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Kroenke K, Linzer M, deGruy FV, Hahn SR. Primary care evaluation of mental disorders (PRIME-MD) New York: Pfizer Pharmaceuticals Group; 1995. [Google Scholar]
- Stone AA, Shiffman S. Ecological momentary assessment (EMA) in behavioral medicine. Annals of Behavioral Medicine. 1994;16:199–202. [Google Scholar]
- Strecher VJ, McClure J, Alexander G, Chakraborty B, Nair V, Konkel J, et al. The role of engagement in a tailored web-based smoking cessation program: randomized controlled trial. Journal of Medical Internet Research. 2008a;10(5):e36. doi: 10.2196/jmir.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strecher VJ, McClure JB, Alexander GL, Chakraborty B, Nair VN, Konkel JM, et al. Web-based smoking-cessation programs: results of a randomized trial. American Journal of Preventative Medicine. 2008b;34(5):373–381. doi: 10.1016/j.amepre.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDHHS. Women and smoking: A report of the surgeon general. Rockville, Maryland: U.S. Department of Health and Human Services, Public Health Service Office of the Surgeon General; 2001. [Google Scholar]
- Walitzer KS, Dearing RL. Gender differences in alcohol and substance use relapse. Clinical Psychology Review. 2006;26(2):128–148. doi: 10.1016/j.cpr.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Wetter DW, Kenford SL, Smith SS, Fiore MC, Jorenby DE, Baker TB. Gender differences in smoking cessation. Journal of Consulting and Clinical Psychology. 1999;67(4):555–562. doi: 10.1037//0022-006x.67.4.555. [DOI] [PubMed] [Google Scholar]


