Abstract
Arterial stiffness is increasingly recognized as an important determinant of cardiovascular risk and may be directly involved in the process of atherosclerosis. As atherosclerosis leads to increased arterial resistance and decrease the flow propagation speed within the arterial lumen, a similar decrease in aortic flow propagation with increased downstream resistance is detected, so aortic flow propagation velocity AVP was evaluated in many studies as a new parameter of aortic stiffness.
Aim
To measure arterial stiffness using the new parameter AVP and compare it to flow mediated dilatation FMD as a parameter of endothelial dysfunction in patients with metabolic syndrome MS.
Methods
AVP (assessed by transthoracic echocardiography) and FMD (assessed by brachial artery reactivity test) were measured in 100 patients with MS (Group 1) and were compared to 14 normal subjects (Group 2).
Results
Patients with MS had significantly lower values of AVP as compared to the normal subjects; 36 ± 5 cm/s vs 57 ± 5, p < 0.05, and lower FMD; 6% ± 1 vs 17 ± 3 p < 0.05 as well, there was significant correlations between AVP and FMD (r = 0.89, p < 0.001).
Conclusion
Transthoracic echocardiographic determination of AVP is a simple practical method and correlates well with FMD in patients with MS.
Abbreviations: AVP, aortic velocity propagation; FMD, flow mediated dilatation; WC, waist circumference; TC, total cholesterol; TG, triglycerides; HDL, high density lipoprotein; SBP, systolic blood pressure; DBP, diastolic blood pressure; FBS, fasting blood sugar; UA, uric acid; EPF, epicardial fat; IVS, interventricular septum; E/e′, filling pressure; AST, aspartate transaminase; ALT, alanine transaminase; CRP, C reactive protein
Keywords: Aortic propagation velocity, Arterial stiffness, AVP, Metabolic syndrome
1. Introduction
Arterial stiffness is a growing epidemic associated with increased risk of cardiovascular events, dementia, and death. Decreased compliance of the central vasculature alters arterial pressure and flow dynamics and impacts cardiac performance and coronary perfusion [1].
Increased central arterial stiffening is not only a hallmark of the aging process [2], but the consequence of many disease states, such as diabetes and chronic renal compromise [3], [4].
In patients with diabetes and metabolic syndrome (MS), arterial stiffening is consistently observed across all age groups. For example, increased arterial stiffness and abnormal endothelial reactivity is already present in obese children with metabolic syndrome [5]. A core feature appears to be insulin resistance, because central arterial stiffness and insulin resistance are positively correlated. Furthermore, the extent of metabolic changes predicts arterial stiffness in a dose-dependent fashion [6]. Chronic hyperglycemia and hyperinsulinemia increases the local activity of the renin-angiotensin-aldosterone system and expression of angiotensin type I receptor in vascular tissue [7], promoting development of wall hypertrophy and fibrosis [8].
A large population-based study of vascular health suggested that hyperglycemia was related to both endothelial dysfunction and arterial stiffness [9]. Greater glycemic variability measured by continuous subcutaneous glucose monitoring is also associated with endothelial dysfunction in adults with normoglycemia, hyperglycemia, and type 2 diabetes [10].
Aortic stiffness has been assessed using parameters, such as pulse-wave velocity, aortic strain, compliance, and distinsibility. These parameters can be measured using applanation tonometry, oscillometry, echocardiography, and velocity-encoded magnetic resonance imaging [11], [12], [13], [14], [15], [16].
Recently, aortic-flow propagation velocity (AVP) in the descending aorta using color M-Mode was used in the assessment of arterial stiffness [17], [18], [19]. Our aim in this study was to measure arterial stiffness using the new parameter (AVP) and compare it to the brachial-artery reactivity test as a parameter of endothelial dysfunction in patients with metabolic syndrome.
2. Materials and methods
One hundred patients meeting the criteria of metabolic syndrome (MS) according to the National Cholesterol Education Program Adult Treatment Panel III [20] were included in the study (Group 1) and were compared to 14 normal individuals (Group 2). All patients were recruited from Tanta University Hospital, Tanta, Egypt from July 2013 to March 2014.
Exclusion criteria: (1) patients who did not meet the criteria of (MS) with isolated primiary hypertension, diabetes mellietus, or dyslipidemia; (2) patients with aortic valve disease, renal disease, and coronary artrey disease; (3) patients with retinopathies and nephropathy; and (4) women taking contraceptive pills. All patients gave informed consent, and the study was approved by the Institutional Ethics Committee (Tanta University Hospital).
Patients and normal individuals were subjected to full clinical examination, electrocardiogram, abdominal ultrasonography to detect fatty liver, echocardiographic examination, full laboratory investigations, including lipid and liver profiles, fasting blood sugar (FBS), uric acid (UA), and c-reactive protein levels (CRP), and a brachial artrey reactivity test (BART). Viral hepatitis serology markers were examined in patients with high liver enzymes.
The echocardiographic examination was performed at rest by an experienced operator who was blinded to the clinical conditions of the participants. Patients were examined in the left lateral decubitus position using a commercially available echocardiographic device (Vivid 7, GE Healthcare, Madison, WI, USA) with a 3.0 MHz transducer to measure left ventricular dimensions and function, diastolic function (graded as normal 0 or 1, 2, 3, and 4), E/e′ as an indication of filling pressure, and septal diameter (IVS).
From the supine position, color M-mode Doppler recordings were obtained with the cursor parallel to the main flow direction in the descending aorta. The Color Doppler Nyquist limit was adapted to 30–50 cm/s and was switched to M-mode with a recorder sweep rate of 200 mm/s. An M-mode spatiotemporal velocity map with the shape of a flame was displayed. If the slope of the flame was unclear, baseline shifting was used to change the aliasing velocity until a clear delineation of isovelocity slope was observed.
AVP was then automatically calculated by dividing the distance between points corresponding to the beginning and end of the propagation slope by the duration between corresponding time points. Thus, AVP corresponded to the velocity at which the flow was propagating down the artery. The mean of at least three measurements was recorded as AVP value. AVP > 60 cm/s was considered normal [21].
BART was performed using a linear 7 MHz transducer (Vivid 7, GE Healthcare), and both groups were directed to abstain from caffeine 12 hours prior to the study. A longitudinal image was used to measure brachial artery diameter (1st baseline image), and a blood-pressure cuff was inflated on the upper arm (2–5 cm above the cubital fossa) to 50 mmHg above systolic pressure for 5 minutes and then deflated after 1 minute. A second longitudinal scan was obtained (from the same position) to calculate the brachial artery diameter (post-occlusion value). Flow-mediated dilation (FMD) was calculated as:
| (1) |
All measurements of the brachial artery lumen diameter were assessed at end diastole.
Statistical analysis was performed using Microsoft Excel (Microsoft Office 2011; Microsoft, Inc., Redmond, WA, USA). All data were expressed as mean ± standard deviation. The difference between two means was statistically analyzed using the Student t test. Pearson’s correlation coefficient (r) was calculated to test the association between two variables. Significance determined as p < 0.05.
3. Results
Eighty-four patients were studied in Group 1, with 16/100 patients having difficult suprasternal echo windows and, therefore, excluded from the study. All normal individuals in Group 2 had feasable echo windows.
All of the patients in Group 1 had large waist circumference (WC), visible epicardial fat, and AVP < 60 cm/s. Forty-five patients had high blood pressure, with 17 being newly diagnosed and only 15 receiving medication (Table 1).
Table 1.
Baseline criteria of patients with metabolic syndrome.
| Number | Total (%) | |
|---|---|---|
| Large WC | 84 | 100 |
| HTN | 45 | 53 |
| Newly diagnosed HTN | 17 | 20 |
| Medications | 15 | |
| FBS > 100 | 56 | 66 |
| Diabetes mellitus | 22 | 26 |
| Newly diagnosed diabetes | 10 | |
| Medications | 5 | |
| TC > 200 | 48 | 57 |
| TG > 150 | 37 | 44 |
| Low HDL | 26 | 30 |
| Newly diagnosed dyslipidemia | 12 | |
| Medications | 30 | |
| Visible epicardial fat | 84 | 100 |
| Fatty liver | 43 | 51 |
| Diastolic dysfunction | 59 | 70 |
| High filling pressure | 10 | 13 |
| IVS > 12 mm | 43 | 51 |
| AVP < 0.6 | 84 | 100 |
| FMD < 10% | 83 | 98 |
| High AST | 22 | 26 |
| High ALT | 23 | 27 |
| High CRP | 13 | 15 |
ALT = alanine transaminase; AST = aspartate transaminase; AVP = aortic velocity propagation; CRP = c-reactive protein; FBS = fasting blood sugar; FMD = flow-mediated dilatation; HDL = high-density lipoprotein; HTN = hypertension; IVS = interventricular septum; TG = triglycerides; TC = total cholesterol; WC = waist circumference.
While 22/84 patients had high AST levels and 23/84 patients had high ALT levels, none had abnormal viral hepatitis markers and all of them had variable degrees of fatty liver as detected by abdominal ultrasonography.
Group 1 WC ranged from 104 cm to 119 cm, with a mean of 116 ± 10 cm. Their TC ranged from 189 mg% to 296 mg%, with a mean of 204 ± 37 mg%. Their TG ranged from 130 mg% to 280 mg%, with a mean of 213 ± 98 mg%. Their HDL levels ranged from 31 mg% to 52 mg%, with a mean of 37 ± 6 mg%, and their systolic blood pressure SBP ranged from 120 mmHg to 160 mmHG, with a mean of 145 ± 16 mmHG. Their DBP ranged from 70 mmHg to 100 mmHg, with a mean of 95 ± 8 mmHG, and their FBS ranged from 80 mg% to 140 mg%, with a mean of 121 ± 8 mg%. Their UA ranged from 4 mg% to 8.8 mg%, with a mean of 7±1 mg%. Their AST ranged from 26 U to 76 U, with a mean of 50 ± 18 U, and their ALT ranged from 20 U to 121 U, with a mean of 57 ± 18 U.
There was no significant differences between the two groups concerning age, gender, or serum creatinine level, while there was significant differences concerning the reminaing criteria (Table 2). Thirteen patients in Group 1 had high CRP, while none of the patients in Group 2 had high CRP.
Table 2.
Comparison of baseline characteristics and laboratory findings in metabolic syndrome patients (Group 1) and controls (Group 2).
| Group 1 (n = 84) | Group 2 (n = 14) | p | Significance | |
|---|---|---|---|---|
| Female (%) | 47 (55) | 7 (50) | 0.7 | NS |
| Age (y) | 44 ± 9 | 39 ± 7 | 0.2 | NS |
| WC (cm) | 116 ± 10 | 101 ± 1 | <0.05 | S |
| TC (mg/dL) | 204 ± 37 | 175 ± 21 | <0.05 | S |
| TG (mg/dL) | 213 ± 98 | 144 ± 22 | <0.05 | S |
| HDL (mg/dL) | 37 ± 6 | 41 ± 6 | <0.05 | S |
| ALT (U/L) | 57 ± 18 | 41 ± 21 | <0.05 | S |
| AST (U/L) | 50 ± 18 | 38 ± 15 | <0.05 | S |
| FBS (mg/dL) | 121 ± 8 | 102 ± 11 | <0.05 | S |
| Serum creatinin (mg/dL) | 0.96 | 0.91 | 0.11 | NS |
| Systolic BP (mmHg) | 145 ± 16 | 120 ± 10 | <0.05 | S |
| diastolic BP (mmHg) | 95 ± 8 | 73 ± 4 | <0.05 | S |
ALT = alanine transaminase; AST = aspartate transaminase; BP = blood pressure; FBS = fasting blood sugar; HDL = high-density lipoprotein; NS = non-significant; S = significant; TG = triglycerides; TC = total cholesterol; WC = waist circumference.
Echo criteria for Group 1 included IVS ranging from 8 mm to 13 mm, with a mean of 11 ± 2 mm, an E/e′ from 6% to 10%, with a mean of 9 ± 1%, an AVP ranging from 29 cm/s to 48 cm/s, with a mean of 36 ± 5 cm/s, and an FMD ranging from 5% to 9%, with a mean of 6 ± 1%.
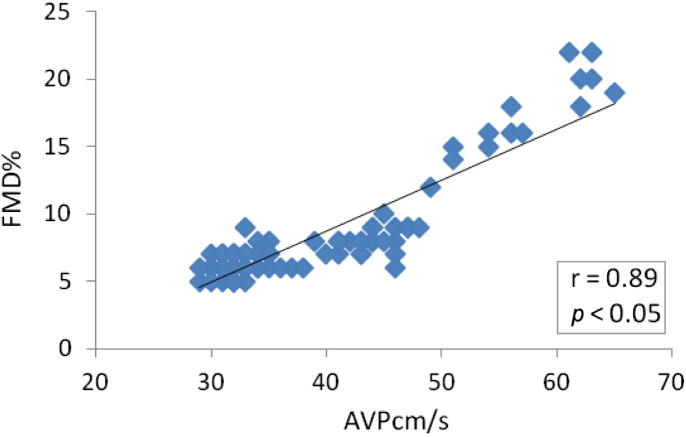
The two groups did not differ significantly concerning EF, while Group 1 had higher IVS and E/e′ and lower AVP and FMD (Table 3). The AVP correlated positevely with FMD (r = 0.89; Fig. 1), AVP correlated negatively with DBP (r = −0.59), WC (r = −0.48), SBP (r = −0.42), CRP (r = −0.44), and E/e′ (r = −0.39). The rest of the parameters did not show significant correlations (Table 4).
Table 3.
Echocardiography and brachial artery reactivity findings.
| Variables | Group 1 | Group 2 | p |
|---|---|---|---|
| LVEF (%) | 62 ± 7 | 64 ± 5 | 0.75 |
| IVS (mm) | 11 ± 2 | 9 ± 1 | <0.05 |
| E/e′ (%) | 9 ± 1 | 7.5 ± 1 | <0.05 |
| AVP (cm/s) | 36 ± 5 | 57 ± 5 | <0.05 |
| FMD (%) | 6 ± 1 | 17 ± 3 | <0.05 |
AVP = aortic velocity propagation; FMD = flow-mediated dilatation; IVS = interventricular septum; LVEF = left ventricular ejection fraction.
Figure 1.
Significant correlation between AVP and FMD. AVP = aortic velocity propagation; FMD = flow-mediated dilatation.
Table 4.
Correlation of AVP and different parameters.
| Parameter | r | p | Significance |
|---|---|---|---|
| FMD | 0.89 | <0.001 | S |
| WC | −0.48 | <0.001 | S |
| TC | −0.23 | 0.09 | NS |
| TG | −0.11 | 0.87 | NS |
| HDL | 0.04 | 0.867 | NS |
| SBP | −0.4 | <0.001 | S |
| DBP | −0.59 | <0.001 | S |
| FBS | −0.14 | 0.134 | NS |
| UA | −0.3 | 0.98 | NS |
| EPF | −0.53 | 0.056 | NS |
| IVS | −0.39357 | 0.745 | NS |
| E/e | −0.37 | <0.001 | S |
| AST | −0.1 | 0.96 | NS |
| ALT | −0.2 | 0.43 | NS |
| CRP | −0.44 | <0.001 | S |
ALT = alanine transaminase; AST = aspartate transaminase; AVP = aortic velocity propagation; CRP = c-reactive protein; DBP = diastolic blood pressure; E/e′ = filling pressure; FBS = fasting blood sugar; FMD = flow-mediated dilatation; EPF = epicardial fat; HDL = high-density lipoprotein; HTN = hypertension; IVS = interventricular septum; SBP = systolic blood pressure; TG = triglycerides; TC = total cholesterol; UA = uric acid; WC = waist circumference.
4. Discussion
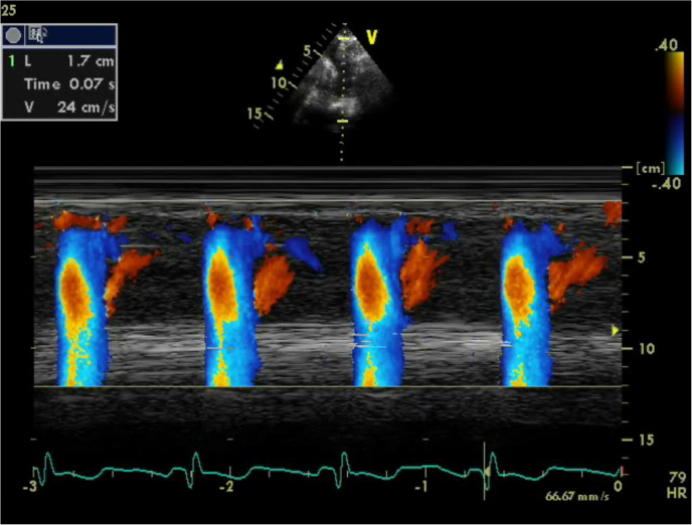
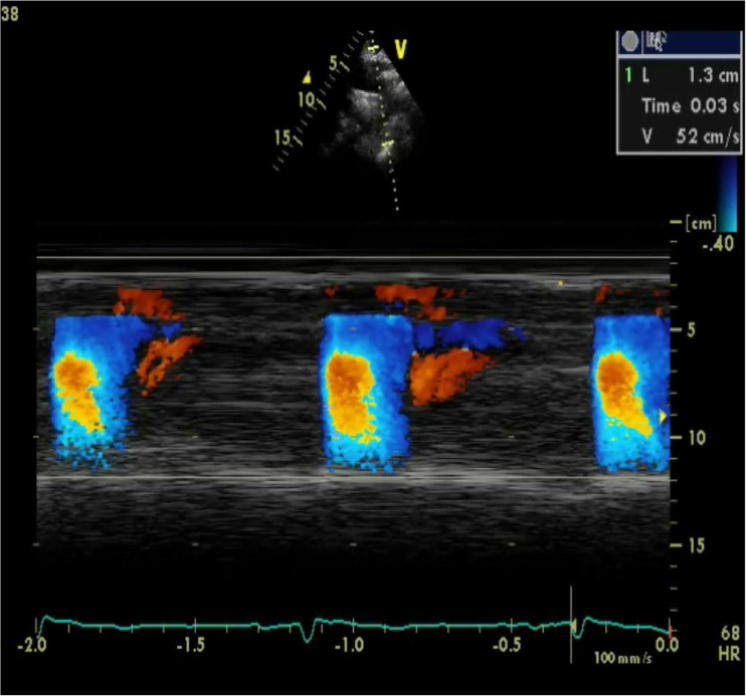
We studied 84 patients with MS. All of them displayed aortic stiffness, as they had significantly reduced AVP (as a parameter of aortic stiffness) as compared to the Group 2. Additionally, they showed reduced FMD, indicating endothelial dysfunction, and 26% of them had high liver enzymes and 15% had high CRP. There was a significant correlation between AVP, FMD, and high CRP, indicationg a possible link between aortic stiffness, endothelial dysfunction, and inflammation (Figure 2, Figure 3).
Figure 2.
Color M-Mode at descending aorta from suprasternal area in a patient with metabolic syndrome. AVP = 24 cm/s. AVP = aortic velocity propagation.
Figure 3.
Color M-Mode at descending aorta from suprasternal area in a normal subject. AVP = 52 cm/s. AVP = aortic velocity propagation.
The existence of a strong association between the presence of MS, arterial stiffness, and inflammation has been shown in many cross-sectional studies [6], [22]. MS is a cluster of common pathologies, including abdominal obesity linked to an excess of visceral fat, insulin resistance, dyslipidemia, and hypertension [1]. At the molecular level, MS is accompanied by abnormal regulation adipokine expression (cytokines and chemokines), making MS an inflammatory condition [22], [23], which may explain the high CRP in some of our patients.
As several cytokines are also produced by adipose tissue [28], it was postulated that an “adipo-vascular” axis [24], [25] may contribute to the increased risk of cardiovascular events in patients with MS. As it expresses itself in lower plasma levels of adiponectin and higher plasma levels of IL-6, this could be linked to the development of arterial stiffness, microvascular dysfunction, and inflammation [26], [27]. Inflammation increases insulin resistance, which, in turn, leads to obesity while propagating diabetes, high blood pressure, prothrombotic states, and dyslipidemia [24].
Early in the course of diabetes and MS, intracellular hyperglycemia due to insulin resistance causes changes in blood flow and increased vascular permeability. This reflects decreased activity of nitric oxide, and increased activity of angiotensin II and endothelin-1. Angiotensin II produces acute vasoconstriction, leading to an increase in blood pressure, and endothelin-1 is an important regulator of vascular tone and has been implicated in the pathogenesis of endothelial dysfunction and atherosclerosis [7], [28], [29].
Additionally, abnormalities of the extracellular matrix contribute to an irreversible increase in vascular permeability. Hyperglycemia itself leads not only to decreased endothelial production of nitric oxide, which represents an anti-atherogenic molecule, but also increased production of a potent inhibitor of fibrinolysis, namely plasminogen activator inhibitor 1 [30]. The risk factors for MS, either together or individually, are also associated with arterial stiffness and endothelial dysfunction both in health and disease [31], [32], [33], [34], [35].
Systemic endothelial function reflects the propensity of arteries to develop atherosclerosis in response to exposure to cardiovascular risk factors. Atherosclerosis leads to increased arterial resistance through thickening and stiffening of the arterial wall. Because the increased aortic resistance acts in a manner to decrease the flow-propagation speed within the arterial lumen, a similar decrease in aortic flow propagation with increased downstream resistance is logical [36], therefore, AVP was studied in many studies as a new parameter of aortic stiffness, endothelial dysfunction, and atherosclerosis. Guneş et al [18] evaluated aortic strain, aortic distensibility, aortofemoral pulse-wave propagation velocity, and AVP in 127 patients undergoing coronary angiography and found that among clinical and echocardiographic variables, AVP was the most significant predictor of coronary artery disease, and that an AVP ⩽ 41 cm/s predicted coronary artery disease with 82.4% sensitivity and 97.2% specificity (positive predictive value: 98.7%; negative predictive value: 68.2%). Guneş et al [37] later measured AVP to predict subclinical atherosclerosis. Sismek et al [38] found that AVP measured by transthoracic echocardiography was decreased in patients with coronary slow flow and significantly correlated with FMD. The correlation between FMD and AVP was r = 0.524 (p < 0.001), which agreed with our results, as we also found correlations between the two parameters (r = 0.89, p < 0.001).
Yildirim et al [39] compared AVP with the Framingham coronary risk score, carotid intima media thickness, and high-sensitive CRP and found out that AVP was significantly lower (p < 0.001) and carotid intima media thickness and high-sensitive CRP were significantly higher in high-risk hypertensive patients. These results aggree with our findings, as we found that AVP was significantly lower and CRP significantly higher in our studied patients.
Guneş et al [40] also found significant associations between AVP, carotid intima media thickness, and FMD in patients having significant coronary atherosclerosis. AVP was also useful in improving the diagnostic accuracy of the exercise electrocardiography test [19]. Twenty-six percent of our patients had high liver enzymes that may have been due to liver inflammation secondary to non-alcoholic fatty liver disease (NAFLD). The progression of NAFLD in MS may be explained by steatosis caused by the initial metabolic disturbance and oxidative stress due to reactive oxygen species formation and cytokine production [41], [42].
Previous studies showed a relationship between NAFLD and atherosclerosis [43], [44], however, we did not find any correlation between AVP, fatty liver, and high liver enzymes, which agrees with findings by Poanta et al [45].
AVP is a new non-invasive method that is simple, easily applicable, inexpensive, and does not require complex insturments. It is also very reliable and correlates significantly with almost all indices of arterial stiffness and atherosclerosis, meaning that it can be used to improve noninvasive assessment of global cardiovascular risk in many situations to improve selection of high-risk individuals for additional investigation.
5. Conclusion
Patients with MS suffer from endothelial dysfunction and arterial stiffness. Transthoracic echocardiographic-detected AVP is a simple, easily applicable method for detection of arterial stiffness that can be used in a wide range of situations for better cardiovascular risk assessment. Our study had limitations: (1) FBS was not measured as glycosylated hemoglobin, as it is not one of the criteria necessary for the diagnosis of MS; (2) we had fewer normal patients in Group 2, because of the difficulty in finding individuals seeking medical help during the interval of the study; (3) CRP was measured to detect inflammation and not high-sensitive CRP, as it was not available in our hospital at the time of the study; and (4) an inadequate suprasternal window was considered a limitation of AVP measurement.
Conflict of interest
The authors declare no conflicts of interest.
Disclosure: Authors have nothing to disclose with regard to commercial support.
Footnotes
Peer review under responsibility of King Saud University.
References
- 1.Chobanian A.V., Bakris G.L., Black H.R., Cushman W.C., Green L.A., Izzo J.L., Jr. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 2.Lakatta E.G., Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 3.Scuteri A., Najjar S.S., Muller D.C., Andres R., Hougaku H., Metter E.J. Metabolic syndrome amplifies the age-associated increases in vascular thickness and stiffness. J Am Coll Cardiol. 2004;43:1388–1395. doi: 10.1016/j.jacc.2003.10.061. [DOI] [PubMed] [Google Scholar]
- 4.Collins A.J., Li S., Gilbertson D.T., Liu J., Chen S.C., Herzog C.A. Chronic kidney disease and cardiovascular disease in the Medicare population. Kidney Int Suppl. 2003;87:S24–S31. doi: 10.1046/j.1523-1755.64.s87.5.x. [DOI] [PubMed] [Google Scholar]
- 5.Tounian P., Aggoun Y., Dubern B., Varille V., Guy-Grand B., Sidi D. Presence of increased stiffness of the common carotid artery and endothelial dysfunction in severely obese children: a prospective study. Lancet. 2001;358:1400–1404. doi: 10.1016/S0140-6736(01)06525-4. [DOI] [PubMed] [Google Scholar]
- 6.Salomaa V., Riley W., Kark J.D., Nardo C., Folsom A.R. Non-insulin-dependent diabetes mellitus and fasting glucose and insulin concentrations are associated with arterial stiffness indexes. The ARIC Study. Atherosclerosis Risk in Communities Study. Circulation. 1995;91:1432–1443. doi: 10.1161/01.cir.91.5.1432. [DOI] [PubMed] [Google Scholar]
- 7.Nickenig G., Roling J., Strehlow K., Schnabel P., Bohm M. Insulin induces upregulation of vascular AT1 receptor gene expression by posttranscriptional mechanisms. Circulation. 1998;98:2453–2460. doi: 10.1161/01.cir.98.22.2453. [DOI] [PubMed] [Google Scholar]
- 8.Rizzoni D., Porteri E., Guelfi D., Muiesan M.L., Valentini U., Cimino A. Structural alterations in subcutaneous small arteries of normotensive and hypertensive patients with non-insulin-dependent diabetes mellitus. Circulation. 2001;103:1238–1244. doi: 10.1161/01.cir.103.9.1238. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell G.F., Guo C.Y., Benjamin E.J., Larson M.G., Keyes M.J., Vita J.A. Cross-sectional correlates of increased aortic stiffness in the community: the Framingham Heart Study. Circulation. 2007;115:2628–2636. doi: 10.1161/CIRCULATIONAHA.106.667733. [DOI] [PubMed] [Google Scholar]
- 10.Buscemi S., Re A., Batsis J.A., Arnone M., Mattina A., Cerasola G. Glycaemic variability using continuous glucose monitoring and endothelial function in the metabolic syndrome and in type 2 diabetes. Diabet Med. 2010;27:872–878. doi: 10.1111/j.1464-5491.2010.03059.x. [DOI] [PubMed] [Google Scholar]
- 11.Asmar R.G., Topouchian J.A., Benetos A., Sayegh F.A., Mourad J.J., Safar M.E. Non-invasive evaluation of arterial abnormalities in hypertensive patients. J Hypertens. 1997;15:S99–S107. doi: 10.1097/00004872-199715022-00010. [DOI] [PubMed] [Google Scholar]
- 12.Asmar R., Benetos A., Topouchian J. Assessment of arterial distensibility by automatic pulse wave velocity measurement. Validation and clinical application studies. Hypertension. 1995;26:485–490. doi: 10.1161/01.hyp.26.3.485. [DOI] [PubMed] [Google Scholar]
- 13.Nelson A.J., Worthley S.G., Cameron J.D. Cardiovascular magnetic resonance-derived aortic distensibility: validation and observed regional differences in the elderly. J Hypertens. 2009;27:535–542. doi: 10.1097/hjh.0b013e32831e4599. [DOI] [PubMed] [Google Scholar]
- 14.Bramwell J.C., Hill A.V., McSwiney B.A. The velocity of the pulse wave in man in relation to age as measured by the hot-wire sphygmograph. Heart. 1923;10:233–255. [Google Scholar]
- 15.Redheuil A., Yu W.C., Wu C.O. Reduced ascending aortic strain and distensibility: earliest manifestations of vascular aging in humans. Hypertension. 2010;55:319–326. doi: 10.1161/HYPERTENSIONAHA.109.141275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson M.R., Stepanek J., Cevette M., Covalciuc M., Hurst R.T., Tajik A.J. Noninvasive measurement of central vascular pressures with arterial tonometry: clinical revival of the pulse pressure waveform? Mayo Clin Proc. 2010;85:460–472. doi: 10.4065/mcp.2009.0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guneş A., Guntekin U., Yıldız S., Kaya B.C., Deveci E., Kaya Z. Association of aortic flow propagation velocity with ankle/brachial blood pressure index in patients with hypertension: an observational study. Anadolu Kardiyol Derg. 2012;12:568–573. doi: 10.5152/akd.2012.186. [DOI] [PubMed] [Google Scholar]
- 18.Guneş Y., Tuncer M., Yıldırım M., Guntekin U., Gumrukcuoğlu H.A., Şahin M. A novel echocardiographic method for the prediction of coronary artery disease. Med Sci Monit. 2008;14:MT42–MT46. [PubMed] [Google Scholar]
- 19.Guneş Y., Gumrukcuoğlu H.A., Kaya Y., Tuncer M. Incremental diagnostic value of color M-mode propagation velocity of the descending thoracic aorta to exercise electrocardiography. Turk Kardiyol Dern Ars. 2010;38:551–557. [PubMed] [Google Scholar]
- 20.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 21.Sen T., Tufekcioglu O., Ozdemir M., Tuncez A., Uygur B., Golbasi Z. A new echocardiographic parameter of aortic stiffness and atherosclerosis in patients with coronary artery disease: aortic propagation velocity. J Cardiol. 2013;62:236–240. doi: 10.1016/j.jjcc.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Dyck D.J., Heigenhauser G.J., Bruce C.R. The role of adipokines as regulators of skeletal muscle fatty acid metabolism and insulin sensitivity. Acta Physiol (Oxf) 2006;6:5–16. doi: 10.1111/j.1748-1716.2005.01502.x. [DOI] [PubMed] [Google Scholar]
- 23.Heilbronn L.K., Campbell L.V. Adipose tissue macrophages, low grade inflammation and insulin resistance in human obesity. Curr Pharm Des. 2008;6:1225–1230. doi: 10.2174/138161208784246153. [DOI] [PubMed] [Google Scholar]
- 24.Festa A., D’Agostino R., Jr., Howard G., Mykkänen L., Tracy R.P., Haffner S.M. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS) Circulation. 2000;102:42. doi: 10.1161/01.cir.102.1.42. [DOI] [PubMed] [Google Scholar]
- 25.Fruhbeck G., Gomez-Ambrosi J., Muruzabal F.J., Burrell M.A. The adipocyte: a model for integration of endocrine and metabolic signaling in energy metabolism regulation. Am J Physiol. 2001;6:E827–E847. doi: 10.1152/ajpendo.2001.280.6.E827. [DOI] [PubMed] [Google Scholar]
- 26.Koh K.K., Han S.H., Quon M.J. Inflammatory markers and the metabolic syndrome insights from therapeutic interventions. J Am Coll Cardiol. 2005;46:1978. doi: 10.1016/j.jacc.2005.06.082. [DOI] [PubMed] [Google Scholar]
- 27.Ridker P.M., Buring J.E., Cook N.R., Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14,719 initially healthy American women. Circulation. 2003;107:391. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- 28.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;6:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 29.Pradhan A.D., Manson J.E., Rifai N., Buring J.E., Ridker P.M. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 30.Festa A., D’Agostino R., Jr., Tracy R.P., Haffner S.M. Elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes. 2002;51:1131. doi: 10.2337/diabetes.51.4.1131. [DOI] [PubMed] [Google Scholar]
- 31.Satoh H., Kishi R., Tsutsui H. Metabolic syndrome is a significant and independent risk factor for increased arterial stiffness in Japanese subjects. Hypertens Res. 2009;6:1067–1071. doi: 10.1038/hr.2009.158. [DOI] [PubMed] [Google Scholar]
- 32.Bots M.L., Dijk J.M., Oren A., Grobbee D.E. Carotid intima-media thickness, arterial stiffness and risk of cardiovascular disease: current evidence. J Hypertens. 2002;6:2317–2325. doi: 10.1097/00004872-200212000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Ahluwalia N., Drouet L., Ruidavets J.B., Perret B., Amar J., Boccalon H. Metabolic syndrome is associated with markers of subclinical atherosclerosis in a French population-based sample. Atherosclerosis. 2006;6:345–353. doi: 10.1016/j.atherosclerosis.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 34.Tuttolomondo A., Di Raimondo D., Di Sciacca R., Pecoraro R., Arnao V., Buttà C. Arterial stiffness and ischemic stroke in subjects with and without metabolic syndrome. Atherosclerosis. 2012;6:216–219. doi: 10.1016/j.atherosclerosis.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 35.Schillaci G., Pirro M., Vaudo G., Mannarino M.R., Savarese G., Pucci G. Metabolic syndrome is associated with aortic stiffness in untreated essential hypertension. Hypertension. 2005;45:1078–1082. doi: 10.1161/01.HYP.0000165313.84007.7d. [DOI] [PubMed] [Google Scholar]
- 36.Bonetto P.O., Leman L.O., Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168–175. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- 37.Simskh H., Sahin M., Guneş Y., Dogan A., Gumrukcuoglu H.A., Tuncer M. A novel echocardiographic method for the detection of subclinical atherosclerosis in newly diagnosed, untreated type 2 diabetes. Echocardiography. 2013;30:644–648. doi: 10.1111/echo.12125. [DOI] [PubMed] [Google Scholar]
- 38.Simesk H., Sahin M., Guneş Y., Akdag S., Akil M.A., Akyol A. A novnel echocardiographic method as an indicator of endothelial dysfunction in patients with coronary slow flow. Eur Rev Med Pharmacol Sci. 2013;17:689–693. [PubMed] [Google Scholar]
- 39.Yıldırım M., Yiginer O., Uzun M., Yilmaz Cingozbay B., Sag C., Kutsi Kabul H. Aortic flow propagation velocity as an early predictor of high coronary risk in hypertensive patients. Med Glas (Zenica) 2012;9:42–48. [PubMed] [Google Scholar]
- 40.Guneş Y., Tuncer M., Guntkin U., Ceylan Y., Simesk H., Sahin M. The relation between the color M-mode propagation velocity of the descending aorta and coronary and carotid atherosclerosis and flow-mediated dilatation. Echocardiography. 2010;27:300–305. doi: 10.1111/j.1540-8175.2009.01019.x. [DOI] [PubMed] [Google Scholar]
- 41.Valtueña S., Pellegrini N., Franzini L., Bianchi M.A., Ardigò D., Del Rio D. Food selection based on total antioxidant capacity can modify antioxidant intake, systemic inflammation, and liver function without altering markers of oxidative stress. Am J Clin Nutr. 2008;87:1290–1297. doi: 10.1093/ajcn/87.5.1290. [DOI] [PubMed] [Google Scholar]
- 42.Targher G. Nonalcoholic fatty liver disease and atherosclerosis. Arterioscler Thromb Vasc Bio. 2005;25:e117–e118. doi: 10.1161/01.ATV.0000170132.91268.a2. [DOI] [PubMed] [Google Scholar]
- 43.Choi S.Y., Kim D., Kang J.H., Park M.J., Kim Y.S., Lim S.H. Nonalcoholic fatty liver disease as a risk factor of cardiovascular disease: relation of non-alcoholic fatty liver disease to carotid atherosclerosis. Korean J Hepatol. 2008;14:77–88. doi: 10.3350/kjhep.2008.14.1.77. [DOI] [PubMed] [Google Scholar]
- 44.Brea A., Mosquera D., Martin E., Arizti A., Cordero J.L., Ros E. Nonalcoholic fatty liver disease is associated with carotid atherosclerosis: a case–control study. Arterioscler Thromb Vasc Biol. 2005;25:1045–1050. doi: 10.1161/01.ATV.0000160613.57985.18. [DOI] [PubMed] [Google Scholar]
- 45.Poanta L.I., Albu A., Fodor D. Association between fatty liver disease and carotid atherosclerosis in patients with uncomplicated type 2 diabetes mellitus. Med Ultrason. 2011;13:215–219. [PubMed] [Google Scholar]