Abstract
Objective
Cardiovascular diseases (CVDs) are the leading cause of mortality in Western countries. Atherosclerosis is a multi-step inflammatory disease characterized at early stages by accumulation of cholesterol in the arterial wall followed by recruitment of immune cells. We sought to determine if pharmacological suppression of RORα/γ activity is beneficial in treatment of atherosclerosis.
Methods
To identify the role of RORα and RORγ in atherosclerosis, we used the LDL-R−/− mouse model of atherosclerosis placed on a high cholesterol diet treated with SR1001, a RORα/γ inverse agonist, for four weeks.
Results
Our results demonstrate that treatment with the ROR inverse agonist substantially decreases plaque formation in vivo. The mechanism of the anti-atherogenic activity of the inhibition of RORα/γ activity appeared to be due to targeting two distinct pathways. SR1001 treatment reduced plasma low density lipoprotein (LDL) level without affecting high density lipoprotein (HDL) via increasing intestinal cholesterol excretion. Treatment with SR1001 also induced an anti-atherogenic immune profile that was characterized by a reduction in Th17 cells and an increase in Treg and Th2 cells. Our data suggest that RORα and RORγ play a critical role in atherosclerosis development by regulating at least two major pathways important in the pathology of this disease: cholesterol flux and inflammation.
Conclusion
Our data suggest that pharmacological targeting of RORα/γ may be an effective method for treatment of atherosclerosis offering a distinct mechanism of action relative to statins.
Keywords: Drug discovery, Nuclear receptor, Atherosclerosis, Cholesterol, Heart disease, ROR
Highlights
-
•
SR1001 decreases atherosclerosis development in a LDL-R−/− mouse model.
-
•
SR1001 promotes Treg and Th2 and decreases Th17 cells differentiation in an atherosclerotic context.
-
•
SR1001 enhances intestinal cholesterol excretion.
1. Introduction
Cardiovascular diseases including atherosclerosis are the leading cause of mortality in Western society. Atherosclerosis is a multi-step disease characterized by accumulation of cholesterol and immune cells within the arterial walls. Lipoproteins particles that pass from the blood streams to the arterial lumen become oxidized resulting in activation of endothelial cells and recruitment of lymphocytes. Lymphocyte signaling promotes the recruitment of macrophages that sequester excess cholesterol and promote its reverse transport to the liver. Over time the macrophages may not sufficiently transport the excess cholesterol leading to accumulation of the lipid and the cells become foam cells and accumulate inside of the arterial lumen. This process culminates in the formation of large lesions that are prone to occlude the vessels leading to myocardial infarction or stroke [1]. Hypercholesterolemia and inflammation have been recognized as critical pathological components in the progression of atherosclerosis.
Macrophages have been recognized as key players in the development of the disease for some time, but more recently the role of lymphocytes has been shown to be critical as well. Naïve lymphocyte CD4+ helper cells differentiate into effector cells that are characterized by their cytokine production profiles and their role in atherogenesis. The majority of lymphocytes T cells found in the atherosclerotic lesions display the characteristics of type 1 inflammatory lymphocytes T-helper (Th1) cells [2]. Th1 cells secrete pro-inflammatory cytokines such as IFNγ and TNFα and membrane CD40-ligand that amplify the immune response by activating macrophages, smooth muscle cells and endothelial cells [3]. In contrast to Th1 cells, type 2 anti-inflammatory lymphocytes T-helper (Th2) cells are rarely found in the atherosclerotic lesions. Th2 cells secrete numerous cytokines including IL-4, IL10 and IL-13. The role of Th2 in atherosclerosis is not completely understood, but within a mouse model resistant to atherosclerosis, an increase of Th2 cells has been shown to protect against early fatty streak development [4]. In the LDL receptor deficient (LDL-R−/−) atherogenic mouse model, deficiency of IL-4 did not affect lesion development [5] however, another study reported a decrease in atherosclerotic lesion formation under these conditions [6]. The role of Th17 lymphocytes, which secrete IL-17, in atherosclerosis are also controversial. Th17 cells are associated with proinflammatory response and have been shown to be associated with increased atherosclerosis [7], but others have suggested that IL-17 is atheroprotective [8]. CD4+CD25+/highFoxp3+ regulatory T cells (Treg cells) are a subpopulation of T lymphocytes involved in the suppression of pathogenic response from the immune system against self or foreign antigens [9]. The depletion of peripheral Treg cells increases atherosclerotic lesion size in atherogenic apolipoprotein E gene deficient (ApoE−/−) mice [10] Atherosclerotic mice have a compromised pool of natural Treg (CD4+/CD25+, nTreg) cells and adoptive transfer of Treg cells into proatherogenic ApoE−/− mice reduces atherosclerosis [11].
Cholesterol is an essential component of cellular plasma membrane and a precursor of bile acids, steroid hormones and vitamin D. Hypercholesterolemia promotes the development of atherosclerosis by increasing accumulation of cholesterol in the arterial lumen. The current standard care for atherosclerosis includes pharmacological intervention and changes in lifestyle. Statins, inhibitors of cholesterol biosynthesis, are the principal drugs used in therapeutic to decrease hypercholesterolemia and slow the progression of atherosclerosis. However, side effects of the statins (such as liver and muscle toxicity) limit the use of these drugs in many patients. Additionally, many individuals with hypercholesterolemia do not reach the LDL lowering goals on statins alone. Thus, there is an unmet medical need for additional classes of therapeutics to treat atherosclerosis.
Many of the new pharmacological strategies include targeting nuclear receptors (NRs). A better understanding of the function of these receptors represents new opportunities for therapeutic development. NRs are a superfamily of ligand-dependent transcription factors that control diverse functions, such as growth, development or metabolism. Ligand binding to the receptor changes the conformation of the protein and leads to modulation of the transcription of NR target genes. RAR-related orphan receptors (ROR) are members of the NR superfamily that are known to be involved in inflammatory and metabolic processes. The RORs represent a subfamily of NRs that includes three members: RORα, RORβ and RORγ. RORs bind DNA as monomers to specific sequences of DNA known as ROR response element (RORE) and act as transcriptional regulators. Both RORα and RORγ are expressed in immune cells as well as other tissues known to be key in regulation of metabolic processes such as the liver and skeletal muscle. We identified a synthetic RORα/γ inverse agonist SR1001 that effectively blocks Th17 cell differentiation and autoimmunity [12], and based on the information described above, we hypothesized that this type of pharmacological agent would be effective in preventing and/or treating atherosclerosis. Here, we used the low-density lipoprotein receptor (LDL-R) knock out mouse model of atherosclerosis to examine the effects of a RORα/γ inverse agonist on atherosclerosis development and progression.
2. Methods
2.1. Animals and treatment
All procedures were approved and conducted in accordance to the St Louis University Institutional Animal Care and Use Committee. Twenty homozygous LDL receptor deficient (LDL-R−/−) male mice were obtained from Jackson Laboratory (Bar Harbor, ME) at the age of 8 weeks and were housed individually. The mice were allowed to acclimate for a week and were maintained on standard chow diet. During the experiment the mice were fed with a high cholesterol diet containing 0.5% (w/w) cholesterol and 42% calories from fat (w/w) (Teklad TD.05305) The ROR inverse agonist SR1001 was formulated in 10%Tween 10% DMSO in PBS. 25 mg/kg SR1001 or vehicle was administered twice a day by intraperitoneal injection for a month. Body weight and food intake was monitored daily. Body composition was analyzed every 2 weeks by NMR using BioSpin LF50 Body Composition Analyzer (Bruker, Germany). Fourteen week-old males RORα flox EIIAcre and their littermate control were fed with a regular chow. All mice were house in a 12 h light/dark cycles. At the end of the experiment, mice were euthanized by CO2 asphyxiation followed by cervical dislocation. Tissues were collected, snap frozen and store at −80 °C for qPCR or western blotting, or fixed in 4% formalin, incubated in 20% sucrose over night before freezing for cryosection.
2.2. Atherosclerotic lesion analysis
At the end of four weeks of SR1001 treatment, mice from both groups were sacrificed. The sections from the aortic root to iliac artery were collected and fixed in 4% paraformaldehyde 20 min at room temperature, washed with PBS, stored overnight in 20% sucrose before freezing. Aortic roots were cryosectioned (15 μM) using Leica cryostat. To carry out en face analysis of aorta, the connective tissues were removed and aorta was cut open longitudinally under dissecting microscope. Plaques in aorta sections were visualized by Oil Red-O staining (Sigma). Aortas were imaged with Leica microscope camera (Leica S6D) and plaque area was quantified as percentage of total aortic surface area using ImageJ.
2.3. Plasma lipid and liver enzyme analysis
Mice were euthanized and blood was collected via cardiac puncture. Concentration of plasma total cholesterol, HDL cholesterol, LDL cholesterol, triglyceride and liver enzymes were assessed using Rx Daytona clinical chemistry analyzer (Randox).
2.4. Quantitative real-time PCR
Total RNA was isolated from mouse tissues using PureLink RNA mini kit (Ambion). RNA was reverse-transcribed to make cDNA using qScript™ cDNA Synthesis Kit (Quanta biosciences) according to the manufacturer's instructions. Real-time PCR was performed using a SYBR-green PCR master mix kit (Roche). Primers purchased from Integrated DNA Technologies.
2.5. Immuno staining
Immuno-histochemistry was conducted using the antibodies CD4 (BD Pharmingen) and Moma-2 (Abcam). Secondary antibodies were purchased from BD Pharmingen. Revelation was performed using ImmunoCruz ABC Staining system (Santa Cruz). Hematoxylin & eosin, Masson Trichrome and Oil Red-O staining were conducted using standard methods.
2.6. Intestinal protein preparation
Intestine tissues were lysed in a lysis buffer (250 mol/l sucrose, 2 mmmol/l MgCl2, 20 mmol/l Tris–HCL, pH7,5) containing protease inhibitor (Protease inhibitor cocktail, Santa Cruz). Lysate was centrifuged at 2000 ×g for 10 min at 4 °C.
2.7. Immunoblotting
The intestinal protein concentration was determined using BCA Kit (Pierce Biotechnology). Proteins (50 μg) were separated by SDS-PAGE and transferred onto PVDF membranes. Antibodies utilized for analysis are listed in Supplementary Table 1.
2.8. Flow cytometry
Spleens and lymph nodes were harvest into a tissue culture dish and tease it apart into a single cell suspension. Cell suspension was passed through a cell 0.22 μM filter and centrifuged (300–400 ×g) at 4 °C. Cell pellet was resuspended in Flow Cytometry Staining Buffer (Biolegend) at the final concentration of 2 × 107/ml. Cell surface and intracellular staining were performed according to Biolegend protocol (Biolegend). Antibodies used for analysis are listed in Supplementary Table 1.
2.9. Statistical analysis
Data are expressed as mean ± SEM. Two-tailed t-test was used to calculate statistical significance. p < 0.05 was considered significant.
3. Results
3.1. RORα deficient mice display an anti-atherosclerotic immune profile
Studies using the staggerer (RORαsg/sg) mouse, a natural mutant containing a deletion in the RORα gene, have demonstrated that RORα plays a critical role in the control of lipid metabolism and in inflammatory and immune responses [13], [14]. Deficiency of RORα results in reduced diet-induced adipose tissue-associated inflammation in mice [13], [14]. Moreover, the relative percentage of proinflammatory M1 macrophages was reduced in RORαsg/sg white adipose tissue (WAT). Furthermore, loss of RORα suppresses Th17 cell differentiation [15] and pharmacological suppression of RORα/γ has been shown to suppress Th17 cell differentiation as well as suppress Th17 cell dependent autoimmunity [12]. This anti-inflammatory profile associated with loss of RORα function appears to conflict with a study that indicated that staggerer mice were susceptible to development atherosclerosis when placed on a high fat diet [16]. Staggerer mice placed on an atherogenic diet for 9-weeks displayed a moderate decrease in HDL-C levels (42–52%) with no change in LDL-C levels and this was associated with an increase in atherogenic lesions [16]. We were particularly intrigued by the identification of significant atherosclerotic lesions in mice that display LDL-C levels in the 40–60 mg/dl range and HDL-C levels in the 30–50 mg/dl range. Determination of whether RORα is proatherogenic or anti-atherogenic is particularly important given recent progress in development of synthetic ligands that can modulate the activity of this receptor.
Unfortunately, the staggerer mouse model displays strong cerebellar ataxia that significantly impairs mobility and feeding activity limiting its usefulness in many types of studies. In order to circumvent this issue, we decided to use a new approach by generating transgenic animals harboring a conditional RORα allele, which decreases RORα expression only after Cre-mediated recombination. We generated mice heterozygous for a transgene encoding Cre recombinase expressed via the gene encoding EIIa (EIIa-Cre), which is expressed in all tissues, and homozygous for loxP-flanked RORα alleles (RORαflox/floxCre+/WT (RORα Hypo)). RORαflox/flox littermates without the EIIa-Cre transgene (RORαWT) served as controls. To verify efficient deletion of RORα, brain, liver and white adipose tissue (WAT) were collected and analyzed by qPCR. We were able to detect a 60% reduction of RORα expression in the brain, 75% in the liver and WAT and 65% in the intestine (Sup Figure 1A). As the deletion is not total this model allows us to study the hypomorphic role of RORα.
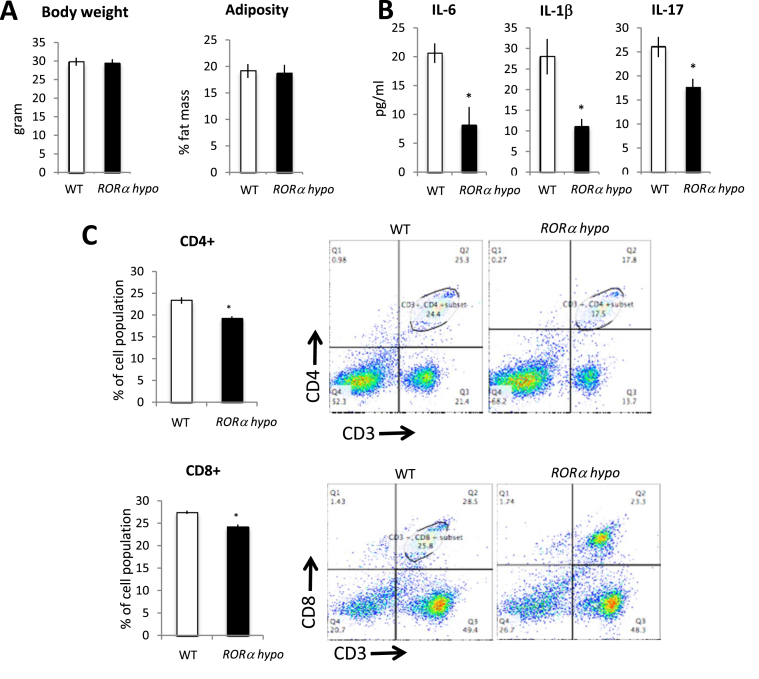
RORα Hypo mice display normal body weight and adiposity similar to that of the RORαWT mice on normal chow (Figure 1A) and no changes in plasma lipid levels (Sup Figure 1B.). Most importantly, no ataxia phenotype was observed in the RORαKO mice suggesting that the limited amount of RORα expressed in these mice may have been sufficient to avoid the cerebellar deficit. Interestingly, RORαKO animals displayed a decrease in plasma levels proinflammatory cytokines such as IL-1β (11.03 pg/ml vs 28 pg/ml), IL-6 (8.1 pg/ml vs 20.6 pg/ml) and IL-17 (17.6 pg/ml vs 26 pg/ml) compared to their WT littermates (Figure 1B). As the spleen is the major site of maturation of lymphocytes, we next examined the T cell population. Consistent with this observation, FACS analysis of splenocytes revealed an anti-inflammatory profile. In the RORαKO spleen, 19.2% of the total population was CD4+ (CD3+ CD4+, CD25−, B220−) compared to 22.3% in the WT littermates (Figure 1C upper panel). The same profile was observed in the peripheral lymph nodes (Sup Figure 1C). Cytotoxic T lymphocytes CD8+ (B220−CD3+CD4−) were also lower in the spleen from RORαKO mice compared to RORαWT (19.2% vs 24.2%) (Figure 1C lower panel). Thus, the RORαKO mice display an immunological profile that is consistent with one that would be expected to be anti-atherogenic.
Figure 1.
RORα deficient mice show an anti-inflammatory profile. (A) Weight and body composition of single housed 12 week-old males RORαWT (white bar, n = 6) or RORα Hypo (black bar, n = 6) littermate. (B) Blood cytokines profile form RORαWT (n = 6) or RORα Hypo (n = 6) male mice. (C) CD4 (upper) or CD8 (lower) expression in splenocytes freshly isolated from the spleen of 12 week-old male RORαWT (white, n = 5) or RORα Hypo (black, n = 5) mice. *p < 0.05, **p < 0.01.
3.2. SR1001 treatment blocks early and late atherosclerosis lesion development
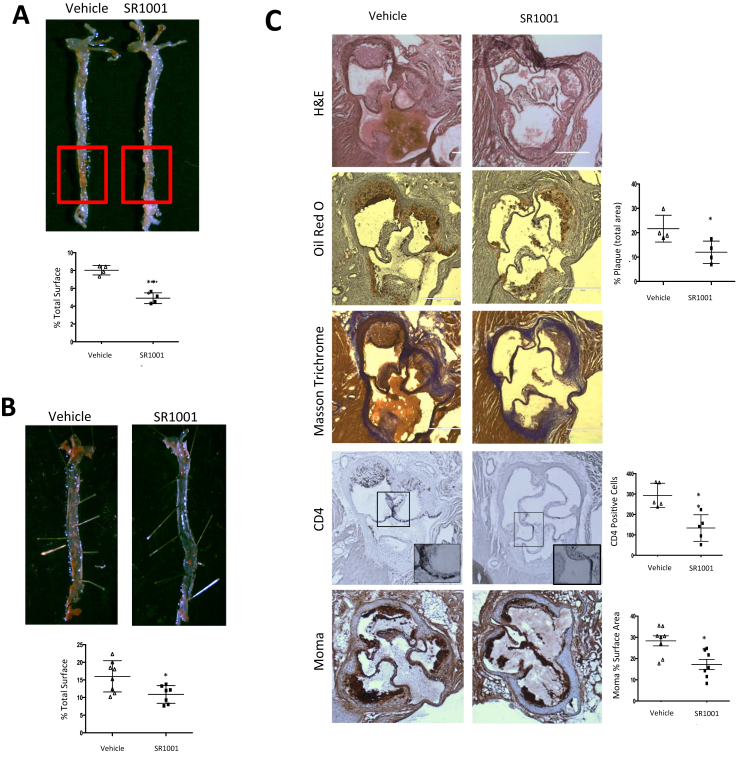
Provided that reduction of RORα or RORγ activity is associated with reduced inflammatory activity, we sought to determine if the RORα/γ inverse agonist we developed (SR1001) [12] would have an effect in a well-characterized mouse model of atherogenesis. We used 10 week-old male LDL-R−/− mice fed with an atherogenic diet (0.5% cholesterol, 21% fat, Tekland) for 10 days and then administered SR1001 (25 mg/kg) twice-per-day for a month. SR1001 treated mice displayed a significant decrease in atherosclerotic lesion progression in aortic surface as evaluated by En face Oil Red-O staining. Quantification of the plaque surface using ImageJ software indicated 40% less staining in SR1001 treated mice vs the vehicle-treated mice (Figure 2A). No weight difference was observed between the two groups. In this paradigm where the mice received the atherogenic diet for only 10 days prior to drug treatment mice the mice developed lesions consistent with early disease. In the subsequent experiment, we sought to examine the effect of SR1001 on more complex plaque associated with later stage disease. We fed LDL-R−/− mice the identical diet for 4 weeks before starting SR1001 administration for one month. Under these conditions, SR1001-treated mice also displayed a reduction in the total plaque (Figure 2B). As illustrated in Figure 2C, aortic roots from SR1001-treated mice display a decrease in lipid deposits vs vehicle-treated animals and no change in collagen cap was observed (Masson Trichrome staining), consistent with a reduction in plaque formation. Fewer surfaces were covered by lesions in SR1001 treated animals compared to vehicle treated mice. Immunostaining for macrophages (Moma2) and lymphocytes (CD4+) were performed on 15 μm cryosections and revealed that SR1001 treated animals exhibited a significant decrease in immune cell infiltration compared to vehicle treated animals. As macrophages are known to play an important role in the progression of atherosclerosis, we assessed the effect of SR1001 on macrophage polarization in vitro and no differences were observed (Sup Figure 2) suggesting that SR1001 does not exert its anti-atherosclerotic activity via alteration of M1 or M2 macrophage levels.
Figure 2.
SR1001 prevents atherosclerosis formation in LDL-R deficient mice. En face aortas of LDL-R−/− mice fed 10 days (A) or a month (B) with a high cholesterol (HC) diet and treated for one moth with SR1001 (n = 9) or vehicle (n = 9). Lipid deposits were stained with Oil Red O and total plaque surface was calculated relative to the total surface of the aorta. (C) Arterial roots from LDL-R−/− mice fed one month with HC diet and then treated with SR1001 (n = 5) or vehicle (n = 5) for a month. Sections were stained for lipid (Oil Red O) and cholesterol (Masson Trichrome) deposit or immune cell infiltration (CD4 or Moma). Quantification of positive staining was done using ImageJ. *p < 0.05, **p < 0.01, ***p < 0.001.
3.3. SR1001 treatment decreases pro-atherosclerotic immune cells and pro-inflammatory cytokines expression
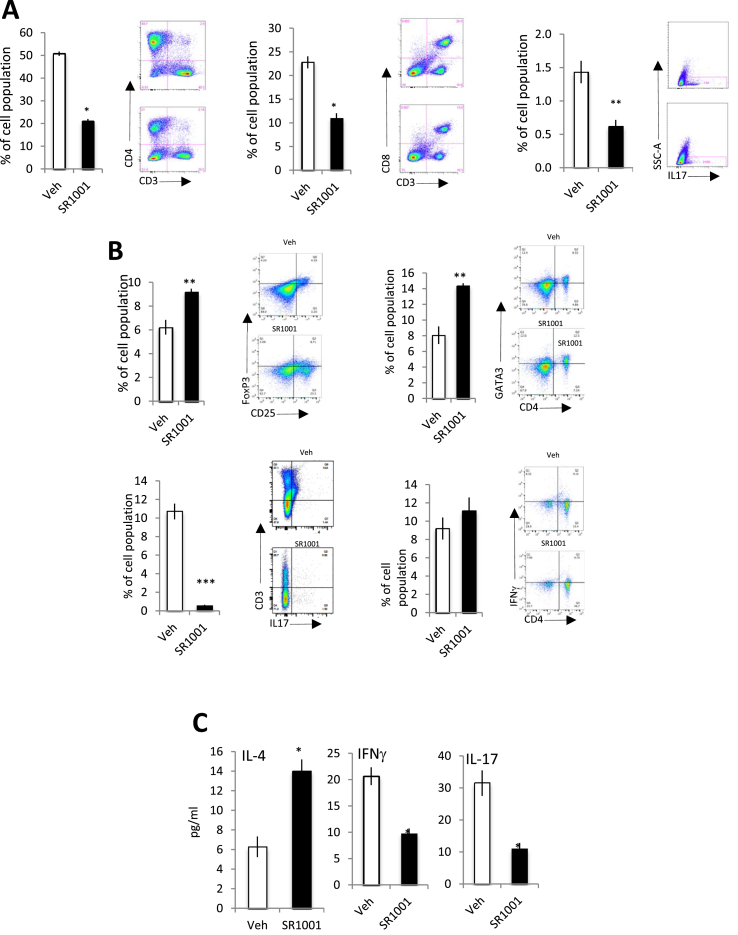
We next sought to assess the role of RORs in the regulation of the immune system in the context of atherosclerosis. Spleens and lymph nodes from the LDL-R−/− mice described in Figure 2A above were analyzed by flow cytometry. Multiple T cells subpopulations were analyzed and, as predicted, SR1001 treatment induced a strong decrease of Th17 (CD3+CD4+IL17A+) differentiation, but in addition, a significant decrease of both CD4+ and CD8+ cells were observed (Figure 3A). Using the mice that had much more extensive atherosclerosis (Figure 2B) we assessed the effect of SR1001 treatment on T cell populations in further detail. We assessed the effect of SR1001 in both spleen and lymph node on Th1 (B220−CD4+CD3+CD25−IFNg+), Th2 (B220−CD3+CD4+CD25−Gata3+), Th17 (B220−CD3+CD4+CD25−IL17A+) and Treg (B220−CD3+CD4+CD25+FoxP3+). For each population, the gating was done on live lymphocytes. Isotype control for each antibody was used to define the background and the negative gating (data not shown).
Figure 3.
SR1001 decreases inflammatory profile in LDL-R−/−mice. Splenocytes from LDL-R−/− mice fed 10 days with a HC diet and treated for one moth with SR1001 (black bar, n = 6) or vehicle (white bar, n = 6) were analyzed by flow cytometry for CD4+ (left panel A), CD8+ (middle panel A) or Th17 (SSC-A/IL-17+) (right panel A). Splenocytes form LDL-R−/− mice fed one month with a HC diet and treated for one moth with SR1001 (black bar n = 6) or vehicle white bar, (n = 6) were analyzed by flow cytometry for Th2 (CD4+ Gata3+ (B, upper right)), Treg (CD4+ FoxP3+ (B, upper left)) or Th17 (CD4+/IL-17+(B, lower left)) or Th1 (CD4+/IFNγ+ (B, lower right)). Plasma form LDL-R−/− mice fed one month with a HC diet and treated for one month with SR1001 (black bar, n = 9) or vehicle (white bar, n = 9) was analyzed by Luminex for pro (IFNγ and IL-17) or anti-inflammatory cytokines (C). *p < 0.05, **p < 0.01.
Both ‘anti-inflammatory’ T cell populations (Th2 and Treg) were significantly induced in the SR1001-treated animals compared to vehicle treated mice in the spleen (Figure 3B) and also in the lymph nodes (Sup Figure 3E). Spleen from SR1001 treated animals display an increase (14.2% vs 8.1%) of Th2 (B220−CD4+CD3+CD25−Gata3+) cells compare to WT. Very surprisingly, we found an increase in the nTreg (B220−CD3+CD4+CD25+Foxp3+) population in the spleen (Figure 3B) and the lymph nodes (Sup Figure 3E) of the SR1001-treated mice compared to vehicle treated mice. This is a particularly important observation since nTreg have been demonstrated to protect against atherosclerotic lesion development in mice [10]. No changes in Th1 (CD3+CD4+IFNγ+) cell population were observed in between the two groups in both spleen and lymph nodes (Figure 3B and Sup Figure 3E). Furthermore, consistent with the T-cell population data, we found a decrease of the pro-atherosclerotic plasma cytokines interferon gamma (IFNγ) and IL-17 in the SR1001-treated vs vehicle-treated mice (Figure 3C). Additionally, IL-4 was increased by SR1001 treatment (Figure 3C). No other changes were observed in a range of plasma cytokines (IL-1β, IL-5, IL-6, IL-10, IL-13). Thus, SR1001 treatment prevents atherosclerosis progression in both early and late stages by decreasing pro-inflammatory signals: suppressing pro-atherogenic cytokines and inducing an anti-inflammatory T cell profile.
3.4. SR1001 treatment enhances cholesterol excretion by the intestine
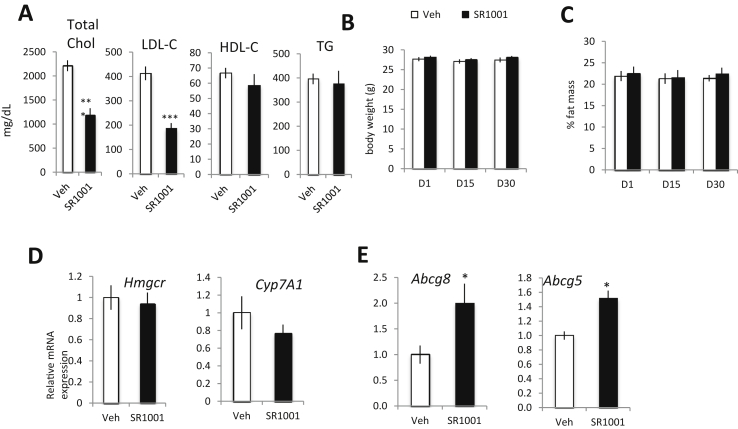
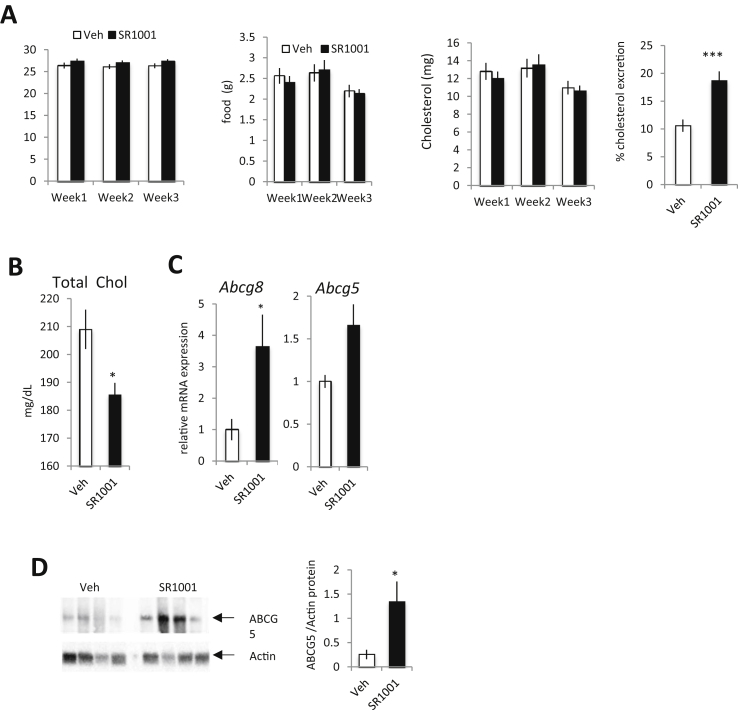
As an elevated level of cholesterol is a major risk factor in atherosclerosis development, we next characterized the effect of SR1001 on hyperlipidemia. We monitored lipids in the plasma of the LDL-R−/− mice described above, within both experimental paradigms (Figure 2A vs Figure 2B). Total cholesterol, LDL cholesterol, HDL cholesterol and triglycerides (TG) were analyzed by chemical clinical analyzer. Both total cholesterol and LDL-cholesterol were decreased upon SR1001 treatment 1 month (Figure 4A) or 10 days (Sup Figure 3A) of diet prior treatment. No changes in HDL-cholesterol or TG levels were detected in SR1001 treated animals compared to vehicle treated mice. No changes in total body weight or fat mass were observed (Figure 4B–C) in SR1001 treated animals compare to vehicle treated animals. In order to determine the mechanism underlying the ability of SR1001 to decrease plasma LDL-C levels we assessed the expression of genes involved in cholesterol metabolism. Examination of Hmgcr and Cyp7A1 expression in the liver revealed no change in expression suggesting that reduction in cholesterol and bile acid synthesis are not a likely candidate (Figure 4D). In the intestine, we observed an increase in the expression of cholesterol transporter gene expression, Abcg5 and Abcg8, induced by SR1001 administration (Figure 4E). Given that increased ABCG5/G8 expression is associated with increased cholesterol excretion [16] these data suggested that SR1001 might enhance cholesterol excretion by the intestine. As we were using the LDL-R−/− mouse model in previous experiments and loss of LDL-R may alter cholesterol excretion, we performed an additional experiment in WT mice. C57Bl/6J mice were used, under high cholesterol diet for 10 days, and treated with SR1001 (25 mg/kg, b.i.d) for 3 weeks. Food uptake and body mass were monitored daily while body composition was assessed weekly by NMR. SR1001 did not affect total food intake nor body mass (Figure 5A). Calculated total cholesterol uptake was not affected by SR1001 (Figure 5A middle panel). We collected feces every day from single housed animals and performed total cholesterol extraction. SR1001 increased the intestinal cholesterol excretion by 40% (Figure 5B right panel). Furthermore, in line with our previous results, total plasma cholesterol was reduced in SR1001-treated animals compared to vehicle treated mice (Figure 5C). Intestinal gene expression was performed by RT-qPCR and both Abcg5 and Abcg8 were found to be increased in SR1001-treated animals vs vehicle (Figure 5E). We also found that both ABCG5 and ABCG8 (data not shown) protein were increased in the intestine from SR1001 treated animals. Given that neither cholesterol nor bile acid biosynthesis appear to be targets of SR1001 (Figure 5D), these data suggest that SR1001 may be decreasing plasma cholesterol by enhancing intestinal cholesterol excretion at least in part via upregulation of ABCG5/G8. However, it is important to emphasize that other regulatory pathways may be modulated as well.
Figure 4.
SR1001 decreases plasma cholesterol levels and increases intestinal cholesterol excretion in mice. Plasma form LDL-R−/− mice fed one month with a HC diet and treated for one month with SR1001 (black bar, n = 9) or vehicle (white bar, n = 9) was analyzed by chemistry analyzer for total (CHOL), LDL, HDL and triglycerides levels (A). Body weight (B) and fat mass (C) of single housed LDL-R−/− mice fed one month with a HC diet over the month of SR1001 (black bar, n = 9) or vehicle (white bar, n = 9) treatment. (D) Liver and (E) intestinal gene expression of cholesterol absorption/excretion form LDL-R−/− mice fed one month with a HC diet and treated for one month with SR1001 (black bar, n = 9) or vehicle (white bar, n = 9). *p < 0.05, **p < 0.01, ***p < 0.001.
Figure 5.
SR1001 decreases plasma cholesterol levels and increases intestinal cholesterol excretion in WT mice. (A) Body weight, food, cholesterol intake and fecal cholesterol excretion of single housed WT mice fed with HC diet for 10 days and then treated with SR1001 (black bar, n = 10) or vehicle (white bar, n = 10) for 3 weeks. (B) Total plasma cholesterol level as quantify in SR1001 (black bar, n = 10) or vehicle (white bar, n = 10) treated animals. Intestinal gene (C) and protein (D) expression of cholesterol excretion. Relative level of ABCG5 protein compare to actin for SR1001 (black bar, n = 4) or vehicle (white bar, n = 4) animals. *p < 0.05.
4. Discussion
Several years ago, our group developed synthetic RORα/γ inverse agonist SR1001 that we used to demonstrate the feasibility of pharmacological targeting of RORα/γ for suppression of autoimmunity via suppression of Th17 cell differentiation and function [12]. In addition to regulation of Th17 cell differentiation, both RORα and RORγ are also known to regulate additional immune functions as well as metabolic pathways that are important in the development of atherosclerosis. Previous studies in using the staggerer mouse suggested that RORα may provide protection against atherosclerosis [17], but our data with the RORα KO mouse (Figure 1) indicated that loss of RORα provided an immune profile may be atheroprotective. Thus, we proceeded with assessment of the RORα/γ inverse agonist SR1001 activity in the LDL-R−/− mouse model of atherosclerosis in order to determine the potential utility of pharmacologically targeting RORs for the treatment of this disease. Our results clearly demonstrate that SR1001 treatment decreases atherosclerosis lesion formation and there are apparently two mechanisms responsible for the efficacy. SR1001 administration reduces plasma total cholesterol and LDL cholesterol levels while also decreasing pro-inflammatory signals and immune cell infiltration within the lesion. It is possible that the reduced inflammation is a secondary effect due to reduced cholesterol accumulation in the plaques, but our data from RORαKO mice indicates that loss of RORα provides an atheroprotective immune profile in the absence of atherosclerosis suggesting that the two mechanisms are independent. RORγ null mice also display an immune phenotype that would be expected to be atheroprotective [18]. In two distinct mouse models, we were able to demonstrate that SR1001 enhances abcg5 and abcg8 expression in the intestine, which is consistent with the increase in fecal cholesterol excretion and decrease in total plasma cholesterol that we have observed.
Recent work has demonstrated that nTreg (CD3+CD4+CD25+ FoxP3+) cells, which have been shown to have an atheroprotective role [11] can be converted into Th17 cells [19], [20]. Treg and Th17 cells are reciprocally regulated through a thigh regulation of the expression of the transcription factor FoxP3. FoxP3 is known to repress RORα and RORγt transcriptional activity by direct interaction [21]. We hypothesize that the loss of RORα or the inhibition of both RORα and RORγ (using SR1001) will lead to a shift from Th17 to Treg differentiation through an upregulation of FoxP3 activity in an atherosclerotic context. Both RORα and RORγ are needed to convert nTreg cells to Th17 cells [15] and by suppressing the activity of these two receptors with SR1001 we may be blocking the conversion of nTreg cells to Th17 cells as well as suppressing the classical differentiation pathway from naïve T cells to Th17 cells. Indeed, we did observe an increase in nTreg cells and a decrease in Th17 cells that is consistent with this mechanism, which would lead to an atheroprotective profile.
In mice, treatment with SR1001 decreases both LDL and total cholesterol levels without decreasing HDL-cholesterol levels (Figure 4A) or raising liver enzyme levels (Sup Figure 3D and [13]). Current clinically approved cholesterol lowering drugs available in the market have side effects such as decreases in HDL-cholesterol and increases in CYP7A1 expression. SR1001 treatment decreases plasma cholesterol level, enhances intestinal cholesterol excretion and without effect on liver cholesterol metabolism in two different mouse-models, LDL-R−/− and WT. A significant caveat is that cholesterol metabolism is considerably different in rodents then in humans and these results may not effectively translate. It appears that at least one mechanism that RORs utilize to regulate intestinal cholesterol expression is via upregulation of the ABCG5/G8 transporters. By inhibiting ROR we were able to increase expression of these two genes. It is unclear if this regulation is direct or indirect, but given that RORs are generally activators of transcription and inhibition of the activity of the RORs led to increased expression of abcg5/g8, the mechanism of regulation must be unique.
We have shown that ROR inverse agonist target key mechanisms involved in atherosclerosis development: regulation of the immune system and intestinal cholesterol excretion. The specific roles of each pathway in the protection against atherosclerosis are not clear and additional, as of yet unidentified, mechanisms may be involved in the anti-atherosclerotic activity of SR1001. We believe that the two mechanisms are independent and further experiment will be necessary to better understand the relationship between regulation of the inflammation and cholesterol metabolism.
Atherosclerosis is a complex disease and mechanisms involved in the development of the disease are different between the mouse-model used and human disease. Cholesterol metabolism in rodent and human differ but the data suggesting the activity of ROR inverse agonists are promising in terms of the potential to translation into a therapeutic.
5. Conclusion
Our data clearly demonstrate that pharmacological suppression RORα and RORγ activity suppresses atherosclerosis in a mouse model. Clearly RORα and RORγ can be targeted to: modulate the immune system and cholesterol excretion, two main risk factors that lead to atherosclerosis. For now it is still unclear if these two mechanisms are linked to each other or independent. Further experiments will be needed to decipher the contribution of ROR in each mechanism during atherosclerosis development. Moreover further experiments will be necessary but we believe that RORs may be a new therapeutic target that regulates distinct pathways relative to the commonly used anti-atherosclerotic statins and that statins and ROR inverse agonists may be able to be combined to increase efficacy.
Authors' contribution
C.B. and T.P.B. conceived the project, designed the experiments, interpreted data, wrote the paper, and are responsible for the integrity of its content. C.B and S.S conducted the experiments.
Acknowledgments
The authors want to acknowledge Sherri Koehm and Joy Eslick for their help in the design of the flow cytometry experiments and Sherry Burris for her work on the histological studies. This work was supported by a grant from the National Institutes of Health to T.P.B. (MH092769).
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molmet.2016.07.001.
Conflict of interest
C.B., T.P.B., and S.S. declare that they have no conflicts of interest.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Glass C.K., Witztum J.L. Atherosclerosis: the road ahead review. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 2.Ketelhuth D., Hansson G. Cellular immunity, low-density lipoprotein and atherosclerosis: break of tolerance in the artery wall. Thrombosis Haemostasis. 2011;106:779–786. doi: 10.1160/TH11-05-0321. [DOI] [PubMed] [Google Scholar]
- 3.Li N. CD4+ T cells in atherosclerosis: regulation by platelets. Thrombosis Haemostasis. 2014;109:980–990. doi: 10.1160/TH12-11-0819. [DOI] [PubMed] [Google Scholar]
- 4.Huber S.A., Sakkinen P., David C., Newell M.K., Tracy R.P.T. helper-cell phenotype regulates atherosclerosis in mice under conditions of mild hypercholesterolemia. Circulation. 2001;103:2610–2616. doi: 10.1161/01.cir.103.21.2610. [DOI] [PubMed] [Google Scholar]
- 5.King V., Cassis L., Daugherty A. Interleukin-4 does not influence development of hypercholesterolemia or angiotensin II-induced atherosclerotic lesions in mice. American Journal of Pathology. 2007;171:2040–2047. doi: 10.2353/ajpath.2007.060857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.King V., Szilvassy S., Daugherty A. Interleukin-4 deficiency promotes gallstone formation. Journal of Lipid Research. 2002;43:768–771. [PubMed] [Google Scholar]
- 7.Ng H., Burris R., Nagarajan S. Attenuated atherosclerotic lesions in apoE-Fcγ-chain-deficient hyperlipidemic mouse model is associated with inhibition of Th17 cells and promotion of regulatory T cells. Journal of Immunology. 2011;187:6082–6093. doi: 10.4049/jimmunol.1004133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taleb S., Romain M., Ramkhelawon B., Uyttenhove C., Pasterkamp G., Herbin O. Loss of SOCS3 expression in T cells reveals a regulatory role for interleukin-17 in atherosclerosis. The Journal of Experimental Medicine. 2009;206:2067–2077. doi: 10.1084/jem.20090545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakaguchi S., Setoguchi R., Yagi H., Nomura T. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in self-tolerance and autoimmune disease. Current Topics in Microbiology and Immunology. 2006;305:55–66. doi: 10.1007/3-540-29714-6_3. [DOI] [PubMed] [Google Scholar]
- 10.Ait-Oufella H., Salomon B.L., Potteaux S., Robertson A.K., Gourdy P., Zoll J. Natural regulatory T cells control the development of atherosclerosis in mice. Nature Medicine. 2006;12:178–180. doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- 11.Mor A., Planer D., Luboshits G., Afek A., Metzger S., Chajek-Shaul T. Role of naturally occurring CD4+ CD25+ regulatory T cells in experimental atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2007;27:893–900. doi: 10.1161/01.ATV.0000259365.31469.89. [DOI] [PubMed] [Google Scholar]
- 12.Solt L.A., Kumar N., Nuhant P., Wang Y., Lauer J.L., Liu J. Suppression of TH17 differentiation and autoimmunity by a synthetic ROR ligand. Nature. 2011;472:491–494. doi: 10.1038/nature10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang H.S., Okamoto K., Takeda Y., Beak J.Y., Gerrish K., Bortner C.D. Transcriptional profiling reveals a role for RORalpha in regulating gene expression in obesity-associated inflammation and hepatic steatosis. Physiological Genomics. 2011;43:818–828. doi: 10.1152/physiolgenomics.00206.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lau P., Fitzsimmons R.L., Pearen M.A., Watt M.J., Muscat G.E. Homozygous staggerer (sg/sg) mice display improved insulin sensitivity and enhanced glucose uptake in skeletal muscle. Diabetologia. 2011;54:1169–1180. doi: 10.1007/s00125-011-2046-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang X.O., Pappu B.P., Nurieva R., Akimzhanov A., Kang H.S., Chung Y. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu L., Li-Hawkins J., Hammer R.E., Berge K.E., Horton J.D., Cohen J.C. Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol. The Journal of Clinical Investigation. 2002;110:671–680. doi: 10.1172/JCI16001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mamontova A., Séguret-Macé S., Esposito B., Chaniale C., Bouly M., Delhaye-Bouchaud N. Severe atherosclerosis and hypoalphalipoproteinemia in the staggerer mouse, a mutant of the nuclear receptor RORalpha. Circulation. 1998;98:2738–2743. doi: 10.1161/01.cir.98.24.2738. [DOI] [PubMed] [Google Scholar]
- 18.Kurebayashi S., Ueda E., Sakaue M., Patel D.D., Medvedev A., Zhang F. Retinoid-related orphan receptor gamma (RORgamma) is essential for lymphoid organogenesis and controls apoptosis during thymopoiesis. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:10132–10137. doi: 10.1073/pnas.97.18.10132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisenstein E., Williams C. The T(reg)/Th17 cell balance: a new paradigm for autoimmunity. Pediatric Research. 2009;65:26R–31R. doi: 10.1203/PDR.0b013e31819e76c7. [DOI] [PubMed] [Google Scholar]
- 20.Gagliani N., Amezcua Vesely M.C., Iseppon A., Brockmann L., Xu H., Palm N.W. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature. 2015;523:221–225. doi: 10.1038/nature14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jetten A.M. Retinoid-related orphan receptors (RORs): critical roles in development, immunity, circadian rhythm, and cellular metabolism. Nuclear Receptor Signaling. 2009;7 doi: 10.1621/nrs.07003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.