Abstract
Objective
Adult neurogenesis in the subgranular zone and subventricular zone is generally accepted, but its existence in other brain areas is still controversial. Circumventricular organs, such as the area postrema (AP) have recently been described as potential neurogenic niches in the adult brain. The AP is the major site of action of the satiating hormone amylin. Amylin has been shown to promote the formation of neuronal projections originating from the AP in neonatal rodents but the role of amylin in adult neurogenesis remains unknown.
Methods
To test this, we first performed an RNA-sequencing of the AP of adult rats acutely injected with either amylin (20 μg/kg), amylin plus the amylin receptor antagonist AC187 (500 μg/kg) or vehicle. Second, animals were subcutaneously equipped with minipumps releasing either amylin (50 μg/kg/day) or vehicle for 3 weeks to assess cell proliferation and differentiation with the 5′-bromo-2-deoxyuridine (BrdU) technique.
Results
Acute amylin injections affected genes involved in pathways and processes that control adult neurogenesis. Amylin consistently upregulated NeuroD1 transcript and protein in the adult AP, and this effect was blocked by the co-administration of AC187. Further, chronic amylin treatment increased the number of newly proliferated AP-cells and significantly promoted their differentiation into neurons rather than astrocytes.
Conclusion
Our findings revealed a novel role of the satiating hormone amylin in promoting neurogenesis in the AP of adult rats.
Keywords: Amylin, Adult neurogenesis, Area postrema, BrdU, Circumventricular organs
Abbreviation: AP, area postrema; bHLH, basic helix-loop-helix; BrdU, 5′-bromo-2-deoxyuridine; CR, calretinin; CTR, calcitonin receptor; CVO, circumventricular organs; EphRs, ephrin receptors; ERK1/2, extracellular signal-regulated kinase 1 and 2; FDR, false discovery rate; GO, gene ontology; ME, median eminence; NeuroD, neuronal differentiation; NeuroD1, neuronal differentiation-1; NGS, next generation sequencing; NSC, neural stem cells; RAMP, receptor activity-modifying protein; Wnt, Wingless-Type MMTV Integration Site Family
Highlights
-
•
Acute amylin affected genes involved in pathways and processes that drive neurogenesis.
-
•
Chronic amylin increased the number of newly proliferating cells in the AP of adult rats.
-
•
Chronic amylin determined neuronal fate over astrocytes in the AP of adult rats.
1. Introduction
Amylin is a pancreatic hormone whose role in the control of food intake and energy metabolism is well characterized [1], [2], [3], [4], [5], [6]. In response to nutrient stimulation, amylin is co-secreted with insulin from the β-cells [2], whereby it acts to slow glucose appearance by reducing food intake, inhibiting glucagon secretion, and slowing gastric emptying [7], [8]. Amylin's effects are primarily mediated via the area postrema (AP), a sensory circumventricular organ (CVO) located at the inferoposterior border of the fourth ventricle in the hindbrain. AP-neurons express amylin receptors, which comprise a calcitonin receptor (CTR) core and one or more members of the receptor activity-modifying proteins (RAMPs) [9], [10], [11], [12]. Amylin treatment activates the extracellular signal-regulated kinase 1 and 2 (ERK 1/2) signaling pathway in CTR-positive AP neurons, which may contribute to its satiating effect [13]. Further, we recently showed that acute amylin differentially regulates the transcription of its own receptor components (RAMPs) and of the leptin receptor in the AP of adult rats [12].
While amylin's role in energy homeostasis is perhaps the most studied, growing evidence suggests that the hormone may have additional beneficial effects in the brain. Neonatal amylin-deficient mice have a reduced density of neuronal projections from the AP, suggesting amylin's potential to function as a trophic factor [14]. Amylin may also exert neuroprotective effects in the brain. Chronic treatment with the amylin analog pramlintide improved memory, cognition, and ameliorated hippocampal pathology in a mouse model of Alzheimer's disease [15]. Chronic treatment with amylin could also rescue reduced cell proliferation observed in the hippocampus and AP following ovariectomy in adult female rats [16].
At present, little is known about the mechanism by which amylin causes these neuronal changes, notably the neurogenic effects observed in the adult brain. In has been shown recently that several CVOs, including the AP, contain the machinery necessary for the birth of new neurons, including neural stem cells (NSC), and that adult neurogenesis constitutively occurs in these nuclei [16], [17], [18], [19]. As observed in the classic neurogenic niches of subgranular zone of the hippocampus [20], [21] and the subventricular zone lining the lateral ventricle [22], adult-born cells in the mammalian brain are guided through proliferation, differentiation and survival by specific extracellular and intracellular programs [23]. Upregulation of the proneural basic helix-loop-helix (bHLH) transcriptional factor NeuroD1 (Neuronal differentiation-1, also known as Beta2) alone is sufficient to induce the differentiation of adult hippocampal neural progenitors into neurons [24], [25], whereas the deletion of NeuroD1 from these neurogenic niches resulted in a significant reduction of new neurons [25]. Further, growing evidence supports the role of Ephrin-signaling and gamma-amino-isobutyric acid (GABA) signaling in the control of multiple steps of adult neurogenesis [26], [27], [28].
It is unclear if these pathways are also involved in adult neurogenesis in the AP, and whether amylin might activate them to promote the birth of new cells. There are several reports, however, demonstrating that signals related to nutrient status can influence adult neurogenesis. Metabolic hormones like insulin, leptin, and ghrelin have all been shown to induce neurogenesis in the hippocampus [29], [30], [31], [32]. It was also shown recently that consumption of a high fat diet increases neurogenesis in the hypothalamic median eminence (ME) [33]. Like insulin and leptin, amylin levels are elevated in states of positive energy balance, and could therefore be another nutritional signal driving the generation of newborn neurons in metabolic centers in the brain [34].
In the present study we therefore asked whether acute and chronic amylin administration might influence adult neurogenesis in the AP. To address these questions, we first performed an RNA-sequencing experiment to determine the genes differentially regulated by acute amylin treatment. Further, we analyzed the chronic amylin-mediated effects on cell proliferation and differentiation in the AP of adult rats by BrdU-labeling and double-fluorescent immunohistochemistry techniques.
2. Animals, materials and methods
2.1. Animals and housing
Adult male Wistar rats (Janvier, Le Genest Saint Isle, France) (220–250 g) were used for the experiments and were single-housed in a temperature-controlled environment (21 ± 1 °C) on an artificial 12 h/12 h light/dark cycle. Rats had ad libitum access to water and standard chow (no. 3430; Provimi Kilba, Gossau, Switzerland), except during fasting periods as described below. All procedures involving animals and their care were approved by the Veterinary Office of the Canton Zurich, Switzerland, and in accordance with the EU Directive 2010/63/EU on the protection of animals used for scientific purposes.
2.2. Drugs
Amylin (Bachem AG, Bubendorf, Switzerland; catalog number: H-9475.1000) was reconstituted in sterile 0.9% NaCl, AC187 (TOCRIS Bioscience, Bristol, UK; catalog number: 3419) was reconstituted in sterile double-distillated water. BrdU (B5002, Sigma Aldrich, Buchs, Switzerland) was dissolved in sterile double-distillated water and heated to 40–50 °C.
2.3. Experiment 1: next generation sequencing (NGS) of the entire AP
Rats (220–250 g; n = 5 per group) were fasted for 12 h during the light phase. At dark onset, rats were acutely treated intra-peritoneally (i.p.) with amylin (20 μg/kg), amylin plus the amylin receptor antagonist AC187 (500 μg/kg) or with vehicle (NaCl). Ninety minutes after drug administration, rats were anesthetized with isoflurane and decapitated; during this 90-min period, rats had no access to food.
2.3.1. RNA extraction
After decapitation, the AP was surgically removed from the hindbrain under a light microscope and RNA was extracted according to manufacturer's instructions (TRI Reagent®; Sigma–Aldrich, Buchs, Switzerland) and then purified following the cleanup protocol of RNeasy® Mini kit (Qiagen, Basel, Switzerland), including the DNase step. The concentration and the integrity of RNA were measured using a nanodrop system (NanoDrop 1000 Spectrophotometer, Thermo Scientific, Waltham, MA, USA).
2.3.2. Strand-specific cDNA library construction
From each sample, 10 ng of total RNA were taken to construct strand-specific cDNA libraries using the Ovation® Single Cell RNA-Seq System (PART NO. 0342, NuGEN; San Carlos, California). The quality of cDNA libraries was tested and quantified cDNA was subjected to purification, elution, end-repair and adapter ligation. A second round of library amplification and purification was performed, following the manufacturer's instruction. Samples were screened by bioanalyzer (Agilent Technologies, Santa Clara, CA, USA) and qPCR. The established cDNA libraries were sequenced using the Illumina HiSeq 2000 platform (Ilumina Inc., San Diego, CA, USA) to generate 100 bp paired-end reads.
2.3.3. Enrichment analysis for pathways, gene ontology (GO) processes and gene network construction
The list of ranked genes for each sequencing batch and the list of susceptibility genes were imported into MetaCore™ software version 6.24 (Thomson Reuters, New York, NY, USA). Enrichment analysis for pathways and GO processes was performed on gene expression data to identify significant pathways and processes affected by our experimental conditions. Enrichment analysis comprised both up- and down-signals, distribution was achieved by intersection setting and the sorting method was based on statistically significant data (threshold = 2 and P-value ≤ 0.01). Analysis was performed via the online platform portal.genego.com version 6.20.66481. To determine the pathways associated with the susceptibility genes, a sub-enrichment analysis was performed on the list of these genes. Pathways are represented by the GO set class of biological processes. Finally, to determine the interactions between susceptibility genes from the different functional groups, gene networks were constructed. Data files were analyzed with MetaCore + MetaDrug® version 6.19 build 65960 scripts 65979.
2.3.4. Quantitative real-time PCR (qPCR)
Total RNA was reverse transcribed with Tetro cDNA Synthesis Kit (Bioline, Elchrom Scientific AG, Cham, Switzerland). Real-time PCR was performed using 7500 Fast system (Applied Biosystem/Life Technologies Carlsbad, CA, USA) with QuantiTect® SYBR® green PCR kit (Qiagen, Basel, Switzerland). Intron-spanning primers were designed with IDT, Integrated DNA Technologies, (http://eu.idtdna.com). The primer sequences were the following: for rat GAPDH, forward: 5′-AGACAGCCGCATCTTCTTGT-3′ and reverse: 5′-CTTGCCGTGCGTAGAGTCAT -3′ (Accession: NM_017008.4); for rat NeuroD1, forward: 5′-GAACACGAGGCAGACAAGAA-3′ and reverse: 5′-TCATCTTCATCCTCCTCCTCTC-3' (Accession: NM_019218.2). 100 ng of cDNA were subjected to an initial heat activation at 95 °C for 15 min, followed by 40 cycles of alternating between 94 °C for 15 s, 60 °C for 30 s, and final extension at 72 °C for 30 s. The fold change in expression of each gene was calculated using the comparative ΔΔCt method, with GAPDH as endogenous control. Each sample was run in duplicate.
2.4. Experiment 2: validation of NGS findings
The second experiment aimed to further validate the difference in NeuroD1 transcript at the protein level. Rats (220–250 g; n = 3 per group) were fasted during the light phase and acutely treated with amylin (20 μg/kg; i.p), or with vehicle (NaCl; i.p). Ninety minutes after drug administration, animals were deeply anesthetized with sodium pentobarbital (100 mg/kg; i.p.) and transcardially perfused using 0.1M PB followed by 4% paraformaldehyde (PFA). During this 90-min period, rats had no access to food.
The brains were collected and maintained overnight in 4% PFA. Subsequently, the brains were transferred to 20% sucrose in PBS for 24 h. Brains were cut into 20-μm coronal sections on a cryostat (Leica microsystem, Wetzlar, Germany) and stored in cryoprotectant (20% glycerol, 30% ethylene glycol, 50% 0.1M PB) until processing for immunohistochemistry.
2.4.1. Immunohistochemistry
Slide-mounted sections (20 μm) were washed in 0.1M PBS (pH 7.4) and then incubated in immunoblocking buffer (2.5% bovine serum albumin [BSA], 0.1% Triton X-100 in PBS) for 1 h at room temperature. Subsequently, sections were incubated with Rabbit anti-NeuroD1 primary antibody (1:250, Abcam, Cambridge, UK). After overnight incubation at 4 °C, the sections were washed in PBS and incubated for 3 h with fluorescent secondary antibody Alexa Fluor 488 Donkey anti-rabbit immunoglobulin G (IgG) (1:400, Invitrogen, MOLECULAR PROBES®, Eugene, Oregon, USA). The nuclear dye DAPI (4′,6-diamidino-2-phenylindole; Sigma–Aldrich, Buchs, Switzerland) was added to secondary antibodies solution for nuclear staining. Sections were then rinsed in PBS and coverslipped with fluorescent mounting medium.
2.5. Experiment 3: effect of chronic amylin treatment on cell proliferation and differentiation in the adult AP
Rats (220–250 g; 4 groups and n = 8 per group) were acclimatized in their home-cage environment. Amylin (50 μg/kg/d) or vehicle was chronically administered peripherally by subcutaneous osmotic minipumps (Alzet®, Model 2004 ensuring up to 3-week constant delivery of the pre-charged solution; Durect Corporation; Cupertino, CA, USA) for seven days or three weeks, respectively. On the day of implantation, the minipumps were filled under sterile conditions with saline or amylin. Rats were initially anesthetized by inhalation of 5% isoflurane (IsoFlo®, Provet AG, Lyssach, Switzerland), then maintained on 2–3% isoflurane and placed on a heating pad to maintain body temperature during surgery. At the site of implantation, rats were shaved and the skin was disinfected with Betadine® (Provet AG, Lyssach, Switzerland). Under sterile conditions, a small incision was made between the scapulae and the minipump was subcutaneously implanted. The wound was closed with interrupted cutaneous sutures. Food intake and body weight were measured daily.
2.5.1. BrdU injection
BrdU was used to track cell proliferation and differentiation. Rats were given twice daily i.p injections of BrdU (75 mg/kg; 12 h apart) for 6 days, starting on the day of minipump implantation. To determine the rate of newborn cells, part of the rats were sacrificed on day 7 (i.e. 12 h after the last BrdU injection); the remaining rats were allowed to survive for an additional two weeks and then sacrificed at the end of the 3-week amylin infusion period, to investigate the fate of the BrdU-labeled cells.
2.5.2. Tissue preparation
Animals were perfused and brains processed following the protocol described in Experiment 2 (Section 2.4), with two exceptions: here, tissues were stored in a solution of 30% sucrose in PBS for 48 h and brains were cut into 30-μm coronal sections.
2.5.3. Double fluorescence immunohistochemistry
Free-floating sections were pretreated to denature the DNA to make the BrdU antibody detectable. Briefly, AP sections were incubated in a solution of 50% formamide for 2 h at 65 °C; then, sections were incubated in 2N HCl for 30 min at 30 °C and subsequently acid neutralized with 0.1 M borate buffer for 10 min. After washing in 0.1M PBS (pH 7.4), sections were incubated in immunoblocking buffer (3% goat serum, 1% BSA, 0.3% Triton X-100 in PBS) for 1 h. Primary antibodies were applied as a cocktail that included rat anti-BrdU primary antibody (1:100, Abcam, Cambridge, UK), mouse anti-HuCD (1:100, Thermo Fisher Scientific, Wahltman, MA, USA), rabbit anti-DCX (1:1000, Abcam, Cambridge, UK), rabbit anti-GFAP (1:1000, Abcam, Cambridge, UK), mouse anti-RECA-1 (1:2000; AbD Serotec, Kidlington, UK) or rabbit anti-calretinin (CR) (1:100, Abcam, Cambridge, UK). After overnight incubation at 4 °C, the sections were washed in PBS and incubated for 4 h with fluorescent secondary antibodies, also applied as a cocktail: Alexa Fluor 488 goat anti-rat immunoglobulin G (IgG) to reveal immunoreactivity of BrdU and Alexa Fluor 555 goat anti-rabbit to reveal immunoreactivity of either GFAP, RECA, DCX and CR IgG, or Alexa 555 goat anti-mouse to reveal immunoreactivity of HuCD (1:400 for all the antibodies; Invitrogen, MOLECULAR PROBES®, Eugene, Oregon, USA). Then, the sections were rinsed in PBS and mounted onto Superfrost glass slides (Thermo Scientific, Waltham, MA USA) and coverslipped with fluorescent mounting medium.
2.6. Cell quantification and analysis
Two AP sections per brain were counted and averaged to determine the number of NeuroD1-positive cells per animal in Experiment 2, and BrdU-labeled cells and the total number of neurons and glia, in Experiment 3. Counting was accomplished at 20× magnification and numerical aperture of 0.5, using a light microscope (Zeiss Imager Z2; Carl Zeiss, Jena, Germany) equipped with a color camera (Zeiss Axiocam; Carl Zeiss, Jena, Germany). The number of NeuroD1-positive cells (i.e; number of particles) and the total number of neuronal and glial cells (i.e; density of particles) was quantified with Image J version 1.49b. BrdU-labeled cells were manually counted by eye. For cell differentiation experiments, fluorescent double-labeled sections with BrdU+/HuCD+ cells and BrdU+/GFAP+ cells were visualized and analyzed on CLSM Leica TCS SP8 upright confocal microscope (Leica Microsystem, Wetzlar, Germany) equipped with an x-y-z motorized stage and three lasers (Argon 488, HeNe 543 and HeNe 633) appropriate to detect DAPI, Alexa 555 and Alexa 488 fluorescence, respectively. Each laser channel was separately scanned using a multitrack PMT configuration to avoid cross-talk between fluorescent labels. A careful examination of all secondary antibodies was performed to avoid any possible cross-talk between fluorescent dyes or any cross-reactivity between secondary antibodies. To evaluate double-labeling, confocal z-stack sectioning was performed at 0.5–1.0-μm intervals using 40 × oil-immersion (NA = 1.30) or 63 × oil-immersion (NA = 1.40) objectives. Images were acquired and 3D-reconstructed using the Zeiss LSM software, cropped and optimized in Imaris Software version 6.4.0 (Bitplane AG, Zurich, Switzerland). Colocalization of BrdU with HuCD or GFAP, respectively, was confirmed by examining multiple optical planes for each cell on the z-axis. The percentage of BrdU-positive cells double-labeled for HuCD or GFAP was determined by eye counting. The experimenter was blinded to the treatment (amylin versus control).
2.7. Statistical analysis
Relative mRNA expression levels were assessed by one-way ANOVA. BrdU-labeled cells and food intake were quantified and then compared using Student's t-test or one-way-ANOVA, as appropriate, by using GraphPad Software version 6.0 (San Diego, CA, USA). All values are expressed as mean ± S.E.M. The level of significance was set to P < 0.05.
3. Results
3.1. Acute amylin treatment affected the regulation of genes involved in pathways and processes that participate in the regulation of neurogenesis
We recently demonstrated that acute amylin treatment regulates the transcription of specific genes in the AP that are involved in amylin signaling at the single cells level [12]. We therefore aimed to determine whether amylin also has the ability to modulate the entire transcriptome of the AP. Pathways analysis performed on the NGS data revealed that Cell adhesion_Ephrin signaling, neurophysiological process_GABAA-receptor life cycle and neurophysiological process_ GABAB-receptor signaling at postsynaptic sides of the synapsis pathways were the top-three pathways which involved genes affected by acute amylin treatment (Table 1).
Table 1.
Enrichment analysis by pathway.
| Pathway maps | *Total | P-value | FDR |
|---|---|---|---|
| Cell adhesion_Ephrin signaling | 45 | 1.384E-07 | 2.076E-05 |
| Neurophysiological process_GABA-A receptor life cycle | 27 | 4.311E-06 | 7.674E-04 |
| Neurophysiological process_GABA-B receptor signaling at postsynaptic sides of synapses | 42 | 2.628E-05 | 2.339E-03 |
| Neurophysiological process_Dopamine D2 receptor signaling in CNS | 47 | 4.125E-05 | 2.448E-03 |
| Signal transduction_PKA signaling | 51 | 5.711E-05 | 2.541 E-03 |
| Neurophysiological process_Receptor-mediated axon growth repulsion | 45 | 1.010E-04 | 7.573E-03 |
| Neurophysiological process_Dopamine D2 receptor transactivation of PDGFR in CNS | 26 | 1.530E-04 | 1.683E-02 |
| Neurophysiological process_HTR1 A receptor signaling in neuronal cells | 26 | 1.530E-04 | 1.683E-02 |
| G-protein signaling_G-Protein alpha-i signaling cascades | 27 | 1.856E-04 | 6.608E-03 |
| Neurophysiological process_Mu-type opioid receptor-mediated analgesia | 30 | 2.554E-04 | 7.578E-03 |
*Total refers to the total number of genes involved in a pathway. False discovery rate (FDR) and P-value refer to pathways involved in the experimental conditions. A P-value <0.01 is considered significant.
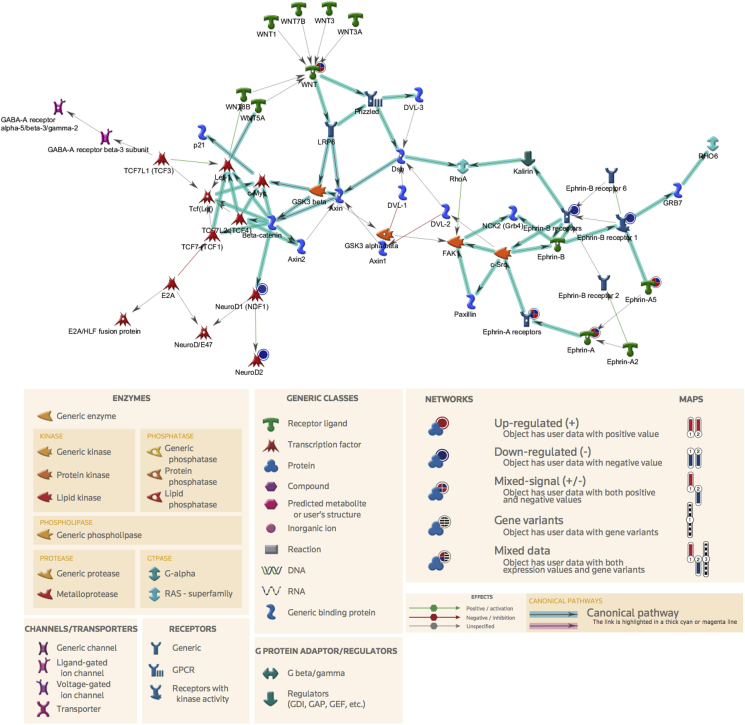
GO analysis strengthened and expanded the results obtained with the pathway analysis. GO analysis described a scenario in which most of the genes that were affected by amylin in the adult AP are involved in processes such as neurogenesis, synaptic transmission, nervous system development, cell–cell signaling and generation of neurons (Table 2). The cross analysis between pathways and GO processes was used to generate a gene network of the major targets affected by our experimental conditions. The resultant gene map confirmed that acute amylin treatment influenced the Ephrin and GABAA gene families. Moreover, the network analysis revealed that NeuroD (Neuronal-differentiation) and Wnt (Wingless-Type MMTV Integration Site Family) gene families are present and integrated in the network of genes affected by amylin in the AP of adult rats (Figure 1).
Table 2.
Enrichment analysis by GO processes.
| Processes | *Total | P-value | FDR |
|---|---|---|---|
| Synaptic transmission | 874 | 6.897E-33 | 2.664E-29 |
| Nervous system development | 2841 | 4.102E-30 | 7.922E-27 |
| Cell–cell signaling | 1293 | 6.233E-28 | 9.147E-25 |
| Neurogenesis | 1969 | 5.902E-21 | 5.698E-18 |
| Neurotransmitter transport | 185 | 1.526E-20 | 1.179E-17 |
| System development | 5219 | 2.760E-20 | 1.776E-17 |
| Neurotransmitter secretion | 133 | 3.607E-20 | 1.990E-17 |
| Regulation of neurotransmitter levels | 190 | 4.640E-19 | 1.792E-16 |
| Generation of neurons | 1871 | 7.046E-19 | 2.474E-16 |
| Neuron differentiation | 1405 | 2.870E-18 | 9.237E-16 |
*Total refers to the total number of genes involved in a process. FDR and P-value refer to processes involved in the experimental conditions. A P-value <0.01 is considered significant.
Figure 1.
Top gene map in response to acute amylin. Network analysis (generated with MetaCore + MetaDrug®) of the genes whose expression was affected by amylin treatment is shown. Major functions of the network are indicated. See the bottom figure legend for details.
3.2. Acute amylin administration significantly upregulated NeuroD1 transcript and protein in the AP of adult rats, and this response was completely blocked by the administration of AC187
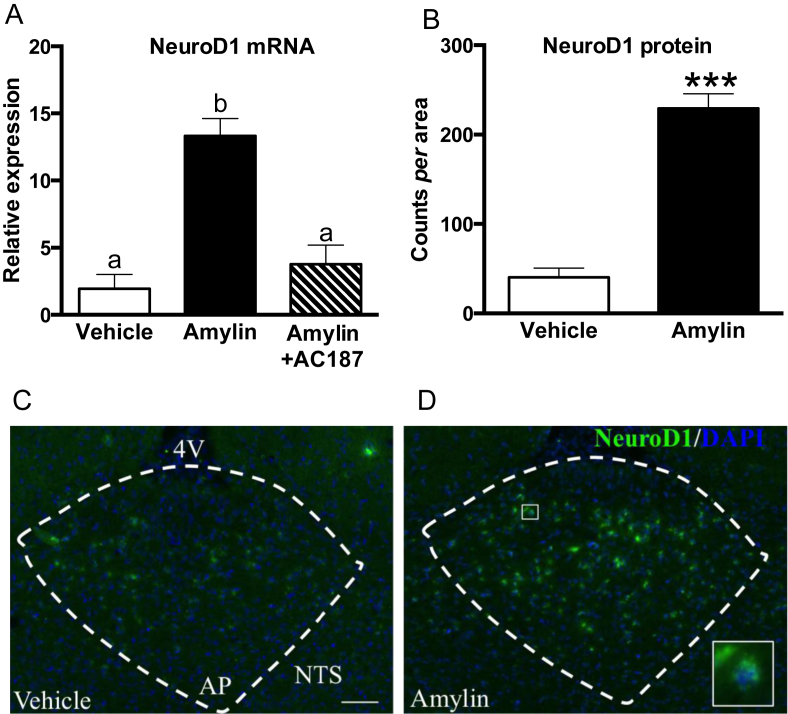
Results from the GO enrichment analysis (Table 2) showed that the majority of the genes identified via the NGS are involved in neurogenesis-like processes; further, the gene network (Figure 1) described a scenario in which NeuroD1 may interact, via canonical and unknown pathways, with Wnt, EphRs and GABAA-receptors signaling to mediate amylin's effect on neurogenesis-like processes. Therefore, we further validated the effect of amylin on NeuroD1 mRNA expression by qPCR and found that acute amylin treatment significantly upregulated NeuroD1 transcript levels about 13 fold. This effect was completely reversed by in vivo co-application of the amylin receptor antagonist AC187 (Figure 2A; One-way ANOVA; F(2,12) = 18.96, ∗∗∗P = 0.0002). We confirmed the upregulation at the protein level and showed that rats (n = 3 per group) that were acutely treated with amylin exhibited a marked upregulation of NeuroD1 compared to controls (Figure 2B–D; Student's t-test; t4 = 9.774, ∗∗∗P = 0.0006).
Figure 2.
NeuroD1 mRNA and protein expression was significantly increased by acute amylin treatment, and reversed by the amylin antagonist AC187. (A) qPCR analysis showing NeuroD1 mRNA expression levels after vehicle, amylin (20 μg/kg) or amylin + AC187 (500 μg/kg) treatments (n = 5 rats per group). Amylin consistently and significantly up-regulated NeuroD1 mRNA expression compared to vehicle. AC187 reversed the amylin effect (One-way ANOVA, ***P = 0.002). mRNA levels are shown as fold change and GAPDH is used as internal control. Data are expressed as mean ± S.E.M. (B) Quantification of NeuroD1 protein. (C) Immunohistochemistry of NeuroD1 in AP sections of a vehicle (C) and amylin (D) treated rat, respectively. The inset shows co-localization of NeuroD1 and DAPI. Scale bar represents 50 μm.
3.3. Chronic amylin treatment significantly increased the number of newly proliferated (BrdU-labeled) cells after one-week treatment in the AP of adult rats
To further investigate a direct role of amylin in adult neurogenesis, animals (n = 8 per group) were chronically treated with amylin (50 μg/kg/d) or vehicle. Food intake was measured daily throughout the experiment; as expected [4], amylin effectively decreased 24-hour food intake (but not body weight) compared to controls (Student's t-test; t14 = 2.574, ∗P < 0.05; Supplementary Figure 1a,b).
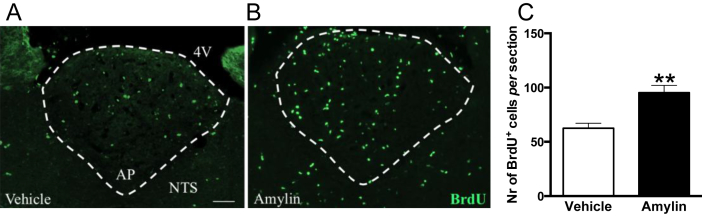
Based on the premise that new cells are continuously generated in the AP of adult rats [17], [18], we examined the number of newly proliferating cells by analyzing BrdU-immunoreactive (BrdU-labeled) cells in vehicle (Figure 3A) and amylin-treated animals (Figure 3B). After one-week of amylin infusion, the number of BrdU-labeled cells was significantly increased compared to controls (Student's t-test; t12 = 3.978, ∗∗P = 0.0018; Figure 3C). This suggests that chronic amylin treatment may be sufficient to affect and enhance the generation rate of adult-born cells.
Figure 3.
Amylin (50 μg/kg/d) increased the number of BrdU-positive cells after one week of treatment. (A) Immunohistochemical staining of BrdU-positive cells in AP sections of vehicle (A) and amylin (B) treated rats, respectively (n = 8 rats per group). (C) Amylin significantly increased the number of BrdU-immunoreactive cells compared to control (Student's t-test; ∗∗P = 0.0018). Data are expressed as mean ± S.E.M. Scale bar represents 50 μm.
3.4. BrdU-labeled cells predominantly differentiated into neurons after three-weeks of chronic amylin treatment in the AP of adult rats
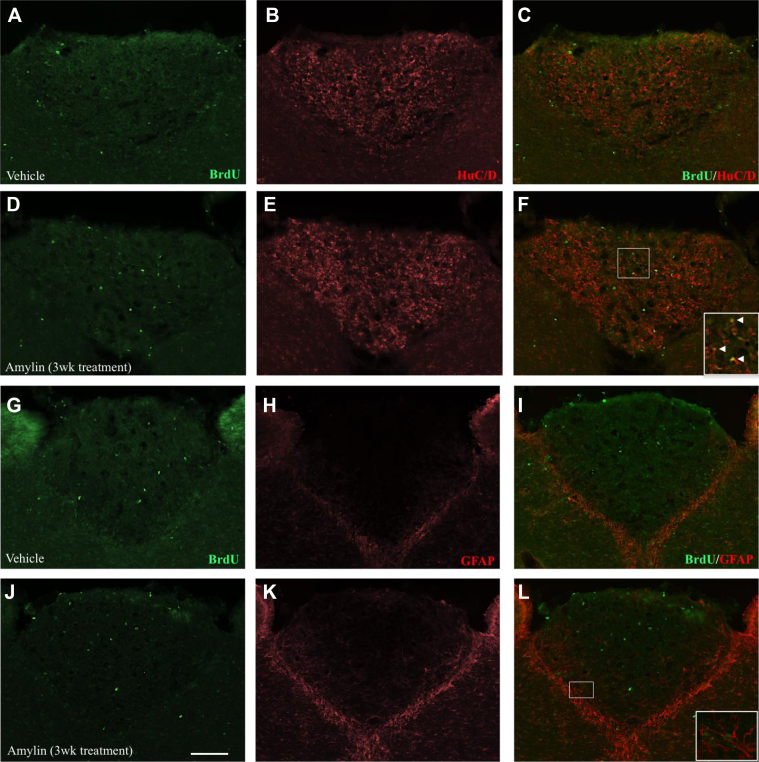
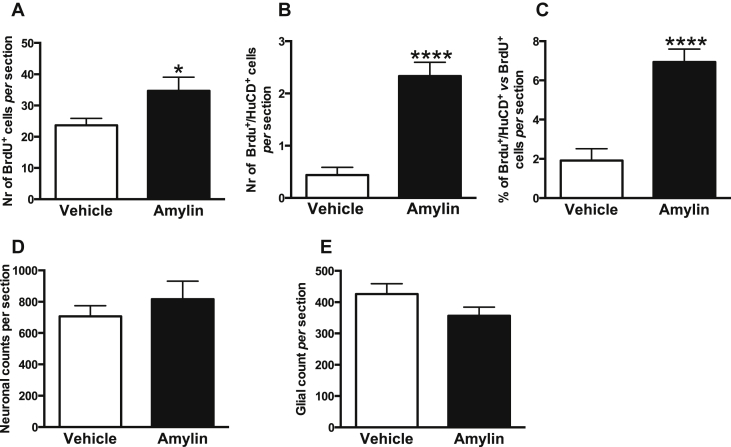
To determine the cell fate of the newly born cells in adult rats, we performed a series of immunohistological double-label staining using different cellular markers after three weeks of amylin treatment (Figure 4). Immature and mature neurons were stained with the neuronal marker HuCD (Figure 4B,E) and BrdU+/HuCD+ double-labeled cells were mainly present in the central region of the AP (Figure 4F). Glial cells were identified with the glial marker GFAP (Figure 4H,K) and BrdU+/GFAP+ double-labeled cells were predominantly located at the borders of the AP (Figure 4I,L). The number of BrdU-labeled cells was higher in rats chronically treated with amylin compared to controls (Student's t-test; t15 = 2.163, ∗P = 0.0471; Figure 5A). Moreover, amylin significantly increased the number of BrdU+/HuCD+ double-labeled cells, compared to control (Student's t-test; t15 = 6.059, ∗P < 0.0001; Figure 5B). Finally amylin significantly raised the percentage of double-labeled cells in respect to the percentage of BrdU-positive cells (Student's t-test; t15 = 5.563, ∗P < 0.0001; Figure 5C).
Figure 4.
BrdU-labeled cells in the AP of vehicle (A,G) and amylin (D,J) treated rats (n = 8 per group); amylin was given for 3 weeks at a dose of 50 μg/kg/d. Sections stained for neuronal (HuCD) and glia (GFAP) markers, respectively, are shown in (B,E,H,K). Newly generated BrdU-positive neurons double labeled for HuCD and GFAP are shown in (C,F) and (I,L), respectively. The insets show examples of colocalization of BrdU and HuCD (F), which represent neurons, or colocalization of BrdU and GFAP (L), which represent glial cells. Scale bar represents 50 μm.
Figure 5.
Quantitative analysis of BrdU+ and double-labeled (BrdU+/HuCD+) AP-cells after 3 weeks of vehicle or amylin (50 μg/kg/d) treatment (n = 8 rats per group). (A) Amylin significantly increased the number of BrdU-labeled cells after 3 weeks of treatment (Student's t-test; ∗P < 0.05). (B) The number of double labeled cells (BrdU+/HuCD+) cells and (C) the percentage of double-labeled BrdU+/HuCD+ cells in respect to the total number of BrdU+ cells were increased after amylin treatment, compared to control (Student's t-test; ∗∗∗∗P < 0.001). (D) No difference in the total number of neurons or (E) glia was detected after 3-weeks of chronic amylin treatment. Data are expressed as mean ± S.E.M.
The number of newly generated cells is not only a function of cell proliferation and migration, but also of cell death. To exclude any potential toxic effects of chronic amylin treatment on neuronal survival, we quantified the total number of mature and immature neurons in the AP of animals after 3-weeks of amylin or vehicle treatment. Our results reported no significant difference in the number of immature and mature neurons comparing amylin versus vehicle-treated animals; suggesting that chronic amylin infusion has no toxic effect on the neuronal survival in the AP of adult rats (Student's t-test; t10 = 0.828, P = 0.4267; Figure 5D). Moreover, chronic amylin treatment did not induce any gliosis in the AP of adult rats (Student's t-test; t13 = 1.607, P = 0.1320; Figure 5E).
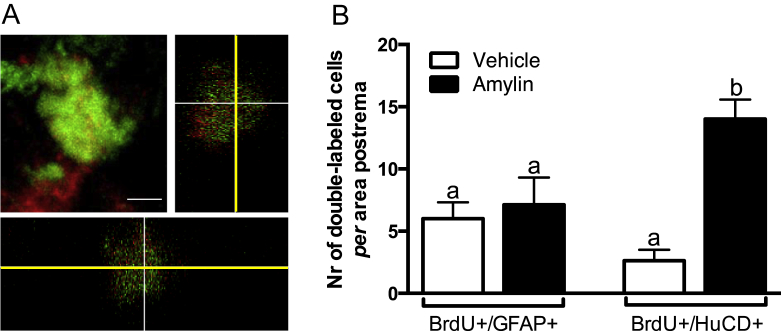
Finally, we further confirmed the double-staining observed under fluorescence microscope (Figure 6A) by confocal quantification. Our results showed that amylin preferentially induced a neuronal rather than glial cell fate (One-way ANOVA; F(3,28) = 27.51; ∗∗∗∗P < 0.0001; Figure 6B).
Figure 6.
Quantification of double-labeled cells. (A) Confocal z-stack image showing the co-localization of BrdU+ and HuCD+ cells. Scale bar represents 5 μm. (B) Number of double-labeled BrdU+/GFAP+ and BrdU+/HuCD+ cells in the AP of adult rats. Adult-born cells preferentially committed to neuronal rather than glial fate after chronic amylin (50 μg/kg/d) treatment (One-way ANOVA, B indicates significant difference, ****P < 0.0001). Data are expressed as mean ± S.E.M.
To confirm a chronic amylin effect, food intake and body weight were monitored daily during the experiment. 24-hours food intake (but not body weight) was significantly decreased by amylin during the three-week treatment period (Student's t-test; t14 = 2.718, ∗P = 0.0167; Supplementary Figure 1c,d). Ultimately, to exclude a possible co-localization of BrdU-labeled cells with interneurons or blood vessels, we performed immunohistochemical analysis for the interneuron marker CR (Supplementary Figure 2a–f) and the blood-vessel marker RECA-1, respectively (Supplementary Figure 2g–l). We did not detect any co-localization of BrdU-labeled cells with CR (Supplementary Figure 2f), indicating that chronic amylin does not seem to promote the differentiation of newborn cells into interneurons in the AP. Abundant RECA-1 staining was found in the AP, confirming its highly vascularized structure (Supplementary Figure 2h,k); however, no co-localization between BrdU and RECA-1 was detected in the AP of adult rats (Supplementary Figure 2i,l).
4. Discussion
The present findings further substantiate amylin's role as a promoter of neurogenesis in the AP of adult rats. First, our NGS analysis demonstrated that acute amylin administration modulates genes involved in pathways and processes that regulate different steps of both post-natal and adult neurogenesis in the AP of adult rats. Second, chronic administration of amylin increased the number of newly proliferated cells in the AP and promoted their differentiation predominantly into neurons rather than astrocytes. Finally, the validation of the NGS results supports the idea that NeuroD1 underlies the neurogenic effects of amylin in the AP.
Specifically, our NGS data showed that signaling pathways that guide all the stages of neurogenesis from birth through maturation, such as Ephrin-, GABA- and Wnt-signaling, are affected by acute amylin treatment. Our results also revealed that amylin triggers the upregulation, and possibly the activation, of NeuroD1 mRNA and protein in the AP of adult rats. Co-administration with the amylin receptor antagonist AC187 blocked this amylin-induced increase of NeuroD1, suggesting a direct role of amylin on NeuroD1 expression in this brain area. As the current study did not investigate whether the upregulation of NeuroD1 is required for neurogenesis in the AP, additional experiments involving mouse models of conditional NeuroD1-deficiency are critically important [25].
Moreover, our study strengthened and expanded previous findings describing that the AP of adult rats, like other CVOs, contains constitutively proliferating cells that differentiate into neurons and astrocytes [17], [33]. The observed in vivo increase in BrdU-labeling in the presence of chronic amylin therefore points to the promoting role of amylin in the control of AP-cell proliferation. Importantly, the amylin-mediated acquisition of a neuronal fate of the adult-born BrdU-labeled cells, adds novel findings to the effects of amylin in the AP. In fact, standard and confocal fluorescent microscopy analysis demonstrated that the majority of newly proliferated cells in the AP of adult rats differentiated into neurons. Only a small percentage of adult-born cells, mainly those located at the edges of the AP, differentiated into astrocytes three weeks after amylin treatment. To exclude the possibility of a neurotoxic role of chronic amylin infusion on AP-cells, we also demonstrated that the total number of mature and immature neurons (HuCD-labeled cells) and astrocytes (GFAP-labeled cells) in the AP of adult rats is not decreased by the administration of amylin. However, since the number of proliferative cells is also function of cell death, further studies investigating apoptotic processes will be required.
The novel actions of amylin characterized in this study raise several questions regarding the functional and physiological role of amylin-mediated increased neurogenesis in the AP of adult rats. At present, the significance of constitutive neurogenesis in the adult AP is poorly understood. A potential explanation could be that the generation of new neurons is required to control homeostasis. Specifically, the ongoing neurogenesis might be responsible for a fine control of food intake and vomiting [1], [35], two main actions controlled by the AP by a large number of physiological or pathological stimuli. Hence, the necessity for the constitutive neurogenesis in the adult AP might reside in the “CVO-nature” of the AP. The lack of a BBB, and the possible negative influence on AP-neurons due to blood-derived toxins might affect the efficiency and survival of AP-neurons. Therefore, the presence of new neurons in this brain area might suggest a protective mechanism of neuronal replacement. Interestingly, other brain areas involved in nutrient sensing and in the control of eating have also been reported to show adult neurogenesis. In the arcuate nucleus of the hypothalamus, the ciliary neurotrophic factor mediates continuous neurogenesis, which has been shown to affect the long-term regulation of energy balance [36]. In the adjacent ME, tanycytes from the ependymal zone generate new neurons that contribute to the control of food intake and development of obesity when mice were fed a high-fat diet [33]. The ME also undergoes structural changes in response to metabolic challenges, like fasting, which alter the accessibility of nutrients, such as glucose, to neurons in the arcuate nucleus [37]. Amylin-induced neurogenesis in the AP, which is structurally similar to the ME, with tanycyte-like cells and fenestrated capillaries, might also facilitate neuronal plasticity and proliferation required to maintain energy homeostasis after metabolic challenges [38], [39].
How amylin exerts the aforementioned effects is unknown at present. Our recent study demonstrated that more than one amylin receptor subtype may be present in AP-neurons [12]. This suggests that different amylin actions (for example, decreased food intake and enhanced neurogenesis) might be mediated by different combinations of the CTR/RAMPs complex. Which of these combinations may be relevant for the neurogenic effect remains to be studied. Moreover, our findings show that exogenous amylin increases neurogenesis, but we did not directly assess the effect of endogenous amylin on adult neurogenesis. Future BrdU-studies that test the effect of AC187 alone on neurogenesis are needed to address this fundamental question.
A potential mechanism used by amylin to enhance adult neurogenesis in the AP might involve amylin-induced activation of the ERK 1/2 cascade. Our own studies showed that acute amylin time and dose-dependently activates ERK 1/2 signaling, by inducing ERK phosphorylation, and that this effect contributes at least in part to amylin's satiating effect [13]. Since pERK-positive AP-neurons partially overlap with the core subunit of the amylin receptor CTR [13], this effect seems to be mediated by CTR-signaling. It has been shown that ERK1/2 knockout mice have impaired neurogenesis (as characterized by abnormal laminar differentiation and marked reduction in neuronal size in hindbrain nuclei), suggesting that ERK1/2 activity is required for the generation of new neurons [40]. Moreover, recent findings demonstrated that Wnt-mediated adult neurogenesis results in the upregulation and activation of NeuroD1, possibly by triggering ERK-signaling [41], [42], [43], [44]. These findings therefore suggest that amylin may influence AP-adult neurogenesis via ERK signaling.
To confirm the presence of an amylin effect, food intake and body weight were monitored daily throughout the course of the chronic experiment. Average daily food intake was significantly reduced in amylin-treated rats compared to control. Unexpectedly, amylin did not reduce body weight. As weight loss per se is known to induce neurogenesis in the hippocampus [45], we can therefore exclude an effect of decreased body weight as a source of increased neurogenesis in the AP of adult rats. However, the exact relevance of amylin-induced neurogenesis in the AP of adult rats in the context of feeding behavior and the control of eating is not known at present. Since our recent work demonstrated that amylin downregulates the subunits of the amylin receptors [12], it seems possible that a chronic amylin treatment might require an increased number of amylin-responsive AP-neurons in order to mediate the satiating effect and thus a prolonged action on the metabolic circuits.
In conclusion, the present data further extend our understanding of the amylin-mediated effects in the rodent AP. Our data demonstrate that amylin regulates genes involved in pathways and processes that drive neurogenesis in the adult mammalian brain. Our results also show an in vivo increase in the number of newly proliferating AP-cells after chronic amylin treatment. Moreover, amylin has the potential to commit the AP adult-born cells to a neuronal fate. Amylin may therefore be a novel target to be used to decipher the interaction between neurogenic and metabolic circuits.
Acknowledgments
This work was supported by the Swiss National Science Foundation (SNF 31003A_156935) and the Center for Integrative Human Physiology of the University of Zurich. The authors gratefully acknowledge the Center for Clinical Studies, the Center for Microscopy and Image Analysis and the Functional Genomic Center Zurich (FGCZ) (University of Zurich, Zurich, Switzerland). The authors specifically acknowledge Barry E. Levin (Rutgers University) and Dr. Christelle Le Foll (University of Zurich) for their precious insights and advice.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molmet.2016.06.015.
Conflict of interest
The authors declare no competing financial interests.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Lutz T.A. Amylinergic control of food intake. Physiology & Behavior. 2006;89(4):465–471. doi: 10.1016/j.physbeh.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Lutz T.A. The role of amylin in the control of energy homeostasis. American Journal of Physiology Regulatory Integrative and Comparative Physiology. 2010;298(6):R1475–R1484. doi: 10.1152/ajpregu.00703.2009. [DOI] [PubMed] [Google Scholar]
- 3.Lutz T.A., Geary N., Szabady M.M., Del Prete E., Scharrer E. Amylin decreases meal size in rats. Physiology & Behavior. 1995;58(6):1197–1202. doi: 10.1016/0031-9384(95)02067-5. [DOI] [PubMed] [Google Scholar]
- 4.Lutz T.A., Mollet A., Rushing P.A., Riediger T., Scharrer E. The anorectic effect of a chronic peripheral infusion of amylin is abolished in area postrema/nucleus of the solitary tract (AP/NTS) lesioned rats. International Journal of Obesity and Related Metabolic Disorders. 2001;25(7):1005–1011. doi: 10.1038/sj.ijo.0801664. [DOI] [PubMed] [Google Scholar]
- 5.Lutz T.A., Rossi R., Althaus J., Del Prete E., Scharrer E. Amylin reduces food intake more potently than calcitonin gene-related peptide (CGRP) when injected into the lateral brain ventricle in rats. Peptides. 1998;19(9):1533–1540. doi: 10.1016/s0196-9781(98)00114-4. [DOI] [PubMed] [Google Scholar]
- 6.Lutz T.A., Del Prete E., Scharrer E. Reduction of food intake in rats by intraperitoneal injection of low doses of amylin. Physiology & Behavior. 1994;55(5):891–895. doi: 10.1016/0031-9384(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 7.Gedulin B.R., Rink T.J., Young A.A. Dose-response for glucagonostatic effect of amylin in rats. Metabolism. 1997;46(1):67–70. doi: 10.1016/s0026-0495(97)90170-0. [DOI] [PubMed] [Google Scholar]
- 8.Young A.A., Gedulin B., Vine W., Percy A., Rink T.J. Gastric emptying is accelerated in diabetic BB rats and is slowed by subcutaneous injections of amylin. Diabetologia. 1995;38(6):642–648. doi: 10.1007/BF00401833. [DOI] [PubMed] [Google Scholar]
- 9.Alexander S.P., Benson H.E., Faccenda E., Pawson A.J., Sharman J.L., Spedding M. The Concise Guide to PHARMACOLOGY 2013/14: G protein-coupled receptors. British Journal of Pharmacology. 2013;170(8):1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christopoulos G., Perry K.J., Morfis M., Tilakaratne N., Gao Y., Fraser N.J. Multiple amylin receptors arise from receptor activity-modifying protein interaction with the calcitonin receptor gene product. Molecular Pharmacology. 1999;56(1):235–242. doi: 10.1124/mol.56.1.235. [DOI] [PubMed] [Google Scholar]
- 11.McLatchie L.M., Fraser N.J., Main M.J., Wise A., Brown J., Thompson N. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393(6683):333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- 12.Liberini C.G., Boyle C.N., Cifani C., Venniro M., Hope B.T., Lutz T.A. Amylin receptor components and the leptin receptor are co-expressed in single rat area postrema neurons. European Journal of Neuroscience. 2016 doi: 10.1111/ejn.13163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Potes C.S., Boyle C.N., Wookey P.J., Riediger T., Lutz T.A. Involvement of the extracellular signal-regulated kinase 1/2 signaling pathway in amylin's eating inhibitory effect. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2012;302(3):R340–R351. doi: 10.1152/ajpregu.00380.2011. [DOI] [PubMed] [Google Scholar]
- 14.Lutz T.A. Roles of amylin in satiation, adiposity and brain development. Forum of Nutrition. 2010;63:64–74. doi: 10.1159/000264394. [DOI] [PubMed] [Google Scholar]
- 15.Adler B.L., Yarchoan M., Hwang H.M., Louneva N., Blair J.A., Palm R. Neuroprotective effects of the amylin analogue pramlintide on Alzheimer's disease pathogenesis and cognition. Neurobiology of Aging. 2014;35(4):793–801. doi: 10.1016/j.neurobiolaging.2013.10.076. [DOI] [PubMed] [Google Scholar]
- 16.Trevaskis J.L., Turek V.F., Wittmer C., Griffin P.S., Wilson J.K., Reynolds J.M. Enhanced amylin-mediated body weight loss in estradiol-deficient diet-induced obese rats. Endocrinology. 2010;151(12):5657–5668. doi: 10.1210/en.2010-0590. [DOI] [PubMed] [Google Scholar]
- 17.Bennett L., Yang M., Enikolopov G., Iacovitti L. Circumventricular organs: a novel site of neural stem cells in the adult brain. Molecular and Cellular Neuroscience. 2009;41(3):337–347. doi: 10.1016/j.mcn.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hourai A., Miyata S. Neurogenesis in the circumventricular organs of adult mouse brains. Journal of Neuroscience Research. 2013;91(6):757–770. doi: 10.1002/jnr.23206. [DOI] [PubMed] [Google Scholar]
- 19.Lin R., Cai J., Nathan C., Wei X., Schleidt S., Rosenwasser R. Neurogenesis is enhanced by stroke in multiple new stem cell niches along the ventricular system at sites of high BBB permeability. Neurobiology of Disease. 2015;74:229–239. doi: 10.1016/j.nbd.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 20.Kempermann G., Gage F.H. Neurogenesis in the adult hippocampus. Novartis Foundation Symposium. 2000;231:220–235. discussion 235–41, 302–6. [PubMed] [Google Scholar]
- 21.Eriksson P.S., Perfilieva E., Bjork-Eriksson T., Alborn A.M., Nordborg C., Peterson D.A. Neurogenesis in the adult human hippocampus. Nature Medicine. 1998;4(11):1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 22.Doetsch F., Caille I., Lim D.A., Garcia-Verdugo J.M., Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97(6):703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 23.Zhao C., Deng W., Gage F.H. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132(4):645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 24.Boutin C., Hardt O., de Chevigny A., Core N., Goebbels S., Seidenfaden R. NeuroD1 induces terminal neuronal differentiation in olfactory neurogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(3):1201–1206. doi: 10.1073/pnas.0909015107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao Z., Ure K., Ables J.L., Lagace D.C., Nave K.A., Goebbels S. Neurod1 is essential for the survival and maturation of adult-born neurons. Nature Neuroscience. 2009;12(9):1090–1092. doi: 10.1038/nn.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fritschy J.M., Panzanelli P. GABAA receptors and plasticity of inhibitory neurotransmission in the central nervous system. European Journal of Neuroscience. 2014;39(11):1845–1865. doi: 10.1111/ejn.12534. [DOI] [PubMed] [Google Scholar]
- 27.Tozuka Y., Fukuda S., Namba T., Seki T., Hisatsune T. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron. 2005;47(6):803–815. doi: 10.1016/j.neuron.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 28.Belluzzi O., Benedusi M., Ackman J., LoTurco J.J. Electrophysiological differentiation of new neurons in the olfactory bulb. The Journal of Neuroscience. 2003;23(32):10411–10418. doi: 10.1523/JNEUROSCI.23-32-10411.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kent B.A., Beynon A.L., Hornsby A.K., Bekinschtein P., Bussey T.J., Davies J.S. The orexigenic hormone acyl-ghrelin increases adult hippocampal neurogenesis and enhances pattern separation. Psychoneuroendocrinology. 2015;51:431–439. doi: 10.1016/j.psyneuen.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garza J.C., Guo M., Zhang W., Lu X.Y. Leptin increases adult hippocampal neurogenesis in vivo and in vitro. The Journal of Biological Chemistry. 2008;283(26):18238–18247. doi: 10.1074/jbc.M800053200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carlson S.W., Madathil S.K., Sama D.M., Gao X., Chen J., Saatman K.E. Conditional overexpression of insulin-like growth factor-1 enhances hippocampal neurogenesis and restores immature neuron dendritic processes after traumatic brain injury. Journal of Neuropathology and Experimental Neurology. 2014;73(8):734–746. doi: 10.1097/NEN.0000000000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiu S.L., Chen C.M., Cline H.T. Insulin receptor signaling regulates synapse number, dendritic plasticity, and circuit function in vivo. Neuron. 2008;58(5):708–719. doi: 10.1016/j.neuron.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee D.A., Bedont J.L., Pak T., Wang H., Song J., Miranda-Angulo A. Tanycytes of the hypothalamic median eminence form a diet-responsive neurogenic niche. Nature Neuroscience. 2012;15(5):700–702. doi: 10.1038/nn.3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bouret S.G., Bates S.H., Chen S., Myers M.G., Jr., Simerly R.B. Distinct roles for specific leptin receptor signals in the development of hypothalamic feeding circuits. The Journal of Neuroscience. 2012;32(4):1244–1252. doi: 10.1523/JNEUROSCI.2277-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller A.D., Leslie R.A. The area postrema and vomiting. Frontiers in Neuroendocrinology. 1994;15(4):301–320. doi: 10.1006/frne.1994.1012. [DOI] [PubMed] [Google Scholar]
- 36.Kokoeva M.V., Yin H., Flier J.S. Neurogenesis in the hypothalamus of adult mice: potential role in energy balance. Science. 2005;310(5748):679–683. doi: 10.1126/science.1115360. [DOI] [PubMed] [Google Scholar]
- 37.Langlet F., Levin B.E., Luquet S., Mazzone M., Messina A., Dunn-Meynell A.A. Tanycytic VEGF-A boosts blood-hypothalamus barrier plasticity and access of metabolic signals to the arcuate nucleus in response to fasting. Cell Metabolism. 2013;17(4):607–617. doi: 10.1016/j.cmet.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Braegger F.E., Asarian L., Dahl K., Lutz T.A., Boyle C.N. The role of the area postrema in the anorectic effects of amylin and salmon calcitonin: behavioral and neuronal phenotyping. European Journal of Neuroscience. 2014;40(7):3055–3066. doi: 10.1111/ejn.12672. [DOI] [PubMed] [Google Scholar]
- 39.Prevot V., Langlet F., Dehouck B. Flipping the tanycyte switch: how circulating signals gain direct access to the metabolic brain. Aging (Albany NY) 2013;5(5):332–334. doi: 10.18632/aging.100557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Satoh Y., Kobayashi Y., Takeuchi A., Pages G., Pouyssegur J., Kazama T. Deletion of ERK1 and ERK2 in the CNS causes cortical abnormalities and neonatal lethality: Erk1 deficiency enhances the impairment of neurogenesis in Erk2-deficient mice. The Journal of Neuroscience. 2011;31(3):1149–1155. doi: 10.1523/JNEUROSCI.2243-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuwabara T., Hsieh J., Muotri A., Yeo G., Warashina M., Lie D.C. Wnt-mediated activation of NeuroD1 and retro-elements during adult neurogenesis. Nature Neuroscience. 2009;12(9):1097–1105. doi: 10.1038/nn.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vanderhaeghen P. Wnts blow on NeuroD1 to promote adult neuron production and diversity. Nature Neuroscience. 2009;12(9):1079–1081. doi: 10.1038/nn0909-1079. [DOI] [PubMed] [Google Scholar]
- 43.Petersen H.V., Jensen J.N., Stein R., Serup P. Glucose induced MAPK signalling influences NeuroD1-mediated activation and nuclear localization. FEBS Letters. 2002;528(1–3):241–245. doi: 10.1016/s0014-5793(02)03318-5. [DOI] [PubMed] [Google Scholar]
- 44.Li Z., Theus M.H., Wei L. Role of ERK 1/2 signaling in neuronal differentiation of cultured embryonic stem cells. Development Growth & Differentiation. 2006;48(8):513–523. doi: 10.1111/j.1440-169X.2006.00889.x. [DOI] [PubMed] [Google Scholar]
- 45.Mattson M.P. Neuroprotective signaling and the aging brain: take away my food and let me run. Brain Research. 2000;886(1–2):47–53. doi: 10.1016/s0006-8993(00)02790-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.