Abstract
Objective
Compared to men, postmenopausal women suffer from a disproportionate burden of many co-morbidities associated with obesity, e.g. cardiovascular disease, cancer, and dementia. The underlying mechanism for this sex difference is not well understood but is believed to relate to absence of the protective effect of estrogen through the action of estrogen receptor alpha (ERα) in the central nervous system. With the recently developed neuron-specific lipoprotein lipase deficient mice (NEXLPL−/−) (Wang et al., Cell Metabolism, 2011 [15]), we set to explore the possible role of lipid sensing in sex differences in obesity development.
Methods
Both male and female NEXLPL−/− mice and littermate WT controls were subjected to pair feeding (pf) where daily food amount given was adjusted according to body weight to match the food intake of ad libitum (ad) fed control WT mice. Food intake and body weight were measured daily, and pair feeding was maintained to 42 wk in male mice and to 38 wk in female mice. Various brain regions of the mice were harvested, and ERα gene expression was examined in both male and female NEXLPL−/− and WT control mice under both ad- and pf-fed conditions.
Results
Although both male and female NEXLPL−/− mice developed obesity similarly on standard chow, male NEXLPL−/− mice still developed obesity under with pair feeding, but on a much delayed time course, while female NEXLPL−/− mice were protected from extra body weight and fat mass gain compared to pair-fed WT control mice. Pair feeding alone induced extra fat mass gain in both male and female WT mice, and this was mostly driven by the reduction in physical activity. LPL deficiency resulted in an increase in ERα mRNA in the hypothalamus of ad-fed female mice, while pair feeding alone also resulted in an increase of ERα in both female WT control and NEXLPL−/− mice. The effect on increasing ERα by pair feeding and LPL deficiency was additive in pair-fed female NEXLPL−/− mice. ERα mRNA levels were not significantly modified in other brain regions examined, nor in the hypothalamus of male NEXLPL−/− mice compared to control mice.
Conclusions
These results suggest that the mechanism underlying ERα regulation of body weight interacts with the LPL-dependent lipid processing in the hypothalamus in a sex specific way. ERα could provide the link between brain lipid sensing and sex differences in obesity development. This study has the potential important clinical implication to provide better management for women who suffer from obesity and obesity-related co-morbidities.
Keywords: Lipoprotein lipase, Pair feeding, Sex-differences, Estrogen receptor, Obesity
Graphical abstract
Highlights
-
•
Male neuronal lipoprotein lipase deficient mice are still obese with pair feeding.
-
•
Female neuronal lipoprotein lipase deficient mice are not obese with pair feeding.
-
•
Neuronal LPL deficiency results in an increase in ERa expression in female mice.
-
•
Pair feeding alone also results in an increase in ERa in both male and female mice.
-
•
ERa provides the link between brain lipid sensing and sex differences in obesity.
1. Introduction
Energy homeostasis is known to be regulated by a complex neuroendocrine system involving both central and peripheral signals. In the central nervous system (CNS), neuronal signals in the hypothalamus cooperatively regulate energy intake and energy expenditure in a coordinate manner with circulating hormones to ensure the balance of energy storage during periods of food scarcity and/or food abundance [1], [2], [3]. Sex differences are well known to exist in the regulation of energy balance in response to calorie shortage or excess [4], [5], [6]. Historically, this is likely due to the different roles played by males and females in survival of the species; for example, males are more responsible for hunting and gathering, while females are responsible for gestation, lactation, and caregiving. Moreover, the reproductive capability of females is importantly related to adipose tissue fuel deposition. Below a critical level of body fat, menarche is absent or delayed, and, when menstruation begins, oligomenorrhea, anovulatory cycles, and subsequent amenorrhea often follow [7]. More recently, various studies have shown that there might be sex-specific molecular mechanisms underlying the sex differences in the regulation of body composition [8], insulin resistance [9], [10] and energy homeostasis [11], [12].
Lipoprotein lipase (LPL) is a gate-keeping enzyme that hydrolyzes triglyceride-rich lipoproteins to release triglyceride (TG) free fatty acids (FFA) for uptake and oxidation in a tissue specific manner [13], [14]. In CNS, LPL has been implied to play a role in regulating lipid uptake and possibly providing important signals in different brain regions to regulate energy balance [14], [15], [16], [17], [18] as well as behavior including learning and memory [14], [19]. Specifically, male neuron-specific LPL deficient mice (NEXLPL−/−) developed obesity on standard chow by 16 wk, with a food intake increase between 12 and 22 wk, and a reduction in energy expenditure at 24 wk and older. Neuropeptides AgRP and NPY, that are known to regulate food intake and energy expenditure, were upregulated in the hypothalamus of NEXLPL before the onset of obesity, and the increase was persistent after obesity developed. Female NEXLPL−/− mice developed obesity in a similar time course as males but gained a higher percentage of excess body weight than the male counterparts by 6 mo [15].
To understand the importance of earlier food intake in obesity development, and to explore the possible sex differences in obesity development, both male and female NEXLPL−/− mice were subjected to pair feeding conditions in which the daily food intake for the experimental mice was limited to the average daily food intake for control ad libitum fed mice. Interesting sex differences in obesity development in response to food scarcity were discovered and reported here.
2. Material and Methods
2.1. Mice and pair feeding conditions
NEXLPL−/− mice and littermate controls were generated as described before [15]. At 10 wk of age, male and female mice were individually caged for a week before being subjected to different pair feeding conditions. All diets were standard chow. To establish the pair feeding conditions, a separate group of wildtype (WT) littermate control mice were first individually caged but fed ad libitum. Food intake was measured daily between 3 and 5 PM for a week, and the daily food intake (FI) normalized per body weight (BW) was calculated for each mouse. The average daily FI/BW was calculated from all control WT ad libitum fed mice (wt-ad), and this was used to determine the amount of food given daily to each individually caged pair-fed mouse. The average daily FI/BW was adjusted every week based on the actual food intake and body weight measured in the previous week for the control wt-ad mice. Food for pair feeding mice (both WT and NEXLPL−/− mice) was given daily between 3 and 5 PM, and the leftover food from previous day was measured and removed from the cage before the new food was given. For the long term pair-feeding experiment, all male mice were fed up to 42 wk of age to observe the significant difference in BW between genotypes, and female mice were fed up to 38 wk of age with no sign of differences in BW. For the short term pair feeding experiment, all mice were fed up to 20 wk of age, at which point no BW differences were observed for all groups.
2.2. Measurement of body weight, body composition, and plasma metabolic parameters
Body weights were monitored on a daily basis between 3 and 5 PM. Body compositions were measured on anesthetized mice by dual-energy X-ray absorptiometry using a mouse densitometer (PIXImus2, Lunar Corp., Madison, WI) at the end of feeding when terminal blood and tissues were collected for analysis. Blood was collected by cardiac puncture, and plasma was stored at −20 °C until further analysis. Plasma glucose was measured using the Cayman Glucose Colorimetric Assay Kit (Caymen Chemical, San Diego CA). TG and FFA were measured using enzymatic, colorimetric assays (Sigma, St. Louis, MO and Wako Chemicals USA, Richmond, VA, respectively), and insulin was measured using a RIA kit (Linco Research, St. Charles, MO). Plasma leptin and adiponectin were measured using specific enzyme-immunoassay kits (ELISA) designed for quantitative determination of mouse plasma samples (Alpco Diagnostics, Salem, NH).
2.3. Indirect calorimetry and physical activity measurements
An open-ended indirect calorimetry system was used to measure oxygen consumption (O2) and carbon dioxide (CO2) production in mice for the calculation of metabolic rate and respiratory quotient (RQ) [20]. Animals at the end of feeding periods were placed in 4 metabolic chambers for measurements taken over three days with free access to food and water. The differential O2 and CO2 concentrations, flow rate, RQ, and metabolic rate (Weir equation) were calculated and stored in a computer configured with data acquisition hardware (Analogic, Wakefield, MA) and software (Labtech, Wilmington, MA). The metabolic rate will be reported in the unit of kcal/day/gram of LBM, where LBM stands for lean body mass. In addition, measurements of physical activity were carried out using the Columbus Instruments Opto M3, a multi-channel activity monitor that utilizes infrared beams to monitor an animal's movement in the X, Y and Z axis. The total physical activity during the last 24 h of calorimetry experiment was determined by adding all the ambulatory counts in the X direction.
2.4. Quantitative real-time PCR
Tissue was collected into RNAlater (Qiagen) from anesthetized mice after a 4 h fast and stored at 4 °C. Total RNA was extracted and reverse transcribed as previously described [15]. Quantitative PCR was performed using primer sets for genes of interest, two reference genes (GAPDH and Ubc) and iQ Supermix or iQ SYBR Supermix (Bio-Rad) following the manufacturer's protocols.
2.5. Statistical analyses
Results are presented as mean ± SEM. One way repeated measure ANOVA was performed for body weight and cumulative food intake data. Two tail, unequal variance t-tests were performed for all the other statistical analysis using the Data Analysis Tool pack in Excel 2010 (Microsoft). A p < 0.05 was considered significant.
3. Results
3.1. Pair feeding delays the obesity development in male NEXLPL−/−mice
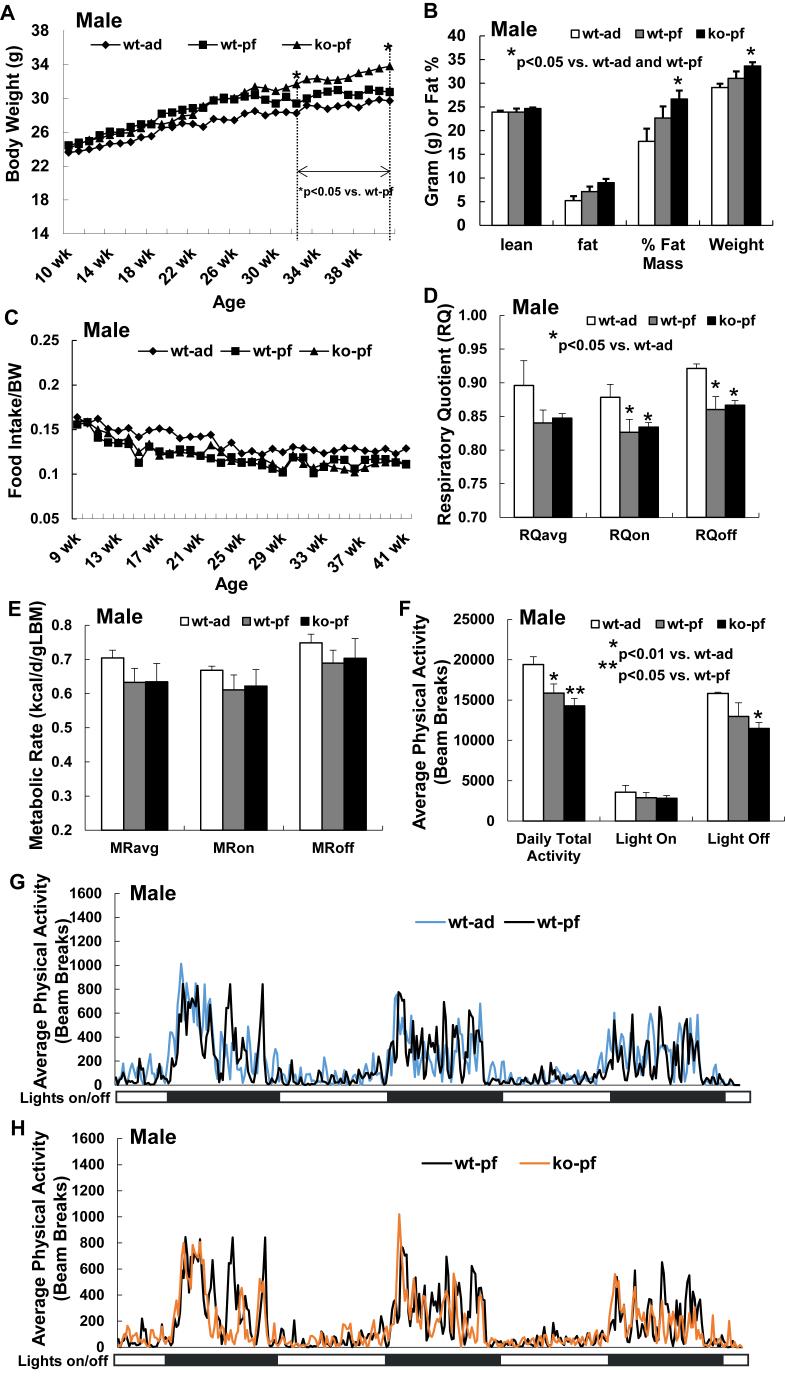
Three separate groups of littermate wild type (WT) and NEXLPL−/− mice were subjected to the following feeding conditions: WT ad libitum feeding (wt-ad), WT pair feeding (wt-pf), and NEXLPL−/− pair feeding (ko-pf). When male mice were allowed to remain on these feeding conditions for up to 42 wk of age, there was no significant difference in body weight for wt-pf mice compared to wt-ad mice (Figure 1A,B), although a trend for higher body weight, higher fat mass and higher % fat mass was consistently observed (Figure 1B). On the other hand, the expected weight gain in ad-fed NEXLPL−/− mice [15] was significantly delayed by pair feeding; however, ko-pf mice eventually experienced additional weight gain starting at 34 wk (Figure 1A). Moreover, both body weight and % fat mass were significantly higher than in wt-pf mice at 42 wk (Figure 1B). It is interesting to note that although the portion of food given daily to pair-fed male mice (both WT and NEXLPL−/−) was based on the average daily food intake normalized to body weight from the wt-ad group, both pair-fed groups appeared to consistently leave food behind, resulting in less FI/BW for both pair-fed groups compared to the wt-ad group (Figure 1C). There were no apparent differences in food intake between the wt-pf and ko-pf mice.
Figure 1.
Delayed obesity development in male pair-fed NEXLPL−/− mice (n = 4 for wt-ad, n = 4 for wt-pf, n = 4 for ko-pf). A. Weekly body weight of male ad-fed WT control mice and pair-fed (pf) WT and NEXLPL−/− mice. B. Body composition of 42 wk male ad-fed WT control mice and pair-fed WT and NEXLPL−/− mice. C. Weekly food intake (FI) normalized by body weight (BW) for male ad-fed WT control mice and pair-fed WT and NEXLPL−/− mice. D. Respiratory quotient (RQ) of 42 wk male ad-fed WT control mice and pair-fed WT and NEXLPL−/− mice. E. Metabolic rate (MR) of 42 wk male ad-fed WT control mice and pair-fed WT and NEXLPL−/− mice. F. Average daily physical activity of 42 wk male ad-fed WT control mice and pair-fed WT and NEXLPL−/− mice. G. Comparison of real time physical activity over the three day calorimetry period between 42 wk male ad-fed and pair-fed WT control mice. H. Comparison of real time physical activity over the three day calorimetry period between 42 wk male pair fed WT and NEXLPL−/− mice.
When male mice were subjected to calorimetry at 42 wk of age, RQ was reduced for both wt-pf and ko-pf mice compared to wt-ad mice (Figure 1D) with no difference between the two pair-fed groups. Pair feeding condition seemed to make both WT and KO mice rely more on fat than on carbohydrate for energy production. Although in ko mice this could be due in part to reduced caloric intake, but, since wt mice also experienced RQ reductions, the pattern of pair feeding would appear to be more explanatory. Moreover, although reductions in energy expenditure with pair-feeding have also been reported [21], metabolic rates trended lower in both pair-fed groups here compared to the wt-ad mice (Figure 1E). Interestingly, physical activity was reduced by pair feeding alone (wt-pf vs. wt-ad, p < 0.01, Figure 1F,G) and was further reduced in pair-fed NEXLPL−/− mice (ko-pf vs. wt-pf, p < 0.05, Figure 1F,H). The reduction in physical activity was all due to reductions in physical activity in lights off period (Figure 1F,H), which might contribute to the RQ reduction observed in both wt-pf and ko-pf mice (Figure 1D).
3.2. Pair feeding prevents the extra body weight gain in female NEXLPL−/− mice
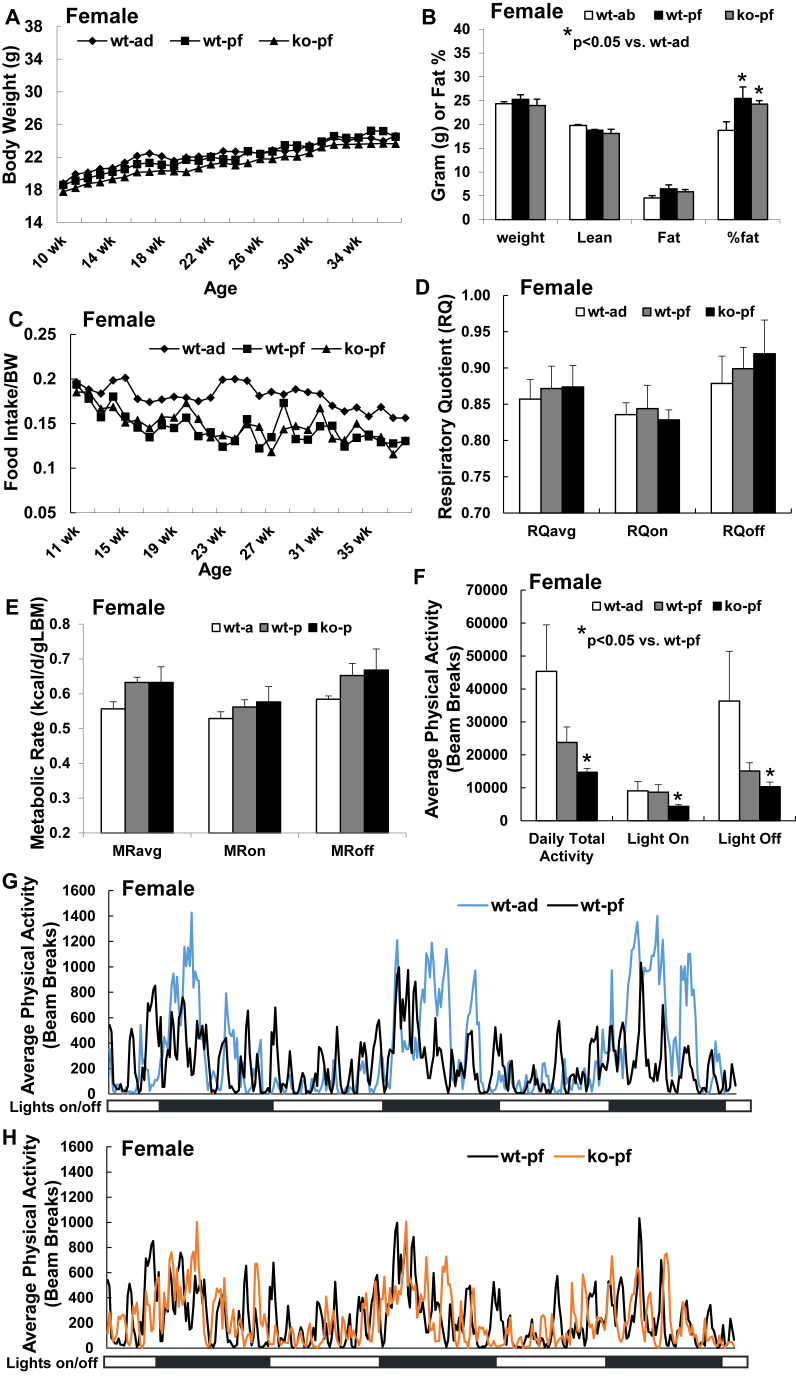
Female mice displayed a quite different response to pair feeding in both WT and NEXLPL−/− mice compared to males. Both wt-pf and ko-pf female mice appeared to gain similar body weight as wt-ad mice (Figure 2A). In other words, pair feeding seemed to block the extra body weight gain in both female WT and NEXLPL−/− mice. But when body composition was examined by DEXA at 38 wk of age, both pair-fed groups displayed higher % fat than the wt-ad group with no difference between the pair-fed WT and NEXLPL−/− mice (Figure 2B). Similar to male mice, both pair-fed female groups had much more leftover food throughout the feeding period (Figure 2C), and, again, there was no difference in food intake between the ko-pf and wt-pf mice. The RQ reduction in both groups of pair-fed male mice (Figure 1D) was not present for pair-fed NEXLPL−/− female mice with also no difference between wt-ad and wt-pf female mice (Figure 2D). And unlike the decrease in RQ (Figure 1D) and trended decrease in metabolic rate (Figure 1E) seen in male mice, both RQ and metabolic rate trended higher in both female pair-fed groups (wt-p and ko-p) (Figure 2D,E). However, the physical activity reduction caused by pair feeding in male mice was still significant in pair-fed female mice, with both lights on and lights off activity reduced in female pair-fed NEXLPL−/− mice (Figure 2F–H). Thus, in female mice, the trended higher metabolic rate but reduced physical activity might result in no significant modification on overall energy expenditure, while in male mice the reduction in energy expenditure and physical activity predisposes to obesity development in pair-fed mice. Moreover, the sex-dependent modification in RQ in pair-fed female vs. male mice may relate to the preferential carbohydrate oxidation and fat storage in female vs male mice in general, a compensation for maintenance of fat storage in the setting of pair-feeding.
Figure 2.
Pair feeding prevents obesity development in female pair-fed NEXLPL−/− mice (n = 4 for wt-ad, n = 4 for wt-pf, n = 4 for ko-pf). A. Weekly body weight of female ad-fed WT control mice and pair-fed (pf) WT and NEXLPL−/− mice. B. Body composition of 38 wk female ad-fed WT control mice and pair-fed WT and NEXLPL−/− mice. C. Weekly food intake (FI) normalized by body weight (BW) for female ad-fed WT control mice and pair-fed WT and NEXLPL−/− mice. D. Respiratory quotient (RQ) of 38 wk female ad-fed WT control mice and pair-fed WT and NEXLPL−/− mice. E. Metabolic rate (MR) of 38 wk female ad-fed WT control mice and pair-fed WT and NEXLPL−/− mice. F. Average daily physical activity of 38 wk female ad-fed WT control mice and pair-fed WT and NEXLPL−/− mice. G. Comparison of real time physical activity over the three day calorimetry period between 38 wk female ad-fed and pair-fed WT control mice. H. Comparison of real time physical activity over the three day calorimetry period between 38 wk female pair fed WT and NEXLPL−/− mice.
In summary, pair feeding alone was associated with extra fat mass gain in both male and female WT mice, and this was driven mostly by the reduction in MR and/or physical activity, since pair-fed mice failed to consume all of their daily food allowance. More importantly, when compared to the pair-fed WT male mice, pair-fed NEXLPL−/− male mice still developed obesity (Figure 1B), whereas pair-fed NEXLPL−/− female mice were protected from this extra body weight and fat mass gain (Figure 2B). Again, in male NEXLPL−/− mice, reductions in MR and physical activity were the major mechanistic explanations of obesity development under pair-fed condition rather than changes in food intake, while in female pair-fed NEXLPL−/− mice, even though there is no significant difference in body weight and fat % compared to the pair-fed WT mice, the reduction in physical activity is still apparent. The reduction in physical activity in male pair-fed mice (regardless of genotypes) seemed to correlate well with the trending reduction in metabolic rate and the reduction in RQ, but this pair feeding effect on fuel selection was lost in female pair-fed mice (again, regardless of genotypes) with trending increase in metabolic rate but persistent reduction in physical activity. This implies a more complex mechanism in females between feeding condition changes and regulation of fuel metabolism. It is important to note that pair feeding did delay obesity development in male NEXLPL−/− mice, implying the importance of sex differences in obesity development in chow-fed NEXLPL−/− mice.
3.3. Hypothalamic gene expression of estrogen receptor alpha was modified by pair feeding in male vs. female NEXLPL−/− mice
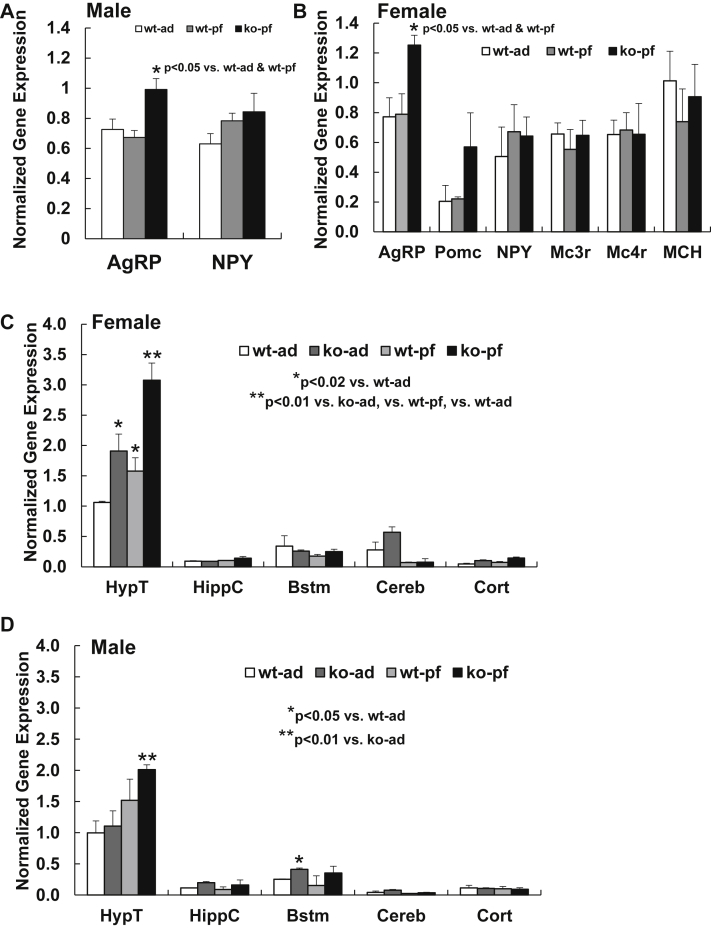
When examining the hypothalamic gene expression of known neuropeptides that regulate energy homeostasis, AgRP gene expression was still elevated in both male (Figure 3A) and female NEXLPL−/− mice (Figure 3B) under the same pair feeding condition, similar to that observed for NEXLPL−/− with chow feeding [15]. This implies that the up-regulation of AgRP in NEXLPL−/− mice is not affected by pair feeding and other neuropeptides related to food intake and energy balance were unchanged (NPY, POMC, Mc3r, Mc4r, MCH, Figure 3B). In addition, the persistent up-regulation of AgRP could still be responsible for the later onset of obesity in pair-fed male NEXLPL−/− starting at 34 wk of age, similar to the second phase of obesity development that was mostly driven by reductions in metabolic rate and physical activity in NEXLPL−/− mice with chow feeding [15].
Figure 3.
Gene expression levels in various brain regions of both male and female ad-fed or pair-fed WT and NEXLPL−/− mice (n = 4 for each group). A. Gene expression of AgRP and NPY in hypothalamus of 42 wk male ad-fed WT control mice and pair-fed WT and NEXLPL−/− mice. B. Gene expression of various neuropeptides in hypothalamus of 38 wk female ad-fed WT control mice and pair-fed WT and NEXLPL−/− mice. C. Gene expression of ERα in various brain regions of 20 wk female ad-fed and pair-fed WT and NEXLPL−/− mice. D. Gene expression of ERα in various brain regions of 20 we male ad-fed and pair-fed WT and NEXLPL−/− mice.
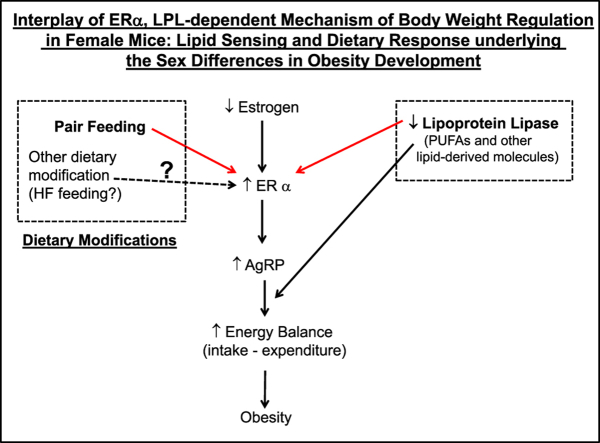
To explore what might be underlying the apparent protective effect of obesity in pair-fed female NEXLPL−/− mice, we examined the gene expression of estrogen receptor alpha (ERα) in different brain regions in both ad-fed and pair-fed female and male WT and NEXLPL−/− mice. This pursuit was based on previous observations that many of the anti-obesity effects of estrogens are believed to be mediated by central mechanisms through the action of ERα [11], [22], [23], [24], [25]. In previous genetically modified murine studies, diminished ERα activity was associated with obesity in both sexes. However, the brain regions where ERα is required to regulate energy balance were different in females from males [23], [25]. Specifically, ERα in SF1 and POMC neurons in the hypothalamus were important to energy balance regulation in female mice [23], [26], whereas ERα in regions outside the hypothalamus, e.g. the amygdala, appeared more important for body weight regulation in male mice [27].
To avoid the possible effect of extra body weight and/or fat mass gain in pair-fed mice on hypothalamic gene expression, we conducted a more abbreviated pair feeding experiment subjecting mice to only 10 wk of pair feeding (or control ad libitum feeding). At 20 wk of age, after the short term pair feeding, all pair-fed male and female WT and NEXLPL−/− mice had similar body weight and body composition compared to wt-ad fed group (data not shown), and the only group of mice that displayed obesity at this age was the ko-ad group, which developed obesity at 16 wk [15]. When ERα gene expression was examined in different brain regions in female mice (Figure 3C), levels were significantly increased in the hypothalamus in NEXLPL−/− mice under ad feeding conditions (ko-ad vs. wt-ad, 80% increase, p < 0.001), and pair feeding alone also resulted in 50% increases n ERα mRNA in WT control mice (wt-pf vs. wt-ad, p = 0.02). Moreover, the effect on increasing ERα by pair feeding and neuronal LPL deficiency was additive in pair-fed female NEXLPL−/− mice (ko-pf vs. ko-ad, 67% increase, p = 0.01; ko-pf vs. wt-pf, 100% increase, p < 0.001; ko-pf vs. wt-ad, 200% increase, p < 0.001). However, ERα mRNA levels were not significantly modified by either pair feeding or LPL deficiency in any of the other brain regions examined (Figure 3C).
In contrast, ERα was increased in the brain stem region of male NEXLPL−/− mice but not in the hypothalamus (Figure 3D), nor in any other brain regions examined, and this increase was not further modified by pair feeding (Figure 3D). It is interesting to note that pair feeding alone also appeared to increase ERα in NEXLPL−/− male mice (ko-pf vs. ko-ad, p < 0.01), but pair-fed NEXLPL−/− male mice did not show a significantly higher level of ERα compared to pair-fed WT male mice, and pair feeding alone did not increase ERα in WT mice (Figure 3D). Since ERα levels in the hypothalamic area of the male do not appear to regulate body weight [23], [25], we do not consider the modifications observed here on ERα in male NEXLPL−/− mice are relevant to the body weight regulation mechanism.
4. Discussion
Men and women differ in the regulation of body composition. For any given body mass index, men have more lean tissue whereas women have more adiposity, and men have more visceral adipose tissue whereas women have more subcutaneous adipose tissue. Yet, when normalized to lean body mass, men and women have similar resting energy expenditure; however, energy expenditure in the form of physical activity is more closely related to percent body fat in men than in women [8], [28]. Although women store more fat, women in general are more insulin sensitive than men [29], [30], [31] and are more protected from obesity-related complications at younger ages. However, postmenopausal women are not consistently protected from hypertension, cardiovascular disease, dementia, and other neurobehavioral disorders as they age. And, as menopause approaches, ovulatory cycles fall, estrogen levels diminish, mensuration ceases, ERα increases, and visceral adipose tissue and insulin resistance increase [32]. More importantly, the estrogen deficiency after menopause has been related to the weight gain [33], [34], and estrogen supplementation can prevent this consistently in animals and less so in humans, but more carefully designed studies are needed before conclusions can be reached [35], [36]. Thus, it is becoming increasingly important to understand the molecular mechanisms underlying the sex differences in body composition and energy balance, and how other nutritional and hormonal factors relate. There is also a need to develop new animal models not only to study underlying mechanisms but also to explore and facilitate the development of new treatments that particularly benefit women.
Our neuronal LPL deficient mice provide an interesting and unique model for studying sex-differences in obesity development. The installation of well controlled pair feeding conditions in both WT and NEXLPL−/− mice revealed a novel, sex-dependent effect on body weight, fat mass, and hypothalamic gene expression. Female mice are generally more sensitive to dietary modifications such as pair feeding, in that limiting total food intake exerts a bigger effect on preventing obesity than that in male mice independent of neuronal LPL levels. For male mice, pair feeding not only reduces the total food intake, but also lowers the metabolic rate and physical activity more in NEXLPL−/− than in controlled WT mice. Thus, in time, the total effect on energy balance is still positive and results in delayed but significant obesity development in pair-fed male NEXLPL−/− mice. These findings recapitulate the clinical observations that metabolic rate and physical activity is more closely related to percent body fat in men than in women [8], [28]. Furthermore, the result from female pair-fed mice implies that limiting total food intake would have more beneficial effect on body weight regulation in women.
The most exciting findings from our study are the modifications of hypothalamic ERα gene expression levels by pair feeding and by neuronal LPL levels in a sex-dependent manner. Specifically, ERα was not different in the hypothalamus in male ad-fed NEXLPL−/− mice vs. WT mice but was increased in female ad libitum fed NEXLPL−/− mice. In addition, pair feeding further increased ERα mRNA in the hypothalamus of both male and female NEXLPL−/− mice as well as control mice. Considering the protective effect of estrogen through the action of ERα and the differential increase of ERα in the hypothalamus in female but not male NEXLPL−/− mice, we hypothesize that the prevention of obesity in female NEXLPL−/− mice by pair feeding is a result of downstream signaling through ERα and is related to LPL-dependent lipid uptake and processing.
It is worthy to point out that although ERα gene expression was already increased in female NEXLPL−/− under ad libitum feeding conditions, the level of increase was not enough to protect female mice from obesity development on ad libitum chow feeding. Yet the additional increase in ERα mRNA with pair feeding was associated with protection from obesity. It is also interesting to note that in recent work by Morselli et al., it was demonstrated that high fat feeding increased the level of sphingolipids and inflammation in the CNS of male mice when compared to female mice and that high fat feeding reduced PGC-1α and ERα in hypothalamic neurons and astrocytes in a sex-specific way [37]. Thus, it would be interesting to propose that in NEXLPL−/− mice, wherein LPL-dependent lipid delivery is reduced [15], [18], the upregulation of ERα could well be important in the energy balance effect of pair-feeding by responding to some lipid-derived signals.
5. Conclusion
In summary, using the novel neuronal-specific LPL deficient mouse and well-controlled pair feeding conditions, this study has provided new and interesting data on how hypothalamic levels of ERα can be modified with discrepant results in male vs. female NEXLPL−/− mice. These results suggest that the mechanism underlying ERα regulation of body weight interacts with the LPL-dependent lipid processing in the hypothalamus and body weight regulation in a sex specific way. It would be both interesting and important to further explore whether ERα gene expression in the hypothalamus can be modified by dietary lipid signals in an LPL-dependent or even independent manner, an effect that could offer innovative approaches to manage obesity and related co-morbidities particularly in women.
Acknowledgments
This study was supported by grants from the ADA (Basic Science Award) and NIH (R01DK089309, K12HD057022). We thank Rachel C. Janssen at the Metabolic Core Laboratory at the University of Colorado Nutrition Obesity Research Center (NIH-P30DK048520) for performing all the real time-PCR reactions and related data analyses.
Conflict of interest
All authors have no conflict of interest to declare.
References
- 1.Stark R., Reichenbach A., Andrews Z.B. Hypothalamic carnitine metabolism integrates nutrient and hormonal feedback to regulate energy homeostasis. Molecular and Cellular Endocrinology. 2015;418(Pt 1):9–16. doi: 10.1016/j.mce.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Seoane-Collazo P., Fernø J., Gonzalez F., Diéguez C., Leis R., Nogueiras R. Hypothalamic-autonomic control of energy homeostasis. Endocrine. 2015;50(2):276–291. doi: 10.1007/s12020-015-0658-y. [DOI] [PubMed] [Google Scholar]
- 3.Mishra A.K., Dubey V., Ghosh A.R. Obesity: an overview of possible role(s) of gut hormones, lipid sensing and gut microbiota. Metabolism. 2016;65(1):48–65. doi: 10.1016/j.metabol.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Mauvais-Jarvis F. Sex differences in metabolic homeostasis, diabetes, and obesity. Biology of Sex Differences. 2015;6:14. doi: 10.1186/s13293-015-0033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi H., Seeley R.J., Clegg D.J. Sexual differences in the control of energy homeostasis. Frontiers in Neuroendocrinology. 2009;30(3):396–404. doi: 10.1016/j.yfrne.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lovejoy J.C., Sainsbury A., Stock Conference Working Group Sex differences in obesity and the regulation of energy homeostasis. Obesity Reviews. 2009;10(2):154–167. doi: 10.1111/j.1467-789X.2008.00529.x. [DOI] [PubMed] [Google Scholar]
- 7.Scott E.C., Johnston F.E. Critical fat, menarche, and the maintenance of menstrual cycles: a critical review. Journal of Adolescent Health Care. 1982;2(4):249–260. doi: 10.1016/s0197-0070(82)80059-4. [DOI] [PubMed] [Google Scholar]
- 8.Karastergiou K., Smith S.R., Greenberg A.S., Fried S.K. Sex differences in human adipose tissues – the biology of pear shape. Biology of Sex Differences. 2012;3(1):13. doi: 10.1186/2042-6410-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beltrán C.J., Candia E., Erranz B., Figueroa C., Gonzalez M.J., Quera R. Peripheral cytokine profile in Chilean patients with Crohn's disease and ulcerative colitis. European Cytokine Network. 2009;20(1):33–38. doi: 10.1684/ecn.2009.0142. [DOI] [PubMed] [Google Scholar]
- 10.Jelenik T., Roden M. How estrogens prevent from lipid-induced insulin resistance. Endocrinology. 2013;154(3):989–992. doi: 10.1210/en.2013-1112. [DOI] [PubMed] [Google Scholar]
- 11.Clegg D.J. Minireview: the year in review of estrogen regulation of metabolism. Molecular Endocrinology. 2012;26(12):1957–1960. doi: 10.1210/me.2012-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varlamov O., Bethea C.L., Roberts C.T., Jr. Sex-specific differences in lipid and glucose metabolism. Frontiers in Endocrinology (Lausanne) 2014;5:241. doi: 10.3389/fendo.2014.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang H., Eckel R.H. Lipoprotein lipase: from gene to obesity. American Journal of Physiology – Endocrinology and Metabolism. 2009;297(2):E271–E288. doi: 10.1152/ajpendo.90920.2008. [DOI] [PubMed] [Google Scholar]
- 14.Wang H., Eckel R.H. Lipoprotein lipase in the brain and nervous system. Annual Review of Nutrition. 2012;32:147–160. doi: 10.1146/annurev-nutr-071811-150703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang H., Astarita G., Taussig M.D., Bharadwaj K.G., DiPatrizio N.V., Nave K.A. Deficiency of lipoprotein lipase in neurons modifies the regulation of energy balance and leads to obesity. Cell Metabolism. 2011;13(1):105–113. doi: 10.1016/j.cmet.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang H., Eckel R.H. What are lipoproteins doing in the brain? Trends in Endocrinology & Metabolism. 2014;25(1):8–14. doi: 10.1016/j.tem.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pellinen J., Wang H., Eckel R.H. Mice with altered brain lipoprotein metabolism display maladaptive responses to environmental challenges that may predispose to weight gain. Metabolic Syndrome and Related Disorders. 2014;12(6):339–346. doi: 10.1089/met.2013.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Libby A.E., Wang H., Mittal R., Sungelo M., Potma E., Eckel R.H. Lipoprotein lipase is an important modulator of lipid uptake and storage in hypothalamic neurons. Biochemical and Biophysical Research Communications. 2015;465(2):287–292. doi: 10.1016/j.bbrc.2015.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu T., Taussig M.D., DiPatrizio N.V., Astarita G., Piomelli D., Bergman B.C. Deficiency of lipoprotein lipase in neurons decreases AMPA receptor phosphorylation and leads to neurobehavioral abnormalities in mice. PLoS One. 2015;10(8):e0135113. doi: 10.1371/journal.pone.0135113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jensen D.R., Gayles E.C., Ammon S., Phillips R., Eckel R.H. A self-correcting indirect calorimeter system for the measurement of energy balance in small animals. Journal of Applied Physiology (1985) 2001;90(3):912–918. doi: 10.1152/jappl.2001.90.3.912. [DOI] [PubMed] [Google Scholar]
- 21.Roth J.D., Hughes H., Kendall E., Baron A.D., Anderson C.M. Antiobesity effects of the beta-cell hormone amylin in diet-induced obese rats: effects on food intake, body weight, composition, energy expenditure, and gene expression. Endocrinology. 2006;147(12):5855–5864. doi: 10.1210/en.2006-0393. [DOI] [PubMed] [Google Scholar]
- 22.Geary N., Asarian L., Korach K.S., Pfaff D.W., Ogawa S. Deficits in E2-dependent control of feeding, weight gain, and cholecystokinin satiation in ER-alpha null mice. Endocrinology. 2001;142(11):4751–4757. doi: 10.1210/endo.142.11.8504. [DOI] [PubMed] [Google Scholar]
- 23.Xu Y., Nedungadi T.P., Zhu L., Sobhani N., Irani B.G., Davis K.E. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metabolism. 2011;14(4):453–465. doi: 10.1016/j.cmet.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao X., Xu P., Oyola M.G., Xia Y., Yan X., Saito K. Estrogens stimulate serotonin neurons to inhibit binge-like eating in mice. Journal of Clinical Investigation. 2014;124(10):4351–4362. doi: 10.1172/JCI74726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saito K., Cao X., He Y., Xu Y. Progress in the molecular understanding of central regulation of body weight by estrogens. Obesity (Silver Spring) 2015;23(5):919–926. doi: 10.1002/oby.21099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu L., Xu P., Cao X., Yang Y., Hinton A.O., Jr., Xia Y. The ERalpha-PI3K Cascade in proopiomelanocortin progenitor neurons regulates feeding and glucose balance in female mice. Endocrinology. 2015;156(12):4474–4491. doi: 10.1210/en.2015-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu P., Cao X., He Y., Zhu L., Yang Y., Saito K. Estrogen receptor-alpha in medial amygdala neurons regulates body weight. Journal of Clinical Investigation. 2015;125(7):2861–2876. doi: 10.1172/JCI80941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geer E.B., Shen W. Gender differences in insulin resistance, body composition, and energy balance. Gender Medicine. 2009;6(Suppl 1):60–75. doi: 10.1016/j.genm.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Donahue R.P., Prineas R.J., DeCarlo D.R., Bean J.A., Skyler J.S. The female ‘insulin advantage’ in a biracial cohort: results from the Miami Community Health Study. International Journal of Obesity and Related Metabolic Disorders. 1996;20(1):76–82. [PubMed] [Google Scholar]
- 30.Sumner A.E., Kushner H., Sherif K.D., Tulenko T.N., Falkner B., Marsh J.B. Sex differences in African-Americans regarding sensitivity to insulin's glucoregulatory and antilipolytic actions. Diabetes Care. 1999;22(1):71–77. doi: 10.2337/diacare.22.1.71. [DOI] [PubMed] [Google Scholar]
- 31.Høeg L., Roepstorff C., Thiele M., Richter E.A., Wojtaszewski J.F., Kiens B. Higher intramuscular triacylglycerol in women does not impair insulin sensitivity and proximal insulin signaling. Journal of Applied Physiology (1985) 2009;107(3):824–831. doi: 10.1152/japplphysiol.91382.2008. [DOI] [PubMed] [Google Scholar]
- 32.Meyer M.R., Clegg D.J., Prossnitz E.R., Barton M. Obesity, insulin resistance and diabetes: sex differences and role of oestrogen receptors. Acta Physiologica (Oxford) 2011;203(1):259–269. doi: 10.1111/j.1748-1716.2010.02237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al-Safi Z.A., Polotsky A.J. Obesity and menopause. Best Practice & Research Clinical Obstetrics & Gynaecology. 2015;29(4):548–553. doi: 10.1016/j.bpobgyn.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 34.Panotopoulos G., Raison J., Ruiz J.C., Guy-Grand B., Basdevant A. Weight gain at the time of menopause. Human Reproduction. 1997;12(Suppl 1):126–133. doi: 10.1093/humrep/12.suppl_1.126. [DOI] [PubMed] [Google Scholar]
- 35.Springer A.M., Foster-Schubert K., Morton G.J., Schur E.A. Is there evidence that estrogen therapy promotes weight maintenance via effects on leptin? Menopause. 2014;21(4):424–432. doi: 10.1097/GME.0000000000000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Biundo B., Gogola M. Estradiol: the emerging evidence for a protective role against insulin resistance and obesity. International Journal of Pharmaceutical Compounding. 2015;19(4):289–293. [PubMed] [Google Scholar]
- 37.Morselli E., Fuente-Martin E., Finan B., Kim M., Frank A., Garcia-Caceres C. Hypothalamic PGC-1alpha protects against high-fat diet exposure by regulating ERalpha. Cell Reports. 2014;9(2):633–645. doi: 10.1016/j.celrep.2014.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]