Abstract
The emerging enteric pathogen Campylobacter concisus is associated with prolonged diarrhea and inflammatory bowel disease. Previous studies have shown that C. concisus strains are very genetically diverse. Nevertheless, C. concisus strains have been divided into two genomospecies, where GS1 strains have been isolated predominantly from healthy individuals, while the GS2 cluster consists of isolates primarily from diarrheic individuals. The aim of the present study was to determine the genetic diversity of C. concisus isolates from Danish diarrheic patients. Multilocus sequence typing using the loci aspA, atpA, glnA, gltA, glyA, ilvD and pgm, as well as genomospecies based on specific differences in the 23S rRNA, was used to characterize 67 isolates (63 fecal and 4 oral), from 49 patients with different clinical presentations (29 with diarrhea, eight with bloody diarrhea, seven with collagenous colitis and five with Crohn’s disease). MLST revealed a high diversity of C. concisus with 53 sequence types (STs), of which 52 were identified as ‘new’ STs. Allele sequences showed more than 90 % similarity between isolates, with only four outliers. Dendrogram profiles of each allele showed a division into two groups, which more or less correlated with genomospecies A and genomospecies B. However, in contrary to previous results, this subgrouping had no association to the clinical severity of disease.
Electronic supplementary material
The online version of this article (doi:10.1186/s13099-016-0126-0) contains supplementary material, which is available to authorized users.
Keywords: Campylobacter concisus, MLST, Diarrhea, Gastroenteritis, Sequencing, Emerging, 23S, Genomospecies, Genetic diversity, Collagenous colitis, Crohn’s disease
Background
Campylobacter jejuni is the most commonly reported bacteriologic agent in gastrointestinal infectious disease [1]. A related species, Campylobacter concisus, was first isolated from human periodontal lesions [2], and is now considered part of the normal human oral microbiota [3]. However, in a recent population based study from Denmark, C. concisus was the most prevalent Campylobacter species in diarrheic stool samples, by cultivation using mCCDA plates as well as a polycarbonate filter technique on blood agar plates [4].
Recent studies have associated C. concisus to prolonged gastroenteritis in children as well as adults [5, 6]. Interestingly, C. concisus infection appears to cause milder, more prolonged diarrhea compared to C. jejuni infection, while C. concisus positive patients still present with the same gastrointestinal complaints following acute gastroenteritis as C. jejuni positive patients [6]. Furthermore, several studies have shown a high prevalence of C. concisus DNA in mucosal biopsies from patients with inflammatory bowel disease (IBD) [7–10] and one study showed a possible association between C. concisus infection and microscopic colitis, especially collagenous colitis [6].
Previous studies have shown that C. concisus is genetically diverse and have described two major clusters or genomospecies (GS), based on both amplified fragment length polymorphism (AFLP) and strain typing using 23S rRNA PCR [11–14]. By use of MLST, Miller et al. [15] also showed that strains of C. concisus could be grouped into two clusters that corresponded to GS A and GS B, however, they did not analyze correlation with different disease presentations. In addition, Mahendran et al. by use of six housekeeping genes [16], showed that oral C. concisus strains also comprised of two GS. Finally, Deshpande et al. sequenced eight strains of C. concisus and showed that C. concisus strains were highly genetically diverse [17, 18].
The aim of the present study was to determine the diversity of C. concisus isolates from Danish diarrheic patients by use of the MLST scheme by Miller et al. [15], and to compare GS with the clinical presentation of disease [6].
Methods
Sixty-seven isolates (63 fecal and 4 oral), from 49 patients (29 females/20 males) with different clinical presentations were analyzed. Twenty-nine with diarrhea (age range: 0–86 years), eight with bloody diarrhea (0–78 years), seven with collagenous colitis (53–80 years), and five with Crohn’s disease (age 2–73 years) were selected for the study.
All strains were isolated at the Department of Clinical Microbiology, Aalborg University Hospital, Denmark and MLST was performed at SSI. C. concisus was isolated using the filter technique on 5 % horse blood agar plates, containing 1 % yeast extract (SSI Diagnostica, Hillerød, Denmark), and incubated at 37 °C in a microaerobic atmosphere with 3 % hydrogen. Final identification was obtained through a species-specific real-time PCR based on the cpn60 gene, as described elsewhere [19], as well as MALDI-TOF analysis (BRUKER DALTONIK GmbH, Bremen, Germany) [20]. All strains were stored in nutrient beef broth with 10 % glycerine (SSI Diagnostica, Hillerød, Denmark) at −80 °C until use.
Isolates were re-cultivated as described above and DNA was extracted using an on-board protocol with the NucliSENS® easyMAG® platform (BioMérieux, Marcy-l’Étoile, France). PCR amplification of the 23S rRNA gene was conducted by use of one forward primer (MUC1) and two reverse primers (CON1 and CON2) used independently, and amplified PCR products were visualized on QiAXcel Advanced screen gel for verification of product size (Qiagen, Hilden, Germany). Isolates amplifying with either MUC1/CON1 or MUC1/CON2 primers were assigned to GS A or B, respectively, according to Kalischuk and Inglis [14].
Seven housekeeping genes (aspA, atpA, glnA, gltA, glyA, ilvD and pgm) were amplified from the 67 isolates using the MLST scheme of Miller et al. [15]. All housekeeping genes were sequenced using both forward and reverse primers and all sequences were evaluated manually. Sequences with low-quality bases in the chromatograms were re-sequenced to give high-quality bases in the chromatograms. BioNumerics version 7.1 (Applied Maths NV, Sint-Martens-Latem, Belgium) was used for sequence analysis and further used to generate an UPGMA tree. All sequences were submitted to the pubmlst.org database and published online (http://pubmlst.org/campylobacter/).
Results
MLST revealed a high diversity of C. concisus with 53 sequence types (STs), of which 52 were identified as ‘new’ STs. The full list of C. concisus strains used in this study is available in the Additional file 1. The high degree of variation across the C. concisus STs was reflected by the large number of alleles within the collection of 67 isolates. The number of alleles were as follows: aspA: 39, atpA: 34, glnA: 37, gltA: 36, glyA: 39, ilvD: 39, pgm: 45.
We found 23 and 38 strains positive only for GS A and GS B, respectively, while six strains were PCR positive for both GS A and GS B. A phylogenetic tree was generated based on the concatenated sequences of the seven housekeeping genes (3345 bp). With the exception of four outliers, isolates were divided into two major clusters (cluster 1 and 2) where all sequences displayed more than 90 % similarity (Fig. 1). The correlation between 23S and MLST was high and almost all strains in cluster 1 were GS A whereas GS B were found in cluster 2 (Figs. 1, 2). The subgrouping had no significant association with clinical disease, since all the different clinical presentations (diarrhea, bloody diarrhea, collagenous colitis, and Crohn’s disease) were distributed within the two clusters (Fig. 1).
Fig. 1.
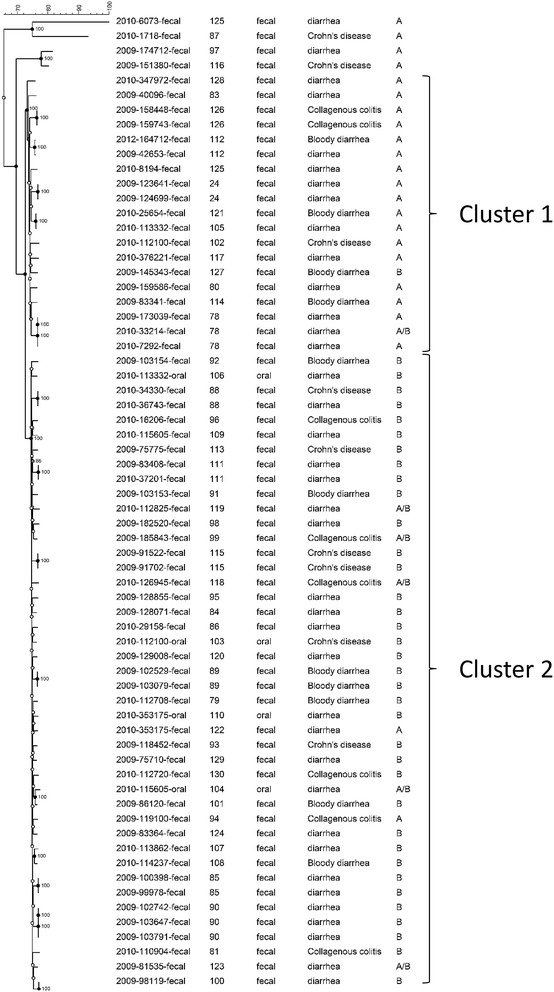
Neighbour joining tree based on merged sequences of the seven housekeeping genes for the C. concisus MLST scheme. ST number, source of the C. concisus strain, the clinical presentation and genomospecies (A, B or A/B) are shown
Fig. 2.
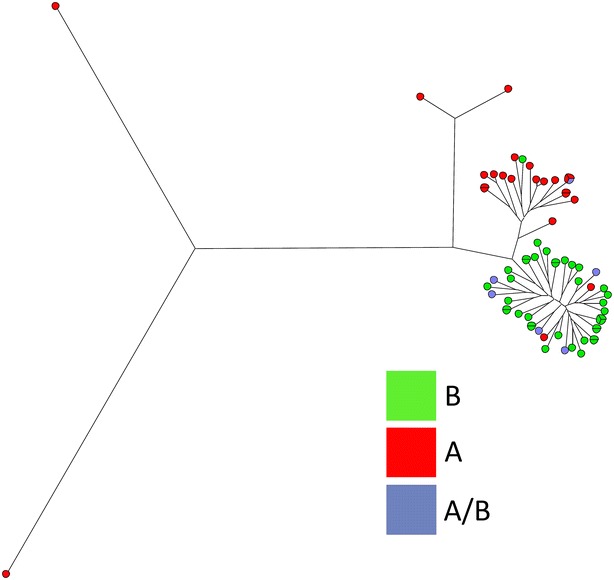
Maximum parsimony tree using concatenated sequences merging of the seven housekeeping genes. The colors represent genomospecies (A, B or A/B) showing a clear division into two main clusters with four outliers
Discussion
In this study, we found that C. concisus isolates from Danish diarrheic patients were highly genetically diverse by use of the MLST-scheme according to Miller et al., an accurate tool for C. concisus genomotyping [15]. We combined the MLST method with 23S and confirmed previous results with division into two groups, almost similar to GS A (cluster 1) and GS B (cluster 2), but found no association with clinical presentation.
We were unable to include fecal isolates from healthy individuals, but our GS A isolates were from patients with different clinical presentations. Previously, Kalischuk and Inglis included five C. concisus isolated from healthy human feces, and four of these isolates were positive for GS A [14]. Those isolates exhibited increased hemolytic ability, apoptotic DNA fragmentation, and IL-8 induction, whereas ALFP cluster 2 isolates, mainly positive for GS B, were predominantly from diarrheic patients, exhibiting higher levels of epithelial invasion and translocation, consistent with factors causing diarrheal disease.
We included four oral strains isolated from patients with clinical disease and not surprisingly, they all had different C. concisus isolates in stools. One of the four oral strains was both GS A and GS B positive and the remaining all belonged to cluster 2 (GS B positive), in accordance with Kalischuk and Inglis, suggesting GS2 to have a higher potential for diarrheic disease [14]. Previous data has shown that both oral and enteric isolates of C. concisus can cause epithelial barrier dysfunction and apoptosis [21], therefore suggesting that C. concisus strains colonizing the intestinal tract originate from oral C. concisus strains [16, 22].
Although, our result showed no significant association with clinical presentation between the two groups, previous findings, as mentioned above, have shown a higher pathogenic potential in GS B positive isolates [14]. In one study, four enteric strains isolated from patients with bloody diarrhea all belonged to AFLP cluster 2 (i.e., genomospecies 2), correlating to GS B [12]. Another study supported this finding, showing that although enteric isolates in general did not form distinct clusters, oral and enteric isolates that were been proven invasive, belonged to the same cluster [16]. We included eight patients with bloody diarrhea of which four strains were in cluster 1, according to the MLST-scheme, however, one of these (ST 127) was positive for GS B instead of GS A. There were also isolates from patients with collagenous colitis and Crohn’s disease in cluster 1, but numbers were limited.
Many molecular sub typing methods have been developed to characterize Campylobacter species, but only a few are commonly used in molecular epidemiology studies. C. concisus has been isolated in high numbers from Danish diarrheic stool samples, and while MLST is suitable for data exchange between different laboratories, it may not be applicable for C. concisus, because the number of STs is very high. Twelve ST-types from 26 strains were represented more than once. However, 17/26 (ST: 24, 85, 89, 90, 100, 115, 125 and 126) were strains that were isolated twice (one triplicate), from patients within the same diarrheic episode and with a minimum of time-span between stool collection. The remaining nine strains (ST: 78, 88, 111 and 112) were isolated from different patients with no obvious relatedness.
A recent study by Kirk et al. [10] showed a relative high isolation rate of C. concisus from saliva samples, intestinal biopsies and stools from both IBD patients and healthy controls. It would be highly interesting to see whether these patients were infected or colonized with different strains, for example by use of the MLST-scheme or by whole genome sequencing technologies.
In conclusion, we have shown, in accordance with previous studies, that C. concisus strains from Danish diarrheic patients are highly genetically diverse. A sequence-based phylogeny resulted in division of the strains into two major groups, almost similar to GS A and GS B, but we found no association with clinical presentation.
Authors’ contributions
HLN and MT collected the data and drafted the manuscript. HLN, HN and MT participated with design of the study and advisory support. MT did the sequencing. HN helped to draft the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors wish to thanks the assistance of Charlotte Hoe in the laboratory at Department for Microbiology and Infection control, Statens Serum Institut, Copenhagen, Denmark; MD Karina Frahm Kirk for proof editing; and William G Miller, United States Department of Agriculture, California, US, the curator of the non-jejuni/coli database at http://pubmlst.org/campylobacter/for uploading the MLST sequences; and all patients who took part in the study. Part of this study was presented at the 18th International workshop on Campylobacter, Helicobacter and Related Organisms (CHRO) 2015, Rotorua, New Zealand.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Data is available in the Additional file and all MLST data are available online at http://pubmlst.org/campylobacter.
Consent for publication
Consent to publish has been obtained from all patients or parents (for children).
Ethics approval and consent to participate
The study adhered to the Declaration of Helsinki, and ethics approval for research was obtained from The Ethics Committee for Region Nordjylland, Denmark (N-20080056). All patients signed written informed consent forms, and parents signed written informed consent forms for children.
Additional file
10.1186/s13099-016-0126-0 All isolates.
Contributor Information
Hans Linde Nielsen, Email: halin@rn.dk.
Henrik Nielsen, Email: henrik.nielsen@rn.dk.
Mia Torpdahl, Email: MTD@ssi.dk.
References
- 1.European centre for disease prevention and control. Annual epidemiological report 2014—food- and waterborne diseases and zoonoses. Campylobacteriosis, p. 20–24. Stockholm. ECDC; 2014.
- 2.Tanner ACR, Badger S, Lai C-H, Listgarten MA, Visconti RA, Socransky SS. Wolinella gen. nov., Wolinella succinogenes (Vibrio succinogenes Wolin et al.) comb. nov., and description of Bacteroides gracilis sp. nov., Wolinella recta sp. nov., Campylobacter concisus sp. nov., and Eikenella corrodens from humans with periodontal disease. Int J Syst Bacteriol. 1981;31:432–445. doi: 10.1099/00207713-31-4-432. [DOI] [Google Scholar]
- 3.Zhang L, Budiman V, Day AS, Mitchell H, Lemberg DA, Riordan SM, Grimm M, Leach ST, Ismail Y. Isolation and detection of Campylobacter concisus from saliva of healthy individuals and patients with inflammatory bowel disease. J Clin Microbiol. 2010;48:2965–2967. doi: 10.1128/JCM.02391-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nielsen HL, Ejlertsen T, Nielsen H. Polycarbonate filtration technique is noninferior to mCCDA for isolation of Campylobacter species from stool samples. Diagn Microbiol Infect Dis. 2015;83:11–12. doi: 10.1016/j.diagmicrobio.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Nielsen HL, Engberg J, Ejlertsen T, Bucker R, Nielsen H. Short-term and medium-term clinical outcomes of Campylobacter concisus infection. Clin Microbiol Infect. 2012;18:E459–E465. doi: 10.1111/j.1469-0691.2012.03990.x. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen HL, Engberg J, Ejlertsen T, Nielsen H. Clinical manifestations of Campylobacter concisus infection in children. Pediatr Infect Dis J. 2013;11:1194–1198. doi: 10.1097/INF.0b013e31829f0aff. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L, Man SM, Day AS, Leach ST, Lemberg DA, Dutt S, Stormon M, Otley A, O’Loughlin EV, Magoffin A, Ng PH, Mitchell H. Detection and isolation of Campylobacter species other than C. jejuni from children with Crohn’s disease. J Clin Microbiol. 2009;47:453–455. doi: 10.1128/JCM.01949-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansen R, Berry SH, Mukhopadhya I, Thomson JM, Saunders KA, Nicholl CE, Bisset WM, Loganathan S, Mahdi G, Kastner-Cole D, Barclay AR, Bishop J, Flynn DM, McGrogan P, Russell RK, El-Omar EM, Hold GL. The microaerophilic microbiota of de-novo paediatric inflammatory bowel disease: the BISCUIT study. PLoS ONE. 2013;8:e58825. doi: 10.1371/journal.pone.0058825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahendran V, Riordan SM, Grimm MC, Tran TA, Major J, Kaakoush NO, Mitchell H, Zhang L. Prevalence of Campylobacter species in adult Crohn’s disease and the preferential colonization sites of Campylobacter species in the human intestine. PLoS ONE. 2011;6:e25417. doi: 10.1371/journal.pone.0025417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirk KF, Nielsen HL, Thorlacius-Ussing O, Nielsen H. Optimized cultivation of Campylobacter concisus from gut mucosal biopsies in inflammatory bowel disease. Gut Pathog. 2016;8:27. doi: 10.1186/s13099-016-0111-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bastyns K, Chapelle S, Vandamme P, Goossens H, De Wachter R. Specific detection of Campylobacter concisus by PCR amplification of 23S rDNA areas. Mol Cell Probes. 1995;9:247–250. doi: 10.1016/S0890-8508(95)90114-0. [DOI] [PubMed] [Google Scholar]
- 12.Aabenhus R, On SL, Siemer BL, Permin H, Andersen LP. Delineation of Campylobacter concisus genomospecies by amplified fragment length polymorphism analysis and correlation of results with clinical data. J Clin Microbiol. 2005;43:5091–5096. doi: 10.1128/JCM.43.10.5091-5096.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engberg J, Bang DD, Aabenhus R, Aarestrup FM, Fussing V, Gerner-Smidt P. Campylobacter concisus: an evaluation of certain phenotypic and genotypic characteritics. Clin Microbiol Infect. 2005;11:288–295. doi: 10.1111/j.1469-0691.2005.01111.x. [DOI] [PubMed] [Google Scholar]
- 14.Kalischuk LD, Inglis GD. Comparative genotypic and pathogenic examination of Campylobacter concisus isolates from diarrheic and non-diarrheic humans. BMC Microbiol. 2011;15:53. doi: 10.1186/1471-2180-11-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller WG, Chapman MH, Yee E, On SL, McNulty DK, Lastovica AJ, Carroll AM, McNamara EB, Duffy G, Mandrell RE. Multilocus sequence typing methods for the emerging Campylobacter species C. hyointestinalis, C. lanienae, C. sputorum, C. concisus, and C. curvus. Front Cell Infect Microbiol. 2012;2:45. doi: 10.3389/fcimb.2012.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahendran V, Octavia S, Demirbas OF, Sabrina S, Ma R, Lan R, Riordan SM, Grimm MC, Zhang L. Delineation of genetic relatedness and population structure of oral and enteric Campylobacter concisus strains by analysis of housekeeping genes. Microbiology. 2015;161:1600–1612. doi: 10.1099/mic.0.000112. [DOI] [PubMed] [Google Scholar]
- 17.Deshpande NP, Kaakoush NO, Mitchell H, Janitz K, Raftery MJ, Li SS, Wilkins MR. Sequencing and validation of the genome of a Campylobacter concisus reveals intra-species diversity. PLoS ONE. 2011;6:e22170. doi: 10.1371/journal.pone.0022170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deshpande NP, Kaakoush NO, Wilkins MR, Mitchell HM. Comparative genomics of Campylobacter concisus isolates reveals genetic diversity and provides insights into disease association. BMC Genom. 2013;14:585. doi: 10.1186/1471-2164-14-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaban B, Musil KM, Himsworth CG, Hill JE. Development of cpn60-based real-time quantitative PCR assays for the detection of 14 Campylobacter species and application to screening of canine fecal samples. Appl Environ Microbiol. 2009;75:3055–3061. doi: 10.1128/AEM.00101-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nielsen HL, Mølvadgaard M, Nielsen H, Kostrzewa M. Identification and differentiation of highly diverse Campylobacter concisus strains using the MALDI biotyper. Clin Microbiol. 2016;5:230. doi: 10.4172/2327-5073.1000230. [DOI] [Google Scholar]
- 21.Nielsen HL, Nielsen H, Ejlertsen T, Engberg J, Gunzel D, Zeitz M, Hering NA, Fromm M, Schulzke JD, Bucker R. Oral and fecal Campylobacter concisus strains perturb barrier function by apoptosis induction in HT-29/B6 intestinal epithelial cells. PLoS ONE. 2011;6(8):e23858. doi: 10.1371/journal.pone.0023858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ismail Y, Mahendran V, Octavia S, Day AS, Riordan SM, Grimm MC, Lan R, Lemberg D, Tran TA, Zhang L. Investigation of the enteric pathogenic potential of oral Campylobacter concisus strains isolated from patients with inflammatory bowel disease. PLoS ONE. 2012;7:e38217. doi: 10.1371/journal.pone.0038217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available in the Additional file and all MLST data are available online at http://pubmlst.org/campylobacter.


