Abstract
Objective
Puberty is a key developmental phenomenon highly sensitive to metabolic modulation. Worrying trends of changes in the timing of puberty have been reported in humans. These might be linked to the escalating prevalence of childhood obesity and could have deleterious impacts on later (cardio-metabolic) health, but their underlying mechanisms remain unsolved. The neuropeptide α-MSH, made by POMC neurons, plays a key role in energy homeostasis by mediating the actions of leptin and likely participates in the control of reproduction. However, its role in the metabolic regulation of puberty and interplay with kisspeptin, an essential puberty-regulating neuropeptide encoded by Kiss1, remain largely unknown. We aim here to unveil the potential contribution of central α-MSH signaling in the metabolic control of puberty by addressing its role in mediating the pubertal effects of leptin and its potential interaction with kisspeptin.
Methods
Using wild type and genetically modified rodent models, we implemented pharmacological studies, expression analyses, electrophysiological recordings, and virogenetic approaches involving DREADD technology to selectively inhibit Kiss1 neurons, in order to interrogate the physiological role of a putative leptin→α-MSH→kisspeptin pathway in the metabolic control of puberty.
Results
Stimulation of central α-MSH signaling robustly activated the reproductive axis in pubertal rats, whereas chronic inhibition of melanocortin receptors MC3/4R, delayed puberty, and prevented the permissive effect of leptin on puberty onset. Central blockade of MC3/4R or genetic elimination of kisspeptin receptors from POMC neurons did not affect kisspeptin effects. Conversely, congenital ablation of kisspeptin receptors or inducible, DREADD-mediated inhibition of arcuate nucleus (ARC) Kiss1 neurons resulted in markedly attenuated gonadotropic responses to MC3/4R activation. Furthermore, close appositions were observed between POMC fibers and ARC Kiss1 neurons while blockade of α-MSH signaling suppressed Kiss1 expression in the ARC of pubertal rats.
Conclusions
Our physiological, virogenetic, and functional genomic studies document a novel α-MSH→kisspeptin→GnRH neuronal signaling pathway involved in transmitting the permissive effects of leptin on pubertal maturation, which is relevant for the metabolic (and, eventually, pharmacological) regulation of puberty onset.
Keywords: α-MSH, Kisspeptin, Leptin, Metabolism, DREADDs, Puberty
Highlights
-
•
Puberty is highly sensitive to metabolic modulation and disturbed by child obesity.
-
•
Altered puberty is linked to adverse metabolic health outcomes via unclear mechanisms.
-
•
The POMC product, α-MSH, transmit leptin-mediated metabolic regulation of puberty.
-
•
A novel α-MSH→kisspeptin→GnRH signaling pathway is involved in the control of puberty
-
•
This pathway is important for the metabolic (and pharmacologic) control of puberty.
1. Introduction
Two possibly interconnected conditions, namely obesity and disordered puberty, are displaying escalating prevalence among adolescents worldwide. Puberty is a crucial transitional period when reproductive capacity is acquired and somatic development completed [1]. The genetic basis and neuroendocrine mechanisms for the control of puberty are attracting growing interest due, in part, to the worrying trend of altered (mostly, earlier) puberty reported recently in girls and boys [2], [3]. Perturbed pubertal timing is associated with adverse health outcomes, including different cancers, as well as gynecological/obstetric, musculoskeletal, and neuro-cognitive disorders [4], together with reduced life expectancy [5]. Recent reports documented that earlier puberty is linked to a higher risk of cardio-metabolic disease, including hypertension and type-2 diabetes, in both men and women [4]. Although the underlying mechanisms remain ill defined, the higher prevalence of childhood obesity has been suggested to underlie the trend of earlier puberty in humans [2], [3]. In turn, puberty onset is frequently coupled to decreased physical activity and behavioral changes, including unhealthy nutritional habits, which predispose to or worsen the development of obesity. These associations warrant a better understanding of the basis of puberty and its deviations.
The timing of puberty is dictated by the concerted activation of the hypothalamic–pituitary–gonadal (HPG) axis, in which a scarce population of hypothalamic neurons secreting gonadotropin-releasing hormone (GnRH) becomes fully active during the pubertal transition to drive pituitary gonadotropin secretion and, thereby, complete gonadal maturation and function [6]. Among the different pubertal regulators, nutritional and metabolic cues have been shown to play a critical role in the central control of puberty [7], [8]. However, strong evidence suggests that despite their hierarchical role, GnRH neurons are not targeted directly by metabolic signals, such as leptin and insulin [9], but are modulated indirectly by mostly unidentified intermediary inputs.
Kisspeptin, the neuropeptide encoded by the Kiss1 gene and signaling through Gpr54 (aka, Kiss1r), has been recognized recently as a master puberty-activating factor [10], [11]. Kiss1 neurons, which in rodents are predominantly located in the arcuate nucleus (ARC) and the rostral periventricular area of the third ventricle (RP3V), are essential upstream afferents to GnRH neurons that play an important role in transmitting the regulatory actions of key signals, including metabolic cues [6], [12]. Indeed, hypothalamic Kiss1/kisspeptin expression is sensitive to conditions of metabolic stress, ranging from subnutrition to obesity [6], [13], [14]. However, Kiss1 neurons apparently do not express substantial amounts of leptin receptors [15], suggesting that leptin does not signal directly to Kiss1 neurons to modulate puberty onset.
Neurons in the ARC expressing the proopiomelanocortin (POMC) gene are essential first-order elements in the central networks governing body weight and energy homeostasis [16]; the major ARC POMC product being α-melanocyte stimulating hormone (α-MSH). The central effects of α-MSH are mediated via interaction with melanocortin receptor (MCR) subtypes, MC3R and MC4R, which are expressed in the hypothalamus and other brain areas [17]. Some evidence in adults suggests that α-MSH signaling is involved in the control of the reproductive axis. For example, POMC neurons are sensitive to a range of metabolic hormones regulating the HPG axis, such as leptin, insulin, and ghrelin [18], and POMC terminals form synapses with GnRH neurons [16]. However, the actual gonadotropic effects of POMC peptides seem to be variable; while α-MSH can activate GnRH neurons and LH secretion, inhibitory effects have been also reported [16], [19]. Mice with inactivation of Pomc, Mc3r or Mc4r not only develop obesity but also subfertility (especially the females) by adulthood [19], [20], [21]. Admittedly, the reproductive phenotype of isolated inactivation of Mc3r or Mc4r is mild, suggesting that the lack of one receptor could be partially compensated by the other. Yet, mice harboring the agouti lethal yellow (Ay) mutant allele that causes over-expression of the agouti-protein, which is similar to the endogenous antagonist of MC3/4R, agouti-related peptide (AgRP), are infertile [22]. Moreover, AgRP has been suggested to interfere the permissive effect of leptin on the reproductive axis [23], [24]. Yet, the pathways mediating the reproductive effects of α-MSH remain unsolved, and its role in the metabolic control of puberty is largely unexplored.
Interactions between POMC and Kiss1 neurons have been suggested, but the data available remain inconclusive. POMC and Kiss1 neurons appear to make mutual contacts in the adult ovine brain, and α-MSH enhanced Kiss1 mRNA levels in the preoptic area of sheep, whereas it decreased Kiss1 expression in the ARC [25]. In mice, a subset of RP3V Kiss1 neurons express the melanocortin receptor, MC4R [26]. Conversely, kisspeptin has been shown to inhibit Pomc gene expression in the ARC of the sheep [27], while ARC POMC neurons displayed increased firing after kisspeptin stimulation in mice [28]. Hence, it is difficult at present to delineate the physiological relevance of α-MSH/kisspeptin interactions in adults and even less so in the context of puberty. We provide herein an integrated analysis of the role of central α-MSH signaling in the metabolic control of puberty and its interactions with kisspeptin pathways.
2. Methods
2.1. Animals
Wistar rats and genetically modified mice, including global Gpr54−/− [29], POMC-specific Gpr54 KO (see below), and Kiss1-CreGFP [30] lines, bred in the vivarium of the University of Cordoba, as well as kisspeptin-IRES-Cre+/−/ROSA26-CAGS-τGFP+/− (Kiss-GFP) mice [31], bred in the animal facilities of the University of Otago, were used. The animals were housed under constant conditions of light (12 h light/dark cycles) and temperature (22 ± 2 °C). The day the litters were born was considered day 1 of age (postnatal day 1; PND-1). Animals were weaned at PND-21 and were provided with a free access to tap water and fed ad libitum with a standard soy-free diet, unless otherwise is indicated. The experiments and animal protocols included in this study were approved by the Ethical Committees of the University of Cordoba and the University of Otago; all experiments were conducted in accordance with European Union normative for the use and care of experimental animals (EU Directive 2010/63/UE, September 2010).
2.2. Drugs
The MC3/4R agonist, Melanotan (MT-II), the selective MC4R agonist, Cyclo (β-Ala-His-d-Phe-Arg-Trp-Glu)-NH2, the MC3/4R antagonist, SHU9119, and kisspeptin110-119-NH2 (termed hereafter kisspeptin-10 or Kp-10) were purchased from Phoenix Pharmaceuticals Inc. (Burlingame, CA, USA). The selective MC3R agonist D-Trp-γ MSH was obtained from American Peptide Company Inc. (Sunnyvale, CA, USA). Recombinant rat leptin was obtained from ProSpec-Tany TechnoGene Ltd. (Ness Ziona, Israel), and 17β-estradiol (E2) and clozapine-N-oxide (CNO) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). All the drugs were dissolved in physiological saline (0.9% NaCl), except E2, which was dissolved in olive oil.
2.3. Treatments and experimental design
The experimental studies included herein were implemented to investigate the putative function of α-MSH signaling in the control of the gonadotropic axis at puberty and to explore their potential interaction with leptin and kisspeptin signaling. Central administration of the different compounds was implemented using standard procedures of cannulation and acute or repeated intracerebroventricular (icv) injection, following previously published procedures [32], [33]. In brief, rats and mice were subjected to icv cannulation 24 h before the beginning of the pharmacological studies; to this end, cannulas (INTRADEMIC polyethylene Tubing, Becton Dickinson, Sparks, MD, USA) were inserted to a depth of 2 mm beneath the surface of the skull, with an insert point at 1 mm posterior and 1.2 mm lateral to Bregma, according to a rat/mouse brain atlas [34]. After cannulation, they were housed in individual cages until the end of the experiments. Blood samples for hormone assays were obtained by jugular venipuncture, unless otherwise is stated.
The following experimental studies, grouped into three major sets, were conducted:
Experimental Set #1: Impact of pharmacological manipulation of central α-MSH signaling on gonadotropin secretion and puberty onset in immature rats. The acute effects of central activation of α-MSH signaling on LH release were explored in infantile and peri-pubertal rats in Experiment 1. To this end, an effective dose of MCR-3/4 agonist Melanotan (MT-II, 1 nmol), defined on the basis of previous references [35], was icv injected to infantile (PND-15) male and female rats, and animals were euthanized 15 min after the injection for trunk blood collection. Similarly, peripubertal male (PND-43) and female (PND-29) rats were icv injected with a single bolus of 1 nmol MT-II, and blood samples were collected before (0) and after 15-, 30- & 60-min (the later time-point, only in males) of MT-II injection. At both age-points, control groups injected with vehicle were included.
Based on initial results, acute LH responses to a similar dose of MT-II were explored in peripubertal male and female rats subjected to metabolic stress, due to short-term fasting (Experiment 2). Food deprivation was applied for a maximum of 48-h, and icv injection of MT-II and serial blood sampling was conducted as for Experiment 1. On the other hand, in Experiment 3, the individual contribution of the major central melanocortin receptors, namely MC3R and MC4R, to LH responses to MT-II was explored. To this end, peripubertal male rats were icv injected with a range of doses (0.2, 1.0 and 5.0 nmol/rat) of the selective MC3R (D-Trp-γ MSH) or MC4R [Cyclo (β-Ala-His-d-Phe-Arg-Trp-Glu)-NH2] agonists, and blood sampling was conducted before (0), and 15-, 30- and 60-min after MT-II injection, as described above.
In addition, the effects of chronic blockade of α-MSH signaling on puberty onset were explored in Experiment 4. Immature female rats received two daily icv injections of the MCR-3/4 antagonist, SHU9119 (1 nmol/12 h/rat), or vehicle between PND-28 to PND-35. The dose of the antagonist was selected based on a previous reference [36]. Since pilot experiments revealed that repeated central administration of SHU9119 resulted in significant increases in food intake and body weight (BW) gain during the pubertal transition, SHU9119-treated animals were pair-fed with vehicle-treated rats in order to avoid the plausible influence of changes in BW on puberty onset. BW and vaginal opening (VO) were daily monitored during the treatment. Animals were sacrificed at PND-35, 60 min after the last injection of SHU9119 or vehicle, and trunk blood, uteri, and ovaries were collected. In the same vein, in Experiment 5, we explored the possible role of central α-MSH signaling in mediating leptin effects on pubertal timing. To this end, immature female rats were subjected from PND-23 to a moderate state of negative energy balance, imposed by 20% restriction of daily calorie intake (under-nutrition: UN group), as a means to decrease endogenous leptin levels, in keeping with previous references [32], [33]. Groups of UN rats received two daily icv injections of vehicle, leptin (3.1 μg/12 h/rat) or leptin in combination with SHU9119 (1 nmol/12 h/rat) between PND-29 and PND-35. Animals fed ad libitum (NN) and injected with vehicle served as controls. BW and VO were daily monitored. On PND-35, animals were killed by decapitation, 60-min after the last injection, and trunk blood, ovaries and uterus were collected.
Experimental Set #2: Assessment of the potential role of central α-MSH signaling as mediator of kisspeptin effects on gonadotropin secretion and puberty onset. In this set, we first evaluated acute LH responses to central administration of Kp-10 after blockade of MC3/4R in Experiment 6. To this end, peripubertal male rats were icv injected with vehicle or SHU9119 (1 nmol/rat), at 120-min and 60-min before administration of a third bolus of vehicle or SHU9119, alone or in combination with 100 pmol/rat Kp-10; the dose of Kp-10 was selected on the basis of previous references to obtain a submaximal LH secretory response [32]. In parallel, groups of SHU9119-treated rats were co-injected with MT-II (1 nmol/rat), as a means to check the efficiency of MC3/4R blockade in our pharmacological setting. Blood samples for hormone assays were obtained at 0-min (basal), and 15-min and 60-min after Kp-10 or MT-II injection.
In addition, in Experiment 7, LH responses to Kp-10 were studied in a novel mouse line with conditional ablation of the kisspeptin receptor Gpr54 in POMC neurons (See Methods 2.4 for details of generation and genotyping of this novel line). Male POMC-Gpr54 null mice and their corresponding WT controls were subjected to icv cannulation, as described above. The animals were icv injected with Kp-10 (50 pmol/mouse) or vehicle, and blood samples were obtained at 15-min for hormone analysis. As a continuation of this initial study, in Experiment 8, pubertal progression was examined in KO and WT female mice. To this end, body weight gain was monitored postnatally, and the animals were examined daily for vaginal opening after weaning; vaginal smears were collected from the day of VO onwards for determination of the occurrence of first estrus by cytological examination, VO and first estrus being consensus sign of puberty onset.
Experimental Set #3: Assessment of the role of kisspeptin signaling as mediator of α-MSH effects on gonadotropin secretion and puberty onset. To cover this goal, we first conducted neuroanatomical studies to identify the potential physical interplay between POMC and Kiss1 neurons. Thus, in Experiment 9, pubertal female rats (PND-33) were perfused with 4% paraformaldehyde (PFA), following standard protocols and the brains were collected upon decapitation for immunohistochemical analyses, as described in detail below. In addition, we assayed the impact of blockade of central α-MSH signaling on hypothalamic Kiss1 mRNA levels was in Experiment 10. This experiment was conducted in pubertal female rats. To avoid the potential confounding factor of changes in circulating estrogen on Kiss1 mRNA expression [37], female rats were subjected on PND-25 to bilateral ovariectomy (OVX) and supplemented with E2 by implanting SILASTIC brand silicon tubing elastomers (Dow Corning, Midland, MI; 10 mm length; inner diameter, 0.062 cm; exterior diameter, 0.125 cm) containing E2 (10 μg/ml in olive oil), in line with previous references [32]. This dose of E2 was selected to achieve moderate levels of circulating E2. Subsequently, the animals received two daily icv injections of SHU9119 (1 nmol/rat) or vehicle between PND-28 and PND-32. SHU9119-treated animals were pair fed to vehicle-treated rats, as was described in Experiment 4. BW was daily monitored and, at the end of the treatment, animals were euthanized and brains were removed, frozen on dry ice and store at −80 °C for posterior in situ hybridization (ISH) analyses, as described in detail below.
To provide further functional evidence for this putative α-MSH→Kiss1/kisspeptin pathway, pointed out by previous experiments, in Experiment 11, we studied the effect of MT-II on LH release in a global Gpr54 KO mouse model, which is defective for kisspeptin signaling [29]. Two-month-old Gpr54 KO male mice and their corresponding WT controls were used. To exclude the possibility that defective gonadotropin response might derive from insufficient pituitary responsiveness to GnRH due to the low endogenous GnRH tone in this mouse line, Gpr54 KO mice were subjected to a protocol of GnRH priming during two days before the test, following a previously published priming protocol [29]. Priming protocols consisted of five successive intra-peritoneal (ip) injections of a low dose of GnRH (0.15 μg/mouse), with the following schedule: at 10:00 h, 17:00 h, and 24:00 h on day 1; and at 08:00 h and 16:00 h on day 2. WT mice injected with vehicle, following the same protocol, served as controls. Forty-eight hours after beginning the priming, Gpr54 KO and WT male mice received a single icv bolus of vehicle or MT-II (1nmol/mouse), and blood samples were collected 15-min after the injection.
In Experiment 12, we studied the effect of MT-II on LH release after silencing ARC kisspeptin neurons by the use of Cre-dependent DREADDs (Designer Receptor Exclusively Activated by Designer Drugs). An adeno-associated virus (AAV) was used to target the pharmacogenetic activity-silencer receptor hM4D [38] to Kiss1 neurons at the ARC. In detail, AAV carrying Cre-dependent hM4D receptor (AAV8-hSyn-DIO-hM4D-(Gi)-mCherry; UNC Vector Core) were injected bilaterally in the ARC (from Bregma AP: −1.6 mm; L: ±0.2 mm; DV: −5.8 mm) of adult male Kiss1-Cre mice [30]. Animals with mistargeted injections of the AAV were used as controls. Following infection, hM4D receptors were activated by ip injection of its agonist, CNO. Mice were subjected to subsequent pharmacological tests, with intervals of four days, starting on week 2 after the AAV injection. In brief, on day 1, blood samples for hormone assays were obtained at basal conditions. On day 5, Kiss1-Cre mice received a single icv injection of MT-II (1nmol/mouse), and blood samples were collected 15-min after the injection. On day 9, animals received two ip injections of CNO (0.3 mg/kg), 30-min and 5-min prior icv administration of a single bolus of MT-II (1 nmol), and blood samples were collected 15-min after MT-II injection. Finally, on day 13, the animals received two ip injections of CNO (0.3 mg/kg), 30-min and 5-min before icv administration of vehicle, and blood samples were collected 15-min later. At the end of the experiment, mice were perfused and the brains were collected for immunohistochemical (IHC) analyses (see below).
Finally, in Experiment 13, electrophysiological analyses were undertaken in acute brain slices to determine the effect of MT-II on the spontaneous action potential firing of ARC Kiss1 neurons. Pre-pubertal (PND 23–26) and adult (2–3-month-old) female Kiss1-IRES-Cre+/−/ROSA26-CAGS-τGFP+/− (Kiss1-GFP) mice [31] were used in these studies. In adult mice, estrous cycle stage was determined by daily vaginal smear each morning, and mice with at least two regular 4- or 5-day estrous cycles were selected for studies conducted on diestrus. Absence of VO in PND23-26 females confirmed that these mice were pre-pubertal. Electrophysiological recordings were performed as described below.
2.4. Generation of a POMC neuron-specific Gpr54 null mouse line
To address the potential role of α-MSH signaling in transmitting kisspeptin effects, mice lacking Gpr54 specifically in POMC neurons were generated by crossing animals carrying Cre recombinase expression under the POMC promoter, the POMC-Cre+/− mouse [39], with mice with LoxP sites flanking the exon two of Gpr54 (Gpr54loxP/loxP mouse [40]). The well-characterized POMC-Cre mice ensure functional Cre recombinase expression in >90% of arcuate POMC neurons, while efficiency of the Gpr54loxP/loxP mouse to allow conditional, Cre-dependent ablation of the kisspeptin receptor is also well proven [29]. Thus, our approach guarantees that double mutant mice will lack Gpr54 expression on the vast majority of ARC POMC neurons.
POMC-Cre+/− mice were initially mated with Gpr54loxP/loxP animals. The resulting genotypes, POMC-Cre+/−; Gpr54loxP/−, were self-crossed to generate all the possible genotypic combinations. For the experiments, only POMC-Cre+/−; Gpr54loxP/loxP (referred as POMC-Gpr54 KO mouse) and POMC-Cre−/−; Gpr54loxP/loxP (referred as controls) were used.
Mice were genotyped using the following combination of primers for POMC-Cre: POMC-Cre-1, 5′-TGG CTC AAT GTC CTT CCT GG-3′; POMC-Cre-2, 5′-CAC ATA AGC TGC ATC GTT AAG-3′; POMC-Cre-3, 5′- GAG ATA TCT TTA ACC CTG ATC-3′ resulting in a band of 1400-bp for the endogenous allele POMC, and a band of 750-bp for the Cre allele. Determination of Gpr54 LoxP alleles was done using the following primers: pr13726, 5′- CTC CCA ATG TGA CCT GGT G-3′; pr14303 5′- CCT CGT GTC CCT CTG TGA TT-3′; pr15168, 5′- AAG ACC GGC TTA GGG AGA TG-3′ resulting in a 577-bp band for wild type, a 663-bp band for the floxed allele, and a 800-bp band following recombination. For further details, see Supplementary Figure S1.
2.5. Kiss1 riboprobe synthesis
For detection of Kiss1 mRNA by in situ hybridization, a specific riboprobe for Kiss1 rat mRNA, spanning 83–371 nt of the cDNA sequence (GenBank NM_181692.1) was generated. First, a DNA template was synthesized by PCR using specific primers for Kiss1 cDNA amplification carrying at their 5′-end sequences for synthetic promoters for bacteriophage-encoded DNA-dependent RNA polymerases (T7 and T3). Primer sequences were as follows: forward primer T3-Kiss sense (5′-CAGAGATGCAATTAACCCTCACTAAAGG GAGATGGTGAACCCTGAACCCACA-3′); reverse primer T7-Kiss as (5′-CCAAGCCTTCTAATACGACTC ACTATAGGGAGAACCTGCCTCCTGCCGTAGCG-3′). For PCR reactions, Go Taq flexi DNA polymerase (Promega Biotech) was used, following the recommendations of the manufacturer. Reactions were performed in an iCycler (Bio-Rad Laboratories, Inc.) using the following protocol: cDNA was denatured for 5 min at 95 °C, and then 4 cycles were performed at 94 °C for 1 min, 54 °C for 2 min and 72 °C for 30 s, followed by 35 cycles at 94 °C for 1 min, 65 °C for 1 min and 72 °C for 30 s. A final extension at 72 °C for 5 min was included. After electrophoresis on a 2% agarose (w/v) gel, a single DNA fragment was obtained of the expected size and gel purified with a QiaQuick gel extraction kit (Qiagen). For the generation of the antisense Kiss1 riboprobe, the product of the PCR was used as template for the transcription reaction, as follows (final volume of 20 μl): 250 Ci [33P]-UTP (Perkin Elmer, Massachusetts, USA), 0.5 μg of template, 2 μl 3NTPs (5 mM rATP, rCTP and rGTP), 1 μl RNasin Ribonuclease Inhibitor (Promega), 4 μl transcription buffer, and 2 μl T7 RNA polymerase (Promega). After 120 min of incubation at 37 °C, another 1 μl of T7 RNA was added to the mix, and the reaction was maintained for an additional 60 min at 37 °C. At the end, the residual DNA was digested with 2 U DNase (Promega), and the reaction was terminated by addition of 3 μl 0.5 M EDTA, pH 8.0. DEPC water was added to a final volume of 50 μl, and the labeled riboprobe was purified using illustra ProbeQuant G-50 Micro Columns (GE Healthcare, UK). For synthesis of sense Kiss1 riboprobe, the same procedure was applied, using T3 RNA polymerase (Promega).
2.6. In situ hybridization (ISH)
For analysis of the neuroanatomical distribution of Kiss1 mRNA expression in the hypothalamus, brains were collected from selected experimental groups and ISH analyses were performed, following previous protocols [32], [33]. Five sets of coronal 20-μm-thick sections were generated and mounted on SuperFrost Plus slides (Thermo Fisher Scientific Inc.). Standard procedures of tissue collection were applied, starting on a fixed coordinate in the rostral hypothalamic area up to ARC, to encompass equivalent areas including anterior and medial portions of this nucleus where Kiss1 neurons are abundantly located [34]. The samples were stored at −80 °C until ISH analyses.
A specific antisense riboprobe for Kiss1 rat mRNA was used (see details above). A single set of sections was used for ISH (adjacent sections 100-μm apart). These tissue sections were: (i) fixed in 4% PFA for 15 min; (ii) stabilized with 0.1 M phosphate buffer (pH 7.4) at room temperature for 10 min; (iii) treated with saline triethanolamine and acetic anhydride to prevent non-specific binding of probes; (iv) washed in 2× saline-sodium citrate (SSC) buffer for 3 min; (v) dehydrated in increasing concentrations of ethanol; (vi) de-lipidated with chloroform; and (vii) air dried at room temperature for 1 h. After these steps, hybridization with Kiss1 riboprobe was performed for 16 h at 55 °C. The hybridization buffer contained (for 40 ml): 25 ml deionized formamide, 10 ml dextran sulfate 50%, 3 ml NaCl 5 M, 0.4 ml Tris base 1M (pH 8), 0.08 ml 0.5M EDTA (pH 8), 1× Denhardt's solution and RNase-free water up to 40 ml Kiss1 riboprobe was added to the hybridization buffer to a final concentration of 0.03 pmol/ml along with yeast tRNA. After hybridization, slides were: (i) washed with 4× SSC for 30 min; (ii) incubated in RNase-A buffer (Roche Biochemical) at 37 °C (32 μg/ml) for 1 h; (iii) equilibrated with 2× SSC for 30 min; (iv) washed in 0.1× SSC for 1 h at 65 °C; (v) dehydrated in increasing ethanol series; and (vi) air dried at room temperature for 1 h. Finally, slides were dipped in Kodak Autoradiography Emulsion type NTB (Eastman Kodak) and exposed for 1 week at 4 °C in the dark. After this, the sections were developed and fixed following the manufacturer instructions (Kodak; Rochester, NY): (i) 4 min in Kodak Developer D-19; (ii) 10 s in distilled water; (iii) 5 min in Kodak Fixer; and (iv) 5 min in distilled water. For mounting, the sections were previously dehydrated and rinsed with Sub-X Clearing Medium (Leica Bio-systems). Then, slices were cover-slipped with Sub-X mounting medium (Leica). For analysis, 50–60 sections from each animal (9–10 slides; 6 sections/slide) were evaluated. Five animals per group were included in the analysis. Slides were read under dark-field illumination with custom-designed software enabled to count the total number of cells (grain clusters). Cells were counted as Kiss1 mRNA positive when the number of silver grains in a cluster exceeded that of background.
2.7. Brain preparation for immunohistochemistry
Mice were anesthetized with ketamine–xylazine and perfused intracardially with saline (0.9% NaCl) followed by 4% PFA in PBS (pH 7.4). Fixed brains were immersed in 30% sucrose and 0.01% sodium azide in PBS at 4 °C for 2–4 days. Next, 3 sets of coronal, 30 μm-thick sections were cut in a freezing microtome Leica CM1850 UV and stored at −20 °C in cryo-protectant. For immunodetection of the different proteins, one set of sections encompassing the whole hypothalamus was used from each animal, and standard procedures for single- or double-label immunohistochemistry was performed (see below).
2.8. Single-label immunohistochemistry
Brains from mice injected with AAV8-hSyn-DIO-hM4D(Gi)-mCherry were assessed for mCherry reporter expression to confirm bilateral infection of ARC Kiss1 neurons with the AVV carrying Cre-dependent hM4D receptor. One set of free-floating sections from each animal was: (i) washed (3 × 10 min) in Tris-buffered saline (TBS) (pH 7.6) at room temperature with gentle agitation; (ii) incubated with a primary rabbit polyclonal anti-mCherry antibody 1:1000 (ab167453, Abcam) at 4 °C for 48 h; (iii) washed (3 × 10 min) in TBS (pH 7.6) at room temperature; (iv) incubated with a secondary biotinilated Donkey Anti-Rabbit antibody 1:200 (JAC-711-066-152, Jackson Immunoresearch) at room temperature for 90 min; (v) washed (3 × 10 min) in TBS (pH 7.6); (vi) incubated with Texas Red Streptavidin 1:200 (SA-5006, Vector laboratories, Inc.) at room temperature for 90 min; and (vii) washed (3 × 10 min) in TBS (pH 7.6). Thereafter, brain sections were mounted on silane-coated slices, air dried, and cover-slipped with Vectashield HardSet Mounting Medium (Vector Laboratories). No signal was detected after applying secondary antibody in absence of primary antibody. Immunoreactivity was visualized in a fluorescence microscope Leica DM2500.
2.9. Double-label immunohistochemistry
For dual detection of POMC and kisspeptin immunoreactivity, one set of brain sections was mounted on SuperFrost Plus slides (VWR International). After drying for 1 h at 37 °C, sections were washed in PBS-T (0.1% Tween-20) for 10 min at room temperature then heated in 10 mM sodium citrate (pH 6) for 10 min at 90 °C to induce antigen retrieval. Afterwards, sections were cooled to room temperature and then washed 5 min in PBS. Sections were blocked in blocking buffer (3% Donkey Serum, 0.4% Triton X-100) in PBS for 45 min at room temperature. Next, sections were incubated overnight at 4 °C with primary sheep anti-rat/mouse kisspeptin antibody 1:10000 (AC #017; gift from Dr Alain Caraty, PRC-INRA, 37380 Nouzilly, France) and with primary rabbit anti-α-MSH antibody 1:1000 (T4434; Peninsula Laboratories International, Inc.). Then, sections were washed in PBS and incubated with secondary antibodies Alexa Fluor 488 Donkey Anti-Sheep 1:500 (A11015, Invitrogen) and Alexa Fluor 555 Donkey Anti-Rabbit 1:500 (A31572, Invitrogen), respectively, for 1 h at 37 °C. Slides were washed in PBS, immersed for 30 s in double distilled water to remove salts and cover-slipped with Vectashield HardSet Mounting Medium (Vector Laboratories). No signal was detected after applying secondary antibodies in the absence of the primary antibody. Immunoreactivity analyses were made on a Nikon A1R FLIM confocal system. Images were viewed and Z stacks condensed to 2D projections using NIS-Elements Viewer software.
2.10. Cell-attached recordings
Coronal brain slices (200 μm thick) including the ARC were cut with a vibratome (VT1000S; Leica) in an ice-cold solution containing (in mM): NaCl 87, KCl 2.5, NaHCO3 25, NaH2PO4 1.25, CaCl2 0.5, MgCl2 6, glucose 25 and sucrose 75. Slices were then incubated at 30 °C for at least 1 h in artificial cerebrospinal fluid (aCSF; in mM): NaCl 120, KCl 3, NaHCO3 26, NaH2PO4 1, CaCl2 2.5, MgCl2 1.2 and glucose 10. All solutions were equilibrated with 95%O2/5%CO2. Electrophysiological recordings of ARC Kiss-GFP neurons were carried out as described previously [41]. Briefly, slices were placed under an upright microscope fitted for epi-fluorescence (Olympus, Tokyo, Japan) and constantly perfused (1.5 ml/min) with warm (∼30 °C) aCSF. GFP-expressing neurons were first visualized by brief fluorescence illumination and subsequently approached using infrared differential interference contrast optics. Spontaneous firing was recorded in voltage clamp mode in the cell-attached configuration. Recording electrodes (3–5 MΩ) were filled with aCSF including 10 mM HEPES. Low resistance seals (10–30 MΩ) were achieved by applying the lowest amount of suction required to detect spontaneous spikes. Electrophysiological signals were recorded using a Multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA) connected to a Digidata 1440A digitizer (Molecular Devices). Signals were low-pass filtered at 3 kHz before being digitized at a rate of 10 kHz and stored on a personal computer. Signal acquisition was carried out with pClamp 10 (Molecular Devices). The spontaneous firing of ARC kisspeptin neurons was monitored in control aCSF for >5 min before MT II was applied in the bath for 1 or 5 min. In some experiments, the extracellular concentration of potassium ([K+]o) was increased by 10 mM to enhance spontaneous firing. Cells displaying no spontaneous firing were excluded from analysis if firing could not be evoked upon stimulation with 20 mM KCl or 30 μM (S)-AMPA. Spontaneous spikes were detected in pClamp 10. To determine the effect of MT II on ARC kisspeptin neuron action potential firing, average firing rates were compared over 1 min periods immediately before and 1–2 min following application of the drug. MT II was considered to have an effect if the firing rate changed by more than twice the standard deviation of the basal firing rate for a period longer than 1 min.
2.11. Hormone measurements
Serum LH levels in mice and rats were determined using double antibody method and RIA kits supplied by the National Institute of Health, NIH (Dr. Parlow, National Institute of Diabetes and Digestive and Kidney Diseases National Hormone and Peptide Program, Bethesda, MD, USA). Hormone provided by the NIH (LH-I-10) was labeled with 125I using Iodo-gen® method (Pierce, Rockford, IL). The hormone concentrations were expressed using reference preparations of LH-RP-3. The sensitivity of the assay was 75 pg/ml. Intra- and inter-assay coefficients of variation (CV) were lower than 8%. The reliability of the hormone determinations was confirmed by measurement of rat serum samples with known concentrations of hormone of interest. Serum leptin levels were measured using RIA kits from Linco Research (St. Charles, MO) following manufacturer indications; the sensitivity of the assays was 0.5 ng/ml.
2.12. Presentation of data and statistics
Data of BW, absolute and relative organ weights, ISH, hormonal levels, and cell-attached recording values are expressed as the mean ± SEM. Both time-course data and integral LH levels of different experimental groups relative to the control groups were estimated as area under the curve (AUC) by the trapezoidal rule. Hormonal determinations were conducted with a minimal total number of 7–12 determinations per group. ISH and IHC assays were conducted with a minimum of 5 animals/group. Results were analyzed using Student t test or ANOVA followed by Student–Newman–Keuls multiple range tests (Prism GraphPad 5.0 software; GraphPad Software Inc., La Jolla, CA). Significance level was set at P ≤ 0.05 and different letters or asterisks indicate statistical significance. For cell-attached recording, comparisons between groups were carried out using Wilcoxon or Mann–Whitney tests with Prism GraphPad Software as appropriate. The sample size used in tests was the number of neurons. Experiments were replicated in at least three different mice in each group, and up to three slices were used in each mouse. Differences were considered significant for P values ≤ 0.05.
3. Results
3.1. Central activation of α-MSH signaling stimulates LH secretion in pubertal but not infantile rats
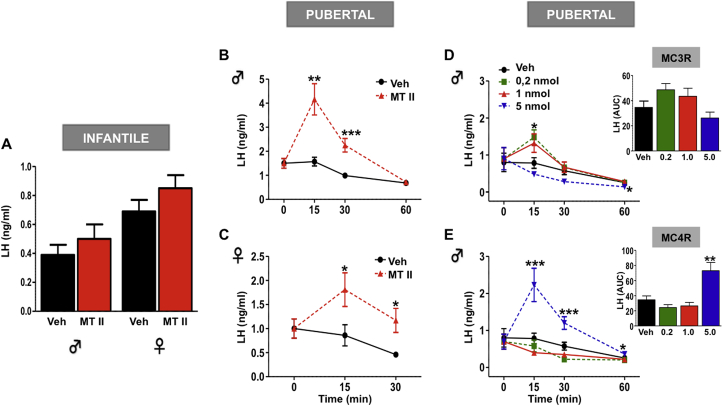
The central effects of α-MSH on the HPG axis were explored during the infantile–pubertal transition in rats by administering the MC3/4R agonist, MT-II, and monitoring LH responses as a surrogate marker of GnRH secretion. Intracerebroventricular (icv; 1 nmol/rat) injection of MT-II to infantile (PND-15) male (n = 10) and female (n = 10) rats failed to change circulating LH levels (Figure 1A). In contrast, icv administration of 1 nmol MT-II to peripubertal male (PND-43; n = 12) and female (PND-29; n = 10) rats evoked significant 2–3 fold increases in LH secretion at 15-min, which persisted for 30-min after injection (Figure 1B–C). In addition, the effects of MT-II were also tested in pubertal animals subjected to 48 h fasting, as this condition is known to suppress gonadotropic function. As expected, LH levels were significantly decreased in fasted male and female rats (n = 12 and 10/group, respectively). Nevertheless, icv MT-II continued to significantly increase LH levels in these pubertal fasted rats (Supplementary Figure S2).
Figure 1.
Central activation of α-MSH signaling, preferentially via MC4R, stimulates LH secretion in pubertal but not infantile rats. (A) The effects of intracerebroventricular (icv) injection of an effective dose (1 nmol) of the MC3/4R agonist MT-II on LH secretion in infantile (PND-15) male and female rats (n = 10/group) are shown. In addition, LH responses to MT-II injection (icv, 1 nmol) in pubertal male (Panel B; n = 12) and female (C; n = 10) rats are presented. (D, E) the effects on LH secretion of the icv injection of a range of doses of selective MC3R (upper) or MC4R (lower) agonists in pubertal male rats (n = 10/group) are depicted. In addition to time-course responses, integral LH responses, calculated as AUC over the 60-min period after injection, are shown in the insets. * P < 0.05, ** P < 0.01 and *** P < 0.001 vs. corresponding time-points in vehicle-injected animals (ANOVA followed by Student–Newman–Keuls multiple range test).
To examine the receptor subtype that mediates the above MT-II effects, we evaluated LH responses to different doses of the selective MC3R (D-Trp-γ MSH) or MC4R [Cyclo (β-Ala-His-d-Phe-Arg-Trp-Glu)-NH2] agonists in pubertal male rats. Both MC3R and MC4R agonists induced significant changes in LH release in a dose-dependent manner. Low doses (0.2- & 1-nmol; n = 10/group) of the MC3R agonist evoked significant (albeit modest) LH responses. In contrast, high doses (5-nmol; n = 10) of the MC4R agonist induced a significant increase in LH levels that was similar in magnitude to that evoked by MT-II (Figure 1D–E).
3.2. Central blockade of α-MSH signaling inhibits puberty onset and prevents the permissive effects of leptin
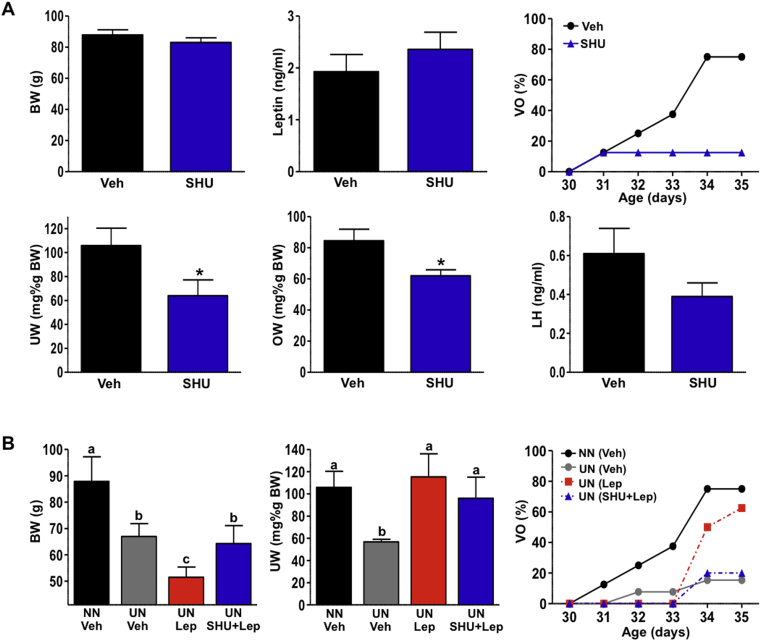
The impact of chronic blockade of central α-MSH signaling on puberty onset was evaluated in female rats by administering the pharmacological MC3/4R antagonist SHU9119 during the pubertal transition. Initial experiments demonstrated that repeated icv injection of SHU9119 (n = 10) caused an increase in daily food intake and body weight (Supplementary Figure S3), illustrating the necessity of endogenous central melanocortins for energy homeostasis during puberty. Thus, to avoid the possible confounding effects of BW changes on puberty onset, for further experiments, SHU9119-treated animals were pair-fed to control animals. Accordingly, no differences in body weight (BW) or circulating leptin levels were observed between both groups at PND-35. Yet, SHU9119 administration from PND-28 to PND-35 caused a marked delay in puberty onset with deferred occurrence of vaginal opening (VO; n = 10). Thus, while >75% of the control, vehicle-treated females displayed VO at PND-35, only <13% of SHU9119-treated animals showed complete VO at this age. This effect was associated with a significant reduction in uterine and ovarian weights, while LH levels also showed a non-significant tendency to be lower in the SHU9119 group (Figure 2A).
Figure 2.
Central blockade of α-MSH signaling inhibits puberty onset and prevents the permissive effects of leptin. (A) The effects of repeated intracerebroventricular (icv) injection of the MC3/4R antagonist SHU9119 or vehicle on pubertal progression in immature female rats (n = 10/group) are shown. Female rats were icv injected with 1 nmol SHU9119 every 12-h between PND-29 to PND-35. Evolution of vaginal opening (VO), as external signal of puberty, together with body weight and leptin levels, as well as uterus (UW) and ovarian (OW) weights and LH concentrations, in terminal (PND-35) samples are shown. (B) The effects of icv administration of repeated doses of leptin, alone or in combination with the MC3/4R antagonist SHU9119 in a rat model of moderate under-nutrition (UN) are displayed (n = 10–14/group). In detail, BW, UW, and VO data from pubertal UN rats treated with vehicle, leptin, or leptin + SHU9119 are shown. Data from age-matched female rats fed ad libitum (normal nutrition: NN) and injected with vehicle are shown for reference purposes (n = 9). For (A) * P < 0.05 vs. corresponding time-points in vehicle-injected animals (Student t-test); for (B) groups with different superscript letters are statistically different (P < 0.05; ANOVA followed by Student–Newman–Keuls multiple range test).
Based on the above data, we evaluated the possibility that central α-MSH signaling may mediate, at least in part, the effects of leptin on puberty onset. This experiment was conducted in a model of chronic, moderate under-nutrition (UN) from PND 23 onwards, in which endogenous leptin levels are decreased and puberty/VO delayed, so that the effects of replacement of leptin, as a permissive signal for puberty onset, could be detected. Food-restricted animals were subjected, from PND 29 to PND 35, to repeated central injections of vehicle or leptin, alone (n = 14 and 10, respectively) or in combination with SHU9119 (n = 10). Females fed ad libitum and chronically treated with vehicle served as controls (n = 9). Chronic UN induced a significant reduction of BW as well as relative uterine and ovarian weights, lowered circulating LH levels, and delayed puberty onset (Figure 2B; ovarian data are not shown). Administration of leptin to UN animals was able to rescue, to a large extent, the negative effect of subnutrition on pubertal timing (estimated by VO) and completely reversed the decrease in relative uterine weight, despite inducing further decreases in BW. Interestingly, concomitant blockade of central MC3/4R signaling prevented the rescue effect of leptin on the timing of VO, without affecting uterine weight (Figure 2B). No significant differences in terminal serum LH levels were detected among the UN groups (data not shown).
3.3. α-MSH signaling does not mediate the stimulatory effects of kisspeptin on puberty/gonadotropic axis
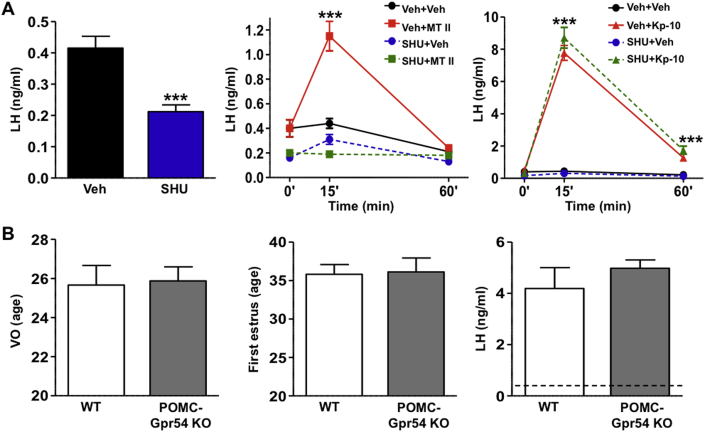
Considering the role of central α-MSH signaling in mediating effects of leptin on puberty onset (our initial data), potential interactions with the puberty-stimulating neuropeptide kisspeptin were explored. Based on fragmentary data from ovine and mouse studies, the hypothesis that kisspeptin might act, at least partially, via α-MSH pathways was initially tested in various rodent models. First, we examined the effect of kisspeptin-10 (Kp-10) on LH release after blockade of central MC3/4R signaling by SHU9119 in pubertal female rats. Central pre-administration of two boluses of SHU9119 caused a significant decrease of LH levels (n = 10), suggesting a role of endogenous α-MSH in the maintenance of basal LH secretion at puberty. However, while SHU9119 administration completely blocked the effect of MT-II on LH secretion (n = 10), blockade of α-MSH signaling did not affect LH responses to kisspeptin (n = 10) (Figure 3A).
Figure 3.
α-MSH signaling does not mediate the stimulatory effects of kisspeptin on puberty/gonadotropic axis. (A) The effects of intracerebroventricular (icv) injection of an effective dose (1 nmol) of the MC3/4R antagonist SHU9119 on basal LH secretion and stimulated responses to MT-II and Kp-10 are presented (n = 9–10/group). While SHU9119 decreased basal LH levels and fully prevented LH responses to the MC3/4R agonist MT-II in pubertal male rats, it did not affect LH responses to a submaximal (50 pmol) dose of Kp-10. (B) A summary of phenotypic and hormonal indices from a mouse model of congenital elimination of kisspeptin receptor Gpr54 from POMC neurons is shown. Neither VO (as external sign of puberty) nor the age of first estrus or the magnitude of LH responses to Kp-10 were altered in mice with congenital ablation of Gpr54 in POMC neurons. Note that basal LH levels were not altered and are denoted as dotted line (right panel). *** P < 0.001 vs. corresponding time-points in vehicle-injected animals (Student t-test or ANOVA followed by Student–Newman–Keuls multiple range test).
To further evaluate this possible kisspeptin→α-MSH pathway, we conducted phenotypic analyses of puberty and LH responses to kisspeptin in a novel mouse model with congenital ablation of the canonical kisspeptin receptor Gpr54 in POMC neurons. Details of generation of this mouse line can be found in the Methods section. POMC-Gpr54 null females failed to display overt alterations of BW postnatally and during the pubertal transition (n = 9). Similarly, pubertal timing, assessed by the occurrence of VO and the age of first estrus, as an additional sign of first ovulation, was not altered by congenital ablation of Gpr54 from POMC neurons (n = 9). Finally, LH responses to a bolus of Kp-10, which generated a >8-fold increase over basal levels, were similar between WT (n = 7) and conditional KO mice (n = 7) (Figure 3B). Together, these data suggest that α-MSH signaling is not likely to mediate the effects of kisspeptin on puberty onset and gonadotropin secretion.
3.4. Kisspeptin signaling is a major mediator for the stimulatory effects of α-MSH on puberty/gonadotropic axis
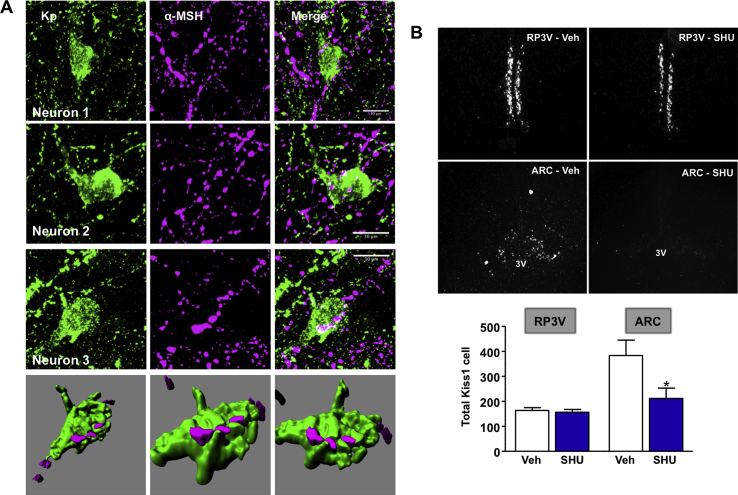
We next tested the hypothesis that α-MSH acts via kisspeptin pathways to modulate puberty and gonadotropin secretion. First, we performed immunohistochemical analyses to identify whether physical contacts between POMC and Kiss1 neurons exist in pubertal female rats (n = 4). As shown in Figure 4A, immunoreactive α-MSH fibers were identified in close apposition to Kiss1 cell bodies, specifically in the ARC of pubertal rats. This was a consistent observation across the different ARC sections containing immunoreactive Kiss1 neurons and the different individuals studied.
Figure 4.
Interplay between POMC/Kiss1 neurons and Kiss1 expression after blockade of α-MSH signaling. (A) Three representative examples are shown of immunohistochemical analyses in different sections of the putative interactions between MSH-positive fibers (in magenta) and kisspeptin (Kp)-positive cell bodies (in green) in the ARC of pubertal female rats. Reconstruction 3-D analysis of the particular example of Neuron-3 is shown at the bottom of panel A. (B) The effects of repeated intracerebroventricular (icv) injection of the MC3/4R antagonist SHU9119 on Kiss1 mRNA expression in the ARC and RP3V of pubertal female rats are presented. Female rats (n = 5) were icv injected with 1 nmol SHU9119 every 12-h between PND-28 to PND-32. Kiss1 expression was assessed by ISH; besides representative photomicrographs, quantitative data are presented in the lower panels. *P < 0.05 vs. corresponding values for each hypothalamic area in vehicle-injected animals (P < 0.05; Student t-test).
In addition, we studied the impact of chronic blockade of MC3/4R signaling during the period of pubertal transition (PND-28 to PND-32) on hypothalamic Kiss1 mRNA expression. To avoid the potential confounding factor of changes in circulating estrogen on Kiss1 expression, immature female rats were subjected to bilateral OVX and supplemented with moderate doses of E2 to mimic physiological levels. In addition, in order to avoid the possible influence of changes of BW on puberty onset, SHU9119-treated animals were pair-fed to control animals. In keeping with previous studies, two main hypothalamic populations of Kiss1-expressing neurons were detected in the ARC and RP3V. Chronic administration of SHU9119 induced a significant suppression (>45%) of Kiss1 mRNA levels in the ARC but not in the RP3V of peripubertal female rats (Figure 4B; n = 5/group).
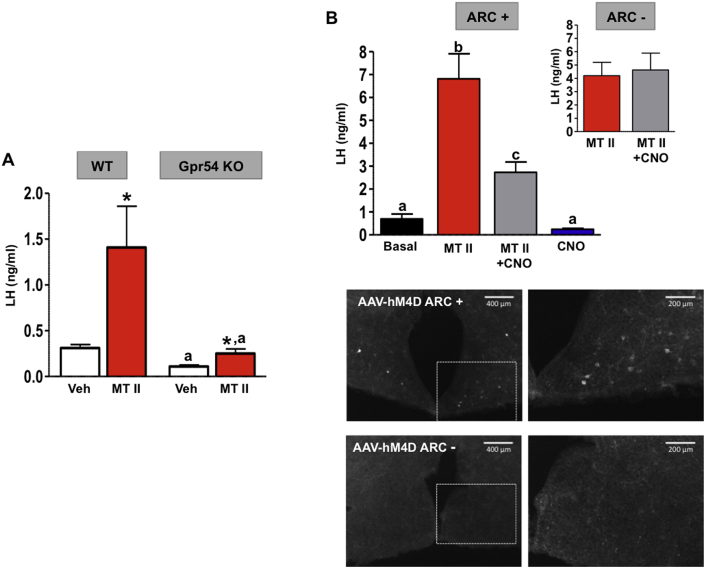
To further document this potential α-MSH→kisspeptin pathway, additional functional and electro-physiological analyses were conducted in different female rodent models. Thus, the capacity of α-MSH to elicit gonadotropic responses in the absence of kisspeptin signaling was evaluated by studying the effect of MT-II on LH secretion in a mouse line with congenital elimination of kisspeptin receptors, the Gpr54 KO mouse line. While WT animals displayed robust LH secretory responses to a single bolus of MT-II (n = 12), LH responses to MT-II were severely blunted, although not totally eliminated, in Gpr54-deficient mice (n = 7) (Figure 5A). Of note, Gpr54 null mice had been subjected to a protocol of GnRH priming to achieve a state of proper pituitary responsiveness, in line with a previous reference [29].
Figure 5.
Kisspeptin signaling is a major mediator for the stimulatory effects of α-MSH on LH secretion. (A) The effects of intracerebroventricular (icv) injection of an effective dose (1 nmol) of the MC3/4R agonist MT-II on LH secretion in WT mice and mice with congenital inactivation of the kisspeptin receptor Gpr54 are shown (n = 7–12/group). LH responses are presented from WT and KO animals, at 15-min after icv injection of MT-II. (B) The effects on LH secretion of the icv injection of MT-II (1-nmol) in mice with DREADD-mediated inhibition of Kiss1 neurons are shown. The first two bars correspond to control animals, infected with mock viral constructs; the following two bars correspond to mice effectively infected with hM4-constructs in the ARC, following receptor activation by CNO administration (n = 7/group). Responses in mice injected with vehicle or MT-II are shown. In addition, in the inset, the magnitude of LH responses to MT-II in animals without effective infection with hM4 in the ARC (in the presence and absence of CNO) is displayed (n = 7/group). In the lower panels, representative photomicrographs of mCherry immunohistochemistry in mice with effective (AAV-hM4D ARC+) or ineffective (AAV-hM4D ARC-) targeting of the ARC nucleus are presented. In panel A, *P < 0.05 vs. corresponding time-points in vehicle-injected animals; aP < 0.05 vs. corresponding WT values. Groups with different superscript letters are statistically different (ANOVA followed by Student–Newman–Keuls multiple range test).
We next tested the ability of α-MSH signaling to stimulate LH secretion after pharmacogenetic silencing of the ARC Kiss1 neurons using DREADD technology. For this purpose, mice with Kiss1-Cre dependent, viral-mediated over-expression of the pharmacogenetic Gi/o-coupled receptor hM4D were injected with an effective dose of MT-II (1 nmol; n = 7), following synaptic silencing of Kiss1 neurons by administration of the hM4D agonist, CNO (n = 7). In line with our initial results, in the absence of CNO, mice injected icv with MT-II displayed elevated levels of LH, 15-min after the injection. In contrast, in the presence of CNO, MT-II was found to be significantly less effective at stimulating LH secretion in mice with effective viral targeting of the ARC, as demonstrated by mCherry immunohistochemistry (Figure 5B). This inhibition was not observed in mice with ineffective hM4D expression in ARC Kiss1 neurons, as documented by the lack of detectable mCherry signal at this site (n = 7). Even assuming that the entire ARC Kiss1 neuronal population might not have been effectively infected with our viral approach (as suggested by the average number of mCherry-positive cells; for a representative example, see Figure 5B), altogether, the above findings strongly suggest that the stimulatory effects of α-MSH on puberty and LH secretion are critically dependent upon the activation of kisspeptin pathways. Of note, DREADD-mediated inhibition of Kiss1 neurons alone resulted in a non-significant tendency to lower basal LH levels (0.24 ± 0.04 vs. 0.77 ± 0.23 ng/ml in reference animals; P = 0.06; n = 7) in pubertal female mice.
Finally, electrophysiological studies were undertaken to examine whether α-MSH directly modulates the electrical excitability of ARC Kiss1 neurons. Female ARC Kiss1 neurons displayed low levels of spontaneous action potential firing with over half of the neurons being completely silent and no differences detected in firing rates between PND23-26 and adult diestrous mice. Because of the large proportion of silent cells, the extracellular [KCl] was elevated to 13 mM (normally 3 mM) in some experiments to evoke a low level of spontaneous activity. With one exception (Supplementary Figure S4E), 1–5 min bath applications of the MC3/4R agonist, MT-II (1 μM), did not change Kiss1 neuronal firing in PND23-26 (Supplementary Figure S4A,C; n = 13) or adult diestrous mice (Supplementary Figure S4B,D; n = 36), regardless of the extracellular [KCl].
4. Discussion
Melanocortin signaling has a key role in the central control of metabolism and energy homeostasis [42], [43]. In the present study, we examined whether the role of α-MSH in signaling leptin levels within the brain extended to the metabolic regulation of puberty onset. Using a variety of techniques in both rat and mouse models, we provide here the first evidence that the permissive influence of leptin on puberty onset is mediated, at least in part, by an α-MSH→Kiss1→GnRH neuron signaling pathway. Notably, while some studies had pharmacologically addressed the roles of α-MSH in the control of gonadotropin secretion in adulthood [44] and direct effects of α-MSH on GnRH neurons had been characterized in adult mice [45], the actual role of melanocortin signaling in metabolic regulation of puberty remained largely unexplored.
Our pharmacological experiments in immature rats, using acute LH responses as a surrogate marker of GnRH neuron activation, have demonstrated that the stimulatory actions of MC3/4R signaling on the gonadotropic axis develop with postnatal age, as potent LH responses to MT-II were detected in peri-pubertal but not in infantile rats. As robust LH responses to melanocortin stimulation were only observed with high doses of the selective MC4R agonist, it is possible that this receptor subtype is primarily responsible for mediating the major stimulatory actions of α-MSH on the gonadotropic axis at puberty. Nevertheless, modest but significant LH-releasing effects of the MC3R agonist were also detected, suggesting that both receptors may be involved. In fact, such differences in the dose-responsiveness to both receptor agonists might reflect a mechanism whereby the melanocortin system could provide a wider range of regulatory actions, depending on α-MSH concentrations, with stimulatory effects being mostly mediated via MC3R or MC4R at low or high concentrations, respectively. In any event, the physiological relevance of such melanocortin signaling was demonstrated by the fact that acute blockade of MC3/4R resulted in the significant lowering of basal LH levels. Furthermore, chronic inhibition of central melanocortin signaling during the pubertal transition resulted in a marked delay of puberty onset, as monitored by a combination of external and internal indices of pubertal maturation. These gain- and loss-of-function experiments are clearly indicative of a stimulatory role for α-MSH signaling in the regulation of puberty in rats. Central administration of the antagonist SHU9119 also caused a significant increase in daily food intake and body weight in pubertal female rats, indicating a dual role for endogenous melanocortin drive in the control of energy homeostasis and gonadotropin secretion during puberty. While similar weight promoting effects of SHU9119 had been previously reported in adult male rats, no discernible impact of central melanocortin blockade on adult gonadotropic function was detected [46], which might suggest that α-MSH signaling plays a selective stimulatory role during pubertal maturation.
Among the metabolic regulators of puberty, the adipocyte hormone leptin is known to play an essential permissive role [47]. The fact that central MC3/MC4 receptor activation could rescue the suppression of basal LH levels in pubertal animals subjected to fasting is compatible with a role of α-MSH signaling as transmitter for the pubertal/reproductive effects of leptin, as its levels are suppressed in conditions of negative energy balance linked to inhibition of reproduction, such as fasting. This was further confirmed by our physiological experiments in female rats that revealed that the permissive effects of leptin on puberty onset, as monitored by VO, could be prevented by the concomitant blockade of central α-MSH signaling. These data indicate that α-MSH is a major down-stream signal for mediating the central effects of leptin on pubertal awakening of the HPG axis. Intriguingly, the reproductive effects of leptin apparently were not blocked by central SHU9119 administration in adult male ob/ob mice [48], which again points to a sex- and/or pubertal-specific role of such leptin–melanocortin pathway in terms of reproductive control.
Fragmentary evidence, mainly from adults, has suggested the interplay between α-MSH and kisspeptin signaling. Our data demonstrate, however, that Kiss1 neurons do not regulate POMC neurons to modulate GnRH neuron activity at the time of puberty. Potent LH responses to even submaximal doses of Kp-10 were fully preserved following MC3/4R blockade, and kisspeptin influence upon pubertal timing and LH secretion was fully maintained in mice engineered to lack Gpr54 selectively in POMC neurons. These observations are in line with recent reports showing that direct kisspeptin actions on GnRH neurons seem to be sufficient to attain puberty [40], [49]. It is interesting to note a prior electrophysiological study that reported effects of kisspeptin on POMC neurons in the mouse [28]. The functional significance of this input is not known but, on the basis of our present experiments, it is unlikely to be involved in the regulation of GnRH neurosecretion and the gonadal axis. It remains possible that direct kisspeptin effects on POMC neurons might serve other functions, as these have been suggested to underlie its eventual anorectic effects [28], [50]. Yet, this also seems improbable as our mice with conditional ablation of Gpr54 from POMC neurons exhibit normal food intake and body weight gain.
In clear contrast, our combination of neuroanatomical, functional, and pharmacogenetic studies has unveiled an important melanocortin→kisspeptin→GnRH regulatory pathway involved in relaying leptin actions and regulating puberty onset. In this pathway, Kiss1 neurons appear to play a prominent role in transmitting the stimulatory effects of melanocortin signaling onto the reproductive centers, as documented by (i) the presence of appositions between α-MSH fibers and Kiss1 neuronal cell bodies in the ARC of pubertal female rats; (ii) the reduction of Kiss1 mRNA expression in the ARC of pubertal females subjected to chronic blockade of MC3/4R; i.e., a protocol that inhibits/delays puberty onset; (iii) the marked attenuation of LH responses to MT-II in mice with congenital inactivation of Gpr54; and, importantly, (iv) the lowering of LH responses to MT-II following DREADD-mediated inhibition of ARC Kiss1 neurons. Considering that most ARC POMC neurons are GABAergic, our data could explain, at least partially, recent findings showing that selective ablation of leptin receptors from all GABA neurons perturbs puberty onset and down-regulates ARC Kiss1 expression in mice [51], [52].
To our knowledge, this is the first report assessing physiological aspects of Kiss1 neuronal function using DREADDs. Interestingly, we noted a trend for suppressed basal LH levels following CNO administration in pubertal female mice with hM4D over-expression in ARC Kiss1 neurons. Although not significant, this observation supports that ARC Kiss1 neurons may be involved in the maintenance of basal gonadotropin secretion at puberty [31]. Moreover, the unambiguous suppression of LH responses to MT-II after DREADD-mediated inhibition of ARC Kiss1 neurons clearly supports the relevance of melanocortin regulation of kisspeptin output for mediating α-MSH actions on GnRH neurons at puberty.
While the cellular mechanisms underlying the potent effects of kisspeptin on GnRH neurons are becoming increasingly well characterized [53], the precise mode of action whereby α-MSH modulates Kiss1 neurons remains unknown. Interestingly, we found that α-MSH did not exert any detectable effects on the firing of ARC Kiss1 neurons, suggesting either that it acts indirectly or that α-MSH regulates ARC Kiss1 neurons without impacting upon their firing rate. Of note, activation of metabotropic pathways or changes in intracellular signaling cascades in Kiss1 neurons might not alter their electrical activity; for instance, neurokinin B can modulate intracellular calcium levels in ARC Kiss1 neurons independent of action potential firing (De Croft & Herbison, unpublished). In addition, our data could be suggestive of the involvement of additional intermediary pathways connecting α-MSH and Kiss1 neurons, which might not be preserved intact in our electrophysiological brain slice preparation. One tenable candidate for such intermediary elements might be nitric oxide (NO) producing neurons, as neurons expressing NO synthase (nNOS) have been recently reported to participate in mediating the permissive effects of leptin on puberty onset in mice [54], and synaptic contacts have been demonstrated between POMC and nNOS neurons [55].
Our findings also suggest a precise maturational program for the melanocortin regulation of the gonadotropic axis. The lack of stimulatory effects of the agonist, MT-II, in infantile rats is in clear contrast to the potent gonadotropin-releasing effects of kisspeptin detected in male and female rats at the same age [56]. This implies that, although the capacity of kisspeptins to activate GnRH neurons is achieved at very early developmental stages, key upstream activators of endogenous kisspeptin pathways such as α-MSH would become operational only at later maturational periods coinciding with pubertal activation. Interestingly, the switch between absent and detectable LH responses to MT-II coincides with the pattern of developmental maturation of Kiss1 neurons, at both ARC and RP3V nuclei, reported in rodents during postnatal maturation [57], [58], [59]. While, as a whole, our findings point to a predominant role of ARC Kiss1 neurons in mediating α-MSH actions on pubertal onset, the eventual contribution of RP3V Kiss1 neurons in this phenomenon cannot be discarded and merits further investigation.
It must be noted that, although severely blunted, detectable LH responses to the MC3/4R agonist were observed in Gpr54 null mice. This suggests that part of the stimulatory effects of α-MSH on GnRH secretion could be independent of kisspeptin signaling. Indeed, direct electrical responses to α-MSH and MT-II have been recorded in GnRH neurons [23], [45]. Our present findings do not refute but rather are compatible with such direct effects. Indeed, the diversity of sites of action indicated by our data, via direct and indirect pathways converging on GnRH neurons, suggests that central melanocortin signaling serves an important evolutionary mechanism that ensures the supply of relevant metabolic information to the reproductive centers in order to exquisitely fit reproductive maturation and fertility with optimal metabolic conditions.
Mice with genetic inactivation of MC3R or MC4R display a variable degree of fertility problems: MC3R KO female mice are sub-fertile, while MC4R null mice, although fertile, are poor breeders and show decreased ovulatory rates [60]. These observations suggest that although α-MSH signaling might be dispensable to achieve reproductive competence, it actually plays a discernible role in the control of some functional aspects of the HPG axis. It must be stressed, though, that detailed characterizations of puberty were not conducted in those mouse lines and, as congenital KO models, they may suffer from developmental compensation, a possibility that is specially tenable in partially redundant systems, such as MC-Rs, when only one element of the family (MC3R or MC4R) is ablated. Notably, mice with functional blockade of both MC3R and MC4R, by over-expression of the agouti protein have been shown to be infertile [22], whereas ablation of AgRP rescues fertility in leptin receptor deficient, db/db mice [23].
5. Conclusions
Our present data in wild type and genetically modified rodent models provide a novel mechanistic insight into the melanocortin regulation of pubertal maturation in the female by documenting a discernible leptin→α-MSH→Kiss1→GnRH pathway, which appears to play an important role in the metabolic control of puberty.
Author contributions
M.M.-L. and J.R. conducted the experiments, evaluated the data, and wrote the manuscript; F.R.-P., D.G.-G., R.P., A.Z., S.L., M.A.S.-G., A.R.-R. and M.J.V. conducted parts of the experiments and evaluated the data; M.M.-L., J.R., C.D. and L.P. participated in the design and/or conducted the experiment and preparation of the manuscript; R.P. and A.E.H. conducted the electrophysiological analyses and revised the manuscript; M.T.-S. designed the study, revised and analyzed the data, and wrote the manuscript. M.T.-S. takes full responsibility for the work as a whole.
Acknowledgements
This work was supported by grants BFU2011-025021 & BFU2014-57581-P (Ministerio de Economía y Competitividad, Spain; co-funded with EU funds from FEDER Program); project PIE-00005 (Flexi-Met, Instituto de Salud Carlos III, Ministerio de Sanidad, Spain); Projects P08-CVI-03788 and P12-FQM-01943 (Junta de Andalucía, Spain); EU research contract DEER FP7-ENV-2007-1 and the New Zealand Health Research Council. CIBER Fisiopatología de la Obesidad y Nutrición is an initiative of Instituto de Salud Carlos III. Senior authors are indebted with Dr. R.A. Steiner (University of Washington, Seattle, USA) and Dr. U. Boehm (University of Saarland School of Medicine, Homburg, Germany) for provision of relevant mouse lines, essential for conduction of some of the experiments included in this study.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molmet.2016.08.003.
Contributor Information
Juan Roa, Email: roarivas@gmail.com.
Manuel Tena-Sempere, Email: fi1tesem@uco.es.
Disclosure statement
The authors have nothing to disclose in relation to the contents of this work.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Parent A.S., Teilmann G., Juul A., Skakkebaek N.E., Toppari J., Bourguignon J.P. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocrine Reviews. 2003;24:668–693. doi: 10.1210/er.2002-0019. [DOI] [PubMed] [Google Scholar]
- 2.Aksglaede L., Juul A., Olsen L.W., Sorensen T.I. Age at puberty and the emerging obesity epidemic. PLoS One. 2009;4:e8450. doi: 10.1371/journal.pone.0008450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Leonibus C., Marcovecchio M.L., Chiavaroli V., de Giorgis T., Chiarelli F., Mohn A. Timing of puberty and physical growth in obese children: a longitudinal study in boys and girls. Pediatric Obesity. 2013;9:292–299. doi: 10.1111/j.2047-6310.2013.00176.x. [DOI] [PubMed] [Google Scholar]
- 4.Day F.R., Elks C.E., Murray A., Ong K.K., Perry J.R. Puberty timing associated with diabetes, cardiovascular disease and also diverse health outcomes in men and women: the UK Biobank study. Scientific Reports. 2015;5:11208. doi: 10.1038/srep11208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lakshman R., Forouhi N.G., Sharp S.J., Luben R., Bingham S.A., Khaw K.T. Early age at menarche associated with cardiovascular disease and mortality. The Journal of Clinical Endocrinology and Metabolism. 2009;94:4953–4960. doi: 10.1210/jc.2009-1789. [DOI] [PubMed] [Google Scholar]
- 6.Pinilla L., Aguilar E., Dieguez C., Millar R.P., Tena-Sempere M. Kisspeptins and reproduction: physiological roles and regulatory mechanisms. Physiological Reviews. 2012;92:1235–1316. doi: 10.1152/physrev.00037.2010. [DOI] [PubMed] [Google Scholar]
- 7.Roa J. Role of GnRH neurons and their neuronal afferents as key integrators between food intake regulatory signals and the control of reproduction. International Journal of Endocrinology. 2013;2013:518046. doi: 10.1155/2013/518046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elias C.F. Leptin action in pubertal development: recent advances and unanswered questions. Trends in Endocrinology and Metabolism. 2012;23:9–15. doi: 10.1016/j.tem.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quennell J.H., Mulligan A.C., Tups A., Liu X., Phipps S.J., Kemp C.J. Leptin indirectly regulates gonadotropin-releasing hormone neuronal function. Endocrinology. 2009;150:2805–2812. doi: 10.1210/en.2008-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seminara S.B., Messager S., Chatzidaki E.E., Thresher R.R., Acierno J.S., Jr., Shagoury The GPR54 gene as a regulator of puberty. New England Journal of Medicine. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 11.de Roux N., Genin E., Carel J.C., Matsuda F., Chaussain J.L., Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clarkson J., Han S.K., Liu X., Lee K., Herbison A.E. Neurobiological mechanisms underlying kisspeptin activation of gonadotropin-releasing hormone (GnRH) neurons at puberty. Molecular and Cellular Endocrinology. 2010;324:45–50. doi: 10.1016/j.mce.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 13.Navarro V.M., Tena-Sempere M. Neuroendocrine control by kisspeptins: role in metabolic regulation of fertility. Nature Reviews Endocrinology. 2012;8:40–53. doi: 10.1038/nrendo.2011.147. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez-Garrido M.A., Ruiz-Pino F., Manfredi-Lozano M., Leon S., Garcia-Galiano D., Castano J.P. Obesity-induced hypogonadism in the male: premature reproductive neuroendocrine senescence and contribution of Kiss1-mediated mechanisms. Endocrinology. 2014;155:1067–1079. doi: 10.1210/en.2013-1584. [DOI] [PubMed] [Google Scholar]
- 15.Donato J., Jr., Cravo R.M., Frazao R., Gautron L., Scott M.M., Lachey J. Leptin's effect on puberty in mice is relayed by the ventral premammillary nucleus and does not require signaling in Kiss1 neurons. Journal of Clinical Investigation. 2011;121:355–368. doi: 10.1172/JCI45106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Y., Faulkner L.D., Hill J.W. Cross-talk between metabolism and reproduction: the role of POMC and SF1 neurons. Frontiers in Endocrinology. 2012;2:98. doi: 10.3389/fendo.2011.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dores R.M., Londraville R.L., Prokop J., Davis P., Dewey N., Lesinski N. Molecular evolution of GPCRs: melanocortin/melanocortin receptors. Journal of Molecular Endocrinology. 2014;52:T29–T42. doi: 10.1530/JME-14-0050. [DOI] [PubMed] [Google Scholar]
- 18.Wilson J.L., Enriori P.J. A talk between fat tissue, gut, pancreas and brain to control body weight. Molecular and Cellular Endocrinology. 2015;418:108–119. doi: 10.1016/j.mce.2015.08.022. [DOI] [PubMed] [Google Scholar]
- 19.Elias C.F., Purohit D. Leptin signaling and circuits in puberty and fertility. Cellular and Molecular Life Sciences. 2012;70:841–862. doi: 10.1007/s00018-012-1095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huszar D., Lynch C.A., Fairchild-Huntress V., Dunmore J.H., Fang Q., Berkemeier L.R. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 21.Butler A.A., Cone R.D. The melanocortin receptors: lessons from knockout models. Neuropeptides. 2002;36:77–84. doi: 10.1054/npep.2002.0890. [DOI] [PubMed] [Google Scholar]
- 22.Granholm N.H., Jeppesen K.W., Japs R.A. Progressive infertility in female lethal yellow mice (Ay/a; strain C57BL/6J) Journal of Reproduction and Fertility. 1986;76:279–287. doi: 10.1530/jrf.0.0760279. [DOI] [PubMed] [Google Scholar]
- 23.Israel D.D., Sheffer-Babila S., de Luca C., Jo Y.H., Liu S.M., Xia Q. Effects of leptin and melanocortin signaling interactions on pubertal development and reproduction. Endocrinology. 2012;153:2408–2419. doi: 10.1210/en.2011-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheffer-Babila S., Sun Y., Israel D.D., Liu S.M., Neal-Perry G., Chua S.C., Jr. Agouti-related peptide plays a critical role in leptin's effects on female puberty and reproduction. American Journal of Physiology. Endocrinology and Metabolism. 2013;305:E1512–E1520. doi: 10.1152/ajpendo.00241.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Backholer K., Smith J., Clarke I.J. Melanocortins may stimulate reproduction by activating orexin neurons in the dorsomedial hypothalamus and kisspeptin neurons in the preoptic area of the ewe. Endocrinology. 2009;150:5488–5497. doi: 10.1210/en.2009-0604. [DOI] [PubMed] [Google Scholar]
- 26.Cravo R.M., Margatho L.O., Osborne-Lawrence S., Donato J., Jr., Atkin S., Bookout A.L. Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience. 2011;173:37–56. doi: 10.1016/j.neuroscience.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Backholer K., Smith J.T., Rao A., Pereira A., Iqbal J., Ogawa S. Kisspeptin cells in the ewe brain respond to leptin and communicate with neuropeptide Y and proopiomelanocortin cells. Endocrinology. 2010;151:2233–2243. doi: 10.1210/en.2009-1190. [DOI] [PubMed] [Google Scholar]
- 28.Fu L.Y., van den Pol A.N. Kisspeptin directly excites anorexigenic proopiomelanocortin neurons but inhibits orexigenic neuropeptide Y cells by an indirect synaptic mechanism. The Journal of Neuroscience. 2010;30:10205–10219. doi: 10.1523/JNEUROSCI.2098-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia-Galiano D., van Ingen Schenau D., Leon S., Krajnc-Franken M.A., Manfredi-Lozano M., Romero-Ruiz A. Kisspeptin signaling is indispensable for neurokinin B, but not glutamate, stimulation of gonadotropin secretion in mice. Endocrinology. 2012;153:316–328. doi: 10.1210/en.2011-1260. [DOI] [PubMed] [Google Scholar]
- 30.Gottsch M.L., Popa S.M., Lawhorn J.K., Qiu J., Tonsfeldt K.J., Bosch M.A. Molecular properties of Kiss1 neurons in the arcuate nucleus of the mouse. Endocrinology. 2011;152:4298–4309. doi: 10.1210/en.2011-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mayer C., Acosta-Martinez M., Dubois S.L., Wolfe A., Radovick S., Boehm U. Timing and completion of puberty in female mice depend on estrogen receptor alpha-signaling in kisspeptin neurons. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:22693–22698. doi: 10.1073/pnas.1012406108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roa J., Garcia-Galiano D., Varela L., Sanchez-Garrido M.A., Pineda R., Castellano J.M. The mammalian target of rapamycin as novel central regulator of puberty onset via modulation of hypothalamic Kiss1 system. Endocrinology. 2009;150:5016–5026. doi: 10.1210/en.2009-0096. [DOI] [PubMed] [Google Scholar]
- 33.Navarro V.M., Ruiz-Pino F., Sanchez-Garrido M.A., Garcia-Galiano D., Hobbs S.J., Manfredi-Lozano M. Role of neurokinin B in the control of female puberty and its modulation by metabolic status. The Journal of Neuroscience. 2012;32:2388–2397. doi: 10.1523/JNEUROSCI.4288-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paxinos G., Franklin K.B.J. Vol. 2. Academic Press; San Diego: 2001. The mouse brain in stereotaxic coordinates. [Google Scholar]
- 35.Watanobe H., Yoneda M., Kakizaki Y., Kohsaka A., Suda T., Schioth H.B. Further evidence for a significant participation of the melanocortin 4 receptor in the preovulatory prolactin surge in the rat. Brain Research Bulletin. 2001;54:521–525. doi: 10.1016/s0361-9230(01)00442-7. [DOI] [PubMed] [Google Scholar]
- 36.Watanobe H., Schioth H.B., Wikberg J.E., Suda T. The melanocortin 4 receptor mediates leptin stimulation of luteinizing hormone and prolactin surges in steroid-primed ovariectomized rats. Biochemical and Biophysical Research. 1999;257:860–864. doi: 10.1006/bbrc.1999.0547. [DOI] [PubMed] [Google Scholar]
- 37.Garcia-Galiano D., Pinilla L., Tena-Sempere M. Sex steroids and the control of the Kiss1 system: developmental roles and major regulatory actions. Journal of Neuroendocrinology. 2012;24:22–33. doi: 10.1111/j.1365-2826.2011.02230.x. [DOI] [PubMed] [Google Scholar]
- 38.Armbruster B.N., Li X., Pausch M.H., Herlitze S., Roth B.L. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Balthasar N., Coppari R., McMinn J., Liu S.M., Lee C.E., Tang V. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 40.Kirilov M., Clarkson J., Liu X., Roa J., Campos P., Porteous R. Dependence of fertility on kisspeptin-Gpr54 signaling at the GnRH neuron. Nature Communications. 2013;4:2492. doi: 10.1038/ncomms3492. [DOI] [PubMed] [Google Scholar]
- 41.de Croft S., Piet R., Mayer C., Mai O., Boehm U., Herbison A.E. Spontaneous kisspeptin neuron firing in the adult mouse reveals marked sex and brain region differences but no support for a direct role in negative feedback. Endocrinology. 2012;153:5384–5393. doi: 10.1210/en.2012-1616. [DOI] [PubMed] [Google Scholar]
- 42.Garfield A.S., Lam D.D., Marston O.J., Przydzial M.J., Heisler L.K. Role of central melanocortin pathways in energy homeostasis. Trends in Endocrinology and Metabolism. 2009;20:203–215. doi: 10.1016/j.tem.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 43.Corander M.P., Coll A.P. Melanocortins and body weight regulation: glucocorticoids, Agouti-related protein and beyond. European Journal of Pharmacology. 2011;660:111–118. doi: 10.1016/j.ejphar.2010.10.103. [DOI] [PubMed] [Google Scholar]
- 44.Reid R.L., Ling N., Yen S.S. Gonadotropin-releasing activity of alpha-melanocyte-stimulating hormone in normal subjects and in subjects with hypothalamic-pituitary dysfunction. The Journal of Clinical Endocrinology and Metabolism. 1984;58:773–777. doi: 10.1210/jcem-58-5-773. [DOI] [PubMed] [Google Scholar]
- 45.Roa J., Herbison A.E. Direct regulation of GnRH neuron excitability by arcuate nucleus POMC and NPY neuron neuropeptides in female mice. Endocrinology. 2012;153:5587–5599. doi: 10.1210/en.2012-1470. [DOI] [PubMed] [Google Scholar]
- 46.Raposinho P.D., Castillo E., d'Alleves V., Broqua P., Pralong F.P., Aubert M.L. Chronic blockade of the melanocortin 4 receptor subtype leads to obesity independently of neuropeptide Y action, with no adverse effects on the gonadotropic and somatotropic axes. Endocrinology. 2000;141:4419–4427. doi: 10.1210/endo.141.12.7842. [DOI] [PubMed] [Google Scholar]
- 47.Roa J., Tena-Sempere M. Connecting metabolism and reproduction: roles of central energy sensors and key molecular mediators. Molecular and Cellular Endocrinology. 2014;397:4–14. doi: 10.1016/j.mce.2014.09.027. [DOI] [PubMed] [Google Scholar]
- 48.Hohmann J.G., Teal T.H., Clifton D.K., Davis J., Hruby V.J., Han G. Differential role of melanocortins in mediating leptin's central effects on feeding and reproduction. The American Journal of Physiology Regulatory Integrative Comparative Physiology. 2000;278:R50–R59. doi: 10.1152/ajpregu.2000.278.1.R50. [DOI] [PubMed] [Google Scholar]
- 49.Leon S., Barroso A., Vazquez M.J., Garcia-Galiano D., Manfredi-Lozano M., Ruiz-Pino F. Direct actions of kisspeptins on GnRH neurons permit attainment of fertility but are insufficient to fully preserve gonadotropic axis activity. Scientific Reports. 2016;6:19206. doi: 10.1038/srep19206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stengel A., Wang L., Goebel-Stengel M., Tache Y. Centrally injected kisspeptin reduces food intake by increasing meal intervals in mice. Neuroreport. 2011;22:253–257. doi: 10.1097/WNR.0b013e32834558df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martin C., Navarro V.M., Simavli S., Vong L., Carroll R.S., Lowell B.B. Leptin-responsive GABAergic neurons regulate fertility through pathways that result in reduced kisspeptinergic tone. The Journal of Neuroscience. 2014;34:6047–6056. doi: 10.1523/JNEUROSCI.3003-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zuure W.A., Roberts A.L., Quennell J.H., Anderson G.M. Leptin signaling in GABA neurons, but not glutamate neurons, is required for reproductive function. The Journal of Neuroscience. 2013;33:17874–17883. doi: 10.1523/JNEUROSCI.2278-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Piet R., de Croft S., Liu X., Herbison A.E. Electrical properties of kisspeptin neurons and their regulation of GnRH neurons. Frontiers in Neuroendocrinology. 2015;36:15–27. doi: 10.1016/j.yfrne.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 54.Bellefontaine N., Chachlaki K., Parkash J., Vanacker C., Colledge W., d'Anglemont de Tassigny X. Leptin-dependent neuronal NO signaling in the preoptic hypothalamus facilitates reproduction. Journal of Clinical Investigation. 2014;124:2550–2559. doi: 10.1172/JCI65928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pu S., Horvath T.L., Diano S., Naftolin F., Kalra P.S., Kalra S.P. Evidence showing that beta-endorphin regulates cyclic guanosine 3′,5′-monophosphate (cGMP) efflux: anatomical and functional support for an interaction between opiates and nitric oxide. Endocrinology. 1997;138:1537–1543. doi: 10.1210/endo.138.4.5086. [DOI] [PubMed] [Google Scholar]
- 56.Castellano J.M., Navarro V.M., Fernandez-Fernandez R., Castano J.P., Malagon M.M., Aguilar E. Ontogeny and mechanisms of action for the stimulatory effect of kisspeptin on gonadotropin-releasing hormone system of the rat. Molecular and Cellular Endocrinology. 2006;257–258:75–83. doi: 10.1016/j.mce.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 57.Overgaard A., Tena-Sempere M., Franceschini I., Desroziers E., Simonneaux V., Mikkelsen J.D. Comparative analysis of kisspeptin-immunoreactivity reveals genuine differences in the hypothalamic Kiss1 systems between rats and mice. Peptides. 2013;45:85–90. doi: 10.1016/j.peptides.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 58.Clarkson J., Herbison A.E. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147:5817–5825. doi: 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clarkson J., Boon W.C., Simpson E.R., Herbison A.E. Postnatal development of an estradiol-kisspeptin positive feedback mechanism implicated in puberty onset. Endocrinology. 2009;150:3214–3220. doi: 10.1210/en.2008-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sandrock M., Schulz A., Merkwitz C., Schoneberg T., Spanel-Borowski K., Ricken A. Reduction in corpora lutea number in obese melanocortin-4-receptor-deficient mice. Reproductive Biology and Endocrinology. 2009;7:24. doi: 10.1186/1477-7827-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.