Abstract
Objective
A major challenge for obesity treatment is the maintenance of reduced body weight. Diet-induced obese mice are resistant to achieving normoweight once the obesogenic conditions are reversed, in part because lowered circulating leptin leads to a reduction in metabolic rate and a rebound of hyperphagia that defend the previously elevated body weight set point. Because hypothalamic POMC is a central leptin target, we investigated whether changes in circulating leptin modify Pomc expression to maintain normal energy balance in genetically predisposed obese mice.
Methods
Mice with reversible Pomc silencing in the arcuate nucleus (ArcPomc−/−) become morbidly obese eating low-fat chow. We measured body composition, food intake, plasma leptin, and leptin sensitivity in ArcPomc−/− mice weight-matched to littermate controls by calorie restriction, either from weaning or after developing obesity. Pomc was reactivated by tamoxifen-dependent Cre recombinase transgenes. Long acting PASylated leptin was administered to weight-reduced ArcPomc−/− mice to mimic the super-elevated leptin levels of obese mice.
Results
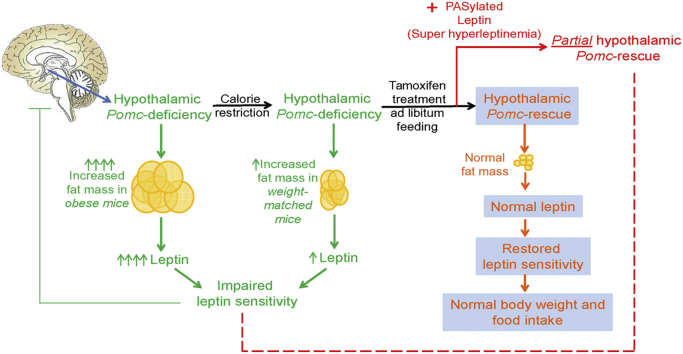
ArcPomc−/− mice had increased adiposity and leptin levels shortly after weaning. Despite chronic calorie restriction to achieve normoweight, ArcPomc−/− mice remained moderately hyperleptinemic and resistant to exogenous leptin's effects to reduce weight and food intake. However, subsequent Pomc reactivation in weight-matched ArcPomc−/− mice normalized plasma leptin, leptin sensitivity, adiposity, and food intake. In contrast, extreme hyperleptinemia induced by PASylated leptin blocked the full restoration of hypothalamic Pomc expression in calorie restricted ArcPomc−/− mice, which consequently regained 30% of their lost body weight and attained a metabolic steady state similar to that of tamoxifen treated obese ArcPomc−/− mice.
Conclusions
Pomc reactivation in previously obese, calorie-restricted ArcPomc−/− mice normalized energy homeostasis, suggesting that their body weight set point was restored to control levels. In contrast, massively obese and hyperleptinemic ArcPomc−/− mice or those weight-matched and treated with PASylated leptin to maintain extreme hyperleptinemia prior to Pomc reactivation converged to an intermediate set point relative to lean control and obese ArcPomc−/− mice. We conclude that restoration of hypothalamic leptin sensitivity and Pomc expression is necessary for obese ArcPomc−/− mice to achieve and sustain normal metabolic homeostasis; whereas deficits in either parameter set a maladaptive allostatic balance that defends increased adiposity and body weight.
Keywords: Body weight set point, Hypothalamus, Leptin, Leptin resistance, Obesity, PASylation, POMC
Graphical abstract
Highlights
-
•
Hypothalamic POMC-deficiency increases adiposity and induces leptin resistance.
-
•
PASylated leptin blocks the normalization of Pomc expression, weight and adiposity.
-
•
Interactions of leptin sensitivity and Pomc expression dictate body weight set point.
1. Introduction
Weight loss in obese humans can be reliably attained by a sufficient reduction in calorie intake below that of calorie expenditure, with or without concomitant increased physical activity. However, significant weight regain over subsequent years is nearly universal [1]. The inability to maintain weight loss has been attributed to multiple factors including reductions in circulating leptin, but regardless of the precise mechanism this phenomenon has prompted vigorous debate over the concept of altered body weight set point and alternatives to dieting for sustained weight loss, including bariatric surgery and an exploration of polypharmaceutical treatment approaches.
The role of leptin in human energy balance and evidence supporting its major function to signal negative energy balance when levels are low has been reviewed comprehensively by Rosenbaum and Leibel [2]. Many clinical studies [3], [4], [5], [6], [7], [8], but not all [9], support the conclusion that weight loss induced by dieting results in reduced circulating leptin associated with decreased satiation and lower than predicted metabolic rate that, at least in part, contribute to the high recidivism rate for weight regain. Although it is well established that leptin treatment is not an effective measure to induce weight loss in patients with common obesity, it may be beneficial in helping to maintain established weight loss following dieting [2] and recent studies suggest that leptin sensitizing agents may be key to employing leptin pharmacotherapy [10].
Diet-induced obesity (DIO) in mice has been employed frequently as a model with high face value for polygenic obesity in humans. DIO C57BL/6J mice switched to low fat chow exhibit significant weight loss but eventually defend a higher body weight and adiposity index than control mice that never were exposed to the high fat diet [11]. This elevated body weight set point may be due to relative hypometabolism, as found in humans, based on indirect calorimetry measurements [12]. Furthermore, DIO mice whose body weight is reduced to that of control mice by calorie restriction regain more weight when subsequently provided ad libitum access to low fat chow than those pair-fed to control mice that had always been fed low fat chow [13]. Another study using DIO C57BL/6J mice, with or without calorie restriction to reduce their body weight below that of sham controls prior to Roux-En-Y gastric bypass (RYGB) surgery, provides evidence for bidirectional defense of body weight set point [14]. Although the precise physiological mechanism was not determined, the final settling points for body weight and adiposity index were strongly correlated with pre-surgical values of the same parameters in each mouse. The previously obese mice that were calorie restricted prior to RYGB surgery actually regained weight and fat mass to final levels that were almost the same as those of the DIO mice without calorie restriction. Chronic hyperleptinemia, per se, associated with DIO adiposity does not appear to explain elevated body weight set point because the same phenomenon did not occur in mice fed a low fat diet and chronically infused with leptin to mimic the high endogenous leptin levels of DIO mice [15]. A caveat with use of the DIO mouse model to study the mechanisms regulating body weight set point, and in particular the role of altered leptin levels [15], is the chronic inflammatory state induced in the liver, adipose tissue and hypothalamus by an excess of fatty acids in the diet [13], [16].
Our laboratory has recently developed an alternative genetic model of obesity in mice fed a standard low fat chow that is based on the conditionally reversible silencing of proopiomelanocortin (Pomc) gene expression in the arcuate nucleus of the hypothalamus (ArcPomc−/−) [17]. POMC regulates energy balance primarily by controlling feeding behavior and energy expenditure [17], [18]. These effects are largely mediated by POMC-derived melanocortin peptides that are agonists for central melanocortin (MC) 3 and/or 4 receptors, see for review [19]. Consequently, ArcPomc-deficiency causes obesity associated with extreme hyperleptinemia by a combination of hyperphagia, reduced locomotor activity and decreased energy expenditure.
Central MC3 and/or 4 receptor signaling is involved in mediating the effects of leptin on energy balance [20], [21], [22], [23], [24], [25]. The actions of leptin to reduce body weight and food intake [26], [27] are attenuated in a genetic model of Mc4r−/− mice [28], [29] and in a pharmacological model of rats treated with the MC3/4 antagonist SHU9119 [30], indicating the importance of the melanocortin system in mediating these functions of leptin. Hypothalamic Pomc expression is upregulated by leptin and reduced in leptin-deficient (ob/ob) or leptin receptor-deficient (db/db) mice [31]. Additionally, restoration of ArcPomc in the leptin receptor-expressing sub-population of hypothalamic POMC neurons was sufficient to normalize energy balance in otherwise ArcPomc−/− mice [32], further supporting an important interaction of ArcPomc and leptin signaling. Moreover, leptin activates POMC neurons to secrete anorexigenic peptides and inhibits NPY/AgRP neurons to attenuate the release of orexigenic signals [33].
We demonstrated previously that genetic restoration of ArcPomc expression at postnatal day 25 prevented excessive weight gain in the obesity-programmed ArcPomc−/− mice [17]. However, ArcPomc reactivation in older and massively obese mice failed to fully reverse the established obesity phenotype and accompanying metabolic disturbances. In the current study, we hypothesized that leptin- and POMC-mediated control of energy homeostasis are reciprocally linked to each other and that reducing the body weight of obese, hyperleptinemic ArcPomc−/− adult mice by calorie restriction prior to reactivation of Pomc expression would restore their body weight set point to that of lean control mice of the same age. Therefore, we measured plasma leptin levels, leptin sensitivity, and body composition in ArcPomc−/− mice at different ages and under prolonged conditions of ad libitum access to chow or calorie restriction. Moreover, we performed a longitudinal study in calorie restricted ArcPomc−/− mice treated with a novel long acting version of leptin using PASylation technology [34], [35] to mimic the hyperleptinemia of obese ArcPomc−/− mice and better understand the association of leptin sensitivity and the central melanocortin system in regulating energy balance.
2. Methods
2.1. Animal husbandry
All animal procedures were approved by the University of Michigan's Institutional Animal Care and Use Committee and followed the National Research Council's “Guide for the Care and Use of Laboratory Animals.” Mice of mixed genotypes derived from ArcPomc+/− breeding pairs that had been back-crossed onto the C57BL/6J background for >12 generations were group housed in ventilated cages on corn cob bedding under controlled temperature (22 °C) and photoperiod (12 h light/12 h dark cycle, lights on from 6 a.m. to 6 p.m.), with tap water and standard laboratory chow (LabDiet, 5L0D; containing 28.5 kcal% protein, 13.5 kcal% fat, and 58.0 kcal% carbohydrate) available ad libitum. Mice were housed individually for studies involving weight-matching by calorie restriction.
2.2. Generation and breeding of mice
Compound ArcPomc−/−;Pomc-Cre-ERT2 and their corresponding control mice were generated by crossing ArcPomc+/− mice with BAC transgenic Pomc-Cre-ERT2 mice [36] (gift from Dr. Joel Elmquist, UT Southwestern Medical Center) followed by a second generation of intercrossing between the resulting compound double heterozygous mice and ArcPomc+/− mice. ArcPomc−/− mice contain a loxP-flanked neomycin resistance cassette, inserted in the neuronal enhancer locus of the Pomc gene, that selectively blocks Pomc transcription in Arc hypothalamic neurons [17], [18]. After tamoxifen administration, the Pomc-Cre-ERT2 transgene specifically and effectively removed the loxP-flanked STOP sequence from the Ai9 reporter allele (B6.Cg-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J; The Jackson Laboratory) as demonstrated by co-localization of red fluorescence in 80–90% of immunohistochemically labeled POMC neurons in the arcuate nucleus (unpublished results). The Pomc-Cre-ERT2 and CAG-Cre-ERT transgenes yielded equivalent tamoxifen induced deletion of the loxP-flanked neomycin cassette from the targeted ArcPomc−/− alleles, and hence reactivation of Pomc expression in the Arc. However, the ArcPomc−/−;Pomc-Cre-ERT2 compound mice had better breeding efficiency than the ArcPomc+/−;CAG-Cre-ERT mice and therefore were used for the majority of experiments.
2.3. Calorie restriction procedure
Individually housed ArcPomc−/− mice were fed 75–80% of the average amount of chow consumed daily by their control littermates starting at age 25-days, shortly after weaning, to continuously match body weights between the two genotypes up to age 16-wk. For these and the following studies, the daily allotment of chow was provided 1 h before lights out.
For the longitudinal studies, compound ArcPomc−/−;Pomc-Cre-ERT2 or ArcPomc−/−;CAG-Cre-ERT mice with free access to chow were allowed to become obese until their body weights stabilized at 5–6 months of age. The obese mutant mice were subsequently fed 55–60% of the average amount of food consumed daily by their respective control (ArcPomc+/+;Cre-ERT transgenic) littermates. It took 8 wk for male and 12 wk for female ArcPomc−/− mice on this calorie restriction protocol to reduce their body weights to match that of their sex- and age-matched controls. Thereafter, the weight-matched mutant mice and their controls received tamoxifen (50 mg/kg, ip) for each of five consecutive days as described previously [17] during which time the mutant mice were maintained on their calorie restricted diet. Following the last tamoxifen injection, all mice had ad libitum access to chow until they were approximately one-year old at the conclusion of the studies.
2.4. General procedures
Intracerebroventricular (icv) cannulae (Plastics One) were placed into the left lateral ventricle and osmotic mini-pumps (Alzet) were implanted subcutaneously (sc) as described previously [37]. Body composition was measured by NMR using a Minispec LF90II instrument (Bruker Optics). Oral glucose tolerance tests were performed as described previously [37].
2.5. Leptin treatment
For acute experiments, recombinant mouse leptin (National Hormone and Peptide Program) was injected either ip (5 mg/kg in PBS, repeated at 8 h intervals for a total of 3 doses) or icv (2 μg in 2 μl PBS, repeated at 8 h intervals for a total of 3 doses) as described previously [37]. For chronic experiments, leptin was administered by osmotic mini-pumps (10 μg/day sc) for 14 days. PASylated leptin [34], [35] (10 mg/kg in PBS) was administered sc at 72 h intervals starting one week prior to tamoxifen injections and ending 72 h after the last tamoxifen dose.
2.6. Leptin and insulin assays
Plasma leptin and insulin levels were measured by ELISAs following the manufacturers' instructions (R&D Systems, MOB00; Crystal Chem, 90080, respectively). PASylated leptin, rather than the standard leptin included in the ELISA kit, was used to construct the standard curve for analyzing plasma leptin levels during PASylated leptin administration.
2.7. Semi-quantitative real-time (qRT)-PCR
Total nucleic acid from tissue blocks was extracted using RNeasy spin columns (Qiagen) for quantification of Lepr or Pomc expression. Contaminating genomic DNA was removed by Turbo DNase treatment (Life Technologies) and total RNA was quantified using a NanoDrop spectrophotometer (ThermoScientific). Reverse transcription to generate cDNA was performed with 500 ng total RNA and random hexamer primers (Goscript RT System, Promega). qRT-PCR was performed on all samples in duplicate using a StepOne Real Time PCR System (Applied Biosystems) and SYBR Green Master Mix (Life Technologies). The primers for detection of all Lepr isoforms were 5′-TGGAAGGAGTTGGAAAACCA-3′ and 5′-CAGTGTTCCGAGCAGTAGGA-3′, and for specific detection of the long-form Leprb were 5′-GGCACAAGGACTGAATTTCC-3′ and 5′-CTGCTGGGACCATCTCATCT-3′. The primers for detection of Pomc were 5′-GAGCTGGTGCCTGGAGAG-3′ and 5′- TTTTCATCAGGGGCTGTTC-3′. All primers were used at a final concentration of 300 nM. The relative quantity of each mRNA was calculated from standard curves spanning 1000-fold change, normalized to the reference gene Hprt and then normalized to the mean of ArcPomc+/+ controls.
2.8. Statistical analyses
Comparisons of one dependent variable between two independent groups were made by Student's unpaired t-tests while comparisons between repeated measures of one dependent variable within a single group were made by Student's paired t-tests. Comparisons among three or more independent groups for one dependent variable were made by one-way ANOVAs followed by Tukey's multiple comparisons test or Dunnett's multiple comparisons test for pair-wise comparisons limited to ArcPomc+/+ controls. Comparisons among two or more independent groups for two dependent variables were made by two-way ANOVAs followed by either Sidak's multiple comparisons test or Dunnett's multiple comparisons test for pair-wise comparisons limited to ArcPomc+/+ controls. Comparisons between two independent groups involving one dependent variable with repeated measures were made by repeated measures two-way ANOVAs (RMANOVA) followed by Bonferroni's multiple comparisons test. All analyses were performed separately for data from male and female mice using Prism 6.0 (Graph Pad) and P < 0.05 was considered significant.
3. Results
3.1. Increased adiposity and hyperleptinemia in juvenile ArcPomc−/− mice
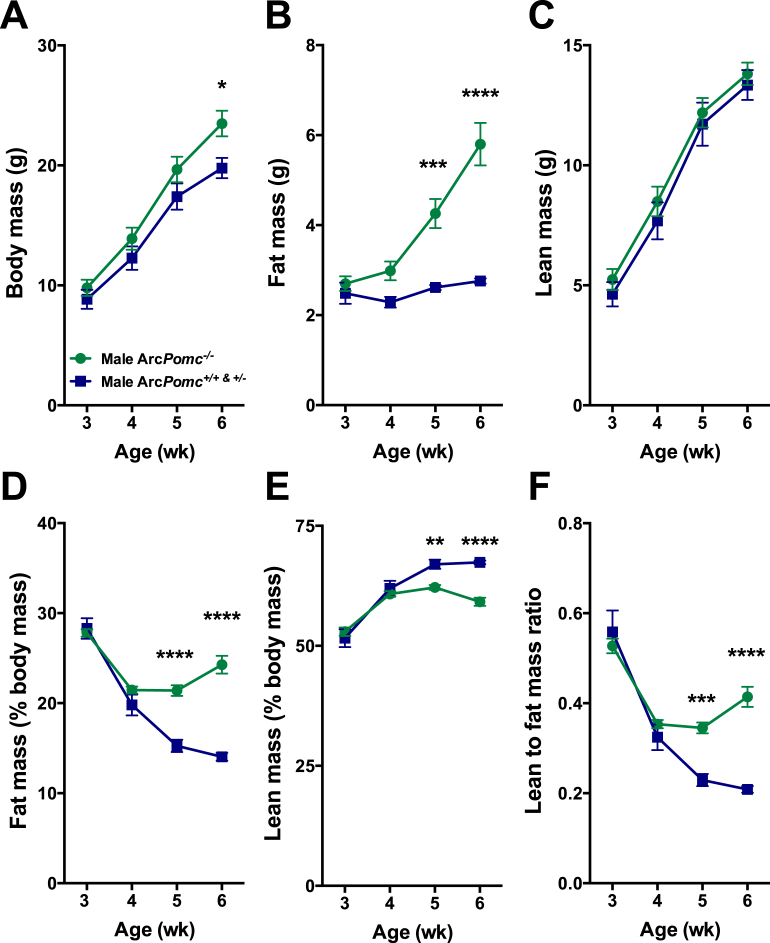
We measured body composition weekly from ages 3 to 6-wk in a cohort of 35 weanlings of all three genotypes and both sexes derived from 9 litters of crosses between ArcPomc+/− heterozygous mice. Data from the male mice are shown in Figure 1. ArcPomc+/+ and ArcPomc+/− mice were indistinguishable from each other and therefore their data were combined for statistical analysis. Two-way ANOVAs for body weight, fat mass, lean mass, percentage of fat mass relative to body mass, percentage of lean mass relative to body mass, and ratio of lean to fat mass revealed significant main effects of genotype for each dependent variable (F1,67 = 9.0, P = 0.0038; F1,67 = 45.4, P < 0.0001; F1,67 = 1.8, P = 0.1902; F1,67 = 67.9, P < 0.0001; F1,67 = 21.6, P < 0.0001; and F1,67 = 29.0, P < 0.0001, respectively) except lean mass. Post-hoc multiple comparisons tests indicated there were no significant differences between the two genotypes of mice at ages 3 and 4 wk for any of the six measures (Figure 1A–F), although trends were evident for divergent body mass and fat mass. However, by age 5-wk, all dependent variables were significantly different between genotypes, except for body mass and lean mass. The results from female mice showed similar patterns compared to males for each of the measures (Supplemental Figure 1). Taken together, these data reveal that the earliest detected metabolic alteration of weanling ArcPomc−/− mice was increased adiposity that was rapidly augmented from age 5-wk onward. In contrast, body weight was only subtly elevated at age 6-wk in ArcPomc−/− mice.
Figure 1.
Altered body composition is the earliest detected metabolic phenotype in ArcPomc−/− mice. A) Body weight, B) Fat mass determined by NMR, C) Lean mass determined by NMR, D) Percentage of fat mass relative to body mass, E) Percentage of lean mass relative to body mass, and F) Ratio of lean to fat mass in male ArcPomc−/− (n = 12) and combined littermate ArcPomc+/+ (n = 4) and ArcPomc+/− (n = 3) mice at ages 3–6 wk. *P < 0.05, **P < 0.01, ***P < 0.005, and ****P < 0.0001; Sidak's post-hoc multiple comparisons tests. Data shown are the mean ± SEM.
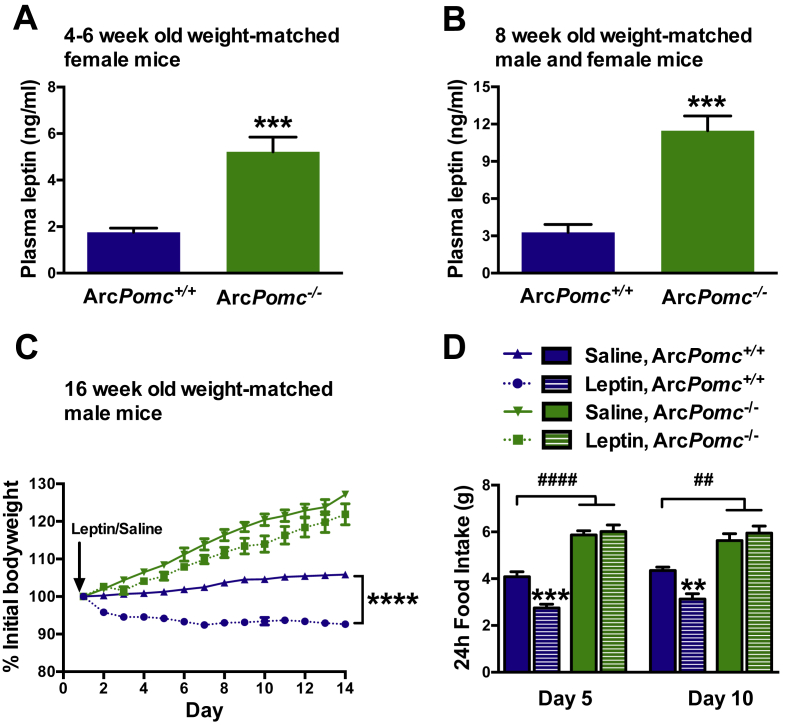
Leptin levels from an independent cohort of 4 to 6-wk old weight-matched females were increased almost 3-fold in ArcPomc−/− compared to their sibling ArcPomc+/+ mice (5.1 ± 0.7 and 1.8 ± 0.2 ng/ml, respectively, P < 0.005) (Figure 2A), consistent with their altered body composition at this early age. These results suggest a direct role of hypothalamic POMC in regulating adiposity and, therefore, plasma leptin levels.
Figure 2.
Elevated plasma leptin levels and leptin insensitivity of calorie restricted ArcPomc−/− mice weight-matched to ArcPomc+/+ mice. A) Plasma leptin levels of 4 to 6-wk old female mice; ***P < 0.005, Student's 2-tailed t-test; n = 6. B) Plasma leptin levels of 8-wk old combined male and female mice; ***P < 0.005, Student's 2-tailed t-test; n = 6. C) Changes in bodyweight of 16-wk old male mice during 14-day sc leptin or saline infusions from osmotic mini-pumps; ****P < 0.0001, leptin vs. saline treated ArcPomc+/+ mice (n = 4–8) at day 14, Bonferroni's multiple comparisons test following two-way RMANOVA (F39,221 = 39, P < 0.0001, interaction of time and treatment). D) Food intake for days 5 and 10 during leptin or saline infusion; **P < 0.01, ***P < 0.005, for leptin vs. saline treated ArcPomc+/+ mice; ##P < 0.01, ####P < 0.0001, for saline and leptin treated ArcPomc−/− vs. saline treated ArcPomc+/+ mice (n = 6), Bonferroni's multiple comparisons tests following 2-way ANOVAs (F3,15 = 71.6, P < 0.0001, main effect of group at day 5; F3,15 = 29.2, P < 0.0001, main effect of group at day 10). Data shown are the mean ± SEM.
3.2. Peripheral and central leptin treatment failed to reduce body weight and food intake in weight-matched ArcPomc−/− mice
Next, we measured plasma leptin levels and leptin sensitivity in weight-matched 8-wk old male ArcPomc−/− mice without any history of overweight. Following weaning, ArcPomc−/− mice were fed 75–80% of the average amount of chow eaten daily by their ArcPomc+/+ littermates to constantly maintain equivalent body weights between the two genotypes. The reduced quantity of food required by ArcPomc−/− mice to achieve weight matching is consistent with their reduced energy expenditure compared to ArcPomc+/+ controls [17]. At age 8 wk, weight-matched ArcPomc−/− mice exhibited moderate hyperleptinemia with levels almost four-fold higher than their ArcPomc+/+ controls (Figure 2B). Furthermore, after a return to ad libitum chow at age 16 wk, weight-matched ArcPomc−/− mice became hyperphagic and two weeks of chronic exposure to peripheral leptin treatment by sc osmotic mini-pump did not prevent further increases in their body weight (Figure 2C) or food intake (Figure 2D) in marked contrast to the reductions of both measures in control mice. In a previous study, we reported that leptin administration at a dose of 10 μg/day delivered by sc mini-pump raised circulating leptin levels of wildtype mice to ∼12 ng/ml [38].
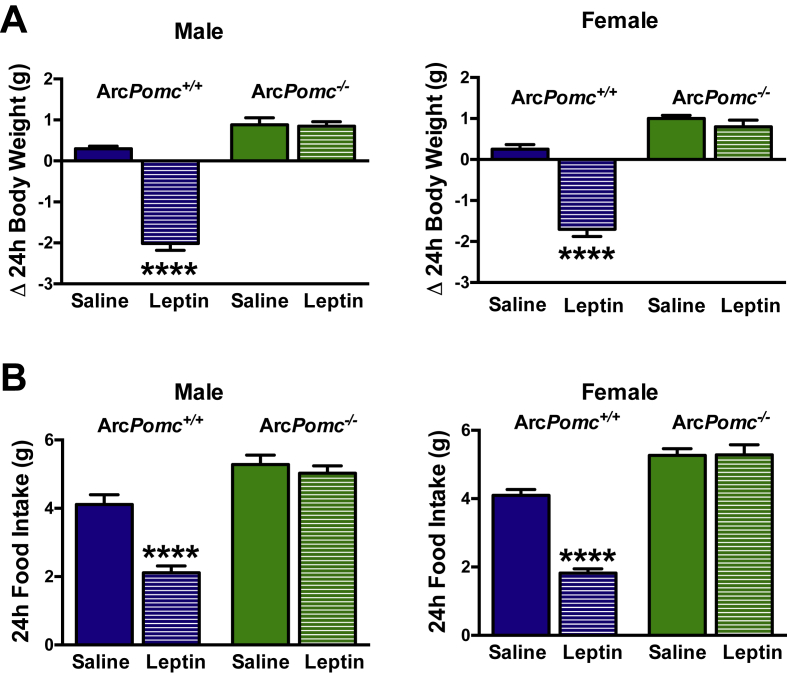
Leptin transport across the blood–brain barrier is required for its effects to decrease food intake and body weight [39], [40], [41]. To address the possibility of decreased leptin transport into the brain of ArcPomc−/− mice, we tested central leptin sensitivity in a separate cohort of 8-wk old weight-matched mice using icv injection of leptin. Like peripheral leptin administration, icv leptin failed to reduce body weight and food intake in both sexes of weight-matched ArcPomc−/− mice (Figure 3A,B). These experiments suggest that reduced leptin transport into the brain was not the underlying cause for impaired leptin sensitivity in the ArcPomc−/− mice. In contrast, we previously showed that acute icv administration of the MC3/4R agonist melanotan II potently reduced food intake and bodyweight in ArcPomc−/− mice [37], [42], indicating that the fidelity of the melanocortin system downstream of the POMC neurons themselves remains intact.
Figure 3.
Central leptin insensitivity of calorie restricted ArcPomc−/− mice weight-matched to ArcPomc+/+ mice. A) Changes in bodyweight and B) 24-h food intake of 8-wk old male and female mice after acute central leptin or saline treatment (three icv injections, 5 p.m., 12 a.m., 8 a.m.); ****P < 0.0001 for leptin vs. saline treated ArcPomc+/+ mice (n = 6), Bonferroni's multiple comparisons tests following 2-way ANOVAs (F3,15 = 78.3, P < 0.0001, main effect of group for male body weight change; F3,15 = 37.3, P < 0.0001, main effect of group for female body weight change; F3,15 = 37.2, P < 0.0001, main effect of group for male food intake; and F3,15 = 72.9, P < 0.0001, main effect of group for female food intake). Data shown are the mean ± SEM.
3.3. Leptin receptor expression is unaltered in the hypothalami of ArcPomc−/− mice
Hypothalamic expression of Lepr total isoforms and specifically the Leprb isoform was normal in the mutant mice (Lepr mRNA: ArcPomc−/− mice, 84 ± 11.5 vs. control, 100 ± 8.0; Leprb mRNA: ArcPomc−/− mice, 95.5 ± 6.5 vs. control, 100 ± 5.1; data are expressed as the percentage relative to the mean of values obtained from ArcPomc+/+ control mice). These results are in agreement with previous studies that showed normal hypothalamic Lepr expression despite leptin resistance in mice with diet-induced obesity [43], [44].
3.4. Reactivation of ArcPomc expression, following weight reduction by calorie restriction, permanently normalizes body weight and food intake in previously obese mice
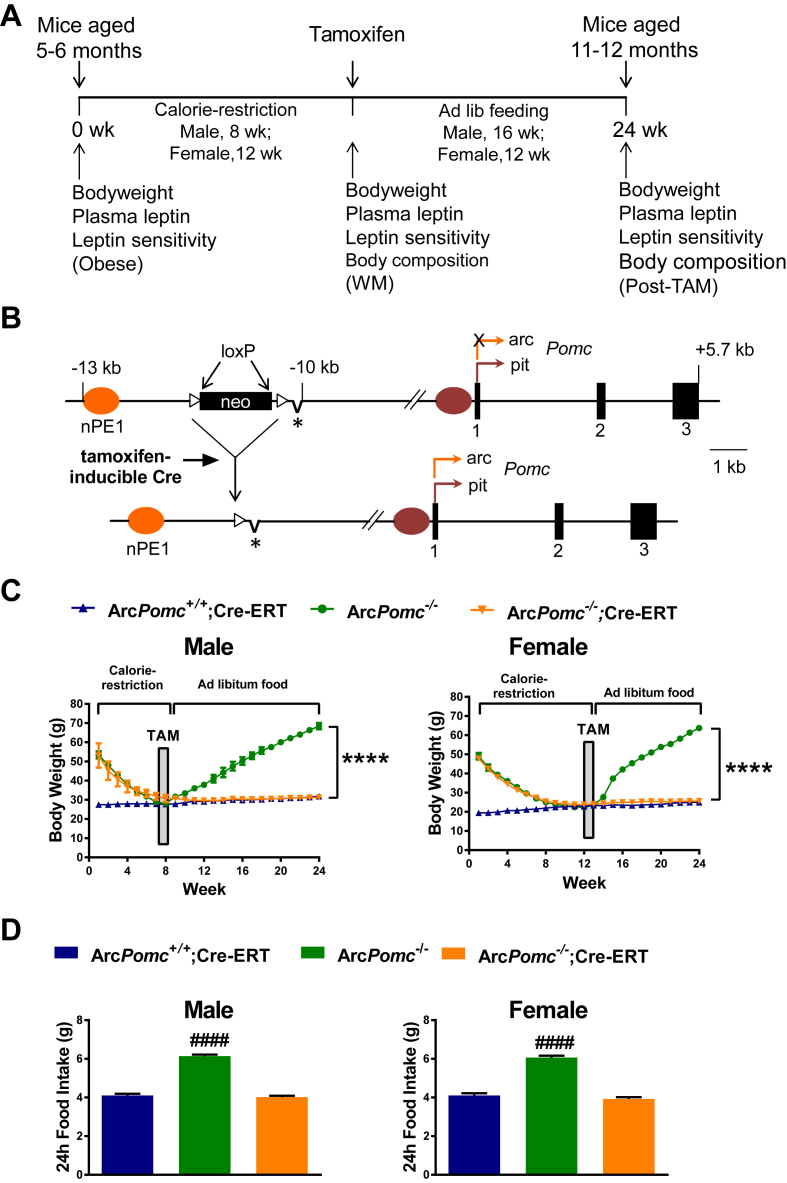
In our previous study, we found that reactivating Pomc expression in obese middle aged ArcPomc−/− mice only partially corrected their weight and metabolic disturbances [17]. To further explore whether this resilience was related to a leptin resistant state, we performed a longitudinal study with repeated physiological measurements (Figure 4A). Specifically, we wanted to determine if reducing body weight, adiposity, and leptin levels in parallel by calorie restriction would improve the efficiency of genetic intervention to reverse obesity. Five to 6-month old, obese ArcPomc−/− mice of both sexes, with or without a Cre-ERT transgene, were calorie restricted for 8 wk (males) or 12 wk (females) to reduce their body weights to the levels of their littermate ArcPomc+/+ controls before reactivating ArcPomc expression by tamoxifen administration (Figure 4B). All mice were provided ad libitum chow access following tamoxifen treatment. We found that body weight and food intake were permanently normalized in compound ArcPomc−/−;Cre-ERT mice due to ArcPomc reactivation in the hypothalamus, while ArcPomc−/− mice without a Cre-ERT transgene rapidly regained weight and exhibited hyperphagia when they were provided ad libitum access to chow after tamoxifen treatment. (Figure 4C,D).
Figure 4.
Reactivation of hypothalamic Pomc expression in ArcPomc−/−;CreERT mice following chronic calorie restriction normalizes food intake and body weight. A) Time line and experimental design of the longitudinal study. Obese mice were 5 to 6-months old at the start of calorie restriction (Time = 0-wk). B) Schematic showing the insertion of a loxP-flanked neomycin (neo) resistance cassette into the upstream flanking region of the Pomc gene to generate the ArcPomc− allele (top). The asterisk indicates the deletion of neural Pomc enhancer 2 (nPE2). Deletion of the neo cassette by a tamoxifen-inducible Cre transgene produces the reactivated Pomc allele containing nPE1, which is sufficient to drive hypothalamic expression of the gene [18] (bottom). C) Serial body weights at the indicated time points, ****P < 0.0001 for ArcPomc−/− vs. ArcPomc+/+;Cre-ERT and ArcPomc−/−;Cre-ERT at 24 wk for male (n = 6–13) and female mice (n = 4–7), Bonferroni's multiple comparisons tests following 2-way RMANOVAs (F46,575 = 69.5, P < 0.0001, interaction of time and male genotype; F46,322 = 161, P < 0.0001, interaction of time and female genotype). D) Ad libitum food intake 2 wk after tamoxifen administration, ####P < 0.0001 for ArcPomc−/− vs. ArcPomc+/+;Cre-ERT and ArcPomc−/−;Cre-ERT for both males (n = 10–17) and females (n = 9–16), Tukey's multiple comparisons test following 1-way ANOVAs (F2,36 = 223, P < 0.0001, main effect of male group; F2,32 = 127, P < 0.0001, main effect of female group). Data shown are the mean ± SEM. For simplicity, ArcPomc−/−;Cre-ERT is used as a common annotation for ArcPomc−/−;CAG-Cre-ERT and ArcPomc−/−;Pomc-Cre-ERT2 compound mice.
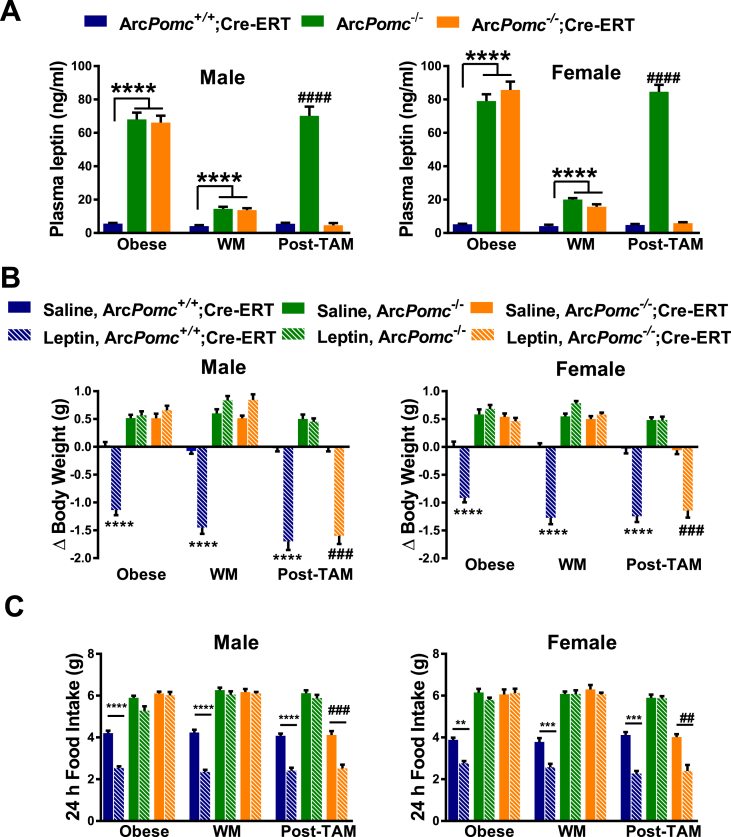
3.5. Reactivation of ArcPomc in calorie restricted ArcPomc−/− mice restores normal energy balance
We predicted that genetic reactivation of ArcPomc in calorie restricted ArcPomc−/− mice would be associated with restoration of leptin sensitivity. To test this hypothesis, we serially measured plasma leptin and leptin sensitivity in ArcPomc−/− mice when they were: i) obese, ii) weight-matched by calorie restriction, and iii) following ArcPomc rescue (Figure 4A). Consistent with other mouse models of severe obesity [28], [43], [45], [46], obese ArcPomc−/− mice had extreme hyperleptinemia (Figure 5A), and neither body weight (Figure 5B) or food intake (Figure 5C) were decreased by acute ip leptin administration. Notably, while the calorie restriction procedure was sufficient to normalize the body weight of ArcPomc−/− mice (Figure 4B), it did not completely reverse their hyperleptinemia and the mice remained resistant to the effects of exogenous leptin to lower body weight or food intake (Figure 5A). Their moderately elevated leptin levels were similar to those found in ArcPomc−/− mice that were calorie restricted following weaning to maintain normal body weight up to age 8 wk (Figure 2B). However, following reactivation of ArcPomc expression in the weight reduced ArcPomc−/−;Cre-ERT2 mice, plasma leptin levels and leptin sensitivity were restored to normal (Figure 5A,B,C).
Figure 5.
Serial plasma leptin levels and functional measurements of leptin sensitivitity in ArcPomc−/−;CreERT mice. A) Serial plasma leptin levels at the indicated experimental stages; ****P < 0.0001, ArcPomc+/+;Cre-ERT vs. ArcPomc−/− and ArcPomc−/−;Cre-ERT and ####P < 0.0001 for ArcPomc−/− vs. ArcPomc+/+;Cre-ERT and ArcPomc−/−;Cre-ERT for males (n = 6–10) and females (n = 8–10), Tukey's multiple comparisons tests following separate 1-way ANOVAs for each combination of sex and experimental stage. B) 24-h changes in body weight and C) Absolute 24-h food intake after i.p. leptin treatment at the indicated experimental stages; ****P < 0.0001, ∗∗∗P < 0.005 for leptin vs. saline treated ArcPomc+/+;Cre-ERT for both sexes; ####P < 0.0001, ###P < 0.005 for leptin vs. saline treated ArcPomc−/−;Cre-ERT for both sexes, paired 2-tailed Student's t-tests, n = 5–8. Data shown are the mean ± SEM. WM, weight matched; Post-TAM, post-tamoxifen. For simplicity, ArcPomc−/−;Cre-ERT is used as a common annotation for either ArcPomc−/−;CAG-Cre-ERT or ArcPomc−/−;Pomc-Cre-ERT2 compound mice.
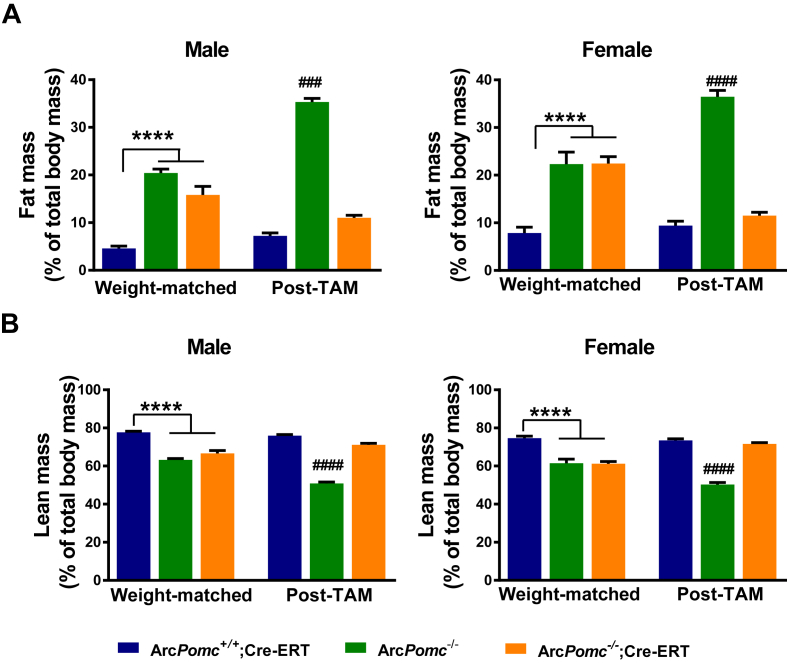
As shown in our previous study [17], the increased body weight of ArcPomc−/− mice was principally due to excessive accumulation of fat mass. Here, we found that calorie restriction to reduce body weight to the equivalent of their age-matched controls was insufficient to normalize adiposity in the ArcPomc−/− mice (Figure 6A,B), thus explaining their residual moderate hyperleptinemia. Remarkably, several weeks after the final tamoxifen dose to reactivate ArcPomc expression, the same mice had normalized fat and lean mass and a fat/lean mass ratio. In contrast, the non-rescued ArcPomc−/− mice quickly reverted to a higher fat mass and fat/lean mass ratio after being allowed to feed ad libitum.
Figure 6.
Reactivation of hypothalamic Pomc expression in ArcPomc-deficient mice following chronic calorie restriction permanently normalizes body composition. A) Fat mass and B) Lean mass as percentages of body mass; ****P < 0.0001 for ArcPomc+/+;Cre-ERT vs. ArcPomc−/− and ArcPomc−/−;Cre-ERT for both male and female mice; ####P < 0.0001, for ArcPomc−/− vs. ArcPomc−/−;Cre-ERT and ArcPomc+/+;Cre-ERT for both male (n = 4–8) and female (n = 6–7) mice post-tamoxifen, Tukey's multiple comparisons tests following 1-way ANOVAs for each combination of sex and experimental stage. Data shown are the mean ± SEM. Post-TAM, post-tamoxifen. For simplicity, ArcPomc−/−;Cre-ERT is used as a common annotation for either ArcPomc−/−;CAG-Cre-ERT or ArcPomc−/−;Pomc-Cre-ERT2 compound mice.
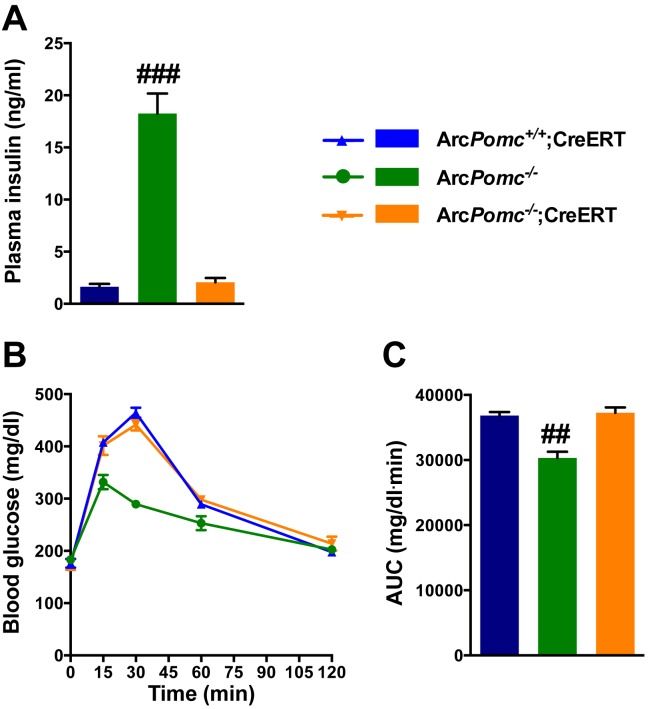
In addition to normalizing food intake, body weight, body composition, and leptin sensitivity, ArcPomc reactivation in weight-reduced ArcPomc−/− mice corrected their hyperinsulinemia (Figure 7A) in contrast to the partial reduction observed previously in identically aged, obese ArcPomc−/−;CAG-Cre-ERT mice treated with tamoxifen [17]. Obese, insulin-resistant ArcPomc−/− mice have paradoxically improved, not worsened, glucose tolerance because of their reduced renal threshold for glycosuria relative to their ArcPomc+/+ littermates [37]. Therefore, ArcPomc-rescue actually caused a deterioration of glucose tolerance along with decreased glycosuria to control levels (Figure 7B,C and Supplemental Table 1), presumably due to restoration of renal sympathetic nervous system activity.
Figure 7.
Normalization of glucose homeostasis in ArcPomc-rescue mice. A) Plasma insulin levels, ####P < 0.0001 for ArcPomc−/− vs. ArcPomc−/−;Cre-ERT and ArcPomc+/+;Cre-ERT mice, Tukey's multiple comparisons test following 1-way ANOVA, n = 6. B) Oral glucose tolerance test (OGTT) and C) corresponding area under the curve (AUC), ####P < 0.0001 for ArcPomc−/− vs. ArcPomc−/−;Cre-ERT and ArcPomc+/+;Cre-ERT mice, Tukey's multiple comparisons test following 1-way ANOVA, n = 6.
3.6. Long-acting PASylated leptin administration prevents normalization of impaired energy balance
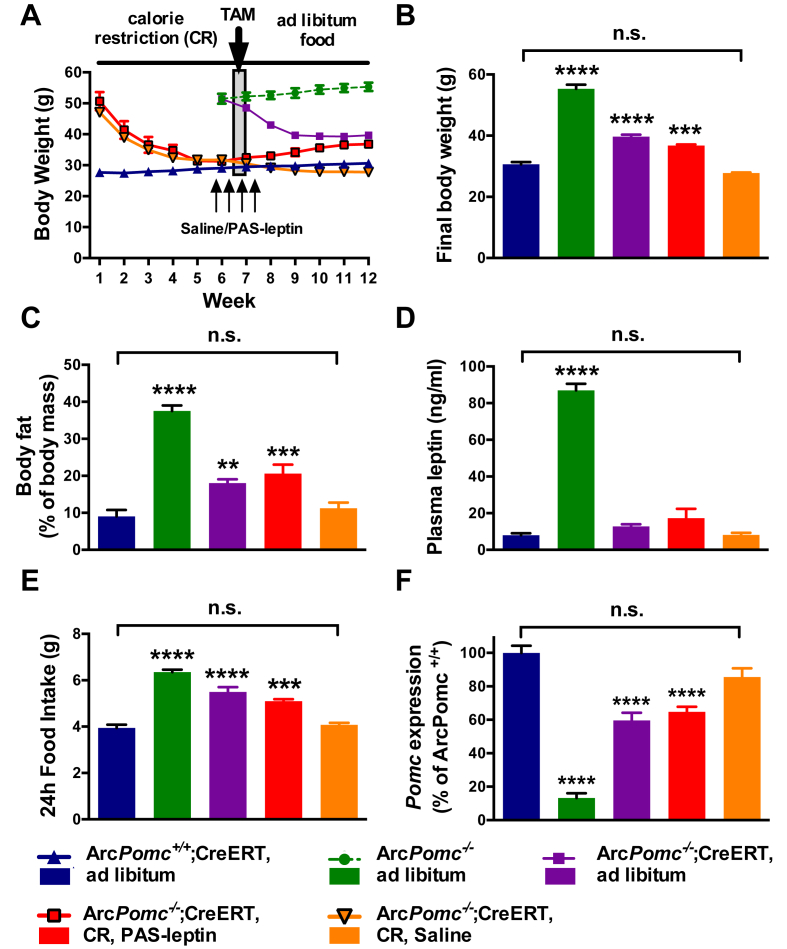
To examine whether the reduction in hyperleptinemia between obese and calorie restricted ArcPomc−/− mice influenced the efficacy of ArcPomc reactivation to restore a normal body weight set point, we performed a second longitudinal experiment using an additional cohort of 5 to 6-month old male mice (Figure 8A). One group of calorie restricted ArcPomc−/−;Pomc-Cre-ERT2 mice was treated with PASylated leptin, a modified form of leptin with extended plasma half-life [34], [35]. The dose was chosen to mimic the extreme hyperleptinemia of obese ArcPomc−/− mice (Figure 5A) and indeed the treatment raised plasma leptin levels to 95 ± 1 ng/ml prior to the first tamoxifen injection and 90 ± 6 ng/ml following the last tamoxifen injection. A second group of calorie restricted ArcPomc−/−;Pomc-Cre-ERT2 mice was treated with saline vehicle and their leptin levels were 15 ± 3 and 17 ± 4 ng/ml measured at the same two time points as the PASylated leptin treated group. Two other groups of non-calorie restricted obese ArcPomc−/− mice, one with the Pomc-Cre-ERT2 transgene and one without, and a group of lean ArcPomc+/+;Pomc-Cre-ERT2 control mice were used for comparison. All five groups of mice received the identical tamoxifen treatment.
Figure 8.
PASylated leptin treatment prevents the normalization of metabolic state in calorie restricted male ArcPomc−/− mice. A) Body weight for each of the five groups of mice over 12 wk. A 2-way RMANOVA for all groups, except ArcPomc−/−, from weeks 9–12 revealed a significant interaction of time and group (F9,42 = 4.3, P < 0.001). B) Final body weight, C) Food intake, D) Fat mass, E) Plasma leptin levels, and F) Hypothalamic Pomc mRNA levels. N = 4–6, **P < 0.01, ***P < 0.005, and ****P < 0.0001 vs. ArcPomc+/+;Pomc-Cre-ERT mice, Dunnett's multiple comparisons test following 1-way ANOVAs for each measurement. CR, calorie restriction; TAM, tamoxifen; PAS-leptin, PASylated leptin. Data shown are the mean ± SEM.
The obese ArcPomc−/− mice were not affected by tamoxifen and continued to gain weight while the obese ArcPomc−/−;Pomc-Cre-ERT2 mice had a rapid decrease in weight over two weeks and then stabilized at a level 60% less than their initial weight, but still significantly higher than age-matched control mice. These two groups replicated our previously published findings [17] using the CAG-Cre-ERT transgenic driver line. PASylated leptin administration, bookending the tamoxifen injections, prevented the normalization of body weight, body composition, and food intake, while each of these parameters was normalized in the saline- and tamoxifen-treated, calorie restricted group to levels indistinguishable from the ArcPomc+/+ mice (Figure 8B–E). Moreover, the PASylated leptin treated mice actually regained 30% of their lost weight. Their final body weights, fat mass, plasma leptin levels, and food intake were almost the same as those of the group of non-calorie restricted obese ArcPomc−/−;Pomc-Cre-ERT2 mice 6 wk following tamoxifen administration (Figure 8B–E).
Next, we assessed hypothalamic Pomc mRNA levels by qRT-PCR in the five experimental groups, 6 wk after tamoxifen treatment (Figure 8F). Pomc expression from the reactivated ArcPomc−/− alleles in the calorie restricted ArcPomc−/−;Pomc-Cre-ERT2 mice was not different from that of the control ArcPomc+/+ mice; whereas it was significantly attenuated in both the obese ArcPomc−/−;Pomc-Cre-ERT2 group and the calorie restricted PASylated leptin treated group following tamoxifen administration (Figure 8F). The relative levels of hypothalamic Pomc mRNA correlated inversely with the measured physiological parameters for each group, suggesting that the former are responsible for the latter.
The specificity of Pomc rescue in the hypothalamus of ArcPomc−/−;Pomc-Cre-ERT2 mice 6 wk following tamoxifen treatment was shown by a lack of change in the minimally detectable levels of Pomc mRNA by qRT-PCR in the hippocampus and brain stem (Supplementary Figure 2) where Pomc expression was 20-fold lower than that in the hypothalamus of ArcPomc+/+ mice and was not different between genotypes. We have never observed POMC peptide immunoreactivity or Pomc mRNA by in situ hybridization in neuronal soma in non-hypothalamic areas of adult mice despite the fact that Pomc-reporter genes often exhibit expression in the brain stem, hippocampus, and amygdala in addition to the Arc [47], [48], [49].
4. Discussion
In this study, we report a direct reciprocal role of hypothalamic POMC and leptin sensitivity in the defense of body weight and adiposity at a given level. Maximal expression of Pomc requires a sensitive leptin signaling pathway to normalize body weight and fat composition and leptin requires POMC to induce its anorectic activity. We found that normal body weight can be restored in aged obese ArcPomc−/− mice only if ArcPomc expression is reinstated after plasma leptin is first reduced below a threshold level by calorie restriction. This observation explains why normal body weight could not be restored in our previous study [17] after reactivation of ArcPomc in obese 6-month old ArcPomc−/− mice, which have significantly higher plasma leptin levels relative to calorie-restricted weight-matched mice (Figure 5A). Therefore, findings from this study support the significance of chronic calorie-restriction [1] and, therefore, relatively lower plasma leptin levels in improving the efficiency of clinical interventions to treat obesity. Our data are also consistent with a recent clinical study that showed significant indirect correlations between cerebrospinal fluid (CSF) levels of POMC and BMI, body fat percentage, CSF leptin, and plasma leptin and insulin [50].
ArcPomc−/− mice, whether obese or weight-matched, with or without a history of obesity, showed no change in food intake and body weight in response to exogenous leptin treatment. These results indicate the necessary role of ArcPomc in mediating the effects of leptin on food intake and body weight. While previous animal studies [43], [45] and clinical observations demonstrate that leptin therapy does not effectively reduce weight in obese subjects [51], we show that leptin treatment is also ineffective in weight-matched mice that lack Pomc in the hypothalamus. Since weight-matched Mc4r−/− mice do respond to leptin treatment [28], it is possible that the blunted leptin action observed in weight-matched ArcPomc-deficient mice is due to a lack of MC3R signaling by melanocortin activation [21]. Moreover, a recent study [29] reported that while endogenous leptin sensitivity is intact in a diet-induced obesity mouse model, obese Mc4r−/− mice do not respond to endogenous leptin, indicating a critical function of the melanocortin system in mediating leptin action during obesity. Altogether, our data support previous observations [21], [28], [29], [30], [43], [52] that melanocortin signaling integrates leptin-mediated effects on energy balance. Our study extends those observations by identifying Pomc expression in the Arc, but not pituitary or hindbrain, as the primary mediator of the actions of leptin to reduce food intake and body weight because ArcPomc-deficient mice have normal Pomc expression in the pituitary and nucleus tractus solitarius [17], [53]. Chronic hyperleptinemia or increased leptin signaling itself contributes to the attenuated ability of leptin to reduce food intake and body weight [54], [55]; nevertheless, in this study, hyperleptinemia is attributable to increased fat mass due to ArcPomc-deficiency even in the absence of hyperphagia in calorie-restricted mice, a feature that is analogous to Mc3r−/− mice [21], [56], [57].
In our previous report [17], we did not measure plasma leptin levels or leptin sensitivity serially in ArcPomc−/− mice. Moreover, it was unclear if the restoration of normal body weight by early, but not delayed, genetic ArcPomc reactivation was associated with restoration of leptin sensitivity. To clarify the role of hypothalamic POMC in leptin sensitivity, we measured plasma leptin levels, and food intake and body weight response after leptin administration in ArcPomc−/− mice. Interestingly, we found that both obese and weight-matched ArcPomc−/− mice exhibited hyperleptinemia. However, the weight-matched mice had significantly lower plasma leptin levels compared to the obese mice. This reduction in leptin apparently contributed to the restoration of normal weight after ArcPomc reactivation in the weight-matched but not obese mice. Both obese and weight-matched ArcPomc-deficient mice did not decrease food intake or body weight in response to exogenous leptin. Remarkably, hyperleptinemia and leptin resistance were corrected after restoration of ArcPomc expression in weight-matched ArcPomc reactivatable mice. These data indicate a direct association of ArcPomc levels with the effects of leptin to reduce food intake and body weight. The longitudinal study also explains why our previous rescue of ArcPomc expression in weight-matched ArcPomc−/−;Cre-ERT mice at age 8 wk [17] permanently maintained their body weight and food intake at normal levels with free access to chow. The prevention of obesity by ArcPomc rescue appears to be linked with relatively lower plasma leptin levels or degree of adiposity compared to massively obese mice at the time of ArcPomc reactivation. This observation suggests a critical threshold of plasma leptin levels during which obesity can be normalized by therapeutic interventions; in contrast, if the leptin levels are higher than the threshold, therapeutic interventions might not completely restore bodyweight as observed by us previously in ArcPomc−/−;Cre-ERT mice [17].
The increased ratio of fat mass to lean mass with normal body weight in the weight-matched ArcPomc−/− mice is reminiscent of the phenotype of Mc3r−/− mice, which have higher fat mass and hyperleptinemia without hyperphagia or hypometabolism [56], [57]. These data confirm that the central melanocortin system directly regulates adiposity, independently of body weight [58]. Remarkably, plasma leptin levels, leptin sensitivity, adiposity, and the ratio of fat mass to lean mass were all corrected after restoration of ArcPomc expression (Figure 5, Figure 6) in otherwise ArcPomc-deficient aged mice that had a history of massive obesity. Therefore, our data indicate that energy balance derangements in ArcPomc−/− mice are not attributable to any developmental or compensatory changes that might have occurred due to lifelong hypothalamic Pomc-deficiency because the derangements were completely reversed after a combination of prior weight loss by calorie restriction followed by ArcPomc-rescue in obese adult mice.
To further clarify the role of baseline leptin levels in influencing the efficiency of genetic intervention to reverse obesity, we treated calorie restricted reactivatable ArcPomc−/− mice with PASylated leptin [34], which was previously shown to reverse obesity in leptin-deficient ob/ob mice [35]. Remarkably, the long-acting leptin treatment prevented maximal rescue of ArcPomc expression and its associated phenotype. Similarly, maximal rescue of Pomc expression was impaired in obese reactivatable ArcPomc−/− mice that have severe endogenous hyperleptinemia. In strong contrast, calorie restricted reactivatable ArcPomc−/− mice not treated with PASylated leptin exhibited a normal metabolic phenotype and reprogramming of their body weight set point after restoration of ArcPomc expression.
In summary, we have demonstrated that ArcPomc-deficiency increases fat mass and the ratio of fat to lean mass, and it impairs the function of leptin to reduce body weight and food intake independently of body weight. Therefore, rescue of ArcPomc expression in weight-matched ArcPomc−/− mice, whether or not there is a history of previous obesity, reestablishes normal energy homeostasis and body weight set point under ad libitum feeding conditions by correcting the abnormal body composition and restoring leptin sensitivity. In contrast, massive hyperleptinemia induced by PASylated leptin administration prevents this normalization by blocking the complete restoration of ArcPomc expression. Therefore, our study reveals a strong reciprocal association between leptin levels and hypothalamic POMC in the regulation of energy homeostasis that is potentially relevant to explain the high recidivism rate for obesity after dieting that is commonly seen in clinical situations. Moreover, our data suggest that reversal of leptin resistance specifically on hypothalamic POMC neurons is a testable mechanism to explain the clinical findings that high dose leptin therapy is effective at maintaining weight loss after calorie restriction and reduction of endogenous leptin, but not for the primary treatment of obesity [2]. Collectively, our study demonstrates that the interaction between hypothalamic leptin sensitivity and Pomc gene expression regulates body weight set point, a phenomenon that can be applied to control obesity more efficiently than targeting either the leptin or POMC signaling pathways alone.
Author contributions
K.H.C and M.J.L. conceived the study, designed experiments, analyzed results, and wrote the manuscript. K.H.C, J.M.A., G.L.J., and M.Y. performed experiments and edited the manuscript. M.S. and A.S. provided the PASylated leptin and edited the manuscript. M.R. discussed results and edited the manuscript for intellectual content. M.J.L. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for integrity of the data and accuracy of its analysis.
Funding
This work was supported by NIH grants R01 DK066604 (M.J.L.), R01 DK068400 (M.J.L. and M.R.), an early stage neurosciences training grant T32 NS076401 (J.M.A. and G.L.J.) and a systems and integrative biology training grant T32 GM008322 (G.L.J.). This work utilized core services provided by the University of Michigan Animal Phenotyping and Chemistry Cores supported by the Michigan Diabetes Research Center and the Michigan Nutrition and Obesity Research Center (NIH grants P30 DK020572 and P30 DK089503).
Disclosure statement
The authors have nothing to disclose.
Acknowledgments
We thank E. Yokosawa and T. Sutton for mouse colony management and genotyping, Dr. E. Na for advice on icv injections, and Dr. M. Myers and members of the Low laboratory for their valuable suggestions on this project. We are grateful to Dr. J. Elmquist, University of Texas Southwestern Medical Center, for providing us with Pomc-Cre-ERT2 mice.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molmet.2016.07.012.
Conflict of interest
A.S. and M.S. are shareholders of XL-protein GmbH, the company that commercializes PASylation technology.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Glycosuria measured with urine glucose test strips during OGTT.
Altered body composition is the earliest detected metabolic phenotype in ArcPomc−/− mice. A) Body weight, B) Fat mass determined by NMR, C) Lean mass determined by NMR, D) Percentage of fat mass relative to body mass, E) Percentage of lean mass relative to body mass, and F) Ratio of lean to fat mass in female ArcPomc−/− (n = 8) and combined littermate ArcPomc+/+ (3) and ArcPomc+/− (n = ) mice at ages 3–6 wk. Two-way ANOVAs revealed significant main effects of genotype for each dependent variable (F1,55 = 16.9, P = 0.0001; F1,55 = 39.2, P < 0.0001; F1,55 = 7.4, P = 0.0089; F1,55 = 57.2, P < 0.0001; F1,55 = 17.5, P = 0.0001; and F1,55 = 31.6, P < 0.0001, respectively). *P < 0.05, **P < 0.01, ***P < 0.005, and ****P < 0.0001; Sidak's post-hoc multiple comparisons tests. Data shown are the mean ± SEM.
Relative Pomc mRNA levels in the hippocampus and brain stem (n = 4–6), ****P < 0.0001 vs. ArcPomc+/+ hypothalamus, Dunnett's multiple comparisons test following 1-way ANOVA (F4,17 = 253, P < 0.0001, main effect of group). Data shown are the mean ± SEM.
References
- 1.Wadden T.A. Treatment of obesity by moderate and severe caloric restriction: results of clinical research trials. Annals of Internal Medicine. 1993;119(7_Part_2):688–693. doi: 10.7326/0003-4819-119-7_part_2-199310011-00012. [DOI] [PubMed] [Google Scholar]
- 2.Rosenbaum M., Leibel R.L. 20 years of leptin: role of leptin in energy homeostasis in humans. Journal of Endocrinology. 2014;223(1):T83–T96. doi: 10.1530/JOE-14-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elliot D.L., Goldberg L., Kuehl K.S., Bennett W.M. Sustained depression of the resting metabolic rate after massive weight loss. The American Journal of Clinical Nutrition. 1989;49(1):93–96. doi: 10.1093/ajcn/49.1.93. [DOI] [PubMed] [Google Scholar]
- 4.Martin C.K., Heilbronn L.K., de Jonge L., DeLany J.P., Volaufova J., Anton S.D. Effect of calorie restriction on resting metabolic rate and spontaneous physical activity. Obesity (Silver Spring) 2007;15(12):2964–2973. doi: 10.1038/oby.2007.354. [DOI] [PubMed] [Google Scholar]
- 5.Byrne N.M., Wood R.E., Schutz Y., Hills A.P. Does metabolic compensation explain the majority of less-than-expected weight loss in obese adults during a short-term severe diet and exercise intervention? International Journal of Obesity (London) 2012;36(11):1472–1478. doi: 10.1038/ijo.2012.109. [DOI] [PubMed] [Google Scholar]
- 6.Kissileff H.R., Thornton J.C., Torres M.I., Pavlovich K., Mayer L.S., Kalari V. Leptin reverses declines in satiation in weight-reduced obese humans. The American Journal of Clinical Nutrition. 2012;95(2):309–317. doi: 10.3945/ajcn.111.012385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knuth N.D., Johannsen D.L., Tamboli R.A., Marks-Shulman P.A., Huizenga R., Chen K.Y. Metabolic adaptation following massive weight loss is related to the degree of energy imbalance and changes in circulating leptin. Obesity (Silver Spring) 2014;22(12):2563–2569. doi: 10.1002/oby.20900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fothergill E., Guo J., Howard L., Kerns J.C., Knuth N.D., Brychta R. Persistent metabolic adaptation 6 years after “The Biggest Loser” competition. Obesity (Silver Spring) 2016;24(8):1612–1619. doi: 10.1002/oby.21538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinsier R.L., Nagy T.R., Hunter G.R., Darnell B.E., Hensrud D.D., Weiss H.L. Do adaptive changes in metabolic rate favor weight regain in weight-reduced individuals? An examination of the set-point theory. The American Journal of Clinical Nutrition. 2000;72(5):1088–1094. doi: 10.1093/ajcn/72.5.1088. [DOI] [PubMed] [Google Scholar]
- 10.Quarta C., Sanchez-Garrido M.A., Tschop M.H., Clemmensen C. Renaissance of leptin for obesity therapy. Diabetologia. 2016;59(5):920–927. doi: 10.1007/s00125-016-3906-7. [DOI] [PubMed] [Google Scholar]
- 11.Guo J., Jou W., Gavrilova O., Hall K.D. Persistent diet-induced obesity in male C57BL/6 mice resulting from temporary obesigenic diets. PLoS One. 2009;4(4):e5370. doi: 10.1371/journal.pone.0005370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ravussin Y., Gutman R., Diano S., Shanabrough M., Borok E., Sarman B. Effects of chronic weight perturbation on energy homeostasis and brain structure in mice. American Journal of Physiology – Regulatory, Integrative and Comparative Physiology. 2011;300(6):R1352–R1362. doi: 10.1152/ajpregu.00429.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmitz J., Evers N., Awazawa M., Nicholls H.T., Bronneke H.S., Dietrich A. Obesogenic memory can confer long-term increases in adipose tissue but not liver inflammation and insulin resistance after weight loss. Molecular Metabolism. 2016;5(5):328–339. doi: 10.1016/j.molmet.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hao Z., Mumphrey M.B., Townsend R.L., Morrison C.D., Munzberg H., Ye J. Reprogramming of defended body weight after Roux-En-Y gastric bypass surgery in diet-induced obese mice. Obesity (Silver Spring) 2016;24(3):654–660. doi: 10.1002/oby.21400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ravussin Y., LeDuc C.A., Watanabe K., Mueller B.R., Skowronski A., Rosenbaum M. Effects of chronic leptin infusion on subsequent body weight and composition in mice: can body weight set point be reset? Molecular Metabolism. 2014;3(4):432–440. doi: 10.1016/j.molmet.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thaler J.P., Guyenet S.J., Dorfman M.D., Wisse B.E., Schwartz M.W. Hypothalamic inflammation: marker or mechanism of obesity pathogenesis? Diabetes. 2013;62(8):2629–2634. doi: 10.2337/db12-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bumaschny V.F., Yamashita M., Casas-Cordero R., Otero-Corchon V., de Souza F.S., Rubinstein M. Obesity-programmed mice are rescued by early genetic intervention. The Journal of Clinical Investigation. 2012;122(11):4203–4212. doi: 10.1172/JCI62543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lam D.D., de Souza F.S., Nasif S., Yamashita M., Lopez-Leal R., Otero-Corchon V. Partially redundant enhancers cooperatively maintain Mammalian pomc expression above a critical functional threshold. PLoS Genetics. 2015;11(2):e1004935. doi: 10.1371/journal.pgen.1004935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Jonghe B.C., Hayes M.R., Bence K.K. Melanocortin control of energy balance: evidence from rodent models. Cellular and Molecular Life Sciences. 2011;68(15):2569–2588. doi: 10.1007/s00018-011-0707-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kask A., Rago L., Wikberg J.E., Schioth H.B. Evidence for involvement of the melanocortin MC4 receptor in the effects of leptin on food intake and body weight. European Journal of Pharmacology. 1998;360(1):15–19. doi: 10.1016/s0014-2999(98)00699-2. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y., Kilroy G.E., Henagan T.M., Prpic-Uhing V., Richards W.G., Bannon A.W. Targeted deletion of melanocortin receptor subtypes 3 and 4, but not CART, alters nutrient partitioning and compromises behavioral and metabolic responses to leptin. FASEB Journal. 2005;19(11):1482–1491. doi: 10.1096/fj.05-3851com. [DOI] [PubMed] [Google Scholar]
- 22.Rahmouni K., Haynes W.G., Morgan D.A., Mark A.L. Role of melanocortin-4 receptors in mediating renal sympathoactivation to leptin and insulin. The Journal of Neuroscience. 2003;23(14):5998–6004. doi: 10.1523/JNEUROSCI.23-14-05998.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goncalves G.H., Li W., Garcia A.V., Figueiredo M.S., Bjorbaek C. Hypothalamic agouti-related peptide neurons and the central melanocortin system are crucial mediators of leptin's antidiabetic actions. Cell Reports. 2014;7(4):1093–1103. doi: 10.1016/j.celrep.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trevaskis J.L., Butler A.A. Double leptin and melanocortin-4 receptor gene mutations have an additive effect on fat mass and are associated with reduced effects of leptin on weight loss and food intake. Endocrinology. 2005;146(10):4257–4265. doi: 10.1210/en.2005-0492. [DOI] [PubMed] [Google Scholar]
- 25.Ceccarini G., Maffei M., Vitti P., Santini F. Fuel homeostasis and locomotor behavior: role of leptin and melanocortin pathways. Journal of Endocrinological Investigation. 2015;38(2):125–131. doi: 10.1007/s40618-014-0225-z. [DOI] [PubMed] [Google Scholar]
- 26.Halaas J.L., Gajiwala K.S., Maffei M., Cohen S.L., Chait B.T., Rabinowitz D. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269(5223):543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 27.Heymsfield S.B., Greenberg A.S., Fujioka K., Dixon R.M., Kushner R., Hunt T. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA. 1999;282(16):1568–1575. doi: 10.1001/jama.282.16.1568. [DOI] [PubMed] [Google Scholar]
- 28.Marsh D.J., Hollopeter G., Huszar D., Laufer R., Yagaloff K.A., Fisher S.L. Response of melanocortin-4 receptor-deficient mice to anorectic and orexigenic peptides. Nature Genetics. 1999;21(1):119–122. doi: 10.1038/5070. [DOI] [PubMed] [Google Scholar]
- 29.Ottaway N., Mahbod P., Rivero B., Norman L.A., Gertler A., D'Alessio D.A. Diet-induced obese mice retain endogenous leptin action. Cell Metabolism. 2015;21(6):877–882. doi: 10.1016/j.cmet.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seeley R.J., Yagaloff K.A., Fisher S.L., Burn P., Thiele T.E., van Dijk G. Melanocortin receptors in leptin effects. Nature. 1997;390(6658):349. doi: 10.1038/37016. [DOI] [PubMed] [Google Scholar]
- 31.Mizuno T.M., Kleopoulos S.P., Bergen H.T., Roberts J.L., Priest C.A., Mobbs C.V. Hypothalamic pro-opiomelanocortin mRNA is reduced by fasting in ob/ob and db/db mice, but is stimulated by leptin. Diabetes. 1998;47(2):294–297. doi: 10.2337/diab.47.2.294. [DOI] [PubMed] [Google Scholar]
- 32.Lam D.D., Attard C.A., Mercer A.J., Myers M.G., Jr., Rubinstein M., Low M.J. Conditional expression of Pomc in the Lepr-positive subpopulation of POMC neurons is sufficient for normal energy homeostasis and metabolism. Endocrinology. 2015;156(4):1292–1302. doi: 10.1210/en.2014-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cowley M.A., Smart J.L., Rubinstein M., Cerdan M.G., Diano S., Horvath T.L. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411(6836):480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- 34.Morath V., Bolze F., Schlapschy M., Schneider S., Sedlmayer F., Seyfarth K. PASylation of murine leptin leads to extended plasma half-life and enhanced in vivo efficacy. Molecular Pharmaceutics. 2015;12(5):1431–1442. doi: 10.1021/mp5007147. [DOI] [PubMed] [Google Scholar]
- 35.Bolze F., Morath V., Bast A., Rink N., Schlapschy M., Mocek S. Long-acting PASylated leptin ameliorates obesity by promoting satiety and preventing hypometabolism in leptin-deficient Lep(ob/ob) mice. Endocrinology. 2016;157(1):233–244. doi: 10.1210/en.2015-1519. [DOI] [PubMed] [Google Scholar]
- 36.Berglund E.D., Liu C., Sohn J.W., Liu T., Kim M.H., Lee C.E. Serotonin 2C receptors in pro-opiomelanocortin neurons regulate energy and glucose homeostasis. The Journal of Clinical Investigation. 2013;123(12):5061–5070. doi: 10.1172/JCI70338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chhabra K.H., Adams J.M., Fagel B., Lam D.D., Qi N., Rubinstein M. Hypothalamic POMC-deficiency improves glucose tolerance despite insulin resistance by increasing glycosuria. Diabetes. 2016;65(3):660–672. doi: 10.2337/db15-0804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mercer A.J., Stuart R.C., Attard C.A., Otero-Corchon V., Nillni E.A., Low M.J. Temporal changes in nutritional state affect hypothalamic POMC peptide levels independently of leptin in adult male mice. American Journal of Physiology. Endocrinology and Metabolism. 2014;306(8):E904–E915. doi: 10.1152/ajpendo.00540.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Banks W.A., DiPalma C.R., Farrell C.L. Impaired transport of leptin across the blood-brain barrier in obesity. Peptides. 1999;20(11):1341–1345. doi: 10.1016/s0196-9781(99)00139-4. [DOI] [PubMed] [Google Scholar]
- 40.Banks W.A., Coon A.B., Robinson S.M., Moinuddin A., Shultz J.M., Nakaoke R. Triglycerides induce leptin resistance at the blood–brain barrier. Diabetes. 2004;53(5):1253–1260. doi: 10.2337/diabetes.53.5.1253. [DOI] [PubMed] [Google Scholar]
- 41.Banks W.A., Farrell C.L. Impaired transport of leptin across the blood-brain barrier in obesity is acquired and reversible. American Journal of Physiology. Endocrinology and Metabolism. 2003;285(1):E10–E15. doi: 10.1152/ajpendo.00468.2002. [DOI] [PubMed] [Google Scholar]
- 42.Tolle V., Low M.J. In vivo evidence for inverse agonism of Agouti-related peptide in the central nervous system of proopiomelanocortin-deficient mice. Diabetes. 2008;57(1):86–94. doi: 10.2337/db07-0733. [DOI] [PubMed] [Google Scholar]
- 43.Enriori P.J., Evans A.E., Sinnayah P., Jobst E.E., Tonelli-Lemos L., Billes S.K. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metabolism. 2007;5(3):181–194. doi: 10.1016/j.cmet.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 44.Munzberg H., Flier J.S., Bjorbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology. 2004;145(11):4880–4889. doi: 10.1210/en.2004-0726. [DOI] [PubMed] [Google Scholar]
- 45.Frederich R.C., Hamann A., Anderson S., Lollmann B., Lowell B.B., Flier J.S. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nature Medicine. 1995;1(12):1311–1314. doi: 10.1038/nm1295-1311. [DOI] [PubMed] [Google Scholar]
- 46.Huszar D., Lynch C.A., Fairchild-Huntress V., Dunmore J.H., Fang Q., Berkemeier L.R. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88(1):131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 47.Overstreet L.S., Hentges S.T., Bumaschny V.F., de Souza F.S., Smart J.L., Santangelo A.M. A transgenic marker for newly born granule cells in dentate gyrus. The Journal of Neuroscience. 2004;24(13):3251–3259. doi: 10.1523/JNEUROSCI.5173-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Appleyard S.M., Bailey T.W., Doyle M.W., Jin Y.H., Smart J.L., Low M.J. Proopiomelanocortin neurons in nucleus tractus solitarius are activated by visceral afferents: regulation by cholecystokinin and opioids. The Journal of Neuroscience. 2005;25(14):3578–3585. doi: 10.1523/JNEUROSCI.4177-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Niikura K., Zhou Y., Ho A., Kreek M.J. Proopiomelanocortin (POMC) expression and conditioned place aversion during protracted withdrawal from chronic intermittent escalating-dose heroin in POMC-EGFP promoter transgenic mice. Neuroscience. 2013;236:220–232. doi: 10.1016/j.neuroscience.2012.12.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Page-Wilson G., Meece K., White A., Rosenbaum M., Leibel R.L., Smiley R. Proopiomelanocortin, agouti-related protein, and leptin in human cerebrospinal fluid: correlations with body weight and adiposity. American Journal of Physiology. Endocrinology and Metabolism. 2015;309(5):E458–E465. doi: 10.1152/ajpendo.00206.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zelissen P.M.J., Stenlof K., Lean M.E.J., Fogteloo J., Keulen E.T.P., Wilding J. Effect of three treatment schedules of recombinant methionyl human leptin on body weight in obese adults: a randomized, placebo-controlled trial. Diabetes, Obesity and Metabolism. 2005;7(6):755–761. doi: 10.1111/j.1463-1326.2005.00468.x. [DOI] [PubMed] [Google Scholar]
- 52.Benoit S.C., Schwartz M.W., Lachey J.L., Hagan M.M., Rushing P.A., Blake K.A. A novel selective melanocortin-4 receptor agonist reduces food intake in rats and mice without producing aversive consequences. The Journal of Neuroscience. 2000;20(9):3442–3448. doi: 10.1523/JNEUROSCI.20-09-03442.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burke L.K., Doslikova B., D'Agostino G., Greenwald-Yarnell M., Georgescu T., Chianese R. Sex difference in physical activity, energy expenditure and obesity driven by a subpopulation of hypothalamic POMC neurons. Molecular Metabolism. 2016;5(3):245–252. doi: 10.1016/j.molmet.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Knight Z.A., Hannan K.S., Greenberg M.L., Friedman J.M. Hyperleptinemia is required for the development of leptin resistance. PLoS One. 2010;5(6):e11376. doi: 10.1371/journal.pone.0011376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gamber K.M., Huo L., Ha S., Hairston J.E., Greeley S., Bjorbaek C. Over-expression of leptin receptors in hypothalamic POMC neurons increases susceptibility to diet-induced obesity. PLoS One. 2012;7(1):e30485. doi: 10.1371/journal.pone.0030485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Renquist B.J., Murphy J.G., Larson E.A., Olsen D., Klein R.F., Ellacott K.L.J. Melanocortin-3 receptor regulates the normal fasting response. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(23):E1489–E1498. doi: 10.1073/pnas.1201994109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen A.S., Marsh D.J., Trumbauer M.E., Frazier E.G., Guan X.M., Yu H. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nature Genetics. 2000;26(1):97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- 58.Nogueiras R., Wiedmer P., Perez-Tilve D., Veyrat-Durebex C., Keogh J.M., Sutton G.M. The central melanocortin system directly controls peripheral lipid metabolism. Journal of Clinical Investigation. 2007;117(11):3475–3488. doi: 10.1172/JCI31743. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Glycosuria measured with urine glucose test strips during OGTT.
Altered body composition is the earliest detected metabolic phenotype in ArcPomc−/− mice. A) Body weight, B) Fat mass determined by NMR, C) Lean mass determined by NMR, D) Percentage of fat mass relative to body mass, E) Percentage of lean mass relative to body mass, and F) Ratio of lean to fat mass in female ArcPomc−/− (n = 8) and combined littermate ArcPomc+/+ (3) and ArcPomc+/− (n = ) mice at ages 3–6 wk. Two-way ANOVAs revealed significant main effects of genotype for each dependent variable (F1,55 = 16.9, P = 0.0001; F1,55 = 39.2, P < 0.0001; F1,55 = 7.4, P = 0.0089; F1,55 = 57.2, P < 0.0001; F1,55 = 17.5, P = 0.0001; and F1,55 = 31.6, P < 0.0001, respectively). *P < 0.05, **P < 0.01, ***P < 0.005, and ****P < 0.0001; Sidak's post-hoc multiple comparisons tests. Data shown are the mean ± SEM.
Relative Pomc mRNA levels in the hippocampus and brain stem (n = 4–6), ****P < 0.0001 vs. ArcPomc+/+ hypothalamus, Dunnett's multiple comparisons test following 1-way ANOVA (F4,17 = 253, P < 0.0001, main effect of group). Data shown are the mean ± SEM.