Abstract
Objective
G protein-coupled receptor (GPCR) signaling regulates insulin secretion and pancreatic β cell-proliferation. While much knowledge has been gained regarding how GPCRs are activated in β cells, less is known about the mechanisms controlling their deactivation. In many cell types, termination of GPCR signaling is controlled by the family of Regulators of G-protein Signaling (RGS). RGS proteins are expressed in most eukaryotic cells and ensure a timely return to the GPCR inactive state upon removal of the stimulus. The aims of this study were i) to determine if RGS16, the most highly enriched RGS protein in β cells, regulates insulin secretion and β-cell proliferation and, if so, ii) to elucidate the mechanisms underlying such effects.
Methods
Mouse and human islets were infected with recombinant adenoviruses expressing shRNA or cDNA sequences to knock-down or overexpress RGS16, respectively. 60 h post-infection, insulin secretion and cAMP levels were measured in static incubations in the presence of glucose and various secretagogues. β-cell proliferation was measured in infected islets after 72 h in the presence of 16.7 mM glucose ± somatostatin and various inhibitors.
Results
RGS16 mRNA levels are strongly up-regulated in islets of Langerhans under hyperglycemic conditions in vivo and ex vivo. RGS16 overexpression stimulated glucose-induced insulin secretion in isolated mouse and human islets while, conversely, insulin secretion was impaired following RGS16 knock-down. Insulin secretion was no longer affected by RGS16 knock-down when islets were pre-treated with pertussis toxin to inactivate Gαi/o proteins, or in the presence of a somatostatin receptor antagonist. RGS16 overexpression increased intracellular cAMP levels, and its effects were blocked by an adenylyl cyclase inhibitor. Finally, RGS16 overexpression prevented the inhibitory effect of somatostatin on insulin secretion and β-cell proliferation.
Conclusions
Our results identify RGS16 as a novel regulator of β-cell function that coordinately controls insulin secretion and proliferation by limiting the tonic inhibitory signal exerted by δ-cell-derived somatostatin in islets.
Keywords: G protein-coupled receptor, Regulator of G protein signaling, Pancreatic beta-cell, Insulin secretion, Proliferation
Abbreviations: GPCR, G protein-coupled receptors; RGS, Regulators of G-protein Signaling; PTX, Bordetella pertussis toxin; SST, Somatostatin
Highlights
-
•
RGS16 is up-regulated under hyperglycemic conditions in islets.
-
•
RGS16 is a key regulator of insulin secretion and β-cell proliferation.
-
•
RGS16 attenuates Gαi/o protein activity downstream of δ-cell derived SST.
1. Introduction
A large number of G protein-coupled receptors (GPCRs) is expressed in islets of Langerhans [1], many of which are critical regulators of insulin secretion from the pancreatic β cell [2]. The therapeutic potential of targeting β-cell GPCRs to enhance insulin secretion for the treatment of type 2 diabetes is exemplified by several marketed agonists of the glucagon-like peptide-1 (GLP-1) receptor. Physiologically, activation of Gαs- and Gαq-coupled GPCRs (e.g., GLP-1 and M3 muscarinic receptors, respectively) increases insulin secretion, whereas agonists of Gαi/o-coupled GPCRs (e.g., somatostatin (SST) and α(2) adrenergic receptors) inhibit insulin release. The importance of Gαi/o-coupled GPCRs in insulin secretion was first described in studies investigating the effect of Bordetella pertussis toxin (PTX), a Gαi/o inhibitor [3]. More recently, transgenic mice specifically expressing PTX in β cells [4] and Gαo2 mutant mice [5] were shown to exhibit a dramatic increase in insulin secretion, enhanced glucose tolerance, and resistance to diet-induced diabetes. The effect of inhibitory G proteins on glucose homeostasis is not only due to their role in controlling insulin secretion but also related to their effect on β-cell proliferation. Indeed, Berger et al. [6] showed that activation of Gαi/o in postnatal β cells compromises β-cell mass and glucose homeostasis in adult mice, whereas inactivation of Gαi/o has the opposite effect. Hence, tonic Gαi/o activity limits β-cell function and mass.
While significant research efforts have been invested in identifying GPCR ligands and their activation mechanisms, much less is known as to how GPCR signaling is terminated in β cells. The duration and amplitude of the signal downstream of GPCRs are controlled by Regulators of G-protein Signaling (RGS), which accelerate the hydrolysis of GTP from Gα-protein subunits [7], [8]. RGS proteins are expressed in most eukaryotic cells, and the role of specific RGS subfamilies has been highlighted in the central nervous system [9], the immune system [10], the cardiovascular system [7], and cancer [11], but knowledge of their involvement in energy homeostasis is still limited. β-cell specific deletion of the RGS4 gene leads to a marked enhancement of insulin secretion in response to M3 muscarinic agonists [12]. RGS8 and RGS16 have been implicated in β-cell development [13], but their function in postnatal β cells is essentially unknown. RGS16 is among the 30 most enriched genes in the β cell [14], [15]. Thus, this study aimed to ascertain whether RGS16 regulates insulin secretion and β-cell proliferation and to identify the underlying mechanisms.
2. Materials and methods
2.1. Reagents and solutions
RPMI-1640 and FBS were from Invitrogen (Burlington, ON, Canada). Dulbecco's modified Eagle's medium (DMEM) was from Hyclone (ThermoFisher Scientific, Burlington, ON, Canada). Glucagon-like peptide-1 (GLP-1) and Somatostatin-14 (SST-14) were from Bachem (Torrance, CA). Cyclosomatostatin and 8-Bromo-cAMP were from Tocris (Minneapolis, MN, USA). Carbachol was from Calbiochem (Etobicoke, ON, Canada). Forskolin and Pertussis Toxin (PTX) were from Sigma (Oakville, ON, Canada).
2.2. Animal experiments
All procedures were approved by the Institutional Committee for the Protection of Animals at the Centre Hospitalier de l’Université de Montréal. Male Wistar rats (6 month-old) and C57BL/6N mice (12–14 week-old) (Charles River, Saint-Constant, QC, Canada) were housed under controlled temperature on a 12-h light/dark cycle with unrestricted access to water and standard laboratory chow. Six month-old Wistar rats were catheterized and infused with glucose or saline for 72 h as described in [16].
2.3. Islet isolation and cell culture
Rat and mouse islets were isolated by collagenase digestion and dextran density gradient centrifugation as described [17] and allowed to recover overnight in RPMI-1640 medium supplemented with 10% (vol/vol) FBS and 11.1 mM glucose. The mouse insulin-secreting MIN6 cell line (passage 20–30; provided by Jun-ichi Miyazaki, Osaka University Graduate School of Medicine, Suita, Osaka, Japan) were cultured in DMEM containing 25 mM glucose, 4 mM l-glutamine and 44 mM NaHCO3 and supplemented with 10% FBS, 1 mM sodium pyruvate and 0.005% β-mercaptoethanol.
2.4. Human islets
The use of human islets was approved by the Institutional Ethics Committee of the Centre Hospitalier de l’Université de Montréal. Isolated islets from non-diabetic human cadaveric donors were provided by the Integrated Islet Distribution Program sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases, the Alberta Diabetes Institute Islet Core at the University of Alberta, and the Clinical Islet Laboratory at the University of Alberta.
2.5. RNA preparation and quantitative real-time PCR
Total RNA was isolated from batches of 100–150 islets each using the RNeasy Qiagen microkit (Qiagen, Toronto, ON, Canada) and from MIN6 cells using TRIzol (ThermoFisher). RNA was quantified by spectrophotometry using NanoDrop 2000, and 1 μg of RNA was reverse-transcribed. PCR was carried out by using QuantiTect SYBR Green PCR kit (Qiagen). Results are expressed as the ratio of target mRNA to housekeeping gene mRNA and normalized to the levels in control islets.
2.6. Adenoviral infection
MIN6 cells were infected with 100 plaque-forming units (pfu) of adenoviruses/cell overnight, after which the medium was replaced with complete medium and cultured for an additional 24 h. Infected cells were then cultured in DMEM supplemented with 10% FBS at 25 mM glucose. Mouse islets were infected with adenoviruses after partial dissociation to ensure penetration of the viruses into the islet core [18]. After isolation, islets were washed twice with 1 ml dissociation buffer (1× Hank's balanced salt solution (HBSS), 20 mM HEPES, 5 mM glucose and 1 mM EGTA) and incubated for 3 min at 37 °C with 0.5 ml dissociating buffer. Islets were then infected with adenovirus (1 × 107 pfu of adenoviruses/100 islets) overnight, after which the medium was replaced with complete medium and cultured for an additional 48 h.
2.7. Perifusions and static incubations
Islet perifusions were performed as described [18]. For static incubations, batches of ten islets each were starved twice in KRB solution containing 0.1% (wt/vol.) BSA and 2.8 mM glucose for 20 min at 37 °C then incubated for 1 h in the presence of various secretagogues as described in Results. For static incubations of MIN6 cells, cells were seeded in 24-well plates at a density of 9 × 105 cells/well the day of infection. 48 h later the cells were washed twice in KRB containing 0.1% (wt/vol) BSA and 2.8 mM glucose for 30 min at 37 °C and then incubated for 1 h in the presence of 10 μM forskolin and various secretagogues as described in Results. Each condition was run in triplicate. Intracellular insulin content was measured after acid–alcohol extraction. Insulin was measured by AlphaLISA (Perkin Elmer, Waltham, MA, USA) for islet static incubations, or radioimmunoassay using a rat insulin RIA kit (Millipore, Billerica, MA) for perifusion and MIN6 cell experiments.
2.8. Proliferation assay
Isolated islets were cultured for 72 h with or without 100 nM SST. The medium was changed every 24 h. At the end of treatment, cells were embedded in OCT compound, frozen, and sectioned. Then, islet sections were fixed and stained for insulin (INS) and the proliferative markers phosphohistone-H3 (PHH3) and Ki67 for mouse and human islets respectively, as described previously [19]. Proliferation was calculated as the percentage of double-positive PHH3+/INS+ or Ki67+/INS+ cells over the total INS+ population (>1500 cells).
2.9. Statistics
Data are expressed as mean ± SEM and were analyzed by one or two-way ANOVA with Bonferroni post-hoc adjustment for multiple comparisons or Student's unpaired t-tests where appropriate. p < 0.05 was considered significant.
3. Results
3.1. RGS16 is up-regulated in islets in hyperglycemic conditions
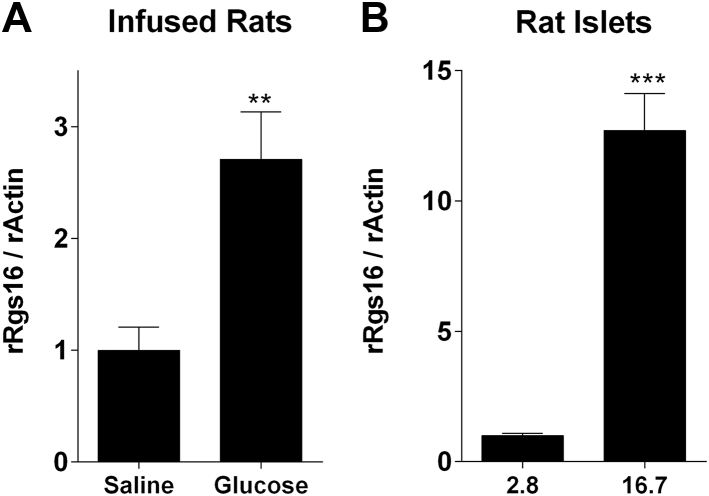
We investigated whether RGS16 gene expression is responsive to hyperglycemic conditions in rodents and humans islets. Adult male Wistar rats infused with glucose for 72 h (average glycemia 12.4 ± 1.2 mM) displayed a 2.7 fold increase of RGS16 mRNA (n = 5) in comparison to saline-infused animals (average glycemia 6.0 ± 0.2 mM) (Figure 1A). Exposure of isolated rat islets to 16.7 mM glucose for 24 h increased RGS16 mRNA 12.7 fold compared to control islets exposed to 2.8 mM glucose (Figure 1B). Thus, RGS16 is up-regulated under conditions of hyperglycemia, suggesting a potential role in the control of β-cell function.
Figure 1.
Glucose increases RGS16 mRNA levels in vitro and in vivo. (A) rRGS16 mRNA was measured in 6-month-old Wistar rats infused with glucose or saline for 72 h (n = 5). (B) rRGS16 mRNA was measured in isolated rat islets exposed to 2.8 or 16.7 mM glucose for 24 h (n = 6). mRNA was determined by qRT-PCR and normalized to rat actin or mouse TATA-binding protein (mTBP) for mouse islets. Data are expressed as mean ± SEM. **p < 0.01, ***p < 0.001, as compared to the corresponding control.
3.2. RGS16 regulates insulin secretion in mouse and human islets
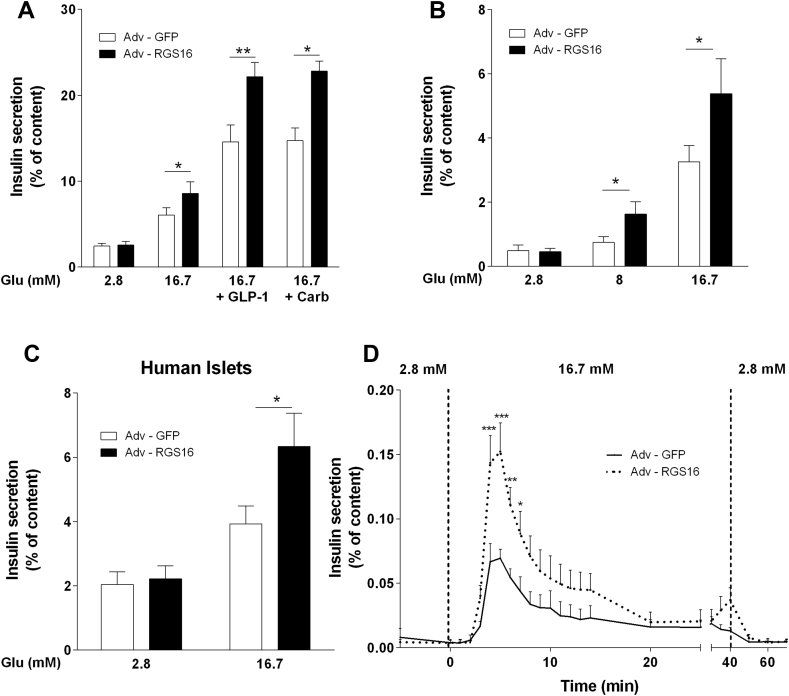
We first overexpressed the human form of RGS16 in mouse islets by adenoviral transduction and assessed insulin secretion in 1-h static incubations in response to 16.7 mM glucose alone or in the presence of GLP-1 (100 nM) or the muscarinic agonist carbachol (0.5 mM). Overexpression of RGS16 significantly increased insulin release in response to 16.7 mM glucose (8.6 ± 1.3 vs. 6.0 ± 0.9% of insulin content in islets infected with the negative control Adv-GFP; n = 7; p < 0.05; Figure 2A). Similarly, the potentiation of glucose-stimulated insulin secretion by GLP-1 and carbachol was enhanced in RGS16-overexpressing islets (Figure 2A). Given that insulin secretion to all secretagogues tested was affected by RGS16 overexpression, we surmised that RGS16 regulates insulin secretion by enhancing the response to glucose. Indeed, RGS16 overexpression amplified the insulin secretory response to intermediate glucose concentrations (Figure 2B). In agreement with the results obtained in mouse islets, adenoviral overexpression of RGS16 in human islets (Figure S1A) also led to a significant increase of insulin release in response to 16.7 mM glucose (6.3 ± 1.0 vs. 3.9 ± 0.6% of insulin content in Adv-GFP-infected islets; n = 6–8; p < 0.05; Figure 2C).
Figure 2.
RGS16 overexpression augments insulin secretion in mouse and human islets. Isolated mouse (n = 4–7) (A, B, D) or human (n = 8) (C) islets were infected with Adv-RGS16 or Adv-GFP as a control. (A–C) Insulin secretion was determined in 1-h static incubations in response to glucose without or with GLP-1 (100 nM) or carbachol (Carb; 0.5 mM). (D) Insulin secretion was assessed in perifusion experiments. Insulin levels are expressed as a percentage of total islet insulin content and are mean ± SEM. *p < 0.05; **p < 0.01 vs. Adv-GFP control.
To determine the effect of RGS16 on the kinectics of insulin release, insulin secretion was measured in perifusion experiments of mouse islets infected with Adv-GFP or Adv-RGS16 (Figure 2D). Islets overexpressing RGS16 showed a significantly higher peak of first phase insulin secretion (0.15 ± 0.02 vs. 0.07 ± 0.01% of insulin content at t = 5 min compared to Adv-GFP infected islets; n = 6; p < 0.001). Taken together, these results indicate that RGS16 is a positive regulator of insulin release.
3.3. RGS16 mitigates SST-induced Gαi/o activity to promote insulin secretion
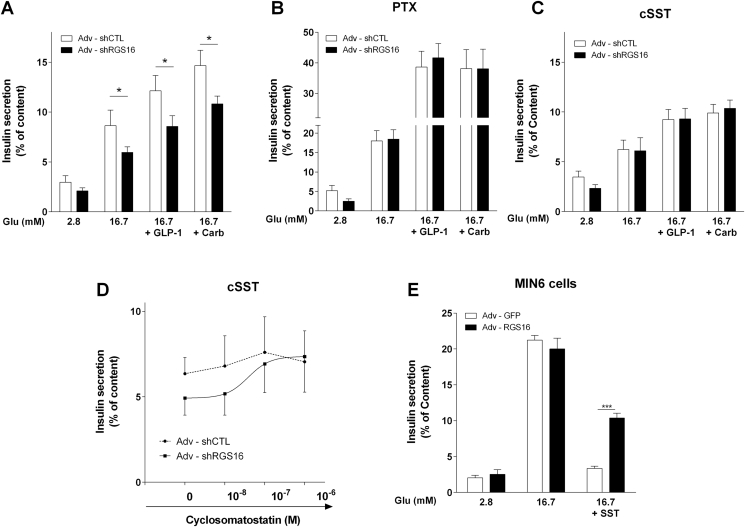
RGS16 is an established negative regulator of Gαi/o [19]. Tonic Gαi/o activity in islets dampens insulin secretion [4], [5], [20]. Therefore, we asked whether RGS16 inhibits Gαi/o to promote insulin secretion. To examine this possibility, we knocked down RGS16 by adenoviral transduction of shRNA in mouse islets, which led to an approximately 60% reduction in RGS16 mRNA levels (Figure S1B). Islets infected with Adv-shRGS16 displayed a significant decrease in glucose-induced insulin release (5.9 ± 0.5 vs. 8.6 ± 1.5% of insulin content in islets infected with the negative control Adv-shCTL; n = 6; p < 0.05) as well as its potentiation by GLP-1 or carbachol (Figure 3A). As expected, pretreatment of islets with PTX led to a large increase in insulin secretion due to alleviation of endogenous inhibitory Gαi/o signaling [21] (Figure 3B). Importantly, PTX pretreatment completely prevented the decrease in insulin secretion upon RGS16 knockdown.
Figure 3.
RGS16 knockdown inhibits insulin secretion and is sensitive to PTX and SSTR antagonism. (A–D) Isolated mouse islets were infected with Adv–shRGS16 or Adv-shCTL (scrambled) as a control. Insulin secretion was assessed in 1-h static incubations in response to glucose without or with GLP-1 (100 nM) or carbachol (Carb; 0.5 mM) in control islets (n = 6–12) (A), after 16 h pretreatment with pertussis toxin (PTX; 100 ng/ml; n = 6) (B), or in the presence of cyclosomatostatin (cSST) at 1 μM (n = 3) (C) or increasing cSST concentrations (n = 4) (D). (E) MIN6 cells were infected with Adv–RGS16 or Adv-GFP as a control and insulin secretion was assessed in 1-h static incubations in response to glucose with or without 100 nM SST-14 (n = 4). Insulin levels are expressed as % of total islet insulin content and are mean ± SEM. *p < 0.05, ***p < 0.001, as compared to the corresponding control.
δ-cell-derived SST is a major contributor to tonic Gαi/o activity in isolated islets [22]. To test whether RGS16 modulates SST signaling, islets were incubated in the presence of the competitive antagonist of all SST receptor (SSTR) isoforms, cyclosomatostatin (cSST; Figure 3C,D). cSST eliminated the ability of RGS16 knock-down to inhibit insulin secretion in response to glucose as well as its potentiation by GLP-1 or carbachol (Figure 3C). These observations suggest that RGS16 limits PTX-sensitive inhibitory G protein activity downstream of SSTR.
As SST secretion from δ-cells is stimulated by glucose [23] and the adenoviral constructs used to manipulate RGS16 expression are active in all islet cell types, we first verified if RGS16 overexpression inhibits SST release by δ-cells. RGS16-overexpressing islets displayed a significant increase in SST secretion in response to 16.7 mM glucose (Figure S2), which would be expected to result in lower insulin secretion in response to glucose. Second, neither RGS16 knock-down nor overexpression changed the mRNA levels of SSTR -2 and -5, the major SSTR isoforms acting in α and β cells, respectively [24] (Figure S3A, B) or of Gαi/o isoforms (Figure S3C, D).
Collectively, the aforementioned results suggest that RGS16 modulates endogenous, δ-cell-derived SST signaling in isolated islets. To further corroborate these findings, we reasoned that RGS16 overexpression in a clonal β-cell line should not affect the insulin secretory response to glucose, given the absence of endogenous SST production. Accordingly, insulin-secreting MIN6 cells infected with Adv-RGS16 displayed a similar level of insulin release in response to 16.7 mM glucose compared to cells infected with the negative control Adv-GFP (Figure 3E). Yet, as expected RGS16 overexpression partially reversed the inhibitory effect of exogenous SST on insulin secretion (10.4 ± 0.7% vs. 3.4 ± 0.3% of insulin content in Adv-GFP infected cells; n = 4; p < 0.001).
3.4. RGS16 promotes β-cell proliferation in mouse and human islets by attenuating the inhibitory effect of SST
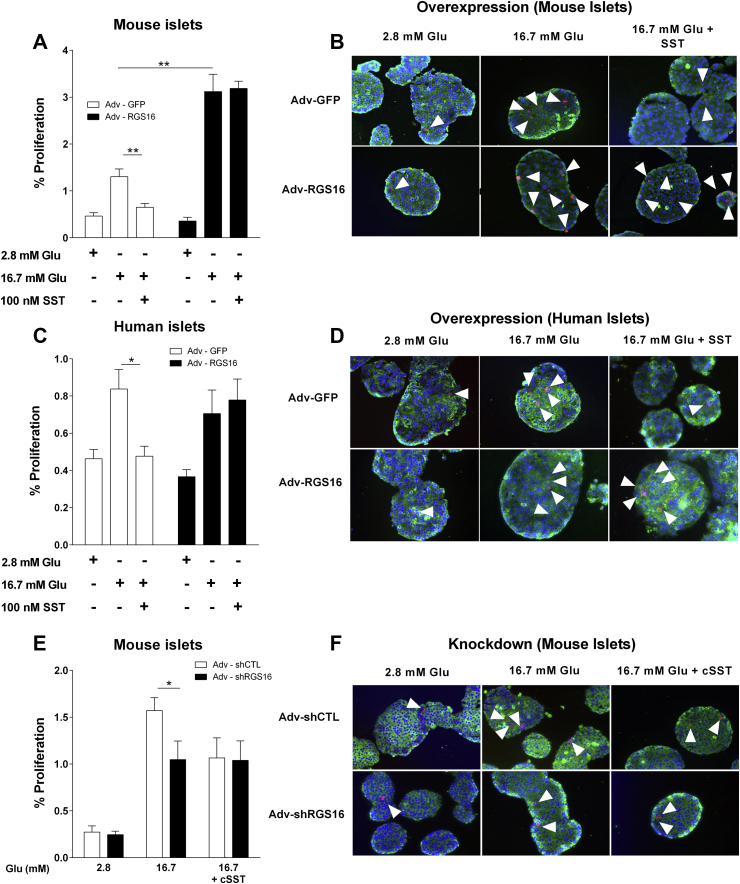
In addition to repressing insulin secretion, tonic PTX-sensitive G protein activity is known to negatively regulate β-cell proliferation [6]. Thus, we investigated the role of RGS16 in β-cell proliferation in mouse and human islets ex vivo in response to glucose in the presence or absence of SST. Whereas a 72-h exposure to 16.7 mM glucose alone induced β-cell proliferation in control, Adv-GFP-infected islets, the proliferative response was essentially eliminated in the presence of SST (0.6 ± 0.1 vs. 1.3 ± 0.2% of proliferative cells in control islets; n = 6; p < 0.01; Figure 4A,B). Islets infected with Adv-RGS16 displayed a significant increase in β-cell proliferation in response to 16.7 mM glucose (3.1 ± 0.4 vs. 1.3 ± 0.2% of proliferative cells in Adv-GFP-infected islets; n = 6; p < 0.01) but were completely insensitive to the addition of SST (Figure 4A,B). Similarly, SST inhibition of glucose-induced β-cell proliferation was eliminated in human islets overexpressing RGS16 (0.8 ± 0.1 vs. 0.5 ± 0.1% of proliferative cells in Adv-GFP infected islets; n = 8; p < 0.05; Figure 4C,D).
Figure 4.
RGS16 promotes β-cell proliferation and mitigates the inhibitory effect of SST. Mouse (n = 6) (A, B, E, F) and human (n = 8) (C, D) islets were infected with Adv-RGS16 (A–D) or Adv-shRGS16 (E, F) or their respective controls (i.e., Adv-GFP or Adv-shCTL, respectively). Islets were cultured in the presence of 2.8 or 16.7 mM with or without 100 nM SST or 1 μM cSST for 72 h. Quantification (A, C, E) and representative images (B, D, F) of phosphohistone H3 (PHH3) (B, F) or Ki67 (D) (red), insulin (green) and Dapi (nuclei; blue) staining. Proliferation rate was calculated as the percentage of double-positive PHH3+/INS+ or Ki67+/INS+ cells over the total INS+ population. Data are mean ± S.E.M. *p < 0.05; **p < 0.01 vs. 2.8 mM glucose condition.
As RGS16 overexpression increased β-cell proliferation in mouse islets, we determined whether RGS16 dampens δ-cell-derived SST signaling to promote β-cell proliferation (Figure 4E,F). Islets infected with Adv-shRGS16 displayed a significant decrease in β-cell proliferation in response to glucose (1.0 ± 0.2 vs. 1.6 ± 0.1% of proliferative cells in Adv-shCTL-infected islets; n = 6; p < 0.05). As observed for insulin secretion (Figure 3C), cSST eliminated the effect of RGS16 knock-down on β-cell proliferation in response to glucose. Taken together, these observations suggest that RGS16 limits inhibitory signaling downstream of SSTR.
3.5. RGS16 regulation of insulin secretion and β-cell proliferation is cAMP-dependent
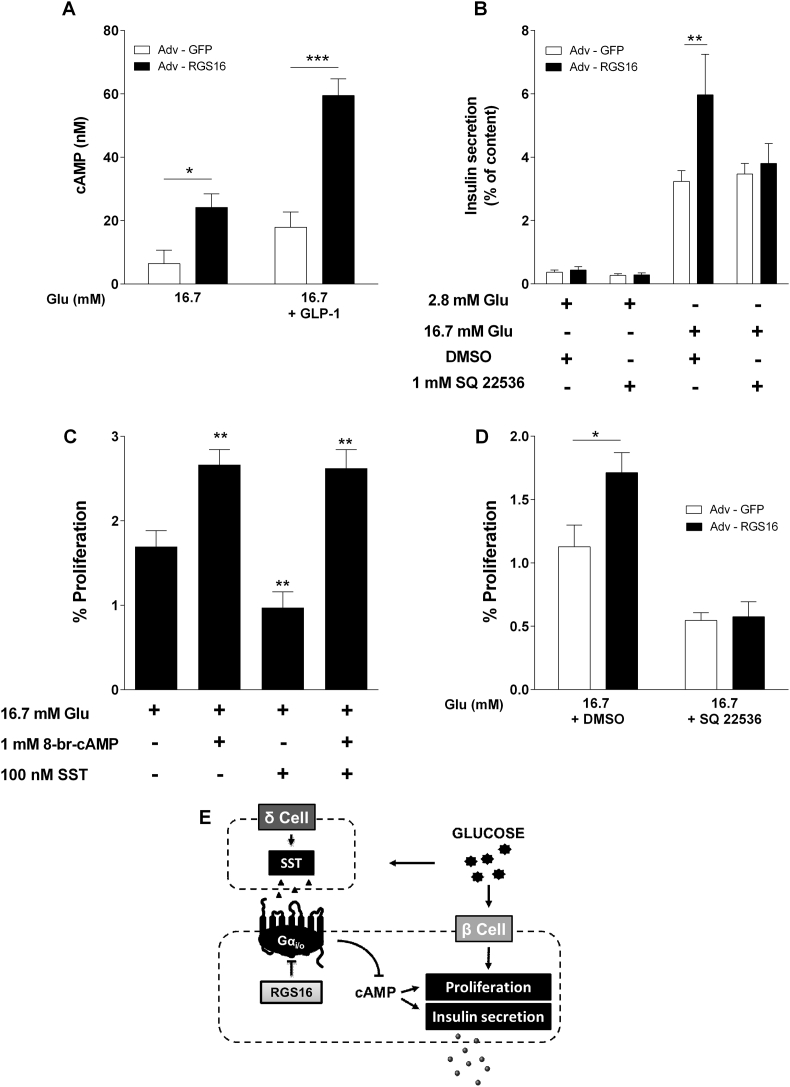
Given that inhibition of insulin secretion by SST involves down-regulation of adenylyl cyclase (AC) activity and consequent reductions in intracellular cAMP levels [23], we asked whether cAMP levels are affected by RGS16 and whether this is required for its effects on insulin secretion and β-cell proliferation. As shown in Figure 5A, islets infected with Adv-RGS16 displayed a significant increase in cAMP levels in response to 16.7 mM glucose (24.2 ± 4.3 vs. 6.4 ± 4.3 nM in Adv-GFP-infected islets; n = 4–7; p < 0.05) and GLP-1 (59.5 ± 5.3 vs. 17.9 ± 4.8 nM in Adv-GFP-infected islets; n = 4–7; p < 0.001). Interestingly, the increase in glucose-induced insulin secretion upon RGS16 overexpression was completely eliminated in the presence of the AC inhibitor SQ22536 (Figure 5B). These observations suggest that RGS16 promotes insulin secretion by increasing intracellular cAMP production.
Figure 5.
RGS16 modulation of insulin secretion and β-cell proliferation involves cAMP. Isolated mouse islets were infected with Adv–RGS16 or Adv-GFP (A, B, D). (A) cAMP levels were measured in response to 16.7 mM glucose with or without 100 nM GLP-1 (n = 4–7). (B) Insulin secretion was assessed in 1-h static incubations in response to glucose in the presence of 1 mM adenylyl cyclase (AC) inhibitor SQ 22536 (n = 6). (C) Islets were cultured for 72 h in the presence of 16.7 mM with or without 100 nM SST and/or 1 mM 8-br-cAMP (n = 6). (D) Islets were cultured in the presence of 16.7 mM with 1 mM SQ 22536 or DMSO for 72 h (n = 6). Insulin levels are expressed as a percentage of total islet insulin content and are mean ± SEM. Proliferation was calculated as the percentage of double-positive PHH3+/INS+ for mouse cells over the total INS+ population. *p < 0.05, **p < 0.01, ***p < 0.001, as compared to the corresponding control. (E) Proposed model for RGS16 action on insulin secretion and β-cell proliferation.
The key role of cAMP in cell growth and proliferation [25] and the cAMP-dependent effects of RGS16 on insulin secretion (Figure 5A,B) suggest that RGS16 might also control β-cell proliferation by regulating cAMP levels. To test this hypothesis, we first asked whether the inhibitory effect of SST on cAMP levels could account for its inhibitory effect on glucose-induced β-cell proliferation. Mouse islets treated with 16.7 mM glucose in the presence of 8-Br-cAMP, a cell-permeable cAMP analog, displayed a significant increase in β-cell proliferation in comparison to glucose alone (2.7 ± 0.2 vs. 1.7 ± 0.2% of proliferating cells in control islets; n = 6; p < 0.01; Figure 5C, Figure S4A). Importantly, SST inhibition of β-cell proliferation was completely prevented when islets were co-incubated with 8-Br-cAMP. Accordingly, blocking cAMP production with the AC inhibitor SQ22536 completely eliminated the ability of RGS16 to stimulate β-cell proliferation (Figure 5D, Figure S4B). These findings demonstrate that RGS16 positively regulates insulin secretion and β-cell proliferation through a cAMP-dependent mechanism.
4. Discussion
This study aimed to determine the role of RGS16 in insulin secretion and β-cell proliferation. We first identified RGS16 as a gene markedly induced in hyperglycemic conditions in islets, both in vivo and in vitro. We then showed that overexpression of RGS16 in isolated islets promotes glucose-stimulated insulin secretion and its potentiation by GPCR agonists. Conversely, knockdown of RGS16 in isolated mouse islets decreases insulin secretion in response to glucose and its potentiation by GLP-1 and carbachol. Importantly, these effects are conserved in human islets. RGS16 effects on insulin secretion are PTX-sensitive, cAMP-dependent, and downstream of SSTR signaling. Finally, RGS16 also controls β-cell proliferation through a cAMP-dependent mechanism. Our findings support the notion that RGS16 limits SST signaling and cAMP production in β cells to promote insulin secretion and proliferation.
Our observation that RGS16 is a glucose-responsive gene is consistent with data from Villasenor et al. [13] demonstrating increased RGS16 expression in islets from hyperglycemic mice. Interestingly, RGS16 expression in insulin-secreting INS 832/13 cells was recently demonstrated to be controlled by Carbohydrate Response Element Binding Protein, a transcription factor regulating the expression of a variety of glucose-responsive genes [26]. Given that RGS16 is among the most highly enriched transcripts in the β cell [15], these observations suggest a potential role for RGS16 in islet function.
RGS16 is an established negative regulator of G protein signaling. More specifically, RGS16 regulates Gαq and Gαi/o subunits [27]. Both pathways play key roles in the regulation of insulin secretion by GPCRs. Specifically, Gαi/o-coupled GPCRs negatively regulate, while Gαq-coupled receptors activate, insulin release [4], [28]. Given that RGS16 overexpression stimulates – and its knockdown inhibits – insulin secretion, we surmised that RGS16 represses Gαi/o-mediated inhibition of insulin secretion rather than Gαq signaling. This possibility is supported by experiments using PTX and led us to conclude that the major targets of RGS16 are PTX-sensitive Gαi/o subunits. We did not investigate the specific Gαi/o subtypes modulated by RGS16 in this context. Although Wang et al. [5] reported that Gαo2 is the isoform responsible for the increased insulin release in PTX-treated β cells, it is unclear whether Gαo2 inhibits adenylyl cyclase [29]. Therefore, the specific β-cell Gαi/o isoforms regulated by RGS16 remain to be identified.
Upon glucose stimulation, several autocrine and paracrine GPCR ligands are released from islet cells [28]. Among these, it is well established that SST secreted by δ cells exerts a tonic inhibition of insulin secretion ex vivo [22]. Ghrelin is also present in α and ε cells in islets [30], [31] and was recently shown to promote SST release from δ cells [32]. Similarly, urocortin 3 released by β cells potentiates SST secretion in response to glucose [33]. Our conclusion that RGS16 primarily represses endogenous SST signaling in islets is supported by several observations: i) as reported by Hauge-Evans et al. [22], exogenous SST failed to inhibit insulin secretion in control islets (data not shown), consistent with tonic inhibition of insulin secretion by endogenous δ-cell-derived SST that cannot be further repressed by exogenous SST; ii) the effect of RGS16 in islets was lost in the presence of the SSTR antagonist cSST; iii) in contrast to isolated islets, in insulin-secreting MIN6 cells, RGS16 overexpression did not alter insulin secretion in response to glucose alone but dampened the inhibitory effect of exogenous SST. These observations establish an important role for RGS16 in intra-islet communication between β and δ cells. Of note, the increase in insulin secretion upon RGS16 overexpression is not merely due to inhibition of SST secretion which, to the contrary, is increased in RGS16-overexpressing islets.
cAMP is a key regulator of insulin exocytosis [34]. Schwede et al. [35] recently reported that first phase insulin secretion is cAMP-dependent in rat and human islets, which is consistent with our perifusion experiments in mouse islets. cAMP is generated by AC activity mostly in response to Gαs-coupled receptor agonists, but also in response to glucose metabolism [36]. The regulation of cAMP levels by RGS16 in islets is entirely consistent with a mechanism of action involving Gαi/o activity. In addition, elimination of RGS16's effects on insulin secretion upon inhibition of AC clearly points to cAMP as an main effector of RGS16 activity in β cells. Thus, our data indicate that RGS16 limits PTX-sensitive Gαi/o signaling, thereby increasing intracellular cAMP levels and amplifying first-phase insulin release. It is also conceivable that RGS16 modulates cAMP levels via G protein-independent mechanisms, since RGS proteins can exert non-canonical functions distinct from the inactivation of Gα subunits [37]. For example, RGS2 can directly interact with and regulate AC activity [38]. In addition, RGS proteins have been described to interact with G protein-activated inwardly rectifying K+ channels [39], which are functional in β cells [40]. In the present study, we have not examined such potential interactions of RGS16 with proteins other than G proteins. However, given the complete absence of RGS16's effects in islets following PTX pretreatment and upon inhibition of AC, it is highly likely that RGS16 primarily modulates insulin secretion via regulation of Gαi/o activity.
In agreement with the study by Yoshitomi et al. [41] showing that SST significantly suppresses MIN6 cell growth, we show here, to our knowledge for the first time, that SST inhibits glucose-induced β-cell proliferation in human islets. This is also consistent with studies in SSTR-1 and -5 knockout mice showing that SST signaling negatively regulates β-cell proliferation and mass [42], [43]. Importantly, our results uniquely demonstrate that the inhibitory effect of SST on β-cell proliferation is regulated by RGS16, both in mouse and human islets. Furthermore by exposing islets to cSST we confirmed that RGS16 controls δ-cell-derived SST signaling to promote β-cell proliferation. Surprisingly, cSST, which alleviates endogenous SST signaling, does not increase β-cell proliferation or insulin secretion in response to glucose. We attribute this unexpected finding to additional, non-specific effects that have been described for this compound [44].
In mouse but not in human islets, RGS16 overexpression induces a large increase in β-cell proliferation in response to glucose, a difference that could be explained by the much lower rate of replication in human β cells and major differences in the control of β-cell proliferation between the two species [45]. Nevertheless, in both human and mouse islets, the inhibitory effect of SST on glucose-induced β-cell proliferation is completely eliminated upon RGS16 overexpression. As observed for insulin secretion, this effect requires cAMP generation. In contrast to the effects shown here on β-cell proliferation, in breast cancer cells, RGS16 was shown to negatively regulate cell proliferation through a G protein-independent mechanism that involves an interaction with the p85α subunit of phosphatidylinositol-3-kinase and inhibition of downstream signaling [46]. This suggests that RGS16 may have significantly different physiological effects and mechanisms of action depending on the cellular context.
5. Conclusion
In summary, our results demonstrate that in pancreatic β cells, RGS16 limits the inhibitory effect of SST on insulin secretion and β-cell proliferation by dampening Gαi/o activity downstream of SSTR and, consequently, increasing intracellular cAMP levels (Figure 5E). Since RGS16 expression is strongly stimulated by glucose, these observations suggest a role for RGS16 in β-cell compensation to increased metabolic demand whereby releasing a tonic “break” on insulin secretion and β-cell proliferation enables the β cell to quickly adjust to a changing metabolic environment. The importance of Gαi/o activity in β-cell compensation is well established. For example, overexpression of the PTX S1 catalytic subunit [4] or genetic deletion of the Gαi/o-coupled free fatty acid receptors −2 and −3 [47] protects mice against diet-induced glucose intolerance. Importantly, a polymorphism in the gene encoding the Gαi-coupled α-(2A) adrenergic receptor in humans is associated with diminished insulin secretion and increased risk for type 2 diabetes [48]. However, whether RGS16 in β-cells contributes to glucose homeostasis and β-cell compensation remains to be examined in vivo.
Author contributions
K.V. conceived and performed the study, researched data, analyzed the results, and wrote the manuscript. V.S.M., B.Z., C.T., A.D.M., H.M. researched data and analyzed the results. J.G. and V.P. conceived the study, analyzed the results, and wrote the manuscript. K.V. and V.P. are the guarantor of this work and, as such, take full responsibility for the work.
Acknowledgments
This study was supported by the Canadian Institutes of Health Research (MOP 86545 to V.P.). V.P. holds the Canada Research Chair in Diabetes and Pancreatic Beta-Cell Function. K.V., V.S.M. and A.D.M. were supported by postdoctoral fellowships from the Fond de Recherche Québec Santé, the CRCHUM, and the Canadian Diabetes Association, respectively. H.M. was supported by a studentship from the Fond de Recherche Québec Santé. We thank M. Guévremont, J. Morin and the Cellular Physiology Service core of the CRCHUM for quantification of AlphaLISA assay; and G. Dodier (CRCHUM) for valuable technical assistance. The authors gratefully acknowledge E. Joly for technical advice on enzymatic assays.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molmet.2016.08.010.
Conflict of interest
None declared.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Amisten S., Salehi A., Rorsman P., Jones P.M., Persaud S.J. An atlas and functional analysis of G-protein coupled receptors in human islets of Langerhans. Pharmacology & Therapeutics. 2013;139:359–391. doi: 10.1016/j.pharmthera.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Ahrén B. Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nature Reviews Drug Discovery. 2009;8:369–385. doi: 10.1038/nrd2782. [DOI] [PubMed] [Google Scholar]
- 3.Katada T. The inhibitory G protein G(i) identified as pertussis toxin-catalyzed ADP-ribosylation. Biological & Pharmaceutical Bulletin. 2012;35:2103–2111. doi: 10.1248/bpb.b212024. [DOI] [PubMed] [Google Scholar]
- 4.Regard J.B., Kataoka H., Cano D.A., Camerer E., Yin L., Zheng Y.W. Probing cell type-specific functions of Gi in vivo identifies GPCR regulators of insulin secretion. Journal of Clinical Investigation. 2007;117:4034–4043. doi: 10.1172/JCI32994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y., Park S., Bajpayee N.S., Nagaoka Y., Boulay G., Birnbaumer L. Augmented glucose-induced insulin release in mice lacking G(o2), but not G(o1) or G(i) proteins. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:1693–1698. doi: 10.1073/pnas.1018903108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berger M., Scheel D.W., Macias H., Miyatsuka T., Kim H., Hoang P. Galphai/o-coupled receptor signaling restricts pancreatic beta-cell expansion. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:2888–2893. doi: 10.1073/pnas.1319378112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kimple A.J., Bosch D.E., Giguère P.M., Siderovski D.P. Regulators of G-protein signaling and their Galpha substrates: promises and challenges in their use as drug discovery targets. Pharmacological Reviews. 2011;63:728–749. doi: 10.1124/pr.110.003038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slep K.C., Kercher M.A., Wieland T., Chen C.K., Simon M.I., Sigler P.B. Molecular architecture of Galphao and the structural basis for RGS16-mediated deactivation. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:6243–6248. doi: 10.1073/pnas.0801569105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson G.R., Posokhova E., Martemyanov K.A. The R7 RGS protein family: multi-subunit regulators of neuronal G protein signaling. Cell Biochemistry and Biophysics. 2009;54:33–46. doi: 10.1007/s12013-009-9052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bansal G., Xie Z., Rao S., Nocka K.H., Druey K.M. Suppression of immunoglobulin E-mediated allergic responses by regulator of G protein signaling 13. Nature Immunology. 2008;9:73–80. doi: 10.1038/ni1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hurst J.H., Hooks S.B. Regulator of G-protein signaling (RGS) proteins in cancer biology. Biochemical Pharmacology. 2009;78:1289–1297. doi: 10.1016/j.bcp.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 12.Ruiz de Azua I., Scarselli M., Rosemond E., Gautam D., Jou W., Gavrilova O. RGS4 is a negative regulator of insulin release from pancreatic beta-cells in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:7999–8004. doi: 10.1073/pnas.1003655107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Villasenor A., Wang Z.V., Rivera L.B., Ocal O., Asterholm I.W., Scherer P.E. Rgs16 and Rgs8 in embryonic endocrine pancreas and mouse models of diabetes. Disease Models & Mechanisms. 2010;3:567–580. doi: 10.1242/dmm.003210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nica A.C., Ongen H., Irminger J.C., Bosco D., Berney T., Antonarakis S.E. Cell-type, allelic, and genetic signatures in the human pancreatic beta cell transcriptome. Genome Research. 2013;23:1554–1562. doi: 10.1101/gr.150706.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dorrell C., Schug J., Lin C.F., Canaday P.S., Fox A.J., Smirnova O. Transcriptomes of the major human pancreatic cell types. Diabetologia. 2011;54:2832–2844. doi: 10.1007/s00125-011-2283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fontés G., Zarrouki B., Hagman D.K., Latour M.G., Semache M., Roskens V. Glucolipotoxicity age-dependently impairs beta cell function in rats despite a marked increase in beta cell mass. Diabetologia. 2010;53:2369–2379. doi: 10.1007/s00125-010-1850-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Latour M.G., Alquier T., Oseid E., Tremblay C., Jetton T.L., Luo J. GPR40 is necessary but not sufficient for fatty acid stimulation of insulin secretion in vivo. Diabetes. 2007;56:1087–1094. doi: 10.2337/db06-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferdaoussi M., Bergeron V., Zarrouki B., Kolic J., Cantley J., Fielitz J. G protein-coupled receptor (GPR)40-dependent potentiation of insulin secretion in mouse islets is mediated by protein kinase D1. Diabetologia. 2012;55:2682–2692. doi: 10.1007/s00125-012-2650-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen C., Zheng B., Han J., Lin S.C. Characterization of a novel mammalian RGS protein that binds to Galpha proteins and inhibits pheromone signaling in yeast. Journal of Biological Chemistry. 1997;272:8679–8685. doi: 10.1074/jbc.272.13.8679. [DOI] [PubMed] [Google Scholar]
- 20.Zhao A., Ohara-Imaizumi M., Brissova M., Benninger R.K., Xu Y., Hao Y. Galphao represses insulin secretion by reducing vesicular docking in pancreatic beta-cells. Diabetes. 2010;59:2522–2529. doi: 10.2337/db09-1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mangmool S., Kurose H. G(i/o) protein-dependent and -independent actions of Pertussis Toxin (PTX) Toxins (Basel) 2011;3:884–899. doi: 10.3390/toxins3070884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hauge-Evans A.C., King A.J., Carmignac D., Richardson C.C., Robinson I.C., Low M.J. Somatostatin secreted by islet delta-cells fulfills multiple roles as a paracrine regulator of islet function. Diabetes. 2009;58:403–411. doi: 10.2337/db08-0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braun M. The somatostatin receptor in human pancreatic beta-cells. Vitamins and Hormones. 2014;95:165–193. doi: 10.1016/B978-0-12-800174-5.00007-7. [DOI] [PubMed] [Google Scholar]
- 24.Patel Y.C. Somatostatin and its receptor family. Frontiers in Neuroendocrinology. 1999;20:157–198. doi: 10.1006/frne.1999.0183. [DOI] [PubMed] [Google Scholar]
- 25.Stork P.J., Schmitt J.M. Crosstalk between cAMP and MAP kinase signaling in the regulation of cell proliferation. Trends in Cell Biology. 2002;12:258–266. doi: 10.1016/s0962-8924(02)02294-8. [DOI] [PubMed] [Google Scholar]
- 26.Sae-Lee C., Moolsuwan K., Chan L., Poungvarin N. ChREBP regulates itself and metabolic genes implicated in lipid accumulation in beta-cell line. PLoS One. 2016;11:e0147411. doi: 10.1371/journal.pone.0147411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Druey K.M., Ugur O., Caron J.M., Chen C.K., Backlund P.S., Jones T.L. Amino-terminal cysteine residues of RGS16 are required for palmitoylation and modulation of Gi- and Gq-mediated signaling. Journal of Biological Chemistry. 1999;274:18836–18842. doi: 10.1074/jbc.274.26.18836. [DOI] [PubMed] [Google Scholar]
- 28.Sassmann A., Gier B., Gröne H.J., Drews G., Offermanns S., Wettschureck N. The Gq/G11-mediated signaling pathway is critical for autocrine potentiation of insulin secretion in mice. Journal of Clinical Investigation. 2010;120:2184–2193. doi: 10.1172/JCI41541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taussig R., Iniguez-Lluhi J.A., Gilman A.G. Inhibition of adenylyl cyclase by Gi alpha. Science. 1993;261:218–221. doi: 10.1126/science.8327893. [DOI] [PubMed] [Google Scholar]
- 30.Date Y., Nakazato M., Hashiguchi S., Dezaki K., Mondal M.S., Hosoda H. Ghrelin is present in pancreatic alpha-cells of humans and rats and stimulates insulin secretion. Diabetes. 2002;51:124–129. doi: 10.2337/diabetes.51.1.124. [DOI] [PubMed] [Google Scholar]
- 31.Raghay K., Gallego R., Scoazec J.Y., Garcia-Caballero T., Morel G. Different ghrelin localisation in adult human and rat endocrine pancreas. Cell and Tissue Research. 2013;352:487–494. doi: 10.1007/s00441-013-1593-y. [DOI] [PubMed] [Google Scholar]
- 32.DiGruccio M.R., Mawla A.M., Donaldson C.J., Noguchi G.M., Vaughan J., Cowing-Zitron C. Comprehensive alpha, beta and delta cell transcriptomes reveal that ghrelin selectively activates delta cells and promotes somatostatin release from pancreatic islets. Molecular Metabolism. 2016;5:449–458. doi: 10.1016/j.molmet.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Meulen T., Donaldson C.J., Cáceres E., Hunter A.E., Cowing-Zitron C., Pound L.D. Urocortin3 mediates somatostatin-dependent negative feedback control of insulin secretion. Nature Medicine. 2015;21:769–776. doi: 10.1038/nm.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tengholm A. Cyclic AMP dynamics in the pancreatic beta-cell. Upsala Journal of Medical Sciences. 2012;117:355–369. doi: 10.3109/03009734.2012.724732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwede F., Chepurny O.G., Kaufholz M., Bertinetti D., Leech C.A., Cabrera O. Rp-cAMPS prodrugs reveal the cAMP dependence of first-phase glucose-stimulated insulin secretion. Molecular Endocrinology. 2015;29:988–1005. doi: 10.1210/me.2014-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dyachok O., Idevall-Hagren O., Sågetorp J., Tian G., Wuttke A., Arrieumerlou C. Glucose-induced cyclic AMP oscillations regulate pulsatile insulin secretion. Cell Metabolism. 2008;8:26–37. doi: 10.1016/j.cmet.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 37.Sethakorn N., Yau D.M., Dulin N.O. Non-canonical functions of RGS proteins. Cellular Signaling. 2010;22:1274–1281. doi: 10.1016/j.cellsig.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roy A.A., Baragli A., Bernstein L.S., Hepler J.R., Hébert T.E., Chidiac P. RGS2 interacts with Gs and adenylyl cyclase in living cells. Cellular Signaling. 2006;18:336–348. doi: 10.1016/j.cellsig.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 39.Doupnik C.A. RGS redundancy and implications in GPCR-GIRK signaling. International Review of Neurobiology. 2015;123:87–116. doi: 10.1016/bs.irn.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 40.Iwanir S., Reuveny E. Adrenaline-induced hyperpolarization of mouse pancreatic islet cells is mediated by G protein-gated inwardly rectifying potassium (GIRK) channels. Pflugers Archiv. 2008;456:1097–1108. doi: 10.1007/s00424-008-0479-4. [DOI] [PubMed] [Google Scholar]
- 41.Yoshitomi H., Fujii Y., Miyazaki M., Nakajima N., Inagaki N., Seino S. Involvement of MAP kinase and c-fos signaling in the inhibition of cell growth by somatostatin. American Journal of Physiology – Endocrinology and Metabolism. 1997;272:E769–E774. doi: 10.1152/ajpendo.1997.272.5.E769. [DOI] [PubMed] [Google Scholar]
- 42.Wang X.P., Norman M.A., Yang J., Cheung A., Moldovan S., Demayo F.J. Double-gene ablation of SSTR1 and SSTR5 results in hyperinsulinemia and improved glucose tolerance in mice. Surgery. 2004;136:585–592. doi: 10.1016/j.surg.2004.05.042. [DOI] [PubMed] [Google Scholar]
- 43.Zhou G., Liu S.H., Shahi K.M., Wang H., Duan X., Lin X. Negative regulation of pancreatic and duodenal homeobox-1 by somatostatin receptor subtype 5. Molecular Endocrinology. 2012;26:1225–1234. doi: 10.1210/me.2012-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benko R., Antwi A., Bartho L. The putative somatostatin antagonist cyclo-somatostatin has opioid agonist effects in gastrointestinal preparations. Life Science. 2012;90:728–732. doi: 10.1016/j.lfs.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 45.Kulkarni R.N., Mizrachi E.B., Ocana A.G., Stewart A.F. Human beta-cell proliferation and intracellular signaling: driving in the dark without a road map. Diabetes. 2012;61:2205–2213. doi: 10.2337/db12-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liang G., Bansal G., Xie Z., Druey K.M. RGS16 inhibits breast cancer cell growth by mitigating phosphatidylinositol 3-kinase signaling. Journal of Biological Chemistry. 2009;284:21719–21727. doi: 10.1074/jbc.M109.028407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tang G., Wang Y., Park S., Bajpayee N.S., Vi D., Nagaoka Y. Go2 G protein mediates galanin inhibitory effects on insulin release from pancreatic beta cells. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:2636–2641. doi: 10.1073/pnas.1200100109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosengren A.H., Jokubka R., Tojjar D., Granhall C., Hansson O., Li D.Q. Overexpression of alpha2A-adrenergic receptors contributes to type 2 diabetes. Science. 2010;327:217–222. doi: 10.1126/science.1176827. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.