Abstract
Frizzleds (FZDs) are unconventional G protein–coupled receptors that belong to the class Frizzled. They are bound and activated by the Wingless/Int-1 lipoglycoprotein (WNT) family of secreted lipoglycoproteins. To date, mechanisms of signal initiation and FZD–G protein coupling remain poorly understood. Previously, we showed that FZD6 assembles with Gαi1/Gαq (but not with Gαs, Gαo and Ga12/13), and that these inactive-state complexes are dissociated by WNTs and regulated by the phosphoprotein Dishevelled (DVL). Here, we investigated the inactive-state assembly of heterotrimeric G proteins with FZD4, a receptor important in retinal vascular development and frequently mutated in Norrie disease or familial exudative vitreoretinopathy. Live-cell imaging experiments using fluorescence recovery after photobleaching show that human FZD4 assembles—in a DVL-independent manner—with Gα12/13 but not representatives of other heterotrimeric G protein subfamilies, such as Gαi1, Gαo, Gαs, and Gαq. The FZD4–G protein complex dissociates upon stimulation with WNT-3A, WNT-5A, WNT-7A, and WNT-10B. In addition, WNT-induced dynamic mass redistribution changes in untransfected and, even more so, in FZD4 green fluorescent protein–transfected cells depend on Gα12/13. Furthermore, expression of FZD4 and Gα12 or Gα13 in human embryonic kidney 293 cells induces WNT-dependent membrane recruitment of p115-RHOGEF (RHO guanine nucleotide exchange factor, molecular weight 115 kDa), a direct target of Gα12/13 signaling, underlining the functionality of an FZD4-Gα12/13-RHO signaling axis. In summary, Gα12/13-mediated WNT/FZD4 signaling through p115-RHOGEF offers an intriguing and previously unappreciated mechanistic link of FZD4 signaling to cytoskeletal rearrangements and RHO signaling with implications for the regulation of angiogenesis during embryonic and tumor development.
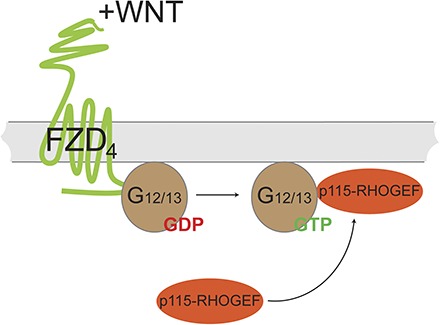
Introduction
Wingless/Int-1 (WNT)–Frizzled (FZD) signaling is initiated through the interaction of class Frizzled (FZD1–10) receptors and their ligands of the WNT family of lipoglycoproteins. WNT signaling holds a central role in embryonic development and the development of human diseases by orchestrating a variety of cellular signaling pathways. The precise mechanisms of signal initiation and specification through WNT binding to the extracellular part of FZD and downstream signal transduction through intracellular signaling partners are so far poorly understood (Nusse, 2003; Angers and Moon, 2009; van Amerongen and Nusse, 2009; Schulte, 2010, 2015; Dijksterhuis et al., 2014).
WNT signaling was historically divided into β-catenin–dependent and -independent pathways. β-Catenin–dependent signals are initiated by WNT binding to FZD and low-density lipoprotein receptor–related protein 5/6 (LRP5/6), recruitment of Dishevelled (DVL), and subsequent inhibition of a destruction complex, resulting in elevated β-catenin levels initiating WNT-target gene transcription (Tamai et al., 2000; Wehrli et al., 2000; He et al., 2004; Macdonald et al., 2007; Clevers and Nusse, 2012). Several β-catenin–independent signaling cascades have been described involving small GTPases, such as RHO, RAC, and Cdc42, as well as heterotrimeric G protein signaling through intracellular mobilization of calcium (Semenov et al., 2007; Schulte, 2010). These pathways regulate, for example, cell movement, cytoskeletal reorganization, and planar cell polarity–like signaling in mammalian systems.
Accumulating mechanistic insight strengthens the concept that FZDs behave as bona fide G protein–coupled receptors (GPCRs) (Slusarski et al., 1997; Liu et al., 1999; Sheldahl et al., 1999; Ahumada et al., 2002; Katanaev et al., 2005; Katanaev and Buestorf, 2009; Kilander et al., 2011, 2014a,b; Koval and Katanaev, 2011; Halleskog et al., 2012; Aznar et al., 2015), whereas other aspects, such as G protein coupling selectivity, ligand-dependent functional selectivity, and the importance of G protein signaling downstream of FZDs, still remain obscure (Dijksterhuis et al., 2015).
Heterotrimeric G proteins interact with GPCRs according to distinct dynamic concepts. On one hand, G proteins and receptors assemble in an inactive state, which would explain receptor–G protein selectivity and the rapid responses observed in cells (Oldham and Hamm, 2008). On the other hand, random collision coupling of G protein and receptors is sufficient to form the fully active, agonist-bound receptor conformation inducing release of GDP from Gα and subsequently a rapid GTP-dependent dissociation of the receptor–G protein complex (Neubig, 1994; Hein et al., 2005; Oldham and Hamm, 2008; Rasmussen et al., 2011; Ayoub et al., 2012); both proposed scenarios are supported experimentally (Galés et al., 2005, 2006; Hein et al., 2005; Nobles et al., 2005; Qin et al., 2011). FZD6 exists in an inactive-state complex with heterotrimeric Gαi and Gαq (Kilander et al., 2014b) similar to what was observed for the muscarinic M3 receptor (Qin et al., 2011). Agonist stimulation of the FZD6–G protein complex is followed by rapid dissociation (Kilander et al., 2014b). The FZD isoform, FZD4, on which this study focuses has so far not been connected to signaling through heterotrimeric G proteins. FZD4 signaling, especially in the physiologic context of retinal vascularization, familial exudative vitreoretinopathy, and Norrie disease, is firmly associated with WNT/β-catenin signaling depending on ligand-induced recruitment of LRP5/6 to FZD4 (Robitaille et al., 2002; Xu et al., 2004; Toomes et al., 2005; Warden et al., 2007; Shastry, 2010). Moreover, it is intriguing that vascularization and angiogenesis, not only limited to the retina, are directly, but independently of each, other linked to FZD4 and G protein signaling through Gα12/13/RHO signaling (Offermanns et al., 1997; Robitaille et al., 2002; Ye et al., 2009; Sivaraj et al., 2013), suggesting a biologically relevant liaison.
In this study, we set out to investigate the ability of FZD4 to mediate heterotrimeric G protein signaling. Based on live-cell imaging experiments, we establish that FZD4 assembles with heterotrimeric Gα12 and Gα13, independently of DVL. Stimulation of FZD4 with WNTs dissociates the receptor–G protein complex, likely leading to G protein activation and subsequent downstream signaling events. We further show that FZD4 mediates dynamic mass redistribution (DMR) and membrane recruitment of p115-RHOGEF (RHO guanine nucleotide exchange factor, molecular weight 115 kDa), a direct downstream target of active Gα12/13, in a Gα12/13- and WNT-dependent manner. Thus, on the basis of our results, we propose a novel WNT-FZD4-Gα12/13-RHO signaling axis offering deeper mechanistic insight into FZD4 signaling to cytoskeletal rearrangements, RHO signaling, and potentially angiogenesis.
Materials and Methods
Cell Culture and Transfections.
Human embryonic kidney 293T (HEK293T) cells (American Type Culture Collection, Manassas, VA) were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovin serum, 1% penicillin/streptomycin, and 1% l-glutamine (all from Invitrogen, Carlsbad, CA) in a humidified CO2 incubator at 37°C. Cell culture plastics were from Sarstedt (Nümbrecht, Germany) or Corning Inc. (Corning, NY) unless otherwise specified. For live-cell imaging, immunochemical, and immunoblot analyses, cells were seeded on 35-mm poly-l-lysine–coated or matrigel-coated (1:300 in starvation medium; Sigma-Aldrich, Stockholm, Sweden) glass-bottom dishes (four-chamber 35-mm glass-bottom dishes; Greiner Bio One, Frickenhausen, Germany). Cells were transfected with Lipofectamine 2000 or 3000 according to the manufacturer’s instructions (Life Technologies, Carlsbad, CA) 24–48 hours before analysis. To diminish the secretion of endogenously expressed WNTs, cells were pretreated over night with 5 µM porcupine inhibitor C59 (2-[4-(2-methylpyridin-4-yl)phenyl]-N-[4-(pyridin-3-yl)phenyl]acetamide; Abcam, Cambridge, UK) where indicated. Recombinant and purified WNT proteins for stimulation experiments were purchased from Bio-Techne/R&D Systems (Minneapolis, MN).
The full-length untagged FZD4 construct was from www.cdna.org (#FZD4000000). Human FZD4 was subcloned into pEGFP-N1, mCherry-N1, or mCerulean-N1 using NheI and BamHI restriction enzymes. Functionality was assessed by recruitment of DVL from cytosolic punctae to the cell membrane upon coexpression (Supplemental Fig. 1). Green fluorescent protein (GFP)-tagged Gas and Gai1 were provided by Mark Rasenick (University of Chicago, Chicago, IL) (Yu and Rasenick, 2002); Gαq-Venus and GαoA-Venus were from Nevin A. Lambert (Georgia Health Sciences University, Augusta, GA) (Digby et al., 2006); and Gα12/13-mCherry were cloned as an N-terminal fusion to the G protein, according to Gα13-RLucII (Yagi et al., 2011). In detail, Gα13-mCherry was cloned from pCEFL MYC GFP10 Gα13 wild type (from Silvio Gutkind), introducing a BglII site with forward primer 5′CCAGATCTGCCACCATGGCGGACTTCCTGCCG 3′ and an EcoRI site with reverse primer 5′ CCGAATTCTCA CTGTAGCATAAGCTGCTT 3′. The Gα13 wild type was excised by BglII and EcoRI digest and inserted into pmCherry-C1 vector. Gα12-mCherry was cloned from Gα12 EE-tagged (internal; GNA120EI00-02 from www.cdna.org) using 5′ ATGAATTCGACCACCATGTCCGGGGTGGT 3′ and 5′ ATGGATCCTCACTGCAGCATGATGTCCTTCAGGTT 3′ to introduce an EcoRI and BamHI site, respectively. The EE-tagged Gα12 was excised by EcoRI and BamHI and inserted into pmCherry-C1. Constructs were confirmed by sequencing. Correct membranous localization and activity of the N-terminally tagged Gα12-mCherry and Gα13-mCherry constructs were verified in HEK293 cells. Membranous localization of the mCherry-tagged constructs indicates correct lipidation of the G proteins (Fig. 1; Supplemental Fig. 2 and 3). Further, we used a p115-RHOGEF-GFP recruitment assay in the presence and absence of overexpressed Gα12- and Gα13-mCherry in combination with lysophosphatidic acid 1 receptor (LPA1 receptor) to further support functionality of the N-terminally tagged G proteins (Supplemental Fig. 2–4). The pCEFL p115-RHOGEF-GFP construct and the extended AU1-tagged regulator of G protein signaling (RGS) domain of p115-RHOGEF (in pCEFL) containing Gα12/13-selective GTPase activating protein/RGS activity were from Silvio Gutkind. The LPA1 receptor–selective antagonist/inverse agonist Ki16425 (3-(4-(4-((1-(2-chlorophenyl)ethoxy)carbonyl)-3-methylisoxazol-5-yl)benzylthio)propanoic acid) (Ohta et al., 2003; Shano et al., 2008) was able to reduce the LPA1 receptor–induced membrane recruitment of p115-RHOGEF-GFP, supporting its dependence on endogenously produced agonists or constitutive activity of the LPA1 receptor upon overexpression (Supplemental Fig. 4). The GFP-KRAS fusion protein of the last 25 amino acid (aa) (RKHKEKMSKDGKKKKKKSKTKCVIM, including the farnesylation site) of KRAS and the fluorescent protein GFP was provided by Nevin A. Lambert (Lan et al., 2011). FLAG-epitope-tagged DVL1 was from Madelon M. Maurice (University Medical Center, Utrecht, The Netherlands), DVL2-MYC was from S. A. Yanagawa (Kyoto University, Kyoto, Japan), and DVL3-FLAG was from Randall T. Moon (University of Washington School of Medicine, Seattle, WA). All constructs were confirmed by sequencing.
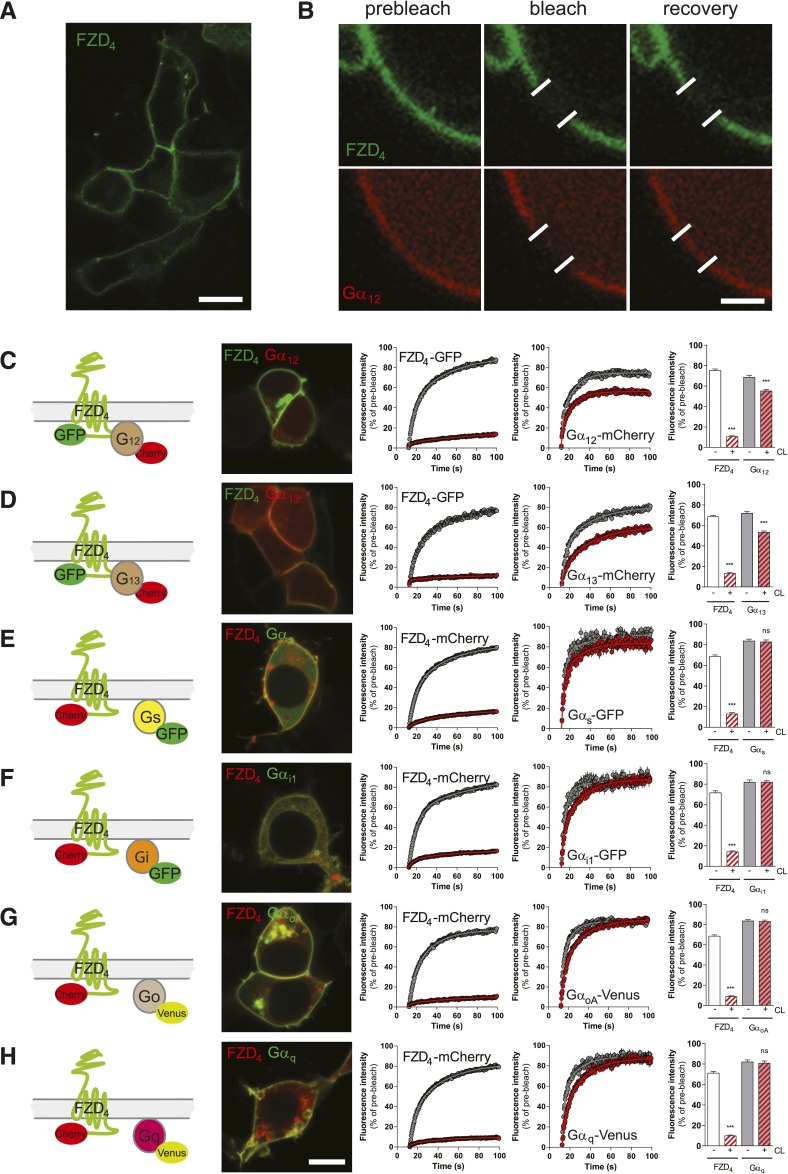
Fig. 1.
dcFRAP in combination with chemical cell surface crosslinking reveals FZD4-Gα12/13 complex formation. (A) HEK293T cells express fluorescently tagged FZD4 predominantly in the cell membrane. Size bar = 10 µm. (B) dcFRAP experiments are done in cells cotransfected with fluorescently tagged FZD4, Gα subunits, and untagged βγ subunits. Micrographs show FZD4-GFP and Gα12-mCherry before, shortly after, and about 100 seconds after the high-laser-power photobleaching in a region of interest (white lines). Surface proteins are chemically crosslinked (CL) by Sulfo-NHS-LC-LC-biotin and avidin. Size bar = 2 µm. (C–H) The figure includes a schematic presentation clarifying the experimental setup, a confocal micrograph showing HEK293T cells coexpressing FZD4 with the respective Gα subunit (size bar = 10 µm), fluorescence intensity curves before (gray) and after (red) CL for both FZD4 and the respective G protein and a bar graph summarizing the mobile fractions of FZD4 and the Gα subunit under each experimental condition. Color code for mobile fractions (consistent throughout the manuscript): white, FZD4 before CL; red hatched, FZD4 after CL; gray, Gα before CL; gray + red hatched, Gα after CL. ***P < 0.001 (n = 3). Error bars provide the S.E.M. ns = not significant.
For human DVL silencing, DVL 1, 2, 3 (panDVL) small interfering RNA (siRNA; AAGUCAACAAGAUCACCUUCU) targeting position 1450–1468 of human DVL1, isoform 1; position 1375–1393 of human DVL1, isoform 2; position 1474–1492 of DVL2; and position 1441–1459 of DVL3 or Xeragon (Qiagen, Sollentuna, Sweden) control nonsilencing siRNA (AAUUCUCCGAACGUGUCACGU) was added simultaneously with plasmids (Bryja et al., 2008). Cells were transfected at a 3:1:1:1 ratio of receptor:Gα:β:γ plasmids or at a 3:1 ratio of receptor:DVL plasmids.
Dual-Color Fluorescence Recovery after Photobleaching.
The procedure was essentially as described in Kilander et al. (2014b), Qin et al. (2011), and Qin et al. (2008). Cells were grown on 35-mm extracellular matrix; coated (1:300; Sigma-Aldrich) glass-bottom dishes and assessed using a Zeiss 710 laser-scanning microscope (Zeiss, Jena, Germany). Membranous, tagged FZD4 was immobilized using avidin-biotin cross-linking. In brief, cells were incubated 0.5 mg/ml NHS-sulfo-LC-LC-biotin followed by 0.1 mg/ml avidin (Thermo Fisher Scientific, Stockholm, Sweden) for 15 minutes each at room temperature and rinsed three times before, between, and after incubations. Washing and incubation steps were performed in cross-linking (CL) buffer (150 mM NaCl, 2.5 mM KCl, 10 mM HEPES, 12 mM glucose, 0.5 mM CaCl2, and 0.5 mM MgCl2, adjusted to pH 8.0). Cellular imaging was performed within 1 hour of avidin exposure. Measurements in which the receptor’s mobility was not sufficiently affected by CL were excluded [cutoff: ≥40% fluorescence recovery after photobleaching (FRAP) recovery]. Images were acquired using a 40×, 1.2 numerical aperture C-Apochromat objective (Zeiss), and the 488- and 561-nm laser lines were used to excite GFP-Venus and mCherry fluorophores, respectively. For all FZD4-Gα subunit combinations, untagged βγ subunits were cotransfected. For FRAP experiments, a 2.86 × 2.86–µm defined area was placed over the cell plasma membrane and monitored using low-intensity illumination. Care was taken to select cells that appeared to have more receptor than G protein expressed, taking into account laser power and gain settings and the differences in the fluorescence yield of the fluorescent proteins. After an initial prebleach period, irreversible photobleaching was performed by increasing the laser intensity to 100%. Fluorescent recovery after bleaching was measured using low illumination again, and was measured for a total of 101 seconds. Average pixel intensity was recorded using the ZEN2013 software (Zeiss), corrected for photobleaching and background fluctuations, and normalized to prebleached intensity. The mobile fraction (Fm) was calculated as Fm = (IP – I0) / (II – I0), where II is the initial intensity measured before bleaching, I0 is the immediate fluorescence intensity after bleaching, and IP is the intensity value after recovery of fluorescence. The mobile fraction was determined by averaging fluorescence intensity values obtained between 55 and 85 seconds.
Förster Resonance Energy Transfer–Photoacceptor Bleaching.
HEK293 cells were seeded on sterile, gelatin-coated coverslips in a 24-well plate and transfected with FZD4-GFP, untagged βγ, and Gα12- or Gα13-mCherry subunits. The cells were treated with or without WNT-7A (300 ng/ml; 5 minutes) prior to fixation in 4% paraformaldehyde. Förster resonance energy transfer (FRET) between FZD4-GFP and Gα12- or Gα13-mCherry subunits was performed on an LSM710 (Zeiss), with a 40× water-immersion objective (C-Apochromat, 1.2 numerical aperture; Zeiss) by acceptor photobleaching with 100% laser power of a 561-nm diode laser for 20 seconds. Signal intensity of the photoacceptor (mCherry) was routinely reduced by 80–90%. Images were acquired before and after photobleaching with excitation/emission ranges of 488/493–545 nm (GFP) and 561/562–681 nm (mCherry). Quantification of the GFP emission before and after the photobleaching was determined with region of interest (ROI) analysis in at least 10 individual cells per experiment and condition using the ZEN2013 software. Data were background corrected and adjusted for fluctuations in intensity with an unbleached ROI as reference. FRET efficiency was calculated as E = [1 – (Ipre/Ipost)] × 100%. Control experiments were performed using FZD4-GFP and myristoylated mCherry to define basal FRET of fluorescent proteins coexpressed in the same cellular compartment.
Dynamic Mass Redistribution.
Label-free measurements were performed using the Epic System (Corning) as described previously in detail (Schröder et al., 2011; Grundmann and Kostenis, 2015). In brief, HEK293 cells were seeded onto fibronectin-coated 384-well biosensor plates at a density of 15,000 cells per well in complete growth medium. After 4 hours, the medium was exchanged for starvation medium lacking fetal bovine serum but supplemented with 5 µM porcupine inhibitor C59 and incubated at 37°C overnight. Cells were washed with Hanks’ balanced salt solution containing 20 mM HEPES, 5 µM C59, and equilibrated at 37°C for at least 1 hour before compound addition and DMR recording. Experiments were performed 24–48 hours after transfection of pcDNA3.1(+) or FZD4-pEGFP-N1 using FuGENE HD (Promega, Madison, WI). HEK293 cells lacking Gα12/13 were a kind gift from Dr. Asuka Inoue (Tohoku University, Sendai City, Japan). Gα12/13, which are encoded by the GNA12 and the GNA13 genes, respectively, were simultaneously targeted by a CRISPR-Cas9 system.
p115-RHOGEF-GFP Recruitment Assay.
HEK293 cells were seeded onto four-chamber glass-bottom dishes (coated with extracellular matrix gel 1:300; Sigma-Aldrich) and transfected with combinations of FZD4-Cerulean, Gα12- or Gα13-mCherry, p115-RHOGEF-GFP, and untagged βγ subunits (ratio 3:1:1:1:1). The next day, the living cells were examined by confocal microscopy (Zeiss LSM510; C-Apochromat 40×/1.2W) to visualize the fluorescently tagged proteins using sequential scanning in combination with 405-nm excitation/420–480 nm emission, 488 nm/long pass 505 nm, and 543 nm/LP 560 nm for Cerulean, GFP, and mCherry, respectively. Cross-talk/bleed-through was controlled using cells expressing the single fluorescent proteins. The Zeiss ZEN2013 software was used to generate fluorescence intensity profiles. For quantification of p115-RHOGEF-GFP membrane recruitment, random images were taken at higher magnification. Cell categories “membranous” and “cytosolic” were defined for p115-RHOGEF-GFP distribution and more than 50 (up to several hundred) cells per condition (+/− FZD4-Cerulean) were counted from three independent experiments. Data are presented as a percentage of all counted p115-RHOGEF-GFP–positive cells showing membranous p115-RHOGEF-GFP distribution.
Western Blot Analysis.
HEK293T cells were plated in a 24-well plate at a density of 150,000 cells/well and grown overnight. Cells were transfected using Lipofectamine 2000 or 3000 according to the manufacturer’s instructions. For lysis, equal amounts of a 2× SDS sample buffer were applied to the cells. Protein lysates were analyzed by standard SDS-PAGE/immunoblotting using the following primary antibodies: mouse anti–β-actin (1:30.000; Sigma-Aldrich), mouse anti-DVL1 (1:500; Santa Cruz Biotechnology, Dallas, TX), rabbit anti-DVL2 (1:1000; Cell Signaling Technology, Danvers, MA), mouse anti-DVL3 (1:500; Santa Cruz), mouse anti-MYC (1:500; Santa Cruz Biotechnology), and mouse anti-FLAG M2 (1:1000; Sigma-Aldrich). Signals were detected by horseradish peroxidase–conjugated secondary antibodies (Thermo Fisher Scientific) and visualized with standard enhanced chemiluminescence detection protocols.
Statistical Analysis.
Statistical and graphical analyses were performed using GraphPad Prism 6 software (GraphPad Software, La Jolla, CA). FRAP data were analyzed by one-way analysis of variance/post hoc Bonferroni’s multiple comparisons or Student’s t test. Curve fitting of FRAP data was achieved with a two-phase association nonlinear function using the least-squares fit. All experiments were repeated at least three times; FRAP data were based on 8–55 ROIs per data point from at least three independent experiments. The number of ROIs per data point varies because some ROIs are excluded from the analysis, such as when visual inspection indicated that cellular movements led to a repositioning of the observed bleached membrane area from the ROI during the recovery phase after photobleaching. The analyzed ROIs originated from individual cells from independent cell transfections. Significance levels are given as follows: *p < 0.05, **p < 0.01, and ***p < 0.001. Data in FRAP curves and FRAP bar graphs are presented as the mean ± S.E.M. Quantitative assessment of protein translocation (DVL1-FLAG and p115-RHOGEF-GFP) was done by manual counting by an observer blinded to the experimental conditions. At least 50 cells in total were counted per experimental condition in three independent experiments. Results are presented in bar graphs as means ± S.E.M. Statistics were done by a one-way analysis of variance with Tukey test for multiple comparisons.
Results
FZD4 Interacts with the Heterotrimeric G Proteins Gα12 and Gα13 but Not Gαi1, Gαo, Gαs, Gαq.
To assess the ability of FZD4 to assemble with heterotrimeric G proteins in an inactive-state complex, we expressed FZD4-GFP or FZD4-mCherry in HEK293T cells. Both constructs showed membrane localization and were able to recruit DVL from a distinct punctuate pattern of cytosolic aggregates to the plasma membrane, similar to the full-length, untagged human FZD4 (Supplemental Fig. 1). Upon coexpression in HEK293T cells, FZD4 colocalized with fluorescently labeled Gαi1, Gαo, Gαs, Gαq, Gα12, and Gα13 subunits predominantly in the plasma membrane (Fig. 1). To assess FZD4–G protein interaction, we took advantage of a dual-color FRAP (dcFRAP) protocol that enables simultaneous assessment of lateral mobility of two fluorescently tagged proteins (Phair et al., 2004; Qin et al., 2008, 2011; Dorsch et al., 2009; Kilander et al., 2014b) (Fig. 1). This experimental setup is based on immobilization of transmembrane proteins by chemical surface CL with sulfo-NHS-LC-LC-biotin and avidin. In contrast to transmembrane receptors, intracellular proteins, such as the heterotrimeric G proteins, are not directly affected by surface CL in this assay. The mobile fraction of intracellular proteins is reduced only upon interaction with CL-immobilized surface proteins (Qin et al., 2008, 2011; Kilander et al., 2014b). In cells transfected with fluorescently tagged FZD4, Gα, and untagged βγ, we found that the mobile fraction of FZD4 was remarkably reduced by surface cross-linking (Fig. 1, C–H). In accordance with an inactive-state assembly of receptor and G protein, we found that the mobile fraction of Gα12 and Gα13, but not that of other representatives of the G protein subfamilies (Gαs, Gαi/o, and Gαq), was affected by surface cross-linking of the receptor (Fig. 1, C–H). To verify that these observed effects were selectively dependent on FZD4 coexpression, and to control for general effects of surface CL on G protein mobility, we previously performed dcFRAP experiments with myristoylated fluorescent proteins as nonreceptor control in combination with fluorescently tagged heterotrimeric Gαi proteins (Kilander et al., 2014b). In Fig. 2, we show that chemical surface cross-linking in the absence of overexpressed FZD4 but in the presence of a membrane-anchored fluorescent protein (GFP-KRAS) did not affect lateral mobility of the heterotrimeric Gα12-mCherry and Gα13-mCherry proteins in the presence of untagged βγ, indicating that cross-linking of endogenously expressed receptors does not perturb the interpretation of our data. This observation is further supported by the finding that the mobile fraction of Gα12-mCherry in the presence of FZD6-GFP is not affected by CL (Kilander et al., 2014b), indicating that even chemical immobilization of an overexpressed transmembrane protein that does not interact with the heterotrimeric G protein leaves the mobile fraction of the G protein unchanged.
Fig. 2.
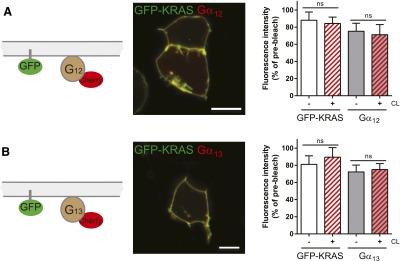
Nonreceptor control ensures that the mobile fraction of Gα12/13-mCherry is not affected by chemical surface crosslinking in the absence of FZD4. HEK293 cells expressing farnesylated GFP-KRAS, untagged βγ subunits, and N-terminally tagged Gα12-mCherry were used for a dcFRAP assay using chemical surface crosslinking (CL) with Sulfo-NHS-LC-LC-biotin and avidin as described in Fig. 1. CL affected neither the mobile fraction of GFP-KRAS nor that of Gα12-mCherry (A) or Gα13-mCherry (B). The figure includes a schematic presentation clarifying the experimental setup, a confocal micrograph showing HEK293T cells coexpressing GFP-KRAS with the Gα12-mCherry (A) or Gα13-mCherry (B; size bars = 10 µm), fluorescence intensity curves before (gray) and after (red) CL for both GFP-KRAS and Gα12/13-mCherry, and a bar graph summarizing the mobile fractions of GFP-KRAS and the Gα subunit under each experimental condition. The data verify that the decrease in G protein mobile fraction observed for Gα12/13-mCherry in the presence of FZD4-GFP is not evoked by CL of endogenously expressed receptors. Error bars provide the S.E.M. ns, not significant. ***P < 0.001. Bar graph summarizes measurements from at least four independent experiments, each including data from several individual cells.
The FZD4-Gα12/13 Complex Dissociates upon WNT Stimulation.
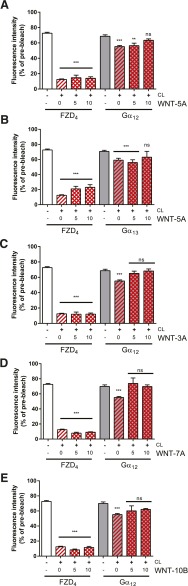
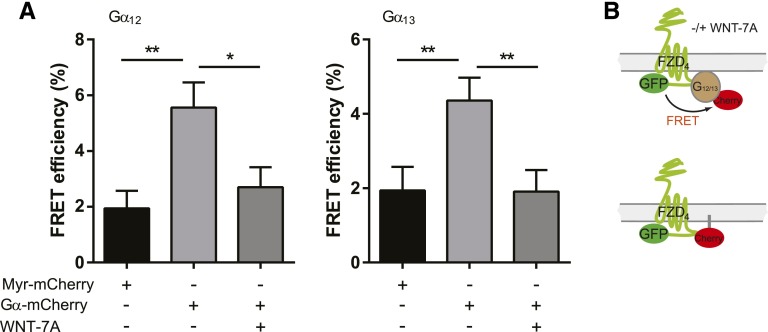
With the purpose of determining whether agonist treatment disrupts the observed FZD4-Gα12/13 complex, we stimulated HEK293T cells expressing FZD4-GFP, untagged βγ, and Gα12/13-mCherry with commercially available, purified WNTs (300 ng/ml; 0, 5, 10 minutes; Fig. 3). As expected from earlier experiments with FZD6 and Gαi1 or Gαq proteins (Kilander et al., 2014b), the previously observed CL-induced reduction of the mobile fraction of the Gα12/13 proteins was abolished by WNT-3A, -5A, -7A, and -10B treatment, indicating dissociation of the inactive-state FZD4-GFP/Gα12/13-mCherry complex. To further support the dcFRAP data on agonist-induced complex dissociation, we used FRET measurements in fixed HEK293 cells transfected with FZD4-GFP, untagged βγ, and Gα12/13-mCherry. Photoacceptor bleaching FRET measurements indicated that energy transfer from FZD4-GFP to Gα12/13-mCherry occurred at baseline, and that FRET decreased with WNT-7A stimulation (300 ng/m; 5 minutes; Fig. 4), indicative of a receptor–G protein dissociation or rearrangement, similar to what was previously observed in the case of FZD6 and Gαi1 or Gαq (Kilander et al., 2014b). The negative control coexpressing FZD4-GFP and myristoylated mCherry defines background levels of basal FRET between fluorescent proteins that are coexpressed in the same cellular compartment but only randomly approaching each other to yield FRET. Importantly, WNT-7A stimulation reduced FRET between FZD4-GFP and Gα12/13-mCherry to basal levels resembling those of the negative control. Thus, both the affinity-based dcFRAP assay and the proximity-based FRET assay support FZD4–G protein interaction and agonist-induced dissociation.
Fig. 3.
FZD4-Gα12/13 complex dissociates upon WNT stimulation. dcFRAP experiments were performed in HEK293T cells expressing FZD4-GFP and Gα12- or Gα13-mCherry. The mobile fractions of the two proteins were determined before and after CL, as well as 5 and 10 minutes after CL/WNT stimulation (all WNTs at 300 ng/ml). Kinetic analysis of the mobile fraction indicates dissociation of the receptor G protein complex upon WNT stimulation. (A and B) For WNT-5A, we investigated WNT-induced dissociation from FZD4-GFP for Gα12- or Gα13-mCherry. (C–E) For the other WNTs (WNT-3A, -7A, 10B), only WNT-induced Gα12-mCherry dissociation was measured. *P < 0.05; **P < 0.01; ***P < 0.001 (n = 3). Error bars provide the S.E.M. ns, not significant. Bar graph summarizes measurements from at least three independent experiments, each including data from several individual cells.
Fig. 4.
FRET analysis supports WNT-evoked FZD4-Gα12/13 complex dissociation. (A) FRET analysis performed in HEK293 cells expressing FZD4-GFP, untagged βγ subunits, and either Gα12- or Gα13-mCherry indicates that FRET between GFP and mCherry decreased in response to WNT-7A stimulation (300 ng/ml; 5 minutes). FRET measurements were performed with photoacceptor bleaching in fixed cells. Light grey bars show FRET between GFP and mCherry in the absence of WNT stimulation. Dark grey bars show FRET upon WNT stimulation. Black bars show FRET efficiency at baseline in cells expressing myristoylated mCherry as negative control. The bar graph summarizes data from three independent experiments with a minimum of 27 ROIs from different cells analyzed per individual experiment and condition. Bars and error bars provide the mean ± S.E.M., respectively. *P < 0.05; **P < 0.001. (B) The experimental setup is illustrated schematically.
The FZD4-Gα12/13 Complex Is Independent of DVL.
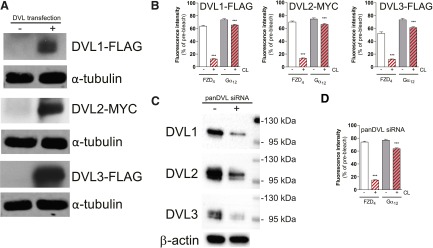
Since we previously identified DVL as a master regulator of FZD6-Gαi1 or FZD6-Gαq association (Kilander et al., 2014b), we also investigated the role of the scaffold protein DVL in the formation of the inactive-state assembled complex between FZD4 and Gα12/13 by in vitro loss- and gain-of-function experiments. On one hand, we overexpressed DVL1, DVL2, and DVL3 in HEK293T cells expressing FZD4 and Gα12-mCherry and untagged βγ subunits at a plasmid ratio of 3:1:1 receptor:Gαβγ:DVL. On the other hand, we downregulated DVL1, DVL2, and DVL3 using pan-DVL siRNA designed to target all three human isoforms of DVL (Fig. 5). Thereby, we created cellular systems with low (pan-DVL siRNA), intermediate (endogenous), and high DVL levels (DVL overexpression). When performing dcFRAP experiments in these cellular setups, we found that changed DVL expression levels did not affect FZD4-Gα12 complex formation (Fig. 5), indicating that the scaffold protein DVL is not required and is dispensable for WNT-FZD4-Gα12/13 inactive-state complex formation.
Fig. 5.
DVL does not play a central role for FZD4-Gα12/13 complex formation. (A and B) DVL1-FLAG, DVL2-MYC, and DVL3-FLAG were coexpressed in HEK293T cells. Cells that were used for dcFRAP were lysed afterward to assess DVL1, DVL2, and DVL3 levels in cellular lysates by immunoblotting using anti-FLAG or anti-MYC antibodies. α-Tubulin was used as the loading control. (B) dcFRAP experiments in cells coexpressing FZD4-GFP and Gα12-mCherry in the presence of DVL1, DVL2, or DVL3. Downregulation of DVL1, DVL2, and DVL3 by using pan-DVL siRNA did not affect FZD4-Gα12/13 assembly. Bar graphs summarize dcFRAP measurements from three independent experiments, each including data from several individual cells. Densitometry analysis of three independent experiments using panDVL siRNA and control indicated that DVL1, DVL2, and DVL3 were routinely reduced by 35–56% [values in percentage reduction in DVL1, DVL2, and DVL3 band intensity by panDVL siRNA compared with control siRNA (mean ± S.E.M.): DVL1 (40 ± 8%); DVL2 (56 ± 2%); DVL3 (35 ± 4%)]. For a graphical presentation of the DVL1, DVL2, and DVL3 levels in control and panDVL siRNA-treated cells (N = 3), see Supplemental Fig. 6. (C) Immunoblotting indicates reduced expression of the three endogenous DVL isoforms in HEK293T cells used for dcFRAP. (D) dcFRAP analysis in cells coexpressing FZD4-GFP and Gα12-mCherry shows no change in the mobile fraction of Gα12-mCherry upon CL in cells with reduced levels of DVL. ***P < 0.001 (n = 3). Error bars provide the S.E.M. Bar graphs summarize measurements from three independent experiments, each including data from several individual cells.
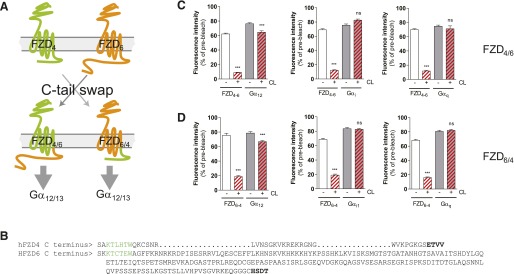
Analysis of G Protein Selectivity of FZD4 and FZD6 Chimeric Receptors.
Given that prediction of receptor G protein selectivity from the primary GPCR structure is still not possible, we aimed to combine our knowledge from FZD6 as a receptor selectively interacting with Gαi and Gαq and FZD4 as a receptor that assembles with Gα12/13 to shed light on domains required for G protein selectivity in FZDs. Both receptors belong to different homology clusters grouping FZD4,9,10 and FZD3,6 (Schulte, 2010), and their C termini differ dramatically in length: the FZD4 C tail comprises 41 aa, and the FZD6 tail consists of 211 aa. The hypothesis for creating FZD4-FZD6 tail and FZD6-FZD4 tail chimera through a classic domain swap of the C-terminal regions was that exchanging C-terminal tails could be accompanied by interchanging G protein selectivity. In Fig. 6, C and D, dcFRAP data are shown, indicating G protein selectivity of the respective chimera. Whereas the G protein–coupling selectivity of the FZD6-FZD4 tail chimera was switched from Gαi/Gαq to Gα12, the FZD4-FZD6 tail chimera still preferentially bound to Gα12 rather than Gαi or Gαq.
Fig. 6.
C-terminal domain swapping between FZD4 and FZD6. (A) Schematic presentation of the exchange strategy of the C termini between FZD4 and FZD6, resulting in FZD4-6 C-terminal tail and FZD6-4 C-terminal tail. (B) The primary structure of human FZD4 and FZD6. Green highlights the conserved KTxxxW sequence involved in DVL binding on the presumptive helix 8. Bold marks the terminal PDZ ligand domain. dcFRAP experiments in HEK293T cells coexpressing FZD4-6-GFP and Gα12-mCherry, FZD4-6-mCherry and Gαi1-GFP, or FZD4-6-mCherry and Gαq-Venus (C) and FZD6-4-GFP and Gα12-mCherry, FZD6-4-mCherry and Gαi1-GFP, or FZD6-4-mCherry and Gαq-Venus (D) reveal predominant assembly with Gα12 of the chimeric receptors. ***P < 0.001 (n = 3). Bar graphs show the mean ± S.E.M. PDZ, PSD-95/dics large/ZO-1 homologous.
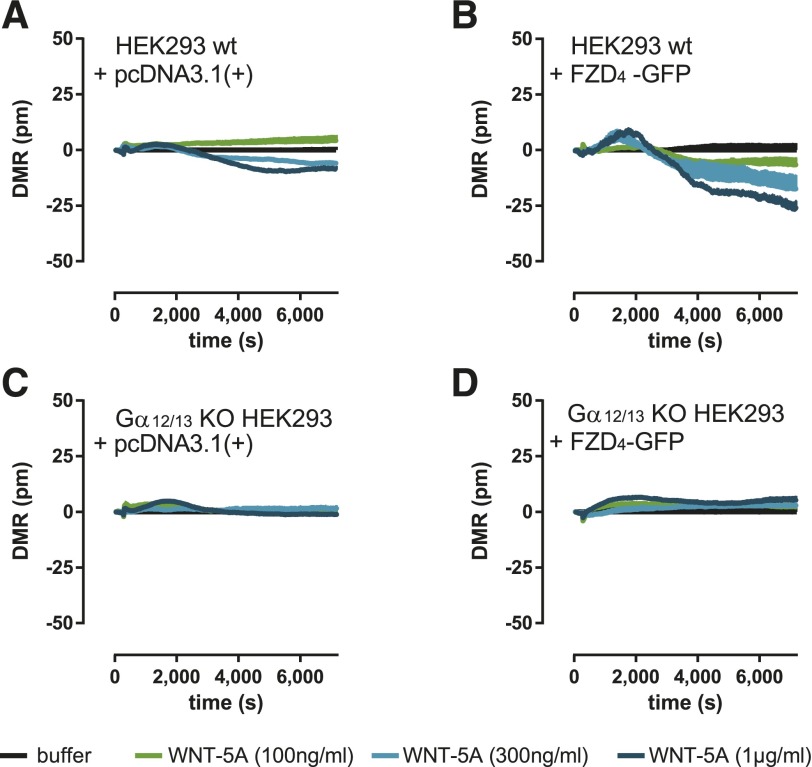
WNT-Induced Dynamic Mass Redistribution in HEK293 Depends on Gα12/13.
Recent developments in label-free technologies, such as DMR measurements, allow global analysis of ligand-induced changes in living cells (Schröder et al., 2011; Grundmann and Kostenis, 2015). DMR in living cells presents a holistic view on changes in multiple cellular processes, such as protein trafficking, morphologic changes, cytoskeletal rearrangement, receptor internalization, or adhesion (Schröder et al., 2010), some of which are connected to Gα12/13 signaling (Worzfeld et al., 2008). To investigate whether DMR technology is competent to visualize the cellular consequences set in motion by WNT-5A upon activation of FZD4-Gα12/13 complexes, we used HEK293 cells genetically deficient or not deficient in Ga12/13 and monitored WNT-induced alterations of global cell activity. We observed negative DMR upon stimulation with WNT-5A in vector-transfected HEK293 wild-type cells (Fig. 7A), indicating interaction of WNT-5A with a molecular target endogenous to the HEK293 cell background. Importantly, overexpression of FZD4-GFP intensified this response, which substantiates a FZD4 receptor–dependent WNT-5A mode-of-action (Fig. 7B). Strikingly, these responses were completely sensitive to Gα12/13 protein knockout (Fig. 7, C and D), indicative of FZD4 receptor signaling via Gα12/13. FZD4 receptor–independent cell responses induced by the direct adenylate cyclase stimulator forskolin, however, remained unaffected by either Gα12/13 protein knockout or FZD4-GFP overexpression (Supplemental Fig. 5).
Fig. 7.
WNT-induced dynamic mass redistribution depends on Gα12/13 and FZD4-GFP. HEK293 wild-type (wt) cells and HEK293 cells lacking Gα12/13 were stimulated with increasing amounts of WNT-5A (100, 300, and 1000 ng/ml). Changes in DMR were recorded over time. The apparent negative, WNT-5A–induced DMR responses in empty vector (A) or FZD4-GFP–transfected (B) HEK293 cells were not observed in the absence of Gα12/13 proteins (C and D). Experiments were done after overnight treatment with the porcupine inhibitor C59 (5 µM) at 37°C. Shown are representative traces (N = 3), buffer-corrected and measured in triplicates + S.E.M. See Supplemental Fig. 5 for expression levels of FZD4-GFP and forskolin-induced DMR responses of wt and Gα12/13-knockout (KO) HEK293 cells.
Signaling along a WNT-FZD4-Gα12/13-p115-RHOGEF Signaling Axis.
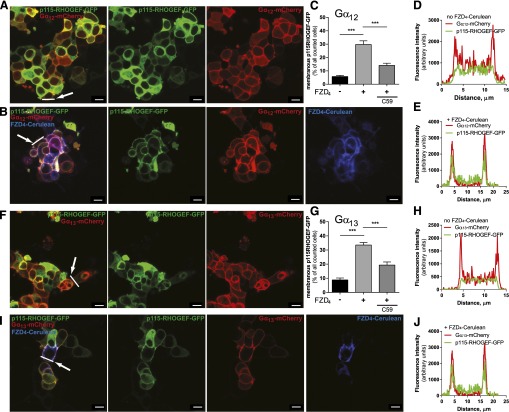
To link the FZD4-Gα12/13 complex functionally to downstream signaling events, and to support the idea that FZD4 indeed mediates the GDP/GTP exchange at Gα12/13 proteins, we expanded on previous findings identifying p115-RHOGEF as a Gα12/13 target that physically interacts with the active, GTP-bound α subunit and stimulates its GTPase activity similar to classic regulators of G protein signaling proteins (Hart et al., 1996, 1998; Meyer et al., 2008; Worzfeld et al., 2008). Most importantly, with regard to the functionality of the tagged Gα12/13-mCherry subunits, correct palmitoylation of Gα12/13 is required for the G protein’s plasma membrane localization and the promotion of p115-RHOGEF recruitment to the plasma membrane by the active, GTP-bound G protein (Bhattacharyya and Wedegaertner, 2000). Comparable to the results with coexpression of the LPA1 receptor and Gα12/13-mCherry (Supplemental Fig. 2-4), FZD4-Cerulean also induced p115-RHOGEF-GFP membrane recruitment in combination with Gα12/13-mCherry (Fig. 8). Neither Gα12/13-mCherry nor FZD4-Cerulean alone was sufficient to translocate p115-RHOGEF-GFP from a predominantly cytosolic expression pattern to a plasma membrane localization (Fig. 8; Supplemental Fig. 2). Substantial p115-RHOGEF-GFP membrane localization was only observed when FZD4-Cerulean was coexpressed with Gα12/13-mCherry, suggesting that receptor-induced activation of Gα12/13 is required for this process.
Fig. 8.
FZD4 induces p115-RHOGEF-GFP membrane recruitment in a Gα12/13- and WNT-dependent manner. (A, B, F, and I) HEK293 cells were cotransfected with combinations of FZD4-Cerulean, Gα12- or Gα13-mCherry, and p115-RHOGEF-GFP and examined by live-cell confocal imaging. p115-RHOGEF-GFP showed an even cytosolic distribution when expressed alone or in combination with either Gα12/Gα13-mCherry or FZD4-Cerulean (see Supplemental Fig. 2). The combination of FZD4-Cerulean and either Gα12- or Gα13-mCherry increased p115-RHOGEF-GFP plasma membrane localization. Quantification of the FZD4-Cerulean–dependent p115-RHOGEF-GFP recruitment was done by counting cells showing membranous versus cytosolic p115-RHOGEF-GFP distribution. Data from three independent experiments (>300 cells from several visual fields counted per condition for each individual experiment) are presented in the bar graphs (C and G). Data represent cells with a membranous p115-RHOGEF-GFP localization calculated as the percentage of total p115-RHOGEF-GFP–positive cells counted. In combination with the porcupine inhibitor C59 (5 µM; overnight treatment), FZD4-Cerulean–induced and Gα12- or Gα13-mCherry–mediated p115-RHOGEF-GFP recruitment was significantly reduced. Error bars provide the S.E.M. ***P < 0.001 (N = 3). Cellular distribution profiles of p115-RHOGEF-GFP (green) and Gα12 or Gα13-mCherry (red) fluorescence intensity along a line drawn over a single cell shown in (A, B, F, and I) (see arrow) in either the absence or presence FZD4-Cerulean are shown in (D, E, H, and J). Size bars = 10 µm.
To dissect the ligand dependence of the response from FZD4 via Gα12/13 to p115-RHOGEF-GFP recruitment, we disrupted endogenous secretion of WNTs by overnight treatment with the pharmacological porcupine inhibitor C59 (Proffitt et al., 2013). Porcupine was identified as a segment polarity gene giving rise to a multispan transmembrane protein catalyzing WNT acylation (Kadowaki et al., 1996). Pharmacological inhibition of porcupine is therefore an efficient way to decrease the amount of endogenously produced, functional WNTs to reduce autocrine stimulation (Dodge et al., 2012). This setup is analogous to the reduction of the LPA1 receptor–induced and Gα12/13-mediated p115-RHOGEF-GFP recruitment by the LPA1 receptor–selective antagonist/inverse agonist Ki16425 (Supplemental Fig. 4). In agreement with this, C59 treatment of cells transfected with FZD4-Cerulean, Gα12/13-mCherry, and p115-RHOGEF-GFP decreased the number of cells showing a membranous localization of p115-RHOGEF-GFP (Fig. 8), indicating that the endogenously secreted WNTs signal through FZD4-GFP and Gα12/13-mCherry to contribute to p115-RHOGEF-GFP recruitment.
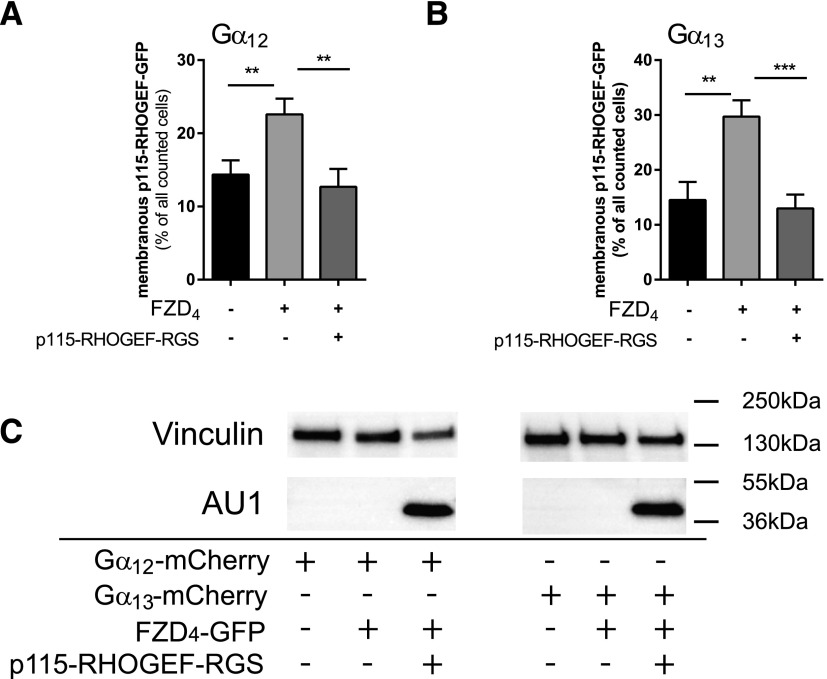
Gα12/13 need to be activated and GTP-bound to physically interact with p115-RHOGEF and simultaneously p115-RHOGEF exerts GTPase activating protein or RGS activity on the heterotrimeric G protein to mediate inactivation as part of a negative feedback loop (Hart et al., 1998; Kozasa et al., 1998). To corroborate the FZD4-mediated activation of Gα12/13, we used the p115-RHOGEF-GFP recruitment as readout in cells cotransfected with the extended AU1-tagged RGS domain of p115-RHOGEF, the domain that promotes hydrolysis of GTP to GDP. Compared with cells transfected with FZD4-GFP, Gα12/13-mCherry, and p115-RHOGEF-GFP, where we observed 23 ± 2% (Gα12)/30 ± 3% (Gα13) (mean ± S.E.M.) cells with membranous p115-RHOGEF-GFP, coexpression of the RGS domain reduced p115-RHOGEF-GFP membrane recruitment to 13 ± 2% (Gα12)/13 ± 3% (Gα13), corresponding to values in the absence of FZD4-GFP transfection [14 ± 2% (Gα12)/15 ± 3% (Gα13)] (Fig. 9).
Fig. 9.
FZD4-induced and Gα12/13-mediated p115-RHOGEF-GFP membrane recruitment depends on activation of the heterotrimeric Gα12/13 proteins. HEK293 cells were transfected with FZD4-Cerulean, Gα12-mCherry (A) or Gα13-mCherry (B), and p115-RHOGEF-GFP either without or with the isolated AU1-tagged RGS domain of p115-RHOGEF-RGS. Cells presenting membranous p115-RHOGEF-GFP localization were counted, and data from four independent experiments were summarized in the bar graph. More than 50 cells were counted for each condition in each independent experiment. Values give the mean ± S.E.M. (C) Cells used for quantification of p115-RHOGEF-GFP recruitment were lysed and immunoblotted for vinculin (loading control) and anti-AU1 to detect the tagged-RGS domain of p115-RHOGEF.
Discussion
In the present study, we used cellular imaging approaches to investigate the interaction of FZD4 with heterotrimeric G proteins in unprecedented depth. Combining the dcFRAP technique with FRET, we were able to identify FZD4 as a receptor assembled with Gα12 and Gα13 based on experiments in a mammalian cellular system. Cellular-imaging experiments were corroborated using innovative technology enabling DMR measurements in living cells to dissect the role of Gα12/13 in WNT-induced cellular responses. In addition, we explored the roles of the scaffold protein DVL in FZD4-G protein assembly and the connection of FZD4, Gα12/13, and signaling to RHO via p115-RHOGEF.
Ever since their discovery in Drosophila melanogaster, FZDs have been postulated as being GPCRs due to structural and functional resemblance to more conventional class A, B, and C GPCRs (Vinson et al., 1989; Park et al., 1994; Schulte and Bryja, 2007). In support of what was previously surmised, a great amount of functional evidence has been gathered over the past two decades confirming that FZDs can signal as GPCRs (Slusarski et al., 1997; Liu et al., 1999, 2001; Ahumada et al., 2002; Sheldahl et al., 2003; Katanaev et al., 2005; Katanaev and Buestorf, 2009; Schulte, 2010; Kilander et al., 2011; Koval and Katanaev, 2011; Halleskog et al., 2012; Dijksterhuis et al., 2014).
In the case of FZD4, a receptor that has been attributed a central role in retinal vascularization and related diseases, such as familial exudative vitreoretinopathy (Robitaille et al., 2002), no evidence suggesting interaction with heterotrimeric G proteins has been reported so far. Based on our data using a live-cell imaging dcFRAP approach, which has been successfully used to study GPCR–G protein interactions in the past (Qin et al., 2008, 2011; Kilander et al., 2014b), we show that FZD4 interacts with Gα12/13 but not representatives of other G protein subfamilies. The observed selectivity of FZD4 for one but not all groups of heterotrimeric G proteins underlines the specificity of the dcFRAP assay and supports the use of overexpressed proteins. Since we were able to detect the inactive-state assembly of FZD4 and Gα12/13 in living cells, we can conclude that this receptor complex is rather stable under the given circumstances. It should be noted, however, that dcFRAP analysis is a population-based assay without single-molecule sensitivity, and that it does not allow definite quantification of the affinity of the receptor to the heterotrimeric G protein. Further, our data do not allow a conclusion about the predominant mode of FZD4–G protein coupling in cells endogenously expressing the proteins, which could be cell-type and receptor expression level dependent. Thus, it remains to be resolved if FZD4 at endogenous expression levels indeed forms an inactive-state complex with Gα12/13 or if the predominant mode of signaling is based on collision coupling.
To understand the structural basis of FZD–G protein selectivity, we performed C-terminal tail-swap experiments with FZD4 and FZD6, of which the latter assembles with Gαi/q (Kilander et al., 2014a,b). Whereas G protein selectivity of FZD6 with the C terminus of FZD4 was changed from predominant Gαi or Gαq to G12/13, FZD4 Gα12/13 selectivity was not affected by exchanging the 41 aa C terminus to the 211 aa C terminus of FZD6 (Fig. 6). Thus, it appears that Gα12/13 assembly can be achieved with structural determinants encoded by the FZD4 and FZD6 core, irrespective of the C-terminal tail. On the other hand, assembly with Gαi or Gαq is not determined by either the FZD6 C terminus or the core alone, but it rather requires the combination of both in the intact FZD6. In this context, it should be noted that some structural features are common to both the FZD4 and the FZD6 C terminus, such as the conserved KTxxxW sequence and the potential ability to form a helix 8 (Schulte, 2010).
Furthermore, we found that this novel FZD4-Gα12/13 liaison neither requires DVL for the formation of an inactive-state-assembled complex nor is sensitive to DVL overexpression. This stands in contrast to previous results, where we defined DVL as an essential component of the FZD6-Gαi/Gαq complex (Kilander et al., 2014b), arguing that the underlying mechanisms of FZDs interacting with heterotrimeric G proteins could be receptor isoform selective.
DMR analysis revealed Gα12/13-dependent WNT effects in both untransfected and FZD4-transfected HEK293 cells. It is well known that HEK293 cells endogenously express FZD4, among other FZDs (Atwood et al., 2011), suggesting that the WNT-Gα12/13 responses measured in the receptor/G protein–overexpression paradigms can occur at endogenous receptor–G protein expression levels. Furthermore, the loss-of-function approach using HEK293 Gα12/13 knockout cells underlines that the observed FZD4-Gα12/13 liaison is not an artifact of receptor/G protein overexpression. On the other hand, these data open the possibility that other class FZD receptors expressed in HEK293 cells could also mediate signaling via Gα12/13.
Heterotrimeric Gα12/13 proteins signal mainly to RHO GTPase-dependent pathways by interacting with several RHOGEFs, regulating, for example, changes in cell shape, migration, and adhesion (Worzfeld et al., 2008). In Figs. 8 and 9, we show that Gα12/13-induced p115-RHOGEF recruitment to the membrane is induced by FZD4. Since p115-RHOGEF interacts with the GTP-bound and active form of Gα12/13 (Hart et al., 1998), which is dissociated from βγ subunits and the GPCR, this finding strongly argues not only that FZD4 passively assembles with Gα12/13, but that FZD4 is indeed capable of functionally coupling to Gα12/13 acting as a GEF on these heterotrimeric G proteins. Given the lack of pharmacological tools for the selective stimulation of or interference with class Frizzled receptors, we instead used an alternative approach to pinpoint the ligand dependence of the FZD4/Gα12/13-mediated p115-RHOGEF-GFP recruitment. Pretreatment with porcupine inhibitors dramatically reduces autocrine WNT secretion (Proffitt et al., 2013), and the negative impact of C59 on p115-RHOGEF-GFP membrane localization argues that FZD4/Gα12/13 signaling is WNT-dependent. In addition, the negative effect of the isolated p115-RHOGEF RGS domain acting as a Gα12/13-selective GTPase-activating protein (Hart et al., 1998; Kozasa et al., 1998) supports the functionality of the WNT-FZD4/Gα12/13-p115-RHOGEF signaling axis and its dependence on FZD4-mediated activation of Gα12/13.
In summary, our results provide the first evidence that FZD4 and Gα12/13 functionally interact, that WNTs can dissociate Gα12/13 from the inactive-state complex, and that FZD4-Gα12/13 mediate RHO signaling through membrane recruitment of p115-RHOGEF. Thus, FZD4 should be seen as a Gα12/13-coupled GPCR, even though the circumstances that specify WNT signaling through the FZD4/Gα12/13/p115-RHOGEF signaling axis over the classic WNT/β-catenin pathway need to be defined in more detail.
Acknowledgments
The authors acknowledge Professor Lars Larsson (Department of Physiology and Pharmacology, Karolinska Institutet, Stockholm, Sweden) for providing generous access to the Zeiss LSM510 confocal microscope. The authors also thank Dr. Asuka Inoue (Graduate School of Pharmaceutical Sciences, Tohoku University) for providing parental and Gα12/13 knockout HEK293 cells. The authors are grateful to Corning Inc. for support on the Epic DMR biosensor.
Abbreviations
- aa
amino acid
- C59
2-[4-(2-methylpyridin-4-yl)phenyl]-N-[4-(pyridin-3-yl)phenyl]acetamide
- CL
cross-linking
- dcFRAP
double-color fluorescence recovery after photobleaching
- DMR
dynamic mass redistribution
- DVL
Disheveled
- FRAP
fluorescence recovery after photobleaching
- FRET
Förster resonance energy transfer
- FZD
Frizzled
- GFP
green fluorescent protein
- GPCR
G protein–coupled receptor
- HEK293
human embryonic kidney 293
- Ki16425
3-(4-(4-((1-(2-chlorophenyl)ethoxy)carbonyl)-3-methylisoxazol-5-yl)benzylthio)propanoic acid
- LPA1
lysophosphatidic acid 1
- pan-DVL
DVL isoforms 1, 2, 3
- p115-RHOGEF
RHO guanine nucleotide exchange factor, molecular weight 115 kDa
- RGS
regulator of G protein signaling
- ROI
region of interest
- siRNA
small interfering RNA
- WNT
Wingless/Int-1 lipoglycoprotein
Authorship Contributions
Participated in research design: Arthofer, Hot, Grundmann, Kostenis, Gutkind, Schulte.
Conducted experiments: Arthofer, Hot, Petersen, Strakova, Jäger, Grundmann.
Performed data analysis: Arthofer, Hot, Petersen, Strakova, Jäger, Grundmann, Schulte.
Wrote or contributed to the writing of the manuscript: Arthofer, Hot, Petersen, Grundmann, Kostenis, Schulte.
Footnotes
The study was financially supported by grants from Karolinska Institutet; Karolinska Institutet’s Eye Disease Research Foundation; the Board of Doctoral Education at Karolinska Institutet (J.P., B.H.); the Swedish Research Council [Grants 2011-2435, 2013-5708, and 2015-02899]; the Swedish Cancer Society [Project Grants CAN 2011/690 and CAN 2014/659]; the Knut and Alice Wallenberg Foundation [Grant KAW2008.0149]; the Karolinska Institutet National Institutes of Health Joint PhD Program in Neuroscience (E.A.); the Czech Science Foundation [Grant 13-32990S]; and the Program “KI-MU” [Grant CZ.1.07/2.3.00/20.0180], cofinanced from European Social Fund and the state budget of the Czech Republic and the Marie Curie ITN WntsApp [608180; www.wntsapp.eu]. This research was supported in part by the Intramural Research Program of the National Institutes of Health National Institute of Dental and Craniofacial Research [Grant Z01DE00551] . S.J. was supported by the ERASMUS+ program.
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Ahumada A, Slusarski DC, Liu X, Moon RT, Malbon CC, Wang HY. (2002) Signaling of rat Frizzled-2 through phosphodiesterase and cyclic GMP. Science 298:2006–2010. [DOI] [PubMed] [Google Scholar]
- Angers S, Moon RT. (2009) Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol 10:468–477. [DOI] [PubMed] [Google Scholar]
- Atwood BK, Lopez J, Wager-Miller J, Mackie K, Straiker A. (2011) Expression of G protein-coupled receptors and related proteins in HEK293, AtT20, BV2, and N18 cell lines as revealed by microarray analysis. BMC Genomics 12:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoub MA, Al-Senaidy A, Pin JP. (2012) Receptor-G protein interaction studied by bioluminescence resonance energy transfer: lessons from protease-activated receptor 1. Front Endocrinol (Lausanne) 3:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aznar N, Midde KK, Dunkel Y, Lopez-Sanchez I, Pavlova Y, Marivin A, Barbazán J, Murray F, Nitsche U, Janssen KP, et al. (2015) Daple is a novel non-receptor GEF required for trimeric G protein activation in Wnt signaling. eLife 4:e07091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya R, Wedegaertner PB. (2000) Galpha 13 requires palmitoylation for plasma membrane localization, Rho-dependent signaling, and promotion of p115-RhoGEF membrane binding. J Biol Chem 275:14992–14999. [DOI] [PubMed] [Google Scholar]
- Bryja V, Schambony A, Cajánek L, Dominguez I, Arenas E, Schulte G. (2008) Beta-arrestin and casein kinase 1/2 define distinct branches of non-canonical WNT signalling pathways. EMBO Rep 9:1244–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H, Nusse R. (2012) Wnt/β-catenin signaling and disease. Cell 149:1192–1205. [DOI] [PubMed] [Google Scholar]
- Digby GJ, Lober RM, Sethi PR, Lambert NA. (2006) Some G protein heterotrimers physically dissociate in living cells. Proc Natl Acad Sci USA 103:17789–17794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijksterhuis JP, Baljinnyam B, Stanger K, Sercan HO, Ji Y, Andres O, Rubin JS, Hannoush RN, Schulte G. (2015) Systematic mapping of WNT-FZD protein interactions reveals functional selectivity by distinct WNT-FZD pairs. J Biol Chem 290:6789–6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijksterhuis JP, Petersen J, Schulte G. (2014) WNT/Frizzled signalling: receptor-ligand selectivity with focus on FZD-G protein signalling and its physiological relevance: IUPHAR Review 3. Br J Pharmacol 171:1195–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge ME, Moon J, Tuladhar R, Lu J, Jacob LS, Zhang LS, Shi H, Wang X, Moro E, Mongera A, et al. (2012) Diverse chemical scaffolds support direct inhibition of the membrane-bound O-acyltransferase porcupine. J Biol Chem 287:23246–23254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsch S, Klotz KN, Engelhardt S, Lohse MJ, Bünemann M. (2009) Analysis of receptor oligomerization by FRAP microscopy. Nat Methods 6:225–230. [DOI] [PubMed] [Google Scholar]
- Galés C, Rebois RV, Hogue M, Trieu P, Breit A, Hébert TE, Bouvier M. (2005) Real-time monitoring of receptor and G-protein interactions in living cells. Nat Methods 2:177–184. [DOI] [PubMed] [Google Scholar]
- Galés C, Van Durm JJ, Schaak S, Pontier S, Percherancier Y, Audet M, Paris H, Bouvier M. (2006) Probing the activation-promoted structural rearrangements in preassembled receptor-G protein complexes. Nat Struct Mol Biol 13:778–786. [DOI] [PubMed] [Google Scholar]
- Grundmann M, Kostenis E. (2015) Label-free biosensor assays in GPCR screening. Methods Mol Biol 1272:199–213. [DOI] [PubMed] [Google Scholar]
- Halleskog C, Dijksterhuis JP, Kilander MB, Becerril-Ortega J, Villaescusa JC, Lindgren E, Arenas E, Schulte G. (2012) Heterotrimeric G protein-dependent WNT-5A signaling to ERK1/2 mediates distinct aspects of microglia proinflammatory transformation. J Neuroinflammation 9:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart MJ, Jiang X, Kozasa T, Roscoe W, Singer WD, Gilman AG, Sternweis PC, Bollag G. (1998) Direct stimulation of the guanine nucleotide exchange activity of p115 RhoGEF by Galpha13. Science 280:2112–2114. [DOI] [PubMed] [Google Scholar]
- Hart MJ, Sharma S, elMasry N, Qiu RG, McCabe P, Polakis P, Bollag G. (1996) Identification of a novel guanine nucleotide exchange factor for the Rho GTPase. J Biol Chem 271:25452–25458. [DOI] [PubMed] [Google Scholar]
- He X, Semenov M, Tamai K, Zeng X. (2004) LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development 131:1663–1677. [DOI] [PubMed] [Google Scholar]
- Hein P, Frank M, Hoffmann C, Lohse MJ, Bünemann M. (2005) Dynamics of receptor/G protein coupling in living cells. EMBO J 24:4106–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki T, Wilder E, Klingensmith J, Zachary K, Perrimon N. (1996) The segment polarity gene porcupine encodes a putative multitransmembrane protein involved in Wingless processing. Genes Dev 10:3116–3128. [DOI] [PubMed] [Google Scholar]
- Katanaev VL, Buestorf S. (2009) Frizzled proteins are bona fide G protein-coupled receptors. Nature Precedings DOI: hdl:10101/npre.2009.2765.1. [Google Scholar]
- Katanaev VL, Ponzielli R, Sémériva M, Tomlinson A. (2005) Trimeric G protein-dependent frizzled signaling in Drosophila. Cell 120:111–122. [DOI] [PubMed] [Google Scholar]
- Kilander MB, Dahlström J, Schulte G. (2014a) Assessment of Frizzled 6 membrane mobility by FRAP supports G protein coupling and reveals WNT-Frizzled selectivity. Cell Signal 26:1943–1949. [DOI] [PubMed] [Google Scholar]
- Kilander MB, Petersen J, Andressen KW, Ganji RS, Levy FO, Schuster J, Dahl N, Bryja V, Schulte G. (2014b) Disheveled regulates precoupling of heterotrimeric G proteins to Frizzled 6. FASEB J 28:2293–2305. [DOI] [PubMed] [Google Scholar]
- Kilander MBC, Dijksterhuis JP, Ganji RS, Bryja V, Schulte G. (2011) WNT-5A stimulates the GDP/GTP exchange at pertussis toxin-sensitive heterotrimeric G proteins. Cell Signal 23:550–554. [DOI] [PubMed] [Google Scholar]
- Koval A, Katanaev VL. (2011) Wnt3a stimulation elicits G-protein-coupled receptor properties of mammalian Frizzled proteins. Biochem J 433:435–440. [DOI] [PubMed] [Google Scholar]
- Kozasa T, Jiang X, Hart MJ, Sternweis PM, Singer WD, Gilman AG, Bollag G, Sternweis PC. (1998) p115 RhoGEF, a GTPase activating protein for Galpha12 and Galpha13. Science 280:2109–2111. [DOI] [PubMed] [Google Scholar]
- Lan TH, Kuravi S, Lambert NA. (2011) Internalization dissociates β2-adrenergic receptors. PLoS One 6:e17361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, DeCostanzo AJ, Liu X, Wang Hy, Hallagan S, Moon RT, Malbon CC. (2001) G protein signaling from activated rat frizzled-1 to the beta-catenin-Lef-Tcf pathway. Science 292:1718–1722. [DOI] [PubMed] [Google Scholar]
- Liu X, Liu T, Slusarski DC, Yang-Snyder J, Malbon CC, Moon RT, Wang H. (1999) Activation of a frizzled-2/beta-adrenergic receptor chimera promotes Wnt signaling and differentiation of mouse F9 teratocarcinoma cells via Galphao and Galphat. Proc Natl Acad Sci USA 96:14383–14388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald BT, Semenov MV, He X. (2007) SnapShot: Wnt/beta-catenin signaling. Cell 131:1204. [DOI] [PubMed] [Google Scholar]
- Meyer BH, Freuler F, Guerini D, Siehler S. (2008) Reversible translocation of p115-RhoGEF by G(12/13)-coupled receptors. J Cell Biochem 104:1660–1670. [DOI] [PubMed] [Google Scholar]
- Neubig RR. (1994) Membrane organization in G-protein mechanisms. FASEB J 8:939–946. [DOI] [PubMed] [Google Scholar]
- Nobles M, Benians A, Tinker A. (2005) Heterotrimeric G proteins precouple with G protein-coupled receptors in living cells. Proc Natl Acad Sci USA 102:18706–18711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R. (2003) Wnts and Hedgehogs: lipid-modified proteins and similarities in signaling mechanisms at the cell surface. Development 130:5297–5305. [DOI] [PubMed] [Google Scholar]
- Offermanns S, Mancino V, Revel JP, Simon MI. (1997) Vascular system defects and impaired cell chemokinesis as a result of Galpha13 deficiency. Science 275:533–536. [DOI] [PubMed] [Google Scholar]
- Ohta H, Sato K, Murata N, Damirin A, Malchinkhuu E, Kon J, Kimura T, Tobo M, Yamazaki Y, Watanabe T, et al. (2003) Ki16425, a subtype-selective antagonist for EDG-family lysophosphatidic acid receptors. Mol Pharmacol 64:994–1005. [DOI] [PubMed] [Google Scholar]
- Oldham WM, Hamm HE. (2008) Heterotrimeric G protein activation by G-protein-coupled receptors. Nat Rev Mol Cell Biol 9:60–71. [DOI] [PubMed] [Google Scholar]
- Park WJ, Liu J, Adler PN. (1994) The frizzled gene of Drosophila encodes a membrane protein with an odd number of transmembrane domains. Mech Dev 45:127–137. [DOI] [PubMed] [Google Scholar]
- Phair RD, Gorski SA, Misteli T. (2004) Measurement of dynamic protein binding to chromatin in vivo, using photobleaching microscopy. Chromatin and Chromatin Remodeling Enzymes. Pt A 375:393–414. [DOI] [PubMed] [Google Scholar]
- Proffitt KD, Madan B, Ke Z, Pendharkar V, Ding L, Lee MA, Hannoush RN, Virshup DM. (2013) Pharmacological inhibition of the Wnt acyltransferase PORCN prevents growth of WNT-driven mammary cancer. Cancer Res 73:502–507. [DOI] [PubMed] [Google Scholar]
- Qin K, Dong C, Wu G, Lambert NA. (2011) Inactive-state preassembly of G(q)-coupled receptors and G(q) heterotrimers. Nat Chem Biol 7:740–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin K, Sethi PR, Lambert NA. (2008) Abundance and stability of complexes containing inactive G protein-coupled receptors and G proteins. FASEB J 22:2920–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen SG, DeVree BT, Zou Y, Kruse AC, Chung KY, Kobilka TS, Thian FS, Chae PS, Pardon E, Calinski D, et al. (2011) Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature 477:549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitaille J, MacDonald ML, Kaykas A, Sheldahl LC, Zeisler J, Dubé MP, Zhang LH, Singaraja RR, Guernsey DL, Zheng B, et al. (2002) Mutant frizzled-4 disrupts retinal angiogenesis in familial exudative vitreoretinopathy. Nat Genet 32:326–330. [DOI] [PubMed] [Google Scholar]
- Schröder R, Janssen N, Schmidt J, Kebig A, Merten N, Hennen S, Müller A, Blättermann S, Mohr-Andrä M, Zahn S, et al. (2010) Deconvolution of complex G protein-coupled receptor signaling in live cells using dynamic mass redistribution measurements. Nat Biotechnol 28:943–949. [DOI] [PubMed] [Google Scholar]
- Schröder R, Schmidt J, Blättermann S, Peters L, Janssen N, Grundmann M, Seemann W, Kaufel D, Merten N, Drewke C, et al. (2011) Applying label-free dynamic mass redistribution technology to frame signaling of G protein-coupled receptors noninvasively in living cells. Nat Protoc 6:1748–1760. [DOI] [PubMed] [Google Scholar]
- Schulte G. (2010) International Union of Basic and Clinical Pharmacology. LXXX. The class Frizzled receptors. Pharmacol Rev 62:632–667. [DOI] [PubMed] [Google Scholar]
- Schulte G. (2015) Frizzleds and WNT/β-catenin signaling--The black box of ligand-receptor selectivity, complex stoichiometry and activation kinetics. Eur J Pharmacol 763 (Pt B):191–195. [DOI] [PubMed] [Google Scholar]
- Schulte G, Bryja V. (2007) The Frizzled family of unconventional G-protein-coupled receptors. Trends Pharmacol Sci 28:518–525. [DOI] [PubMed] [Google Scholar]
- Semenov MV, Habas R, Macdonald BT, He X. (2007) SnapShot: Noncanonical Wnt Signaling Pathways. Cell 131:1378. [DOI] [PubMed] [Google Scholar]
- Shano S, Hatanaka K, Ninose S, Moriyama R, Tsujiuchi T, Fukushima N. (2008) A lysophosphatidic acid receptor lacking the PDZ-binding domain is constitutively active and stimulates cell proliferation. Biochim Biophys Acta 1783:748–759. [DOI] [PubMed] [Google Scholar]
- Shastry BS. (2010) Genetic susceptibility to advanced retinopathy of prematurity (ROP). J Biomed Sci 17:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldahl LC, Park M, Malbon CC, Moon RT. (1999) Protein kinase C is differentially stimulated by Wnt and Frizzled homologs in a G-protein-dependent manner. Curr Biol 9:695–698. [DOI] [PubMed] [Google Scholar]
- Sheldahl LC, Slusarski DC, Pandur P, Miller JR, Kühl M, Moon RT. (2003) Dishevelled activates Ca2+ flux, PKC, and CamKII in vertebrate embryos. J Cell Biol 161:769–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaraj KK, Takefuji M, Schmidt I, Adams RH, Offermanns S, Wettschureck N. (2013) G13 controls angiogenesis through regulation of VEGFR-2 expression. Dev Cell 25:427–434. [DOI] [PubMed] [Google Scholar]
- Slusarski DC, Corces VG, Moon RT. (1997) Interaction of Wnt and a Frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature 390:410–413. [DOI] [PubMed] [Google Scholar]
- Tamai K, Semenov M, Kato Y, Spokony R, Liu C, Katsuyama Y, Hess F, Saint-Jeannet JP, He X. (2000) LDL-receptor-related proteins in Wnt signal transduction. Nature 407:530–535. [DOI] [PubMed] [Google Scholar]
- Toomes C, Downey LM, Bottomley HM, Mintz-Hittner HA, Inglehearn CF. (2005) Further evidence of genetic heterogeneity in familial exudative vitreoretinopathy; exclusion of EVR1, EVR3, and EVR4 in a large autosomal dominant pedigree. Br J Ophthalmol 89:194–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Amerongen R, Nusse R. (2009) Towards an integrated view of Wnt signaling in development. Development 136:3205–3214. [DOI] [PubMed] [Google Scholar]
- Vinson CR, Conover S, Adler PN. (1989) A Drosophila tissue polarity locus encodes a protein containing seven potential transmembrane domains. Nature 338:263–264. [DOI] [PubMed] [Google Scholar]
- Warden SM, Andreoli CM, Mukai S. (2007) The Wnt signaling pathway in familial exudative vitreoretinopathy and Norrie disease. Semin Ophthalmol 22:211–217. [DOI] [PubMed] [Google Scholar]
- Wehrli M, Dougan ST, Caldwell K, O’Keefe L, Schwartz S, Vaizel-Ohayon D, Schejter E, Tomlinson A, DiNardo S. (2000) arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature 407:527–530. [DOI] [PubMed] [Google Scholar]
- Worzfeld T, Wettschureck N, Offermanns S. (2008) G(12)/G(13)-mediated signalling in mammalian physiology and disease. Trends Pharmacol Sci 29:582–589. [DOI] [PubMed] [Google Scholar]
- Xu Q, Wang Y, Dabdoub A, Smallwood PM, Williams J, Woods C, Kelley MW, Jiang L, Tasman W, Zhang K, et al. (2004) Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell 116:883–895. [DOI] [PubMed] [Google Scholar]
- Yagi H, Tan W, Dillenburg-Pilla P, Armando S, Amornphimoltham P, Simaan M, Weigert R, Molinolo AA, Bouvier M, Gutkind JS. (2011) A synthetic biology approach reveals a CXCR4-G13-Rho signaling axis driving transendothelial migration of metastatic breast cancer cells. Sci Signal 4:ra60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Wang Y, Cahill H, Yu M, Badea TC, Smallwood PM, Peachey NS, Nathans J. (2009) Norrin, frizzled-4, and Lrp5 signaling in endothelial cells controls a genetic program for retinal vascularization. Cell 139:285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JZ, Rasenick MM. (2002) Real-time visualization of a fluorescent G(alpha)(s): dissociation of the activated G protein from plasma membrane. Mol Pharmacol 61:352–359. [DOI] [PubMed] [Google Scholar]