Abstract
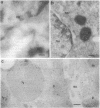
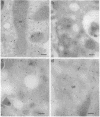
The intracellular localization of human copper,zinc superoxide dismutase (Cu,Zn-SOD; superoxide:superoxide oxidoreductase, EC 1.15.1.1) was evaluated by using EM immunocytochemistry and both isolated human cell lines and human tissues. Eight monoclonal antibodies raised against either native or recombinant human Cu,Zn-SOD and two polyclonal antibodies raised against either native or recombinant human Cu,Zn-SOD were used. Fixation with 2% paraformaldehyde/0.2% glutaraldehyde was found necessary to preserve normal distribution of the protein. Monoclonal antibodies were less effective than polyclonal antibodies in recognizing the antigen after adequate fixation of tissue. Cu,Zn-SOD was found widely distributed in the cell cytosol and in the cell nucleus, consistent with it being a soluble cytosolic protein. Mitochondria and secretory compartments did not label for this protein. In human cells, peroxisomes showed a labeling density slightly less than that of cytoplasm.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi T., Usami Y., Kishi T., Hirano K., Hayashi K. An enzyme immunoassay for cuprozinc superoxide dismutase using monoclonal antibodies. Application for pharmacokinetic study. J Immunol Methods. 1988 Apr 22;109(1):93–101. doi: 10.1016/0022-1759(88)90446-2. [DOI] [PubMed] [Google Scholar]
- Chang L. Y., Slot J. W., Geuze H. J., Crapo J. D. Molecular immunocytochemistry of the CuZn superoxide dismutase in rat hepatocytes. J Cell Biol. 1988 Dec;107(6 Pt 1):2169–2179. doi: 10.1083/jcb.107.6.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaunsi G. S., Gulati S., Singh A. K., Orak J. K., Asayama K., Singh I. Demonstration of Cu-Zn superoxide dismutase in rat liver peroxisomes. Biochemical and immunochemical evidence. J Biol Chem. 1992 Apr 5;267(10):6870–6873. [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Adv Enzymol Relat Areas Mol Biol. 1986;58:61–97. doi: 10.1002/9780470123041.ch2. [DOI] [PubMed] [Google Scholar]
- Keller G. A., Gould S., Deluca M., Subramani S. Firefly luciferase is targeted to peroxisomes in mammalian cells. Proc Natl Acad Sci U S A. 1987 May;84(10):3264–3268. doi: 10.1073/pnas.84.10.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller G. A., Warner T. G., Steimer K. S., Hallewell R. A. Cu,Zn superoxide dismutase is a peroxisomal enzyme in human fibroblasts and hepatoma cells. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7381–7385. doi: 10.1073/pnas.88.16.7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberley T. D., Gonzalez A., Lauchner L. J., Oberley L. W., Li J. J. Characterization of early kidney lesions in estrogen-induced tumors in the Syrian hamster. Cancer Res. 1991 Apr 1;51(7):1922–1929. [PubMed] [Google Scholar]
- Ono K., Kimura S., Nakano M., Naruse T. Detection of heterogeneity of Cu, Zn-superoxide dismutase with monoclonal antibodies and the establishment of a highly sensitive fluorescence sandwich enzyme-linked immunosorbent assay. FEBS Lett. 1991 Apr 22;282(1):115–118. doi: 10.1016/0014-5793(91)80457-e. [DOI] [PubMed] [Google Scholar]
- Porstmann T., Wietschke R., Schmechta H., Grunow R., Porstmann B., Bleiber R., Pergande M., Stachat S., von Baehr R. A rapid and sensitive enzyme immunoassay for Cu/Zn superoxide dismutase with polyclonal and monoclonal antibodies. Clin Chim Acta. 1988 Jan 15;171(1):1–10. doi: 10.1016/0009-8981(88)90285-9. [DOI] [PubMed] [Google Scholar]
- Slot J. W., Geuze H. J., Freeman B. A., Crapo J. D. Intracellular localization of the copper-zinc and manganese superoxide dismutases in rat liver parenchymal cells. Lab Invest. 1986 Sep;55(3):363–371. [PubMed] [Google Scholar]
- Slot J. W., Geuze H. J., Gigengack S., Lienhard G. E., James D. E. Immuno-localization of the insulin regulatable glucose transporter in brown adipose tissue of the rat. J Cell Biol. 1991 Apr;113(1):123–135. doi: 10.1083/jcb.113.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanders R. J., Denis S. Identification of superoxide dismutase in rat liver peroxisomes. Biochim Biophys Acta. 1992 Jan 23;1115(3):259–262. doi: 10.1016/0304-4165(92)90063-z. [DOI] [PubMed] [Google Scholar]