Abstract
Lysophosphatidic acid (LPA) is a lipid mediator that mediates cellular effects via G protein–coupled receptors (GPCRs). Epidermal growth factor (EGF) is a peptide that acts via a receptor tyrosine kinase. LPA and EGF both induce proliferation of prostate cancer cells and can transactivate each other’s receptors. The LPA receptor LPA1 is particularly important for LPA response in human prostate cancer cells. Previous work in our laboratory has demonstrated that free fatty acid 4 (FFA4), a GPCR activated by ω-3 fatty acids, inhibits responses to both LPA and EGF in these cells. One potential mechanism for the inhibition involves negative interactions between FFA4 and LPA1, thereby suppressing responses to EGF that require LPA1. In the current study, we examined the role of LPA1 in mediating EGF and FFA4 agonist responses in two human prostate cancer cell lines, DU145 and PC-3. The results show that an LPA1-selective antagonist inhibits proliferation and migration to both LPA and EGF. Knockdown of LPA1 expression, using silencing RNA, blocks responses to LPA and significantly inhibits responses to EGF. The partial response to EGF that is observed after LPA1 knockdown is not inhibited by FFA4 agonists. Finally, the role of arrestin-3, a GPCR-binding protein that mediates many actions of activated GPCRs, was tested. Knockdown of arrestin-3 completely inhibits responses to both LPA and EGF in prostate cancer cells. Taken together, these results suggest that LPA1 plays a critical role in EGF responses and that FFA4 agonists inhibit proliferation by suppressing positive cross-talk between LPA1 and the EGF receptor.
Introduction
Cross-talk between cell membrane receptors can take many forms, from heterologous desensitization (negative cross-talk) to transactivation (positive cross-talk). Our group previously demonstrated that bidirectional positive cross-talk occurs between G protein–coupled receptors (GPCRs) for lysophosphatidic acid (LPA) and receptor tyrosine kinase receptors (RTKs) for epidermal growth factor (EGF) in ovarian cancer cells (Snider et al., 2010). These interactions were initially attributed to the widely demonstrated transactivation of the EGF receptor (EGFR) by LPA (e.g., Kue et al., 2002; Zhao et al., 2006), as well as to transactivation of LPA receptors (LPARs) via EGF-mediated generation of LPA (Snider et al., 2010); however, the mechanism(s) have not been fully elucidated. We have also reported that LPA antagonists suppress responses to both LPA and EGF in prostate cancer (Liu et al., 2015) and breast cancer (Hopkins et al., 2016) cell lines.
In a recent study, we showed that agonists for free fatty acid receptor 4 (FFA4) inhibit responses to both LPA and EGF in prostate cancer cells (Liu et al., 2015). In other words, negative cross-talk occurs between FFA4 and receptors for LPA and/or EGF. Agonists for FFA4 include the dietary ω-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Similar findings were reported by our group for breast cancer, although in this case the receptor implicated was FFA1, another member of the free fatty acid receptor (FFAR) family (Hopkins et al., 2016). The profound inhibitory effects of FFAR agonists on cancer cell proliferation and migration suggest that FFA1 and FFA4 are potential therapeutic targets for cancer prevention or therapy (Hopkins and Meier, 2016). These findings prompted us to examine negative receptor cross-talk in more detail. The current study explores the mechanisms underlying the inhibitory effects of FFAR agonists on growth factor–induced proliferation and migration.
The observation that FFA4 agonists inhibit responses to both LPA and EGF raised the hypothesis that FFA4 might be inhibiting EGF action indirectly via an LPAR. In the current study, we therefore explored the requirement of the EGFR for an LPAR (positive cross-talk). Our hypothesis regarding FFA4 action (negative cross-talk) is based in part on the known ability of GPCRs to inhibit the activation of other GPCRs. Of the LPA receptors, LPA1 has been particularly implicated in LPA-mediated proliferation in prostate cancer cells (Guo et al., 2006; Gibbs et al., 2009). Since LPA antagonists inhibit EGF response in prostate cancer cells (Liu et al., 2015), FFAR-mediated negative cross-talk with an LPA receptor could likewise interfere with EGF actions.
In general, the mechanisms of heterologous desensitization of GPCRs are poorly understood. There are several hypotheses, the most prevalent of which is that downstream signaling by the activated GPCR causes conformational changes in the unoccupied heterologous receptor (Bektas et al., 2005; Kelly et al., 2008). Another mechanism of desensitization involves GPCR heterodimerization since GPCRs can form both homodimers and heterodimers (Milligan, 2004). With respect to positive cross-talk, LPA receptors can interact with the EGFR, a RTK (Liebmann and Bohmer, 2000; Bektas et al., 2005). GPCR-EGFR positive modulation has been noted in many systems and is of particular interest with respect to cancer progression (Bhola and Grandis, 2008). This has been a continuing topic for research as more detailed mechanistic explanations are sought (Pyne and Pyne, 2011; George et al., 2013).
FFA4 can signal through Gq/11 and/or arrestin-3 (β-arrestin-2). The pathway that is activated depends on several factors, including the cell type, agonist, and FFA4 phosphorylation patterns (Magalhaes et al., 2012; Luttrell, 2013; Alghamdi et al., 2014; Prihandoko et al., 2016). Although arrestin-3 is not the only GPCR effector potentially involved, a prior study strongly implicates this particular arrestin in inhibitory responses mediated by both FFA4 and FFA1 (Yan et al., 2013). Moreover, arrestin-3 is involved in transactivation of the EGFR in response to various GPCRs (Tilley et al., 2009; Esposito et al., 2011; Salazar et al., 2014). Thus, arrestin-3 may be involved in both positive and negative GPCR-EGFR cross-talk.
In this study, we focused first on the role of LPA1 in EGF-induced proliferation and migration in human prostate cancer cells and then tested whether LPA1 is required for the inhibitory effects of FFA4 agonists. Finally, we explored the role of arrestin-3 in responses to LPA and EGF.
Materials and Methods
Materials
EPA (prepared in ethanol) was from Cayman Chemical (Ann Arbor, MI). The FFAR agonist TUG-891 (4-[(4-fluoro-4′-methyl[1,1′-biphenyl]-2-yl)methoxy]-benzenepropanoic acid, prepared in DMSO) was from Millipore (Billerica, MA). AM966 (2-[4-[4-[4-[[(1R)-1-(2-chlorophenyl)ethoxy]carbonylamino]-3-methyl-1,2-oxazol-5yl] phenyl]phenyl]acetic acid) was purchased predissolved in dimethylsulfoxide (DMSO) from MedChem Express (Monmouth Junction, NJ). Ki16425 (3-[[[4-[4-[[[1-(2-chlorophenyl)ethoxy] carbonyl]amino]-3-methyl-5-isoazoly]phenyl]phenyl]methyl]thio]-propanoic acid; prepared in DMSO) was purchased from Cayman Chemical (Ann Arbor, MI). Vehicle controls for FFAR agonists were used at a final concentration of 0.03% (v/v) ethanol. LPA (18:1; oleoyl) was obtained from Avanti Polar Lipids (Birmingham, AL) and was delivered to cells as a 1000× stock solution prepared in 4 mg/ml fatty acid-free bovine serum albumin. Vehicle controls for LPA had a final concentration of 4 µg/ml bovine serum albumin. EGF was from Sigma (St. Louis, MO). Anti-LPA1 (EPR9710) and anti-arrestin-3 (ab54790) were purchased from Abcam (Cambridge, MA) and used at 1:1000 dilutions. Anti-actin, obtained from BD Transduction Laboratories (Lexington, KY) (lot no. 51711), was used at a 1:5000 dilution. Goat anti-rabbit secondary antibody (lot no. 083M4752) was purchased from Sigma and used at 1:20,000 dilution, whereas goat anti-mouse secondary antibody (lot no. 1124907A) was purchased from Invitrogen/Life Technologies (Grand Island, NY) and used at a 1:5000 dilution.
Cell Culture
DU145 and PC-3 cells were obtained from the American Type Culture Collection (Manassas, VA). The cells were grown in RPMI 1640 medium with 10% fetal bovine serum (FBS) (Hyclone/Thermo-Fisher Scientific, Waltham, MA). Both cell lines were grown in an incubator at 37°C in 5% CO2 on standard tissue culture plastic.
Cell Proliferation Assays
Cells were seeded in six-well plates at 3 × 105 cells/well in serum-containing medium. After 1 day, the medium was changed to RPMI 1640 without serum. On the next day, the medium was changed to RPMI 1640 with 10% FBS, 10 µM LPA, or 10 nM EGF, in the absence or presence of 100 nM AM966, 10 μM Ki16425, 20 µM EPA, or 1 µM TUG-891. Control cells were incubated with the appropriate vehicle (0.03% ethanol, v/v). Duplicate wells were prepared for each experimental condition. Cell numbers were evaluated after 24, 48, and 72 hours by removing medium, incubating cells with trypsin/EDTA for 5 minutes, adding trypan blue, and counting the suspended live cells (excluding trypan blue) using a hemacytometer.
Cell Migration Assays.
Cell migration was assessed using a modified Boyden chamber method, as previously described (Liu et al., 2015). Cells were serum starved for 24 hours and then seeded in serum-free medium at 2.5 × 104 cells per insert in the upper chambers of 8-μm transwell inserts (BD Biosciences, San Jose, CA). Cells were then treated with 10% FBS, 100 nM AM966, 20 μM EPA, 1 μM TUG-891, 10 μM LPA, or 10 nM EGF, either alone or in combination. Serum-free medium was added to the lower wells. After a 6-hour migration period, the insert membranes were fixed and stained using methanol and crystal violet. Cells that invaded the lower chambers were counted using light microscopy.
Immunoblotting.
Cells were rinsed twice with ice-cold phosphate-buffered saline, harvested by scraping into 1 ml ice-cold phosphate-buffered saline, collected by centrifugation at 10,000xg for 10 minutes at 4°C, and resuspended in ice-cold lysis buffer (20 mM HEPES [pH 7.4]), 1% Triton X-100, 50 mM NaCl, 2 mM EGTA, 5 mM β−glycerophosphate, 30 mM sodium pyrophosphate, 100 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 10 µg/ml aprotinin, 10 µg/ml leupeptin). Insoluble debris was removed after centrifugation. Whole-cell extracts containing equal amounts of protein (30 µg) were separated by SDS-PAGE on 10% Laemmli gels, transferred to nitrocellulose, and incubated with primary (overnight at 4°C) and then secondary (one to two hours at room temperature) antibodies. Blots were developed using enhanced chemiluminescence reagents (GE Healthcare, Little Chalfont, UK), and imaged using a Gel Doc system (BioRad Laboratories, Hercules, CA). Protein expression was quantified by densitometry using Quantity One software (Bio-Rad). Results were normalized to the actin loading control, and then to the value obtained for untreated control cells.
RNA Interference.
Predesigned human Silencer Select siRNA specific to LPA1 (no. 4050) and human Stealth siRNA specific to arrestin-3 (no. 28129220) were purchased from Life Technologies (Grand Island, NY), along with Opti-MEM reduced serum medium and LipofectAMINE 3000 reagent. The siRNA was resuspended in RNase-free water at a final concentration of 60 µM and stored at −20°C. AllStars Negative Control siRNA oligos were purchased from Qiagen (Hilden, Germany) and were used for all negative controls. According to the manufacturer’s directions for RNAi transfections, cells were plated in 1.7 ml of medium per well in RPMI medium plus 10% FBS without antibiotics in six-well plates one day before transfection. At the time of transfection, cells were approximately 50% confluent. For each transfection, two mixtures were prepared: 1) duplex siRNA added to 150 µl Opti-MEM, and 2) 4 µl of LipofectAMINE 3000 added to 150 µl Opti-MEM. The two solutions were gently mixed and then incubated at room temperature for 5 minutes. Final concentrations of siRNA were 100 nM and 250 nM for LPA1 and arrestin-3, respectively. The mixtures were added to each well and cells were cultured for an additional 24–72 hours. For LPA- and EGF-treated cells, the same procedure was followed, except cells were seeded in six-well plates 2 days before transfection and serum starved overnight the day before transfection.
Statistical Analysis
Data were analyzed by two-way analysis of variance (ANOVA) followed by Tukey's multiple comparisons test. The only exceptions were assays in which there was only one time point (e.g., migration assays); these data were analyzed by one-way ANOVA followed by Tukey’s multiple comparisons test. All analyses were done using Prism software (Graphpad, San Diego, CA).
Results
Effects of LPA1 Inhibition on Prostate Cancer Cell Proliferation.
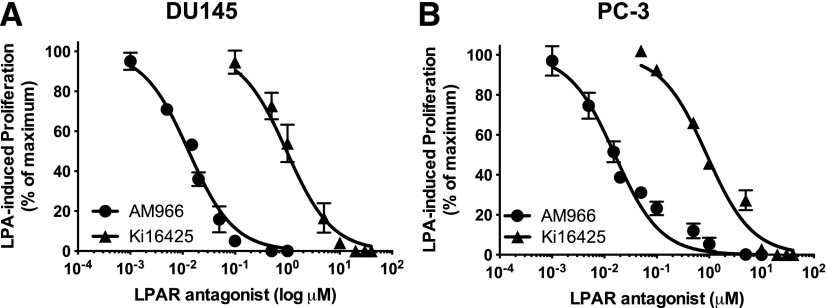
Previous studies in our laboratory have shown that several LPA receptors, including LPA1, are expressed in prostate cancer cell lines (Gibbs et al., 2009) and that LPA antagonists can interfere with LPA-induced proliferation in these cells (Liu et al., 2015). We selected two LPA receptor pharmacologic antagonists to further study the role of LPA1 in EGF response. The first drug, Ki16425, is a selective inhibitor of LPA1 and LPA3 (Ohta et al., 2003) that we have used in previous studies of prostate cancer cells (Liu et al., 2015). The second agent, AM966, is an LPA1-selective antagonist (Swaney et al., 2010). Dose-response studies were performed on DU145 and PC-3 cells in a 48-hour proliferation assay to establish appropriate drug concentrations. Figure 1 shows that Ki16425 inhibited LPA-induced proliferation in DU145 with an IC50 of 966 nM, and in PC-3 cells with an IC50 of 900 nM. The LPA1-selective antagonist AM966 also inhibited proliferation in response to LPA, with an IC50 of 13 nM in DU145 and 16 nM in PC-3.
Fig. 1.
Dose-response for the effects of LPA receptor antagonists on DU145 cell proliferation. Serum-starved DU145 (A) and PC-3 (B) cells were incubated for 48 hours with and without LPA in the absence and presence of the indicated concentrations of Ki16425 and AM966. The number of live cells achieved in response to LPA in the absence of other additions was defined as 100% response; the number of cells present in the absence of LPA was defined as 0% response. Each point represents mean ± S.E.M. (n = 4) of values from two separate experiments, each of which was performed using separate replicate wells of cells.
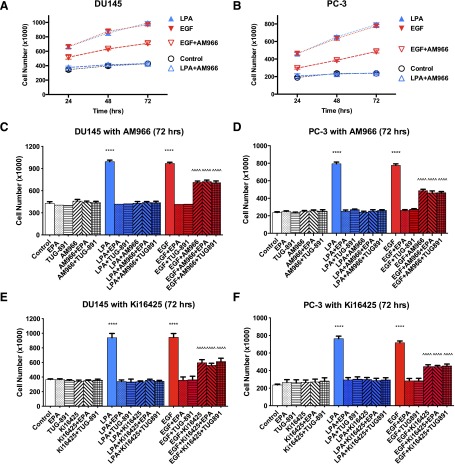
Next, we investigated whether the effects of LPA antagonists extend to EGF-induced proliferation (Fig. 2). In addition, the effects of the FFA4 agonists EPA and TUG-891, in the absence and presence of LPAR antagonists, were tested on proliferation induced by LPA or EGF. Both DU145 and PC-3 cells were used in this series of experiments. Panels A and B of Fig. 2 show the time course of proliferation in both cell lines. As shown previously, cell number did not decrease over the course of 72 hours for control cells maintained without serum, indicating that cell death was not influencing the results of the assay. LPA and EGF stimulated proliferation to similar extents, as evidenced by the increase in cell numbers. Panels C–F present the results from the 72-hour time point so that all experimental controls can be presented in the same panels. FFAR agonists and LPA antagonists had no effect on proliferation when added alone. The LPAR antagonists (10 µM Ki16425; 100 nM AM966) blocked LPA-induced proliferation in both cell lines, as expected. Each LPAR antagonist partially inhibited EGF-induced proliferation (45%–60% inhibition), to a significant extent (P < 0.0001). These results extend our previously published results (separate experiments) for DU145 cells (Liu et al., 2015) to now include PC-3 cells. LPAR antagonists eliminated the inhibitory effects of FFAR agonists on EGF-induced proliferation, in both cell lines.
Fig. 2.
Effects of LPAR antagonists on human prostate cancer cell proliferation. Proliferation assays were conducted using serum-starved DU145 (A, C, and E) or PC-3 (B, D, and F) cells. Cells were incubated with or without 10 μM LPA or 10 nM EGF in the absence and presence of 100 nM AM966 (A–D), 10 μM Ki16425 (E, F), 20 μM EPA, or 1 μM TUG-891 for the indicated times. (A, B) Full 72-hour time courses. (C–F) show only the 48-hour time point. AM966 and Ki16425 alone had no significant effect on cell numbers at any of the time points tested (shown for 72 hours). Each data point represents the mean ± S.E.M. (n ≥ 4) of values from at least two separate experiments. ****P < 0.0001; **P < 0.01; *P < 0.05 versus control; ^^^^P < 0.0001 versus control or EGF. Data were analyzed using two-way ANOVA, followed by Tukey’s multiple comparisons test.
Effects of LPA1 Inhibition on Prostate Cancer Cell Migration.
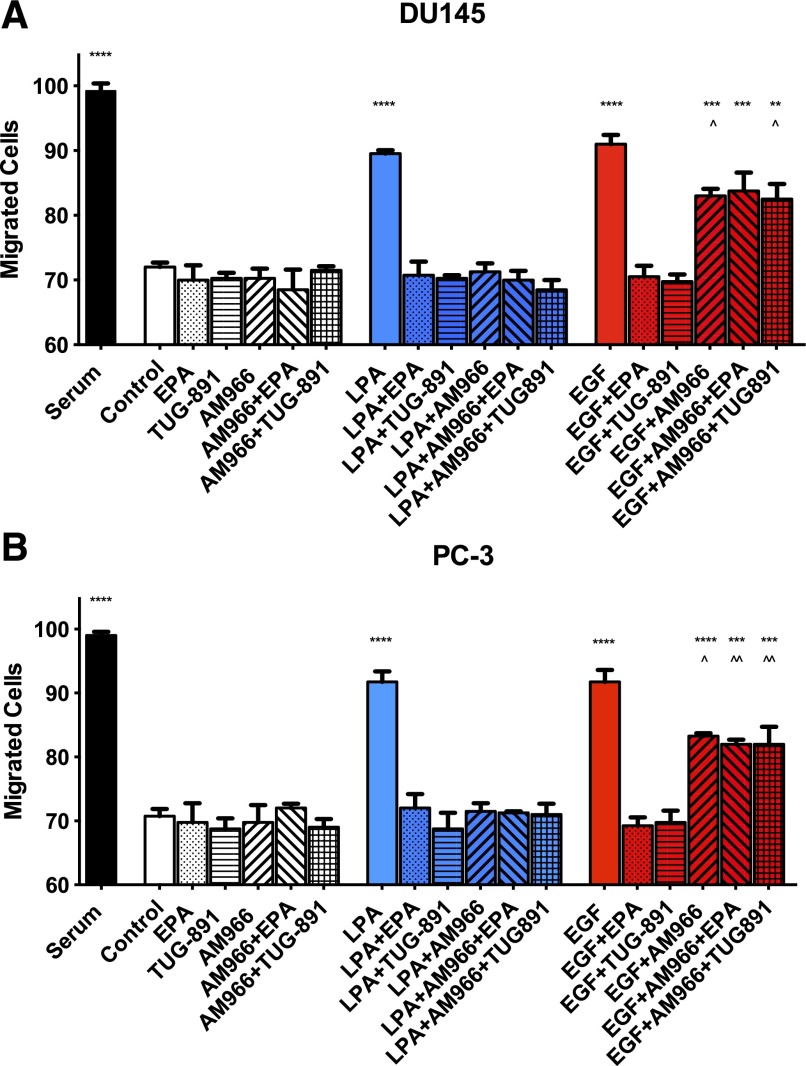
We next evaluated the effects of LPA1 antagonists on cell migration. We used the LPA1-selective antagonist AM966 for these experiments. As shown in Fig. 3, both EGF and LPA stimulated migration in a chemokinetic assay, to a lesser extent than the positive control (10% FBS). This finding is consistent with results reported previously (Liu et al., 2015). AM966 (100 nM) blocked LPA-induced migration. In addition, AM966 partially inhibited EGF-induced migration (42% inhibition in DU145; 46% inhibition in PC-3). In both cell lines, the residual EGF response was significantly different from both the control and from EGF alone. The FFA4 agonists EPA and TUG-891 inhibited migration in response to either LPA or EGF in both cell lines, as reported previously (Liu et al., 2015). Similar to the proliferation results (Fig. 2), the LPA1 antagonist blocked the inhibitory effects of EPA and TUG-891 on migration induced by EGF in both DU145 (Fig. 3A) and PC-3 (Fig. 3B).
Fig. 3.
Effects of LPAR agonists on human prostate cancer cell migration. Serum-starved DU145 (A) and PC-3 (B) cells were treated with 100 nM AM966, 20 μM EPA, 1 μM TUG-891, 10 μM LPA, or 10 nM EGF, either alone or in combination. After a 6-hour migration period, cells were analyzed as described in Materials and Methods. Each bar represents the mean ± S.E.M. (n = 4) of values obtained from two separate experiments, each of which was performed using separate replicate wells of cells. ****P < 0.0001; ***P < 0.001; **P < 0.01 versus control; ^^P < 0.01; ^P < 0.05 versus EGF). Data were analyzed using one-way ANOVA, followed by Tukey’s multiple comparisons test.
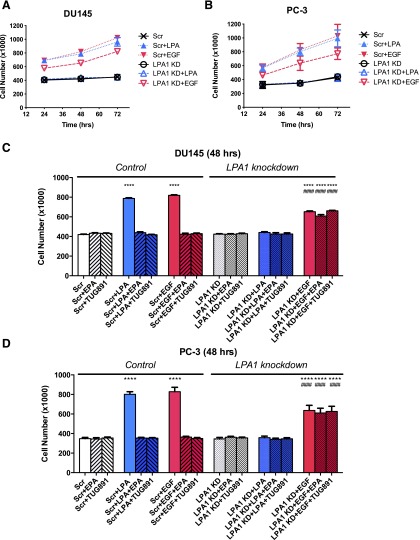
Effects of LPA1 Knockdown on Human Prostate Cancer Cell Proliferation.
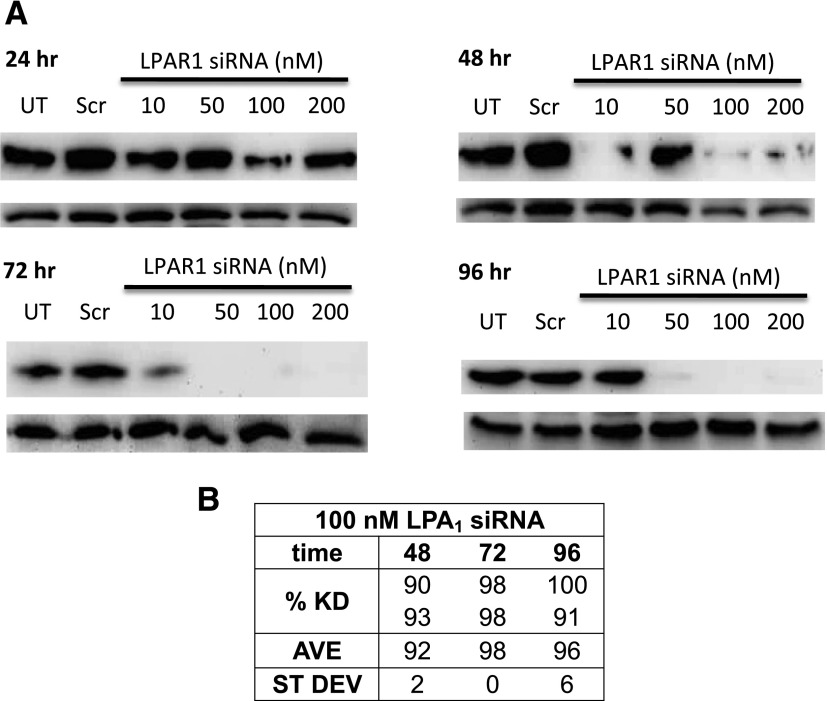
As another approach to test whether LPA1 is required for the effects of FFA4 agonists, siRNA was used to inhibit LPA1 expression in human prostate cancer cells. The siRNA treatment was quite effective, with LPA1 knockdown detected by 48 hours after transfection in DU145 cells (Fig. 4). This effect was maintained for at least 96 hours, as quantified in the table within Fig. 4.
Fig. 4.
LPA1 knockdown in prostate cancer cells. (A, B) DU145 cells were incubated with scrambled siRNA (negative control) or various concentrations of LPA1 siRNA for the indicated times. Whole-cell extracts were immunoblotted for LPA1 and actin (loading control) using siRNA (A). Representative blots for each time point are shown. (B) Densitometric analysis of the results of two separate experiments was performed using Quantity One software. The percent knockdown (KD) was calculated after quantification by densitometry, comparison with the Scr control for the same time point, and normalization to actin (n = 2).
Next, we tested whether FFAR agonists can inhibit LPA- or EGF-induced proliferation in cells deficient in LPA1 (Fig. 5). In these experiments, LPA or EGF was added 24 hours after siRNA treatment. As shown in Fig. 5, A and B, LPA1 knockdown alone had no effect on cell number in serum-starved DU145 or PC-3 cells in the absence of LPA or EGF and thus did not affect basal proliferation over a 72-hour time course; however, there was no response to LPA in either cell line after LPA1 knockdown. LPA1 knockdown only partially inhibited EGF-induced proliferation, consistent with the partial effects of LPA antagonists as shown earlier in Fig. 2. The results presented in Fig. 5, C and D, also demonstrate that FFA4 agonists significantly inhibited LPA-induced proliferation at 48 hours in DU145 and PC-3 cells treated with scrambled siRNA (negative control) but had no additional effect on cell number in cells treated with LPA1 siRNA. We tested the effects of the FFA4 agonists EPA and TUG-891 on EGF-induced proliferation in the absence and presence of LPA1. The EGF response remaining after LPA1 knockdown was refractory to inhibition by both EPA and TUG-891.
Fig. 5.
Effects of LPA1 knockdown on human prostate cancer cell proliferation. Serum-starved DU145 (A and C) and PC-3 (B and D) cells were incubated with control siRNA (“Scr”) or LPA1 siRNA (LPA1 KD) as described in Materials and Methods. (A) The proliferation time course is shown for DU145 cells, in response to 10 µM LPA or 10 nM EGF, with and without LPA1 knockdown. Then, 24 hours after transfection, cells were treated with and without 20 μM EPA or 1 μM TUG-891 and then with 10 μM LPA or 10 nM EGF. (A and B) Results from a full 72-hour time course. (C and D) Results from the 48-hour time point. Data points represent mean ± S.E.M. (n = 4) of values from two separate experiments, each of which was performed using separate replicate wells of cells. ****P < 0.0001 versus control siRNA Scr; ####P < 0.0001 vs. Scr + EGF) indicate values that were significantly different (two-way ANOVA followed by Tukey’s multiple comparisons test).
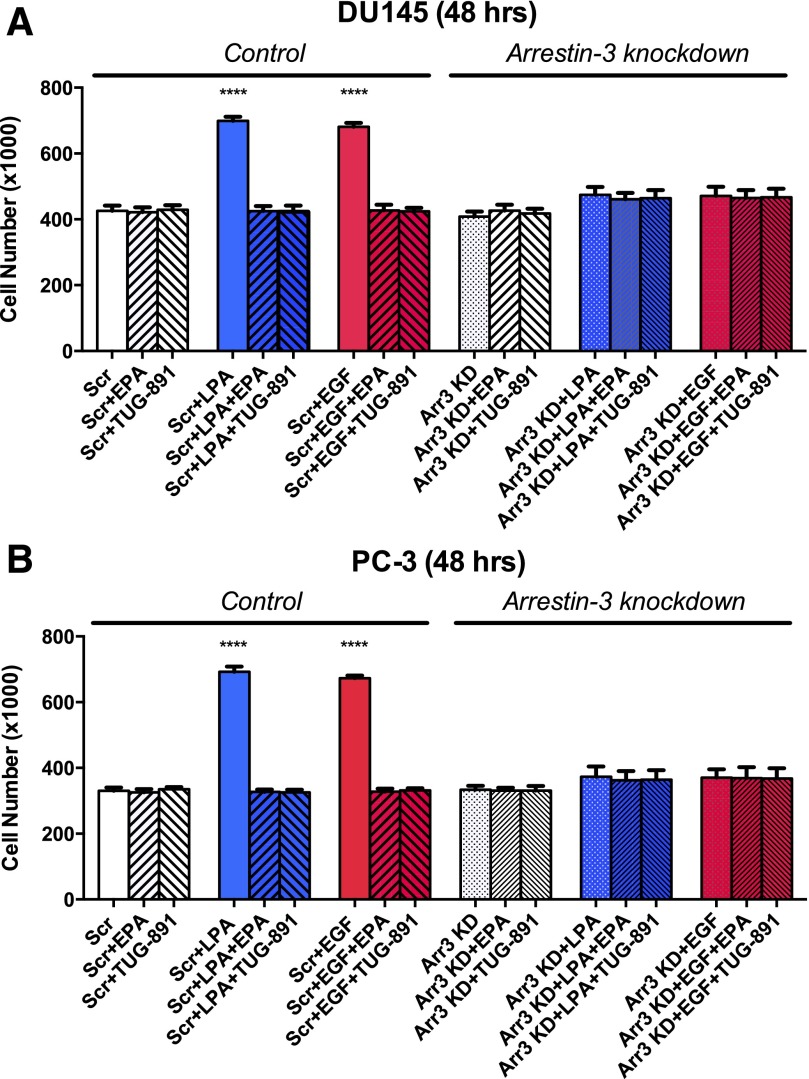
Effects of Arrestin-3 Knockdown on Human Prostate Cancer Cell Proliferation.
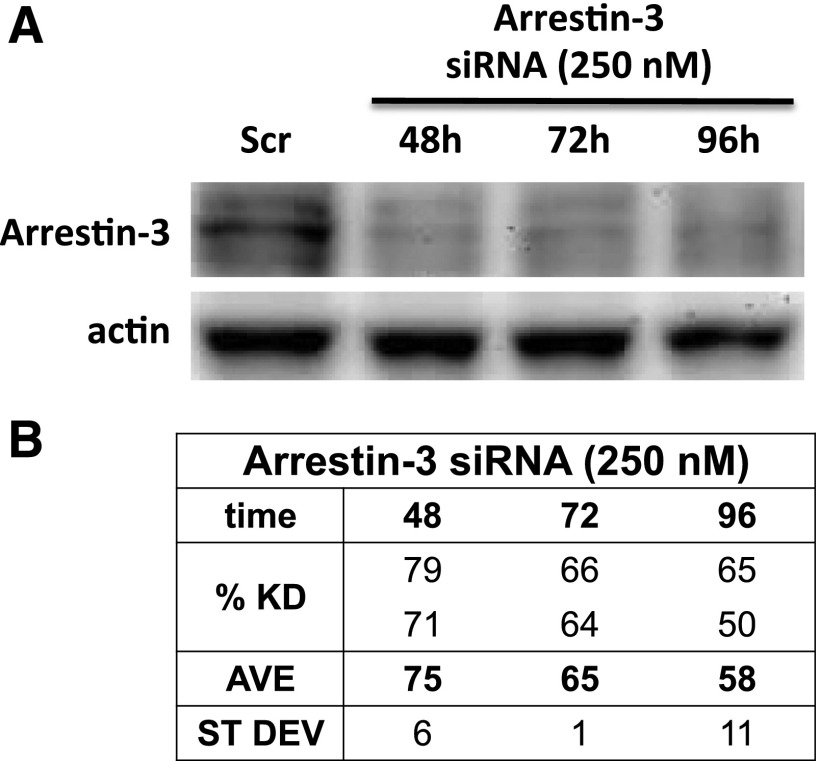
We next investigated whether an arrestin-mediated signaling pathway mediates the action of FFA4, as well as cross-talk between LPA1 and the EGFR. Human FFA4 exists in two alternatively spliced isoforms; both can signal through the arrestin pathway, but only one signals directly through G proteins (Hudson et al., 2013). Although both arrestin-2 and arrestin-3 are expressed in prostate cancer cells (Zhang et al., 2011), FFA4 is likely signaling through arrestin-3, with which it interacts strongly (reviewed in Hopkins and Meier, 2016). We therefore proceeded to perform knockdown of arrestin-3 and then test for effects of FFA4 agonists and growth factors.
Figure 6 illustrates successful knockdown of arrestin-3 in DU145 cells using siRNA. The knockdown persisted at greater than 50% through 96 hours after transfection. The effects of arrestin-3 knockdown on prostate cancer cell proliferation and migration were then examined (Fig. 7). After arrestin-3 knockdown, neither LPA nor EGF significantly increased proliferation in either DU145 or PC-3 cells. These results made it unfeasible to use the knockdown approach to discern the role of arrestin-3 in the response to FFA4 agonists; however, there was a slight, but not statistically significant, proliferation response to LPA and EGF after arrestin-3 knockdown. EPA and TUG-891 did not further inhibit this residual response, suggesting that FFA4 also signals through arrestin-3. Additional experiments will be required to determine whether FFA4 activation inhibits growth factor responses in an arrestin-dependent manner. Nonetheless, an important conclusion drawn from this experiment is that arrestin-3 is critical for the actions of both LPA and EGF and is thus likely to participate in both positive and negative cross-talk between GPCRs and the EGFR.
Fig. 6.
Knockdown of arrestin-3 in prostate cancer cells. (A) DU145 cells were incubated with scrambled (Scr) siRNA (negative control; 48 hours) or arrestin-3 (Ai) siRNA for the indicated times. Whole-cell extracts were immunoblotted for arrestin-3 and actin (loading control). A representative blot is shown. (B) Densitometric analysis was performed using Quantify One software. The percent knockdown (KD) was calculated after quantification of the results of two separate experiments by densitometry, comparison with the Scr control for the same time point, and normalization to actin (n = 2).
Fig. 7.
Effects of arrestin-3 knockdown on human prostate cancer cell proliferation. Serum- starved DU145 cells (A) and PC-3 (B) were incubated with scrambled siRNA (Scr) or arrestin-3 siRNA (Arr3 KD) as described in Materials and Methods. Twenty-four hours post-transfection, cells were treated with and without 20 μM EPA or 1 μM TUG-891 and then with 10 μM LPA (A) or 10 nM EGF (B). Data points represent the mean ± S.E.M. (n = 4) of values from two separate experiments, each of which was performed using separate replicate wells of cells. ****P < 0.0001 indicates values that were significantly different from control (two-way ANOVA followed by Tukey’s multiple comparisons test).
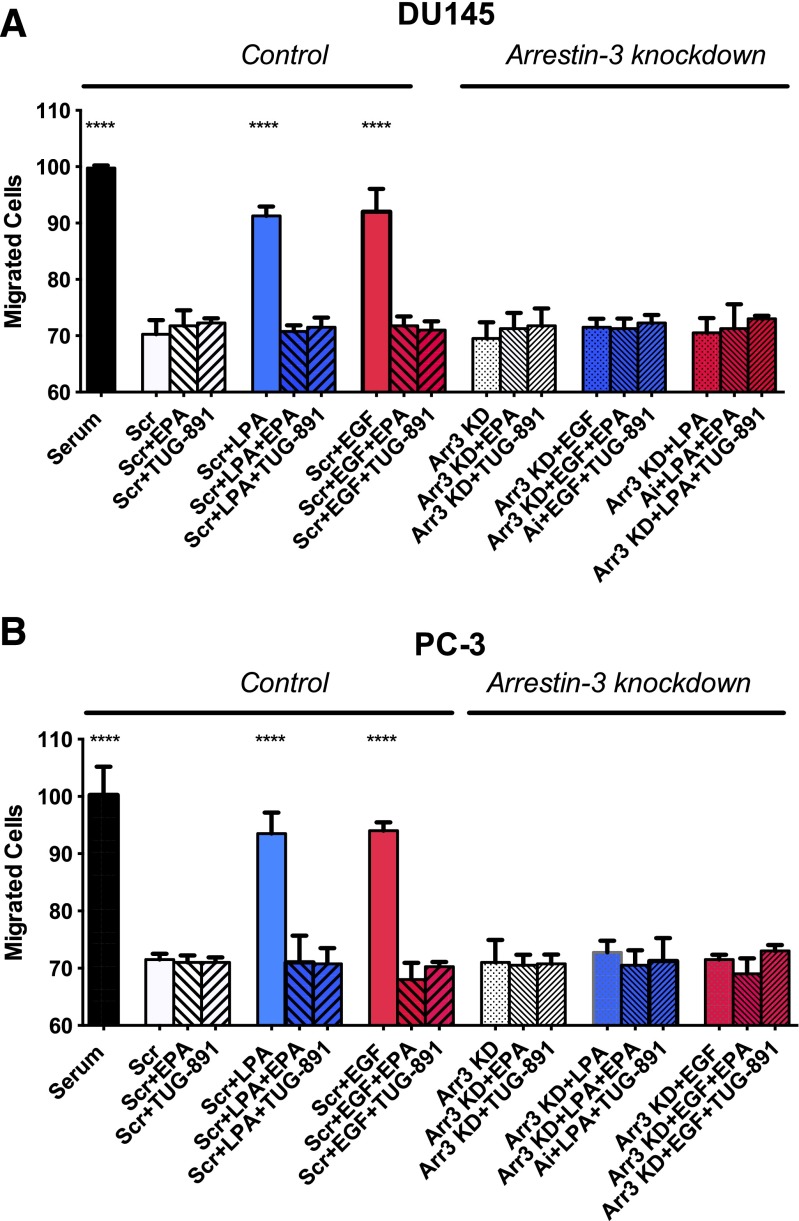
Effects of Arrestin-3 Knockdown on Human Prostate Cancer Cell Migration.
We next tested the role of arrestin-3 in prostate cancer cell migration (Fig. 8). Unlike the situation for proliferation (Fig. 7), there was complete inhibition of the migration response to LPA and EGF in both DU145 and PC-3 cells, with no detectable residual response, when arrestin-3 was knocked down. These results underscore the importance of arrestin-3 in both LPA- and EGF-induced signaling.
Fig. 8.
Effects of arrestin-3 knockdown on human prostate cancer cell migration. Serum-starved DU145 (A) and PC-3 (B) cells were incubated with scrambled siRNA (“Scr”) or arrestin-3 siRNA (Arr3 KD) as described in Materials and Methods. Forty-eight hours post-transfection, cells were treated with and without 20 μM EPA or 1 μM TUG-891 and then with 10 μM LPA or 10 nM EGF. Data points represent the mean ± S.E.M. (n = 4) of values from two separate experiments, each of which was performed using separate replicate wells of cells. Asterisks indicate values that were significantly different from control (one-way ANOVA followed by Tukey’s multiple comparisons test) from the control.
Discussion
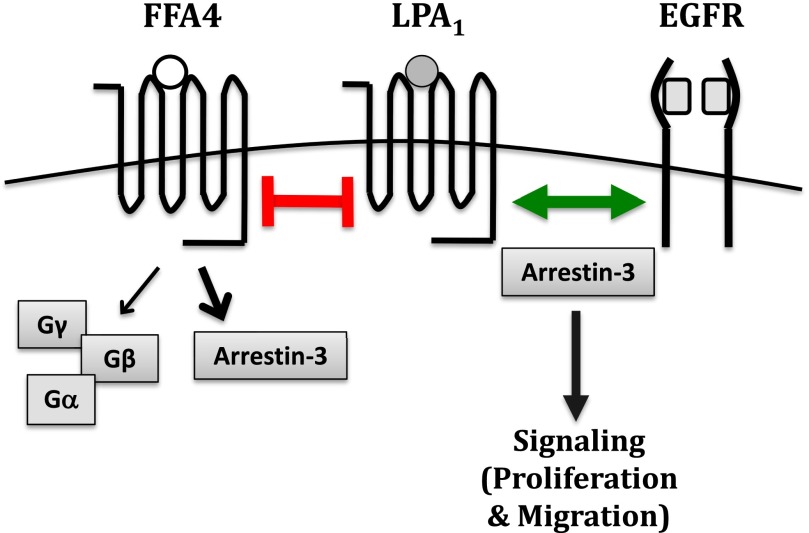
In this study, we explored the mechanism by which FFA4 activation causes inhibition of LPA- and EGF-mediated proliferation and migration in prostate cancer cells. The inhibition could be accomplished by either FFAR inhibiting LPAR(s), and thereby the EGFR, or by FFAR directly inhibiting both LPAR(s) and the EGFR. In our studies, we show that LPA1 inhibition and knockdown inhibit LPA-mediated proliferation and migration but also partially inhibit EGF-mediated proliferation and migration. The FFA4 agonists EPA and TUG-891 have no further effect on EGF-mediated signaling when LPA1 is unavailable. This result suggests that FFA4 activation acts indirectly on EGF-mediated signaling via effects on LPA1. We have therefore determined that the first scenario, in which FFA4 activation directly inhibits LPA-mediated signaling and thereby indirectly inhibits EGF-mediated signaling through LPA1, is most likely (Fig. 9).
Fig. 9.
Effects of FFA4 activation on LPAR activation and growth factor cross-talk. FFA4 activation by an FFA4 agonist (e.g., EPA, TUG-891) inhibits LPA1-mediated signaling. Activation of LPA1, is required for EGF-mediated proliferation and migration. Arrestin-3 interacts with both FFA4 and LPA1 and is required for responses to LPA and EGF; however, the role of arrestin-3 in receptor cross-talk remains to be elucidated.
The possibility remains that FFA4 activation inhibits LPA and EGF signaling through a downstream response pathway (e.g., protein phosphorylation) that is initiated by a second messenger produced via G-protein activation; however, based on our previous work with prostate cancer cells (Liu et al., 2015), the magnitude of the inhibition, the inhibition of multiple LPA-mediated downstream signaling pathways, the consistency of the inhibition in two different cell lines, and the duration of the inhibitory response, argue against this interpretation (Liu et al., 2015).
Our data confirm that LPA1 plays a critical role in EGF-induced proliferation in prostate cancer cells, despite the fact that DU145 and PC-3 cells also express LPA2, LPA3, and other LPA receptors. Literature in the field (e.g., Daaka, 2002) suggests that LPA1 is uniquely involved in facilitating EGFR response, but the mechanistic basis for this unique role has not yet been elucidated.
With respect to the roles of FFARs, three previous studies have shown that ω-3 fatty acids and other FFAR agonists can inhibit LPA signaling (Kim et al., 2014; Liu et al., 2015; Hopkins et al., 2016). In our previous study, we showed that these effects were mediated via FFA4, and not an LPAR, through the following experiments: 1) both ω-3 fatty acids and two different synthetic FFAR agonists (not fatty acids) had similar effects, and 2) FFA4 knockdown eliminated the inhibitory effects of FFAR agonists. There are several mechanisms by which FFA4 agonists may inhibit signaling through LPA1. First, agonist-bound FFA4 and LPA1 may heterodimerize in a manner that inhibits LPA1 action. It is well documented that GPCRs can form oligomers (Szidonya et al., 2008; Palczewski, 2010; Gonzalez-Maeso, 2011; Ferre et al., 2014), so it is plausible that this may be the case for FFA4 and LPA1. LPA1 has previously been shown to heterodimerize with sphingosine-1-phosphate receptors, other LPA receptors, and other GPCRs, as well as to form homodimers with itself (Zaslavsky et al., 2006). Second, FFA4 and LPA1 may participate in negative cross-talk via desensitization. This can occur through a variety of different signaling pathways, such as protein kinase C activation (Kelly et al., 2008; Delom and Fessart, 2011); however, this explanation does not seem particularly plausible given the duration of the inhibitory effect, its magnitude, and the fact that similar inhibition was observed in two different cell lines. A third alternative is that FFA4 and LPA1 compete for a pool of arrestin-3. For example, if FFA4 prevents LPA1 from interacting with arrestin-3, it can conceivably cause loss of response to LPA, since our data show that LPA1 response is dependent on arrestin-3.
As shown in Figs. 2, 3, and 5, EGF action is significantly, but only partially, inhibited when LPA1 is inhibited or knocked down. There are several mechanisms by which LPA1 may participate positively in EGFR response. It is well documented that LPA can transactivate the EGFR in prostate cancer cells (Daaka, 2002; Kue et al., 2002; Bektas et al., 2005), as well as in other cell types (Snider et al., 2010; Cattaneo et al., 2014). Although the LPA receptors have been called the “’masters’ of EGFRs” (Daaka, 2002), they are not the only GPCRs that can positively modulate the EGFR (Cattaneo et al., 2014). For example, it was recently reported that growth hormone-releasing hormone receptors transactivate EGFR and HER2 in PC-3 cells (Munoz-Moreno et al., 2014). However, the type of positive modulation observed in the current study, in which LPA1 is required for response to exogenous EGF, is distinct from the type that requires the release of EGFR ligands. The fact that LPA antagonists and LPA1 knockdown only partially block EGF response suggests that additional receptors can serve as “partners” to support EGFR activity in human prostate cancer cells.
Our group recently published the results of a related study concerning the dependence of EGF response on LPA receptors in breast cancer cell lines (Hopkins et al., 2016). In breast cancer cells, LPA antagonists and FFAR agonists likewise inhibited proliferation in response to LPA or EGF; however, as there was no significant residual response to EGF in the presence of LPA antagonists in these cells, we were unable to test whether the inhibition of EGF response in the presence of FFAR agonists was dependent or independent of LPARs. In the breast cancer study, we did not use knockdown approaches to test roles for LPARs or arrestin-3.
Ligand-dependent EGFR transactivation is generally thought to involve matrix metalloproteinase activation that in turn causes shedding of heparin-binding EGF (Cattaneo et al., 2014). In the studies reported herein, in which a maximal dose of exogenous EGF was added directly to the cells, transactivation mediated by release of EGFR ligands should not play a significant role in overall EGF response. A second mechanism, ligand-independent EGFR transactivation, can transpire when a ligand-bound GPCR activates EGFR intracellularly via a second messenger or effector (e.g., ROS, Ca2+, Src, arrestin). This causes rapid tyrosine phosphorylation of the EGFR (Audigier et al., 2013). A third mechanism of EGFR transactivation involves oligomerization of GPCR(s) and EGFR (Auigier et al., 2013; Cattaneo et al., 2014; Sur and Agarwal, 2014). A commonly cited example of GPCR-EGFR oligomerization is the heterodimerization of the β2-adrenergic receptor with EGFR, which results in EGFR transactivation (Audigier et al., 2013). This oligomerization is dependent on arrestin-3 recruitment, as is the case for some other GPCR-EGFR oligomers. Previous reports have indicated that GPCRs can physically associate with tyrosine kinase receptors (Moughal et al., 2006; Chung and Walker, 2007) and that EGFR can phosphorylate GRK2 (a GPCR kinase) (Chen et al., 2008). As reviewed by Berasain and colleagues (2011), GPCRs can both positively and negatively regulate the EGFR through mechanisms that do not involve shedding of EGFR ligands. This type of mechanism is most likely involved in the requirement for LPA1 in EGF response, as observed in the current study.
It is interesting to note that, although FFA4 agonists completely inhibit EGF response in human prostate cancer cells, the residual response to EGF that is seen after LPA1 inhibition or knockdown is not inhibited by FFA4 agonists. This result suggests that, in the absence of LPA1, the EGFR is able to interact with other partners that are not susceptible to FFA4 inhibition. An alternative explanation for the residual partial response is that there is enhanced diversification of the initial signal between ErbB family receptors when LPA1 is not available to enhance the signal. The ErbB family members interact in ways that often enhance signaling between the four receptors (Yarden and Sliwkowski, 2001; Normanno et al., 2006). One of these mechanisms, or a combination of them, is likely responsible for the partial EGF response observed when LPA1 is unavailable.
Our results have established that FFA4 activation inhibits LPA1-mediated signaling and the crosstalk between LPA1 and the EGFR, also rendering EGFR-mediated proliferation and migration inactive. Our results show that arrestin-3 knockdown completely inhibits LPA- and EGF-induced proliferation and migration (Fig. 8). This result indicates that EGFR response is dependent on arrestin-3. We therefore hypothesize that arrestin-3 mediates the inhibitory effects of FFA4. Since: 1) FFA4 is known to signal through arrestin-3, 2) EGFR is dependent on LPA1, and 3) both LPA1 and EGFR are dependent on arrestin-3, a negative modulatory effect of FFAR-arrestin-3 on LPA1 could explain the observed inhibition of EGF response.
Other groups have previously reported that arrestins, and particularly arrestin-3, play crucial roles in GPCR-mediated signaling relevant to proliferation, such as Erk activation (Ahn et al., 2003; Rajagopal et al., 2010). Arrestin-2 (β-arrestin-1) has been shown to be necessary for transactivation of the EGFR by prostaglandin E2 in colorectal carcinoma (Buchanan et al., 2006). Silencing of arrestin-3 has been reported to decrease cell migration and invasion in some cell types, including human breast cancer cells (Li et al., 2009; Rajagopal et al., 2010; Alemayehu et al., 2013). In the current study, the complete inhibition of LPA response in the absence of arrestin-3 was somewhat surprising, since pertussis toxin (an inhibitor of Gi/o protein signaling) also blocks LPA action in these cells (Guo et al., 2006). The complete inhibition of EGF response by arrestin-3 knockdown, as observed in our study, was unexpected. In the most pertinent published example, Miyatake and colleagues (2009) examined the inhibitory effects of a GPCR (µ-opioid receptor) on EGF signaling. Similar to the results reported herein, these authors found that arrestin-3 was required for signaling by exogenous EGF. A molecular mechanism has not yet been established for this effect.
Association of arrestin-3 and the EGFR can occur in either a ligand-dependent or -independent manner and is proposed to play a functional role in GPCR-EGFR complexes (Salazar et al., 2014; Sur and Agrawal, 2014); however, there are relatively few published studies of the roles of arrestins in EGF signaling, and most of these concern arrestin-3-dependent positive modulation of the EGFR by GPCRs (Tilley et al., 2009; Esposito et al., 2011; Salazar et al., 2014). In an interesting twist, one group recently showed that arrestin-3 functions as a tumor suppressor by negatively modulating chemokine receptor-induced EGFR activation in LnCAP prostate cancer cells (Kallifatidis et al., 2016). Although our studies show the importance of arrestins in GPCR-induced proliferation, further analysis of the role of arrestin-3 is still needed, particularly with regard to potential receptor-receptor interactions.
In summary, the results of this study suggest the provocative possibility that certain agonist-activated GPCRs function as “allosteric switches” to positively and negatively regulate the function of other receptors, including GPCRs and RTKs. This model is consistent with the known abilities of both GPCRs and RTKs to bind multiple protein partners, some of which are scaffold proteins that facilitate the formation of multiprotein complexes. The protein-protein interactions involved in the modulatory interactions remain to be determined. Finally, the data presented herein provide evidence that FFA4, LPA1, and arrestin-3 are all potential therapeutic targets in prostate cancer.
Abbreviations
- ANOVA
analysis of variance
- DMSO
dimethylsulfoxide
- EGF
epidermal growth factor
- EGFR
EGF receptor
- EPA
eicosapentaeneoic acid
- FA
fatty acid
- FBS
fetal bovine serum
- FFA
free fatty acid
- FFAR
free fatty acid receptor
- GPCR
G protein–coupled receptor
- LPA
lysophosphatidic acid
- LPAR
LPA receptor
- RTK
receptor tyrosine kinase receptor
Authorship Contributions
Participated in research design: Hopkins, Meier.
Conducted experiments: Hopkins, Liu.
Performed data analysis: Hopkins, Meier.
Wrote or contributed to the writing of the manuscript: Hopkins, Liu, Meier.
Footnotes
This work was supported by the College of Pharmacy at Washington State University (WSU). M.M.H., a member of the Graduate Program in Pharmaceutical Sciences in the College of Pharmacy, also received support from the National Institutes of Health Protein Biotechnology Training Program at WSU [Grant T32 GM008336].
References
- Ahn S, Nelson CD, Garrison TR, Miller WE, Lefkowitz RJ. (2003) Desensitization, internalization, and signaling functions of β-arrestins demonstrated by RNA interference. Proc Natl Acad Sci USA 100:1740–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alemayehu M, Dragan M, Pape C, Siddiqui I, Sacks DB, Di Guglielmo GM, Babwah AV, Bhattacharya M. (2013) β-Arrestin2 regulates lysophosphatidic acid-induced human breast tumor cell migration and invasion via Rap1 and IQGAP1. PLoS One 8:e56174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alghamdi F, Guo M, Abdulkhalek S, Crawford N, Amith SR, Szewczuk MR. (2014) A novel insulin receptor-signaling platform and its link to insulin resistance and type 2 diabetes. Cell Signal 26:1355–1368. [DOI] [PubMed] [Google Scholar]
- Audigier Y, Picault F-X, Chaves-Almagro C, Masri B. (2013) G protein-coupled receptors in cancer: biochemical interactions and drug design. Prog Mol Biol Transl Sci 115:143–173. [DOI] [PubMed] [Google Scholar]
- Bektas M, Payne SG, Liu H, Goparaju S, Milstien S, Spiegel S. (2005) A novel acylglycerol kinase that produces lysophosphatidic acid modulates cross talk with EGFR in prostate cancer cells. J Cell Biol 169:801–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berasain C, Ujue Latasa M, Urtasun R, Goñi S, Elizalde M, Garcia-Irigoyen O, Azcona M, Prieto J, Avila MA. (2011) Epidermal growth factor receptor (EGFR) crosstalks in liver cancer. Cancers (Basel) 3:2444–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhola NE, Grandis JR. (2008) Crosstalk between G-protein-coupled receptors and epidermal growth factor receptor in cancer. Front Biosci 13:1857–1865. [DOI] [PubMed] [Google Scholar]
- Buchanan FG, Gorden DL, Matta P, Shi Q, Matrisian LM, DuBois RN. (2006) Role of β-arrestin 1 in the metastatic progression of colorectal cancer. Proc Natl Acad Sci USA 103:1492–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo F, Guerra G, Parisi M, De Marinis M, Tafuri D, Cinelli M, Ammendola R. (2014) Cell-surface receptors transactivation mediated by g protein-coupled receptors. Int J Mol Sci 15:19700–19728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Long H, Wu Z, Jiang X, Ma L. (2008) EGF transregulates opioid receptors through EGFR-mediated GRK2 phosphorylation and activation. Mol Biol Cell 19:2973–2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung KY, Walker JW. (2007) Interaction and inhibitory cross-talk between endothelin and ErbB receptors in the adult heart. Mol Pharmacol 71:1494–1502. [DOI] [PubMed] [Google Scholar]
- Daaka Y. (2002) Mitogenic action of LPA in prostate. Biochim Biophys Acta 1582:265–269. [DOI] [PubMed] [Google Scholar]
- Delom F, Fessart D. (2011) Role of phosphorylation in the control of clathrin-mediated internalization of GPCR. Int J Cell Biol 2011:246954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito G, Perrino C, Cannavo A, Schiattarella GG, Borgia F, Sannino A, Pironti G, Gargiulo G, Di Serafino L, Franzone A, et al. (2011) EGFR trans-activation by urotensin II receptor is mediated by β-arrestin recruitment and confers cardioprotection in pressure overload-induced cardiac hypertrophy. Basic Res Cardiol 106:577–589. [DOI] [PubMed] [Google Scholar]
- Ferré S, Casadó V, Devi LA, Filizola M, Jockers R, Lohse MJ, Milligan G, Pin J-P, Guitart X. (2014) G protein-coupled receptor oligomerization revisited: functional and pharmacological perspectives. Pharmacol Rev 66:413–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George AJ, Hannan RD, Thomas WG. (2013) Unravelling the molecular complexity of GPCR-mediated EGFR transactivation using functional genomics approaches. FEBS J 280:5258–5268. [DOI] [PubMed] [Google Scholar]
- Gibbs TC, Rubio MV, Zhang Z, Xie Y, Kipp KR, Meier KE. (2009) Signal transduction responses to lysophosphatidic acid and sphingosine 1-phosphate in human prostate cancer cells. Prostate 69:1493–1506. [DOI] [PubMed] [Google Scholar]
- González-Maeso J. (2011) GPCR oligomers in pharmacology and signaling. Mol Brain 4:20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo R, Kasbohm EA, Arora P, Sample CJ, Baban B, Sud N, Sivashanmugam P, Moniri NH, Daaka Y. (2006) Expression and function of lysophosphatidic acid LPA1 receptor in prostate cancer cells. Endocrinology 147:4883–4892. [DOI] [PubMed] [Google Scholar]
- Hopkins MM and Meier KE(2016) Free fatty acid receptors and cancer: from nutrition to pharmacology, in Free Fatty Acid Receptors, Handbook of Experimental Pharmacology, (G. Milligan G, Kimura I eds), Springer Publishing, New York. [DOI] [PubMed] [Google Scholar]
- Hopkins MM, Zhang Z, Liu Z, Meier KE. (2016) Omega-3 fatty acid and other free fatty acid receptor agonists inhibit LA- and EGF-induced proliferation of breast cancer cells. J Clin Med 5:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson BD, Murdoch H, Milligan G. (2013) Minireview: the effects of species ortholog and SNP variation on receptors for free fatty acids. Mol Endocrinol 27:1177–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallifatidis G, Munoz D, Singh RK, Salazar N, Hoy JJ, Lokeshwar BL. (2016) Beta-arrestin-2 counters CXCR7-mediated EGFR transactivation and proliferation. Mol Cancer Res 14:493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly E, Bailey CP, Henderson G. (2008) Agonist-selective mechanisms of GPCR desensitization. Br J Pharmacol 153(Suppl 1):S379–S388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EK, Ha JM, Kim YW, Jin SY, Ha HK, Bae SS. (2014) Inhibitory role of polyunsaturated fatty acids on lysophosphatidic acid-induced cancer cell migration and adhesion. FEBS Lett 588:2971–2977. [DOI] [PubMed] [Google Scholar]
- Kue PF, Taub JS, Harrington LB, Polakiewicz RD, Ullrich A, Daaka Y. (2002) Lysophosphatidic acid-regulated mitogenic ERK signaling in androgen-insensitive prostate cancer PC-3 cells. Int J Cancer 102:572–579. [DOI] [PubMed] [Google Scholar]
- Li TT, Alemayehu M, Aziziyeh AI, Pape C, Pampillo M, Postovit LM, Mills GB, Babwah AV, Bhattacharya M. (2009) Beta-arrestin/Ral signaling regulates lysophosphatidic acid-mediated migration and invasion of human breast tumor cells. Mol Cancer Res 7:1064–1077. [DOI] [PubMed] [Google Scholar]
- Liebmann C, Böhmer F-D. (2000) Signal transduction pathways of G protein-coupled receptors and their cross-talk with receptor tyrosine kinases: lessons from bradykinin signaling. Curr Med Chem 7:911–943. [DOI] [PubMed] [Google Scholar]
- Liu Z, Hopkins MM, Zhang Z, Quisenberry CB, Fix LC, Galvan BM, Meier KE. (2015) Omega-3 fatty acids and other FFA4 agonists inhibit growth factor signaling in human prostate cancer cells. J Pharmacol Exp Ther 352:380–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttrell LM. (2013) Arrestin pathways as drug targets. Prog Mol Biol Transl Sci 118:469–497. [DOI] [PubMed] [Google Scholar]
- Magalhaes AC, Dunn H, Ferguson SSG. (2012) Regulation of GPCR activity, trafficking and localization by GPCR-interacting proteins. Br J Pharmacol 165:1717–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G. (2004) G protein-coupled receptor dimerization: function and ligand pharmacology. Mol Pharmacol 66:1–7. [DOI] [PubMed] [Google Scholar]
- Miyatake M, Rubinstein TJ, McLennan GP, Belcheva MM, Coscia CJ. (2009) Inhibition of EGF-induced ERK/MAP kinase-mediated astrocyte proliferation by mu opioids: integration of G protein and beta-arrestin 2-dependent pathways. J Neurochem 110:662–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moughal NA, Waters CM, Valentine WJ, Connell M, Richardson JC, Tigyi G, Pyne S, Pyne NJ. (2006) Protean agonism of the lysophosphatidic acid receptor-1 with Ki16425 reduces nerve growth factor-induced neurite outgrowth in pheochromocytoma 12 cells. J Neurochem 98:1920–1929. [DOI] [PubMed] [Google Scholar]
- Muñoz-Moreno L, Arenas MI, Carmena MJ, Schally AV, Prieto JC, Bajo AM. (2014) Growth hormone-releasing hormone antagonists abolish the transactivation of human epidermal growth factor receptors in advanced prostate cancer models. Invest New Drugs 32:871–882. [DOI] [PubMed] [Google Scholar]
- Normanno N, De Luca A, Bianco C, Strizzi L, Mancino M, Maiello MR, Carotenuto A, De Feo G, Caponigro F, Salomon DS. (2006) Epidermal growth factor receptor (EGFR) signaling in cancer. Gene 366:2–16. [DOI] [PubMed] [Google Scholar]
- Ohta H, Sato K, Murata N, Damirin A, Malchinkhuu E, Kon J, Kimura T, Tobo M, Yamazaki Y, Watanabe T, et al. (2003) Ki16425, a subtype-selective antagonist for EDG-family lysophosphatidic acid receptors. Mol Pharmacol 64:994–1005. [DOI] [PubMed] [Google Scholar]
- Palczewski K. (2010) Oligomeric forms of G protein-coupled receptors (GPCRs). Trends Biochem Sci 35:595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prihandoko R, Alvarez-Curto E, Hudson BD, Butcher AJ, Ulven T, Miller AM, Tobin AB, Milligan G. (2016) Distinct phosphorylation clusters determine the signaling outcome of free fatty acid receptor 4/G protein-coupled receptor 120. Mol Pharmacol 89:505–520. [DOI] [PubMed] [Google Scholar]
- Pyne NJ, Pyne S. (2011) Receptor tyrosine kinase-G-protein-coupled receptor signalling platforms: out of the shadow? Trends Pharmacol Sci 32:443–450. [DOI] [PubMed] [Google Scholar]
- Rajagopal S, Kim J, Ahn S, Craig S, Lam CM, Gerard NP, Gerard C, Lefkowitz RJ. (2010) β-arrestin- but not G protein-mediated signaling by the “decoy” receptor CXCR7. Proc Natl Acad Sci USA 107:628–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar N, Muñoz D, Kallifatidis G, Singh RK, Jordà M, Lokeshwar BL. (2014) The chemokine receptor CXCR7 interacts with EGFR to promote breast cancer cell proliferation. Mol Cancer 13:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider AJ, Zhang Z, Xie Y, Meier KE. (2010) Epidermal growth factor increases lysophosphatidic acid production in human ovarian cancer cells: roles for phospholipase D2 and receptor transactivation. Am J Physiol Cell Physiol 298:C163–C170. [DOI] [PubMed] [Google Scholar]
- Sur S, Agrawal DK. (2014) Transactivation of EGFR by G protein-coupled receptor in the pathophysiology of intimal hyperplasia. Curr Vasc Pharmacol 12:190–201. [DOI] [PubMed] [Google Scholar]
- Swaney JS, Chapman C, Correa LD, Stebbins KJ, Bundey RA, Prodanovich PC, Fagan P, Baccei CS, Santini AM, Hutchinson JH, et al. (2010) A novel, orally active LPA(1) receptor antagonist inhibits lung fibrosis in the mouse bleomycin model. Br J Pharmacol 160:1699–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szidonya L, Cserzo M, Hunyady L. (2008) Dimerization and oligomerization of G-protein-coupled receptors: debated structures with established and emerging functions. J Endocrinol 196:435–453. [DOI] [PubMed] [Google Scholar]
- Tilley DG, Kim I-M, Patel PA, Violin JD, Rockman HA. (2009) β-Arrestin mediates β1-adrenergic receptor-epidermal growth factor receptor interaction and downstream signaling. J Biol Chem 284:20375–20386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Jiang W, Spinetti T, Tardivel A, Castillo R, Bourquin C, Guarda G, Tian Z, Tschopp J, Zhou R. (2013) Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity 38:1154–1163. [DOI] [PubMed] [Google Scholar]
- Yarden Y, Sliwkowski MX. (2001) Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2:127–137. [DOI] [PubMed] [Google Scholar]
- Zaslavsky A, Singh LS, Tan H, Ding H, Liang Z, Xu Y. (2006) Homo- and hetero-dimerization of LPA/S1P receptors, OGR1 and GPR4. Biochim Biophys Acta 1761:1200–1212. [DOI] [PubMed] [Google Scholar]
- Zhang P, He X, Tan J, Zhou X, Zou L. (2011) β-arrestin2 mediates β-2 adrenergic receptor signaling inducing prostate cancer cell progression. Oncol Rep 26:1471–1477. [DOI] [PubMed] [Google Scholar]
- Zhao Y, He D, Saatian B, Watkins T, Spannhake EW, Pyne NJ, Natarajan V. (2006) Regulation of lysophosphatidic acid-induced epidermal growth factor receptor transactivation and interleukin-8 secretion in human bronchial epithelial cells by protein kinase Cdelta, Lyn kinase, and matrix metalloproteinases. J Biol Chem 281:19501–19511. [DOI] [PMC free article] [PubMed] [Google Scholar]