Abstract
The inflammatory bowel diseases (IBDs) are chronic inflammatory disorders with a complex etiology. IBD is thought to arise in genetically susceptible individuals in the context of aberrant interactions with the intestinal microbiota and other environmental risk factors. Recently, the pregnane X receptor (PXR) was identified as a sensor for microbial metabolites, whose activation can regulate the intestinal epithelial barrier. Mutations in NR1I2, the gene that encodes the PXR, have been linked to IBD, and in animal models, PXR deletion leads to barrier dysfunction. In the current study, we sought to assess the mechanism(s) through which the PXR regulates barrier function during inflammation. In Caco-2 intestinal epithelial cell monolayers, tumor necrosis factor-α/interferon-γ exposure disrupted the barrier and triggered zonula occludens-1 relocalization, increased expression of myosin light-chain kinase (MLCK), and activation of c-Jun N-terminal kinase 1/2 (JNK1/2). Activation of the PXR [rifaximin and [[3,5-Bis(1,1-dimethylethyl)-4-hydroxyphenyl]ethenylidene]bis-phosphonic acid tetraethyl ester (SR12813); 10 μM] protected the barrier, an effect that was associated with attenuated MLCK expression and JNK1/2 activation. In vivo, activation of the PXR [pregnenolone 16α-carbonitrile (PCN)] attenuated barrier disruption induced by toll-like receptor 4 activation in wild-type, but not Pxr−/−, mice. Furthermore, PCN treatment protected the barrier in the dextran-sulfate sodium model of experimental colitis, an effect that was associated with reduced expression of mucosal MLCK and phosphorylated JNK1/2. Together, our data suggest that the PXR regulates the intestinal epithelial barrier during inflammation by modulating cytokine-induced MLCK expression and JNK1/2 activation. Thus, targeting the PXR may prove beneficial for the treatment of inflammation-associated barrier disruption in the context of IBD.
Introduction
Although the etiology of the inflammatory bowel diseases (IBDs) has yet to be completely elucidated, the current paradigm suggests that these diseases are triggered by the integration of multiple factors, including specific genetic variants that govern host processes, the environment, the microbiota, and dysregulation of immune responses (Xavier and Podolsky, 2007). The intestinal mucosa, particularly the epithelium, provides a physical barrier that prevents noxious compounds and the microbiota from entering the internal tissues and inappropriately activating the mucosal immune system (Turner, 2009; Iacucci and Ghosh, 2011). Although increased permeability is observed in patients with IBD, it is well established that inflammatory processes can drive barrier dysfunction, thus it is difficult to establish causation (Teshima et al., 2012). However, data from a variety of studies suggest that compromised intestinal barrier function may contribute to the pathogenesis of IBD (Hollander et al., 1986; Hollander, 1988; Katz et al., 1989; Clayburgh et al., 2004). Although insufficient to cause disease on its own (Cunningham and Turner, 2012), barrier dysfunction is associated with relapse and recurrence in IBD patients (D'Inca et al., 1999; Tibble et al., 2000). Thus, therapies designed to re-establish or preserve barrier function, through the direct modulation of intestinal epithelial cell (IEC) function or damping of mucosal immune responses, would prove beneficial in the treatment of IBD.
We, and others, have reported that the pregnane X receptor (PXR), a ligand-activated nuclear receptor that senses and responds to a variety of chemical stimuli, including intestinal microbial metabolites (Venkatesh et al., 2014), plays a key role in the intestinal epithelium, regulating inflammatory responses (Ma et al., 2007; Mencarelli et al., 2010, 2011), enhancing wound healing (Terc et al., 2014), and maintaining barrier function under basal (Venkatesh et al., 2014) and inflammatory conditions (Terc et al., 2014). Interestingly, genetic studies have identified single-nucleotide polymorphisms in NR1I2, the gene that encodes the PXR, which are associated with increased susceptibility for developing Crohn’s disease and ulcerative colitis (Langmann et al., 2004; Dring et al., 2006; Martinez et al., 2007; Glas et al., 2011). Furthermore, others have reported that selective activation of the PXR attenuates nuclear factor κ-light-chain-enhancer of activated B cells (NFκB)–dependent inflammatory signaling in the intestinal epithelium (Shah et al., 2007; Mencarelli et al., 2010, 2011), an effect associated with protection in experimental models of colitis (Ma et al., 2007; Shah et al., 2007; Dou et al., 2012, 2013; Terc et al., 2014). In the current study, we sought to assess whether stimulation of the PXR in IECs regulates the intracellular signaling pathways that modulate barrier function in the context of inflammation. We hypothesized that the PXR protects the epithelial barrier through its purported ability to regulate NFκB and c-Jun N-terminal kinase 1/2 (JNK1/2) signaling, key pathways that regulate tight-junction function in the context of cellular stress and inflammation.
Herein, we report that stimulation of the PXR in Caco-2 IECs prevented tumor necrosis factor-α (TNFα)/interferon-γ (IFNγ)–induced barrier disruption, an effect that was associated with a preservation of tight junctions. PXR stimulation attenuated NFκB signaling and reduced TNFα/IFNγ-induced expression of myosin light-chain kinase (MLCK) and activation of JNK1/2. In vivo, using two distinct mouse models, we found that activation of the PXR blocked the barrier disruption evoked by toll-like receptor 4 (TLR4) activation and during dextran sulfate sodium (DSS)–induced experimental colitis, an effect that was associated with an attenuation of the expression of MLCK and activation of JNK1/2 in the intestinal mucosa.
Materials and Methods
Reagents.
The selective human PXR agonists rifaximin (Ma et al., 2007) and [[3,5-Bis(1,1-dimethylethyl)-4-hydroxyphenyl]ethenylidene]bis-phosphonic acid tetraethyl ester (SR12813) (Watkins et al., 2001) (Sigma-Aldrich, St. Louis, MO) were dissolved in sterile dimethylsulfoxide (DMSO) and added to culture media to reach the appropriate experimental concentration (10 μM) (Ma et al., 2007; Lau et al., 2012; Sharma et al., 2013; Terc et al., 2014). The concentration of SR12813 (10 μM) is a maximal PXR-activating concentration (Jones et al., 2000), whereas the concentration of rifaximin (10 μM) is an effective concentration in activating the PXR (Ma et al., 2007); for comparative purposes, equimolar concentrations were used throughout our studies. Rifaximin was chosen based on its potential clinical efficacy in IBD (Prantera et al., 2006, 2012; Isaacs et al., 2007), whereas SR12813 was chosen due to its extensive characterization as a selective human PXR agonist (Jones et al., 2000; Watkins et al., 2001). Matching volumes of sterile DMSO were added to culture media for experimental vehicle controls. Hexahydro-1-[(5-iodo-1-naphthalenyl)sulfonyl]-1H-1,4-diazepine hydrochloride (ML-7, to inhibit MLCK; Sigma-Aldrich) and anthra[1-9-cd]pyrazol-6(2H)-one (SP600125, to inhibit JNK1/2; Sigma-Aldrich) were dissolved in sterile DMSO. Volumes of stock solutions were added to culture media to reach the appropriate experimental concentration (1 μM). Inhibitors were added 1 hour prior to the addition of TNFα/IFNγ (TNFα, 10 ng/ml; IFNγ, 20 ng/ml; R&D Solutions, Minneapolis, MN). TNFα and IFNγ concentrations and exposure time (16 hours) were based on values/ranges reported previously (Zolotarevsky et al., 2002; Bruewer et al., 2003; Wang et al., 2005, 2006). Ultra-pure lipolysaccharide (LPS; InvivoGen, San Diego, CA) was dissolved in sterile LPS-free water and added to culture media to achieve the appropriate experimental concentration (100 ng/ml).
To activate the mouse PXR in our in vivo experiments, we used the rodent selective PXR agonist pregnenolone 16α-carbonitrile (PCN; Sigma-Aldrich), which was dissolved in sterile corn oil. Each mouse was administered PCN via i.p. injection at a dose of 25 mg/kg, as we have published previously (Terc et al., 2014). Control mice were administered matching volumes of sterile corn oil via i.p. injection.
Cell Culture.
Caco-2 cells [adult human colonic epithelial cell line derived from a colorectal adenocarcinoma; American Type Culture Collection (ATCC), Manassas, VA] were cultured in Dulbecco's modified Eagle’s medium supplemented with 20% heat-inactivated fetal bovine serum (FBS) and penicillin-streptomycin (100 μg/ml, 1 nM; Invitrogen, Carlsbad, CA). All experiments were performed on cell passages 15–25.
THP-1-Blue NFκB cells which express an NFκB-driven secreted embryonic alkaline phosphatase (SEAP) reporter gene (InvivoGen) were propagated in RPMI 1640 media supplemented with 10% FBS, 1% penicillin-streptomycin, 1% sodium pyruvate, and β-mercaptoethanol (to a final concentration of 0.05 mM). Prior to exposure to LPS, THP-1-Blue NFκB cells were plated at a density of 100,000 cells/well (96-well plate) and differentiated into macrophages by exposing them to phorbol-12-myristate-13-acetate (50 ng/ml; 24 hours). All experiments were performed in OptiMEM media (Invitrogen) supplemented with 5% FBS.
HepG2 (human hepatocellular carcinoma cells; ATCC) and LS180 cells (human colon adenocarcinoma cells; ATCC) were cultured in minimum essential medium supplemented with 2 mM l-glutamine, 1% penicillin-streptomycin, and 10% FBS, as described previously (Lau and Chang, 2015).
Fluorescein Isothiocyanate-Dextran Flux Barrier Studies.
To assess barrier function in vitro, fluorescein isothiocyanate-dextran (FITC-dextran) flux permeability assays were performed as previously described (Hansen et al., 2013; Schenck et al., 2013). In brief, Caco-2 IECs were plated on Transwell permeable inserts (Corning Inc., Corning, NY) and given fresh medium every other day. Monolayers were grown up to 14 days postconfluence to allow for polarization and then used in our experiments. To assess permeability, FITC-dextran (0.5 mg/ml; Sigma-Aldrich) was added to the apical compartment of the Transwell insert. Cells were pretreated with PXR agonists (rifaximin or SR12813) or inhibitors (ML-7 or SP600125) 1 hour prior to exposure to TNFα/IFNγ (TNFα, 10 ng/ml; IFNγ, 20 ng/ml) (Zolotarevsky et al., 2002; Bruewer et al., 2003; Wang et al., 2005, 2006) and media sampled from the basolateral compartment at 16 hours post-TNFα/IFNγ stimulation (Hansen et al., 2013; Schenck et al., 2013). The movement of FITC-dextran from the apical to the basolateral compartment was quantified by a fluorescence plate reader (excitation 490 nm, emission 520 nm).
Zonula Occludens-1 Immunofluorescence Staining.
Caco-2 IECs were plated at 1.2 × 105 cells/ml on 8-well chamber slides (Thermo Fisher Scientific, Mississauga, Canada). Cells were grown for 14 days postconfluence and then used for our experiments. Cells were pretreated with PXR agonists (rifaximin or SR12813) 1 hour prior to exposure to TNFα/IFNγ (TNFα, 10 ng/ml; IFNγ, 20 ng/ml) for 16 hours (Zolotarevsky et al., 2002; Bruewer et al., 2003; Wang et al., 2005, 2006). At the end of the experiment, the cells were stained, as we have described previously (Hansen et al., 2013; Schenck et al., 2013). In brief, cells were rinsed twice with ice-cold phosphate-buffered saline (PBS) and then fixed with ice-cold methanol for 30 minutes at 4°C. Following fixation, the cells were blocked with normal donkey serum (15 minutes at room temperature) and then incubated with rabbit anti–zonula occludens-1 (anti–ZO-1) primary antibody (1:100 dilution, 1 hour at 37°C; Invitrogen). Cells were then rinsed with PBS twice, incubated with Cy5-conjugated secondary goat anti-rabbit IgG (1:500 dilution, 1 hour at 37°C; Jackson ImmunoResearch, West Grove, PA), rinsed three times with PBS, and affixed on coverslips using Fluorosave Reagent (Calbiochem, San Diego, CA). Cells were viewed by fluorescence microscopy, and images were captured with a digital DS-Fi1 camera (Nikon, Tokyo, Japan).
Western Blots.
To isolate cellular lysates, culture medium was removed, and the cells were washed with ice-cold PBS. Cell lysis buffer was added [150 mM NaCl, 20 mM Tris (pH 7.5), 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, and protease inhibitor cocktail; phosphatase inhibitor cocktail: Complete Minitab; Complete PhoStop (Laval, Canada), Roche]. Total protein was quantified using the Precision Red Advanced Protein Assay (Cytoskeleton, Denver, CO) and sample protein concentration equalized. Samples were resolved and transferred to nitrocellulose membranes (0.2-μm pores; Bio-Rad, Hercules, CA) and blotted with the following antibodies: PXR (TA312120; OriGene, Rockville, MD), MLCK (SAV1300116; Sigma-Aldrich), JNK1/2 (9258; Cell Signaling, Danvers, MA), phosphorylated JNK1/2 (4668; Cell Signaling). Blots were imaged using a ChemiDoc XRS (Bio-Rad) and band intensity quantified using ImageJ (National Institutes of Health, Bethesda, MD).
PXR-Dependent Reporter Gene Assay.
HepG2 and LS180 cells were transfected as described previously (Lau and Chang, 2015). In brief, cells were transfected within 6 hours of seeding by treating them with FuGENE 6 transection reagent (Madison, WI) and plasmids (human PXR overexpression plasmid: pCMV6-XL4-human PXR; PXR reporter plasmid: pGL3-basic-CYP3A4-xenobiotic responsive enhancer module (XREM)-luc; reference Renilla reniformis luciferase: pGL4.74[hRluc/TK]) dissolved in serum-free OptiMEM (Thermo Fisher Scientific). After 24 hours, culture medium was replaced with fresh supplemented growth medium, and the selective PXR agonists rifaximin (10 μM) and SR12813 (10 μM) or DMSO vehicle control was added for an additional 24 hours. At the end of the experiment, the cells were lysed and the firefly luciferase and R. reniformis luciferase activities quantified. Data are expressed as a ratio of firefly luciferase activity to R. reniformis luciferase activity.
NFκB Reporter Assays.
Phorbol-12-myristate-13-acetate –differentiated THP-1-Blue NFκB cells were pretreated with rifaximin 1 hour prior to exposure to LPS (100 ng/ml; 24 hours). NFκB activity was assessed by quantifying NFκB-dependent SEAP production, released into the culture supernatant following addition of QUANTI-Blue SEAP detection reagent (InvivoGen). Following 24 hours of LPS exposure, SEAP activity was assessed via spectrophotometer (620–655 nm).
TLR4-Induced Small Intestinal Barrier Disruption.
All experiments outlined in this section were approved by the Albert Einstein College of Medicine Animal Institute Committee (protocol #20070715, 20100711) and performed in accordance with institutional and national guidelines. Pxr−/− mice were kindly provided by Dr. Jeff Staudinger (University of Kansas, Lawrence, KS). Disruption of the small intestinal mucosal barrier was accomplished by administering di[3-deoxy-d-manno-octulosonyl]-lipid A (KDO2), a selective TLR4 ligand, directly into the jejunum, as we have published previously (Venkatesh et al., 2014). In brief, male mice were pretreated with 200 μl of 10% sodium bicarbonate (15 minutes prior to KDO2 administration) to decrease gastric acidity. After this, 200 μg of KDO2 (Avanti Polar Lipids, Inc., Alabaster, AL) was delivered to each mouse via sterile injection directly into the jejunum. Twenty-four hours after KDO2 administration, intestinal permeability was assessed by FITC-dextran flux assay. At the end of the experiment, mice were administered FITC-dextran (4 kDa, 10 mg/mouse, oral gavage; Sigma-Aldrich) 4 hours prior to sacrifice, exsanguinated via cardiac puncture, and euthanized by cervical dislocation. The movement of FITC-dextran into the serum, a measure of intestinal permeability, was quantified by a fluorescence plate reader (excitation 490 nm, emission 520 nm).
Experimental Colitis.
All experiments outlined in this section were approved by the Health Sciences Animal Care Committee, University of Calgary, and conform to the guidelines set by the Canadian Council on Animal Care (protocol #AC12-0146). Male C57Bl/6 were used between 8 and 10 weeks of age. Colitis was induced by the addition of DSS [2.5% (w/v); molecular weight, 40,000; ICN Biomedical, Irvine, CA] to the drinking water as described previously (Hirota et al., 2011; Terc et al., 2014). Animals were assessed and body weights were recorded daily. Mice were given a daily administration of PCN (mouse-specific PXR agonist; 25 mg/kg, i.p.) throughout the 7-day course of DSS, starting 1 day prior to commencement. At the end of the experiment, intestinal permeability was assessed by FITC-dextran flux assay (as described in the previous section). Colonic tissues were harvested, and a macroscopic damage score was given to each animal. Colons were opened longitudinally and placed on a flat surface. The mucosal and serosal surfaces of each colon were assessed for the presence of any of the following gross lesions: erythema, hemorrhage, edema, fecal blood, fecal mucous, diarrhea, strictures, ulcerations, and adhesions. Each lesion was given a score of 1, and lesions were summed to produce an overall macroscopic score. In addition to this analysis, colonic sections were isolated and tissue myeloperoxidase (MPO) levels quantified, and the mucosal expression of MLCK and activation (phosphorylation) of JNK1/2 were assessed by immunohistochemical staining.
Tissue Myeloperoxidase Assay.
Colonic tissue MPO activity, an inflammatory index of granulocyte infiltration, was determined following a published protocol (Diaz-Granados et al., 2000). MPO activity was reported as units per milligram of tissue, where one unit of MPO was defined as the amount of enzyme needed to degrade 1 µmol of H2O2 per minute at room temperature.
Immunohistochemical Staining.
Freshly dissected colonic tissue was fixed overnight in 4% formaldehyde in phosphate-buffered saline and embedded in paraffin as a “Swiss roll.” MLCK and phosphorylated/active JNK1/2 were detected by immunohistochemical staining. In brief, tissue sections were deparaffinized/rehydrated and antigen retrieval performed (Tris/EDTA pH 9.0 in a vegetable steamer at 95–100°C for 20 minutes). Sections were then blocked (Serum Blocking Reagent, Avidin Blocking Reagent, Biotin Blocking Reagent; R&D Systems, Minneapolis, MN) and then incubated with primary antibodies [MLCK: #Ab76092, 1:100 (Abcam, Cambridge, UK); phospho-JNK1/2: #AF1205, 1:100 (R&D Systems)] diluted in incubation buffer (1% bovine serum albumin, 1% normal donkey serum, 0.3% Triton X-100, 0.01% sodium azide in PBS). Sections were then incubated with biotin-conjugated secondary antibodies, high sensitivity streptavidin-horseradish peroxidase, and DAB Chromogen (R&D Systems). Following the chromogen reaction, sections were counterstained with Gill-2 hematoxylin and dehydrated, and coverslips were mounted with xylene-based mounting media.
Results
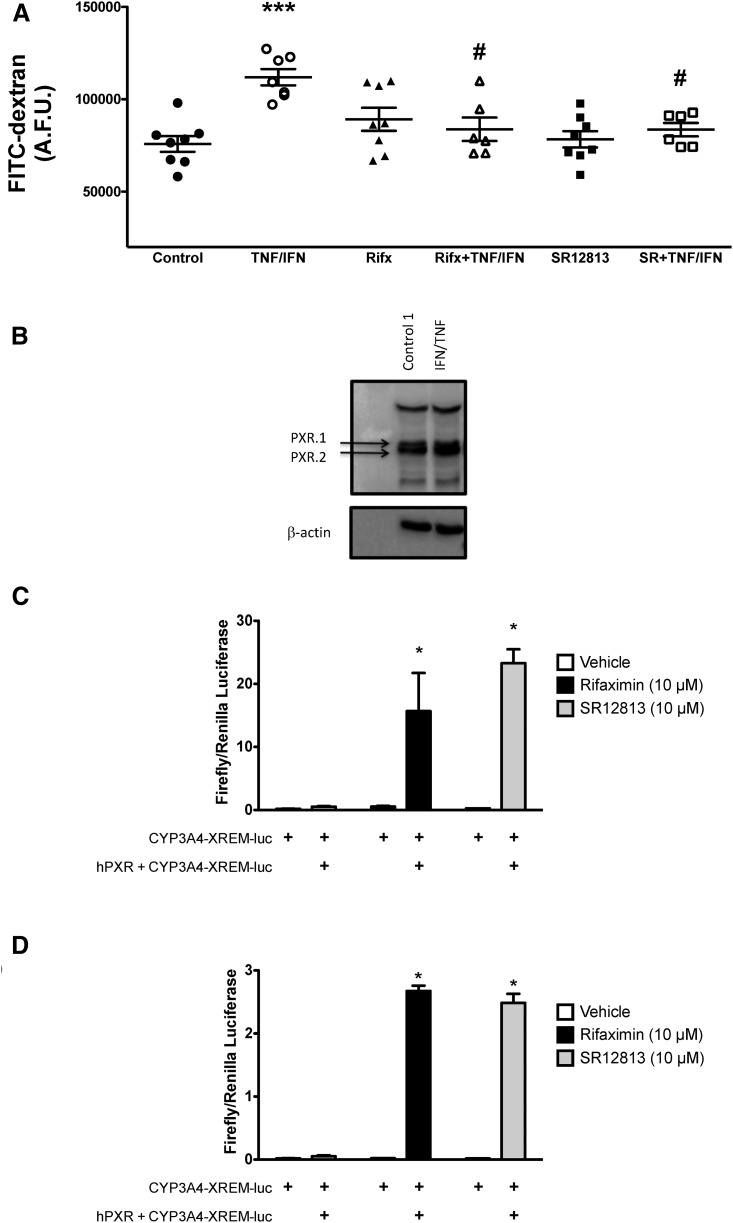
To model the intestinal epithelium experimentally, we plated Caco-2 IECs on Transwell inserts and cultured them for 14 days postconfluence. Caco-2 monolayers were then exposed to TNFα (10 ng/ml) and IFNγ (20 ng/ml) to model key aspects of the inflammatory milieu of the intestinal mucosa thought to contribute to the barrier dysfunction observed in patients with IBD (Turner, 2009). To assess barrier function, we quantified the movement of FITC-dextran (4 kDa) from the apical to the basolateral compartment of the Transwell insert, as we have done previously (Hansen et al., 2013; Schenck et al., 2013). TNFα/IFNγ exposure (16 hours) significantly increased FITC-dextran flux across the intestinal epithelial monolayer (Fig. 1A), indicating barrier dysfunction, as has been reported previously (Ma et al., 2005; Ye et al., 2006). TNFα/IFNγ had no effect on the expression of the PXR in these polarized monolayers (both the PXR.1 and PXR.2 isoforms; Fig. 1B). Pretreatment with the selective human PXR agonists rifaximin (10 μM) or SR12813 (10 μM) significantly reduced the FITC-dextran flux induced by TNFα/IFNγ exposure (Fig. 1A), suggesting that PXR stimulation can afford barrier protection by exerting a direct effect on the intestinal epithelium.
Fig. 1.
Selective activation of the PXR preserves barrier function in Caco-2 IEC monolayers. Caco-2 monolayers grown for 14 days postconfluence were pretreated with selective PXR agonists [rifaximin (Rifx) or SR12813 (SR), each at 10 μM) for 1 hour prior to exposure to TNFα (10 ng/ml) and IFNγ (20 ng/ml) for 16 hours. (A) The barrier function of each monolayer was quantified by assessing FITC-dextran flux (4 kDa). ***P < 0.001 compared with NoRx (vehicle control); #P < 0.05 compared with TNFα/IFNγ (n = 6–8). Rifaximin and SR12813 are selective PXR agonists. Overexpression of the human PXR (hPXR) in LS180 IECs and HepG2 cells enhances the activation of PXR-responsive CYP3A4-XREM–driven luciferase when exposed to rifaximin and SR12813. (B) The PXR is expressed in Caco-2 IECs grown for 14 days postconfluence on Transwell inserts (blots are representative of four separate experiments). LS180 (C) and HepG2 cells (D) transfected with the hPXR and treated with rifaximin (10 μM) and SR12813 (10 μM) exhibit enhanced CYP3A4-XREM–driven luciferase activity compared with the vehicle control (DMSO). *P < 0.05 compared with the group not transfected with the hPXR construct (n = 3). A.F.U., arbitrary fluorescence units.
Whereas rifaximin (Ma et al., 2007; Cheng et al., 2010; Mencarelli et al., 2010, 2011) and SR12813 (Ekins and Erickson, 2002; Lemaire et al., 2007) have been widely reported to act as selective agonists of the PXR, we confirmed their ability to elicit PXR-dependent effects. Rifaximin and SR12813 did not significantly activate an XREM-containing PXR-responsive reporter gene construct in LS180 and HepG2 cells (Fig. 1, C and D). However, overexpression of the human PXR significantly increased rifaximin- and SR12813-induced XREM-containing reporter activation (Fig. 1, B and C), suggesting that these compounds activate PXR-driven processes.
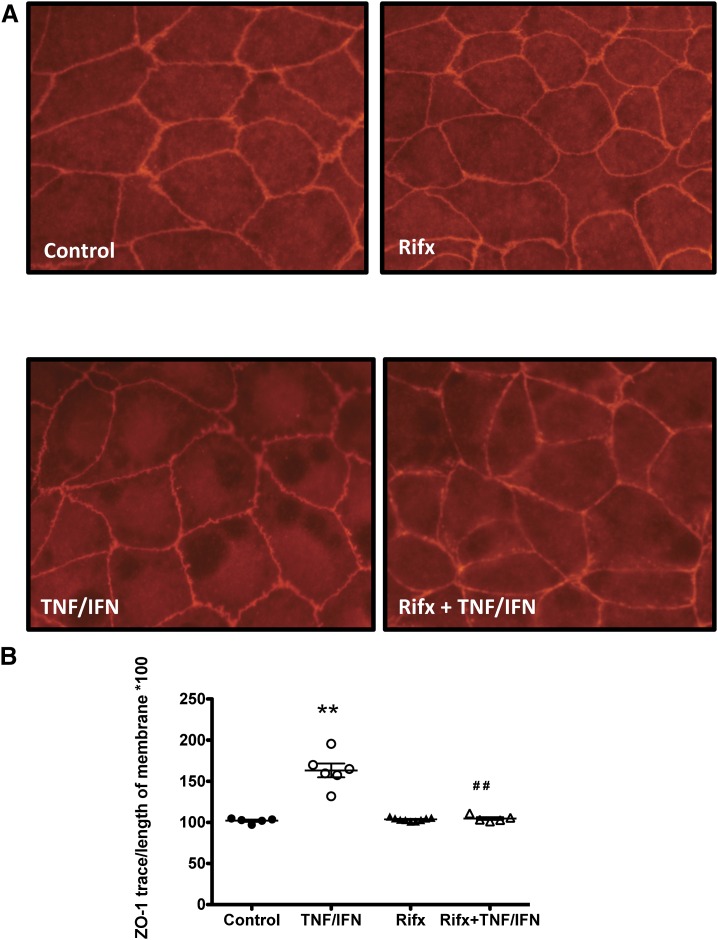
Next, we sought to determine the mechanism through which PXR stimulation preserves barrier function in intestinal epithelial monolayers exposed to inflammatory mediators. We have previously visualized ZO-1 and assessed its localization at cell-cell contacts as a measure of tight-junction integrity (Hansen et al., 2013; Schenck et al., 2013). Caco-2 monolayers treated with TNFα/IFNγ (TNFα, 10 ng/ml; IFNγ, 20 ng/ml; 16 hours) exhibited ruffling of the peripheral ZO-1 staining (Fig. 2A), an observation that was quantified by expressing the measured distance of the ruffling as a percentage of total cell-cell contact (Fig. 2B). When quantified, this ratio was significantly increased in TNFα/IFNγ-treated monolayers when compared with cell monolayers treated with vehicle or the selective PXR agonist rifaximin (10 μM) alone (Fig. 2B). Interestingly, pretreatment with rifaximin attenuated the ZO-1 ruffling (Fig. 2A) and blocked the increased ruffling/cell-cell contact ratio triggered by TNFα/IFNγ treatment (Fig. 2B), suggesting that PXR activation can directly modulate the response of IECs to inflammatory stress and preserve tight-junction integrity.
Fig. 2.
Selective activation of the PXR on Caco-2 IECs maintains tight-junction integrity in epithelial monolayers. (A) Caco-2 monolayers grown for 14 days postconfluence were pretreated with rifaximin (Rifx; 10 μM), a selective PXR agonist, for 1 hour prior to exposure to TNFα (10 ng/ml) and IFNγ (20 ng/ml) for 16 hours. (B) The degree of ZO-1 mislocalization, an indicator of tight-junction disruption, was quantified by using ImageJ to measure the length of ZO-1 staining and expressing it as a ratio of the length of total cell-cell contact. A value of 100 indicates ZO-1 staining is maintained over the entire cell-cell interface. A value >100 indicates ZO-1 staining is disrupted over the cell-cell interface. **P < 0.005 compared with control; ##P < 0.005 compared with TNFα/IFNγ group (n = 5–9 replicates from four separate experiments).
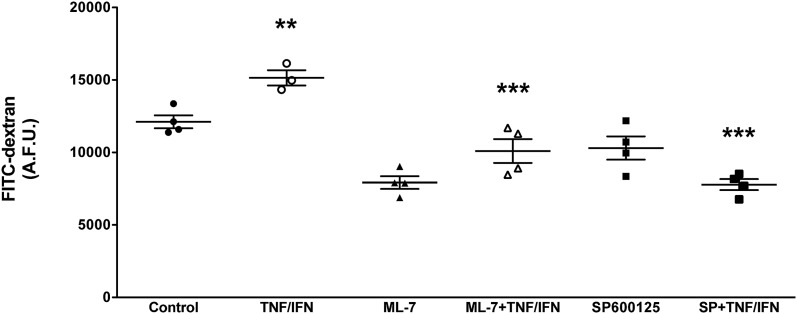
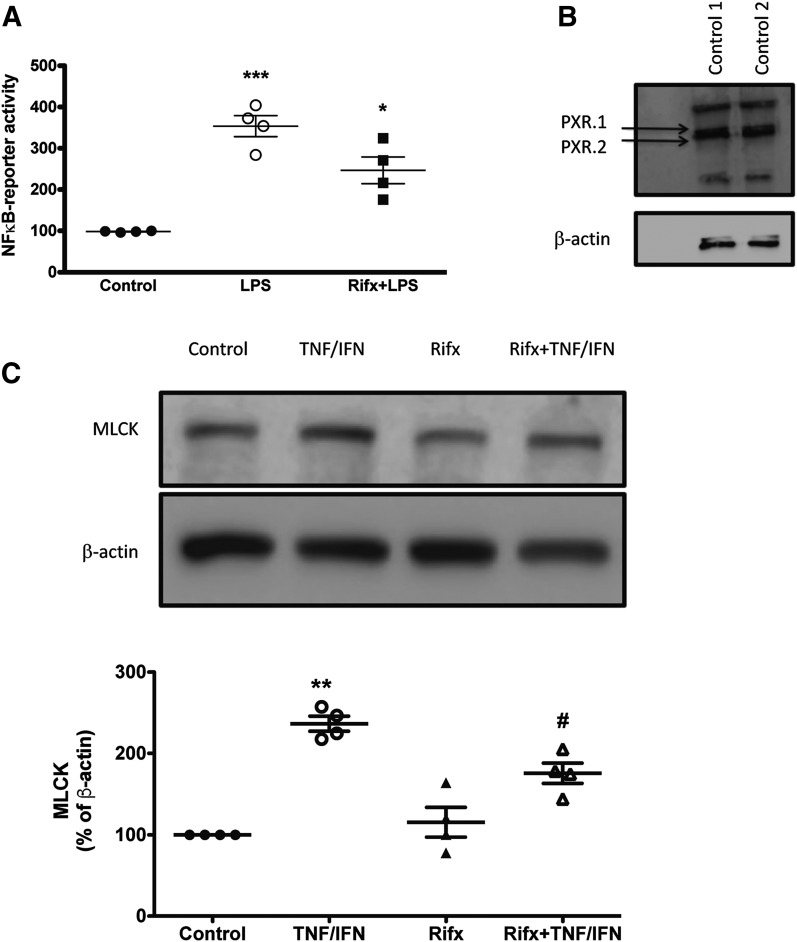
The disrupted localization of ZO-1 that is associated with intestinal epithelial barrier dysfunction has been linked to a variety of intracellular signaling pathways. Some have reported that TNFα-induced activation of NFκB and subsequent upregulation of MLCK triggers cytoskeletal activation, disruption of tight junctions, and enhanced permeability (Ma et al., 2005; Ye et al., 2006; Al-Sadi et al., 2016). Others have reported that ZO-1 relocalization can be driven by the activation of JNK1/2 signaling (Samak et al., 2014, 2015; Zhang et al., 2015). Interestingly, activation of the PXR has been reported to interact with both of these pathways (Zhou et al., 2006; Wang et al., 2010). Furthermore, selective deletion of the PXR in IECs leads to a defect in intestinal epithelial barrier function in mice (Venkatesh et al., 2014). Thus, we sought to determine whether activation of the PXR affords barrier protection by inhibiting these two intracellular signaling pathways. First, we confirmed that MLCK and JNK1/2 activity drives TNFα/IFNγ-induced barrier dysfunction in Caco-2 monolayers. Indeed, inhibition of MLCK (with ML-7) and JNK1/2 signaling (with SP600125) attenuated the barrier dysfunction triggered by TNFα/IFNγ exposure (Fig. 3). As reported previously (Ma et al., 2005; Ye et al., 2006), TNFα/IFNγ exposure increased the expression of MLCK in Caco-2 IECs (Fig. 4A). However, pretreatment of the monolayers with rifaximin attenuated this response (Fig. 4A).
Fig. 3.
Inhibition of MLCK and JNK1/2 attenuate TNFα/IFNγ-induced barrier dysfunction. Caco-2 monolayers grown for 14 days postconfluence were pretreated with ML-7 (to inhibit MLCK; 1 μM) or SP600125 (SP; to inhibit JNK1/2 signaling; 1 μM) for 1 hour prior to exposure to TNFα (10 ng/ml) and IFNγ (20 ng/ml) for 16 hours. **P < 0.01 compared with control (NoRx); ***P < 0.005 compared with TNF/IFN (n = 4). A.F.U., XXX.
Fig. 4.
Activation of the PXR attenuates TNFα/IFNγ-induced upregulation of MLCK and inhibits NFκB signaling. (A) Rifaximin (Rifx) attenuates LPS-induced NFκB activation. THP-1-Blue NFκB cells, which express an NFκB-driven SEAP reporter gene, were pretreated with rifaximin (10 μM) for 1 hour prior to exposure to LPS (100 ng/ml). SEAP activity was assessed in the culture supernatant after 16 hours of LPS exposure. ***P < 0.005 compared with control; *P < 0.05 compared with LPS group (n = 4). (B) THP-1-Blue NFκB cells express the PXR (PXR1.1 and PXR1.2 isoforms). (C) Rifaximin attenuates TNFα/IFNγ-induced upregulation of MLCK. Caco-2 monolayers grown for 14 days postconfluence were pretreated with selective rifaximin (10 μM) for 1 hour prior to exposure to TNFα (10 ng/ml) and IFNγ (20 ng/ml) for 16 hours. **P < 0.01 compared with control; #P < 0.05 compared with TNFα/IFNγ (n = 4).
It is well established that selective activation of the PXR inhibits NFκB signaling in a variety of cell types, including IECs (Mencarelli et al., 2010, 2011). Using a THP-1–based TLR4/NFκB-reporter system, we then sought to confirm that activation of the PXR, with rifaximin, could modulate NFκB signaling. In our THP-1–based reporter system, rifaximin inhibited LPS-induced NFκB signaling (Fig. 4A), an observation that is in agreement with previous reports characterizing the anti-inflammatory properties of rifaximin and other PXR agonists (Ma et al., 2007; Shah et al., 2007; Mencarelli et al., 2010, 2011).
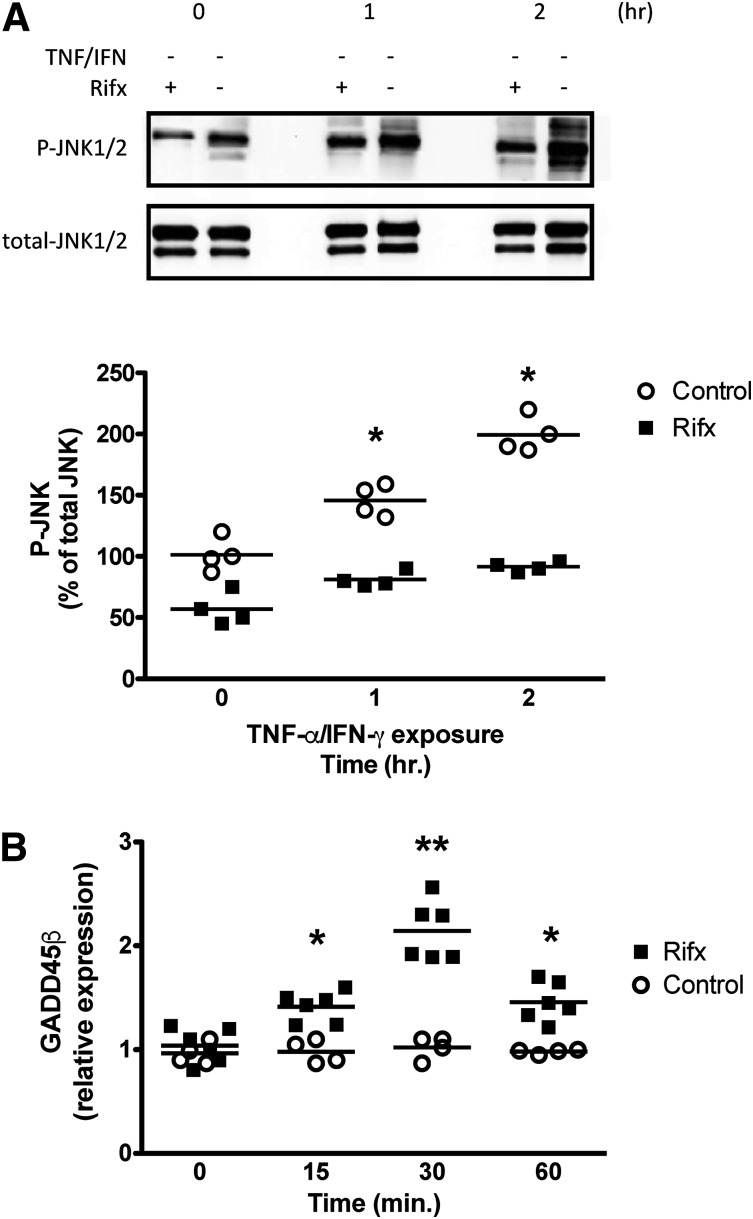
Whereas NFκB-driven MLCK expression contributed to the barrier disruption in our study, inhibition of JNK1/2 signaling also protected against TNFα/IFNγ exposure (Fig. 3). Activation of the PXR has been reported to increase the expression of growth arrest and DNA damage–inducible 45β (GADD45β) (Kodama and Negishi, 2011), a protein that can block JNK1/2 activation by inhibiting its upstream kinase MKK7 (De Smaele et al., 2001; Papa et al., 2004; Larsen et al., 2006). Thus, we assessed whether PXR activation could modulate TNFα/IFNγ-induced JNK1/2 activation in IEC monolayers. Selective activation of the PXR in IEC monolayers attenuated TNFα/IFNγ-induced JNK1/2 activation (Fig. 5A). This was associated with a significant increase in GADD45β transcript expression (Fig. 5B). Taken together, our in vitro data suggest that the selective activation of the PXR affords intestinal epithelial barrier protection by preserving tight-junction integrity by attenuating TNFα/IFNγ-induced upregulation of MLCK and inhibiting JNK1/2 signaling.
Fig. 5.
Selective activation of the PXR upregulates the expression of GADD45β and attenuates the activation of JNK1/2 in confluent Caco-2 IECs. (A) Confluent Caco-2 cells were treated with rifaximin (Rifx; 10 μM) or vehicle (DMSO), and the expression of GADD45β was assessed by quantitative real-time polymerase chain reaction. **P < 0.01 between rifaximin and vehicle at the indicated time point; *P < 0.05 between rifaximin and vehicle at the indicated time point (n = 4–6). (B) Rifaximin attenuates JNK1/2 activation. Caco-2 monolayers grown for 14 days postconfluence were pretreated with selective PXR agonist rifaximin (10 μM) for 1 hour prior to exposure to TNFα (10 ng/ml) and IFNγ (20 ng/ml). *P < 0.05 between rifaximin and control at the indicated time point (n = 4). P-JNK, phorphorylated JNK.
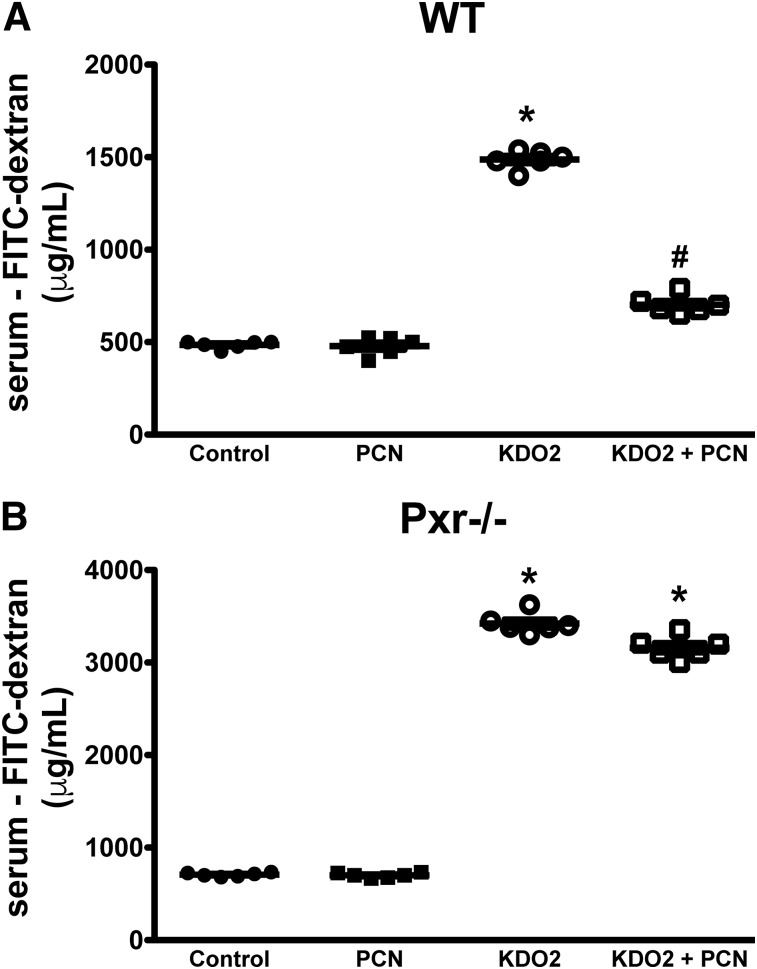
In the final set of experiments, we sought to confirm our in vitro findings using two distinct mouse models of barrier disruption. First, we assessed the role of the PXR in TLR4-induced small intestinal barrier disruption, using a model we have used previously (Venkatesh et al., 2014). Jejunal administration of KDO2, a selective TLR4 agonist, triggers the expression of mucosal Tnf-a and disrupts the small intestinal mucosal barrier (Venkatesh et al., 2014). In wild-type (WT) and Pxr−/− mice, administration of KDO2 triggered a significant increase in permeability, as evidenced by increased FITC-dextran detected in the serum (Fig. 6, A and B). Pretreating mice with PCN (25 mg/kg, i.p.), a rodent-specific PXR agonist, attenuated KDO2-induced barrier disruption in WT mice, but not Pxr−/− mice, highlighting the selectivity of this agonist in our system. These data indicate that activation of the PXR can afford barrier protection in a model system where barrier disruption is associated with increased TNFα expression (Venkatesh et al., 2014).
Fig. 6.
Activation of the PXR attenuates intestinal permeability induced by TLR4 activation in WT mice (A), but not Pxr−/− mice (B). Male WT and Pxr−/− mice were pretreated with the rodent-specific PXR agonist PCN (25 mg/kg, i.p.), then administered the selective TLR4 agonist KDO2 (200 μg per mouse). After 24 hours, intestinal permeability was assessed by FITC-dextran flux assay. *P < 0.05 compared with control and PCN-treated mice; #P < 0.05 compared with KDO2 group (n = 5–6 per group).
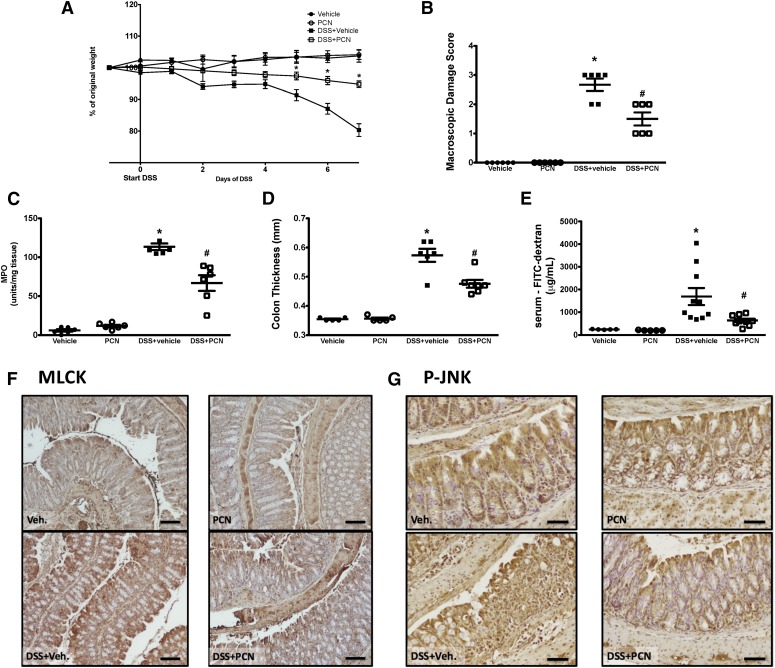
In the next set of experiments, we used a mouse model of experimental colitis to assess the role of the PXR in regulating barrier function. We reported previously that stimulation of the PXR, with PCN, reduced barrier dysfunction in the acute and recovery phase of experimental colitis (Terc et al., 2014). In the latter case, this was independent of tissue inflammation. Thus, we hypothesized that the barrier protection afforded by activation of the PXR could be due to its ability to dampen inflammation-associated expression of MLCK and activation of JNK1/2 in the intestinal mucosa. Pretreating mice with PCN (25 mg/kg, i.p., daily) significantly reduced the macroscopic damage, colonic thickening, and barrier dysfunction triggered during DSS-induced colitis [2.5% (w/v) in drinking water, 7 days; Fig. 7, A–C], a finding that supports our previously published work (Terc et al., 2014). The preservation of barrier function by selective activation of the mouse PXR was associated with reduced colonic mucosal expression of MLCK (Fig. 7D) and the levels of phosphorylated/active JNK1/2, as assessed by immunohistochemical staining (Fig. 7E).
Fig. 7.
Activation of the PXR reduces the clinical severity of colitis and attenuates intestinal damage and barrier dysfunction in mice exposed to DSS [2.5% (w/v) in the drinking water, 7 days], an effect that is associated with reduced mucosal expression of MLCK and activation of JNK1/2, as assessed by detecting phosphorylated JNK1/2. Treating male mice with PCN (25 mg/kg, i.p., daily) reduces colitis-associated body weight loss (A), macroscopic colonic tissue damage (B), colonic MPO levels (C), mucosal thickening, (D) and attenuates inflammation-associated barrier dysfunction, as assessed by FITC-dextran flux assay (E). (A)*P < 0.05 compared with DSS+vehicle group. (B–E) *P < 0.05 compared with naïve and PCN-treated mice; #P < 0.05 compared with DSS+vehicle group (n = 5–10). PXR activation reduces colonic mucosal MLCK expression (scale bar: 100 μm) (F) and JNK1/2 phosphorylation (P-JNK; scale bar: 50 μm) (G) induced by DSS exposure. Veh., vehicle.
Taken together, our in vitro and in vivo data suggest that PXR activation can preserve intestinal epithelial barrier function in the context of inflammation by directly modulating intracellular signaling within IECs, ultimately maintaining tight-junction integrity.
Discussion
In the current study, we sought to assess the mechanism(s) through which the PXR regulates the intestinal epithelium, protecting against intestinal inflammation and tissue damage during colitis. We, and others, have reported previously that the PXR plays an important role in maintaining intestinal mucosal homeostasis (Zhou et al., 2006; Terc et al., 2014; Venkatesh et al., 2014), and its activation dampens inflammation and mucosal damage in experimental colitis (Ma et al., 2007; Shah et al., 2007; Dou et al., 2012, 2013, 2014; Terc et al., 2014). In our previous report, we found that treating mice with PCN attenuated colonic mucosal neutrophil infiltration and afforded barrier protection (Terc et al., 2014). However, we were unable to delineate whether the protection observed was driven by a direct effect of PXR signaling within the intestinal epithelium, or was due to an overall reduction in the inflammatory burden in the intestinal mucosa. We reported recently that selective deletion of the PXR in the intestinal epithelium increases mucosal permeability, suggesting that this receptor regulates the biology of the intestinal epithelial barrier (Venkatesh et al., 2014). Compromised barrier function has been associated with IBD; however, it is still unclear whether it plays a direct role in driving/initiating the disease or is secondary to tissue inflammation. Increased intestinal permeability is associated with IBD relapse and recurrence (Hollander et al., 1986; Katz et al., 1989; Wyatt et al., 1993; D'Inca et al., 1999; Tibble et al., 2000; Teshima et al., 2012), and inflammatory mediators, such as TNFα and IFNγ, which are elevated in the tissues of IBD patients (Turner, 2009), can directly disrupt the intestinal epithelial barrier (Turner, 2009; Cunningham and Turner, 2012). Thus, in the current study, we began with an in vitro approach to test whether selective activation of the PXR could regulate signaling pathways that contribute to intestinal epithelial barrier dysfunction in conditions that model aspects of the mucosal inflammation found in IBD. We found that stimulation of the PXR preserved the barrier function of IEC monolayers exposed to TNFα/IFNγ. This effect was associated with the preservation of tight-junction integrity, attenuated TNFα/IFNγ-induced expression of MLCK, and inhibition of JNK1/2 activity. These data were supported by our in vivo findings, where selective activation of the PXR significantly reduced inflammation-induced intestinal barrier dysfunction evoked by TLR4 activation or DSS exposure, the latter associated with a reduction in MLCK expression and activation of mucosal JNK1/2.
Highly expressed in the liver and intestinal epithelium, the PXR has been previously characterized as a master regulator of xenobiotic metabolism and clearance (Watkins et al., 2002; Staudinger et al., 2006; Shukla et al., 2011; Dou et al., 2013; Pondugula and Mani, 2013). Acting as a sensor of exogenous chemicals, the PXR’s activation leads to the expression of a variety of target genes related to detoxification and efflux pathways. In addition to enhancing xenobiotic gene transcription, PXR activation has been reported to prevent NFκB-DNA interactions and inhibit the release of proinflammatory mediators (Zhou et al., 2006; Shah et al., 2007; Mencarelli et al., 2010, 2011). In agreement with these reports, we found that pretreating cells with rifaximin, a selective human PXR agonist, attenuated LPS-induced NFκB reporter activity. In our IEC monolayers, we observed the selective activation of the PXR with SR12813 and rifaximin protected against cytokine-induced barrier disruption. TNFα and IFNγ decrease transepithelial resistance, in part, by inducing NFκB-dependent expression of MLCK, activation of the cytoskeleton, ZO-1 redistribution, and disruption of cell-cell contacts (Ma et al., 2005; Ye et al., 2006). We found that the barrier protection afforded by PXR activation was associated with attenuated TNFα/IFNγ-induced MLCK expression and a preservation of tight-junction integrity. Interestingly, in their initial report, Ma et al. (2005) used curcumin to selectively inhibit NFκB, an approach that abrogated TNFα-induced MLCK expression and barrier disruption. Curcumin has been reported to activate the PXR, an effect thought to contribute to its anti-inflammatory activity in the gastrointestinal tract (Kluth et al., 2007; Nones et al., 2009). Taken together, these data suggest that the PXR can afford barrier protection, in part, by attenuating NFκB-dependent cytokine-induced expression of MLCK.
In addition to interacting with NFκB, the PXR has been reported to modulate the function of other signaling pathways. Previously, we reported that activation of the human PXR, with a variety of selective agonists, enhanced IEC migration and accelerated intestinal epithelial wound healing in a p38 mitogen-activated protein kinase–dependent fashion (Terc et al., 2014). Others have reported that PXR-dependent activation of p38 mitogen-activated protein kinase signaling is driven by increased expression of GADD45β, an early response gene that regulates a variety of intracellular signaling cascades (Kodama and Negishi, 2011). Relevant to our current study, Papa et al. (2007) reported GADD45β inhibits JNK1/2 activation by directly interacting with its upstream kinase MKK7, reducing its catalytic function. Others have reported that GADD45β contributes to anti-inflammatory signaling by inhibiting TNFα-induced JNK1/2 signaling (Papa et al., 2004; Bortoff et al., 2010). We found that activation of the PXR in IECs inhibited TNFα/IFNγ-induced JNK1/2 signaling, an effect that was associated increased expression of GADD45β.
To translate our in vitro findings, we sought to assess whether selective activation of the PXR in mice could protect the intestinal mucosal barrier in two different models of inflammation-driven barrier disruption. In the first set of experiments, we found that WT mice, but not Pxr−/− mice, pretreated with PCN (a selective rodent PXR agonist) were protected against KDO2-induced TLR4-driven intestinal barrier dysfunction, supporting our in vitro data and reinforcing PCN’s selectivity. In the second set of experiments, we sought to recapitulate key findings from our in vitro experiments. Specifically, we sought to determine whether PXR activation could afford barrier protection in the context of inflammation by regulating MLCK expression and JNK1/2 activity. DSS-exposed mice exhibited increased intestinal permeability, an effect that was attenuated when they were pretreated with PCN. Furthermore, in agreement with our in vitro findings, the barrier protection afforded by PXR activation was associated with reduced expression of mucosal MLCK and reduced JNK1/2 activity. Although the role of intestinal epithelial MLCK in regulating mucosal permeability has been characterized extensively (Cunningham and Turner, 2012), participation of JNK1/2 in this process is less clear. Some have reported that deletion of JNK1 or JNK2 (or both) increases the susceptibility of mice in experimental models of colitis (Chromik et al., 2007; Kersting et al., 2013b); however, others have reported that selective inhibition of JNK1/2 is protective, reducing IEC apoptosis and modulating mucosal inflammatory mediator expression (Assi et al., 2006; Mitsuyama et al., 2006; Kersting et al., 2013a). Interestingly, in Crohn’s disease patients, the expression of activated JNK is increased in the intestinal epithelium and mucosal immune cells (Bantel et al., 2002; Mitsuyama et al., 2006), and its selective inhibition reduces the expression of inflammatory mediators in colonic mucosal biopsies (Mitsuyama et al., 2006).
The findings that PXR stimulation protects the intestinal barrier during inflammation are in line with previous studies highlighting its role in regulating intestinal mucosal homeostasis. Deletion of the PXR leads to the development of mild spontaneous intestinal inflammation (Zhou et al., 2006) and decreased barrier function (Venkatesh et al., 2014). Furthermore, selective activation of the PXR reduces intestinal mucosal inflammation and tissue damage (Shah et al., 2007). Although the endogenous ligand(s) for the PXR in the gastrointestinal tract has yet to be completely elucidated, members of our team reported recently that the PXR could be activated by indole-propionic acid, a microbial metabolite, suggesting that this receptor may play a role in host-microbe interactions at the intestinal epithelium (Venkatesh et al., 2014).
Our study may provide insight into how PXR dysfunction may contribute to IBD susceptibility (Langmann et al., 2004; Dring et al., 2006; Martinez et al., 2007; Glas et al., 2011). Whereas others have characterized the anti-inflammatory effects of PXR signaling in the gut, our in vitro and in vivo data suggest that selective activation of the PXR affords protection of the intestinal epithelial barrier in the context of inflammation. In addition, our findings also provide insight into the potential non-antimicrobial mechanism(s) of action by which rifaximin elicits its clinical efficacy in the treatment of enteric infections and IBD, both of which are associated with disruption of the intestinal epithelial barrier. Rifaximin is a luminal antibiotic and selective human PXR agonist (Ma et al., 2007), which has shown promise in the treatment of IBD (Prantera et al., 2012). Future studies should be designed to assess the therapeutic efficacy of PXR agonists in a variety of preclinical models, assessing the anti-inflammatory and probarrier effects after the induction of chronic inflammation, to better model the clinical course of human IBD.
Although the pathogenesis of IBD has yet to be completely elucidated, barrier dysfunction and increased epithelial permeability are strongly associated with disease relapse and recurrence. Thus, our data suggest that targeting the PXR may prove effective in the treatment of IBD by targeting the intestinal epithelium and enhancing its barrier function in the context of inflammation.
Acknowledgments
The authors thank J. Staudinger (University of Kansas) for providing Pxr−/− (Nr1i2−/−) mice.
Abbreviations
- ATCC
American Type Culture Collection
- DSS
dextran sulfate sodium
- FBS
fetal bovine serum
- FITC-dextran
fluorescein isothiocyanate-dextran
- GADD45β
growth arrest and DNA damage–inducible 45β
- IBD
inflammatory bowel diseases
- IEC
intestinal epithelial cell
- IFNγ
interferon-γ
- JNK1/2
c-Jun N-terminal kinase 1/2
- KDO2
di[3-deoxy-d-manno-octulosonyl]-lipid A
- LPS
lipopolysaccharide
- ML-7
hexahydro-1-[(5-iodo-1-naphthalenyl)sulfonyl]-1H-1,4-diazepine hydrochloride
- MLCK
myosin light-chain kinase
- MPO
myeloperoxidase
- NFκB
nuclear factor κ-light-chain-enhancer of activated B cells
- PBS
phosphate-buffered saline
- PCN
pregnenolone 16α-carbonitrile
- PXR
pregnane X receptor
- SEAP
secreted embryonic alkaline phosphatase
- SP600125
anthra[1-9-cd]pyrazol-6(2H)-one
- SR12813
[[3,5-Bis(1,1-dimethylethyl)-4-hydroxyphenyl]ethenylidene]bis-phosphonic acid tetraethyl ester
- TLR4
toll-like receptor 4
- TNFα
tumor necrosis factor-α
- UC
ulcerative colitis
- WT
wild type
- XREM
XXX
- ZO-1
zonula occludens-1
Authorship Contributions
Participated in research design: Lau, Chang, Mani, Hirota.
Conducted experiments: Garg, Zhao, Erickson, Mukherjee, Lau, Alston, Hirota.
Contributed new reagents or analytic tools: Chang, Mani, Hirota.
Performed data analysis: Garg, Zhao, Erickson, Mukherjee, Lau, Alston, Mani, Hirota.
Wrote or contributed to the writing of the manuscript: Chang, Mani, Hirota.
Footnotes
S.A.H.’s salary is covered by the Canadian Institutes of Health Research’s Canada Research Chair (CRC) program (Tier II CRC in Host-Microbe Interactions and Chronic Disease); S.A.H.’s laboratory is supported by an infrastructure grant provided by the Canadian Foundation for Innovation John R. Evans Leaders Fund, operating funds from Crohn’s and Colitis Canada (S.A.H. and T.K.H.C. coinvestigators), the Dr. Lloyd Sutherland Investigatorship in IBD/GI Research. Sridhar Mani’s laboratory is supported by the National Institutes of Health [Grants CA127231 and CA161879] and the Broad Medical Research Program–Crohn’s and Colitis Foundation Investigator Award [Proposal No. 262520].
References
- Al-Sadi R, Guo S, Ye D, Rawat M, Ma TY. (2016) TNF-α Modulation of Intestinal Tight Junction Permeability Is Mediated by NIK/IKK-α Axis Activation of the Canonical NF-κB Pathway. Am J Pathol 186:1151–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assi K, Pillai R, Gómez-Muñoz A, Owen D, Salh B. (2006) The specific JNK inhibitor SP600125 targets tumour necrosis factor-alpha production and epithelial cell apoptosis in acute murine colitis. Immunology 118:112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantel H, Schmitz ML, Raible A, Gregor M, Schulze-Osthoff K. (2002) Critical role of NF-kappaB and stress-activated protein kinases in steroid unresponsiveness. FASEB J 16:1832–1834. [DOI] [PubMed] [Google Scholar]
- Bortoff KD, Keeton AB, Franklin JL, Messina JL. (2010) Anti-Inflammatory Action of Insulin via Induction of Gadd45-β Transcription by the mTOR Signaling Pathway. Hepat Med 2001:79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruewer M, Luegering A, Kucharzik T, Parkos CA, Madara JL, Hopkins AM, Nusrat A. (2003) Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol 171:6164–6172. [DOI] [PubMed] [Google Scholar]
- Cheng J, Shah YM, Ma X, Pang X, Tanaka T, Kodama T, Krausz KW, Gonzalez FJ. (2010) Therapeutic role of rifaximin in inflammatory bowel disease: clinical implication of human pregnane X receptor activation. J Pharmacol Exp Ther 335:32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chromik AM, Muller AM, Korner J, Belyaev O, Holland-Letz T, Schmitz F, Herdegen T, Uhl W, Mittelkotter U. (2007) Genetic deletion of JNK1 and JNK2 aggravates the DSS-induced colitis in mice. J Invest Surg 20:23–33. [DOI] [PubMed] [Google Scholar]
- Clayburgh DR, Rosen S, Witkowski ED, Wang F, Blair S, Dudek S, Garcia JG, Alverdy JC, Turner JR. (2004) A differentiation-dependent splice variant of myosin light chain kinase, MLCK1, regulates epithelial tight junction permeability. J Biol Chem 279:55506–55513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham KE, Turner JR. (2012) Myosin light chain kinase: pulling the strings of epithelial tight junction function. Ann N Y Acad Sci 1258:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smaele E, Zazzeroni F, Papa S, Nguyen DU, Jin R, Jones J, Cong R, Franzoso G. (2001) Induction of gadd45beta by NF-kappaB downregulates pro-apoptotic JNK signalling. Nature 414:308–313. [DOI] [PubMed] [Google Scholar]
- Diaz-Granados N, Howe K, Lu J, McKay DM. (2000) Dextran sulfate sodium-induced colonic histopathology, but not altered epithelial ion transport, is reduced by inhibition of phosphodiesterase activity. Am J Pathol 156:2169–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Incà R, Di Leo V, Corrao G, Martines D, D’Odorico A, Mestriner C, Venturi C, Longo G, Sturniolo GC. (1999) Intestinal permeability test as a predictor of clinical course in Crohn’s disease. Am J Gastroenterol 94:2956–2960. [DOI] [PubMed] [Google Scholar]
- Dou W, Mukherjee S, Li H, Venkatesh M, Wang H, Kortagere S, Peleg A, Chilimuri SS, Wang ZT, Feng Y, et al. (2012) Alleviation of gut inflammation by Cdx2/Pxr pathway in a mouse model of chemical colitis. PLoS One 7:e36075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou W, Zhang J, Li H, Kortagere S, Sun K, Ding L, Ren G, Wang Z, Mani S. (2014) Plant flavonol isorhamnetin attenuates chemically induced inflammatory bowel disease via a PXR-dependent pathway. J Nutr Biochem 25:923–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou W, Zhang J, Zhang E, Sun A, Ding L, Chou G, Wang Z, Mani S. (2013) Chrysin ameliorates chemically induced colitis in the mouse through modulation of a PXR/NF-κB signaling pathway. J Pharmacol Exp Ther 345:473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dring MM, Goulding CA, Trimble VI, Keegan D, Ryan AW, Brophy KM, Smyth CM, Keeling PW, O’Donoghue D, O’Sullivan M, et al. (2006) The pregnane X receptor locus is associated with susceptibility to inflammatory bowel disease. Gastroenterology 130:341–348, quiz 592. [DOI] [PubMed] [Google Scholar]
- Ekins S, Erickson JA. (2002) A pharmacophore for human pregnane X receptor ligands. Drug Metab Dispos 30:96–99. [DOI] [PubMed] [Google Scholar]
- Glas J, Seiderer J, Fischer D, Tengler B, Pfennig S, Wetzke M, Beigel F, Olszak T, Weidinger M, Göke B, et al. (2011) Pregnane X receptor (PXR/NR1I2) gene haplotypes modulate susceptibility to inflammatory bowel disease. Inflamm Bowel Dis 17:1917–1924. [DOI] [PubMed] [Google Scholar]
- Hansen A, Alston L, Tulk SE, Schenck LP, Grassie ME, Alhassan BF, Veermalla AT, Al-Bashir S, Gendron FP, Altier C, et al. (2013) The P2Y6 receptor mediates Clostridium difficile toxin-induced CXCL8/IL-8 production and intestinal epithelial barrier dysfunction. PLoS One 8:e81491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota SA, Ng J, Lueng A, Khajah M, Parhar K, Li Y, Lam V, Potentier MS, Ng K, Bawa M, et al. (2011) NLRP3 inflammasome plays a key role in the regulation of intestinal homeostasis. Inflamm Bowel Dis 17:1359–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander D. (1988) Crohn’s disease--a permeability disorder of the tight junction? Gut 29:1621–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander D, Vadheim CM, Brettholz E, Petersen GM, Delahunty T, Rotter JI. (1986) Increased intestinal permeability in patients with Crohn’s disease and their relatives. A possible etiologic factor. Ann Intern Med 105:883–885. [DOI] [PubMed] [Google Scholar]
- Iacucci M, Ghosh S. (2011) Looking beyond symptom relief: evolution of mucosal healing in inflammatory bowel disease. Therap Adv Gastroenterol 4:129–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs KL, Sandler RS, Abreu M, Picco MF, Hanauer SB, Bickston SJ, Present D, Farraye FA, Wolf D, Sandborn WJ, Crohn’s and Colitis Foundation of America Clinical Alliance (2007) Rifaximin for the treatment of active pouchitis: a randomized, double-blind, placebo-controlled pilot study. Inflamm Bowel Dis 13:1250–1255. [DOI] [PubMed] [Google Scholar]
- Jones SA, Moore LB, Shenk JL, Wisely GB, Hamilton GA, McKee DD, Tomkinson NC, LeCluyse EL, Lambert MH, Willson TM, et al. (2000) The pregnane X receptor: a promiscuous xenobiotic receptor that has diverged during evolution. Mol Endocrinol 14:27–39. [DOI] [PubMed] [Google Scholar]
- Katz KD, Hollander D, Vadheim CM, McElree C, Delahunty T, Dadufalza VD, Krugliak P, Rotter JI. (1989) Intestinal permeability in patients with Crohn’s disease and their healthy relatives. Gastroenterology 97:927–931. [DOI] [PubMed] [Google Scholar]
- Kersting S, Behrendt V, Kersting J, Reinecke K, Hilgert C, Stricker I, Herdegen T, Janot MS, Uhl W, Chromik AM. (2013a) The impact of JNK inhibitor D-JNKI-1 in a murine model of chronic colitis induced by dextran sulfate sodium. J Inflamm Res 6:71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersting S, Reinecke K, Hilgert C, Janot MS, Haarmann E, Albrecht M, Müller AM, Herdegen T, Mittelkötter U, Uhl W, et al. (2013b) Knockout of the c-Jun N-terminal Kinase 2 aggravates the development of mild chronic dextran sulfate sodium colitis independently of expression of intestinal cytokines TNFα, TGFB1, and IL-6. J Inflamm Res 6:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluth D, Banning A, Paur I, Blomhoff R, Brigelius-Flohé R. (2007) Modulation of pregnane X receptor- and electrophile responsive element-mediated gene expression by dietary polyphenolic compounds. Free Radic Biol Med 42:315–325. [DOI] [PubMed] [Google Scholar]
- Kodama S, Negishi M. (2011) Pregnane X receptor PXR activates the GADD45beta gene, eliciting the p38 MAPK signal and cell migration. J Biol Chem 286:3570–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmann T, Moehle C, Mauerer R, Scharl M, Liebisch G, Zahn A, Stremmel W, Schmitz G. (2004) Loss of detoxification in inflammatory bowel disease: dysregulation of pregnane X receptor target genes. Gastroenterology 127:26–40. [DOI] [PubMed] [Google Scholar]
- Larsen CM, Døssing MG, Papa S, Franzoso G, Billestrup N, Mandrup-Poulsen T. (2006) Growth arrest- and DNA-damage-inducible 45beta gene inhibits c-Jun N-terminal kinase and extracellular signal-regulated kinase and decreases IL-1beta-induced apoptosis in insulin-producing INS-1E cells. Diabetologia 49:980–989. [DOI] [PubMed] [Google Scholar]
- Lau AJ, Chang TK. (2015) 3-Hydroxyflavone and structural analogues differentially activate pregnane X receptor: Implication for inflammatory bowel disease. Pharmacol Res 100:64–72. [DOI] [PubMed] [Google Scholar]
- Lau AJ, Yang G, Yap CW, Chang TK. (2012) Selective agonism of human pregnane X receptor by individual ginkgolides. Drug Metab Dispos 40:1113–1121. [DOI] [PubMed] [Google Scholar]
- Lemaire G, Benod C, Nahoum V, Pillon A, Boussioux AM, Guichou JF, Subra G, Pascussi JM, Bourguet W, Chavanieu A, et al. (2007) Discovery of a highly active ligand of human pregnane x receptor: a case study from pharmacophore modeling and virtual screening to “in vivo” biological activity. Mol Pharmacol 72:572–581. [DOI] [PubMed] [Google Scholar]
- Ma TY, Boivin MA, Ye D, Pedram A, Said HM. (2005) Mechanism of TNF-alpha modulation of Caco-2 intestinal epithelial tight junction barrier: role of myosin light-chain kinase protein expression. Am J Physiol Gastrointest Liver Physiol 288:G422–G430. [DOI] [PubMed] [Google Scholar]
- Ma X, Shah YM, Guo GL, Wang T, Krausz KW, Idle JR, Gonzalez FJ. (2007) Rifaximin is a gut-specific human pregnane X receptor activator. J Pharmacol Exp Ther 322:391–398. [DOI] [PubMed] [Google Scholar]
- Martínez A, Márquez A, Mendoza J, Taxonera C, Fernández-Arquero M, Díaz-Rubio M, de la Concha EG, Urcelay E. (2007) Role of the PXR gene locus in inflammatory bowel diseases. Inflamm Bowel Dis 13:1484–1487. [DOI] [PubMed] [Google Scholar]
- Mencarelli A, Migliorati M, Barbanti M, Cipriani S, Palladino G, Distrutti E, Renga B, Fiorucci S. (2010) Pregnane-X-receptor mediates the anti-inflammatory activities of rifaximin on detoxification pathways in intestinal epithelial cells. Biochem Pharmacol 80:1700–1707. [DOI] [PubMed] [Google Scholar]
- Mencarelli A, Renga B, Palladino G, Claudio D, Ricci P, Distrutti E, Barbanti M, Baldelli F, Fiorucci S. (2011) Inhibition of NF-κB by a PXR-dependent pathway mediates counter-regulatory activities of rifaximin on innate immunity in intestinal epithelial cells. Eur J Pharmacol 668:317–324. [DOI] [PubMed] [Google Scholar]
- Mitsuyama K, Suzuki A, Tomiyasu N, Tsuruta O, Kitazaki S, Takeda T, Satoh Y, Bennett BL, Toyonaga A, Sata M. (2006) Pro-inflammatory signaling by Jun-N-terminal kinase in inflammatory bowel disease. Int J Mol Med 17:449–455. [PubMed] [Google Scholar]
- Nones K, Dommels YE, Martell S, Butts C, McNabb WC, Park ZA, Zhu S, Hedderley D, Barnett MP, Roy NC. (2009) The effects of dietary curcumin and rutin on colonic inflammation and gene expression in multidrug resistance gene-deficient (mdr1a-/-) mice, a model of inflammatory bowel diseases. Br J Nutr 101:169–181. [DOI] [PubMed] [Google Scholar]
- Papa S, Monti SM, Vitale RM, Bubici C, Jayawardena S, Alvarez K, De Smaele E, Dathan N, Pedone C, Ruvo M, et al. (2007) Insights into the structural basis of the GADD45beta-mediated inactivation of the JNK kinase, MKK7/JNKK2. J Biol Chem 282:19029–19041. [DOI] [PubMed] [Google Scholar]
- Papa S, Zazzeroni F, Bubici C, Jayawardena S, Alvarez K, Matsuda S, Nguyen DU, Pham CG, Nelsbach AH, Melis T, et al. (2004) Gadd45 beta mediates the NF-kappa B suppression of JNK signalling by targeting MKK7/JNKK2. Nat Cell Biol 6:146–153. [DOI] [PubMed] [Google Scholar]
- Pondugula SR, Mani S. (2013) Pregnane xenobiotic receptor in cancer pathogenesis and therapeutic response. Cancer Lett 328:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prantera C, Lochs H, Campieri M, Scribano ML, Sturniolo GC, Castiglione F, Cottone M. (2006) Antibiotic treatment of Crohn’s disease: results of a multicentre, double blind, randomized, placebo-controlled trial with rifaximin. Aliment Pharmacol Ther 23:1117–1125. [DOI] [PubMed] [Google Scholar]
- Prantera C, Lochs H, Grimaldi M, Danese S, Scribano ML, Gionchetti P; Retic Study Group (2012) Rifaximin-extended intestinal release induces remission in patients with moderately active Crohn's disease. Gastroenterology 142:473–481. [DOI] [PubMed] [Google Scholar]
- Samak G, Chaudhry KK, Gangwar R, Narayanan D, Jaggar JH, Rao R. (2015) Calcium/Ask1/MKK7/JNK2/c-Src signalling cascade mediates disruption of intestinal epithelial tight junctions by dextran sulfate sodium. Biochem J 465:503–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samak G, Gangwar R, Crosby LM, Desai LP, Wilhelm K, Waters CM, Rao R. (2014) Cyclic stretch disrupts apical junctional complexes in Caco-2 cell monolayers by a JNK-2-, c-Src-, and MLCK-dependent mechanism. Am J Physiol Gastrointest Liver Physiol 306:G947–G958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenck LP, Hirota SA, Hirota CL, Boasquevisque P, Tulk SE, Li Y, Wadhwani A, Doktorchik CT, Macnaughton WK, Beck PL, MacDonald JA. (2013) Attenuation of Clostridium difficile toxin-induced damage to epithelial barrier by ecto-5′-nucleotidase (CD73) and adenosine receptor signaling. Neurogastroenterol Motil 25:e441–e453. [DOI] [PubMed] [Google Scholar]
- Shah YM, Ma X, Morimura K, Kim I, Gonzalez FJ. (2007) Pregnane X receptor activation ameliorates DSS-induced inflammatory bowel disease via inhibition of NF-kappaB target gene expression. Am J Physiol Gastrointest Liver Physiol 292:G1114–G1122. [DOI] [PubMed] [Google Scholar]
- Sharma D, Lau AJ, Sherman MA, Chang TK. (2013) Agonism of human pregnane X receptor by rilpivirine and etravirine: comparison with first generation non-nucleoside reverse transcriptase inhibitors. Biochem Pharmacol 85:1700–1711. [DOI] [PubMed] [Google Scholar]
- Shukla SJ, Sakamuru S, Huang R, Moeller TA, Shinn P, Vanleer D, Auld DS, Austin CP, Xia M. (2011) Identification of clinically used drugs that activate pregnane X receptors. Drug Metab Dispos 39:151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudinger JL, Ding X, Lichti K. (2006) Pregnane X receptor and natural products: beyond drug-drug interactions. Expert Opin Drug Metab Toxicol 2:847–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terc J, Hansen A, Alston L, Hirota SA. (2014) Pregnane X receptor agonists enhance intestinal epithelial wound healing and repair of the intestinal barrier following the induction of experimental colitis. Eur J Pharm Sci 55:12–19. [DOI] [PubMed] [Google Scholar]
- Teshima CW, Dieleman LA, Meddings JB. (2012) Abnormal intestinal permeability in Crohn’s disease pathogenesis. Ann N Y Acad Sci 1258:159–165. [DOI] [PubMed] [Google Scholar]
- Tibble JA, Sigthorsson G, Bridger S, Fagerhol MK, Bjarnason I. (2000) Surrogate markers of intestinal inflammation are predictive of relapse in patients with inflammatory bowel disease. Gastroenterology 119:15–22. [DOI] [PubMed] [Google Scholar]
- Turner JR. (2009) Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 9:799–809. [DOI] [PubMed] [Google Scholar]
- Venkatesh M, Mukherjee S, Wang H, Li H, Sun K, Benechet AP, Qiu Z, Maher L, Redinbo MR, Phillips RS, et al. (2014) Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4. Immunity 41:296–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Graham WV, Wang Y, Witkowski ED, Schwarz BT, Turner JR. (2005) Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol 166:409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Schwarz BT, Graham WV, Wang Y, Su L, Clayburgh DR, Abraham C, Turner JR. (2006) IFN-gamma-induced TNFR2 expression is required for TNF-dependent intestinal epithelial barrier dysfunction. Gastroenterology 131:1153–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Damjanov I, Wan YJ. (2010) The protective role of pregnane X receptor in lipopolysaccharide/D-galactosamine-induced acute liver injury. Lab Invest 90:257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins RE, Noble SM, Redinbo MR. (2002) Structural insights into the promiscuity and function of the human pregnane X receptor. Curr Opin Drug Discov Devel 5:150–158. [PubMed] [Google Scholar]
- Watkins RE, Wisely GB, Moore LB, Collins JL, Lambert MH, Williams SP, Willson TM, Kliewer SA, Redinbo MR. (2001) The human nuclear xenobiotic receptor PXR: structural determinants of directed promiscuity. Science 292:2329–2333. [DOI] [PubMed] [Google Scholar]
- Wyatt J, Vogelsang H, Hübl W, Waldhöer T, Lochs H. (1993) Intestinal permeability and the prediction of relapse in Crohn’s disease. Lancet 341:1437–1439. [DOI] [PubMed] [Google Scholar]
- Xavier RJ, Podolsky DK. (2007) Unravelling the pathogenesis of inflammatory bowel disease. Nature 448:427–434. [DOI] [PubMed] [Google Scholar]
- Ye D, Ma I, Ma TY. (2006) Molecular mechanism of tumor necrosis factor-alpha modulation of intestinal epithelial tight junction barrier. Am J Physiol Gastrointest Liver Physiol 290:G496–G504. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wu S, Ma J, Xia Y, Ai X, Sun J. (2015) Bacterial protein AvrA stabilizes intestinal epithelial tight junctions via blockage of the C-Jun N-terminal kinase pathway. Tissue Barriers 3:e972849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Tabb MM, Nelson EL, Grün F, Verma S, Sadatrafiei A, Lin M, Mallick S, Forman BM, Thummel KE, et al. (2006) Mutual repression between steroid and xenobiotic receptor and NF-kappaB signaling pathways links xenobiotic metabolism and inflammation. J Clin Invest 116:2280–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolotarevsky Y, Hecht G, Koutsouris A, Gonzalez DE, Quan C, Tom J, Mrsny RJ, Turner JR. (2002) A membrane-permeant peptide that inhibits MLC kinase restores barrier function in in vitro models of intestinal disease. Gastroenterology 123:163–172. [DOI] [PubMed] [Google Scholar]