Abstract
Following administration at subanesthetic doses, (R,S)-ketamine (ketamine) induces rapid and robust relief from symptoms of depression in treatment-refractory depressed patients. Previous studies suggest that ketamine’s antidepressant properties involve enhancement of dopamine (DA) neurotransmission. Ketamine is rapidly metabolized to (2S,6S)- and (2R,6R)-hydroxynorketamine (HNK), which have antidepressant actions independent of N-methyl-d-aspartate glutamate receptor inhibition. These antidepressant actions of (2S,6S;2R,6R)-HNK, or other metabolites, as well as ketamine’s side effects, including abuse potential, may be related to direct effects on components of the dopaminergic (DAergic) system. Here, brain and blood distribution/clearance and pharmacodynamic analyses at DA receptors (D1–D5) and the DA, norepinephrine, and serotonin transporters were assessed for ketamine and its major metabolites (norketamine, dehydronorketamine, and HNKs). Additionally, we measured electrically evoked mesolimbic DA release and decay using fast-scan cyclic voltammetry following acute administration of subanesthetic doses of ketamine (2, 10, and 50 mg/kg, i.p.). Following ketamine injection, ketamine, norketamine, and multiple hydroxynorketamines were detected in the plasma and brain of mice. Dehydronorketamine was detectable in plasma, but concentrations were below detectable limits in the brain. Ketamine did not alter the magnitude or kinetics of evoked DA release in the nucleus accumbens in anesthetized mice. Neither ketamine’s enantiomers nor its metabolites had affinity for DA receptors or the DA, noradrenaline, and serotonin transporters (up to 10 μM). These results suggest that neither the side effects nor antidepressant actions of ketamine or ketamine metabolites are associated with direct effects on mesolimbic DAergic neurotransmission. Previously observed in vivo changes in DAergic neurotransmission following ketamine administration are likely indirect.
Introduction
A single subanesthetic dose of (R,S)-ketamine (ketamine) produces rapid and sustained antidepressant effects in humans (Berman et al., 2000; Zarate et al., 2006; 2012; aan het Rot et al., 2010; Diazgranados et al., 2010; Murrough et al., 2013). Although the discovery of ketamine’s antidepressant efficacy was a major breakthrough, ketamine’s clinical use is limited due to its serious side effects, which include dissociation and abuse potential (Krystal et al., 1994; Morgan and Curran, 2012).
Evidence suggests that ketamine’s antidepressant actions might involve its actions on the dopaminergic (DAergic) system. Administration of ketamine reversed depression-related deficits in dopamine (DA)-dependent synaptic plasticity, which was associated with its ability to reverse helpless behavior in rats (Belujon and Grace, 2014). In addition, haloperidol, presumably acting as a D2 receptor antagonist, blocked the antidepressant effects of ketamine in the mouse forced-swim test, whereas activation of D2/D3 receptors enhanced its antidepressant effects (Li et al., 2015). These findings, along with the well established role of the DAergic system in the pathophysiology and treatment of depression (Willner et al., 2005; Papakostas, 2006; Dunlop and Nemeroff, 2007; Grace, 2016), suggest that ketamine might act via the regulation of the DAergic neurotransmission to exert its antidepressant effects. In fact, several agents that directly act on the DAergic system, including pramipexole (a D2/D3 receptor agonist), bupropion (DA reuptake inhibitor), and monoamine oxidase inhibitors, have shown efficacy in the treatment of depression (Nieuwstraten and Dolovich, 2001; Zarate et al., 2004; Shulman et al., 2013). Since we have recently demonstrated that ketamine’s antidepressant effects in mice are the result of the metabolite (2S,6S;2R,6R)-hydroxynorketamine (HNK) (Zanos et al., 2016), we hypothesize that direct effects of ketamine and/or its metabolites at DA receptors and monoamine transporters might exist and contribute to these antidepressant properties or side effects.
The possible mechanistic link between ketamine, the DA system, and behavioral changes is further supported by previous studies in rodents. Both haloperidol and destruction of catecholaminergic terminals with 6-hydroxydopamine attenuated ketamine-induced hyperlocomotion in mice (Irifune et al., 1991). Additionally, ketamine reversed haloperidol-induced catalepsy in rats (Lannes et al., 1991) and enhanced D2 agonist quinpirole-induced hyperlocomotion (Witkin et al., 2016). Haloperidol and the D2 antagonist raclopride reversed the disruptive effect of ketamine on spatial delayed alternation performance (Verma and Moghaddam, 1996). Furthermore, pretreatment with a D1/D5 receptor antagonist prevented ketamine-induced hippocampal synaptic depression and its associated spatial memory deficits in freely moving rats (Duan et al., 2013). Administration of ketamine also enhanced the interoceptive stimulus properties of methamphetamine in a rat drug discrimination paradigm (Wooters et al., 2011). In situ receptor binding studies indicated that ketamine has a strong affinity for the rat D2 receptor (Kapur and Seeman, 2002). However, positron emission tomography has not shown any effects of ketamine administration on these receptors in humans (Aalto et al., 2002).
Ketamine administration was recently shown to increase the number of spontaneously active DA neurons in the ventral tegmental area (VTA) (Belujon and Grace, 2014; Witkin et al., 2016), and to increase the firing rate and burst firing of these cells (Belujon and Grace, 2014). Similarly, in humans, ketamine enhances amphetamine-induced augmentation of striatal DA release (Kegeles et al., 2000). However, conflicting data exist as well. Ketamine has been reported to increase (Irifune et al., 1991; Verma and Moghaddam, 1996; Witkin et al., 2016), to have no effect (Lannes et al., 1991; Micheletti et al., 1992), or to decrease (Rao et al., 1989) striatal DA turnover, or extracellular DA dialysate levels. Stereoselective effects of ketamine on DA release in rat striatal slices have been reported (Hancock and Stamford, 1999; Tso et al., 2004). Thus, although overall changes in extracellular DA concentrations have been assessed previously, there is no consensus effect, and the low temporal resolution of microdialysis does not permit a determination of the relative contributions of DA release by axon terminals or the dynamics of DA reuptake.
Here, we used fast-scan cyclic voltammetry (FSCV) to assess the effects of ketamine treatment on the magnitude and temporal dynamics of DA release, and the reuptake of extracellular DA, in the nucleus accumbens (NAc) core in vivo. We also performed an in vitro pharmacological affinity screening of (S)- and (R)-enantiomers of ketamine and its principal metabolites, (R)- and (S)-norketamine, (2S,6S)-HNK, (2R,6R)-HNK, (2S,6R)-HNK, (2R,6S)-HNK, and (R)- and (S)-dehydronorketamines (DHNKs) on DA D1–D5 receptors and monoamine transporters. Finally, we functionally examined possible agonist and antagonist actions of the stereoisomers of ketamine and metabolites on DA receptors and DA transporters (DATs), norepinephrine transporters (NETs) and serotonin transporters (SERTs).
Materials and Methods
Animals
Male C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME), ages 11–12 weeks old at the time of the experiments, were housed five per cage in an animal room at a constant temperature (22 ± 1°C) and a 12-hour light/dark cycle (lights on/off at 0700/1900), with food and water provided ad libitum. Experiments were performed in the light phase of the cycle. All experimental procedures were approved by the University of Maryland, Baltimore, Animal Care and Use Committee, and were conducted in full accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Drugs/Compounds
(R,S)-ketamine hydrochloride (ketamine; Sigma-Aldrich, St. Louis, MO) was dissolved in 0.9% saline and injected i.p. with an injection volume of 7.5 ml/kg at doses of 2, 10, or 50 mg/kg. Quinpirole hydrochloride (Sigma-Aldrich) was dissolved in 7.5 ml/kg saline and injected i.p. at a dose of 0.5 mg/kg. For the binding and functional studies, (S)-ketamine, (R)-ketamine, (S)-norketamine, (R)-norketamine, (S)-DHNK, (R)-DHNK, (2S,6S)-HNK, (2R,6R)-HNK, (2R,6S)-HNK, and (2S,6R)-HNK were synthesized as previously described (Moaddel et al., 2010; Zanos et al., 2016).
Tissue Distribution and Clearance Measurements of Ketamine and Its Metabolites
C57BL/6J male mice received a single i.p. injection of ketamine (10 mg/kg). Mice were exposed to 3% isoflurane for 30 seconds and decapitated 10, 30, 60, 240, or 480 minutes following ketamine injection. Trunk blood was collected in EDTA-containing tubes and centrifuged at 8000 rpm for 6 minutes at 4°C. Plasma was collected and stored at −80°C until analysis. Whole brains were simultaneously collected, rinsed with phosphate-buffered saline, immediately frozen on dry ice, and stored at −80°C until analysis.
The concentrations of ketamine and its metabolites in plasma and brain tissue were determined by achiral liquid chromatography–tandem mass spectrometry using a previously described protocol with slight modifications (Paul et al., 2014; Moaddel et al., 2015). For plasma samples, the calibration standards for (R,S)-ketamine, (R,S)-norketamine, (2R,6R;2S,6S)-HNK, and (R,S)-DHNK ranged from 10,000 to 19.53 ng/ml. The quantification of (R,S)-ketamine, (R,S)-norketamine, (R,S)-DHNK, and the HNK stereoisomers was accomplished by calculating area ratios using D4-ketamine (10 μl of 10 μg/ml solution) as the internal standard. Whole brains were suspended in 990 μl of water:methanol (3:2, v/v), D4-ketamine (10 μl of 10 μg/ml) was added, and the resulting mixture was homogenized on ice with a polytron homogenizer and centrifuged at 21,000 × g for 30 minutes. The supernatant was collected and processed using 1-ml Oasis HLB solid-phase extraction cartridges (Waters Corp., Waltham, MA). The cartridges were preconditioned with 1 ml of methanol, followed by 1 ml of water and then 1 ml ammonium acetate (10 mM, pH 9.5). The supernatants were added to the cartridges, followed by 1 ml of water, and the compounds were eluted with 1 ml of methanol. The eluent was transferred to an autosampler vial for analysis. Quality control standards were prepared at 78.125, 625, and 2,500 ng/ml.
Fast-Scan Cyclic Voltammetry
Electrodes for measuring extracellular DA concentration were constructed by inserting a carbon fiber (7-µm diameter; Goodfellow, Huntingdon, UK) into a glass capillary tube (1.2-mm outer diameter; A-M Systems, Sequim, WA), pulled with a micropipette puller (Narishige, Tokyo, Japan). Carbon fibers were then cut at approximately 100 µM past the glass tip (Heien et al., 2004). Mice were anesthetized with urethane (1.5 g/kg, i.p.), and their heads were positioned in a stereotaxic frame (David Kopf Instruments, Tujunga, CA). Body temperature was continuously regulated with a rectal thermoregulator and maintained at 37°C during surgery. Burr holes were drilled in the skull for the implantation of three electrodes (recording, stimulating, and reference) in the brain. The recording electrode was placed at the level of the NAc core (+1.2 anterior-posterior, +1.1 medial-lateral, and −3.4 dorsal-ventral). A bipolar steel stimulation electrode (Plastics1, Roanoke, VA) was positioned ipsilaterally in the VTA (−3.1 anterior-posterior, +0.7 medial-lateral, and +4.8 dorsal-ventral). An Ag/AgCl reference electrode (0.5-mm diameter; Acros Organics, Springfield, NJ) was placed in the cortex contralateral to the recording and stimulating electrodes. Both recording and stimulating electrodes were slowly lowered into target locations until evoked DA release was maximized. Electrode placements were finalized once maximal evoked DA output was reached, and the locations of electrodes were kept unchanged throughout the remainder of the experiment. Recording electrodes were conditioned by applying an inverted V wave form (−0.4V to +1.3V to −0.4V, 400 V/s) at 60 Hz for 10 minutes, after which the frequency of the wave form was changed to 10 Hz and kept constant during the subsequent procedures. In all experiments, we recorded the “background currents” produced by the inverted V wave form applied to the recording electrode (Heien et al., 2004). This background current was subtracted from the “faradic currents” recorded after each VTA stimulation to derive the current attributable to DA release.
Electrical stimulation (60 Hz, 60 biphasic square pulses, 2 ms/phase, 300 µA) was applied with a constant-current isolator (A-M Systems) to evoke DA release every 3 minutes for 30 minutes. Baseline recordings of the extracellular concentration of DA evoked by VTA stimulation ([DA]) were made every 3 minutes until the peak amplitude was stabilized with less than 15% variance. Immediately following recording of the last baseline response, each mouse received an i.p. injection of the single drug and dose combination that it was assigned to. Cyclic voltammograms were recorded every three minutes for a total period of 30 minutes and analyzed with TarHeel CV and Demon Voltammetry software (University of North Carolina, Chapel Hill, NC and Wake Forest University, Winston-Salem, NC,). The peak extracellular concentration of DA evoked by VTA stimulation ([DA]max) obtained in the last predrug stimulation was used as baseline, and the [DA]max following all subsequent stimulations was calculated as a percentage change from this baseline for each individual mouse. For each stimulation, the duration of the rising phase of the response was calculated by measuring the time that it took to reach [DA]max starting from the initiation of electrical stimulation. The time constant of decay (tau) was calculated by fitting the falling phase of each response to a single exponential decay function (Yorgason et al., 2011). These release and decay values were then normalized to the average predrug baseline for each individual animal and reported as percentages.
Radioligand Binding Assays
Binding profiles of (S)-, and (R)-ketamine, as well as (S)-norketamine, (R)-norketamine, (S)-DHNK, (R)-DHNK, (2S,6S)-HNK, (2R,6R)-HNK, (2R,6S)-HNK, and (2S,6R)-HNK for DA receptors and monoamine transporters were performed by the National Institute of Mental Health Psychoactive Drug Screening Program (NIMH PDSP; University of North Carolina, Chapel Hill, NC). For all radioligand receptor assay protocols, see the Psychoactive Drug Screening Program web site (http://pdsp.med.unc.edu) and previously published protocols (Besnard et al., 2012). The data were used to calculate binding affinities expressed as Ki values.
Monoamine Transporters and Dopamine Receptor Functional Assays
Monoamine transporter functional assays were performed by the NIMH PDSP (for details, see http://pdsp.med.unc.edu) using a neurotransmitter transporter uptake assay kit (Molecular Devices, Sunnyvale, CA). Agonist and antagonist activity at DA receptors (D1–D5) was determined using GPCR Tango assays, also performed by the NIMH PDSP, using previously published protocols with modifications (Kroeze et al., 2015). In brief, HTLA cells were transfected with DA receptor Tango constructs (D1–D5) overnight and plated in Poly-l-Lys–coated 384-well white clear-bottom cell culture plates using Dulbecco’s modified Eagle’s medium supplemented with 1% dialyzed fetal bovine serum at a density of 15,000 cells in 40 μl per well. Compounds (concentration responses) were then added to cells 6 hours after plating. For antagonist activity, reference agonist at about EC80 concentration was added 30 minutes after testing compounds. After overnight stimulation, medium and drug solutions were removed, and BrightGlo reagents (Promega, Madison, WI) were added to cells (20 μl/well). Luminescence was counted on a luminescence counter after plates were incubated for 20 minutes in the dark at room temperature.
Agonist Activity.
EC50 values were determined using an agonist dose-response formula: response = basal activity + [(Emax − basal activity)/(1 + 10(LogEC50 − agonist concentration) × Hill slope)].
Antagonist Activity.
Antagonist responses were measured at a fixed EC80 concentration of the corresponding agonist in the presence of antagonist dilutions. IC50 values were determined using a concentration-response formula: response = basal activity + [(Emax − basal activity)/(1 + 10(LogIC50 − agonist concentration) × Hill slope)]. IC50 was then converted to Ki using the Cheng-Prusoff equation: Ki = IC50/[1 + (L/EC50)], where Ki is the binding affinity derived from an antagonist concentration-response assay, IC50 is the antagonist concentration where 50% inhibition is reached, L is the reference agonist concentration used in the assay (i.e., EC50 to EC80 concentration of the reference agonist), and EC50 is the known potency of the reference agonist (Cheng and Prusoff, 1973).
Statistical Analysis
Data are presented as the mean ± S.E.M. Statistical analyses were performed by GraphPad Prism v6 (GraphPad Software, La Jolla, CA). FSCV data were analyzed with two-way repeated-measures analysis of variance (ANOVA). Correction for multiple comparisons was done by Holm-Šídák post-hoc test. The criterion for statistical significance was p < 0.05.
Results
Plasma and Brain Tissue Distribution and Clearance of Ketamine and Major Metabolites.
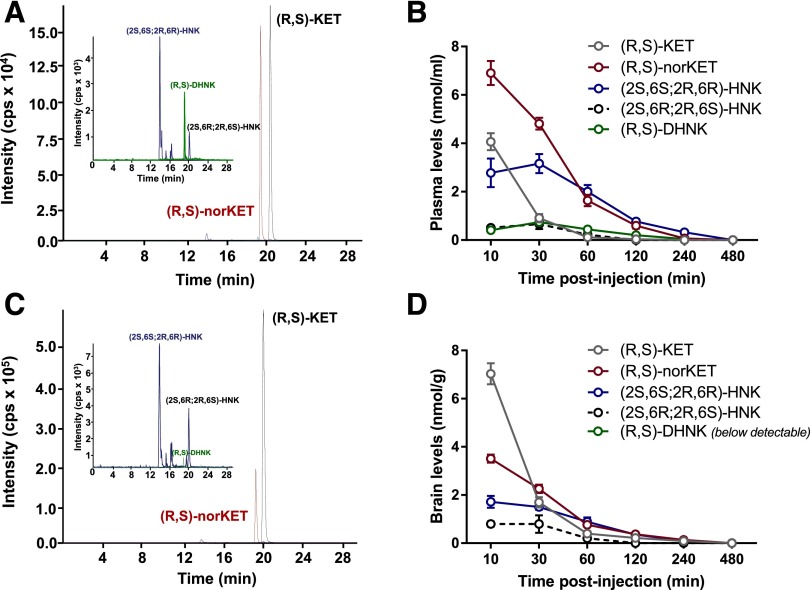
Ketamine is extensively and stereoselectively transformed by multiple hepatic cytochrome P450 isoforms into multiple metabolites (Adams et al., 1981; Desta et al., 2012). We first sought to quantify and compare brain and plasma concentrations of ketamine and ketamine’s major metabolites in the C57BL/6J mouse strain that would be used subsequently for FSCV. As shown in the representative chromatographic trace, quantifiable plasma concentrations of (R,S)-ketamine, (R,S)-norketamine, (2S,6S;2R,6R)-HNKs, (2S,6R;2R,6S)-HNK, and (R,S)-DHNK were identified within 10 minutes of an i.p. injection of ketamine (10 mg/kg) (Fig. 1A). The plasma concentration-time curves are presented in Fig. 1B. The maximum plasma concentration of (R,S)-ketamine (4.07 ± 0.3 nmol/ml) was observed at the 10-minute time point, and the concentration rapidly declined, reaching 0.03 ± 0.03 nmol/ml at 120 minutes postinjection. The maximum plasma concentration of (R,S)-norketamine (6.90 ± 0.5 nmol/ml) was also observed at the 10-minute time point, indicating the rapid and extensive N-demethylation of (R,S)-ketamine. The clearance of (R,S)-norketamine was slower than (R,S)-ketamine, as the minimum quantifiable concentration (0.07 ± 0.07 nmol/ml) was measured in the plasma sample obtained at 240 minutes postadministration. The peak plasma concentration of (2S,6S;2R,6R)-HNK (3.16 ± 0.4 nmol/ml) was observed at 30 minutes following ketamine injection and then declined to 0.32 ± 0.1 nmol/ml in the 240-minute plasma samples. The longer time to peak concentration and slower clearance of (2S,6S;2R,6R)-HNK are consistent with the primary formation of the metabolite from ring hydroxylation of (R,S)-norketamine (Desta et al., 2012). For (2S,6R; 2R,6S)-HNK, maximum plasma levels were observed 30 minutes following ketamine injection (0.66 ± 0.2 nmol/ml) and were rapidly cleared within 60 minutes (0.23 ± 0.2 nmol/ml). The maximum plasma concentration of (R,S)-DHNK (0.74 ± 0.08 nmol/ml) was also reached at 30 minutes postketamine administration and declined to a concentration of 0.04 ± 0.04 nmol/ml in the 120-minute plasma sample. Plasma levels of HNK metabolites (2S,5S;2R,5R)-HNK, (2S,4S;2R,4R)-HNK, (2S,4R;2R,4S)-HNK, and (2S,5R;2R,5S)-HNK were below quantification limits at every time point.
Fig. 1.
Plasma and brain concentrations of ketamine and its metabolites following systemic ketamine administration. Representative chromatograms from the 10-minute time point from plasma (A) and brain (C). Concentration versus time relationship for plasma (B) and brain tissue concentrations (D) of (R,S)-ketamine, (R,S)-norketamine, (R,S)-dehydronorketamine, (2S,6R;2R,6R)-hydroxynorketamine, and (2S,6S;2R,6R)-hydroxynorketamine after intraperitoneal administration of (R,S)-ketamine (10 mg/kg). The measured analyte concentrations in the brain were normalized according to tissue weight and are reported as umol/g of tissue. Data are the mean ± S.E.M. (n = 4/time point). KET, ketamine.
A representative chromatographic trace from the analysis of brain tissue obtained after an i.p. injection of ketamine (10 mg/kg) is presented in Fig. 1C. The relationships between time following injection and measured concentrations of (R,S)-ketamine, (R,S)-norketamine, (2S,6S;2R,6R)-HNK, and (2S,6R;2R,6S)-HNK are presented in Fig. 1D. The peak brain tissue concentration of (R,S)-ketamine (7.03 ± 0.4 nmol/g) was observed 10 minutes following (R,S)-ketamine administration and then declined to 0.09 ± 0.05 nmol/g in the 240-minute samples. The maximum brain tissue concentrations of (R,S)-norketamine (3.51 ± 0.17 nmol/g) and (2S,6S;2R,6R)-HNK (1.72 ± 0.25 nmol/g) were also observed at the 10-minute time point and then decreased to 0.14 ± 0.03 nmol/g and 0.09 ± 0.02 nmol/g, respectively, at 240 minutes. Peak brain levels of the (2S,6R;2R,6S)-HNK metabolite were observed 10 minutes following ketamine administration (0.8 ± 0.1 nmol/g) and decreased to 0.20 ± 0.04 nmol/g within 60 minutes. The maximum brain tissue concentration of (R,S)-ketamine was 73% higher than the corresponding plasma concentration, whereas the brain tissue concentrations of (R,S)-norketamine and (2S,6S;2R,6R)-HNK were 49 and 45% lower than the corresponding maximum plasma concentrations. These data are consistent with previous observations that (R,S)-ketamine rapidly accumulates in the brain of rats (Moaddel et al., 2015). Although (R,S)-DHNK was also detected in brain tissues, its levels were below quantification. This may in part be due to circulating plasma levels of (R,S)-DHNK that are significantly (4–8 times) lower than the other metabolites, but also due to poor crossing of the blood-brain barrier. A potential explanation for the low circulating and brain tissue concentrations of (R,S)-DHNK was provided by a recent study reporting that (R,S)-DHNK rapidly and irreversibly partitions into rat red blood cells, reducing the available plasma concentrations to less than 20% of the spiked values (Moaddel et al., 2016). The same effect was not observed with (R,S)-ketamine, (R,S)-norketamine, or (2S,6S;2R,6R)-HNK (Hijazi et al., 2001; Moaddel et al., 2016). Brain levels of (2S,5S;2R,5R)-HNK, (2S,4S;2R,4R)-HNK, (2S,4R;2R,4S)-HNK, and (2S,5R;2R,5S)-HNK were below quantification at every time point.
Effects of Ketamine Administration on the Kinetics of Electrically Evoked Dopamine Release.
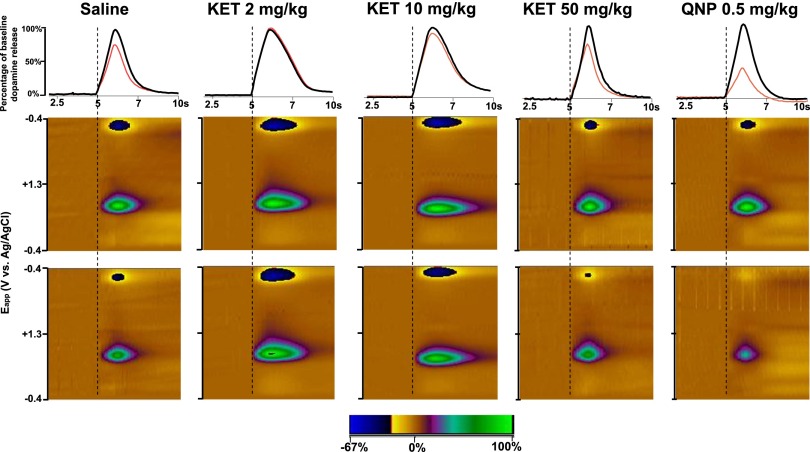
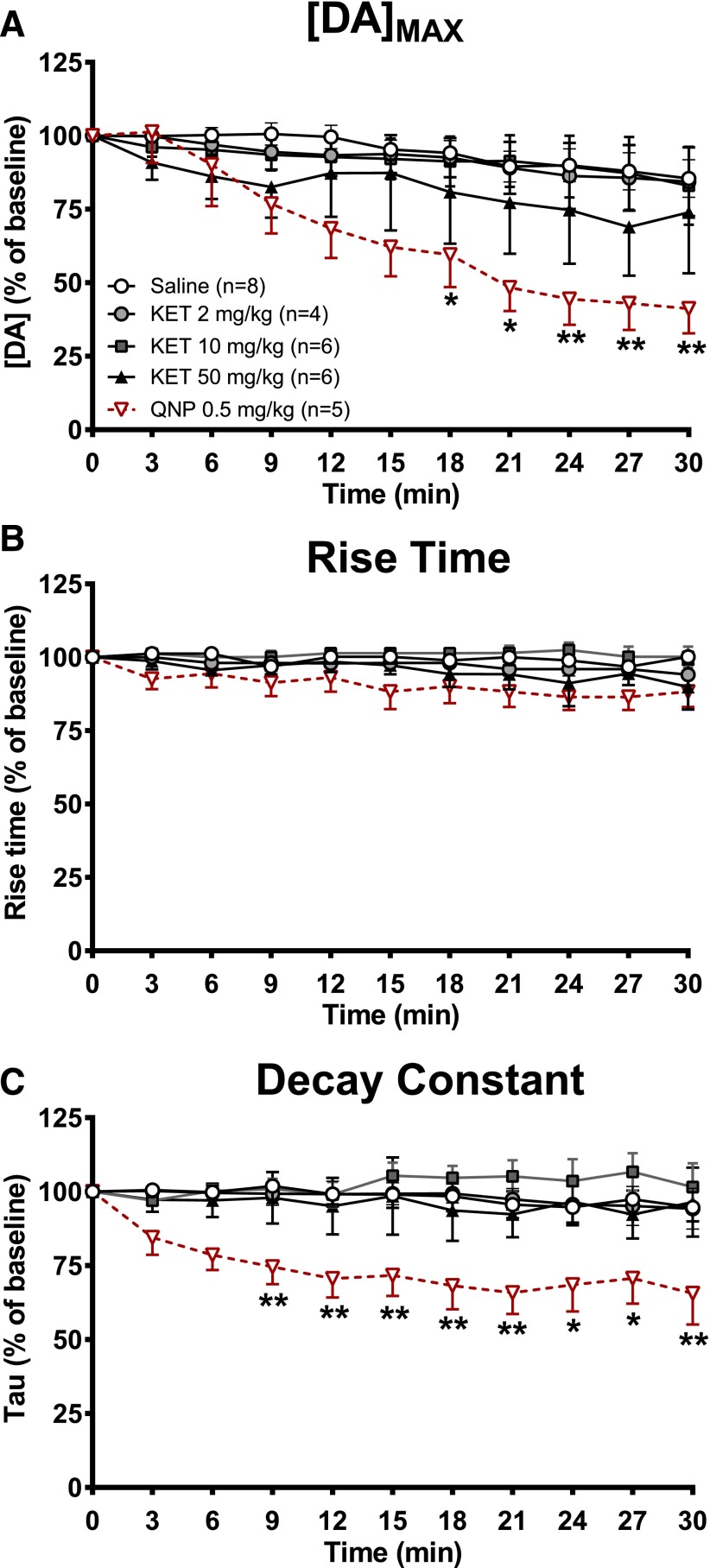
We used FSCV to assess the magnitude and temporal dynamics of DA release and reuptake at subsecond temporal resolution. We electrically stimulated the VTA and recorded the resulting changes in extracellular DA concentration in the NAc core. Individual animals were either administered (i.p.) vehicle (saline) or ketamine at 2, 10, or 50 mg/kg, which represents the range of subanesthetic doses that produce antidepressant actions in rodent behavioral models (Browne and Lucki, 2013), and received VTA stimulation once every 3 minutes for a 30-minute period. Changes in extracellular dopamine concentration [DA] evoked by electrical stimulation in mice representing each treatment group are presented in Fig. 2. Ketamine did not change DA release at any of the doses that were tested. In contrast, quinpirole administration (0.5 mg/kg) induced a marked decrease in the peak amplitude of evoked DA ([DA]max) values from the baseline (Fig. 2), as previously reported (Stamford et al., 1991; Maina and Mathews, 2010) and consistent with its agonist actions on D2 autoreceptors. Group data on the effects of treatments on DA release are presented in Fig. 3A. A two-way repeated-measures ANOVA revealed a significant main effect of “time” (F9,216 = 16.25, p < 0.0001) and an interaction of time × drug treatment (F36,216 = 2.52, p < 0.0001) but no significant “drug treatment” effect (F4,24 = 1.88, p = 0.146). Holm-Šídák post-hoc comparisons of the effects of quinpirole administration indicated that [DA]max values were significantly lower compared with saline, starting at the 18th minute and lasting until the end of data collection (Fig. 3A). No statistically significant differences between saline- and ketamine-treated groups were observed at any time point (p > 0.05). Furthermore, ketamine administration did not significantly alter [DA]max values at any time point after injection (Fig. 3A).
Fig. 2.
Changes in extracellular dopamine concentration in mice that received saline, ketamine (2, 10, or 50 mg/kg), or quinpirole (0.5 mg/kg). Black traces of the upper row and upper color plots of the middle row show a representative data from each treatment group. Red traces of the upper row and the color plots of the lower row show the last recording from the same animal 30 minute after the drug treatment. Time scale shown on the x-axes of the first row also applies to the x-axes of the color plots. The y-axes of the color plots indicates the potential applied to the recording electrode. The pseudo-color plot scale (z-axis) of the color plots indicates the percentage change from the baseline DA concentration, where DA signals are represented by the color change in near the center (∼0.6 V) of the rising phase of the voltage ramp. KET, ketamine; QNP, quinpirole.
Fig. 3.
Effects of ketamine on parameters of dopamine release. (A) Percentage changes in the [DA]max of stimulation-evoked DA release at each stimulation after the drug treatment (predrug baseline = 100). (B) Percentage changes in rise time (the amount of time it takes to reach peak DA concentrations) at each stimulation during the release of DA (predrug baseline = 100). (C) Percentage changes in decay constant (tau) values at each stimulation during the falling phase of evoked DA (predrug baseline = 100). Two-way repeated-measures ANOVA; *p < 0.05, **p < 0.01 compared with the saline group, Holm-Šídák post-hoc test. Data are the mean ± S.E.M. (saline: n = 8; KET 2 mg/kg : n = 4; KET 10 mg/kg: n = 6; KET 50 mg/kg: n = 6; QNP: n = 5). KET, ketamine; QNP, quinpirole.
A two-way repeated-measures ANOVA performed on changes in rise-time values [time that it takes for evoked DA concentrations to reach their maximal values ([DA]max) after the start of each electrical stimulation] indicated no main effect of drug treatment (F4,24 = 2.01, p > 0.05), but a significant main effect of time (F9,216 = 3.08, p < 0.01) and no interaction between these factors (F36,216 = 0.91, P > 0.05) (Fig. 3B). Two-way repeated-measures ANOVA on decay constants revealed no main effect of time (F9,216 = 1.82, p > 0.05), but there was a main effect of drug treatment (F4,24 = 4.04, p < 0.05) (Fig. 3C). Although the ANOVA interaction between these variables was not statistically significant (F36,216 = 1.2, p > 0.05), Holm-Šídák post-hoc pairwise comparisons between saline and all other treatment groups were performed to assess whether decay constants were differentially altered between treatment groups. These comparisons revealed that the decrease in decay constants was significantly higher in the quinpirole treatment group compared with saline group at all times from the ninth minute onward, whereas there was no significant difference in change in decay constants in ketamine treatment groups and the saline group (Fig. 3C).
Overall, our results reveal that ketamine does not change electrically evoked DA release or alter DA release and decay kinetics in the NAc core. However, quinpirole, a D2 receptor agonist, decreases DA release as expected from activation of presynaptic D2 autoreceptors (Stamford et al., 1991; Maina and Mathews, 2010).
Dopamine Receptor Binding Affinity and Functional Activity.
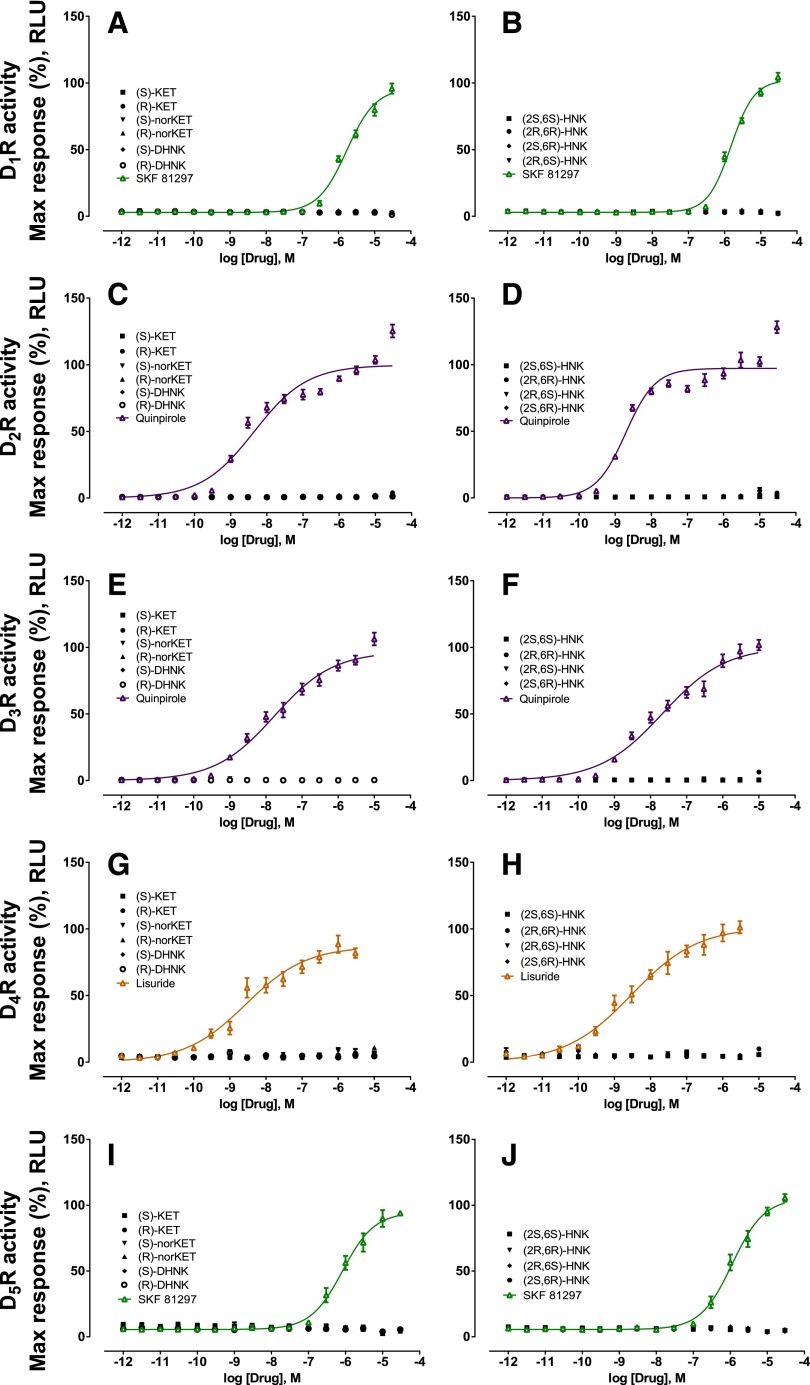
An in vitro affinity screening was performed to assess affinity of (S)-ketamine, (R)-ketamine, or their metabolites (S)-norketamine, (R)-norketamine, (S)-DHNK, (R)-DHNK, (2S,6S)-HNK, (2R,6R)-HNK, (2R,6S)-HNK, and (2S,6R)-HNK on DA D1, D2, D3, D4, or D5 receptors. In the primary receptor screening, at 10 μM concentrations, none of ketamine’s enantiomers or metabolites showed more than 50% inhibition of any of the DA receptor subtypes (Table 1). Thus, no Ki determinations were performed.
TABLE 1.
Binding potency of ketamine’s enantiomers and metabolites at dopamine receptors
Percent values are the mean of two to five experiments, with four replicates each, and were derived from a single concentration (10 μM). Negative values are represented as 0%.
| % Inhibition |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (S)-KET | (R)-KET | (S)-norKET | (R)-norKET | (S)-DHNK | (R)-DHNK | (2S,6S)-HNK | (2R,6R)-HNK | (2R,6S)-HNK | (2S,6R)-HNK | |
| Target | ||||||||||
| D1R | 12 | 4 | 0 | 7 | 0 | 0 | 9 | 0 | 26 | 0 |
| D2R | 1 | 12 | 0 | 2 | 5 | 17 | 0 | 0 | 9 | 0 |
| D3R | 10 | 8 | 0 | 2 | 0 | 3 | 0 | 0 | 0 | 0 |
| D4R | 4 | 0 | 0 | 0 | 8 | 0 | 0 | 0 | 6 | 0 |
| D5R | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 2 |
D1R, dopamine receptor D1; D2R, dopamine receptor D2; D3R, dopamine receptor D3; D4R, dopamine receptor D4; D5R, dopamine receptor D5; KET, ketamine; norKET, norketamine.
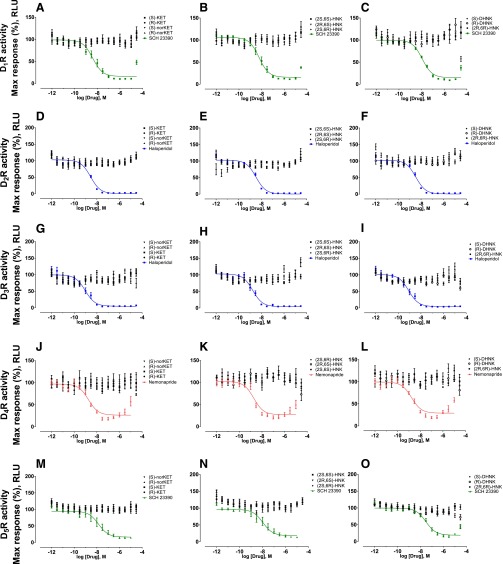
In vitro agonist and antagonist functional assays were performed at DA D1, D2, D3, D4, and D5 receptors for (S)-ketamine, (R)-ketamine, and their metabolites using the GPCR Tango assays to measure agonist-mediated β-arrestin translocation (Kroeze et al., 2015). No agonist (Fig. 4) or antagonist (Fig. 5) actions on DA receptors were observed.
Fig. 4.
Functional agonist activity of ketamine and ketamine metabolites at dopamine D1–5 receptors compared with positive control compounds. Functional assays of (S)-ketamine, (R)-ketamine, (S)-norketamine, (R)-norketamine, (S)-DHNK, (R)-DHNK, (2S,6S)-HNK, (2R,6R)-HNK, (2R,6S)-HNK, and (2S,6R)-HNK at DA (A-B) D1, (C-D) D2, (E-F) D3, (G-H) D4, and (I-J) D5 receptors indicated lack of agonist action. Positive control data for each experiment are indicated by colored lines. Data are the mean ± S.E.M. (n = 9; three independent experiments and three replicates per experiment). KET, ketamine; RLU, relative light units; SKF 81297, (±)-6-Chloro-2,3,4,5-tetrahydro-1-phenyl-1H-3-benzazepine hydrobromide.
Fig. 5.
Functional antagonist activity of ketamine and ketamine metabolites at dopamine D1–5 receptors compared with positive control compounds. Functional assays of (S)-ketamine, (R)-ketamine, (S)-norketamine, (R)-norketamine, (S)-DHNK, (R)-DHNK, (2S,6S)-HNK, (2R,6R)-HNK, (2R,6S)-HNK and (2S,6R)-HNK at DA (A-C) D1, (D-F) D2, (G-I) D3, (J-L) D4, and (M-O) D5 receptors indicated lack of antagonistic action. Data are the mean ± S.E.M. (n = 9; three independent experiments and three replicates per experiment). KET, ketamine; RLU, relative light units; SCH 23390, (R)-(+)-7-Chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride.
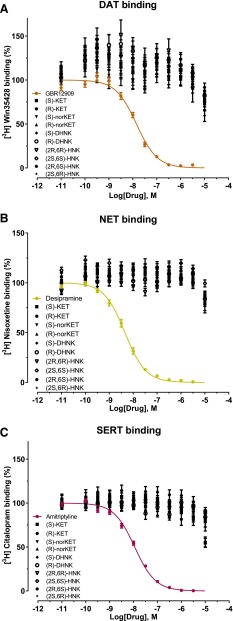
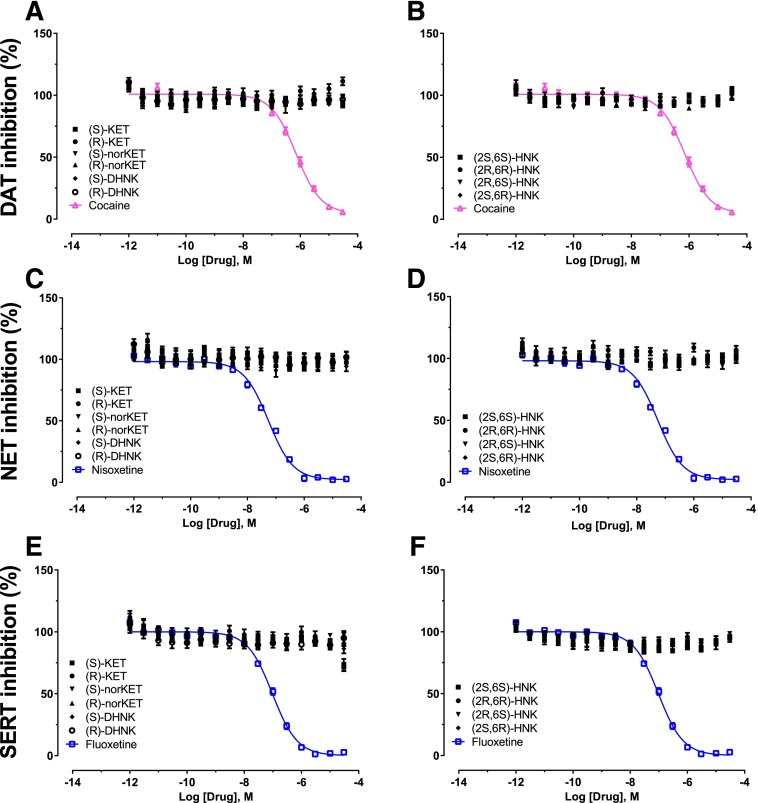
Binding Affinity and Functional Activity at Monoamine Transporters.
In vitro binding assays were performed to assess binding affinity of (S)-ketamine, (R)-ketamine, and their metabolites to the monoamine transporters. No inhibition of binding by ketamine enantiomers and metabolites was observed at concentrations up to 10 μM at DAT, NET, or SERT (Fig. 6). We also determined effects of ketamine enantiomers and metabolites at DAT, NET, and SERT and found that they showed no inhibitory effect on neurotransmitter transporter activity (Fig. 7) at up to 10 μM.
Fig. 6.
Competition binding profiles for ketamine’s enantiomers and metabolites against the DATs (A), NETs (B), and SERTs (C). Binding displacement by (S)-ketamine, (R)-ketamine, (S)-norketamine, (R)-norketamine, (S)-DHNK, (R)-DHNK, (2S,6S)-HNK, (2R,6R)-HNK, (2R,6S)-HNK, and (2S,6R)-HNK was determined at DAT using 3H-Win35428 [β-Carbomethoxy-3-β-(4-fluorophenyl)tropane)] with GBR12909 (-[2-[Bis-(4-fluorophenyl)methoxy]ethyl]-4-(3-phenylpropyl)piperazine dihydrochloride) as a reference ligand, at NET using 3H-nisoxetine with desipramine as a reference ligand, and at SERT using 3H-citalopram with amitriptyline as a reference ligand. Data are the mean ± S.E.M. (n ≥ 9; three or more independent experiments and three to four replicates per experiment). KET, ketamine.
Fig. 7.
Functional activity of ketamine and ketamine metabolites at monoamine transporters compared with positive control compounds. Functional assays of (S)-ketamine, (R)-ketamine, (S)-norketamine, (R)-norketamine, (S)-DHNK, (R)-DHNK, (2S,6S)-HNK, (2R,6R)-HNK, (2R,6S)-HNK, and (2S,6R)-HNK at (A-B) DAT, (C-D) NET, and (E-F) SERT indicated lack of inhibitory activity. Positive control data for each experiment are indicated by colored lines. Data are the mean ± S.E.M. (n = 11; three independent experiments and three to four replicates per experiment). KET, ketamine.
Discussion
We used FSCV to study the effects of acute, subanesthetic doses of ketamine on the magnitude and kinetics of electrical stimulation-evoked DA release in the NAc core of anesthetized mice. We also conducted a comprehensive in vitro pharmacological screening of the binding and functional activity of ketamine and its metabolites on DA receptors and monoamine transporters. We observed no significant effects of acute, systemic administration of ketamine on the magnitude or kinetics of the electrically evoked DA concentrations. This contrasts with the finding by Hancock and Stamford (1999), who observed that a 100 μM concentration of ketamine [and in particular, (S)-ketamine] increased NAc DA release, as measured by FSCV in rat slices. However, the concentration of ketamine used in this previous study is higher than brain exposure under our conditions relevant to the antidepressant actions of ketamine. Our observed lack of direct effects of ketamine on DA release in vivo is supported by the pharmacological/functional activity profile of ketamine and its metabolites, which also showed no significant affinity or agonist/antagonist activity on DA D1–5 receptors or DAT, NET, or SERT.
Previous studies showed that ketamine has an affinity (Ki = 0.05–0.5 μM) for DA D2-type receptors in vitro (Kapur and Seeman, 2002; Seeman et al., 2005), which was not identified in our study (Table 1). We also did not find any agonist or antagonist functional activity at the D2 receptor (Figs. 4 and 5). Nishimura and colleagues (1998) reported that ketamine inhibits NET (66.8 μM), DAT (62.9 μM), and SERT (162 μM) transporters expressed in human embryonic kidney 293 cells. In a follow-up study, they reported that (S)-ketamine shows a greater inhibition at DAT than (R)-ketamine (Ki = 46.9 vs. 390 μM) (Nishimura and Sato, 1999). In contrast, ketamine exhibited no stereoselectivity for NET and SERT (Nishimura and Sato, 1999). Consistent with these results, we did not observe any binding of ketamine’s enantiomers or metabolites to DAT, NET, or SERT when tested up to 10 μM. Moreover, we demonstrate no functional inhibition of ketamine’s enantiomers or primary metabolites on DAT, NET, or SERT (up to a maximum concentration of 10 μΜ). Our FSCV studies confirmed the lack of activity of ketamine on monoamine transporters and DA receptors, since we did not observe a statistically significant effect of different doses of ketamine on extracellular NAc DA kinetics, whereas the D2 receptor agonist quinpirole significantly decreased the evoked release of DA. Our pharmacological/functional screening similarly did not provide any evidence for direct effects of ketamine and its metabolites on DA receptor function. Taken together, our data indicate that ketamine does not affect electrically evoked DA release in the NAc core of anesthetized mice, and that ketamine and its metabolites exert no significant effect on the DAergic receptors and transporters. Additionally, no functional effects on any of these receptors and transporters were observed in vitro at concentrations that are relevant to the antidepressant effects of ketamine.
Ketamine is a noncompetitive N-methyl-d-aspartate glutamate receptor (NMDAR) antagonist [Ki = 0.2–1.6 μM, with (S)-ketamine approximately 4-fold more potent than (R)-ketamine] (Parsons et al., 1995; Ebert et al., 1997; Moaddel et al., 2013). (S)- and (R)-norketamine are less potent NMDAR antagonists (Ki = 1.7–2.25 and 13–26 μM, respectively) than ketamine, whereas (2S,6S)-HNK and (2R,6R)-HNK show limited, if any, in vitro binding or in situ functional activity at the NMDAR (Moaddel et al., 2013; Zanos et al., 2016). We have recently shown that the antidepressant actions of (2R,6R)-HNK involves potentiation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (Zanos et al., 2016). Our current findings cannot preclude indirect effects of ketamine administration on DA neurotransmission in vivo, since glutamatergic signaling plays an important role in the regulation of the mesolimbic DAergic system. In fact, DAergic neurons in the VTA express both NMDAR and AMPA receptors, and these neurons receive direct glutamatergic inputs from numerous cortical (e.g. hippocampus and prefrontal cortex) and subcortical (laterodorsal tegmental and pedunculopontine tegmental nuclei, the bed nucleus of the stria terminalis and the superior colliculus) brain regions (Morikawa and Paladini, 2011). Activation of NMDARs in the VTA increases DA neuron burst firing rates (Overton and Clark, 1992; Chergui et al., 1993), whereas NMDAR antagonists decrease spontaneous bursts of VTA DAergic neurons after the activation of glutamatergic inputs to the VTA (Overton and Clark, 1992, 1997; Chergui et al., 1994; Morikawa and Paladini, 2011). In a similar fashion, in mutant mice lacking NMDARs specifically on DAergic neurons, burst firing of VTA DAergic neurons and striatal DA release are attenuated after electrical stimulation of the pedunculopontine tegmental nucleus, which projects glutamatergic inputs to the VTA (Zweifel et al., 2009). Ketamine-induced VTA neuronal activation in vivo has been shown to be glutamate-dependent, since administration of the AMPA receptor antagonist NBQX blocked this effect of ketamine (Witkin et al., 2016). Moreover, indirect modulation of the DAergic neurotransmission in other brain regions by ketamine might also exist. The results of previous studies indicate that systemic subanesthetic doses of ketamine increase both DA and glutamate release in the prefrontal cortex as measured by microdialysis or DA turnover quantification (Rao et al., 1989; Verma and Moghaddam, 1996; Lindefors et al., 1997; Moghaddam et al., 1997; Lorrain et al., 2003). Stereoselective effects of (S)- and (R)-ketamine on increased electrically evoked DA release in the caudate putamen and bed nucleus of stria terminalis in rat brain slices have also been reported (Tso et al., 2004). However, this occurred at a ketamine concentration of 100 μM, much higher than concentrations (<10 nmol/g) found in the brain following an antidepressant effective dose (Fig. 1).
Other NMDAR antagonists have been shown to modulate the midbrain DAergic system as well. In particular, systemic administration of (5S,10R)-(+)-5-Methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine (MK-801), a selective noncompetitive NMDAR antagonist, increases extracellular levels of DA and DA metabolism in the striatum and prefrontal cortex (Liljequist et al., 1991; Loscher et al., 1991; Bristow et al., 1993; Wolf et al., 1993). Similarly, phencyclidine, another noncompetitive NMDAR antagonist, increases extracellular levels of DA or DA metabolism in the NAc, amygdala, and prefrontal cortex (Rao et al., 1989; Bristow et al., 1993; Hondo et al., 1994). Although these findings show that inhibition of the NMDAR induces changes in the DA system, they do not clarify whether these effects are due to indirect effects of NMDAR antagonists via glutamatergic signaling or direct effects over DA receptors and transporters. For example, Carlsson and Carlsson (1989) showed that MK-801-induced hyperlocomotion is not affected by monoamine depletion in mice, indicating that this behavioral response does not depend on the DAergic system. We also note that haloperidol did not reduce ketamine-induced perceptual changes, negative symptoms, or euphoria in healthy subjects (Krystal et al., 1999).
In addition to NMDAR activity, ketamine has reported effects on other receptors, including μ-opioid receptors (Ki = 26.8 μM), σ-opioid receptors (Ki = 26.8 μM), κ-opioid receptors (Ki = 85.2 μM), δ-opioid receptors (Ki = 101 μM) (Smith et al., 1987), M1 muscarinic receptors (Ki = 200 μM) (Durieux, 1995), serotonin (5-hydroxytryptamine) receptors subtype 2 (Ki = 15 μM) (Kapur and Seeman, 2002), and nicotinic receptors (Yamakura et al., 2000; Weber et al., 2005; Moaddel et al., 2013). Although the binding or uptake of ketamine, or its metabolites, to these membrane constituents might contribute to its clinical profile (Mathew et al., 2012), these Ki values are higher than peak concentrations found in the brain or plasma (∼8 nmol/g or 8 nmol/ml, respectively) following an antidepressant effective dose (Fig. 1).
These results extend our knowledge of ketamine’s actions on mesolimbic DA release and highlight the need for the study of potential indirect interactions between glutamatergic and DAergic neurotransmitter systems to obtain a complete picture of ketamine’s actions relevant to both therapeutic actions and side effects (see Grace, 2016 for a recent review).
Acknowledgments
The authors thank Craig J. Thomas and Patrick J. Morris from the National Institutes of Health, National Center for Advancing Translational Medicine for providing (2S,6S)- and (2R,6R)-hydroxynorketamine. Receptor binding profiles and Ki determinations were generously provided by the NIMH Psychoactive Drug Screening Program, Contract # HHSN-271-2008-025C, directed by Dr. Bryan Roth (University of North Carolina, Chapel Hill, NC) in conjunction with Jamie Driscoll (NIMH, Bethesda, MD).
Abbreviations
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- ANOVA
analysis of variance
- DA
dopamine
- DAergic
dopaminergic
- [DA]max
peak amplitude of evoked dopamine
- DAT
dopamine transporter
- DHNK
dehydronorketamine
- FSCV
fast-scan cyclic voltammetry
- HNK
hydroxynorketamine
- MK-801
(5S,10R)-(+)-5-Methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine
- NAc
nucleus accumbens
- NET
norepinephrine transporter
- NIMH PDSP
National Institute of Mental Health Psychoactive Drug Screening Program
- NMDAR
N-methyl-d-aspartate glutamate receptor
- SERT
serotonin transporter
- VTA
ventral tegmental area
Authorship Contributions
Participated in research design: Can, Zanos, Moaddel, Cheer, Frost, Huang, Gould.
Conducted experiments: Can, Zanos, Kang, Dossou, Huang.
Contributed new reagents or analytic tools: Wainer.
Performed data analysis: Can, Zanos, Moaddel, Kang, Huang.
Wrote or contributed to the writing of the manuscript: Can, Zanos, Wainer, Frost, Gould.
Footnotes
This study was supported by the Intramural Research Program of the National Institutes of Health National Institute of Mental Health [Grant MH107615 to T.D.G.] and the National Institute on Aging. Receptor binding profiles, Ki determinations, and functional assays (H.J.K. and X.-P.H.) were supported by the National Institute of Mental Health Psychoactive Drug Screening Program [Contract # HHSN-271-2008-025C to Bryan L. Roth, University of North Carolina] in conjunction with Jamie Driscoll (National Institute of Mental Health, Bethesda, MD).
Ruin Moaddel and Irving W. Wainer are listed as coinventors on a patent for the use of (2R,6R)-hydroxynorketamine, (S)-dehydronorketamine, and other stereoisomeric dehydro- and hydroxylated metabolites of (R,S)-ketamine metabolites in the treatment of depression and neuropathic pain. Panos Zanos, Ruin Moaddel, Irving W. Wainer, and Todd D. Gould are listed as coinventors on a patent application for the use of (2R,6R)-hydroxynorketamine and (2S,6S)-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation, and post-traumatic stress disorders. Ruin Moaddel and Irving W. Wainer have assigned their patent rights to the U.S. government but will share a percentage of any royalties that may be received by the government. Panos Zanos and Todd D. Gould have assigned their patent rights to the University of Maryland, Baltimore, but will share a percentage of any royalties that may be received by the University of Maryland Baltimore. All other authors declare no competing interests.
References
- Aalto S, Hirvonen J, Kajander J, Scheinin H, Någren K, Vilkman H, Gustafsson L, Syvälahti E, Hietala J. (2002) Ketamine does not decrease striatal dopamine D2 receptor binding in man. Psychopharmacology (Berl) 164:401–406. [DOI] [PubMed] [Google Scholar]
- aan het, Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, Mathew SJ. (2010) Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry 67:139–145. [DOI] [PubMed] [Google Scholar]
- Adams JD, Jr, Baillie TA, Trevor AJ, Castagnoli N., Jr (1981) Studies on the biotransformation of ketamine. 1-Identification of metabolites produced in vitro from rat liver microsomal preparations. Biomed Mass Spectrom 8:527–538. [DOI] [PubMed] [Google Scholar]
- Belujon P, Grace AA. (2014) Restoring mood balance in depression: ketamine reverses deficit in dopamine-dependent synaptic plasticity. Biol Psychiatry 76:927–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47:351–354. [DOI] [PubMed] [Google Scholar]
- Besnard J, Ruda GF, Setola V, Abecassis K, Rodriguiz RM, Huang XP, Norval S, Sassano MF, Shin AI, Webster LA, et al. (2012) Automated design of ligands to polypharmacological profiles. Nature 492:215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristow LJ, Hutson PH, Thorn L, Tricklebank MD. (1993) The glycine/NMDA receptor antagonist, R-(+)-HA-966, blocks activation of the mesolimbic dopaminergic system induced by phencyclidine and dizocilpine (MK-801) in rodents. Br J Pharmacol 108:1156–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne CA, Lucki I. (2013) Antidepressant effects of ketamine: mechanisms underlying fast-acting novel antidepressants. Front Pharmacol 4:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson M, Carlsson A. (1989) The NMDA antagonist MK-801 causes marked locomotor stimulation in monoamine-depleted mice. J Neural Transm 75:221–226. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. (1973) Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol 22:3099–3108. [DOI] [PubMed] [Google Scholar]
- Chergui K, Akaoka H, Charléty PJ, Saunier CF, Buda M, Chouvet G. (1994) Subthalamic nucleus modulates burst firing of nigral dopamine neurones via NMDA receptors. Neuroreport 5:1185–1188. [DOI] [PubMed] [Google Scholar]
- Chergui K, Charléty PJ, Akaoka H, Saunier CF, Brunet JL, Buda M, Svensson TH, Chouvet G. (1993) Tonic activation of NMDA receptors causes spontaneous burst discharge of rat midbrain dopamine neurons in vivo. Eur J Neurosci 5:137–144. [DOI] [PubMed] [Google Scholar]
- Desta Z, Moaddel R, Ogburn ET, Xu C, Ramamoorthy A, Venkata SL, Sanghvi M, Goldberg ME, Torjman MC, Wainer IW. (2012) Stereoselective and regiospecific hydroxylation of ketamine and norketamine. Xenobiotica 42:1076–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, Kammerer WA, Quezado Z, Luckenbaugh DA, Salvadore G, et al. (2010) A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry 67:793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan TT, Tan JW, Yuan Q, Cao J, Zhou QX, Xu L. (2013) Acute ketamine induces hippocampal synaptic depression and spatial memory impairment through dopamine D1/D5 receptors. Psychopharmacology (Berl) 228:451–461. [DOI] [PubMed] [Google Scholar]
- Dunlop BW, Nemeroff CB. (2007) The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry 64:327–337. [DOI] [PubMed] [Google Scholar]
- Durieux ME. (1995) Inhibition by ketamine of muscarinic acetylcholine receptor function. Anesth Analg 81:57–62. [DOI] [PubMed] [Google Scholar]
- Ebert B, Mikkelsen S, Thorkildsen C, Borgbjerg FM. (1997) Norketamine, the main metabolite of ketamine, is a non-competitive NMDA receptor antagonist in the rat cortex and spinal cord. Eur J Pharmacol 333:99–104. [DOI] [PubMed] [Google Scholar]
- Grace AA. (2016) Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat Rev Neurosci 17:524–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock PJ, Stamford JA. (1999) Stereospecific effects of ketamine on dopamine efflux and uptake in the rat nucleus accumbens. Br J Anaesth 82:603–608. [DOI] [PubMed] [Google Scholar]
- Heien MLAV, Johnson MA, Wightman RM. (2004) Resolving neurotransmitters detected by fast-scan cyclic voltammetry. Anal Chem 76:5697–5704. [DOI] [PubMed] [Google Scholar]
- Hijazi Y, Bolon M, Boulieu R. (2001) Stability of ketamine and its metabolites norketamine and dehydronorketamine in human biological samples. Clin Chem 47:1713–1715. [PubMed] [Google Scholar]
- Hondo H, Yonezawa Y, Nakahara T, Nakamura K, Hirano M, Uchimura H, Tashiro N. (1994) Effect of phencyclidine on dopamine release in the rat prefrontal cortex; an in vivo microdialysis study. Brain Res 633:337–342. [DOI] [PubMed] [Google Scholar]
- Irifune M, Shimizu T, Nomoto M. (1991) Ketamine-induced hyperlocomotion associated with alteration of presynaptic components of dopamine neurons in the nucleus accumbens of mice. Pharmacol Biochem Behav 40:399–407. [DOI] [PubMed] [Google Scholar]
- Kapur S, Seeman P. (2002) NMDA receptor antagonists ketamine and PCP have direct effects on the dopamine D(2) and serotonin 5-HT(2)receptors-implications for models of schizophrenia. Mol Psychiatry 7:837–844. [DOI] [PubMed] [Google Scholar]
- Kegeles LS, Abi-Dargham A, Zea-Ponce Y, Rodenhiser-Hill J, Mann JJ, Van Heertum RL, Cooper TB, Carlsson A, Laruelle M. (2000) Modulation of amphetamine-induced striatal dopamine release by ketamine in humans: implications for schizophrenia. Biol Psychiatry 48:627–640. [DOI] [PubMed] [Google Scholar]
- Kroeze WK, Sassano MF, Huang XP, Lansu K, McCorvy JD, Giguère PM, Sciaky N, Roth BL. (2015) PRESTO-Tango as an open-source resource for interrogation of the druggable human GPCRome. Nat Struct Mol Biol 22:362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, D’Souza DC, Karper LP, Bennett A, Abi-Dargham A, Abi-Saab D, Cassello K, Bowers MB, Jr, Vegso S, Heninger GR, et al. (1999) Interactive effects of subanesthetic ketamine and haloperidol in healthy humans. Psychopharmacology (Berl) 145:193–204. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr, Charney DS. (1994) Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 51:199–214. [DOI] [PubMed] [Google Scholar]
- Lannes B, Micheletti G, Warter JM, Kempf E, Di Scala G. (1991) Behavioural, pharmacological and biochemical effects of acute and chronic administration of ketamine in the rat. Neurosci Lett 128:177–181. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhu ZR, Ou BC, Wang YQ, Tan ZB, Deng CM, Gao YY, Tang M, So JH, Mu YL, et al. (2015) Dopamine D2/D3 but not dopamine D1 receptors are involved in the rapid antidepressant-like effects of ketamine in the forced swim test. Behav Brain Res 279:100–105. [DOI] [PubMed] [Google Scholar]
- Liljequist S, Ossowska K, Grabowska-Andén M, Andén NE. (1991) Effect of the NMDA receptor antagonist, MK-801, on locomotor activity and on the metabolism of dopamine in various brain areas of mice. Eur J Pharmacol 195:55–61. [DOI] [PubMed] [Google Scholar]
- Lindefors N, Barati S, O’Connor WT. (1997) Differential effects of single and repeated ketamine administration on dopamine, serotonin and GABA transmission in rat medial prefrontal cortex. Brain Res 759:205–212. [DOI] [PubMed] [Google Scholar]
- Lorrain DS, Baccei CS, Bristow LJ, Anderson JJ, Varney MA. (2003) Effects of ketamine and N-methyl-D-aspartate on glutamate and dopamine release in the rat prefrontal cortex: modulation by a group II selective metabotropic glutamate receptor agonist LY379268. Neuroscience 117:697–706. [DOI] [PubMed] [Google Scholar]
- Löscher W, Annies R, Hönack D. (1991) The N-methyl-D-aspartate receptor antagonist MK-801 induces increases in dopamine and serotonin metabolism in several brain regions of rats. Neurosci Lett 128:191–194. [DOI] [PubMed] [Google Scholar]
- Maina FK, Mathews TA. (2010) A functional fast scan cyclic voltammetry assay to characterize dopamine D2 and D3 autoreceptors in the mouse striatum. ACS Chem Neurosci 1:450–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew SJ, Shah A, Lapidus K, Clark C, Jarun N, Ostermeyer B, Murrough JW. (2012) Ketamine for treatment-resistant unipolar depression: current evidence. CNS Drugs 26:189–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheletti G, Lannes B, Haby C, Borrelli E, Kempf E, Warter JM, Zwiller J. (1992) Chronic administration of NMDA antagonists induces D2 receptor synthesis in rat striatum. Brain Res Mol Brain Res 14:363–368. [DOI] [PubMed] [Google Scholar]
- Moaddel R, Abdrakhmanova G, Kozak J, Jozwiak K, Toll L, Jimenez L, Rosenberg A, Tran T, Xiao Y, Zarate CA, et al. (2013) Sub-anesthetic concentrations of (R,S)-ketamine metabolites inhibit acetylcholine-evoked currents in α7 nicotinic acetylcholine receptors. Eur J Pharmacol 698:228–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moaddel R, Sanghvi M, Dossou KS, Ramamoorthy A, Green C, Bupp J, Swezey R, O’Loughlin K, Wainer IW. (2015) The distribution and clearance of (2S,6S)-hydroxynorketamine, an active ketamine metabolite, in Wistar rats. Pharmacol Res Perspect 3:e00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moaddel R, Sanghvi M, Ramamoorthy A, Jozwiak K, Singh N, Green C, O’Loughlin K, Torjman M, Wainer IW. (2016) Subchronic administration of (R,S)-ketamine induces ketamine ring hydroxylation in Wistar rats. J Pharm Biomed Anal 127:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moaddel R, Venkata SL, Tanga MJ, Bupp JE, Green CE, Iyer L, Furimsky A, Goldberg ME, Torjman MC, Wainer IW. (2010) A parallel chiral-achiral liquid chromatographic method for the determination of the stereoisomers of ketamine and ketamine metabolites in the plasma and urine of patients with complex regional pain syndrome. Talanta 82:1892–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D. (1997) Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci 17:2921–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CJ, Curran HV, Independent Scientific Committee on Drugs (2012) Ketamine use: a review. Addiction 107:27–38. [DOI] [PubMed] [Google Scholar]
- Morikawa H, Paladini CA. (2011) Dynamic regulation of midbrain dopamine neuron activity: intrinsic, synaptic, and plasticity mechanisms. Neuroscience 198:95–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, Iqbal S, Pillemer S, Foulkes A, Shah A, et al. (2013) Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry 170:1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwstraten CE, Dolovich LR. (2001) Bupropion versus selective serotonin-reuptake inhibitors for treatment of depression. Ann Pharmacother 35:1608–1613. [DOI] [PubMed] [Google Scholar]
- Nishimura M, Sato K. (1999) Ketamine stereoselectively inhibits rat dopamine transporter. Neurosci Lett 274:131–134. [DOI] [PubMed] [Google Scholar]
- Nishimura M, Sato K, Okada T, Yoshiya I, Schloss P, Shimada S, Tohyama M. (1998) Ketamine inhibits monoamine transporters expressed in human embryonic kidney 293 cells. Anesthesiology 88:768–774. [DOI] [PubMed] [Google Scholar]
- Overton P, Clark D. (1992) Iontophoretically administered drugs acting at the N-methyl-D-aspartate receptor modulate burst firing in A9 dopamine neurons in the rat. Synapse 10:131–140. [DOI] [PubMed] [Google Scholar]
- Overton PG, Clark D. (1997) Burst firing in midbrain dopaminergic neurons. Brain Res Brain Res Rev 25:312–334. [DOI] [PubMed] [Google Scholar]
- Papakostas GI. (2006) Dopaminergic-based pharmacotherapies for depression. Eur Neuropsychopharmacol 16:391–402. [DOI] [PubMed] [Google Scholar]
- Parsons CG, Quack G, Bresink I, Baran L, Przegalinski E, Kostowski W, Krzascik P, Hartmann S, Danysz W. (1995) Comparison of the potency, kinetics and voltage-dependency of a series of uncompetitive NMDA receptor antagonists in vitro with anticonvulsive and motor impairment activity in vivo. Neuropharmacology 34:1239–1258. [DOI] [PubMed] [Google Scholar]
- Paul RK, Singh NS, Khadeer M, Moaddel R, Sanghvi M, Green CE, O’Loughlin K, Torjman MC, Bernier M, Wainer IW. (2014) (R,S)-Ketamine metabolites (R,S)-norketamine and (2S,6S)-hydroxynorketamine increase the mammalian target of rapamycin function. Anesthesiology 121:149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao TS, Kim HS, Lehmann J, Martin LL, Wood PL. (1989) Differential effects of phencyclidine (PCP) and ketamine on mesocortical and mesostriatal dopamine release in vivo. Life Sci 45:1065–1072. [DOI] [PubMed] [Google Scholar]
- Seeman P, Ko F, Tallerico T. (2005) Dopamine receptor contribution to the action of PCP, LSD and ketamine psychotomimetics. Mol Psychiatry 10:877–883. [DOI] [PubMed] [Google Scholar]
- Shulman KI, Herrmann N, Walker SE. (2013) Current place of monoamine oxidase inhibitors in the treatment of depression. CNS Drugs 27:789–797. [DOI] [PubMed] [Google Scholar]
- Smith DJ, Bouchal RL, deSanctis CA, Monroe PJ, Amedro JB, Perrotti JM, Crisp T. (1987) Properties of the interaction between ketamine and opiate binding sites in vivo and in vitro. Neuropharmacology 26:1253–1260. [DOI] [PubMed] [Google Scholar]
- Stamford JA, Kruk ZL, Millar J. (1991) Differential effects of dopamine agonists upon stimulated limbic and striatal dopamine release: in vivo voltammetric data. Br J Pharmacol 102:45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso MM, Blatchford KL, Callado LF, McLaughlin DP, Stamford JA. (2004) Stereoselective effects of ketamine on dopamine, serotonin and noradrenaline release and uptake in rat brain slices. Neurochem Int 44:1–7. [DOI] [PubMed] [Google Scholar]
- Verma A, Moghaddam B. (1996) NMDA receptor antagonists impair prefrontal cortex function as assessed via spatial delayed alternation performance in rats: modulation by dopamine. J Neurosci 16:373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M, Motin L, Gaul S, Beker F, Fink RH, Adams DJ. (2005) Intravenous anaesthetics inhibit nicotinic acetylcholine receptor-mediated currents and Ca2+ transients in rat intracardiac ganglion neurons. Br J Pharmacol 144:98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P, Hale AS, Argyropoulos S. (2005) Dopaminergic mechanism of antidepressant action in depressed patients. J Affect Disord 86:37–45. [DOI] [PubMed] [Google Scholar]
- Witkin JM, Monn JA, Schoepp DD, Li X, Overshiner CD, Mitchell SN, Carter G, Johnson B, Rasmussen K, Rorick-Kehn LM. (2016) The rapidly acting antidepressant ketamine and the mGlu2/3 receptor antagonist LY341495 rapidly engage dopaminergic mood circuits. J Pharmacol Exp Ther 358:71–82. [DOI] [PubMed] [Google Scholar]
- Wolf ME, White FJ, Hu XT. (1993) Behavioral sensitization to MK-801 (dizocilpine): neurochemical and electrophysiological correlates in the mesoaccumbens dopamine system. Behav Pharmacol 4:429–442. [PubMed] [Google Scholar]
- Wooters TE, Dwoskin LP, Bardo MT. (2011) Discriminative stimulus effects of NMDA, AMPA, and mGluR5 glutamate receptor ligands in methamphetamine-trained rats. Behav Pharmacol 22:516–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakura T, Chavez-Noriega LE, Harris RA. (2000) Subunit-dependent inhibition of human neuronal nicotinic acetylcholine receptors and other ligand-gated ion channels by dissociative anesthetics ketamine and dizocilpine. Anesthesiology 92:1144–1153. [DOI] [PubMed] [Google Scholar]
- Yorgason JT, España RA, Jones SR. (2011) Demon voltammetry and analysis software: analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. J Neurosci Methods 202:158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, et al. (2016) NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533:481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, Selter J, Marquardt CA, Liberty V, Luckenbaugh DA. (2012) Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry 71:939–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Payne JL, Singh J, Quiroz JA, Luckenbaugh DA, Denicoff KD, Charney DS, Manji HK. (2004) Pramipexole for bipolar II depression: a placebo-controlled proof of concept study. Biol Psychiatry 56:54–60. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. (2006) A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63:856–864. [DOI] [PubMed] [Google Scholar]
- Zweifel LS, Parker JG, Lobb CJ, Rainwater A, Wall VZ, Fadok JP, Darvas M, Kim MJ, Mizumori SJY, Paladini CA, et al. (2009) Disruption of NMDAR-dependent burst firing by dopamine neurons provides selective assessment of phasic dopamine-dependent behavior. Proc Natl Acad Sci USA 106:7281–7288. [DOI] [PMC free article] [PubMed] [Google Scholar]