Abstract
Trichloroethylene (TCE) and perchloroethylene or tetrachloroethylene (PCE) are high–production volume chemicals with numerous industrial applications. As a consequence of their widespread use, these chemicals are ubiquitous environmental contaminants to which the general population is commonly exposed. It is widely assumed that TCE and PCE are toxicologically similar; both are simple olefins with three (TCE) or four (PCE) chlorines. Nonetheless, despite decades of research on the adverse health effects of TCE or PCE, few studies have directly compared these two toxicants. Although the metabolic pathways are qualitatively similar, quantitative differences in the flux and yield of metabolites exist. Recent human health assessments have uncovered some overlap in target organs that are affected by exposure to TCE or PCE, and divergent species- and sex-specificity with regard to cancer and noncancer hazards. The objective of this minireview is to highlight key similarities, differences, and data gaps in target organ metabolism and mechanism of toxicity. The main anticipated outcome of this review is to encourage research to 1) directly compare the responses to TCE and PCE using more sensitive biochemical techniques and robust statistical comparisons; 2) more closely examine interindividual variability in the relationship between toxicokinetics and toxicodynamics for TCE and PCE; 3) elucidate the effect of coexposure to these two toxicants; and 4) explore new mechanisms for target organ toxicity associated with TCE and/or PCE exposure.
Exposures: Sources, Occurrence, Occupational, Environmental, and Coexposures
Trichloroethylene (TCE) and tetrachloroethylene (perchloroethylene; PCE) are halogenated olefin solvents that are high-volume production chemicals with a broad range of industrial uses. The main uses of both TCE and PCE are in metal degreasing and as a feed stock in the production of chlorinated chemicals (Guha et al., 2012). Since the 1950s, the use of TCE as a dry cleaning solvent has ceased, while PCE is still widely used for that application (IARC, 2014). Human exposure to TCE and PCE is assumed to be mostly through inhalation, owing to their low solubility in water and high vapor pressures. As volatile chemicals with very little water solubility, TCE and PCE are common contaminants in ambient and urban air, although they are also commonly found in ground and drinking water (IARC, 2014). At National Priority List sites in the United States, TCE is the most commonly found groundwater contaminant, and cocontamination with TCE and PCE is common (Fay and Mumtaz, 1996). In a relatively recent evaluation of contaminants found at hazardous waste sites in the United States, TCE and PCE were the most commonly found cocontaminants (11.6% of sites evaluated) out of all chemical combinations assessed (Pohl et al., 2008). Although dermal exposure through direct contact with contaminated water is possible (e.g., during showering), this is not thought to be a major route of exposure (US EPA, 2011b; US EPA, 2011a). Data from the National Health and Nutrition Examination Survey (NHANES) from 1999-2000 show that mean concentrations (in μg/m3) of TCE and PCE in exhaled air from human subjects were similar (3.48 and 3.16, respectively) (Jia et al., 2012). Concentrations of PCE in the blood were about 10-fold higher than TCE (0.138 ± 0.023 and 0.013 ± 0.002 ng/ml, respectively; mean ± S.E.). Similarly, in the most recent NHANES data from 2005-2006, blood PCE levels were higher than blood TCE levels (0.289 ± 0.087 and 0.116 ± 0.041 ng/mL, respectively; mean ± S.E.) (CDC, 2011).
Recent Human Health Assessments of TCE and PCE
Recently, human cancer and noncancer risks of TCE and PCE were evaluated by the US Environmental Protection Agency (US EPA), the International Agency for Research on Cancer (IARC), the National Toxicology Program (NTP), and the California Environmental Protection Agency via the Office of Environmental Health Hazard Assessment (OEHHA) Air Toxics Hot Spots Program. Results are summarized below and in Tables 1 and 3.
TABLE 1.
Cancer and noncancer toxicity values for TCE and PCE
| TCEa | PCEb | ||
|---|---|---|---|
| Cancer risk | Effect | Kidney cancer (human), adjusted for liver/hematopoietic cancer risk | Hepatocellular tumors (mouse) |
| Oral slope factor | 5 × 10−2 per mg/kg-day | 2 × 10−3 per mg/kg-day | |
| Inhalation unit risk | 2 × 10−2 per ppm | 2 × 10−3 per ppm | |
| Noncancer risk | Effect | Cardiac malformations (mouse), immunologic effects (mouse), developmental immunotoxicity (mouse), toxic nephropathy (rat) | Visuospatial deficits (human) |
| RfD | 5 × 10−4 mg/kg-day | 6 × 10−3 mg/kg-day | |
| RfC | 4 × 10−4 ppm | 5.8 × 10−3 ppm |
TABLE 3.
Strength of evidence for tissue-specific cancer hazard effects associated with exposure to TCE or PCEa
| Cancer Type | Human |
Evidence in Rodents |
Summary of Evidence by US EPA |
|||
|---|---|---|---|---|---|---|
| TCE | PCE | TCE | PCE | TCEb | PCE | |
| Kidney cancer; renal cell carcinoma; renal tubular cell adenoma or adenocarcinoma | Sufficient | Inadequate | + | + c | “Convincing evidence of a causal association” | “Limited” epidemiologic evidenced |
| Liver cancer; hepatocellular carcinoma/adenoma | Limited | Inadequate | + | + | “More limited” [compared with kidney cancer] | “Suggestive but limited evidence”e |
| Non-Hodgkin lymphoma | Limited | Inadequate | + | +f | Evidence is “strong…but less convincing than for kidney cancer” | Evidence “indicate an elevated risk”e |
| Bladder cancer | N/A | Limited | — | — | N/A | “Pattern of evidence associating tetrachloroethylene exposure” and bladder cancere |
| Overall human cancer hazard classification | IARC: Group 1g | IARC: Group 2Ag | ||||
| US EPA: Knowng | US EPA: Probablyg | |||||
| NTP: Known to be a human carcinogeng | ||||||
Sufficient defined as “a causal relationship has been established between exposure to the agent and human cancer” (IARC, 2006); Limited defined as “a positive association has been observed between exposure to the agent and cancer for which a causal interpretation is considered to be credible, but chance, bias, or confounding could not be ruled out with reasonable confidence.” (IARC, 2006); Inadequate defined as “the available studies are of insufficient quality, consistency or statistical power to permit a conclusion regarding the presence or absence of a causal association between exposure and cancer, or no data on cancer in humans are available” (IARC, 2006); +, a statistically significant increased incidence of benign or malignant neoplasms was observed in at least one study; N/A, not applicable.
Tissues shown represent those for which limited or sufficient evidence of human cancer hazard effects associated with TCE or PCE were available, as determined by IARC (IARC, 2014)
Section 6.3 “Overall characterization of TCE Hazard and Dose Response” (US EPA, 2011b)
Renal cell adenoma/carcinoma observed in rats exposed to PCE for two years, however this was only statistically significant when compared with historical controls (NTP, 1986).
Section 4.2.1.2.2. Summary of results of Kidney Cancer in Humans (US EPA, 2011a)
Section 4.10.4. Synthesis of Epidemiologic Studies for Summary of Hazard Identification (US EPA, 2011a)
Evidence of other hematopoietic cancers observed in rodents (e.g., mononuclear cell leukemia in studies of PCE)
Group 1, carcinogenic to humans (IARC); Group 2A, probably carcinogenic to humans (IARC, 2006); Known, known human carcinogen (US EPA); Likely, likely carcinogenic to human (US EPA).
The cancer hazard of TCE was evaluated by IARC (IARC, 2014), the US EPA (US EPA, 2011b), and NTP (NTP, 2015). The conclusion of all three assessments is that there is sufficient evidence that TCE is a human carcinogen, with the kidney being the target tissue. Limited evidence, on the basis of positive associations observed with TCE exposure, was found for non-Hodgkin lymphoma (NHL) and related cancers (including Chronic Lymphocytic Leukemia, Multiple Myeloma, Hairy Cell Leukemia) and liver cancer. Using the risk for renal cell carcinoma in humans, adjusted for the potential risk of NHL and liver cancer associated with TCE exposure, the US EPA established an inhalation unit risk of 2 × 10−2 per ppm (5 × 10−2 per mg/kg-day via oral exposure).
The US EPA also evaluated the noncancer toxicity associated with TCE exposure (US EPA, 2011b). In their risk assessment, they identified central nervous system, kidney, liver, immune system, male reproductive system, and developmental toxicity associated with TCE exposure. There was also some evidence of TCE-associated toxicity in the respiratory tract and female reproductive tract. The most sensitive of these organs or systems to noncancer effects of TCE, based on reference concentration in the air (RfC) or reference dose via oral ingestion (RfD), are the kidney, the immune system, and the developing fetus (RfC = 0.0004 ppm, RfD = 0.0005 mg/kg-day).
The cancer hazard of PCE has also recently been evaluated by IARC (IARC, 2014), US EPA (US EPA, 2011a), and OEHHA (http://www.oehha.ca.gov/air/hot_spots/pdf/finalpublicreviewPCE_UR_TSD02162016.pdf). Limited epidemiologic data exist to support an association between PCE exposure and cancer of the bladder. No consistent pattern was observed for cancer of the esophagus, kidney, or cervix, and for NHL. Combined with sufficient evidence of carcinogenicity in experimental animals, PCE was classified by IARC as a probable human carcinogen [Group 2A, (IARC, 2014)]. Based on rodent liver tumor data, adjusted for interspecies differences in metabolism, the US EPA derived a cancer inhalation unit risk of 1.8 × 10−3 per ppm (2.1 × 10−3 per mg/kg-day via oral exposure) (US EPA, 2011a). The OEHHA draft risk assessment of PCE is currently under public review and has proposed an inhalation unit risk factor of 4.1 × 10−2 per ppm (2.1 × 10−2 per mg/kg-day via oral exposure) (CalEPA, 2016). For noncancer toxicity in humans, the US EPA evaluated neurotoxicity as the most sensitive pathway associated with PCE exposure. Specific effects of PCE used in deriving the RfC and RfD were visuospatial deficits, including changes in color vision and reaction time in exposed humans (RfC = 0.0058 ppm, RfD = 0.006 mg/kg-day).
Metabolism and Metabolites
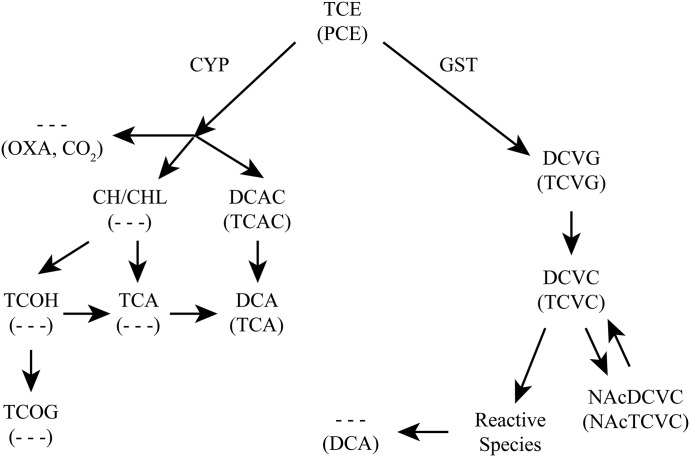
A simplified schematic of TCE and PCE metabolism is shown in Fig. 1. Additionally, Table 2 presents metabolites, their formation in target tissues, and indicates whether they are systemically available.
Fig. 1.
Metabolic pathways of TCE and PCE. Major metabolites of TCE (top) and PCE (bottom, in parentheses) are shown through P450-mediated oxidation and GSH conjugation. CYP, cytochrome P450; OXA, oxalate; TCAC, trichloroacetyl chloride; TCOG, trichloroethanol O-glucuronide.
TABLE 2.
Tissue specificity of TCE and PCE metabolites
| Target Tissue | Oxidative Pathway |
GSH Conjugation Pathway |
||
|---|---|---|---|---|
| TCE | PCE | TCE | PCE | |
| Liver: local formation | CH/CHL | DCVG | TCVG | |
| TCA | TCA | DCVC | TCVC | |
| TCOH | NAcDCVC | NAcTCVC | ||
| TCOG | ||||
| DCAC | TCAC | |||
| DCA | ||||
| Kidney: local formation | — | — | DCVG | TCVG |
| DCVC | TCVC | |||
| NAcDCVC | NAcTCVC | |||
| Reactive metabolites | Reactive metabolites | |||
| DCA | ||||
| Lung: local formation | CH/CHL | — | — | |
| TCA | TCA | |||
| TCOH | ||||
| DCAC | TCAC | |||
| DCA | ||||
| Testes: local formation | CH/CHL | — | — | |
| TCA | TCA | |||
| TCOH | ||||
| DCAC | TCAC | |||
| DCA | ||||
| Systemically available | CH/CHL | DCVG | TCVC | |
| TCA | TCA | DCVC | TCVC | |
| TCOH | NAcDCVC | NAcTCVC | ||
| TCOG | DCA | |||
| DCA | ||||
TCOG, trichloroethanol O-glucuronide.
Absorption and Distribution.
As small, lipophilic chemicals, TCE and PCE can readily cross biologic membranes. TCE and PCE are also slightly soluble in human blood, with blood/air partition coefficients ranging from approximately 10 (TCE) to approximately 15 (PCE), as summarized in the recent IARC monograph (IARC, 2014). As both chemicals have low solubility in water, this partitioning into the blood is mediated by binding to other blood components, such as lipids. Dermal absorption of TCE and PCE vapor and liquid is considered a limited pathway for exposure to either chemical for most individuals (McDougal et al., 1990). Pulmonary uptake of both chemicals is also rapid, with steady-state levels being attained within a few hours after the start of exposure (IARC, 2014). Both TCE and PCE rapidly partition into lipid-containing tissue beds upon absorption into the body. This is attributable to their extremely high fat/blood partition coefficients, with the fat/blood partition coefficients ranging from 52-64 for TCE and 125 for PCE, as summarized in the IARC monograph (IARC, 2014).
Oxidative Metabolism.
As shown in Fig. 1, TCE and PCE have qualitatively similar metabolic schemes in rodents and humans (IARC, 2014). Metabolism of TCE and PCE can result in both intoxication and detoxication. Hepatic cytochrome P450 2E1 (CYP2E1) is proposed to be the main contributor to oxidative metabolism of TCE, although other P450s are also involved (Lash and Parker, 2001; Lash et al., 2014). Oxidative metabolism of PCE has also been attributed primarily to CYP2E1 activity owing to its structural similarity to other CYP2E1 substrates (Lash and Parker, 2001), although this is yet to be confirmed experimentally. Although the liver is the main contributor to oxidation of both TCE and PCE (Table 2), extrahepatic oxidative metabolism can occur, for instance in the testes and lungs (Forkert et al., 1985; Forkert et al., 2002). For TCE, the first step in its oxidative metabolism is formation of an unstable intermediate (TCE-O–P450) that can lead to production of N-hydroxy-acetylaminoethanol, chloral/chloral hydrate (CHL/CH), or TCE-epoxide (TCE-O). TCE-O can spontaneously form dichloroacetyl chloride (DCAC) or oxalic acid. DCAC can dechlorinate spontaneously to dichloroacetic acid (DCA). The fate of CHL/CH is either reduction by aldehyde dehydrogenases or P450s to trichloroethanol (TCOH), or oxidation to trichloroacetic acid (TCA). TCA can also be formed from P450-mediated oxidation of TCOH. TCOH exists as both the free alcohol and TCOH-glucuronide. Although some overlap exists in the oxidative metabolism of TCE and PCE, formation of CH/CHL, TCOH (glucuronidated and free), and DCA via this pathway is observed only following TCE toxicity. In PCE-exposed rodents, DCA is formed locally in the kidney as a result of the glutathione (GSH) conjugation pathway (Fig. 1).
Conjugative Metabolism.
Both liver and kidney have the capacity to conjugate GSH with TCE or PCE (Lash et al., 1998a,b). For both TCE and PCE, metabolic flux through oxidation has been shown to greatly exceed that through GSH conjugation in experimental animals. The specific glutathione S-transferases (GSTs) involved in hepatic or renal conjugation of GSH to TCE or PCE are unknown. It is suggested that GST polymorphisms could play a role in interindividual variability in susceptibility to TCE- (and likely PCE-) associated toxicity. Polymorphisms in several isoforms of GST from GST-μ (GSTM), GST-θ (GSTT), and GST-π (GSTP) families have been implicated in increased susceptibility to TCE toxicity, however inconsistencies exist in the literature (US EPA, 2011b). Di- and trichlorovinyl-l-glutathione (DCVG and TCVG) are thought to be primarily formed in the liver. After leaving the liver, the GSH conjugates are hydrolyzed by γ-glutamyltransferase (GGT) and cysteinylglycine dipeptidase on the brush-border membrane of the proximal tubule of the kidney. The cleaved products, di- or trichlorovinyl-l-cysteine (DCVC or TCVC), are formed from DCVG or TCVG, respectively. DCVC and TCVC can then be enzymatically converted to reactive metabolites, via the cysteine conjugate β-lyase (CCBL) or flavin-containing monooxygenases, which are hypothesized to be involved in renal injury associated with exposure to TCE and PCE (Lash and Parker, 2001; Lash et al., 2001, 2002, 2007). DCVC and TCVC can also be converted to mercapturic acids via N-acetylation and subsequent urinary elimination, which is generally a detoxification pathway; however, a deacetylase can regenerate the cysteine conjugate.
Comparison of Oxidative and Conjugative Pathways.
In humans and experimental animals, the total flux of TCE and PCE through oxidative metabolism is thought to considerably exceed that through conjugative metabolism. For TCE, major metabolites are TCOH and TCA; for PCE, TCA is the major metabolite. This is based on human data (Bernauer et al., 1996; Volkel and Dekant, 1998; Lash et al., 1999) and in vivo and in vitro experimental rodent data (Lash and Parker, 2001; Bradford et al., 2011; Lash et al., 2014; Yoo et al., 2015a,b). In both humans and experimental animals, it is evident that exposure to TCE or PCE results in larger urinary levels of oxidative TCE/PCE metabolites compared with mercapturic acids derived from the GSH conjugation pathway. However, as conjugative metabolites of TCE and PCE can form reactive metabolites that may rapidly bind to cellular macromolecules, specifically in the kidney, there is potential to considerably underestimate the flux of TCE or PCE through conjugative metabolism in humans and laboratory rodents (Chiu et al., 2007, 2014; Chiu and Ginsberg, 2011). For this reason, considerable uncertainty exists in estimates of conjugation of TCE (Chiu et al., 2009) or PCE (Chiu and Ginsberg, 2011), particularly in humans at environmental exposure levels.
Excretion.
Exhalation of parent TCE or PCE after exposure is a major route of excretion. For TCE, human physiologically based pharmacokinetic (PBPK)-model estimates predict 60–70% of the dose is cleared via exhaled air (Chiu et al., 2007) in humans. For PCE, human PBPK-model estimates predict 90–99% of the inhaled dose or 81–99% of the ingested dose is excreted into the air (Chiu and Ginsberg, 2011). The increased absorption of TCE relative to PCE is probably the result of the increased capacity for the liver to oxidize TCE relative to PCE. Additionally, at higher exposures when metabolism becomes saturated, the fraction of excretion via exhalation of the parent compound increases.
Gaps in Data on Metabolic Fate of TCE and PCE.
Although the metabolism of TCE and PCE has been widely studied, data gaps still exist in the specific forms of P450s, GSTs, and other enzyme families that contribute to the metabolism of these toxicants. Additionally, although there is evidence that factors such as sex, age, genotype, lifestyle, body composition, and coexposure to other environmental chemicals will modify the toxicokinetics and toxicodynamics of organic solvents such as TCE and PCE (Lof and Johanson, 1998; Pastino et al., 2000; National Research Council, 2009), the effect of these factors remain poorly characterized quantitatively. Future work on TCE and PCE could clarify the quantitative contribution of these factors to interindividual variability in toxicokinetics of these chemicals.
Cancer
Table 3 summarizes the target organ carcinogenesis associated with TCE or PCE exposure. These data are derived from previous reports for TCE (Zhu et al., 2008; US EPA, 2011b; Chiu et al., 2013; IARC, 2014; National Toxicology Program, 2015) and PCE (US EPA, 2011a; IARC, 2014; Guyton et al., 2014).
Kidney Cancer.
TCE has been classified as a known human carcinogen by the US EPA (US EPA, 2011b), “carcinogenic to humans (Group 1)” by IARC (IARC, 2014), and “known to be a human carcinogen” by the NTP (National Toxicology Program, 2015). All three agencies independently used as a basis for their overall hazard classification of TCE the strength of the epidemiologic evidence for kidney cancer and were able to exclude chance, bias, and confounding with reasonable confidence as an explanation for a causal association. The IARC Group 1 classification of TCE was also supported by strong mechanistic evidence.
The epidemiologic data consistently demonstrate an increased risk of kidney cancer associated with exposure to TCE irrespective of the study design (case-control and cohort studies), geographic region in which the study was conducted, or exposure setting. Exposure settings include occupational exposures in the aerospace and screw-cutting industries and exposure to the general population occurs through contaminated air, water, and soil. An increased risk of kidney cancer was reported in all of the studies in which there was a moderate to very high level of estimated TCE exposure (Henschler et al., 1995; Morgan et al., 1998; Vamvakas et al., 1998; Brüning et al., 2003; Raaschou-Nielsen et al., 2003; Zhao et al., 2005; Boice et al., 2006; Charbotel et al., 2006; Radican et al., 2008; Moore et al., 2010; Hansen et al., 2013), with most risk estimates being statistically significant. Additionally, positive exposure-response relationships were reported in several case-control (Charbotel et al., 2006; Moore et al., 2010) and cohort studies (Zhao et al., 2005; Raaschou-Nielsen et al., 2003) using different exposure metrics. In Moore et al. (2010), statistically significant associations between occupational exposure to TCE and renal cell carcinoma were only observed in individuals with an active GSTT1 genotype and certain CCBL1 genotypes. Two meta-analyses (Scott and Jinot, 2011; Karami et al., 2012) support the observations from individual studies, reporting an approximately 30% increased risk of kidney cancer associated with TCE exposure as well as a positive exposure-response relationship. The meta-relative risks [1.27 (95% CI, 1.13–1.43) in Scott and Jinot (2011) and 1.32 (95% CI, 1.17-1.50) in Karami et al. (2012)] were robust with respect to sensitivity analyses, and there was no evidence of publication bias or significant heterogeneity across studies. The consistency of the positive association across study designs, geographic locations and exposure settings, as well as the positive exposure-response relationship and the results of the meta-analyses argue against chance as a possible explanation for the causal association.
Tobacco smoking is unlikely to confound the association between TCE exposure and kidney cancer because: 1) it is a relatively weak risk factor for kidney cancer, 2) the relative risks for lung cancer were not elevated in most cohorts, and 3) a meta-analysis showed no association between TCE exposure and lung cancer (Scott and Jinot, 2011). Confounding by other lifestyle factors or occupational coexposures (e.g., mineral oils) can also be ruled out because adjustment for these variables had little impact on the risk estimates and they are not known or suspected renal carcinogens. Selection bias could not be ruled out in two of the studies reporting the highest risk estimates (Henschler et al., 1995, Vamvakas et al., 1998); however, these studies do not affect the overall conclusion as they were not included in the meta-analyses.
In experimental animals tumors were observed in multiple tissues of both male and female mice and rats following a two-year TCE exposure. Renal cell tubular-cell adenomas or adenocarcinomas were reported in male and female rats after chronic exposure to TCE (Maltoni et al., 1988; National Toxicology Program, 1988, 1990).
Similarly, PCE-induced renal cell tubular-cell adenoma or adenocarcinoma was seen in male rats (National Toxicology Program, 1986). In humans, however, evidence for PCE-induced kidney cancer is inadequate (US EPA, 2011a; IARC, 2014) owing to inconsistencies in the results within and across studies and limitations with study methodology. Generally, the cohort studies did not demonstrate an association, whereas all of the case-control studies of kidney cancer reported a positive association with PCE exposure.
Most of the informative cohorts focused on dry-cleaning and related occupations, where PCE was the primary exposure. The results from the cohort studies were inconsistent: the risk estimates for kidney cancer associated with ever being exposed to PCE were above one in two studies (Anttila et al., 1995; Calvert et al., 2011), fewer than one in three studies (Boice et al., 1999; Selden and Ahlborg, 2011; Silver et al., 2014), and equal to one in one study (Blair et al., 2003; although the relative risk was elevated in workers with medium/high exposure). Increased mortality from kidney cancer was associated with PCE exposure through contaminated drinking water at Camp LeJeune (Bove et al., 2014). The largest cohort study conducted in four Nordic countries, which linked occupation from censuses to National Cancer Registry data (Vlaanderen et al., 2013) and included 76,130 cases of kidney cancer (1,022 exposed to PCE), showed no association between PCE exposure and kidney cancer.
All of the seven case-control studies of kidney cancer (Asal et al., 1988, Delahunt et al., 1995; Mandel et al., 1995; Dosemeci et al., 1999, Pesch et al., 2000, Karami et al., 2012, Christensen et al., 2013) reported positive associations with PCE exposure (primarily from work in dry-cleaning), in men, women, or both; however, selection bias and limitations in the exposure assessment were concerns. Only three of these studies reported statistically significant results (Delahunt et al., 1995; Mandel et al., 1995; Pesch et al., 2000) and only one study reported positive monotonic trends (Karami et al., 2012).
Cancer of the Lympho-Hematopoietic System.
A modest increase in the risk of NHL and related B-cell lymphomas (including chronic lymphocytic leukemia, hairy cell leukemia, and multiple myeloma) associated with TCE exposure has been observed in several studies, across study designs and geographic locations. Most studies reported relative risks above one (Raaschou- Morgan et al., 1998; Persson and Fredrikson, 1999;Raaschou-Nielsen et al., 2003; Radican et al., 2008; Wang et al., 2009; Lipworth et al., 2011; Christensen et al., 2013; Cocco et al., 2013; Hansen et al., 2013). However, no association was found in some studies, which could be attributed to methodological limitations in the design or analysis or low prevalence of TCE exposure [Boice et al., 2006 (based on 1 case); Bahr et al., 2011; Vlaanderen et al., 2013; Bove et al., 2014; Silver et al., 2014]. Although the results for NHL are less consistent than for kidney cancer, it should be noted that interpretation of the published literature is complicated by the change over time in the classification and coding systems of NHL and its related subtypes (American Cancer Society, 2016).
The pooled analysis of case-control studies from the large International Lymphoma Epidemiology Consortium (Cocco et al., 2013) provided the strongest evidence of an association: the odds ratio for high probability of TCE exposure was 1.4 (95% CI 0.9–2.1) and there was a positive exposure-response relationship (P value for trend = 0.009). Two recent meta-analyses (Scott and Jinot, 2011; Karami et al., 2012), neither of which included the most recent publications discussed above (Hansen et al., 2013; Vlaanderen et al., 2013; Bove et al., 2014; Silver et al., 2014), also reported statistically significant increased risks for NHL associated with TCE exposure. The meta–relative risk estimates of 1.23 (95% CI 1.07–1.42) (Scott and Jinot, 2011) and 1.32 (95% CI 1.14–1.54) (Karami et al., 2012) were relatively robust compared to various sensitivity analyses, although there was a small amount of heterogeneity between studies and some evidence of publication bias. Potential confounding by occupational coexposures and other factors could not be ruled out with reasonable confidence but would probably not explain the association given that none of the documented coexposures have been identified as risk factors for NHL (e.g., organic solvents) and because a significant exposure-response was observed in the International Lymphoma Epidemiology Consortium study, in which there was no evidence that other potential coexposures were highly correlated with TCE exposure.
Reports of PCE-associated cancer of the lympho-hematopoietic system, particularly NHL, were inconsistent (IARC, 2014) from several cohort and case-control studies. In rodent studies, mononuclear cell leukemia in male and female rats increased following chronic exposure to PCE (National Toxicology Program, 1986; Japanese Industrial Safety Association, 1993).
Liver Cancer.
Several cohort studies and one case-control study (Christensen et al., 2013) have assessed the association between TCE exposure and liver cancer, with a relative risk above one being reported in several studies (Morgan et al., 1998; Raaschou-Nielsen et al., 2003; Boice et al., 2006; Radican et al., 2008; Christensen et al., 2013; Hansen et al., 2013) and a meta-analysis (Scott and Jinot, 2011). However, the evidence was limited, as some studies were underpowered to detect an effect, because liver cancer is a rare outcome or because the prevalence of TCE exposure was low, failed to adjust for potential confounders (e.g., alcohol consumption), did not establish an exposure-response relationship, or nondifferential exposure misclassification was a concern (which would bias risk estimates toward the null) (IARC, 2014).
For PCE, the epidemiologic evidence was sparse and the data were considered to be inadequate (IARC, 2014). Six cohort studies (Bond et al., 1990; Blair et al., 2003; Calvert et al., 2011; Selden and Ahlborg, 2011; Vlaanderen et al., 2013; Silver et al., 2014) and two case-control studies reported on this association (Suarez et al., 1989; Christensen et al., 2013), but the findings were inconsistent.
In rodents, hepatocellular carcinoma and/or adenoma (HCC/HCA) have been observed in both sexes of mice following exposure to TCE (National Toxicology Program, 1976, 1988, 1990; Maltoni et al., 1988) or PCE (National Toxicology Program, 1977, 1986; Japanese Industrial Safety Association, 1993). Males are generally the more sensitive sex to liver carcinogenicity induced by TCE or PCE.
Bladder Cancer.
The bladder has been identified as a potential target tissue for PCE-induced carcinogenesis in several cohort and case-control studies conducted in different geographic locations, most of which adequately controlled for confounding by tobacco smoking. However, the evidence was judged as limited because of the relatively crude exposure assessment (employment as a dry-cleaner was the only indicator of PCE exposure in most studies), the small number of exposed cases, and the lack of an exposure-response relationship (IARC, 2014). Similar conclusions were drawn by the US EPA (US EPA, 2011a). A meta-analysis also demonstrated an increased risk of bladder cancer among dry-cleaners overall (meta-relative risk, 1.47; 95% CI, 1.16–1.85) and also when restricted to studies that adjusted for smoking (meta-relative risk, 1.50; 95% CI, 0.80–2.84) (Vlaanderen et al., 2014). Although dry-cleaners incur mixed exposures, PCE could be responsible for the excess bladder cancer risk because it is the primary solvent used and the only chemical commonly used by dry-cleaners that is currently identified as a possible bladder carcinogen. No evidence for urinary bladder carcinogenesis was observed in studies in experimental animals for PCE (or TCE), and there is currently no mechanistic evidence to provide biologic plausibility in support of a hypothesis of PCE-associated bladder cancer.
Other Cancer Sites.
In female rats, chronic PCE exposure induced mammary gland fibroadenomas only in the lowest dose group (Japanese Industrial Safety Association, 1993). Testicular interstitial cell tumors were observed in male rats following TCE (Maltoni et al., 1988; National Toxicology Program, 1988) or PCE (National Toxicology Program, 1986) exposure. PCE also induced brain gliomas in male rats (National Toxicology Program, 1986). In mice, bronchiolar adenomas were induced by TCE (Fukuda et al., 1983; Maltoni et al., 1988).
Noncancer Toxicity
As with the cancer response, a great degree of chemical, tissue, species, and sex specificity exists in the noncancer responses to TCE and PCE exposure, which are summarized in Table 4. Exposure to TCE or PCE is associated with noncancer toxicity in various target organs (US EPA, 2011a,b; IARC, 2014).
TABLE 4.
Tissue-specific noncancer hazard effects associated with exposure to TCE or PCE
Overall strength of evidence was developed on the basis of the US EPA’s Integrative Risk Information System [IRIS; (US EPA, 2011a,b)], whereby the overall strength of human evidence used as a basis data derived from studies in humans and rodents. +, evidence for a positive effect; blank, limited evidence.
| Human |
Rat |
Mouse |
|||||
|---|---|---|---|---|---|---|---|
| Effect | TCE | PCE | TCE | PCE | TCE | PCE | |
| Liver | Hepatitis | + | |||||
| Elevated serum enzymes | + | + | + | + | + | ||
| Hepatomegaly | + | + | + | + | + | ||
| Elevated serum bile acids | + | ||||||
| Lipid alterations | + | + | + | ||||
| Peroxisome proliferation | + | + | + | ||||
| Degeneration and necrosis | + | + | + | ||||
| Karyomegaly | + | ||||||
| Hypertrophy | + | + | + | ||||
| Altered hepatic sonography | + | ||||||
| Overall strength of evidence: | + | + | + | + | + | + | |
| Kidney | Proximal tubular dysfunction | + | + | + | + | ||
| End-stage renal disease | + | + | |||||
| Nephritis/nephrosis | + | + | + | + | |||
| Chronic kidney disease | + | ||||||
| Cytotoxicity | + | + | |||||
| Cytomegaly | + | + | + | + | |||
| Karyomegaly | + | + | + | + | |||
| Meganucleocytosis | + | + | |||||
| Hyaline droplets | |||||||
| Overall strength of evidence: | + | + | + | + | + | + | |
| Central nervous system | Altered trigeminal nerve function | + | |||||
| Visuospatial deficit | + | + | + | + | + | ||
| Demyelination | + | ||||||
| Impaired nerve regeneration | + | + | |||||
| Neurochemical changes | + | + | + | + | |||
| Overall strength of evidence: | + | + | + | + | + | + | |
| Respiratory tract | Dyspnea | + | |||||
| Pulmonary vasculitis | + | ||||||
| Clara cell vacuolation/cytotoxicity | + | ||||||
| Overall strength of evidence: | + | + | |||||
| Hematopoietic system | Hypersensitivity | + | + | + | |||
| Immunosuppression | + | + | + | + | |||
| Autoimmunity | + | + | + | ||||
| Increased serum cytokines | + | ||||||
| Increased T- and NK cells | + | ||||||
| Increased total WBC | + | + | |||||
| Decreased RBC/Hb levels | + | ||||||
| Overall strength of evidence: | + | + | + | + | |||
| Female reproductive tract | Decreased fertility | + | |||||
| Abnormal menstruation | + | + | |||||
| Spontaneous abortion | + | ||||||
| Overall strength of evidence: | |||||||
| Male reproductive tract | Decreased semen quantity/quality | + | + | + | + | + | |
| Altered sexual drive/function | + | + | |||||
| Altered serum testosterone levels | + | + | |||||
| Gynecomastia | + | ||||||
| Pathologic changes | + | + | |||||
| Altered fertilization capacity | + | + | |||||
| Overall strength of evidence: | + | + | + | ||||
| Developmental effects | Cardiac malformations | + | + | ||||
| Prenatal losses/perinatal death | + | + | + | ||||
| Decreased growth | + | + | + | + | + | + | |
| Low birth weight | + | + | + | + | + | + | |
| CNS effects | + | + | + | ||||
| Oral cleft and other musculoskeletal effects | + | + | + | + | + | ||
| Childhood cancers | + | ||||||
| Immunotoxicity | + | ||||||
| Overall strength of evidence: | + | + | + | + | + | + | |
CNS, Central nervous system; Hb, hemoglobin; RBC, red blood cell; WBC, white blood cell.
Kidney Noncancer Toxicity.
Several studies have examined the effect of TCE exposure on risk of noncancer renal injury in humans, as determined by nonspecific urinary protein markers (e.g., α1-microglobulin, albumin, N-acetyl-β-d-glucosaminidase, GST-α, etc.) (Rasmussen et al., 1993b; Brüning et al., 1999a,b; Bolt et al., 2004; Green et al., 2004; National Research Council, 2006). In a recent study conducted in metal degreasing factories in the Guangdong Province of China, occupational exposure to a concentration of TCE (22 ± 35 ppm; mean ± SD) over a mean of 2 years was associated with an increase in urinary excretion of the proximal tubule–specific marker kidney injury molecule-1 (KIM-1) (Vermeulen et al., 2012). However, no association was observed between TCE exposure and urinary levels of N-acetyl-β-d-glucosaminidase or vascular endothelial growth factor. Epidemiologic evidence suggests that PCE is a human nephrotoxicant, based on findings including decreased kidney function (Hake and Stewart, 1977; Franchini et al., 1983; Vyskocil et al., 1990; Solet and Robins, 1991; Mutti et al., 1992; Verplanke et al., 1999; Trevisan et al., 2000) and end-stage renal disease (Calvert et al., 2011).
Laboratory studies using rodents found that both mice and rats of both sexes are susceptible to renal injury following TCE or PCE exposure (US EPA, 2011a,b; IARC, 2014). In vitro evidence suggests that male rats are more sensitive to TCE-associated renal injury than female rats and mice of both sexes (Lash et al., 2001), and PCE is relatively more toxic compared with TCE (Lash et al., 2007). The species- and sex-specificity of injury observed in vitro are in concordance with chronic in vivo studies, wherein the degree of renal cytomegaly following a 2-year exposure to TCE was greater in male rats compared with female rats and male and female mice (National Toxicology Program, 1990). PCE-associated chronic nephropathy, including karyomegaly, occurs in rats (National Toxicology Program, 1977, 1986) of both sexes after chronic exposure. Renal injury associated with TCE or PCE exposure is probably mediated through GSH=conjugative metabolites (i.e., DCVG/TCVG and DCVC/TCVC) (Lash and Parker, 2001; Lash et al., 2014; Rusyn et al., 2014).
Liver Noncancer Toxicity.
There is some epidemiologic evidence for TCE- and PCE- associated noncancer hepatotoxicity (EPA, 2011a,b; Guyton et al., 2014). In most studies of TCE, liver dysfunction was monitored by serum liver enzyme levels and/or serum bile acid levels (Driscoll et al., 1992; Nagaya et al., 1993; Rasmussen et al., 1993b; Neghab et al., 1997; Davis et al., 2005; Kamijima et al., 2007; Xu et al., 2009). Hypersensitivity reactions to TCE that involve the skin (toxic epidermal necrolysis) and other organs, including the liver (jaundice, hepatomegaly, hepatosplenomegaly, hepatitis) have been reported (Kamijima et al., 2007; Kamijima et al., 2008). For PCE, hepatomegaly, hepatocellular damage, and hepatobiliary impairment have been observed in humans after exposure (Coler and Rossmiller, 1953; Meckler and Phelps, 1966; Saland, 1967; Bagnell and Ellenberger, 1977; Hake and Stewart, 1977; Lauwerys et al., 1983; Cai et al., 1991; Gennari et al., 1992; Brodkin et al., 1995). Experimental evidence supports characterization of TCE and PCE as hepatotoxicants to rats and mice, with mice being the more sensitive species. On the basis of the epidemiologic evidence, there is similar evidence that humans can experience hepatotoxicity following exposure to TCE or PCE. Humans are expected to be less sensitive than rodents to hepatic effects of PCE owing to interspecies differences in hepatic oxidative metabolism (Chiu and Ginsberg, 2011). In contrast, interspecies differences in TCE hepatotoxicity are expected to be small because oxidative metabolism is hepatic blood-flow–limited (Chiu et al., 2009).
Noncancer Neurotoxicity.
In humans, changes in trigeminal nerve function or morphology (Triebig et al., 1982, 1983; Ruijten et al., 1991; Feldman et al., 1992; Kilburn and Warshaw, 1992; Rasmussen et al., 1993a; Kilburn, 2002; Mhiri et al., 2004) and vestibular dysfunction (Rasmussen et al., 1993c; Burg and Gist, 1995, 1999) have been identified as neurotoxic phenotypes associated with TCE exposure. For PCE, neurotoxicity is the most sensitive outcome of exposure in humans, where toxicity is observed even at low-exposure concentrations. For instance, perturbations in visual contrast sensitivity have been observed with chronic exposure to as little as 0.3 ppm PCE (Schreiber et al., 2002; New York State Department of Health, 2010), whereas effects in other organs (e.g., liver or kidney) are not observed until airborne concentrations of PCE are at least two to three orders-of-magnitude higher (US EPA, 2011a). A very recent study reported an increased risk of epilepsy in humans exposed to PCE through contaminated public water supplies during gestation or early childhood (Aschengrau et al., 2015). In addition to the epidemiologic evidence, there are many well-documented neurotoxic effects of both PCE and TCE in experimental animals. These studies range from the effects of these chemicals on narcosis, motor activity, neurotransmitter levels, and other neurochemical and neurotoxicological examinations (EPA, 2011a,b).
Developmental Cardiotoxicity.
Developmental cardiotoxicity has been associated with TCE exposure in humans and consists of congenital cardiac malformations. However, most of the epidemiologic data come from studies with relatively small numbers of cases, thus complicating interpretation of any individual study (US EPA, 2011b). Nonetheless, as noted by the National Research Council (National Research Council, 2006), the compilation of studies report that “…the effect size of a 2- to 3-fold increase in risk is similar across multiple studies.” In particular, one study (and its follow up study) examining birth defects in Endicott, New York, from the years 1983–2000 reported an increased incidence of developmental cardiac defects in TCE-exposed humans (http://www.atsdr.cdc.gov/HAC/pha//endicottareainvestigation/endicottHealthStatsReviewHC052606.pdf; http://www/atsdr.cdc.gov/hac/pha//endicottareainvestigationfollowup/endicottareahc051508.pdf; Forand et al., 2012). A more recent analysis of the same region of New York State found that maternal residence in an area contaminated with TCE or PCE was associated with cardiac birth effects, but these effects were only significant for the TCE-exposed group (Forand et al., 2012). In contrast, a study in Texas found weak to no evidence of an association between TCE or PCE exposure and developmental cardiotoxicity (Brender et al., 2014). There is evidence of TCE-induced cardiotoxicity in experimental animals, particularly avian in vivo (Elovaara et al., 1979; Bross et al., 1983; Drake et al., 2006a,b; Rufer et al., 2010) and in vitro studies (embryonic chick atrioventricular canal cushion cells) (Boyer et al., 2000), whereas the few rodent studies are inconsistent (US EPA, 2011b).
Noncancer Toxicity of the Lympho-Hematopoietic System.
Epidemiologic data support the association of TCE exposure with an increased risk of autoimmune disease, and suggest TCE causes a generalized hypersensitivity syndrome, as reviewed in Cooper et al. (2009). Specifically, two reports (Kamijima et al., 2007, 2008) document a high prevalence of severe hypersensitivity skin disorders. Most cases were in the Guangdong Province of China, where workers were occupationally exposed to TCE in factories in which metal degreasing occurred. In some cases, this severe skin disorder was complicated by hepatitis. A number of studies in occupationally exposed workers in China also reported clinical signs of immunosuppression (Lan et al., 2010; Hosgood et al., 2012; Bassig et al., 2013). The epidemiologic evidence is more sparse for an association of exposure to PCE and an augmented Th2 responsiveness (Andrys et al., 1997; Emara et al., 2010). However, it has been shown in one human study that red blood cell and hemoglobin counts are decreased after exposure to PCE (Emara et al., 2010).
A number of studies in experimental rodents have found associations between TCE exposure and immunosuppression, hypersensitivity, and autoimmunity in a species-, dose-, age-, and route-of-administration–dependent fashion (US EPA, 2011b). For example, developmental immunotoxicity (Gilbert et al., 2014b) and autoimmune hepatitis (Gilbert et al., 2014a) have been observed in a mouse strain prone to autoimmune disorders (MRL+/+) after TCE exposure. Compared with TCE, less experimental evidence is available on the noncancer effects of PCE on the lympho-hematopoietic system.
Mechanisms of Toxicity
The mechanisms of action for cancer and noncancer toxicity associated with TCE or PCE exposure are dependent on multiple factors, including tissue and species. Considerable evidence supports the fact that oxidative and conjugative metabolites of TCE and PCE are involved in both genotoxic and nongenotoxic mechanisms of toxicity.
Genotoxicity.
Epidemiologic evidence for chromosomal aberrations, sister chromatid exchange, and von Hippel-Lindau (VHL) gene mutations associated with TCE exposure in humans does not provide conclusive evidence of TCE-associated genotoxicity in humans (IARC, 2014). In in vivo experimental animal models, a low level of covalent binding with DNA from rat and mouse tissues (liver, kidney, lung, and stomach) has been observed (Mazzullo et al., 1992). TCE has shown little or weak genotoxicity in in vitro model systems devoid of metabolic capacity. Upon addition of human or rodent microsomes and appropriate cofactors, covalent binding of radiolabeled 14C-TCE (via TCE-epoxide) to both protein and DNA is enhanced (Miller and Guengerich, 1983; Cai and Guengerich, 2001). Aside from binding assays, a number of in vitro, ex vivo, and in vivo assays using nonmammalian (namely bacteria, fungi, and yeast) and rodent models have evaluated the mutagenic and cytogenetic effects, and effects of TCE on other types of DNA damage (e.g., unscheduled DNA synthesis) (US EPA, 2011b; IARC, 2014). Collectively, the evidence to suggest that unmetabolized TCE or its oxidative pathway metabolites are genotoxic is weak. An important exception is CH, for which there is strong evidence of genotoxicity. CH can induce mutations, chromosomal aberrations, and micronuclei, both in vivo and in vitro, in mammalian and other test systems (IARC, 2014). A significant increase in micronuclei was observed in peripheral blood lymphocytes in infants administered chloral hydrate orally as a sedative (Ikbal et al., 2004).
No statistically significant effects of PCE exposure on chromosomal aberrations or sister chromatid exchange were detected in the few relatively small cross-sectional studies evaluated (Ikeda et al., 1980; Seiji et al., 1990; Tucker et al., 2011). Experimental evidence suggests that both mouse and rat liver and kidney are sensitive to the binding of [14C] PCE to DNA. Similar to findings with TCE, PCE only showed evidence of in vitro DNA binding when metabolism occurred through either oxidation and/or GSH conjugation. PCE did increase the frequency of micronucleus formation in human lymphoblastoid cells (Doherty et al., 1996; White et al., 2001), although no sister chromatid exchange was observed in human blood cells at subcytotoxic and cytotoxic doses (Hartmann and Speit, 1995). In mutagenesis assays (Ames) using bacteria (Salmonella typhimurium), PCE caused mutations when rat liver GST, kidney microsomes, and GSH were added to the culture to mimic the in vivo conjugation of TCE with GSH via hepatic GST and subsequent generation of reactive metabolites via renal GGT and CCBL (Vamvakas et al., 1989a). Thus, as with TCE, the parent compound PCE exhibits minimal (if any) genotoxic potential.
Certain metabolites of TCE and PCE may be genotoxic. As noted above, oxidative metabolism of TCE does not lead to genotoxicity. Specifically, TCA does not exhibit significant genotoxic potential in vitro or in vivo. The evidence for DCA-associated genotoxicity is weak to moderate, on the basis of both in vitro and in vivo experimental evidence. The few available experimental data for the genotoxic potential of TCOH preclude its classification as genotoxic or nongenotoxic.
On the other hand, DCVG and TCVG are both positive for genotoxicity, as assessed by induction of unscheduled DNA synthesis (Vamvakas et al., 1989b) and mutagenicity in the Ames assay (S. typhimurium) (Vamvakas et al., 1988c) when the culture medium is supplemented with GSH and kidney, but not liver, subcellular fractions. These data suggest that renal metabolism (via GGT and CCBL) of TCE or PCE has the potential to generate genotoxic metabolites. DCVC (Dekant et al., 1986; Vamvakas et al., 1988a) and TCVC (Dekant et al., 1986; Dreessen et al., 2003) both yield positive results in the Ames assay. Genotoxicity is enhanced with addition of kidney subcellular fractions and diminished by chemical inhibition of CCBL activity. DCVC has been reported to induce DNA strand breaks in rabbits in vivo and ex vivo (Jaffe et al., 1985) and unscheduled DNA synthesis in Syrian hamster embryo fibroblasts (Vamvakas et al., 1988b), increase transformation in isolated primary rat (Eker) kidney epithelial cells (Mally et al., 2006), and increase DNA strand breaks in proximal tubules of the kidney in rats (Clay, 2008). TCVC exposure resulted in a dose-dependent increase in unscheduled DNA synthesis in porcine kidney cells, an effect that was abolished by inhibiting CCBL activity (Vamvakas et al., 1989b). Taken together, these data suggest that DCVC and TCVC both have significant genotoxic potential and that the genotoxicity is probably mediated by reactive metabolites generated via the CCBL. NAcDCVC and NAcTCVC are both positive for mutagenic potential (Ames assay) without exogenous activation; kidney cytoplasm enhanced the genotoxic effect of either chemical, whereas inhibition of CCBL activity diminished the effect (Vamvakas et al., 1987).
Kidney Nongenotoxic Mechanisms of Toxicity.
Accumulation of alpha2u-globulin is a distinct histopathological finding specific to the male rat following chronic exposure to many xenobiotics. Chemicals hypothesized to produce kidney tumors in male rats through alpha2u-globulin accumulation are not thought to be a cancer hazard to humans. Experimental evidence does not suggest that TCE or PCE act solely through this proposed mechanism, although in some studies accumulation of alpha2u-globulin was observed (IARC, 2014).
Substantial evidence exists for TCE- and PCE-induced renal toxicity in humans and experimental animals (see above). Most of the experimental evidence suggests that the renal noncancer toxicity observed in humans and rodents is mediated by cytotoxicity followed by sustained chronic nephrotoxicity without accumulation of alpha2u-globulin in the kidney.
Activation of peroxisome proliferator–activated receptor alpha (PPARα) in renal tissue has been observed following TCE (Goldsworthy and Popp, 1987; Yoo et al., 2015b,c) and PCE (Goldsworthy and Popp, 1987; Odum et al., 1988) exposure. However, the evidence in support of a PPARα-dependent mechanism of toxicity for both TCE- and PCE-associated kidney injury (cancer or noncancer) is considered relatively weak (EPA, 2011a,b; IARC, 2014).
Liver Nongenotoxic Mechanisms of Toxicity.
Exposure to TCE or PCE is associated with hepatotoxicity and oxidative stress in humans and animals (EPA, 2011a,b; IARC, 2014). Hepatotoxicity is usually assessed by elevated serum liver enzyme levels such as alanine transaminase, which has been observed in humans occupationally exposed to TCE or PCE. However, the epidemiologic evidence is not consistent concerning the noncancer hepatotoxicity of either solvent. In experimental animals, both TCE and PCE have also been shown to elicit hepatotoxicity, typically at doses at or exceeding 100 mg/kg (e.g., Buben and O’Flaherty, 1985; Philip et al., 2007; Yoo et al., 2015a). In addition to elevation of serum liver enzymes, hyperbilirubinemia, cholestatic liver injury, oxidative damage to DNA and cell membranes, changes in lipid metabolism, lipid accumulation (steatosis), and hepatomegaly have all been reported in rodents exposed to TCE or PCE (EPA, 2011a,b; IARC, 2014).
Cell proliferation, apoptosis, and clonal expansion is another potential mechanism of TCE- or PCE-associated hepatotoxicity. All of the evidence is derived from experimental studies in rodents. Cell proliferation has been observed in the livers of rodents exposed to TCE or PCE. In most cases, this was determined by immunohistochemical staining for proliferating cell nuclear antigen-, Ki-67 antigen-, or 5-bromo-2-deoxyuridine-positive cells (EPA, 2011a,b; IARC, 2014). There are conflicting reports of increased apoptosis in the liver after TCE exposure in rodents (Dees and Travis, 1993; Channel et al., 1998; Sano et al., 2009), and there is no experimental evidence for PCE-associated apoptosis in the liver, or TCE- or PCE-associated clonal expansion of hepatocytes.
The contribution of PPARα activation to hepatocellular carcinomas and/or adenomas in the mouse after chronic exposure to TCE and PCE has been postulated (Corton, 2008). TCE and PCE induce PPARα in rodent studies, likely through formation of TCA and DCA (Bull, 2000; Corton, 2008; Maloney and Waxman, 1999). One study reported that PCE induces PPARα after acute and subacute (less than 14 days) exposure but not after 30 days of exposure (Philip et al., 2007); however, the overall database on the role of PPARα in the effects of PCE is limited. Studies of mice deficient in PPARα have shown that they are more sensitive to TCE-induced hepatosteatosis (Ramdhan et al., 2010) and have lower levels of TCA in their urine (Ramdhan et al., 2010) and in serum, liver, and kidney (Yoo et al., 2015c) compared with wild-type mice.
Epigenetic effects are recognized for their potential influence on carcinogenesis of environmental chemicals. No epidemiologic or experimental evidence is available for TCE- or PCE-induced epigenetic effects in the liver after exposure to either parent chemical. However, in the mouse, TCA or DCA administered via drinking water can lead to DNA hypomethylation in liver tumor tissue in N-nitroso-N-methylurea-initiated hepatocellular adenomas/carcinomas (Tao et al., 1998, 2000, 2004). This epigenetic effect was reversed upon cessation of DCA, but not TCA, administration (Tao et al., 1998).
Other Organs/Systems.
Little to no evidence is available in support of nongenotoxic (or genotoxic) mechanisms of toxicity for TCE or PCE in tissues other than liver and kidney. For central nervous system toxicity, it is known that PCE, TCE, and other volatile, lipophilic solvents can change brain neurochemistry and result in neurotoxicity, although the mechanisms of toxicity are unclear (EPA, 2011a,b).
Perspectives and Conclusions
TCE and PCE remain environmental toxicants of utmost public health concern owing to widespread exposure across the population and their high–production volume, persistence in the environment, and potential for causing severe adverse health effects. Although the toxic responses to TCE and PCE have been widely studied, significant gaps in our knowledge still exist.
Interindividual Variability.
Interindividual variability in susceptibility to environmental toxicants complicates human health assessments. Very few data are available on interindividual variability in response to TCE or PCE. For both these chemicals, understanding population-level variability in toxicokinetics and toxicodynamics (and the relationship between the two) is critical to understanding and predicting risk. Several computational studies using population PBPK models have examined TCE or PCE toxicokinetic variability by combining data from historical human controlled exposure studies (Chiu and Bois 2006; Chiu et al., 2009). A more recent experimental study used a multistrain panel of inbred mice to examine interindividual variability in susceptibility to TCE-associated toxicity (Bradford et al., 2011). This study examined oxidative and conjugative metabolism and the transcriptomic response of the livers of 14 inbred strains of mice to a single high dose of TCE. The authors reported substantial interindividual variability in levels of TCA, DCA, DCVG, and DCVC in the serum of mice. Interestingly, in the context of strain, serum levels of TCA were significantly correlated with induction of the PPARα pathway. Furthermore, this study, and others (Harrill et al., 2009), reported that the genetic background was the strongest determinant of hepatic gene expression, having a greater effect than the toxicant examined.
As genetic background can greatly influence xenobiotic metabolism and susceptibility to the toxic response to chemical exposure, it is important to consider this variability within the population. Accordingly, toxicokinetic data from population-based studies in rodents can be used as a surrogate for predicting variability within the human population. This approach has recently been demonstrated for TCE in a population PBPK modeling–based analysis (Chiu et al., 2014) using data derived from (Bradford et al., 2011). Future population-based studies examining the contribution of factors other than genetics—including underlying disease—are also warranted, and could similarly be used to better clarify key sources of variability.
Comparative and Coexposure Studies.
The literature on the comparative toxicity of chlorinated solvents is relatively sparse. Almost all such studies have relied on insensitive measures of toxicity [e.g., lethal dose, 50% (LD50) values, serum liver enzyme levels, urinary proteins, etc.]. Further, existing studies have not performed multiple-comparison statistical tests between vehicle-, TCE-, and PCE-exposed groups. Therefore, it is hoped that this review will encourage future work on directly comparing the adverse health effects of TCE and PCE by rigorous evaluation of sensitive biomolecular data.
Conclusions.
After decades of toxicological research on TCE and PCE, our knowledge remains incomplete concerning how these chemicals induce toxicity. Toxic effects in experimental systems are not fully characterized, and the human relevance of these effects is poorly understood. In the kidney, GSH-mediated conjugative pathways can yield genotoxic metabolites, as is supported by epidemiologic evidence on TCE (Moore et al., 2010). In other tissues, the role of target organ metabolism in ultimate adverse response is unclear. Clarification of the specific isozymes or enzymes responsible for TCE and PCE metabolism will provide useful mechanistic information for extrapolation of data derived from rodent studies to human health assessments. Although TCE is a widely studied chemical, considerably less experimental and epidemiologic evidence is available for PCE, one of the most widely used chlorinated solvents. Few studies have directly compared toxic responses to the two chemicals. Furthermore, although cocontamination of and coexposure to TCE and PCE are common, relatively few studies have evaluated the effects of coexposure to these chemicals. Finally, although data are available on the effect of genetic background on toxicokinetics and toxicodynamics of TCE, other potential contributors to interindividual variability, such as lifestyle factors, which may lead to underlying disease, have not yet been experimentally evaluated. Future studies are warranted to narrow the knowledge gaps addressed above to better inform risk management decisions for TCE and PCE.
Acknowledgments
The authors acknowledge the IARC v106 Working Group, the IARC Secretariat, and the coauthors of the US Environmental Protection Agency risk assessments.
Abbreviations
- CCBL
cysteine conjugate β-lyase
- CHL
chloral
- CH
chloral hydrate
- DCA
dichloroacetate
- DCAC
dichloroacetyl chloride
- DCVC
S-(trans-1,2,-dichlorovinyl)-l-cysteine
- DCVG
S-(1,2-dichlorovinyl)glutathione
- GGT
γ-glutamyltransferase
- GSH
glutathione
- GST
glutathione S-transferase
- IARC
International Agency for Research on Cancer
- NAcDCVC
N-acetyl-S-(1,2-dichlorovinyl)- l-cysteine
- NAcTCVC
N-acetyl-S-(1,2-trichlorovinyl)- l-cysteine
- NHL
non-Hodgkin lymphoma
- NTP
National Toxicology Program
- OEHHA
Office of Environmental Health Hazard Assessment
- P450
cytochrome P450
- PBPK
physiologically-based pharmacokinetic
- PCE
perchloroethylene or tetrachloroethylene
- PPARα
peroxisome proliferator–activated receptor alpha
- RfC
reference concentration in the air
- RfD
reference dose via oral ingestion
- TCA
trichloroacetate
- TCE
trichloroethylene
- TCE-O
TCE-epoxide
- TCOH
trichloroethanol
- TCVC
S-(1,2,2-trichlorovinyl)-l-cysteine
- TCVG
S-(1,2,2-trichlorovinyl)glutathione
- US EPA
US Environmental Protection Agency
Authorship Contributions
Wrote or contributed to the writing of the article: Cichocki, Guyton, Guha, Chiu, Rusyn, Lash.
Footnotes
The IARC Monographs are supported in part by the National Institutes of Health National Cancer Institute [Cooperative Agreement U01 CA33193]. J.A.C. is supported by a Ruth L. Kirschstein National Research Service Award from the National Institute of Environmental Health Sciences [1F32ES026005-01].
References
- American Cancer Society (2016). Types of Non-Hodgkin lymphoma. Retrieved on April 18, 2016 from http://www.cancer.org/cancer/non-hodgkinlymphoma/detailedguide/non-hodgkin-lymphoma-types-of-non-hodgkin-lymphoma
- Andrýs C, Hanovcová I, Chýlková V, Tejral J, Eminger S, Procházková J. (1997) Immunological monitoring of dry-cleaning shop workers--exposure to tetrachloroethylene. Cent Eur J Public Health 5:136–142. [PubMed] [Google Scholar]
- Anttila A, Pukkala E, Sallmén M, Hernberg S, Hemminki K. (1995) Cancer incidence among Finnish workers exposed to halogenated hydrocarbons. J Occup Environ Med 37:797–806. [DOI] [PubMed] [Google Scholar]
- Asal NR, Geyer JR, Risser DR, Lee ET, Kadamani S, Cherng N. (1988) Risk factors in renal cell carcinoma. II. Medical history, occupation, multivariate analysis, and conclusions. Cancer Detection Prevention 13:263–279. [PubMed] [Google Scholar]
- Aschengrau A, Winter MR, Vieira VM, Webster TF, Janulewicz PA, Gallagher LG, Weinberg J, Ozonoff DM. (2015) Long-term health effects of early life exposure to tetrachloroethylene (PCE)-contaminated drinking water: a retrospective cohort study. Environ Health 14:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnell PC, Ellenberger HA. (1977) Obstructive jaundice due to a chlorinated hydrocarbon in breast milk. Can Med Assoc J 117:1047–1048. [PMC free article] [PubMed] [Google Scholar]
- Bahr DE, Aldrich TE, Seidu D, Brion GM, Tollerud DJ, Paducah Gaseous Diffusion Plant Project Team. Muldoon S, Reinhart N, Youseefagha A, McKinney P, Hughes T, et al. (2011) Occupational exposure to trichloroethylene and cancer risk for workers at the Paducah Gaseous Diffusion Plant. Int J Occup Med Environ Health 24:67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassig BA, Zhang L, Tang X, Vermeulen R, Shen M, Smith MT, Qiu C, Ge Y, Ji Z, Reiss B, et al. (2013) Occupational exposure to trichloroethylene and serum concentrations of IL-6, IL-10, and TNF-alpha. Environ Mol Mutagen 54:450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernauer U, Birner G, Dekant W, Henschler D. (1996) Biotransformation of trichloroethene: dose-dependent excretion of 2,2,2-trichloro-metabolites and mercapturic acids in rats and humans after inhalation. Arch Toxicol 70:338–346. [DOI] [PubMed] [Google Scholar]
- Blair A, Petralia SA, Stewart PA. (2003) Extended mortality follow-up of a cohort of dry cleaners. Ann Epidemiol 13:50–56. [DOI] [PubMed] [Google Scholar]
- Boice JD, Jr, Marano DE, Cohen SS, Mumma MT, Blot WJ, Brill AB, Fryzek JP, Henderson BE, McLaughlin JK. (2006) Mortality among Rocketdyne workers who tested rocket engines, 1948-1999. J Occup Environ Med 48:1070–1092. [DOI] [PubMed] [Google Scholar]
- Boice JD, Jr, Marano DE, Fryzek JP, Sadler CJ, McLaughlin JK. (1999) Mortality among aircraft manufacturing workers. Occup Environ Med 56:581–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolt HM, Lammert M, Selinski S, Brüning T. (2004) Urinary alpha1-microglobulin excretion as biomarker of renal toxicity in trichloroethylene-exposed persons. Int Arch Occup Environ Health 77:186–190. [DOI] [PubMed] [Google Scholar]
- Bond GG, McLaren EA, Sabel FL, Bodner KM, Lipps TE, Cook RR. (1990) Liver and biliary tract cancer among chemical workers. Am J Ind Med 18:19–24. [DOI] [PubMed] [Google Scholar]
- Bove FJ, Ruckart PZ, Maslia M, Larson TC. (2014) Evaluation of mortality among marines and navy personnel exposed to contaminated drinking water at USMC base Camp Lejeune: a retrospective cohort study. Environ Health 13:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer AS, Finch WT, Runyan RB. (2000) Trichloroethylene inhibits development of embryonic heart valve precursors in vitro. Toxicol Sci 53:109–117. [DOI] [PubMed] [Google Scholar]
- Bradford BU, Lock EF, Kosyk O, Kim S, Uehara T, Harbourt D, DeSimone M, Threadgill DW, Tryndyak V, Pogribny IP, et al. (2011) Interstrain differences in the liver effects of trichloroethylene in a multistrain panel of inbred mice. Toxicol Sci 120:206–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brender JD, Shinde MU, Zhan FB, Gong X, Langlois PH. (2014) Maternal residential proximity to chlorinated solvent emissions and birth defects in offspring: a case-control study. Environ Health 13:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodkin CA, Daniell W, Checkoway H, Echeverria D, Johnson J, Wang K, Sohaey R, Green D, Redlich C, Gretch D, et al. (1995) Hepatic ultrasonic changes in workers exposed to perchloroethylene. Occup Environ Med 52:679–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bross G, DiFranceisco D, Desmond ME. (1983) The effects of low dosages of trichloroethylene on chick development. Toxicology 28:283–294. [DOI] [PubMed] [Google Scholar]
- Brüning T, Mann H, Melzer H, Sundberg AG, Bolt HM. (1999a) Pathological excretion patterns of urinary proteins in renal cell cancer patients exposed to trichloroethylene. Occup Med (Lond) 49:299–305. [DOI] [PubMed] [Google Scholar]
- Brüning T, Pesch B, Wiesenhütter B, Rabstein S, Lammert M, Baumüller A, Bolt HM. (2003) Renal cell cancer risk and occupational exposure to trichloroethylene: results of a consecutive case-control study in Arnsberg, Germany. Am J Ind Med 43:274–285. [DOI] [PubMed] [Google Scholar]
- Brüning T, Sundberg AG, Birner G, Lammert M, Bolt HM, Appelkvist EL, Nilsson R, Dallner G. (1999b) Glutathione transferase alpha as a marker for tubular damage after trichloroethylene exposure. Arch Toxicol 73:246–254. [DOI] [PubMed] [Google Scholar]
- Buben JA, O'Flaherty EJ. (1985) Delineation of the role of metabolism in the hepatotoxicity of trichloroethylene and perchloroethylene: A dose-effect study. Toxicol Appl Pharmacol 78:105–122. [DOI] [PubMed] [Google Scholar]
- Bull RJ. (2000) Mode of action of liver tumor induction by trichloroethylene and its metabolites, trichloroacetate and dichloroacetate. Environ Health Perspect 108 (Suppl 2):241–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg JR, Gist GL. (1995) The National Exposure Registry: procedures for establishing a registry of persons environmentally exposed to hazardous substances. Toxicol Ind Health 11:231–248. [DOI] [PubMed] [Google Scholar]
- Burg JR, Gist GL. (1999) Health effects of environmental contaminant exposure: an intrafile comparison of the Trichloroethylene Subregistry. Arch Environ Health 54:231–241. [DOI] [PubMed] [Google Scholar]
- Cai H, Guengerich FP. (2001) Reaction of trichloroethylene and trichloroethylene oxide with cytochrome P450 enzymes: inactivation and sites of modification. Chem Res Toxicol 14:451–458. [DOI] [PubMed] [Google Scholar]
- Cai SX, Huang MY, Chen Z, Liu YT, Jin C, Watanabe T, Nakatsuka H, Seiji K, Inoue O, Ikeda M. (1991) Subjective symptom increase among dry-cleaning workers exposed to tetrachloroethylene vapor. Ind Health 29:111–121. [DOI] [PubMed] [Google Scholar]
- CalEPA (California Environmental Protection Agency) (2016). Perchloroethylene Inhalation Cancer Unit Risk Factor Technical Support Document for Cancer Potency Factors, Appendix B, Public Review Draft (February, 2016). Air Toxics Hot Spots Program. Retrieved March 16, 2016 from http://www.oehha.ca.gov/air/hot_spots/pdf/finalpublicreviewPCE_UR_TSD02162016.pdf
- Calvert GM, Ruder AM, Petersen MR. (2011) Mortality and end-stage renal disease incidence among dry cleaning workers. Occup Environ Med 68:709–716. [DOI] [PubMed] [Google Scholar]
- CDC (Center for Disease Control National Center for Health Statistics) (2011). National Health and Nutrition Examination Survey Data. Hyattsville, MD: US Department of Health and Human Services, Centers for Disease Control and Prevention, 2005–2006. Retrieved March 16, 2016 from http://wwwn.cdc.gov/nchs/nhanes/2005–2006/VOCWB_D.htm
- Channel SR, Latendresse JR, Kidney JK, Grabau JH, Lane JW, Steel-Goodwin L, Gothaus MC. (1998) A subchronic exposure to trichloroethylene causes lipid peroxidation and hepatocellular proliferation in male B6C3F1 mouse liver. Toxicol Sci 43:145–154. [DOI] [PubMed] [Google Scholar]
- Charbotel B, Fevotte J, Hours M, Martin JL, Bergeret A. (2006) Case-control study on renal cell cancer and occupational exposure to trichloroethylene. Part II: Epidemiological aspects. Ann Occup Hyg 50:777–787. [DOI] [PubMed] [Google Scholar]
- Chiu WA, Bois FY. (2006) Revisiting the population toxicokinetics of tetrachloroethylene. Arch Toxicol 80:382–385. [DOI] [PubMed] [Google Scholar]
- Chiu WA, Campbell JL, Jr, Clewell HJ, 3rd, Zhou YH, Wright FA, Guyton KZ, Rusyn I. (2014) Physiologically based pharmacokinetic (PBPK) modeling of interstrain variability in trichloroethylene metabolism in the mouse. Environ Health Perspect 122:456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu WA, Ginsberg GL. (2011) Development and evaluation of a harmonized physiologically based pharmacokinetic (PBPK) model for perchloroethylene toxicokinetics in mice, rats, and humans. Toxicol Appl Pharmacol 253:203–234. [DOI] [PubMed] [Google Scholar]
- Chiu WA, Jinot J, Scott CS, Makris SL, Cooper GS, Dzubow RC, Bale AS, Evans MV, Guyton KZ, Keshava N, et al. (2013) Human health effects of trichloroethylene: key findings and scientific issues. Environ Health Perspect 121:303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu WA, Micallef S, Monster AC, Bois FY. (2007) Toxicokinetics of inhaled trichloroethylene and tetrachloroethylene in humans at 1 ppm: empirical results and comparisons with previous studies. Toxicol Sci 95:23–36. [DOI] [PubMed] [Google Scholar]
- Chiu WA, Okino MS, Evans MV. (2009) Characterizing uncertainty and population variability in the toxicokinetics of trichloroethylene and metabolites in mice, rats, and humans using an updated database, physiologically based pharmacokinetic (PBPK) model, and Bayesian approach. Toxicol Appl Pharmacol 241:36–60. [DOI] [PubMed] [Google Scholar]
- Christensen KY, Vizcaya D, Richardson H, Lavoué J, Aronson K, Siemiatycki J. (2013) Risk of selected cancers due to occupational exposure to chlorinated solvents in a case-control study in Montreal. J Occup Environ Med 55:198–208. [DOI] [PubMed] [Google Scholar]
- Clay P. (2008) Assessment of the genotoxicity of trichloroethylene and its metabolite, S-(1,2-dichlorovinyl)-L-cysteine (DCVC), in the comet assay in rat kidney. Mutagenesis 23:27–33. [DOI] [PubMed] [Google Scholar]
- Cocco P, Vermeulen R, Flore V, Nonne T, Campagna M, Purdue M, Blair A, Monnereau A, Orsi L, Clavel J, et al. (2013) Occupational exposure to trichloroethylene and risk of non-Hodgkin lymphoma and its major subtypes: a pooled InterLymph [correction of IinterLlymph] analysis. Occup Environ Med 70:795–802. [DOI] [PubMed] [Google Scholar]
- Coler HR, Rossmiller HR. (1953) Tetrachlorethylene exposure in a small industry. AMA Arch Ind Hyg Occup Med 8:227–233. [PubMed] [Google Scholar]
- Cooper GS, Makris SL, Nietert PJ, Jinot J. (2009) Evidence of autoimmune-related effects of trichloroethylene exposure from studies in mice and humans. Environ Health Perspect 117:696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corton JC. (2008) Evaluation of the role of peroxisome proliferator-activated receptor alpha (PPARalpha) in mouse liver tumor induction by trichloroethylene and metabolites. Crit Rev Toxicol 38:857–875. [DOI] [PubMed] [Google Scholar]
- Davis SI, Laszlo Pallos L, Wu JQ, Sapp JH, 2nd, Cusack C. (2005) ATSDR’s trichloroethylene subregistry methods and results: 1989-2000. Arch Environ Occup Health 60:130–139. [DOI] [PubMed] [Google Scholar]
- Dees C, Travis C. (1993) The mitogenic potential of trichloroethylene in B6C3F1 mice. Toxicol Lett 69:129–137. [DOI] [PubMed] [Google Scholar]
- Dekant W, Vamvakas S, Berthold K, Schmidt S, Wild D, Henschler D. (1986) Bacterial beta-lyase mediated cleavage and mutagenicity of cysteine conjugates derived from the nephrocarcinogenic alkenes trichloroethylene, tetrachloroethylene and hexachlorobutadiene. Chem Biol Interact 60:31–45. [DOI] [PubMed] [Google Scholar]
- Delahunt B, Bethwaite PB, Nacey JN. (1995) Occupational risk for renal cell carcinoma. A case-control study based on the new zealand cancer registry. Brit J Urol 75:578–582. [DOI] [PubMed] [Google Scholar]
- Doherty AT, Ellard S, Parry EM, Parry JM. (1996) An investigation into the activation and deactivation of chlorinated hydrocarbons to genotoxins in metabolically competent human cells. Mutagenesis 11:247–274. [DOI] [PubMed] [Google Scholar]
- Dosemeci M, Cocco P, Chow WH. (1999) Gender differences in risk of renal cell carcinoma and occupational exposures to chlorinated aliphatic hydrocarbons. Am J Ind Med 36:54–59. [DOI] [PubMed] [Google Scholar]
- Drake VJ, Koprowski SL, Hu N, Smith SM, Lough J. (2006a) Cardiogenic effects of trichloroethylene and trichloroacetic acid following exposure during heart specification of avian development. Toxicol Sci 94:153–162. [DOI] [PubMed] [Google Scholar]
- Drake VJ, Koprowski SL, Lough J, Hu N, Smith SM. (2006b) Trichloroethylene exposure during cardiac valvuloseptal morphogenesis alters cushion formation and cardiac hemodynamics in the avian embryo. Environ Health Perspect 114:842–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreessen B, Westphal G, Bünger J, Hallier E, Müller M. (2003) Mutagenicity of the glutathione and cysteine S-conjugates of the haloalkenes 1,1,2-trichloro-3,3,3-trifluoro-1-propene and trichlorofluoroethene in the Ames test in comparison with the tetrachloroethene-analogues. Mutat Res 539:157–166. [DOI] [PubMed] [Google Scholar]
- Driscoll TR, Hamdan HH, Wang G, Wright PF, Stacey NH. (1992) Concentrations of individual serum or plasma bile acids in workers exposed to chlorinated aliphatic hydrocarbons. Br J Ind Med 49:700–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elovaara E, Hemminki K, Vainio H. (1979) Effects of methylene chloride, trichloroethane, trichloroethylene, tetrachloroethylene and toluene on the development of chick embryos. Toxicology 12:111–119. [DOI] [PubMed] [Google Scholar]
- Emara AM, Abo El-Noor MM, Hassan NA, Wagih AA. (2010) Immunotoxicity and hematotoxicity induced by tetrachloroethylene in egyptian dry cleaning workers. Inhal Toxicol 22:117–124. [DOI] [PubMed] [Google Scholar]
- Fay RM, Mumtaz MM. (1996) Development of a priority list of chemical mixtures occurring at 1188 hazardous waste sites, using the HazDat database. Food Chem Toxicol 34:1163–1165. [DOI] [PubMed] [Google Scholar]
- Feldman RG, Niles C, Proctor SP, Jabre J. (1992) Blink reflex measurement of effects of trichloroethylene exposure on the trigeminal nerve. Muscle Nerve 15:490–495. [DOI] [PubMed] [Google Scholar]
- Forand SP, Lewis-Michl EL, Gomez MI. (2012) Adverse birth outcomes and maternal exposure to trichloroethylene and tetrachloroethylene through soil vapor intrusion in New York State. Environ Health Perspect 120:616–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forkert PG, Lash LH, Nadeau V, Tardif R, Simmonds A. (2002) Metabolism and toxicity of trichloroethylene in epididymis and testis. Toxicol Appl Pharmacol 182:244–254. [DOI] [PubMed] [Google Scholar]
- Forkert PG, Sylvestre PL, Poland JS. (1985) Lung injury induced by trichloroethylene. Toxicology 35:143–160. [DOI] [PubMed] [Google Scholar]
- Franchini I, Cavatorta A, Falzoi M, Lucertini S, Mutti A. (1983) Early indicators of renal damage in workers exposed to organic solvents. Int Arch Occup Environ Health 52:1–9. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Takemoto K, Tsuruta H. (1983) Inhalation carcinogenicity of trichloroethylene in mice and rats. Ind Health 21:243–254. [DOI] [PubMed] [Google Scholar]
- Gennari P, Naldi M, Motta R, Nucci MC, Giacomini C, Violante FS, Raffi GB. (1992) gamma-Glutamyltransferase isoenzyme pattern in workers exposed to tetrachloroethylene. Am J Ind Med 21:661–671. [DOI] [PubMed] [Google Scholar]
- Gilbert KM, Reisfeld B, Zurlinden TJ, Kreps MN, Erickson SW, Blossom SJ. (2014a) Modeling toxicodynamic effects of trichloroethylene on liver in mouse model of autoimmune hepatitis. Toxicol Appl Pharmacol 279:284–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert KM, Woodruff W, Blossom SJ. (2014b) Differential immunotoxicity induced by two different windows of developmental trichloroethylene exposure. Autoimmune Dis 2014:982073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsworthy TL, Popp JA. (1987) Chlorinated hydrocarbon-induced peroxisomal enzyme activity in relation to species and organ carcinogenicity. Toxicol Appl Pharmacol 88:225–233. [DOI] [PubMed] [Google Scholar]
- Green T, Dow J, Ong CN, Ng V, Ong HY, Zhuang ZX, Yang XF, Bloemen L. (2004) Biological monitoring of kidney function among workers occupationally exposed to trichloroethylene. Occup Environ Med 61:312–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha N, Loomis D, Grosse Y, Lauby-Secretan B, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Baan R, Mattock H, Straif K, International Agency for Research on Cancer Monograph Working Group (2012) Carcinogenicity of trichloroethylene, tetrachloroethylene, some other chlorinated solvents, and their metabolites. Lancet Oncol 13:1192–1193. [DOI] [PubMed] [Google Scholar]
- Guyton KZ, Hogan KA, Scott CS, Cooper GS, Bale AS, Kopylev L, Barone S, Makris SL, Glenn B, Subramaniam RP, et al. (2014) Human health effects of tetrachloroethylene: key findings and scientific issues. Environ Health Perspect 122:325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hake CL, Stewart RD. (1977) Human exposure to tetrachloroethylene: inhalation and skin contact. Environ Health Perspect 21:231–238.612448 [Google Scholar]
- Hansen J, Sallmén M, Seldén AI, Anttila A, Pukkala E, Andersson K, Bryngelsson IL, Raaschou-Nielsen O, Olsen JH, McLaughlin JK. (2013) Risk of cancer among workers exposed to trichloroethylene: analysis of three Nordic cohort studies. J Natl Cancer Inst 105:869–877. [DOI] [PubMed] [Google Scholar]
- Harrill AH, Ross PK, Gatti DM, Threadgill DW, Rusyn I. (2009) Population-based discovery of toxicogenomics biomarkers for hepatotoxicity using a laboratory strain diversity panel. Toxicol Sci 110:235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann A, Speit G. (1995) Genotoxic effects of chemicals in the single cell gel (SCG) test with human blood cells in relation to the induction of sister-chromatid exchanges (SCE). Mutat Res 346:49–56. [DOI] [PubMed] [Google Scholar]
- Henschler D, Vamvakas S, Lammert M, Dekant W, Kraus B, Thomas B, Ulm K. (1995) Increased incidence of renal cell tumors in a cohort of cardboard workers exposed to trichloroethene. Arch Toxicol 69:291–299. [DOI] [PubMed] [Google Scholar]
- Hosgood HD, 3rd, Zhang L, Tang X, Vermeulen R, Qiu C, Shen M, Smith MT, Ge Y, Ji Z, Xiong J, et al. (2012) Decreased numbers of CD4(+) naive and effector memory T cells, and CD8(+) naïve t cells, are associated with trichloroethylene exposure. Front Oncol 1:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC (2006) IARC Monographs on the Evaluation of Carcinogenic Risks to Humans (Preamble.) International Agency for Research on Cancer, Lyon, France. [Google Scholar]
- IARC (2014) IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Trichloroethylene, Tetrachloroethylene and Some Other Chlorinated Agents (Vol. 106). International Agency for Research on Cancer, Lyon, France. [PMC free article] [PubMed] [Google Scholar]
- Ikbal M, Tastekin A, Dogan H, Pirim I, Ors R. (2004) The assessment of genotoxic effects in lymphocyte cultures of infants treated with chloral hydrate. Mutat Res 564:159–164. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Koizumi A, Watanabe T, Endo A, Sato K. (1980) Cytogenetic and cytokinetic investigations on lymphocytes from workers occupationally exposed to tetrachloroethylene. Toxicol Lett 5:251–256. [DOI] [PubMed] [Google Scholar]
- Jaffe DR, Hassall CD, Gandolfi AJ, Brendel K. (1985) Production of DNA single strand breaks in rabbit renal tissue after exposure to 1,2-dichlorovinylcysteine. Toxicology 35:25–33. [DOI] [PubMed] [Google Scholar]
- Japanese Industrial Safety Association (1993) Carcinogenicity study of tetrachloroethylene by inhalation in rats and mice, Japanese Industrial Safety Association, Kanagawa.
- Jia C, Yu X, Masiak W. (2012) Blood/air distribution of volatile organic compounds (VOCs) in a nationally representative sample. Sci Total Environ 419:225–232. [DOI] [PubMed] [Google Scholar]
- Kamijima M, Hisanaga N, Wang H, Nakajima T. (2007) Occupational trichloroethylene exposure as a cause of idiosyncratic generalized skin disorders and accompanying hepatitis similar to drug hypersensitivities. Int Arch Occup Environ Health 80:357–370. [DOI] [PubMed] [Google Scholar]
- Kamijima M, Wang H, Huang H, Li L, Shibata E, Lin B, Sakai K, Liu H, Tsuchiyama F, Chen J, et al. (2008) Trichloroethylene causes generalized hypersensitivity skin disorders complicated by hepatitis. J Occup Health 50:328–338. [DOI] [PubMed] [Google Scholar]
- Karami S, Lan Q, Rothman N, Stewart PA, Lee KM, Vermeulen R, Moore LE. (2012) Occupational trichloroethylene exposure and kidney cancer risk: a meta-analysis. Occup Environ Med 69:858–867. [DOI] [PubMed] [Google Scholar]
- Kilburn KH. (2002) Is neurotoxicity associated with environmental trichloroethylene (TCE)? Arch Environ Health 57:113–120. [DOI] [PubMed] [Google Scholar]
- Kilburn KH, Warshaw RH. (1992) Prevalence of symptoms of systemic lupus erythematosus (SLE) and of fluorescent antinuclear antibodies associated with chronic exposure to trichloroethylene and other chemicals in well water. Environ Res 57:1–9. [DOI] [PubMed] [Google Scholar]
- Lan Q, Zhang L, Tang X, Shen M, Smith MT, Qiu C, Ge Y, Ji Z, Xiong J, He J, et al. (2010) Occupational exposure to trichloroethylene is associated with a decline in lymphocyte subsets and soluble CD27 and CD30 markers. Carcinogenesis 31:1592–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lash LH, Chiu WA, Guyton KZ, Rusyn I. (2014) Trichloroethylene biotransformation and its role in mutagenicity, carcinogenicity and target organ toxicity. Mutat Res Rev Mutat Res 762:22–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lash LH, Parker JC. (2001) Hepatic and renal toxicities associated with perchloroethylene. Pharmacol Rev 53:177–208. [PubMed] [Google Scholar]
- Lash LH, Putt DA, Brashear WT, Abbas R, Parker JC, Fisher JW. (1999) Identification of S-(1,2-dichlorovinyl)glutathione in the blood of human volunteers exposed to trichloroethylene. J Toxicol Environ Health A 56:1–21. [DOI] [PubMed] [Google Scholar]
- Lash LH, Putt DA, Huang P, Hueni SE, Parker JC. (2007) Modulation of hepatic and renal metabolism and toxicity of trichloroethylene and perchloroethylene by alterations in status of cytochrome P450 and glutathione. Toxicology 235:11–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lash LH, Qian W, Putt DA, Desai K, Elfarra AA, Sicuri AR, Parker JC. (1998a) Glutathione conjugation of perchloroethylene in rats and mice in vitro: sex-, species-, and tissue-dependent differences. Toxicol Appl Pharmacol 150:49–57. [DOI] [PubMed] [Google Scholar]
- Lash LH, Qian W, Putt DA, Hueni SE, Elfarra AA, Krause RJ, Parker JC. (2001) Renal and hepatic toxicity of trichloroethylene and its glutathione-derived metabolites in rats and mice: sex-, species-, and tissue-dependent differences. J Pharmacol Exp Ther 297:155–164. [PubMed] [Google Scholar]
- Lash LH, Qian W, Putt DA, Hueni SE, Elfarra AA, Sicuri AR, Parker JC. (2002) Renal toxicity of perchloroethylene and S-(1,2,2-trichlorovinyl)glutathione in rats and mice: sex- and species-dependent differences. Toxicol Appl Pharmacol 179:163–171. [DOI] [PubMed] [Google Scholar]
- Lash LH, Qian W, Putt DA, Jacobs K, Elfarra AA, Krause RJ, Parker JC. (1998b) Glutathione conjugation of trichloroethylene in rats and mice: sex-, species-, and tissue-dependent differences. Drug Metab Dispos 26:12–19. [PubMed] [Google Scholar]
- Lauwerys R, Herbrand J, Buchet JP, Bernard A, Gaussin J. (1983) Health surveillance of workers exposed to tetrachloroethylene in dry-cleaning shops. Int Arch Occup Environ Health 52:69–77. [DOI] [PubMed] [Google Scholar]
- Lipworth L, Sonderman JS, Mumma MT, Tarone RE, Marano DE, Boice JD, Jr, McLaughlin JK. (2011) Cancer mortality among aircraft manufacturing workers: an extended follow-up. J Occup Environ Med 53:992–1007. [DOI] [PubMed] [Google Scholar]
- Löf A, Johanson G. (1998) Toxicokinetics of organic solvents: a review of modifying factors. Crit Rev Toxicol 28:571–650. [DOI] [PubMed] [Google Scholar]
- Mally A, Walker CL, Everitt JI, Dekant W, Vamvakas S. (2006) Analysis of renal cell transformation following exposure to trichloroethene in vivo and its metabolite S-(dichlorovinyl)-L-cysteine in vitro. Toxicology 224:108–118. [DOI] [PubMed] [Google Scholar]
- Maloney EK, Waxman DJ. (1999) trans-Activation of PPARalpha and PPARgamma by structurally diverse environmental chemicals. Toxicol Appl Pharmacol 161:209–218. [DOI] [PubMed] [Google Scholar]
- Maltoni C, Lefemine G, Cotti G, Perino G. (1988) Long-term carcinogenicity bioassays on trichloroethylene administered by inhalation to Sprague-Dawley rats and Swiss and B6C3F1 mice. Ann N Y Acad Sci 534:316–342. [DOI] [PubMed] [Google Scholar]
- Mandel JS, McLaughlin JK, Schlehofer B, Mellemgaard A, Helmert U, Lindblad P, McCredie M, Adami HO. (1995) International renal-cell cancer studyIv. Occupation. Int J Cancer 61:601–605. [DOI] [PubMed] [Google Scholar]
- Mazzullo M, Bartoli S, Bonora B, Colacci A, Lattanzi G, Niero A, Silingardi P, Grilli S. (1992) In vivo and in vitro interaction of trichloroethylene with macromolecules from various organs of rat and mouse. Res Commun Chem Pathol Pharmacol 76:192–208. [PubMed] [Google Scholar]
- McDougal JN, Jepson GW, Clewell HJ, 3rd, Gargas ML, Andersen ME. (1990) Dermal absorption of organic chemical vapors in rats and humans. Fundam Appl Toxicol 14:299–308. [DOI] [PubMed] [Google Scholar]
- Meckler LC, Phelps DK. (1966) Liver disease secondary to tetrachloroethylene exposure. A case report. JAMA 197:662–663. [PubMed] [Google Scholar]
- Mhiri C, Choyakh F, Ben Hmida M, Feki I, Ben Messaud M, Zouari N. (2004) Trigeminal somatosensory evoked potentials in trichloroethylene-exposed workers. Neurosciences (Riyadh) 9:102–107. [PubMed] [Google Scholar]
- Miller RE, Guengerich FP. (1983) Metabolism of trichloroethylene in isolated hepatocytes, microsomes, and reconstituted enzyme systems containing cytochrome P-450. Cancer Res 43:1145–1152. [PubMed] [Google Scholar]
- Moore LE, Boffetta P, Karami S, Brennan P, Stewart PS, Hung R, Zaridze D, Matveev V, Janout V, Kollarova H, et al. (2010) Occupational trichloroethylene exposure and renal carcinoma risk: evidence of genetic susceptibility by reductive metabolism gene variants. Cancer Res 70:6527–6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RW, Kelsh MA, Zhao K, Heringer S. (1998) Mortality of aerospace workers exposed to trichloroethylene. Epidemiology 9:424–431. [PubMed] [Google Scholar]
- Mutti A, Alinovi R, Bergamaschi E, Biagini C, Cavazzini S, Franchini I, Lauwerys RR, Bernard AM, Roels H, Gelpi E, et al. (1992) Nephropathies and exposure to perchloroethylene in dry-cleaners. Lancet 340:189–193. [DOI] [PubMed] [Google Scholar]
- Nagaya T, Ishikawa N, Hata H, Otobe T. (1993) Subclinical and reversible hepatic effects of occupational exposure to trichloroethylene. Int Arch Occup Environ Health 64:561–563. [DOI] [PubMed] [Google Scholar]
- National Toxicology Program (1976) Carcinogenesis bioassay of trichloroethylene. Natl Cancer Inst Carcinog Tech Rep Ser 2:1–215. [PubMed] [Google Scholar]
- National Toxicology Program (1977) Bioassay of tetrachloroethylene for possible carcinogenicity. Natl Cancer Inst Carcinog Tech Rep Ser 13:1–83. [PubMed] [Google Scholar]
- National Toxicology Program (1986) NTP Toxicology and Carcinogenesis Studies of Tetrachloroethylene (Perchloroethylene) (CAS No. 127-18-4) in F344/N Rats and B6C3F1 Mice (Inhalation Studies). Natl Toxicol Program Tech Rep Ser 311:1–197. [PubMed] [Google Scholar]
- National Toxicology Program (1988) NTP Toxicology and Carcinogenesis Studies of Trichloroethylene (CAS No. 79-01-6) in Four Strains of Rats (ACI, August, Marshall, Osborne-Mendel) (Gavage Studies). Natl Toxicol Program Tech Rep Ser 273:1–299. [PubMed] [Google Scholar]
- National Toxicology Program (1990) NTP Carcinogenesis Studies of Trichloroethylene (Without Epichlorohydrin) (CAS No. 79-01-6) in F344/N Rats and B6C3F1 Mice (Gavage Studies). Natl Toxicol Program Tech Rep Ser 243:1–174. [PubMed] [Google Scholar]
- National Toxicology Program (2015) Report on Carcinogens: Monograph on Trichloroethylene. US Department of Health and Human Services, Public Health Service, Research Triangle Park, NC. [Google Scholar]
- National Research Council (2006) Assessing the Human Health Risks of Trichloroethylene: Key Scientific Issues, The National Academies Press, Washigton, DC. [Google Scholar]
- National Research Council (2009) Contaminated Water Supplies at Camp Lejeune: Assessing Potential Health Effects, National Academies Press, Washington, DC. [PubMed] [Google Scholar]
- Neghab M, Qu S, Bai CL, Caples J, Stacey NH. (1997) Raised concentration of serum bile acids following occupational exposure to halogenated solvents, 1,1,2-trichloro-1,2,2-trifluoroethane and trichloroethylene. Int Arch Occup Environ Health 70:187–194. [DOI] [PubMed] [Google Scholar]
- New York State Department of Health (2010) Tetrachloroethylene (PERC) exposure and visual contrast sensitivity (VCS) teset performance in adults and children residing in buildings with or without a dry cleaner.
- Odum J, Green T, Foster JR, Hext PM. (1988) The role of trichloracetic acid and peroxisome proliferation in the differences in carcinogenicity of perchloroethylene in the mouse and rat. Toxicol Appl Pharmacol 92:103–112. [DOI] [PubMed] [Google Scholar]
- Pastino GM, Yap WY, Carroquino M. (2000) Human variability and susceptibility to trichloroethylene. Environ Health Perspect 108 (Suppl 2):201–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson B, Fredrikson M. (1999) Some risk factors for non-Hodgkin’s lymphoma. Int J Occup Med Environ Health 12:135–142. [PubMed] [Google Scholar]
- Pesch B, Haerting J, Ranft U, Klimpel A, Oelschlägel B, Schill W. (2000) Occupational risk factors for renal cell carcinoma: agent-specific results from a case-control study in Germany. MURC Study Group. Multicenter urothelial and renal cancer study. Int J Epidemiol 29:1014–1024. [DOI] [PubMed] [Google Scholar]
- Philip BK, Mumtaz MM, Latendresse JR, Mehendale HM. (2007) Impact of repeated exposure on toxicity of perchloroethylene in Swiss Webster mice. Toxicology 232:1–14. [DOI] [PubMed] [Google Scholar]
- Pohl HR, Tarkowski S, Buczynska A, Fay M, De Rosa CT. (2008) Chemical exposures at hazardous waste sites: Experiences from the United States and Poland. Environ Toxicol Pharmacol 25:283–291. [DOI] [PubMed] [Google Scholar]
- Raaschou-Nielsen O, Hansen J, McLaughlin JK, Kolstad H, Christensen JM, Tarone RE, Olsen JH. (2003) Cancer risk among workers at Danish companies using trichloroethylene: a cohort study. Am J Epidemiol 158:1182–1192. [DOI] [PubMed] [Google Scholar]
- Radican L, Blair A, Stewart P, Wartenberg D. (2008) Mortality of aircraft maintenance workers exposed to trichloroethylene and other hydrocarbons and chemicals: extended follow-up. J Occup Environ Med 50:1306–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramdhan DH, Kamijima M, Wang D, Ito Y, Naito H, Yanagiba Y, Hayashi Y, Tanaka N, Aoyama T, Gonzalez FJ, et al. (2010) Differential response to trichloroethylene-induced hepatosteatosis in wild-type and PPARalpha-humanized mice. Environ Health Perspect 118:1557–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen K, Arlien-Søborg P, Sabroe S. (1993a) Clinical neurological findings among metal degreasers exposed to chlorinated solvents. Acta Neurol Scand 87:200–204. [DOI] [PubMed] [Google Scholar]
- Rasmussen K, Brogren CH, Sabroe S. (1993b) Subclinical affection of liver and kidney function and solvent exposure. Int Arch Occup Environ Health 64:445–448. [DOI] [PubMed] [Google Scholar]
- Rasmussen K, Jeppesen HJ, Sabroe S. (1993c) Psychometric tests for assessment of brain function after solvent exposure. Am J Ind Med 24:553–565. [DOI] [PubMed] [Google Scholar]
- Rufer ES, Hacker TA, Flentke GR, Drake VJ, Brody MJ, Lough J, Smith SM. (2010) Altered cardiac function and ventricular septal defect in avian embryos exposed to low-dose trichloroethylene. Toxicol Sci 113:444–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruijten MW, Verberk MM, Sallé HJ. (1991) Nerve function in workers with long term exposure to trichloroethene. Br J Ind Med 48:87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusyn I, Chiu WA, Lash LH, Kromhout H, Hansen J, Guyton KZ. (2014) Trichloroethylene: Mechanistic, epidemiologic and other supporting evidence of carcinogenic hazard. Pharmacol Ther 141:55–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saland G. (1967) Accidental exposure to perchloroethylene. N Y State J Med 67:2359–2361. [PubMed] [Google Scholar]
- Sano Y, Nakashima H, Yoshioka N, Etho N, Nomiyama T, Nishiwaki Y, Takebayashi T, Oame K. (2009) Trichloroethylene liver toxicity in mouse and rat: microarray analysis reveals species differences in gene expression. Arch Toxicol 83:835–849. [DOI] [PubMed] [Google Scholar]
- Schreiber JS, Hudnell HK, Geller AM, House DE, Aldous KM, Force MS, Langguth K, Prohonic EJ, Parker JC. (2002) Apartment residents’ and day care workers’ exposures to tetrachloroethylene and deficits in visual contrast sensitivity. Environ Health Perspect 110:655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott CS, Jinot J. (2011) Trichloroethylene and cancer: systematic and quantitative review of epidemiologic evidence for identifying hazards. Int J Environ Res Public Health 8:4238–4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiji K, Jin C, Watanabe T, Nakatsuka H, Ikeda M. (1990) Sister chromatid exchanges in peripheral lymphocytes of workers exposed to benzene, trichloroethylene, or tetrachloroethylene, with reference to smoking habits. Int Arch Occup Environ Health 62:171–176. [DOI] [PubMed] [Google Scholar]
- Seldén AI, Ahlborg G., Jr (2011) Cancer morbidity in Swedish dry-cleaners and laundry workers: historically prospective cohort study. Int Arch Occup Environ Health 84:435–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver SR, Pinkerton LE, Fleming DA, Jones JH, Allee S, Luo L, Bertke SJ. (2014) Retrospective cohort study of a microelectronics and business machine facility. Am J Ind Med 57:412–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solet D, Robins TG. (1991) Renal function in dry cleaning workers exposed to perchloroethylene. Am J Ind Med 20:601–614. [DOI] [PubMed] [Google Scholar]
- Suarez L, Weiss NS, Martin J. (1989) Primary liver cancer death and occupation in Texas. Am J Ind Med 15:167–175. [DOI] [PubMed] [Google Scholar]
- Tao L, Kramer PM, Ge R, Pereira MA. (1998) Effect of dichloroacetic acid and trichloroacetic acid on DNA methylation in liver and tumors of female B6C3F1 mice. Toxicol Sci 43:139–144. [DOI] [PubMed] [Google Scholar]
- Tao L, Li Y, Kramer PM, Wang W, Pereira MA. (2004) Hypomethylation of DNA and the insulin-like growth factor-II gene in dichloroacetic and trichloroacetic acid-promoted mouse liver tumors. Toxicology 196:127–136. [DOI] [PubMed] [Google Scholar]
- Tao L, Yang S, Xie M, Kramer PM, Pereira MA. (2000) Effect of trichloroethylene and its metabolites, dichloroacetic acid and trichloroacetic acid, on the methylation and expression of c-Jun and c-Myc protooncogenes in mouse liver: prevention by methionine. Toxicol Sci 54:399–407. [DOI] [PubMed] [Google Scholar]
- Trevisan A, Maccà I, Rui F, Carrieri M, Battista Bartolucci G, Manno M. (2000) Kidney and liver biomarkers in female dry-cleaning workers exposed to perchloroethylene. Biomarkers 5:399–409. [DOI] [PubMed] [Google Scholar]
- Triebig G, Bestler W, Baumeister P, Valentin H. (1983) [Neurotoxicity of workplace substances. IV. Determination of motor and sensory nerve conduction velocity in persons exposed to solvent mixtures]. Int Arch Occup Environ Health 52:139–150. [DOI] [PubMed] [Google Scholar]
- Triebig G, Trautner P, Weltle D, Saure E, Valentin H. (1982) [Investigations on neurotoxicity of chemical substances at the workplace. III. Determination of the motor and sensory nerve conduction velocity in persons occupationally exposed to trichloroethylene] [Article in German]. Int Arch Occup Environ Health 51:25–34. [DOI] [PubMed] [Google Scholar]
- Tucker JD, Sorensen KJ, Ruder AM, McKernan LT, Forrester CL, Butler MA. (2011) Cytogenetic analysis of an exposed-referent study: perchloroethylene-exposed dry cleaners compared to unexposed laundry workers. Environ Health 10:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US EPA (2011a) Toxicological Review of Tetrachloroethylene (CAS No. 127-18-4) in Support of Summary Information on the Integrated Risk Information System (IRIS), US Environmental Protection Agency, Washington, DC. [Google Scholar]
- US EPA (2011b) Toxicological Review of Trichloroethylene (CAS No. 79-01-6) in Support of Summary Information on the Integrated Risk Information System (IRIS), US Environmental Protection Agency, Washington, DC. [Google Scholar]
- Vamvakas S, Berthold K, Dekant W, Henschler D. (1988a) Bacterial cysteine conjugate beta-lyase and the metabolism of cysteine S-conjugates: structural requirements for the cleavage of S-conjugates and the formation of reactive intermediates. Chem Biol Interact 65:59–71. [DOI] [PubMed] [Google Scholar]
- Vamvakas S, Brüning T, Thomasson B, Lammert M, Baumüller A, Bolt HM, Dekant W, Birner G, Henschler D, Ulm K. (1998) Renal cell cancer correlated with occupational exposure to trichloroethene. J Cancer Res Clin Oncol 124:374–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vamvakas S, Dekant W, Berthold K, Schmidt S, Wild D, Henschler D. (1987) Enzymatic transformation of mercapturic acids derived from halogenated alkenes to reactive and mutagenic intermediates. Biochem Pharmacol 36:2741–2748. [DOI] [PubMed] [Google Scholar]
- Vamvakas S, Dekant W, Henschler D. (1989a) Assessment of unscheduled DNA synthesis in a cultured line of renal epithelial cells exposed to cysteine S-conjugates of haloalkenes and haloalkanes. Mutat Res 222:329–335. [DOI] [PubMed] [Google Scholar]
- Vamvakas S, Dekant W, Henschler D. (1989b) Genotoxicity of haloalkene and haloalkane glutathione S-conjugates in porcine kidney cells. Toxicol In Vitro 3:151–156. [DOI] [PubMed] [Google Scholar]
- Vamvakas S, Dekant W, Schiffmann D, Henschler D. (1988b) Induction of unscheduled DNA synthesis and micronucleus formation in Syrian hamster embryo fibroblasts treated with cysteine S-conjugates of chlorinated hydrocarbons. Cell Biol Toxicol 4:393–403. [DOI] [PubMed] [Google Scholar]
- Vamvakas S, Elfarra AA, Dekant W, Henschler D, Anders MW. (1988c) Mutagenicity of amino acid and glutathione S-conjugates in the Ames test. Mutat Res 206:83–90. [DOI] [PubMed] [Google Scholar]
- Vermeulen R, Zhang L, Spierenburg A, Tang X, Bonventre JV, Reiss B, Shen M, Smith MT, Qiu C, Ge Y, et al. (2012) Elevated urinary levels of kidney injury molecule-1 among Chinese factory workers exposed to trichloroethylene. Carcinogenesis 33:1538–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verplanke AJ, Leummens MH, Herber RF. (1999) Occupational exposure to tetrachloroethene and its effects on the kidneys. J Occup Environ Med 41:11–16. [DOI] [PubMed] [Google Scholar]
- Vlaanderen J, Straif K, Pukkala E, Kauppinen T, Kyyrönen P, Martinsen JI, Kjaerheim K, Tryggvadottir L, Hansen J, Sparén P, et al. (2013) Occupational exposure to trichloroethylene and perchloroethylene and the risk of lymphoma, liver, and kidney cancer in four Nordic countries. Occup Environ Med 70:393–401. [DOI] [PubMed] [Google Scholar]
- Vlaanderen J, Straif K, Ruder A, Blair A, Hansen J, Lynge E, Charbotel B, Loomis D, Kauppinen T, Kyyronen P, et al. (2014) Tetrachloroethylene exposure and bladder cancer risk: a meta-analysis of dry-cleaning-worker studies. Environ Health Perspect 122:661–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Völkel W, Dekant W. (1998) Chlorothioketene, the ultimate reactive intermediate formed by cysteine conjugate beta-lyase-mediated cleavage of the trichloroethene metabolite S-(1,2-Dichlorovinyl)-L-cysteine, forms cytosine adducts in organic solvents, but not in aqueous solution. Chem Res Toxicol 11:1082–1088. [DOI] [PubMed] [Google Scholar]
- Vyskocil A, Emminger S, Tejral J, Fiala Z, Ettlerova E, Cermanová A. (1990) Study on kidney function in female workers exposed to perchlorethylene. Hum Exp Toxicol 9:377–380. [DOI] [PubMed] [Google Scholar]
- Wang R, Zhang Y, Lan Q, Holford TR, Leaderer B, Zahm SH, Boyle P, Dosemeci M, Rothman N, Zhu Y, Qin Q, Zheng T. (2009) Occupational exposure to solvents and risk of non-hodgkin lymphoma in connecticut women. Am J Epidemiol 169:176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White IN, Razvi N, Gibbs AH, Davies AM, Manno M, Zaccaro C, De Matteis F, Pähler A, Dekant W. (2001) Neoantigen formation and clastogenic action of HCFC-123 and perchloroethylene in human MCL-5 cells. Toxicol Lett 124:129–138. [DOI] [PubMed] [Google Scholar]
- Xu X, Yang R, Wu N, Zhong P, Ke Y, Zhou L, Yuan J, Li G, Huang H, Wu B. (2009) Severe hypersensitivity dermatitis and liver dysfunction induced by occupational exposure to trichloroethylene. Ind Health 47:107–112. [DOI] [PubMed] [Google Scholar]
- Yoo HS, Bradford BU, Kosyk O, Shymonyak S, Uehara T, Collins LB, Bodnar WM, Ball LM, Gold A, Rusyn I. (2015a) Comparative analysis of the relationship between trichloroethylene metabolism and tissue-specific toxicity among inbred mouse strains: liver effects. J Toxicol Environ Health A 78:15–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo HS, Bradford BU, Kosyk O, Uehara T, Shymonyak S, Collins LB, Bodnar WM, Ball LM, Gold A, Rusyn I. (2015b) Comparative analysis of the relationship between trichloroethylene metabolism and tissue-specific toxicity among inbred mouse strains: kidney effects. J Toxicol Environ Health A 78:32–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo HS, Cichocki JA, Kim S, Venkatratnam A, Iwata Y, Kosyk O, Bodnar W, Sweet S, Knap A, Wade T, et al. (2015c) The Contribution of Peroxisome Proliferator-Activated Receptor Alpha to the Relationship Between Toxicokinetics and Toxicodynamics of Trichloroethylene. Toxicol Sci 147:339–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Krishnadasan A, Kennedy N, Morgenstern H, Ritz B. (2005) Estimated effects of solvents and mineral oils on cancer incidence and mortality in a cohort of aerospace workers. Am J Ind Med 48:249–258. [DOI] [PubMed] [Google Scholar]
- Zhu H, Rusyn I, Richard A, Tropsha A. (2008) Use of cell viability assay data improves the prediction accuracy of conventional quantitative structure-activity relationship models of animal carcinogenicity. Environ Health Perspect 116:506–513. [DOI] [PMC free article] [PubMed] [Google Scholar]