Abstract
BACKGROUND
The Renal Optimization Strategies Evaluation in Acute Heart Failure (ROSE AHF) trial found that as compared to placebo, neither low-dose dopamine (2 ug/kg/min) nor low-dose nesiritide (0.005 µg/kg/min without bolus) enhanced decongestion or preserved renal function in AHF patients with renal dysfunction. However, there may be differential responses to vasoactive agents in AHF patients with reduced versus preserved ejection fraction (EF). This post-hoc analysis examined potential interaction between treatment effect and EF group (EF ≤ 40% vs > 40%) on the ROSE AHF endpoints.
METHODS AND RESULTS
ROSE AHF enrolled AHF patients (n=360; any EF) with renal dysfunction. The co-primary endpoints were cumulative urine volume and the change in serum cystatin-C from over 72 hours. The effect of dopamine (interaction p=0.001) and nesiritide (interaction p=0.039) on urine volume varied by EF group. In HFrEF, urine volume was higher with active treatment versus placebo whereas in HFpEF, urine volume was lower with active treatment. The effect of dopamine and nesiritide on weight change, sodium excretion and incidence of AHF treatment failure also varied and in a similar manner by EF group (interaction p<0.05 for all). There was no interaction between either vasoactive treatment’s effect and EF group on change in cystatin-C. Compared to placebo, dopamine was associated with improved clinical outcomes in HFrEF and worse clinical outcomes in HFpEF. With nesiritide, there were no differences in clinical outcomes as compared to placebo in both HFrEF and HFpEF.
CONCLUSIONS
In this post-hoc analysis of ROSE AHF, the response to vasoactive therapies differed in patients with HFrEF and HFpEF. Investigation of AHF therapies should assess the potential for differential responses in AHF with preserved versus reduced EF.
CLINICAL TRIAL REGISTRATION
URL: http://www.clinicaltrials.gov. Unique identifier: NCT01132846.
Keywords: heart failure, renal function, diastolic heart failure, inotropes, vasodilator
Acute heart failure (AHF) accounts for over one million hospital admissions annually.1 There remains a paucity of randomized clinical trial data to guide treatment for hospitalized patients with AHF.2 Approximately fifty percent of AHF patients have preserved (HFpEF) ejection fraction (EF), and HFpEF patients are more likely to be older, female and hypertensive compared to AHF patients with AHF and reduced EF (HFrEF).3 These unique clinical features and fundamental differences in ventricular structure and function in HFpEF versus HFrEF may predispose to differential responses to treatments for AHF.
The Renal Optimization Strategies Evaluation in Acute Heart Failure (ROSE AHF) found that in patients with AHF and renal dysfunction, low-dose dopamine or low-dose nesiritide did not improve renal function or enhance decongestion when compared with standard diuretic therapy alone.4 Previous small studies which investigated the renal protective effects of low-dose dopamine included only subjects with HFrEF5, 6 or did not examine the interaction between treatment allocation and EF.7, 8 The Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure (ASCEND-HF) trial of nesiritide in AHF revealed no differential effect of nesiritide on symptom relief or outcomes in AHF patients with HFrEF or HFpEF, but tested standard dose nesiritide and did not examine the interaction between treatment effect and EF group on decongestion and renal preservation.1, 9
The objective of this post-hoc analysis of the ROSE AHF study was to determine if there are differential responses to low-dose dopamine or low-dose nesiritide in AHF patients with HFpEF versus HFrEF.
METHODS
Study Overview
The ROSE AHF study design and results have been previously described.4, 10 ROSE AHF was a double-blind, placebo-controlled, randomized clinical trial designed by and performed within the National Heart, Lung, and Blood Institute sponsored Heart Failure Research Network. The study was approved by each participating center’s institutional review board, and all participants provided written informed consent.
Study Design
Patients (n=360) hospitalized with AHF and renal dysfunction were enrolled within 24 hours of hospital admission. Heart failure (HF) was defined by at least one HF symptom plus at least one HF sign. Renal dysfunction was defined as a glomerular filtration rate of 15–60 mL/min/1.73m2 estimated by the Modification of Diet in Renal Disease equation.11, 12
Patients were initially randomized in an open, 1:1 manner to the dopamine or nesiritide strategy. Within each strategy, patients were randomized in a double-blind, 2:1 manner to the active therapy versus placebo group. The dopamine subjects received a 2 ug/kg/min infusion for 72 hours. The nesiritide subjects received a 0.005 ug/kg/min infusion for 72 hours with no loading dose of nesiritide. The placebo subjects were pooled across the two strategies and compared to the active therapy groups. All patients received open-label intravenous loop diuretic therapy with a recommended initial dose of 2.5 times the patient’s outpatient oral loop diuretic, a 2000-mg sodium diet and a 2000-mL fluid restriction. Double randomization was performed to limit the number of patients requiring central lines for dopamine administration and to test the two independent hypotheses of dopamine and nesiritide. There was one placebo, which differed by route of administration to match the active treatment it was paired with, with blocks as small as possible given the trial design. The trial includes 26 participating centers across North America.
This subgroup analysis retrospectively analyzed the co-primary and secondary outcomes as defined in the ROSE AHF study by subdividing the dopamine, nesiritide, and pooled placebo groups based on EF ≤ 40% (HFrEF) or > 40% (HFpEF). In the original ROSE AHF analysis, the pre-specified subgroup analysis used 50% as the EF cutoff in defining HFrEF and HFpEF.4, 10 However, in this analysis, we chose to use a cutoff of 40% so as to capture those with significantly impaired systolic function; recognizing that several large AHF studies have used a partition value of 40% when comparing AHF patients with HFrEF versus HFpEF.3, 13 Additionally, given the baseline characteristics and mean EF of the enrolled population, a cutoff of 40% allowed for more appropriate sample size in both EF groups. A sensitivity analysis was performed using 50% as the partition value for HFpEF and HFrEF, and comparison of results based on a 50% versus 40% cutoff can be found in the supplemental materials.
Outcome Measurements
The co-primary endpoints were decongestion, as measured by 72-hour cumulative urine volume, and renal function preservation, as measured by change in cystatin C from randomization to 72 hours.
Secondary outcomes included additional measures of decongestion (weight change, cumulative sodium excretion, change in NT-proBNP and incidence of treatment failure at 72 hours). Treatment failure was defined as the development of type 1 cardiorenal syndrome (CRS), worsening or persistent HF with need for additional vasoactive agents, ultrafiltration, or mechanical respiratory support, significant hypotension or significant tachycardia. Secondary measures of changes in renal function included changes in creatinine and incidence of CRS (increase in creatinine of greater than 0.3 mg/dL) at 72 hours. Symptom relief was assessed by the patient's global assessment of symptoms, measured with the use of a visual-analogue scale and quantified as the area under the curve (AUC VAS) of serial assessments from baseline to 72 hours with a larger value indicating greater symptom relief.14 Clinical outcomes including mortality (60 and 180 day) and mortality or HF re-hospitalization by 60 days were assessed.
Statistical Methods
Unless otherwise noted, data are presented as median (25th, 75th percentile) for continuous variables and as percent for categorical variables. Categorical baseline characteristics were compared with chi-square tests or with Fisher’s exact tests in the case of small cell counts. Continuous variables were compared with Wilcoxon rank sum tests. Continuous endpoints were modeled with linear regression models that included randomized treatment, EF group, and the treatment-by-EF group interaction. In the cases of weight, creatinine, cystatin-C, NTproBNP, and VAS AUC change, models were also adjusted for the baseline measure. The c-index is the area under the receiver operating characteristic curve from logistic regression where models include the treatment effect, the binary EF category, and the treatment-by-EF category interaction. In the case of cardiorenal syndrome, the model also adjusts for baseline creatinine value. For time to event endpoints, Harrell’s c-index is presented.
Multiple imputation was used to account for missing data in linear regression models. One hundred imputed datasets were created for each continuous endpoint where the following variables were included when creating the imputed datasets: age, sex, treatment, and values of the endpoint at baseline, 24, 48, and 72 hours. Linear regression was done for each dataset and results of the 100 models combined. This method assumes that data are missing at random. Categorical endpoints at 72 hours were analyzed with logistic regression models that included randomized treatment, EF group, and the treatment-by-EF group interaction. The model for incidence of CRS was adjusted for baseline creatinine. Longer term categorical endpoints (death and HF rehospitalization) were analyzed with Cox proportional hazards regression models. The summary statistics presented for 60 and 180 day endpoints are number of events/number non-missing follow-up time (Kaplan-Meier rate). P-values for treatment effects within EF group are only shown if there is a significant interaction (p<0.05 for primary analysis and p<0.10 for sensitivity analysis). Data were analyzed with SAS version 9.4.
RESULTS
Patient population
There were 360 study participants enrolled in ROSE AHF between September 2010 and March 2013 at 26 sites in the United States and Canada. In this subgroup analysis, only 358 patients were included as baseline EF data was missing for 2 subjects. Of these, 222 (62%) had HFrEF and 136 (38%) had HFpEF. As compared to the HFrEF group, subjects in the HFpEF group were older, with a greater proportion being female and of white race (Table 1). The HFpEF subjects also had higher body mass index (BMI), systolic blood pressure and prevalence of atrial fibrillation but fewer implantable cardiac devices (ICD) or recent HF hospitalizations. The HFrEF subjects were more likely to be on standard HF therapies than HFpEF patients. Subjects in the HFpEF group had higher plasma cystatin C with equivalent creatinine and eGFR, and lower NT-proBNP compared to the HFrEF group.
Table 1.
Baseline characteristics by ejection fraction group
| Variable | EF ≤ 40 (N=222*) | EF > 40 (N=136*) | p |
|---|---|---|---|
| Age (years) | 68.5 (61, 77) | 74 (65, 83) | 0.0004 |
| Male sex | 178 (80) | 86 (63) | 0.0004 |
| White race | 159 (72) | 112 (82) | 0.0216 |
| Body Mass Index (kg/m2) | N=219 | N=132 | 0.0029 |
| 30.1 (25.8, 35.5) | 33.0 (27.5, 39.1) | ||
| Systolic Blood Pressure (mmHg) | 111 (100, 123) | 119 (110, 135) | <0.0001 |
| Edema ≥ 2+ | 154/221 (70) | 95/134 (71) | 0.81 |
| Orthopnea | 194/212 (92) | 111/129 (86) | 0.11 |
| JVP ≥ 8 cm water | 203/212 (96) | 123/130 (95) | 0.63 |
| Rales | 128/219 (58) | 68/133 (51) | 0.18 |
| Last Ejection Fraction (%) | 25 (18, 30) | 55 (50, 60) | <0.0001 |
| HF Hospitalization in last year | 158/221 (71) | 81/135 (60) | 0.0251 |
| Ischemic HF etiology | 135 (61) | 73 (54) | 0.18 |
| Diabetes | 120 (54) | 79 (58) | 0.46 |
| Atrial fibrillation/flutter | 124 (56) | 91 (67) | 0.0382 |
| Hypertension | 177 (80) | 119 (88) | 0.0593 |
| ICD | 140 (63) | 17 (13) | <0.0001 |
| Cardiac Medications | |||
| ACEI / ARB | 121 (55) | 57 (42) | 0.0207 |
| Hydralazine | 41 (18) | 27 (20) | 0.75 |
| Nitrates | 66 (30) | 24 (18) | 0.0105 |
| Beta blocker | 194 (87) | 104 (76) | 0.0073 |
| Aldosterone antagonist | 73 (33) | 36 (26) | 0.20 |
| Digoxin | 68 (31) | 21 (15) | 0.0012 |
| Loop diuretic | 209 (94) | 130 (96) | 0.55 |
| Laboratory Values | |||
| Plasma cystatin C (mg/L) | N=212 | N=132 | 0.0455 |
| 1.66 (1.40, 2.10) | 1.83 (1.49, 2.24) | ||
| Creatinine (mg/dL) | N=212 | N=132 | 0.13 |
| 1.64 (1.39, 1.98) | 1.62 (1.23, 1.99) | ||
| eGFR (mL/min/1.73m2) | N=212 | N=132 | 0.66 |
| 44.6 (34.8, 55.7) | 44.1 (30.8, 55.5) | ||
| BUN (mg/dL) | N=220 | N=136 | 0.74 |
| 36.5 (28.0, 51.0) | 38.0 (27.0, 50.8) | ||
| NT-proBNP value (pg/mL) | N=212 | N=132 | <0.0001 |
| 6671 (3347, 11469) | 3324 (1542, 7079) |
If data are missing for a variable, the number with non-missing data is presented as a denominator for categorical variables or on a separate row for continuous variables.
Data are n (%) or Median (50th, 75th percentile).
Abbreviations: JVP, jugular venous pulse; ICD, implantable cardiac defibrillator; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker: eGFR, estimated glomerular filtration rate; BUN, blood urea nitrogen; HF, heart failure
In the HFrEF group, there were 76, 74 and 72 subjects in the placebo, dopamine and nesiritide groups respectively. In the HFpEF group, there were 43, 46 and 47 subjects in the placebo, dopamine, and nesiritide groups respectively. The baseline characteristics were similar between the treatment groups within each EF group (Supplemental Table 1).
Low-Dose Dopamine Strategy
There was a differential effect of dopamine on decongestion between EF groups, including endpoints of 72-hour cumulative urine output (Figure 1), sodium excretion, and treatment failure (interaction p<0.05 for all; Table 2). However, there was no differential effect of dopamine on 72-hour change in cystatin-c (interaction p=0.66; Figure 1), or NT-proBNP. There was also differential effect on clinical endpoints, including death at 60 or 180 days and death or HF rehospitalization at 60 days (interaction p<0.05 for all; Table 2).
Figure 1.
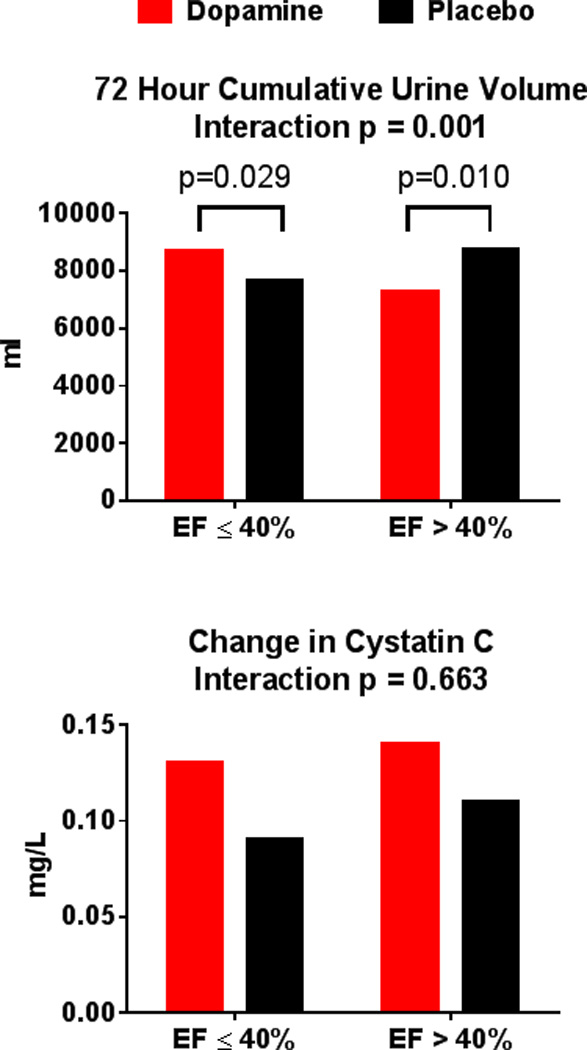
Co-Primary Endpoints in Dopamine Strategy according to Ejection Fraction Group
Before imputation, 13 had missing urine volume and 36 had missing cystatin C.
Table 2.
Secondary Outcomes for Dopamine Strategy
| Endpoint* | N missing before imputation |
Placebo | Dopamine | Treatment difference, dopamine – placebo (95% CI) |
P value† | Interaction P Value |
|---|---|---|---|---|---|---|
| Weight Change (lbs) | 0.0001 | |||||
| EF ≤ 40% | 9 | −5.1 (−10.0, −2.4) | −8.9 (−13, −4.0) | −2.8 (−5.1, −0.5) | 0.0161 | |
| EF > 40% | 8 | −9.4 (−14.0, −5.1) | −6.0 (−9.0, −1.5) | 4.6 (1.4, 7.9) | 0.0056 | |
| 72-hr Na Excretion (mmol) | 0.0094 | |||||
| EF ≤ 40% | 30 | 445 (308, 672) | 536 (328, 763) | 65 (−33, 163) | 0.20 | |
| EF > 40% | 20 | 548 (350, 720) | 396 (261, 546) | −150 (−280, −20) | 0.0235 | |
| AUC VAS Change | 0.0564 | |||||
| EF ≤ 40% | 8 | 4860 (3698, 5616) | 4547 (3728, 5615) | 71 (−273, 416) | n/a | |
| EF > 40% | 7 | 5084 (4366, 5863) | 4651 (3505, 5412) | −468 (−884, −51) | n/a | |
| NT-proBNP Change (pg/mL) | 0.16 | |||||
| EF ≤ 40% | 19 | −1611 (−3764, −175) | −2698 (−5162, −710) | −772 (−1838, 293) | n/a | |
| EF > 40% | 17 | −706 (−1531, −95) | −585 (−2264, 497) | 252 (−929, 1433) | n/a | |
| Creatinine Change (mg/dL) | 0.28 | |||||
| EF ≤ 40% | 19 | 0.04 (−0.13, 0.24) | 0.02 (−0.29, 0.25) | −0.06 (−0.19, 0.08) | n/a | |
| EF > 40% | 17 | −0.05 (−0.22, 0.11) | −0.03 (−0.26, 0.21) | 0.07 (−0.09, 0.23) | n/a | |
|
Odds ratio for dopamine v. placebo (95% CI) |
||||||
| Treatment failure at 72 hr (c=0.61) | 0.0190 | |||||
| EF ≤ 40% | 4 | 28/75 (37.3%) | 21/71 (29.6%) | 0.7 (0.4, 1.4) | 0.32 | |
| EF > 40% | 5 | 4/40 (10.0%) | 13/44 (29.5%) | 3.8 (1.1, 12.8) | 0.0327 | |
| CRS Incidence (c=0.67) | 0.0532 | |||||
| EF ≤ 40% | 13 | 20/71 (28.2%) | 14/66 (21.2%) | 0.7 (0.3, 1.5) | n/a | |
| EF > 40% | 12 | 4/39 (10.3%) | 9/38 (23.7%) | 3.1 (0.8, 11.4) | n/a | |
|
Hazard ratio for dopamine v. placebo (95% CI) |
||||||
| Death at 60 d (c=0.69) | 0.0053 | |||||
| EF ≤ 40% | 0 | 10/76 (13.7%) | 2/74 (2.8%) | 0.2 (0.04, 0.9) | 0.0328 | |
| EF > 40% | 0 | 2/43 (4.8%) | 8/46 (18.2%) | 4.2 (0.9, 19.8) | 0.0696 | |
| Death at 180 d (c=0.63) | 0.0025 | |||||
| EF ≤ 40% | 0 | 19/76 (26.5%) | 9/74 (12.7%) | 0.4 (0.2, 0.9) | 0.0323 | |
| EF > 40% | 0 | 5/43 (11.9%) | 14/46 (29.5%) | 3.1 (1.1, 8.6) | 0.0306 | |
| Death or HF ReHsp at 60d (c=0.59) | 0.0173 | |||||
| EF ≤ 40% | 2 | 22/75 (27.8%) | 16/73 (22.9%) | 0.7 (0.4, 1.3) | 0.23 | |
| EF > 40% | 3 | 7/42 (16.9%) | 16/44 (37.3%) | 2.6 (1.1, 6.2) | 0.0379 |
Data are median (25th, 75th percentile) or n/n with data (%) unless noted. Continuous endpoints use multiple imputation data and categorical variables use non-missing data.
Independent variables included in each model are EF category, randomized treatment, and the EF category-by-treatment interaction. In addition, models for change in weight, VAS, NT-proBNP, and creatinine also adjusted for the baseline value. Baseline creatinine was used for adjustment in the model for cardiorenal syndrome over 72 hours. The C-index is shown in parentheses for the binary and time to event endpoints.
P-values for treatment effects within EF categories are only shown if there is a significant interaction.
Abbreviations: AUC VAS, Area under the curve at 72 hours for Global Well Being Visual Analog Score; CRS, Cardiorenal Syndrome; d, day; HF, heart failure; hr, hour; ReHsp, re-hospitalization
In the HFrEF group, there was greater urine volume in the dopamine (8703 {7070, 10970} mL) versus placebo (7650 {5803, 10123} mL, p = 0.029) group. Additionally, in the HFrEF dopamine versus placebo group, there was greater weight reduction, but no significant difference in VAS AUC or incidence of CRS (Table 2). For clinical endpoints, in the HFrEF dopamine versus placebo group, there were fewer deaths at 60 or 180 days (Table 2).
In contrast, in the HFpEF group, urine volume was lower in the dopamine (7300 {5450, 8950} mL) versus placebo (8750 {7250, 11400} mL, p = 0.010) group. Additionally, in the HFpEF dopamine versus placebo group, there was less weight reduction, lower 72-hour sodium excretion, greater incidence of treatment failure, lower difference in VAS AUC, and higher incidence of CRS (Table 2). For clinical endpoints, in the HFpEF dopamine versus placebo group, there were significantly more deaths at 180 days, and higher incidence of death or HF rehospitalization at 60 days (Table 2).
Low-Dose Nesiritide Strategy
There was a differential effect of nesiritide on decongestion between EF groups, including endpoints of 72-hour cumulative urine volume (Figure 2), weight change, sodium excretion, and treatment failure (interaction p<0.05 for all; Table 3). However, there was no differential effect of nesiritide on 72-hour change in cystatin-c (interaction p=0.37; Figure 2), or NT-proBNP.
Figure 2.
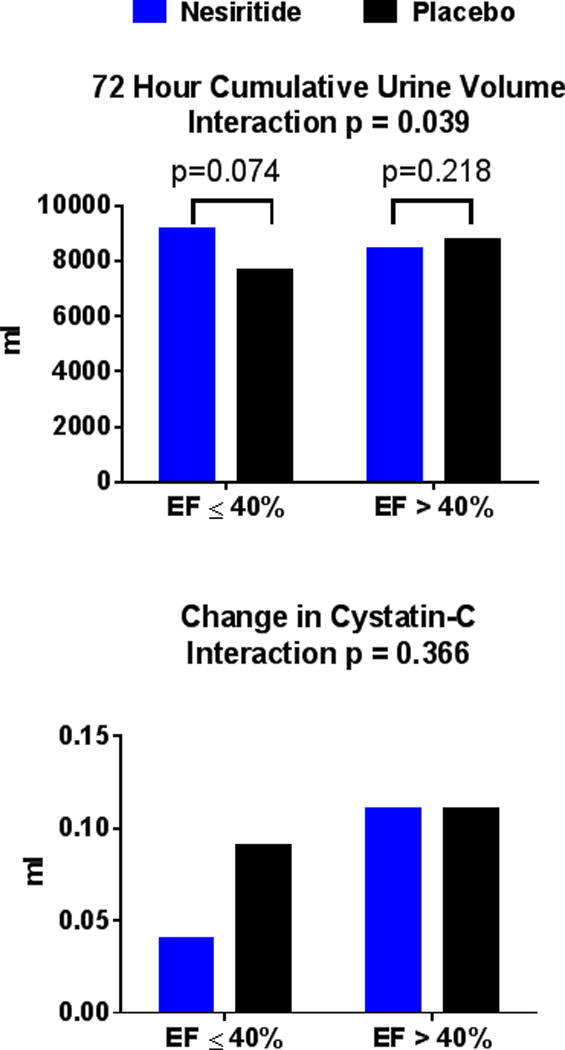
Co-Primary Endpoints in Nesiritide Strategy according to Ejection Fraction Group
Before imputation, 17 had missing urine volume and 30 had missing cystatin C.
Table 3.
Secondary Outcomes for Nesiritide Strategy
| Endpoint* | N missing before imputation |
Placebo | Nesiritide | Treatment difference, nesiritide – placebo (95% CI) |
P value† | Interaction P Value |
|---|---|---|---|---|---|---|
| Weight Change (lbs) | 0.0039 | |||||
| EF ≤ 40% | 10 | −5.1 (−10, −2.4) | −7.3 (−11, −3.5) | −1.6 (−3.9, 0.6) | 0.15 | |
| EF > 40% | 11 | −9.4 (−14, −5.1) | −6.2 (−9.5, −3.1) | 3.8 (0.5, 7.1) | 0.0242 | |
| 72-hr Na Excretion (mmol) | 0.0301 | |||||
| EF ≤ 40% | 30 | 445 (308, 672) | 517 (373, 720) | 39 (−53, 130) | 0.41 | |
| EF > 40% | 18 | 548 (350, 720) | 381 (310, 550) | −130 (−254, −5) | 0.0411 | |
| AUC VAS Change | 0.0901 | |||||
| EF ≤ 40% | 6 | 4860 (3698, 5616) | 4674 (3823, 5560) | 93 (−203, 388) | n/a | |
| EF > 40% | 5 | 5084 (4366, 5863) | 4382 (3624, 5180) | −408 (−794, −23) | n/a | |
| NT-proBNP Change (pg/mL) | 0.9060 | |||||
| EF ≤ 40% | 18 | −1611 (−3764, −175) | −1592 (−4461, −512) | −659 (−1720, 402) | n/a | |
| EF > 40% | 12 | −706 (−1531, −95) | −750 (−2149, −171) | −539 (−1415, 338) | n/a | |
| Creatinine Change (mg/dL) | 0.0671 | |||||
| EF ≤ 40% | 18 | 0.04 (−0.13, 0.24) | −0.08 (−0.26, 0.25) | −0.08 (−0.22, 0.06) | n/a | |
| EF > 40% | 12 | −0.05 (−0.22, 0.11) | 0.07 (−0.13, 0.27) | 0.11 (−0.02, 0.24) | n/a | |
|
Odds ratio for nesiritide v. placebo (95% CI) |
||||||
| Treatment failure at 72 hr (c=0.61) | 0.0266 | |||||
| EF ≤ 40% | 1 | 28/75 (37.3%) | 30/72 (41.7%) | 1.2 (0.6, 2.3) | 0.59 | |
| EF > 40% | 3 | 4/40 (10.0%) | 18/47 (38.3%) | 5.6 (1.7, 18.3) | 0.0046 | |
| CRS Incidence (c=0.60) | 0.0854 | |||||
| EF ≤ 40% | 10 | 20/71 (28.2%) | 17/67 (25.4%) | 0.8 (0.4, 1.8) | n/a | |
| EF > 40% | 7 | 4/39 (10.3%) | 11/44 (25.0%) | 3.0 (0.9, 10.6) | n/a | |
|
Hazard ratio for nesiritide v. placebo (95% CI) |
||||||
| Death at 60 d (c=0.65) | 0.72 | |||||
| EF ≤ 40% | 0 | 10/76 (13.7%) | 7/72 (10.0%) | 0.7 (0.3, 1.9) | n/a | |
| EF > 40% | 0 | 2/43 (4.8%) | 1/47 (2.1%) | 0.5 (0.04, 4.9) | n/a | |
| Death at 180 d (c=0.64) | 0.39 | |||||
| EF ≤ 40% | 0 | 19/76 (26.5%) | 20/72 (28.8%) | 1.1 (0.6, 2.0) | n/a | |
| EF > 40% | 0 | 5/43 (11.9%) | 3/47 (4.4%) | 0.5 (0.1, 2.2) | n/a | |
| Death or HF ReHsp at 60d (c=0.62) | 0.25 | |||||
| EF ≤ 40% | 2 | 22/75 (27.8%) | 20/71 (28.1%) | 0.9 (0.5, 1.6) | n/a | |
| EF > 40% | 1 | 7/42 (16.9%) | 3/47 (6.4%) | 0.4 (0.1, 1.4) | n/a |
Data are median (25th, 75th percentile) or n/n with data (%) unless noted. Continuous endpoints use multiple imputation data and categorical variables use non-missing data.
Independent variables included in each model are EF category, randomized treatment, and the EF category-by-treatment interaction. In addition, models for change in weight, VAS, NT-proBNP, and creatinine also adjusted for the baseline value. Baseline creatinine was used for adjustment in the model for cardiorenal syndrome over 72 hours. The C-index is shown in parentheses for the binary and time to event endpoints.
P-values for treatment effects within EF categories are only shown if there is a significant interaction.
Abbreviations: AUC VAS, Area under the Global Well Being Visual Analog Score; CRS, Cardiorenal Syndrome; d, day; HF, heart failure; hr, hour; ReHsp, re-hospitalization
In HFrEF, there was a non-significant trend for greater 72-hour cumulative urine volume in the nesiritide (9135 {6300, 10325} mL) versus placebo (7650 {5803, 10123} mL, p = 0.074) group. Additionally, in the HFrEF nesiritide versus placebo group, there was no difference in weight loss, 72-hour sodium excretion or incidence of treatment failure.
In the HFpEF group, there was no difference in 72-hour cumulative urine volume in the nesiritide (8400 {5730, 10450} mL) versus placebo (8750 {7250, 11400} mL, p = 0.22) group. Additionally, in the HFpEF nesiritide versus placebo group, there was less weight reduction, lower 72-hour sodium excretion, and greater incidence of treatment failure (Table 3).
There was no differential effect of nesiritide on clinical outcomes between the EF groups (Table 3), and there was no differential effect of nesiritide on symptom relief (VAS AUC) (interaction p=0.090) (Table 3).
In summary, the results suggest that low dose dopamine is associated with a beneficial effect on both surrogate and clinical outcomes in HFrEF patients. In contrast, there is a signal for more adverse clinical outcomes with dopamine in patients with HFpEF. Furthermore, the effects of nesiritide on surrogate outcomes were better in HFrEF than HFpEF, but there was no differential effect on clinical outcomes.
Sensitivity Analysis
Findings of this analysis were not meaningfully different when an EF of 50% was used as the partition value for HFrEF versus HFpEF (Supplemental Tables 2–5). Patient characteristics between treatment groups in HFrEF and HFpEF did not differ (SSupplemental Table 2). Similar to analysis using EF≤40% or >40% to define HFrEF and HFpEF, there were significant (p<0.05) interactions between treatment allocation and EF group for both dopamine and nesiritide on the co-primary 72 hour cumulative urine volume endpoint but not on the co-primary change in cystatin C endpoint (Supplemental Table 3). Differential effects on secondary endpoints were also consistent with the primary subgroup analysis findings (Supplemental Tables 4 and 5).
Analysis of patients with hypotension versus no hypotension on admission regarding interaction with intermediate variables and clinical outcomes demonstrated no clear pattern or evidence of differential effect by blood pressure. Through analysis of 3-way interaction of EF-by-treatment-by-hypotension, we do not see any evidence that the EF-by-treatment interaction is confounded or influenced by systolic blood pressure.
DISCUSSION
The ROSE AHF trial did not find any significant enhancement in decongestion, renal function or longer term outcomes with low-dose dopamine or low-dose nesiritide as compared to placebo.4 In this post-hoc analysis, there were differential responses to both agents on endpoints reflective of decongestion according to EF where dopamine enhanced decongestion in HFrEF but had an adverse effect on decongestion in HFpEF. Despite differential effects on decongestion, there was no interaction between treatment allocation and EF group on changes in renal function. There was also evidence of a differential effect of dopamine (but not nesiritide) on survival at 60 and 180 days where relative to placebo, dopamine therapy was associated with improved outcomes in HFrEF but adverse outcomes in HFpEF. While there were similar trends for differential effects on decongestion according to EF for nesiritide, the results with nesiritide were modest and unassociated with effects on outcomes in either HFrEF or HFpEF. While differential response to chronic HF therapies in HFrEF and HFpEF is well established, these hypothesis generating data suggest that investigation of AHF therapies should be powered to assess the potential for differential responses in AHF with preserved versus reduced EF.
Differential response to decongestive therapies in HFrEF and HFpEF
Decongestion with diuretics or ultrafiltration is the cornerstone of AHF therapy. As recently reviewed, few studies have compared the efficacy of AHF treatments in HFrEF versus HFpEF.15 The limited data available from AHF registries or cohort studies suggest that AHF patients with HFrEF or HFpEF respond similarly to diuretics3, 16 and ultrafiltration.17 Thus, the differential effect of both low-dose dopamine and low-dose nesiritide on decongestion in diuretic treated AHF patients with HFrEF or HFpEF is of interest.
There is a possibility that EF may be a surrogate for blood pressure as a variable in the differential effect findings. However, upon three-way analysis, there was no evidence of confounding blood pressure influence on the differential effect findings.
Differential effects of low-dose dopamine on decongestion in HFpEF and HFrEF
Dopamine exerts its actions via two families of cell surface, G protein-coupled receptors: D1-like receptors (D1 and D5) stimulate adenylyl cyclases, while D2-like receptors (D2, D3, and D4) inhibit adenylyl cyclases. The dopamine receptors are present in the kidney and renal vasculature where the endogenous dopaminergic system plays an important role in regulation of sodium excretion and blood pressure control.18 Thus, dopamine infusion may augment natriuresis via effects on renal hemodynamics or tubular sodium reabsorption. With brief (3–5 minutes) infusion of dopamine at 2 ug/kg/min in patients with HFrEF, renal blood flow increased and renal and systemic vascular resistance decreased.19 While the renal hemodynamic response to dopamine has not been characterized in human HFpEF, dopaminergic receptor function is impaired in animal models of hypertension and oxidative stress, both common in HFpEF.20 Thus, in the HFpEF patients, attenuated direct renal vascular and tubular effects may have contributed to the lack of enhanced diuresis and natriuresis as observed with dopamine in HFrEF.
Metra et al demonstrated that in HFrEF patients, dopamine at 2 ug/kg/min (for 30 minutes) increased stroke volume and decreased systemic vascular resistance.21 Rajfer et al compared the inotropic and vasodilatory effects of brief (10 minute) infusion of incremental doses (2–10 ug/kg/min) of dopamine and dobutamine and demonstrated that the load-independent inotropic effects of dopamine and dobutamine were similar at similar doses whereas lower doses of dopamine also produced systemic vasodilatation and overall, significant increases in cardiac index.22 These inotropic and vasodilatory systemic hemodynamic effects of low-dose dopamine may be more dramatic with sustained infusion as utilized in ROSE AHF and may contribute to the enhanced diuresis and natriuresis with dopamine in AHF patients with HFrEF. Indeed, in ROSE AHF, hypotension was less common in dopamine treated patients suggestive of inotropic effects.4 To our knowledge, no studies have characterized the inotropic or vasodilatory effects of dopamine in HFpEF. However, Schwartzenberg et al compared the effect of vasodilatation with sodium nitroprusside in HFpEF and HFrEF.23 In HFrEF, nitroprusside resulted in minimal decreases in blood pressure and a marked increase in stroke volume. However, similar nitroprusside doses in HFpEF patients resulted in greater blood pressure reduction and blunted increases in stroke volume as compared to HFrEF with a significant proportion (35%) of HFpEF patients experiencing a reduction in stroke volume with acute vasodilator administration. These findings are consistent with the markedly steeper end-systolic pressure volume relationship in HFpEF versus HFrEF. Thus, in HFpEF, differential systemic hemodynamic response to the inotropic and vasodilatory effects of dopamine may have contributed to the adverse effect on decongestion observed in HFpEF.
Differential effects of low-dose nesiritide on decongestion in HFpEF and HFrEF
The understanding of the effects of vasodilation between HFrEF and HFpEF subjects remain limited.24 As noted above, Schwartzenberg et al demonstrated that acute vasodilatation in HFpEF patients resulted in greater blood pressure reduction and blunted increases (and frequently decreases) in stroke volume as compared to HFrEF. In the Relaxin in AHF (RELAX-AHF) trial of AHF patients with normal or increased blood pressure, the potent vasodilator serelaxin improved symptoms and reduced longer term mortality with no differential effect on these endpoints in patients with EF≤50% vs >50%.25, 26 Overall, serelaxin and placebo treated patients had similar weight loss at five days but with significantly lower diuretic doses in the serelaxin group. There was a greater diuretic sparing effect seen in the HFrEF group compared to the HFpEF group. These trends may suggest that the “diuretic sparing” effect of serelaxin was not as apparent in HFpEF. However, the relatively small numbers of HFpEF patients in both studies and differences in population characteristics (different EF partition value and much higher blood pressure and lower cystatin C levels in RELAX-AHF as compared to ROSE AHF) hinder comparisons.
Differential effects of low-dose dopamine or nesiritide on outcomes in HFrEF and HFpEF
As compared to the pooled placebo group, dopamine treated HFrEF patients had improved survival at 60 and 180 days where as dopamine treated HFpEF patients had worse survival. In the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) trial, HFrEF patients with aggressive decongestion had much lower 180 day post-discharge mortality (despite greater increases in creatinine) as compared to the group with less decongestion.27 Thus, the differential effects of dopamine on outcomes may be related to the differential effects on decongestion. However, similar differences in outcomes with nesiritide in HFrEF and HFpEF were not observed despite differences in decongestion, albeit less dramatic, with nesiritide in the two EF groups. Further, the absolute number of events was low and these findings may be due to chance.
Definition of HFpEF
Studies have varied as to the EF value used to discriminate HFrEF from HFpEF in AHF.3, 13, 25 Patients with an EF between 40% and 50% are problematic but generally are felt to have more of a HFpEF phenotype and guidelines do not endorse use of standard HFrEF therapies in patients with an EF>40%.24 Thus, for the purposes of this analysis, the partition value of 40% was used. This provided larger HFpEF sample size and more statistical power. Our sensitivity analysis indicates directionally and statistically concordant findings when an EF of 50% was used to define HFpEF.
Study Limitations
As noted above, the ROSE AHF study was not powered to detect differential treatment effects between the HFrEF and HFpEF groups, and the findings of this post-hoc analysis could be due to chance alone. Our analyses did not transform continuous outcome variables. In a sensitivity analysis with log-transformed NT-proBNP as the outcome variable, the lack of a statistically significant interaction between treatment and EF group remained.
Mechanisms accounting for observed interactions are not defined in the current study. Future studies may consider the measurement of other important biomarkers as the degree of NT-proBNP change in this study does not appear to correlate with the dramatic clinical outcome differences. Given the dramatic clinical outcomes difference between the placebo and dopamine groups in HFrEF, there is a possibility that the results may be due to chance, and these hypothesis-generating findings should prompt further prospective studies given the physiological differences between HFrEF and HFpEF.
CONCLUSIONS
In this post-hoc analysis of ROSE AHF, there were differential responses to low-dose dopamine and low-dose nesiritide based on EF in patients with AHF and renal dysfunction. As differential response to chronic HF therapies in HFrEF and HFpEF is well established, these hypothesis generating data suggest that investigation of AHF therapies should also assess the potential for differential responses in AHF with preserved versus reduced EF. The specific finding that adjunctive low-dose dopamine enhanced decongestion and was associated with improved post-hospitalization outcomes among AHF patients with HFrEF group merits reexamination in a separate patient cohort. Furthermore, the findings of this study highlight the need for greater mechanistic understanding of the different phenotypes present in AHF, and suggest that further prospective studies may benefit from greater characterization of AHF phenotypes in trial design when testing therapy.
Supplemental Data
CLINICAL PERSPECTIVE.
In this post-hoc analysis of ROSE AHF, there were differential responses to low-dose dopamine and low-dose nesiritide based on EF in patients with AHF and renal dysfunction. Adjunctive low-dose dopamine enhanced decongestion and was associated with improved post-hospitalization outcomes among AHF patients with HFrEF. Differential findings with nesiritide were modest and without any associated clinical outcomes improvement. The hypothesis-generating findings of this study highlight the importance of greater mechanistic understanding of the different phenotypes between HFrEF and HFpEF in AHF. Future investigations of AHF therapies should assess the potential for differential responses in AHF with preserved versus reduced EF.
Acknowledgments
Sources of Funding: Supported by grants from the NHLBI: Coordinating Center: U10 HL084904; Regional Clinical Centers: U01 HL084861, U10 HL110312, U109 HL110337, U01 HL084889, U01HL084890, U01 HL084891, U10 L110342, U10 HL110262, U01 HL084931, U10 HL110297,U10 HL110302, U10 HL110309, U10 HL110336, U10 HL110338. This work is also supported by the National Center for Advancing Translational Sciences (NCATS):UL1TR000454, UL1 TR000135, UL1RR025008, UL1TR 000439; and the National Institute on Minority Health and Health Disparities (NIMHD): 8 U54 MD007588.
Disclosures: SHW: None; SRS: None; BAB: None; KJA: None; AD: Research support as site PI for a multicenter trial from Novartis; GMF: Grant support from NHLBI, Novartis, Amgen, Roche Diagnostics; Consulting for Novartis, Amgen, Trevena, Otsuka; MMG: None; BAB: None; WHWT: None; MMR: None; HHC: Ownership Interest: Co-founder of Zumbro Discovery; Patent designer natriuretic peptides; Loyalties from Capricor therapeutics, Anexon Inc and Up-to-date.
REFERENCES
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB. Heart disease and stroke statistics-2015 update: A report from the american heart association. Circulation. 2015;131:e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Gheorghiade M, Zannad F, Sopko G, Klein L, Pina IL, Konstam MA, Massie BM, Roland E, Targum S, Collins SP, Filippatos G, Tavazzi L. Acute heart failure syndromes: Current state and framework for future research. Circulation. 2005;112:3958–3968. doi: 10.1161/CIRCULATIONAHA.105.590091. [DOI] [PubMed] [Google Scholar]
- 3.Yancy CW, Lopatin M, Stevenson LW, De Marco T, Fonarow GC. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: A report from the acute decompensated heart failure national registry (adhere) database. J Am Coll Cardiol. 2006;47:76–84. doi: 10.1016/j.jacc.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 4.Chen HH, Anstrom KJ, Givertz MM, Stevenson LW, Semigran MJ, Goldsmith SR, Bart BA, Bull DA, Stehlik J, LeWinter MM, Konstam MA, Huggins GS, Rouleau JL, O'Meara E, Tang WH, Starling RC, Butler J, Deswal A, Felker GM, O'Connor CM, Bonita RE, Margulies KB, Cappola TP, Ofili EO, Mann DL, Davila-Roman VG, McNulty SE, Borlaug BA, Velazquez EJ, Lee KL, Shah MR, Hernandez AF, Braunwald E, Redfield MM. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: The rose acute heart failure randomized trial. Jama. 2013;310:2533–2543. doi: 10.1001/jama.2013.282190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cotter G, Weissgarten J, Metzkor E, Moshkovitz Y, Litinski I, Tavori U, Perry C, Zaidenstein R, Golik A. Increased toxicity of high-dose furosemide versus low-dose dopamine in the treatment of refractory congestive heart failure. Clin Pharmacol Ther. 1997;62:187–193. doi: 10.1016/S0009-9236(97)90067-9. [DOI] [PubMed] [Google Scholar]
- 6.Varriale P, Mossavi A. The benefit of low-dose dopamine during vigorous diuresis for congestive heart failure associated with renal insufficiency: Does it protect renal function? Clin Cardiol. 1997;20:627–630. doi: 10.1002/clc.4960200709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giamouzis G, Butler J, Starling RC, Karayannis G, Nastas J, Parisis C, Rovithis D, Economou D, Savvatis K, Kirlidis T, Tsaknakis T, Skoularigis J, Westermann D, Tschope C, Triposkiadis F. Impact of dopamine infusion on renal function in hospitalized heart failure patients: Results of the dopamine in acute decompensated heart failure (dad-hf) trial. J Card Fail. 2010;16:922–930. doi: 10.1016/j.cardfail.2010.07.246. [DOI] [PubMed] [Google Scholar]
- 8.Triposkiadis FK, Butler J, Karayannis G, Starling RC, Filippatos G, Wolski K, Parissis J, Parisis C, Rovithis D, Koutrakis K, Skoularigis J, Antoniou CK, Chrysohoou C, Pitsavos C, Stefanadis C, Nastas J, Tsaknakis T, Mantziari L, Giannakoulas G, Karvounis H, Kalogeropoulos AP, Giamouzis G. Efficacy and safety of high dose versus low dose furosemide with or without dopamine infusion: The dopamine in acute decompensated heart failure ii (dad-hf ii) trial. Int J Cardiol. 2014;172:115–121. doi: 10.1016/j.ijcard.2013.12.276. [DOI] [PubMed] [Google Scholar]
- 9.O'Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, Heizer GM, Komajda M, Massie BM, McMurray JJ, Nieminen MS, Reist CJ, Rouleau JL, Swedberg K, Adams KF, Jr, Anker SD, Atar D, Battler A, Botero R, Bohidar NR, Butler J, Clausell N, Corbalan R, Costanzo MR, Dahlstrom U, Deckelbaum LI, Diaz R, Dunlap ME, Ezekowitz JA, Feldman D, Felker GM, Fonarow GC, Gennevois D, Gottlieb SS, Hill JA, Hollander JE, Howlett JG, Hudson MP, Kociol RD, Krum H, Laucevicius A, Levy WC, Mendez GF, Metra M, Mittal S, Oh BH, Pereira NL, Ponikowski P, Tang WH, Tanomsup S, Teerlink JR, Triposkiadis F, Troughton RW, Voors AA, Whellan DJ, Zannad F, Califf RM. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365:32–43. doi: 10.1056/NEJMoa1100171. [DOI] [PubMed] [Google Scholar]
- 10.Chen HH, AbouEzzeddine OF, Anstrom KJ, Givertz MM, Bart BA, Felker GM, Hernandez AF, Lee KL, Braunwald E, Redfield MM. Targeting the kidney in acute heart failure: Can old drugs provide new benefit? Renal optimization strategies evaluation in acute heart failure (rose ahf) trial. Circ Heart Fail. 2013;6:1087–1094. doi: 10.1161/CIRCHEARTFAILURE.113.000347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of diet in renal disease study group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G. National kidney foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 13.Hernandez AF, O'Connor CM, Starling RC, Reist CJ, Armstrong PW, Dickstein K, Lorenz TJ, Gibler WB, Hasselblad V, Komajda M, Massie B, McMurray JJ, Nieminen M, Rouleau JL, Swedberg K, Califf RM. Rationale and design of the acute study of clinical effectiveness of nesiritide in decompensated heart failure trial (ascend-hf) Am Heart J. 2009;157:271–277. doi: 10.1016/j.ahj.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 14.Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, LeWinter MM, Deswal A, Rouleau JL, Ofili EO, Anstrom KJ, Hernandez AF, McNulty SE, Velazquez EJ, Kfoury AG, Chen HH, Givertz MM, Semigran MJ, Bart BA, Mascette AM, Braunwald E, O'Connor CM. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364:797–805. doi: 10.1056/NEJMoa1005419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bishu K, Redfield MM. Acute heart failure with preserved ejection fraction: Unique patient characteristics and targets for therapy. Curr Heart Fail Rep. 2013;10:190–197. doi: 10.1007/s11897-013-0149-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fonarow GC, Stough WG, Abraham WT, Albert NM, Gheorghiade M, Greenberg BH, O'Connor CM, Sun JL, Yancy CW, Young JB. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: A report from the optimize-hf registry. J Am Coll Cardiol. 2007;50:768–777. doi: 10.1016/j.jacc.2007.04.064. [DOI] [PubMed] [Google Scholar]
- 17.Jefferies JL, Bartone C, Menon S, Egnaczyk GF, O'Brien TM, Chung ES. Ultrafiltration in heart failure with preserved ejection fraction: Comparison with systolic heart failure patients. Circ Heart Fail. 2013;6:733–739. doi: 10.1161/CIRCHEARTFAILURE.112.000309. [DOI] [PubMed] [Google Scholar]
- 18.Carey RM. The intrarenal renin-angiotensin and dopaminergic systems: Control of renal sodium excretion and blood pressure. Hypertension. 2013;61:673–680. doi: 10.1161/HYPERTENSIONAHA.111.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elkayam U, Ng TM, Hatamizadeh P, Janmohamed M, Mehra A. Renal vasodilatory action of dopamine in patients with heart failure: Magnitude of effect and site of action. Circulation. 2008;117:200–205. doi: 10.1161/CIRCULATIONAHA.107.737106. [DOI] [PubMed] [Google Scholar]
- 20.Zeng C, Villar VA, Yu P, Zhou L, Jose PA. Reactive oxygen species and dopamine receptor function in essential hypertension. Clin Exp Hypertens. 2009;31:156–178. doi: 10.1080/10641960802621283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Metra M, Missale C, Spano PF, Cas LD. Dopaminergic drugs in congestive heart failure: Hemodynamic and neuroendocrine responses to ibopamine, dopamine, and dihydroergotoxine. J Cardiovasc Pharmacol. 1995;25:732–740. doi: 10.1097/00005344-199505000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Rajfer SI, Borow KM, Lang RM, Neumann A, Carroll JD. Effects of dopamine on left ventricular afterload and contractile state in heart failure: Relation to the activation of beta 1-adrenoceptors and dopamine receptors. J Am Coll Cardiol. 1988;12:498–506. doi: 10.1016/0735-1097(88)90426-3. [DOI] [PubMed] [Google Scholar]
- 23.Schwartzenberg S, Redfield MM, From AM, Sorajja P, Nishimura RA, Borlaug BA. Effects of vasodilation in heart failure with preserved or reduced ejection fraction implications of distinct pathophysiologies on response to therapy. J Am Coll Cardiol. 2012;59:442–451. doi: 10.1016/j.jacc.2011.09.062. [DOI] [PubMed] [Google Scholar]
- 24.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 accf/aha guideline for the management of heart failure: A report of the american college of cardiology foundation/american heart association task force on practice guidelines. Circulation. 2013;128:e240–327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 25.Filippatos G, Teerlink JR, Farmakis D, Cotter G, Davison BA, Felker GM, Greenberg BH, Hua T, Ponikowski P, Severin T, Unemori E, Voors AA, Metra M. Serelaxin in acute heart failure patients with preserved left ventricular ejection fraction: Results from the relax-ahf trial. Eur Heart J. 2014;35:1041–1050. doi: 10.1093/eurheartj/eht497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teerlink JR, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Ponikowski P, Unemori E, Voors AA, Adams KF, Jr, Dorobantu MI, Grinfeld LR, Jondeau G, Marmor A, Masip J, Pang PS, Werdan K, Teichman SL, Trapani A, Bush CA, Saini R, Schumacher C, Severin TM, Metra M. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (relax-ahf): A randomised, placebo-controlled trial. Lancet. 2013;381:29–39. doi: 10.1016/S0140-6736(12)61855-8. [DOI] [PubMed] [Google Scholar]
- 27.Testani JM, Chen J, McCauley BD, Kimmel SE, Shannon RP. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation. 2010;122:265–272. doi: 10.1161/CIRCULATIONAHA.109.933275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


