Abstract
Voltage-gated sodium (Nav) channels are responsible for the depolarizing phase of the action potential in most nerve cells, and Nav channel localization to the axon initial segment is vital to action potential initiation. Nav channels in the soma play a role in the transfer of axonal output information to the rest of the neuron and in synaptic plasticity, although little is known about Nav channel localization and dynamics within this neuronal compartment. This study uses single-particle tracking and photoactivation localization microscopy to analyze cell-surface Nav1.6 within the soma of cultured hippocampal neurons. Mean-square displacement analysis of individual trajectories indicated that half of the somatic Nav1.6 channels localized to stable nanoclusters ∼230 nm in diameter. Strikingly, these domains were stabilized at specific sites on the cell membrane for >30 min, notably via an ankyrin-independent mechanism, indicating that the means by which Nav1.6 nanoclusters are maintained in the soma is biologically different from axonal localization. Nonclustered Nav1.6 channels showed anomalous diffusion, as determined by mean-square-displacement analysis. High-density single-particle tracking of Nav channels labeled with photoactivatable fluorophores in combination with Bayesian inference analysis was employed to characterize the surface nanoclusters. A subpopulation of mobile Nav1.6 was observed to be transiently trapped in the nanoclusters. Somatic Nav1.6 nanoclusters represent a new, to our knowledge, type of Nav channel localization, and are hypothesized to be sites of localized channel regulation.
Introduction
Voltage-gated sodium (Nav) channels are responsible for the initiation and conduction of most neuronal action potentials. Nav channels are composed of a large pore-forming α-subunit of ∼1900 amino acids and smaller auxiliary β-subunits (1). Of the nine Nav α-subunits (Nav1.1–1.9), Nav1.1, Nav1.2, Nav1.3, and Nav1.6 are the major isoforms within the central nervous system (2) where the differential expression and distribution of Nav isoforms within the somatodendritic and axonal compartments determine the action potential waveform (3, 4, 5). Thus, the number, type, and location of channels must be tightly regulated to ensure proper neuronal function. Nav localization to the axon initial segment (AIS) has been extensively studied, since this domain is vital to action potential initiation (3, 6, 7, 8). In contrast, little is known about Nav channel localization and dynamics within the neuronal cell body even though somatic Nav channels play a role in synaptic plasticity and in the transfer of axonal output information to the rest of the neuron (9, 10).
Multiple studies have revealed that the cell surface is highly compartmentalized such that restricted movement and localization of surface proteins enhances signaling by altering diffusion-limited biochemical reactions (11, 12, 13). Furthermore, this compartmentalization is dynamic and highly regulated. Some of the best examples deal with the diffusion of neurotransmitter receptors into the postsynaptic membrane where they can become transiently tethered to intracellular scaffolds (14, 15). For example, single-molecule studies of AMPA and glycine receptors indicate that receptor diffusion and tethering at the synapse can be highly regulated (16, 17, 18). Whether similar diffusion patterns exist for other neuronal proteins, such as Nav1.6, and within extrasynaptic compartments, is the focus of this study.
In addition to axon localization, Nav channels are present in both somatic and dendritic compartments, as demonstrated by functional methods including electrophysiology and fluorescent Na+ indicators (4, 10, 19, 20). Localized somatic application of sodium channel blockers diminishes action potential back-propagation, suggesting that these channels relay information about axon output to the rest of the neuron. In addition, somatic spiking has been postulated to regulate synaptic plasticity in the absence of back-propagating action potentials (9). Furthermore, there is precedence that Nav channels in the AIS and somatodendritic compartments are functionally distinct and differentially regulated. In neocortical neurons, slowly inactivating, persistent current is derived from AIS channels as opposed to those in the soma (21). Activation of D1/D5 dopamine receptors in prefrontal-cortex pyramidal neurons preferentially modulates Nav channels in the soma and proximal dendrites and increases the amount of persistent current (19). Despite the functional importance of Nav channels in the cell body, the distribution and dynamics of somatodendritic Nav channels has remained elusive because traditional immunofluorescence-based assays are not sensitive enough to detect the sparse Nav channel distribution in the soma (4, 8, 22). Quantitative electron microscopy using immunogold-labeled SDS-digested freeze-fracture replica labeling has so far provided the best demonstration that Nav channels are present on the soma and dendrites of hippocampal CA1 pyramidal cells, although at a density ∼40 times lower than that in the AIS (23). However, this high-resolution approach provides no information concerning dynamics and potential interactions of Nav channels on the cell surface.
This article focuses on the surface localization and diffusion of Nav1.6 channels on the soma of live rat hippocampal neurons. The Nav1.6 isoform was chosen for our current studies because it is abundant in the central nervous system, may have location-specific biophysical properties, since it is present within both the somato-dendritic and axonal compartments, and is directly linked to human pathologies such as ataxia (24, 25), epilepsy (26), multiple sclerosis (27), and stroke (28). Using fluorescent protein- and extracellular epitope-tagged Nav1.6 constructs in conjunction with high-density single-particle tracking, we found that somatic Nav1.6 channels localized to stable nanoclusters ∼230 nm in diameter. The nanoscale organization of Nav channels was further elucidated by analyzing single-molecule trajectories via quantitative Bayesian inference methods. These nanoclusters were found to be ankyrin-, actin-, and clathrin-independent and, as such, represent a new type of molecular organization of Nav channels on the neuronal surface. We postulate that Nav1.6 nanoclusters represent sites of channel regulation, potentially contributing to the functional differences seen between somatic and axonal Nav channels.
Materials and Methods
Cell culture
Rat hippocampal neurons were cultured as previously described (29). Animals were used according to protocols approved by the Institutional Animal Care and Use Committee of Colorado State University (Animal Welfare Assurance Number A3572-01). Embryonic hippocampal tissue was collected after anesthesia with isoflurane followed by decapitation. E18 rat hippocampal neurons were plated on glass-bottom 35 mm dishes with No. 1.5 coverslips (MatTek, Ashland, MA) that were coated with poly-L-lysine (Sigma-Aldrich, St. Louis, MO). Neurons were grown in Neurobasal Medium (Gibco/Thermo Fisher Scientific, Waltham, MA) with penicillin/streptomycin antibiotics (Cellgro/Mediatech, Manassas, VA), GlutaMAX (Gibco/Thermo Fisher Scientific), and NeuroCult SM1 Neuronal Supplement (STEMCELL Technologies, Vancouver, BC, Canada). For imaging, the cultures were incubated in neuronal imaging saline consisting of 126 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 0.6 mM MgSO4, 0.15 mM NaH2PO4, 0.1 mM ascorbic acid, 8 mM glucose, and 20 mM HEPES (pH 7.4).
Transfection
Wild-type and mutant Nav1.6 containing GFP and an extracellular biotin acceptor domain (BAD) (Nav1.6-BAD-GFP and Nav1.6-BAD-dABM) were constructed and functionally validated as previously described (29). Nav1.6-Dendra2 was constructed by replacing the GFP from Nav1.6-GFP with Dendra2 using KpnI and PacI restriction sites. Neuronal transfections were performed after days in vitro (DIV) 4–6 in culture as indicated for each experiment using Lipofectamine 2000 (Invitrogen, Life Technologies, Grand Island, NY) and either Nav1.6-BAD, Nav1.6-Dendra2, or Nav1.6-BAD-GFP (1 μg), human β1 in pcDNA3.1Mygro(+), and rat β2 in pcDNA3.1VS-HisTopoTA, as indicated. For the Nav1.6-BAD-GFP and Nav1.6-BAD constructs, pSec-BirA (bacterial biotin ligase) was cotransfected to biotinylate the channel. Plasmids encoding clathrin-light-chain-GFP, Kv2.1-GFP, photoactivatable-GFP-actin, and Ruby-Lifeact were used as previously described (30, 31).
Live-cell surface labeling
For experiments using the Nav1.6 construct containing the extracellular BAD, labeling of the surface channel was performed before imaging. Neurons were rinsed with neuronal imaging saline to remove the Neurobasal media and then incubated for 10 min at 37°C with either streptavidin-conjugated Alexa Fluor 594 (Thermo Fisher Scientific) or CF640R-streptavidin (Biotium, Hayward, CA) diluted 1:1000 in neuronal imaging saline. Excess label was removed by rinsing with imaging saline. CF640R was used for far-red imaging instead of streptavidin-conjugated Alexa Fluor 647, since we found that the latter does not label Nav1.6-BAD efficiently. Alexa Fluor 647 has a higher molecular weight than either Alexa Fluor 594 or CF640R, suggesting that the biotin within the BAD domain is only accessible to smaller dye conjugates. For single-particle tracking photoactivated localization microscopy (spt-PALM) and actin super-resolution experiments, 0.1 μm TetraSpeck beads (Thermo Fisher Scientific) were used as fiduciary markers to correct for drift. Beads were diluted 1:1000 in imaging saline and applied to the cultures for 10 min to place several beads within the field of view.
Microscopy
Total internal reflection fluorescence (TIRF) images were acquired using a Nikon Eclipse Ti fluorescence microscope equipped with a Perfect-Focus system, a Nikon photoactivation unit (PAU), acousto-optic-tunable-filter (AOTF)-controlled 405, 488, 561, and 647 nm diode lasers, 100 mW each split equally between the TIRF and PAU pathways, an Andor iXon EMCCD DU-897 camera, and a Plan Apo TIRF 100×, NA 1.49 objective. Emission was collected through a filter wheel containing the appropriate bandpass filters. For excitation, an incident angle of 63° was used that gives an estimated penetration depth of 144 nm at a wavelength λ = 488 nm. Before TIRF imaging, differential interference contrast (DIC) and wide-field fluorescence imaging were used to distinguish transfected neurons from the relatively flat glia. Neurons were readily identified based on the characteristic soma morphology and localization of Nav1.6 to the axon initial segment. All imaging was performed at 37°C using a heated stage and objective heater.
Mobility of Nav1.6 puncta
The stability of Nav1.6 puncta was assessed using a custom algorithm implemented in LabView. First, puncta were identified using a Fourier bandpass filter followed by threshold segmentation. Then, we used the puncta in the first frame of the video as a mask for all subsequent images such that only puncta that survived the Fourier filter and threshold segmentation and overlapped with puncta in the first frame were analyzed. This image processing algorithm provided a tool to quantify the fraction of channels that remained confined to a diffraction-limited spot after a given imaging time.
Fluorescence recovery after photobleaching
Neurons transfected with Nav1.6-BAD and the biotin ligase were labeled with CF640R before TIRF imaging. The cells were imaged every 5 s for 2 min to establish a baseline. The microscope PAU was used to apply high-intensity illumination to a small region of the soma membrane until the initial fluorescence was photobleached (∼10 s). After photobleaching, images were acquired every 5 s for 30 min to observe fluorescence recovery. Time-lapse microscopy at a low rate minimized photobleaching during the recovery period.
Single-molecule tracking
DIV10 rat hippocampal neurons expressing biotinylated Nav1.6-BAD or Nav1.6-BAD-GFP were surface-labeled with SA-CF640R and imaged at 20 frames/s using TIRF microscopy as described above. Images were background subtracted and filtered using a Gaussian kernel with a standard deviation of 0.7 pixels in ImageJ. Tracking of individual fluorophores was performed in MATLAB using the U-track algorithm developed by Jaqaman et al. (32). Manual inspection confirmed accurate single-molecule detection and tracking. The tracks were corrected for drift using TetraSpeck beads as fiduciary markers, with custom-written LabView codes.
Analysis of diffusion and potential energy landscapes
The dynamics of Nav channels were mapped on the cell surface in terms of their diffusion and potential energy by using high-density single-particle tracking of Nav channels labeled with photoactivatable fluorophores (33) in conjunction with InferenceMAP, a software package based on Bayesian inference (34). DIV10 rat hippocampal neurons expressing Nav1.6-Dendra2 were imaged using TIRF microscopy as described above. Images of the unconverted Dendra2 fluorescence and DIC images of the neurons were acquired both pre- and postimaging. The image of the unconverted Dendra2 fluorescence confirmed that the neuronal membrane was within the TIRF excitation field. Dendra2 was photoconverted with a low-intensity 405 nm laser, and photoconverted molecules were excited, imaged, and subsequently photobleached using the 561 nm laser (50 mW). The 405 laser intensity was adjusted in the range 0.05 to 0.5 mW such that an appropriate density of photoconverted Dendra2 molecules was present. Image sequences of 10,000 frames were acquired at 20 frames/s for each cell. Single-molecule tracks were assembled using U-track and analyzed with InferenceMAP.
Results
Somatic Nav1.6 has a heterogeneous distribution
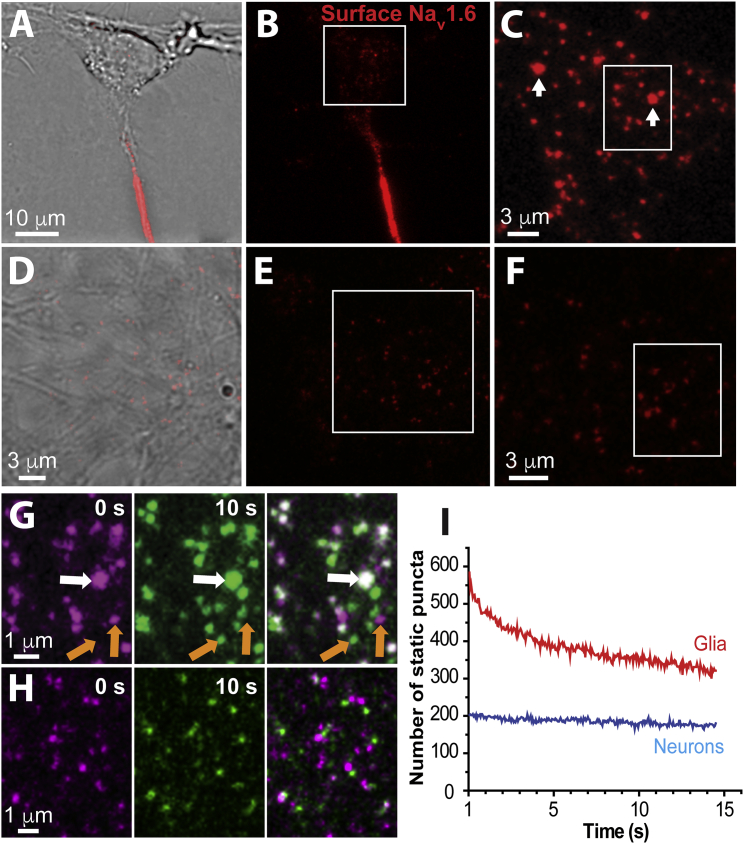
Our previous studies of Nav1.6 examined the directed trafficking of nascent channels to the AIS of hippocampal neurons (29). In these studies, we transfected cultured rat hippocampal neurons with a modified Nav1.6 construct, Nav1.6-BAD, that contained an extracellular biotin acceptor domain (BAD), thus allowing live-cell labeling of surface channels using streptavidin-conjugated fluorophores. Fig. 1 A shows DIC and TIRF images of a cultured hippocampal neuron transfected with Nav1.6-BAD, labeled with streptavidin-conjugated CF640R. In addition to the expected high-density surface localization within the AIS, Nav1.6 localized to small surface puncta on the soma. As illustrated by the higher magnification of the soma shown in Fig. 1 C, the somatic channels are distributed nonuniformly, with single channels being either dispersed across the surface or aggregated into bright nanoclusters as indicated by the white arrows. Note that the Fig. 1 C image represents a different time point from that in Fig. 1 B, thus accounting for the nonidentical Nav1.6 localization patterns. The contrast in Fig. 1 C is enhanced to allow visualization of the nanoclusters, which are dim compared to the high density of Nav1.6 within the AIS. We use the term nanocluster here to refer to nanoscale-sized static structures that are smaller than the traditional light microscopy diffraction limit and include at least two channels (see the data in Figs. 4 and 5 for nanocluster size). Single-channel intensities can be derived from freely diffusing particles very likely representing single Nav1.6 channels bound to one fluorophore-labeled streptavidin. Using this approach, the number of channels per nanocluster was found to be in the range 2–20. However, the uncertainty associated with the extent of streptavidin binding to the biotinylated channels and the efficiency of channel biotinylation by the cotransfected biotin ligase makes it difficult to determine the exact number of channels within each nanocluster. In contrast to what was observed in neurons, Nav1.6 nanoclustering was not seen in transfected glial cells present within the neuronal cultures (Fig. 1 F). In addition, the distribution of surface puncta intensities illustrated in Fig. S1 in the Supporting Material shows high-intensity values, i.e., Nav1.6 nanoclusters, in neurons that are absent from glia. Thus, the nanoclustering is a function of the neuronal surface as opposed to being induced by the surface labeling or GFP moiety of the Nav1.6 construct. Fig. 1 G shows time-lapse imaging that indicates that the nanoclusters are stably localized over at least 10 s on the neuronal surface, whereas the non-clustered channels are mobile. Fig. 1 G, left, pseudocolored in magenta, shows t = 0; Fig. 1 G, middle, pseudocolored in green, indicates t = 10 s; and Fig. 1 G, right, shows the merge of these frames. The presence of two channel populations, mobile and nanoclustered, is also evident in Movie S1. Interestingly, this image sequence suggests that Nav1.6 can exchange between the nanoclusters and the mobile population. Note that the low intensity and mobile puncta in Movie S1 are interpreted as representing single channels. Fig. 1 H illustrates the enhanced mobility of Nav1.6 channels that was observed in glial cells, where most of the channels are mobile. This enhanced movement is also illustrated in Movie S2. Occasionally static puncta were observed (small white spots), but none of these were nanoclusters in that the fluorescent intensity corresponded to only a single channel. Fig. 1 I summarizes the mobility of all surface channels in both neurons and glia. Here, all puncta (without discerning between single channels or nanoclusters) detected in frame 1 of movies from either glia or neurons were analyzed as to whether they remained in their initial localization in subsequent frames. The number of puncta remaining in place relative to the first localization is plotted against time in Fig. 1 I. Note that in glia, the number of Nav1.6 puncta that remained within the same location decreased much more rapidly than in neurons, indicating greater Nav1.6 mobility relative to that observed in neurons. Photobleaching was minimal for the 15 s and the total number of detected puncta in each frame remained constant over this time.
Figure 1.
Nav1.6 is distributed heterogeneously in the somatic membrane. (A) Nav1.6-BAD surface expression in DIV10 rat hippocampal neurons is highly enriched at the AIS, as indicated by live-cell labeling with SA-CF640R (red). (B) Same image as (A) but without the DIC overlay. Channels on the somatic surface are barely visible at this contrast. (C) An enlargement of the boxed region shown in (B). The white arrows point to Nav1.6-BAD surface nanoclusters. The image contrast is enhanced relative to that in (B) and a different time point is presented. (D) DIC and surface labeling of a transfected glial cell within the neuronal culture. (E) The surface expression pattern for Nav1.6-BAD in the glial cell shown in (D). (F) An enlargement of the boxed region in (E). Note the absence of the neuronal nanoclusters. The heterogeneity in single-channel intensity is due to variability in CF640R labeling of the streptavidin in addition to single-fluorophore photobleaching during imaging. (G) Left: An enlargement of the white box in (C) with the CF640R fluorescence pseudocolored magenta. Contrast has been enhanced to visualize individual (orange arrows) and clustered (white arrow) somatic channels. Middle: The same field imaged 10 s later and with the SA-CF640R fluorescence now pseudocolored green. Right: Overlay of the two time frames, where colocalization appears white. The brightest punctum, i.e., nanocluster, appears in the same location in both image sequences (white arrow), whereas smaller puncta demonstrate mobility (orange arrows). (H) Same temporal analysis as in (G) but performed with the glial cell shown in (D)–(F). Note that most particles moved during the 10 s time period. (I) Stability of Nav1.6 puncta. Puncta were detected in frame 1, and in subsequent frames the fraction of puncta remaining in the same location was determined. Summed data from three neurons and three glial cells are presented. The total puncta number in both cell types remained constant during this 15 s imaging period.
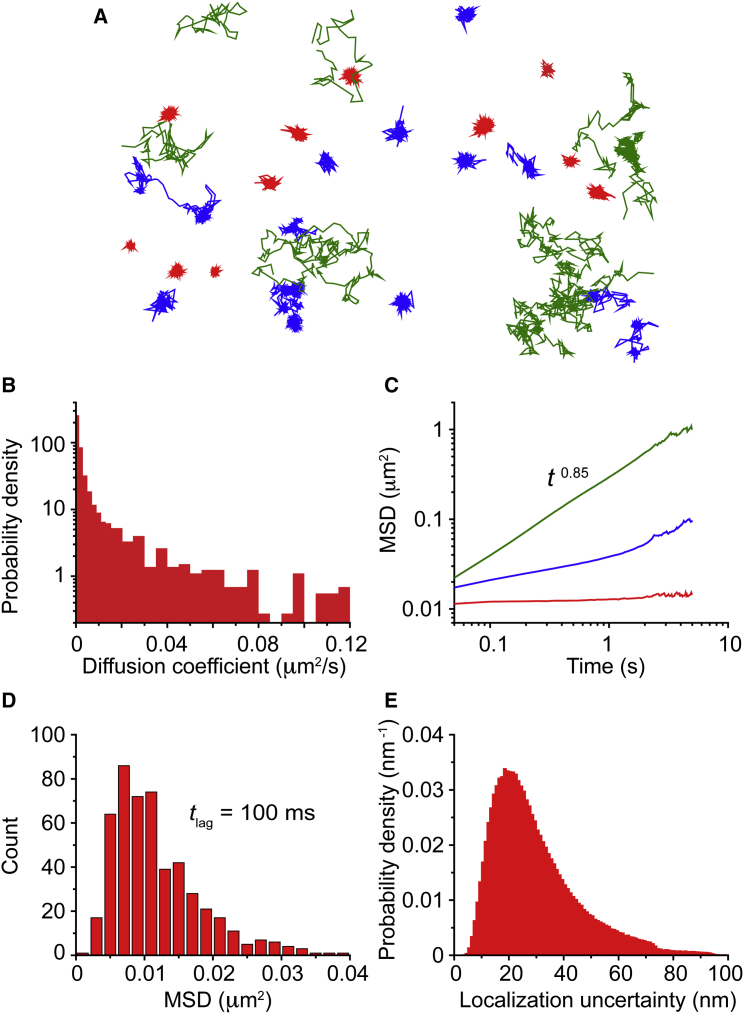
Figure 4.
Single-molecule tracking reveals distinct distributions of somatic Nav1.6 mobility. (A) Thirty-three representative single-molecule trajectories in an 8 μm × 11 μm window after surface labeling of Nav1.6 with CF640 in DIV10 hippocampal neurons and tracking of individual channels. Imaging was performed at 20 Hz using TIRF microscopy. The red, blue, and green colors represent tracks with low, intermediate, or high diffusivity, respectively. (B) Histogram of effective diffusion coefficients of 1478 particles from four cells obtained from a linear regression of the MSD at lag times up to 500 ms (10 frames). (C) MSD as a function of lag time for three different populations. Trajectories were placed into three different pools according to their effective diffusion coefficients using ad hoc thresholds. Specifically, trajectories were placed into 1) a low-diffusivity regime, with D < 0.001 μm2/s, 2) an intermediate regime, with 0.001 μm2/s < D < 0.03 μm2/s, and 3) a high-diffusivity regime. These three populations are color-coded as in (A). (D) Histogram of the MSDs at lag time for the trajectories in the low-diffusivity regime, which correspond to the molecules that remain confined during the whole observation time. (E) Distribution of localization uncertainty of all localized particles, from which 29 ± 15 nm (mean ± SD).
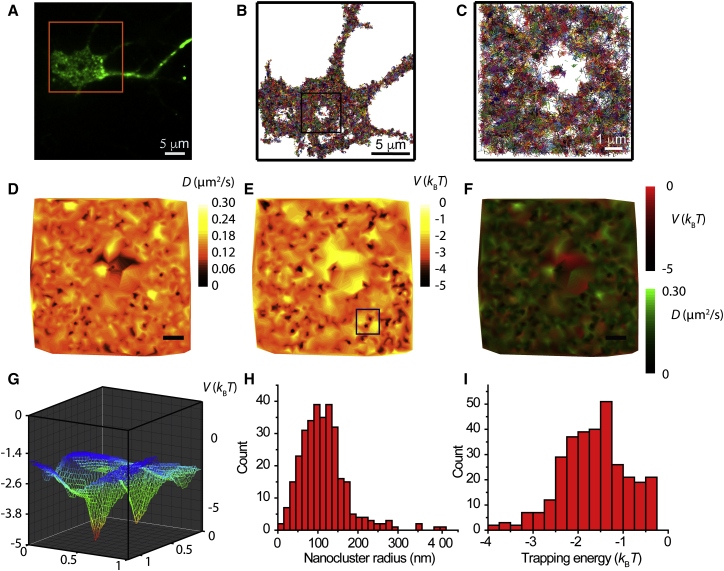
Figure 5.
spt-PALM of Nav1.6-Dendra2 shows heterogeneity in the diffusion and energy landscapes. (A) DIV 10 hippocampal neuron expressing Nav1.6-Dendra2, showing unconverted Dendra2 as imaged in TIRF. (B) Ensemble of tracks from individual Nav1.6-Dendra2 particles in the somatic region (orange box in (A)). Dendra2 particles were stochastically activated to allow visualization of individual particles while image sequences of 10,000 frames were acquired at 20 Hz. Molecules were detected and connected into tracks using U-track. Each colored line represents a track from an individual particle. The boxed region alone represents 114,923 Dendra2 detections. (C) An enlargement of the boxed region in (B). (D) Diffusion landscape of the membrane region illustrated in (C). The cell surface was divided into regions based on adaptive meshing, such that each section contains a similar number of localizations. The step sizes of particle tracks within these regions were used to determine the diffusion coefficient within each grid. (E) Potential energy landscape. Information about both the mobility of channels and the direction of movement were used to determine the potential energy. Energy wells appear as the dark puncta. (F) Overlay of the diffusion and potential energy maps. Dark spots indicate regions where both the energy and the diffusivity are lower than in the rest of the membrane. (G) 3D representation of the two energy wells indicated by the box in (E). (H) Distribution of energy well radii measured in 158 domains. The radius was defined as the standard deviation of the Gaussian fit and two radii were measured for each nanocluster, σx and σy. From the energy wells, the nanocluster radii were found to be 114 ± 58 nm (mean ± SD). (I) Distribution of the energy well depths. The measured trapping energy was −1.6 ± 0.7 kBT.
Nav1.6 somatic nanoclusters are ankyrin-G independent
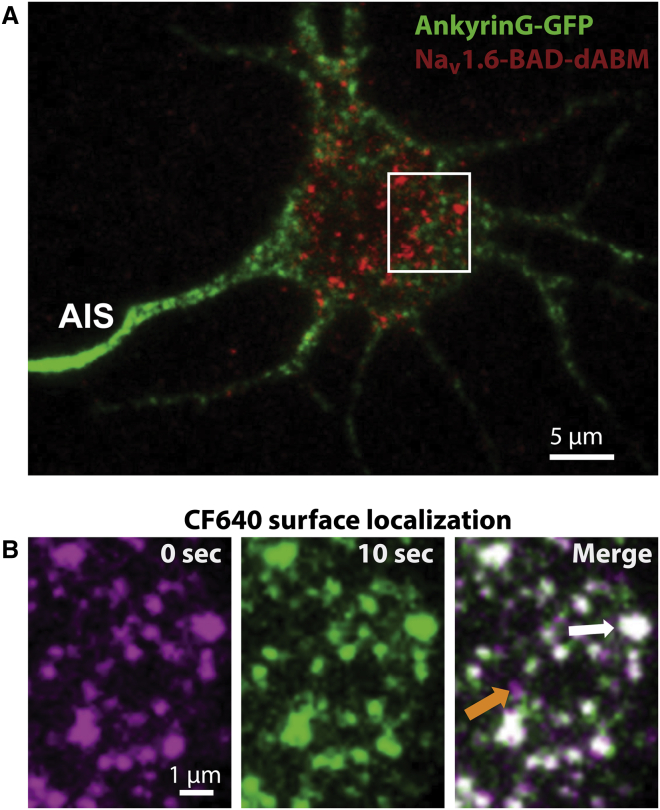
The stable Nav1.6 nanoclusters shown in Fig. 1 suggest Nav1.6 interactions with an intracellular binding partner. The only known mechanism underlying Nav localization in neurons involves cytoskeletal tethering via interactions with ankyrin-G (ankG) and loss of ankG-binding prevents localization of Nav1.6 channels to the AIS without altering channel function (35). To investigate the role of ankG-binding in the somatic nanoclustering of Nav1.6, we examined the somatic distribution of a Nav1.6 mutant channel in which the ankyrin-binding motif (ABM) was removed (Nav1.6-BAD-dABM). We have previously shown that, in contrast to the wild-type channel (Fig.1), this ankyrin-G-binding mutant does not localize to the ankyrin-rich AIS (29). Fig. 2 shows a DIV10 neuron expressing Nav1.6-BAD-dABM and ankG-GFP, which marks the AIS. In contrast to the wild-type channel that colocalizes with ankG at the AIS, the mutant channel does not concentrate within this region (29). However, Nav1.6-BAD-dABM still localized to the somatic nanoclusters (Fig. 2 B). To visually display the mobility of nanoclusters and individual channels, we again overlaid two frames from an image sequence of channels labeled with CF640 on the somatic membrane (Fig. 2 B). When the first frame (Fig. 2 B, left) and a frame 10 s later (Fig. 2 B, middle) were overlaid we again saw that large puncta did not move over this time (white arrow), whereas some of the smaller puncta (orange arrow), presumably single channels, did. Thus, the dABM mutant still produces both a mobile channel population and stable nanoclusters, similar to that seen for the full-length Nav1.6 protein (Fig. 1 B). Since the somatic Nav1.6 nanoclusters represent, to our knowledge, a new mechanism for Nav channel localization in a neuronal compartment where Nav1.6 localization has not been previously appreciated, we next quantified the stability of these structures in greater detail.
Figure 2.
Nav1.6 somatic distribution is ankyrin-G independent. (A) DIV10 rat hippocampal neuron expressing a mutant Nav1.6 channel lacking the ankyrin-binding motif (Nav1.6-BAD-dABM) labeled with CF640R. This channel localizes to the somatic region but does not show a high density of channels within the AIS, which is marked by ankyrin-G-GFP. (B) Enlargement of the area identified by the white box in (A), showing two frames of an image sequence spaced 10 s apart and a merged image (right). The first frame (magenta) and a frame 10 s later (green) are overlaid in the merged image. Colocalization appears white. Large bright puncta (white arrow) appear in the same location in both image sequences, whereas the smaller puncta are mobile (orange arrow).
Nav1.6 localizes to stable somatic nanoclusters
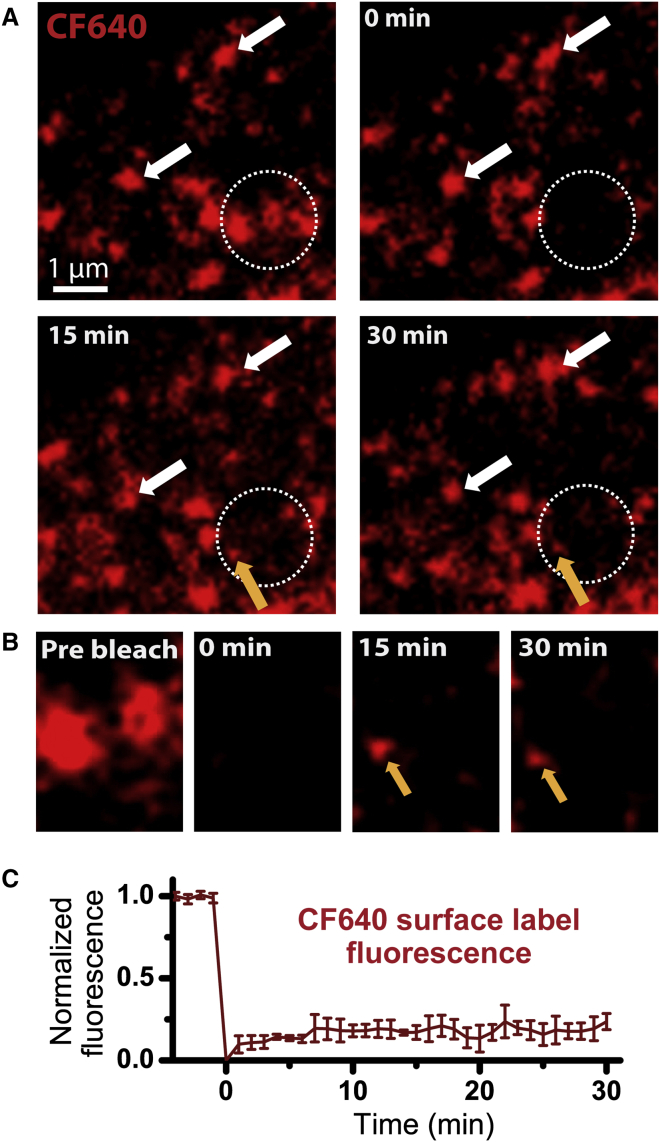
To gain a quantitative understanding of nanocluster maintenance, we used time-lapse imaging and fluorescence recovery after photobleaching (FRAP). Fig. 3 A shows surface channels labeled with streptavidin-conjugated CF640 in a region within the soma. Three nanoclusters within the region of interest indicated by the dotted circle were photobleached. Then the cell was imaged for 30 min with images acquired at low frequency (every 5 s) to minimize photobleaching during fluorescence recovery. As illustrated in Fig. 3 A, very little recovery was seen for the bleached nanoclusters over this time frame. At 15 min (Fig. 3, A and B, third image), a small spot appeared at one of the bleached clusters and remained stationary over the next 15 min (orange arrows). Due to its low intensity relative to the unbleached nanoclusters, this spot most likely represents a single Nav1.6 channel that diffused into this region and was captured into the nanocluster. This single-molecule recovery suggests that although exchange does occur between the clustered and nonclustered Nav1.6 populations, it is relatively slow. Several of the bright puncta outside of the photobleached region persisted throughout the image sequence (Fig. 3 A, white arrows) suggesting that the Nav1.6 somatic nanocluster domains are stably localized for more than 30 min. This assay is not sensitive to new channels being delivered to the plasma membrane (PM), since only the surface channels at the beginning of imaging were fluorescently labeled. Fig. 3 C shows the average fluorescence recovery of Nav1.6 nanoclusters after photobleaching (n = 5 nanoclusters from three cells). Within 30 min measurements, the clusters are observed to recover 23% ± 9% (mean ± SD) of the original fluorescence intensity, indicating that in this timescale, only one in four original clustered channels is exchanged by surface diffusion.
Figure 3.
Nav1.6 somatic nanoclusters are stable. Cell-surface Nav1.6-BAD in DIV10 rat hippocampal neurons was detected with CF640. (A) A representative FRAP time course where the bleach was applied to the region of intereset indicated by the white dotted circle. Somatic nanoclusters outside of the bleached region show stable localization throughout the image sequence (white arrows). (B) Enlargement of a portion of the bleached region of interest in (A), showing fluorescence before photobleaching, immediately after photobleaching, and 15 and 30 min postbleach. Note the stable addition of a single Nav1.6 channel at the 15 min time point (orange arrows in both A and B). (C) Average normalized FRAP over 30 min for CF640-labeled Nav1.6 nanoclusters. On average, there was a 23% ± 9% (n = 5; mean ± SD) recovery. Fluorescence loss for channels outside of the bleached region during the experiment was <10%.
Single-particle tracking of somatic Nav1.6 channels
The data presented thus far imply that two populations of Nav1.6 channels exist on the somatic surface, one being mobile and one stably anchored within nanoclusters. Although the ankG-independent nanocluster locations are stable over >30 min, there appears to be a slow exchange between the two populations, as illustrated in Fig. 3 B. To gain quantitative insights into the kinetics of Nav1.6 channels, we examined the motion of somatic Nav1.6 using single-particle tracking and analyzed 1478 trajectories in terms of their mean-square displacement (MSD). Fig. 4 A shows a set of trajectories obtained by imaging Nav1.6 channels labeled with CF640R in an 8 μm × 11 μm window. The behavior of the trajectories is highly heterogeneous, with some molecules exploring large membrane regions and others showing tight confinement within small domains. The trajectories have been color-coded based on their associated diffusion coefficients, as discussed below.
The conventional way to characterize the mobility of individual molecules is by means of the time-averaged MSD, ,
where is the lag time, T the observation time, and r is the two-dimensional position of the particle. For particles undergoing Brownian motion, the MSD is linear in lag time. In particular, in two dimensions, . Thus, the MSD yields an effective diffusion coefficient. Fig. 4 B shows a histogram of effective diffusion coefficients obtained from a linear regression of the MSD at lag times up to 500 ms (10 frames). The effective diffusion coefficient is observed to have a broad distribution that spans more than two orders of magnitude. At least two populations are evident, a narrow peak at low diffusivities and a broad shoulder extending to large values. We placed trajectories into three different pools according to their effective diffusion coefficient using ad hoc thresholds, obtained from visual examination of the distribution. Namely, we arranged the trajectories into 1) a low-diffusivity regime with D < 0.001 μm2/s, 2) an intermediate regime with 0.001 μm2/s < D < 0.03 μm2/s, and 3) a high-diffusivity regime. The low-diffusivity regime consists of 41% of the total trajectories, the intermediate regime 47%, and the high regime 11%. The trajectories in Fig. 4 A are colored red, blue, and green according to whether they have low, intermediate, or high diffusivity, respectively. As seen in the figure, the molecules with low diffusivity are strongly confined within nanoscale domains. The trajectories with intermediate diffusivity are partially confined, where part of the trajectory shows unconfined mobility. Usually the molecules exhibit intermittent behavior, alternating between phases of confinement and phases of unconfined diffusion. In some cases, the molecule is trapped again in the same domain from where it has escaped, and in others it is captured into a different domain. Lastly, the molecules in the high-diffusivity regime are not affected by the trapping domains and they do not exhibit any apparent confinement. Although this method of finding an effective diffusion coefficient is an efficient characterization tool for the mobility of the molecules, it does not necessarily represent the diffusion coefficient of the molecules given that it does not account for anomalous diffusion processes.
In addition to the diffusion coefficient, the MSD provides further information on protein dynamics. For example, a Brownian particle confined to a circular domain exhibits an MSD that is linear at short times but saturates at long times such that , where is the radius of the domain. Furthermore, the PM is often characterized by subdiffusive behavior (36, 37), with , where is the generalized diffusion coefficient with units cm2/sα, and is the anomalous exponent. We expect different populations of Nav channels to be described by different types of MSDs. Fig. 4 C shows the MSD averaged for all trajectories within each diffusivity regime. In each of these regimes, the MSD has very distinctive features. For the molecules with low effective diffusion coefficient, the MSD rapidly converges to a value of μm2/s, which is characteristic of confined particles. The high-diffusivity regime shows anomalous diffusion with during the whole observation time. On the other hand, the intermediate regime exhibits two different behaviors. At short lag times, the MSD appears to saturate, as in confined motion, but at longer times, the MSD increases again. This behavior is expected for molecules that are transiently confined but are able to escape from the trapping environment until they are captured again into a new domain. The inflection point in the MSD indicates that the characteristic trapping time of these molecules is of the order of 1 s.
When particles are confined, the size of the domain can be estimated from the saturation in the MSD. Fig. 4 D shows a histogram of the MSDs at lag time for the trajectories in the low-diffusivity regime, which correspond to the molecules that remain confined during the whole observation time. The MSD at 100 ms, 0.012 ± 0.009 μm2 (mean ± SD), is a good indicator of the saturation value. However, two different features can affect the MSD saturation, the radius of the domain and the localization error. In practice, , where σ is the localization standard error and, assuming that the domain is circular, is its radius. Fig. 4 E shows the distribution of localization uncertainty of all localized particles, from which we find 29 ± 15 nm (mean ± SD). Taking this localization uncertainty into account, we can infer the radii of the confinement domains to be 130 ± 90 nm. Note that this size is at or below the diffraction limit of traditional imaging. Thus, size cannot be inferred from the images presented in Figs. 1, 2, and 3.
Diffusion and energy landscapes of Nav1.6 on the soma
Considering that a subpopulation of channels is localized to stable nanocluster domains, we sought to map the two-dimensional diffusion and energy landscapes of Nav1.6 channels using high-density single-particle tracking and Bayesian inference methods. A suitable method for measuring single-particle trajectories at high densities consists of labeling the molecules with photoactivatable fluorophores so that at any given time only a small fraction of the molecules are in their active fluorescence state. This technique, known as spt-PALM (33), allows the sampling of hundreds of thousands of short trajectories within a single cell. We used Nav1.6 tagged with Dendra2 on the C-terminus (Nav1.6-Dendra2). Dendra2 is a monomeric protein that emits green fluorescence in its unconverted state and irreversibly switches to red emission upon irradiation with violet light (38). Fig. 5 A shows a TIRF image of a rat hippocampal neuron expressing Nav1.6-Dendra2, acquired with 488 nm excitation to observe the total expression before photoconversion. Here, the unconverted green Dendra2 shows all surface channels and fluorescence from any intracellular channels excited by the TIR excitation field. The surface-specific labeling shown in Fig. 1 B is sparse by comparison, due not only to the low surface density on the soma but also to the fact that channel biotinylation and streptavidin binding are not 100% efficient. Using a low-intensity 405 nm laser, a sparse subset of photoconverted molecules were continuously maintained in the field of view. The photoconverted molecules were imaged under 561 nm excitation and tracked until they photobleached. Fig. 5 B shows the tracks obtained from spt-PALM during 8 min (10,000 frames), where each colored line represents a track from an individual photoconverted Dendra2 molecule. The precision of localization as determined by Gaussian fitting was σ = 39 ± 18 nm. Further details can be observed in the 8 μm × 8 μm enlarged region shown in Fig. 5 C, defined by the dark square in Fig. 5 B. Note that the central region in Fig. 5 C that is devoid of single-molecule tracks most likely represents a part of the soma membrane that was not in direct contact with the coverslip surface and thus was outside the TIRF illumination field.
The high-density single-molecule data were used to obtain large-scale maps of the diffusivity and energy landscapes using InferenceMAP, an inference software based on Bayesian tools (34). Unsupervised learning was used to mesh the surface of cells according to local density. This strategy leads to a Voronoi tessellation of Nav1.6 channel localization with higher resolution in dense regions such as nanocluster domains. Voronoi tessellation ensures a regularized amount of information spread over the complete surface of the cell. The diffusivity, D, and potential energy, V, of each subdomain are estimated from the displacements within the observed trajectories (39, 40). The system is not assumed to be in thermodynamic equilibrium, and thus, the energy is computed from the molecular translocations and not from the channel density. Fig. 5 D shows the diffusivity map determined from the trajectories in Fig. 5 C. Although most of the surface has a diffusivity D = 0.13 ± 0.02 μm2/s, small dark pockets of lower diffusivity where D < 0.06 μm2/s are apparent. Fig. 5, E and F, shows the potential energy landscape (E) and an overlay of diffusivity (green) and energy (red) (F). The energy landscape also exhibits lower energy wells where Nav1.6 channels aggregate, i.e., at nanoclusters. The overlaid image shows many black spots where both the energy and the diffusivity are lower than in the rest of the membrane. These spots indicate that the diffusivity in the nanoclusters is smaller than that in the rest of the cell. Two energy wells, indicated by a box in Fig. 5 E, are shown in Fig. 5 G.
Given that the energy wells observed on the cell surface show the location and morphology of Nav1.6 nanocluster domains, we took advantage of the energy landscape to map and characterize the nanoclusters in detail. The nanoclusters were identified by thresholding the energy landscape and then their size and energy depth were found by a Gaussian fit across the horizontal and vertical axes. We found an average of 3 nanoclusters/μm2, but these domains were not uniformly distributed across the surface. Although some regions had a large concentration of nanoclusters, others seemed to be devoid of them. Fig. 5 H shows the distribution of nanocluster radii measured in 158 domains. The radius was defined as the standard deviation of the Gaussian fit and two radii were measured for each nanocluster, σx and σy. From the energy wells, the nanocluster radii were found to be 114 ± 58 nm (mean ± SD). This value agrees well with the 130 ± 90 nm radii of the confinement domains as determined from the MSD analysis of Fig. 4. Fig. 5 I shows the distribution of the depths of the energy wells. The measured trapping energy was found to be −1.6 ± 0.7 kBT. Such a shallow energy depth is not consistent with molecules being confined within nanoclusters during long observation times as seen in Fig. 3. The discrepancy is due to the fact that all the molecules were employed to map the diffusion and energy landscapes, but only a molecular subpopulation (41%) were efficiently trapped into nanoclusters. Thus, although the trapped molecules allowed us to determine the nanocluster size with superior accuracy relative to the MSD analysis presented in Fig. 4, the nonclustering mobile molecules effectively lowered the calculated energy depth of the wells.
Nav1.6 nanoclusters are actin independent
Since the localization mechanism of somatic Nav1.6 is not due to ankyrin binding, we hypothesized that other cytoskeletal components may be involved. Indeed, actin regulates the formation of Kv2.1 K+ channel domains in neurons (41) as well as the clustering of different membrane proteins in other cell types (42, 43, 44). Thus, we sought to determine whether Nav1.6 nanoclusters are also stabilized by cortical actin. To this end, we imaged the cortical actin cytoskeleton with PALM superresolution while simultaneously observing Nav1.6 localization. Actin was labeled with photoactivatable GFP and surface Nav1.6 with CF640R. Superresolution imaging of F-actin as illustrated in Fig. S2 A failed to colocalize cortical actin filaments with the Nav1.6 nanoclusters, although the two were often in close proximity. To further address whether actin is involved in Nav1.6 localization, we imaged the distribution of Nav1.6 in the presence of 200 nM swinholide A (swinA), a drug that both severs F-actin and sequesters G-actin (45). Fig. S3 shows that the intensity and location of the Nav1.6 nanoclusters is not perturbed over 75 min after addition of swinA. Nanocluster number and location did not change after actin depolymerization (p > 0.65).
Nav1.6 nanoclusters do not localize with clathrin-coated pits, mitochondria, or Kv2.1-induced endoplasmic-reticiulum-PM junctions
Since neither ankyrin-G nor actin seem to play an important role in the maintenance of the somatic nanoclusters, we next investigated whether Nav1.6 colocalized with several scaffold/organelle markers. Since we have previously described that clathrin-coated pits transiently immobilize Kv2.1 K+ channels (31), we coexpressed clathrin light chain tagged with GFP (CLC-GFP) with Nav1.6. However, CLC-GFP did not colocalize with Nav1.6-BAD nanoclusters labeled with CF640R (see Fig. S4 A), indicating that the nanoclusters are not clathrin-mediated endocytic platforms. This is consistent with the very long lifetime of the nanoclusters, since clathrin-coated pits are shorter-lived (seconds to minutes) (31, 46). However, individual Nav1.6 channels may interact with clathrin-coated pits for clathrin-mediated internalization.
Mitochondria, which localize near membrane-bound proteins and regulate them through calcium and oxidative signaling (47), were also evaluated as a candidate involved in the regulation of Nav1.6 nanoclusters. MitoTracker-labeled mitochondria adjacent to the plasma membrane were imaged in TIRF mode and compared to the distribution of Nav1.6-BAD labeled with streptavidin-AlexaFluor488. Again, no apparent relationship between mitochondria and the somatic nanoclusters was observed (Fig. S4 B).
We next looked at the correlation between Nav1.6 nanoclusters and the delayed rectifier voltage-gated potassium channel, Kv2.1, which forms large, micron-sized clusters on both the soma and AIS of hippocampal neurons (48, 49). These channels were recently found to mediate the formation of junctions between the endoplasmic reticulum and PM, which act as membrane trafficking hubs (50). As illustrated in Fig. S4 C, Nav1.6-BAD nanoclusters were excluded from the large Kv2.1 clusters. This exclusion from Kv2.1-induced endoplasmic-reticulum-PM junctions is likely due to the large intracellular mass of the Nav1.6 channel and may explain the small regions of soma membrane that were consistently devoid of Nav1.6 single-molecule tracks, as illustrated in Fig. 5 C.
Discussion
Due to the low numbers of somatic Nav1.6 channels and lack of tools to visualize them, the cell surface distribution of this protein has not been previously visualized in living neurons. Here, with the use of Nav1.6 constructs allowing the specific labeling of Nav1.6 surface channels combined with the high sensitivity of TIRF microscopy, we were able to visualize with single-molecule sensitivity the compartmental distribution of somatic Nav1.6 channels in live cells for the first time, to our knowledge. Specifically, we find a novel localization pattern of Nav1.6 in cultured hippocampal neurons where channels are anchored within long-lived (>30 min) nanoclusters with radii of 114 nm. Nanoclustering was detected using three distinct experimental strategies, saturation labeling of all surface Nav1.6 with fluorescent streptavidin (Fig. 1), single-particle tracking of a Nav1.6 subpopulation labeled with fluorescent streptavidin (Fig. 4), and spt-PALM using a fluorescent protein tag without surface streptavidin labeling (Fig. 5). The consistency of the protein distribution between these methods argues that these structures are not an artifact of the fluorescent protein or epitope tagging of Nav1.6, nor are they due to streptavidin labeling. In addition, the Nav1.6 nanoclusters were not observed in glial cells, which suggests that Nav1.6 nanoclustering is dependent on a protein or lipid component present in neurons but not glial cells. Importantly, Nav channel localization to these membrane domains is ankyrin independent, which is a striking discovery, since this is the only known ankyrin-independent mechanism for Nav channel localization in neurons.
The detection of Nav1.6 channel localization with single-molecule sensitivity in living cells enabled analysis of channel mobility. This is important, since both location and dynamics provide insights into protein regulation, for tethering into macromolecular complexes and molecular encounters govern most biological signaling. Our current understanding is that the plasma membrane is structured such that molecular movement is influenced in a manner that increases the likelihood of relevant biochemical interactions. This organization is achieved through several different mechanisms, including compartmentalization by the actin cytoskeleton and protein-protein interactions, as well as through effects of local lipid environments (36). Our analysis of long trajectories provides information on the heterogeneity of individual Nav1.6 behavior that is lost in ensemble techniques such as FRAP. Using spt-PALM data in combination with Bayesian inference tools, we were able to describe the diffusion and potential energy landscapes both surrounding and within stable membrane nanostructures. The strength of this approach lies in its ability to investigate the entire population of Nav1.6 surface protein within a substantial timescale (8 min) and with high resolution.
Three distinct behaviors were observed in the dynamics of somatic Nav1.6 channels. Single-particle tracking data indicate that 41% of channels are efficiently confined within nanoclusters for long times and a second population (11%) diffuses freely without interacting with the nanoclusters. Also apparent in the single-molecule data is a third population (47%) for which capturing interactions are weak and thus result in transient confinements. These observations suggest that Nav1.6 channels undergo posttranslational modifications that alter interactions and localization within the plasma membrane.
We hypothesize that functional differences exist between clustered and nonclustered channels, perhaps in a fashion similar to the behavior of Kv2.1 channels where clustered channels are held in a nonconducting state (51). Thus, modifications that regulate Nav1.6 clustering would allow the effective regulation of Nav1.6 function without the need of protein internalization to reduce voltage-dependent Na+ currents. Alternatively, clustered Nav1.6 channels could have biophysical properties distinct from the mobile population. Another possibility is that nanoclustering is linked to the function of other ion channels such as Na+-dependent K+ channels. Nav1.6-dependent Na+ influx is likely to only activate Na+-dependent K+ channel activity if the two channels are in very close proximity (52, 53). However, it is possible that Nav1.6 nanoclustering exists simply to enhance signaling fidelity. Theoretical work has demonstrated that 5–10 signaling molecules within a nanocluster can achieve an optimal signal/noise ratio by digitizing an analog signal (54). The physiological role of Nav channels is to transduce the analog-based membrane potential into the digital action potential. When Nav channels are clustered, it becomes much more likely that depolarizing stimuli will generate an action potential, for the localized depolarization caused by a single channel opening is more likely to activate other channels in its close vicinity before being dissipated.
What could be the mechanism responsible for the stable nanoclustering of Nav1.6? The channels appear to be corralled within 114- to 130-nm-radius domains, which suggests an interaction with the cortical cytoskeleton. However, the actin cytoskeleton is not involved, given the actin imaging and depolymerization experiments shown in Figs. S2 and S3. It is possible that cytoplasmic regions of Nav1.6 tether to intracellular scaffolds such as the recently described Kidins220/ARMS scaffolding protein that interacts with Nav1.2 and modulates its activity (55). Alternatively, another possible mechanism involves Nav1.6 glycosylation. Indeed, in Chinese hamster ovary cell lines expressing a dendritic cell membrane receptor (DC-SIGN), N-linked glycan-mediated interactions influence the overall lateral mobility of the protein (12). Nav channels can carry up to 40% of their mass in extracellular carbohydrate (56), and glycosylation is required for stable surface expression (57). Perhaps interaction of Nav1.6 carbohydrate with extracellular structures influences the surface distribution in a fashion analogous to cytoskeletal interactions.
Conclusions
Despite the fact that Nav channels were discovered decades ago and their central importance to neuronal function has been long accepted, knowledge of Nav cell biology is surprisingly lacking relative to that regarding other ion channels. This has been especially true for the Nav1.6 isoform that is perhaps the most abundant Nav channel in the mammalian brain. Our current study provides insight into the dynamics of Nav1.6 on the cell surface and raises new questions such as how somatic localization and function are linked and how localized regulation of these channels may influence overall neuronal physiology. Importantly, understanding the complex diffusion and energy landscape of the neuronal surface is essential to furthering our understanding of basic neuronal cell biology and the molecular regulation of electrical activity in the brain.
Author Contributions
E.J.A., M.M.T., and D.K. designed experiments. E.J.A., L.S., and B.J. performed the experiments and analyzed data. J.B.M. and M.B. assisted with the InferenceMAP analysis and wrote updated code. E.J.A., M.M.T., and D.K. analyzed data and wrote the manuscript. L.S., B.J., and J.B.M. edited the manuscript.
Acknowledgments
The authors thank Aubrey Weigel and Sanaz Sadegh for helpful discussions with regard to single-particle detection and tracking.
This work was supported by National Science Foundation Graduate Research Fellowship award DGE-1321845 to E.J.A., National Science Foundation grant 1401432 to D.K., and National Institutes of Health grant RO1 NS085142 to M.M.T.
Editor: Anne Kenworthy.
Footnotes
Supporting Materials and Methods, four figures and two movies are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(16)30704-4.
Contributor Information
Diego Krapf, Email: krapf@engr.colostate.edu.
Michael M. Tamkun, Email: michael.tamkun@colostate.edu.
Supporting Citations
References 58, 59 appear in the Supporting Material.
Supporting Material
Surface channels were labeled with SA-CF640R and imaged at 10 Hz using TIRF microscopy. The yellow arrows point to static nanoclusters while the white arrow points to a region that transiently accumulates individual channels.
Surface channels labeled with SA-CF640R were imaged at 10 Hz using TIRF microscopy.
References
- 1.Isom L.L., De Jongh K.S., Catterall W.A. Primary structure and functional expression of the β1 subunit of the rat brain sodium channel. Science. 1992;256:839–842. doi: 10.1126/science.1375395. [DOI] [PubMed] [Google Scholar]
- 2.Catterall W.A., Goldin A.L., Waxman S.G. International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol. Rev. 2005;57:397–409. doi: 10.1124/pr.57.4.4. [DOI] [PubMed] [Google Scholar]
- 3.Boiko T., Van Wart A., Matthews G. Functional specialization of the axon initial segment by isoform-specific sodium channel targeting. J. Neurosci. 2003;23:2306–2313. doi: 10.1523/JNEUROSCI.23-06-02306.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu W., Tian C., Shu Y. Distinct contributions of Nav1.6 and Nav1.2 in action potential initiation and backpropagation. Nat. Neurosci. 2009;12:996–1002. doi: 10.1038/nn.2359. [DOI] [PubMed] [Google Scholar]
- 5.Lorincz A., Nusser Z. Cell-type-dependent molecular composition of the axon initial segment. J. Neurosci. 2008;28:14329–14340. doi: 10.1523/JNEUROSCI.4833-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou D., Lambert S., Bennett V. AnkyrinG is required for clustering of voltage-gated Na channels at axon initial segments and for normal action potential firing. J. Cell Biol. 1998;143:1295–1304. doi: 10.1083/jcb.143.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meeks J.P., Mennerick S. Action potential initiation and propagation in CA3 pyramidal axons. J. Neurophysiol. 2007;97:3460–3472. doi: 10.1152/jn.01288.2006. [DOI] [PubMed] [Google Scholar]
- 8.Kole M.H., Ilschner S.U., Stuart G.J. Action potential generation requires a high sodium channel density in the axon initial segment. Nat. Neurosci. 2008;11:178–186. doi: 10.1038/nn2040. [DOI] [PubMed] [Google Scholar]
- 9.Myoga M.H., Beierlein M., Regehr W.G. Somatic spikes regulate dendritic signaling in small neurons in the absence of backpropagating action potentials. J. Neurosci. 2009;29:7803–7814. doi: 10.1523/JNEUROSCI.0030-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams S.R., Stuart G.J. Action potential backpropagation and somato-dendritic distribution of ion channels in thalamocortical neurons. J. Neurosci. 2000;20:1307–1317. doi: 10.1523/JNEUROSCI.20-04-01307.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cambi A., Lakadamyali M., Garcia-Parajo M.F. Meeting report—Visualizing signaling nanoplatforms at a higher spatiotemporal resolution. J. Cell Sci. 2013;126:3817–3821. doi: 10.1242/jcs.137901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torreno-Pina J.A., Castro B.M., Garcia-Parajo M.F. Enhanced receptor-clathrin interactions induced by N-glycan-mediated membrane micropatterning. Proc. Natl. Acad. Sci. USA. 2014;111:11037–11042. doi: 10.1073/pnas.1402041111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torreno-Pina J.A., Manzo C., Garcia-Parajo M.F. The actin cytoskeleton modulates the activation of iNKT cells by segregating CD1d nanoclusters on antigen-presenting cells. Proc. Natl. Acad. Sci. USA. 2016;113:E772–E781. doi: 10.1073/pnas.1514530113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choquet D., Triller A. The dynamic synapse. Neuron. 2013;80:691–703. doi: 10.1016/j.neuron.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 15.Kneussel M., Triller A., Choquet D. SnapShot: receptor dynamics at plastic synapses. Cell. 2014;157:1738. doi: 10.1016/j.cell.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Renner M., Schweizer C., Lévi S. Diffusion barriers constrain receptors at synapses. PLoS One. 2012;7:e43032. doi: 10.1371/journal.pone.0043032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rust M.B., Gurniak C.B., Witke W. Learning, AMPA receptor mobility and synaptic plasticity depend on n-cofilin-mediated actin dynamics. EMBO J. 2010;29:1889–1902. doi: 10.1038/emboj.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Specht C.G., Grünewald N., Triller A. Regulation of glycine receptor diffusion properties and gephyrin interactions by protein kinase C. EMBO J. 2011;30:3842–3853. doi: 10.1038/emboj.2011.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gorelova N., Seamans J.K. Cell-attached single-channel recordings in intact prefrontal cortex pyramidal neurons reveal compartmentalized D1/D5 receptor modulation of the persistent sodium current. Front. Neural Circuits. 2015;9:4. doi: 10.3389/fncir.2015.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleidervish I.A., Lasser-Ross N., Ross W.N. Na+ imaging reveals little difference in action potential-evoked Na+ influx between axon and soma. Nat. Neurosci. 2010;13:852–860. doi: 10.1038/nn.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Astman N., Gutnick M.J., Fleidervish I.A. Persistent sodium current in layer 5 neocortical neurons is primarily generated in the proximal axon. J. Neurosci. 2006;26:3465–3473. doi: 10.1523/JNEUROSCI.4907-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tian C., Wang K., Shu Y. Molecular identity of axonal sodium channels in human cortical pyramidal cells. Front. Cell. Neurosci. 2014;8:297. doi: 10.3389/fncel.2014.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lorincz A., Nusser Z. Molecular identity of dendritic voltage-gated sodium channels. Science. 2010;328:906–909. doi: 10.1126/science.1187958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharkey L.M., Cheng X., Meisler M.H. The ataxia3 mutation in the N-terminal cytoplasmic domain of sodium channel Nav1.6 disrupts intracellular trafficking. J. Neurosci. 2009;29:2733–2741. doi: 10.1523/JNEUROSCI.6026-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trudeau M.M., Dalton J.C., Meisler M.H. Heterozygosity for a protein truncation mutation of sodium channel SCN8A in a patient with cerebellar atrophy, ataxia, and mental retardation. J. Med. Genet. 2006;43:527–530. doi: 10.1136/jmg.2005.035667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meisler M.H., Kearney J.A. Sodium channel mutations in epilepsy and other neurological disorders. J. Clin. Invest. 2005;115:2010–2017. doi: 10.1172/JCI25466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Craner M.J., Newcombe J., Waxman S.G. Molecular changes in neurons in multiple sclerosis: altered axonal expression of Nav1.2 and Nav1.6 sodium channels and Na+/Ca2+ exchanger. Proc. Natl. Acad. Sci. USA. 2004;101:8168–8173. doi: 10.1073/pnas.0402765101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hinman J.D., Rasband M.N., Carmichael S.T. Remodeling of the axon initial segment after focal cortical and white matter stroke. Stroke. 2013;44:182–189. doi: 10.1161/STROKEAHA.112.668749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akin E.J., Solé L., Tamkun M.M. Preferential targeting of Nav1.6 voltage-gated Na+ channels to the axon initial segment during development. PLoS One. 2015;10:e0124397. doi: 10.1371/journal.pone.0124397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fox P.D., Haberkorn C.J., Tamkun M.M. Plasma membrane domains enriched in cortical endoplasmic reticulum function as membrane protein trafficking hubs. Mol. Biol. Cell. 2013;24:2703–2713. doi: 10.1091/mbc.E12-12-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weigel A.V., Tamkun M.M., Krapf D. Quantifying the dynamic interactions between a clathrin-coated pit and cargo molecules. Proc. Natl. Acad. Sci. USA. 2013;110:E4591–E4600. doi: 10.1073/pnas.1315202110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jaqaman K., Loerke D., Danuser G. Robust single-particle tracking in live-cell time-lapse sequences. Nat. Methods. 2008;5:695–702. doi: 10.1038/nmeth.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manley S., Gillette J.M., Lippincott-Schwartz J. High-density mapping of single-molecule trajectories with photoactivated localization microscopy. Nat. Methods. 2008;5:155–157. doi: 10.1038/nmeth.1176. [DOI] [PubMed] [Google Scholar]
- 34.El Beheiry M., Dahan M., Masson J.B. InferenceMAP: mapping of single-molecule dynamics with Bayesian inference. Nat. Methods. 2015;12:594–595. doi: 10.1038/nmeth.3441. [DOI] [PubMed] [Google Scholar]
- 35.Gasser A., Ho T.S.-Y., Dib-Hajj S.D. An ankyrinG-binding motif is necessary and sufficient for targeting Nav1.6 sodium channels to axon initial segments and nodes of Ranvier. J. Neurosci. 2012;32:7232–7243. doi: 10.1523/JNEUROSCI.5434-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krapf D. Mechanisms underlying anomalous diffusion in the plasma membrane. Curr. Top. Membr. 2015;75:167–207. doi: 10.1016/bs.ctm.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 37.Metzler R., Jeon J.H., Barkai E. Anomalous diffusion models and their properties: non-stationarity, non-ergodicity, and ageing at the centenary of single particle tracking. Phys. Chem. Chem. Phys. 2014;16:24128–24164. doi: 10.1039/c4cp03465a. [DOI] [PubMed] [Google Scholar]
- 38.Chudakov D.M., Lukyanov S., Lukyanov K.A. Tracking intracellular protein movements using photoswitchable fluorescent proteins PS-CFP2 and Dendra2. Nat. Protoc. 2007;2:2024–2032. doi: 10.1038/nprot.2007.291. [DOI] [PubMed] [Google Scholar]
- 39.Masson J.-B., Dionne P., Dahan M. Mapping the energy and diffusion landscapes of membrane proteins at the cell surface using high-density single-molecule imaging and Bayesian inference: application to the multiscale dynamics of glycine receptors in the neuronal membrane. Biophys. J. 2014;106:74–83. doi: 10.1016/j.bpj.2013.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Türkcan S., Alexandrou A., Masson J.B. A Bayesian inference scheme to extract diffusivity and potential fields from confined single-molecule trajectories. Biophys. J. 2012;102:2288–2298. doi: 10.1016/j.bpj.2012.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Connell K.M., Rolig A.S., Tamkun M.M. Kv2.1 potassium channels are retained within dynamic cell surface microdomains that are defined by a perimeter fence. J. Neurosci. 2006;26:9609–9618. doi: 10.1523/JNEUROSCI.1825-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bakker G.J., Eich C., Garcia-Parajo M.F. Lateral mobility of individual integrin nanoclusters orchestrates the onset for leukocyte adhesion. Proc. Natl. Acad. Sci. USA. 2012;109:4869–4874. doi: 10.1073/pnas.1116425109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goswami D., Gowrishankar K., Mayor S. Nanoclusters of GPI-anchored proteins are formed by cortical actin-driven activity. Cell. 2008;135:1085–1097. doi: 10.1016/j.cell.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Zanten T.S., Cambi A., Garcia-Parajo M.F. Hotspots of GPI-anchored proteins and integrin nanoclusters function as nucleation sites for cell adhesion. Proc. Natl. Acad. Sci. USA. 2009;106:18557–18562. doi: 10.1073/pnas.0905217106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bubb M.R., Spector I., Korn E.D. Swinholide A is a microfilament disrupting marine toxin that stabilizes actin dimers and severs actin filaments. J. Biol. Chem. 1995;270:3463–3466. doi: 10.1074/jbc.270.8.3463. [DOI] [PubMed] [Google Scholar]
- 46.Loerke D., Mettlen M., Schmid S.L. Cargo and dynamin regulate clathrin-coated pit maturation. PLoS Biol. 2009;7:e57. doi: 10.1371/journal.pbio.1000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chaplin N.L., Nieves-Cintrón M., Amberg G.C. Arterial smooth muscle mitochondria amplify hydrogen peroxide microdomains functionally coupled to L-type calcium channels. Circ. Res. 2015;117:1013–1023. doi: 10.1161/CIRCRESAHA.115.306996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sarmiere P.D., Weigle C.M., Tamkun M.M. The Kv2.1 K+ channel targets to the axon initial segment of hippocampal and cortical neurons in culture and in situ. BMC Neurosci. 2008;9:112. doi: 10.1186/1471-2202-9-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tamkun M.M., O’Connell K.M., Rolig A.S. A cytoskeletal-based perimeter fence selectively corrals a sub-population of cell surface Kv2.1 channels. J. Cell Sci. 2007;120:2413–2423. doi: 10.1242/jcs.007351. [DOI] [PubMed] [Google Scholar]
- 50.Fox P.D., Haberkorn C.J., Tamkun M.M. Induction of stable ER-plasma-membrane junctions by Kv2.1 potassium channels. J. Cell Sci. 2015;128:2096–2105. doi: 10.1242/jcs.166009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O’Connell K.M., Loftus R., Tamkun M.M. Localization-dependent activity of the Kv2.1 delayed-rectifier K+ channel. Proc. Natl. Acad. Sci. USA. 2010;107:12351–12356. doi: 10.1073/pnas.1003028107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hage T.A., Salkoff L. Sodium-activated potassium channels are functionally coupled to persistent sodium currents. J. Neurosci. 2012;32:2714–2721. doi: 10.1523/JNEUROSCI.5088-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salkoff L., Butler A., Wei A. High-conductance potassium channels of the SLO family. Nat. Rev. Neurosci. 2006;7:921–931. doi: 10.1038/nrn1992. [DOI] [PubMed] [Google Scholar]
- 54.Roob E., 3rd, Trendel N., Mugler A. Cooperative clustering digitizes biochemical signaling and enhances its fidelity. Biophys. J. 2016;110:1661–1669. doi: 10.1016/j.bpj.2016.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cesca F., Satapathy A., Scholz-Starke J. Functional interaction between the scaffold protein Kidins220/ARMS and neuronal voltage-gated Na+ channels. J. Biol. Chem. 2015;290:18045–18055. doi: 10.1074/jbc.M115.654699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marban E., Yamagishi T., Tomaselli G.F. Structure and function of voltage-gated sodium channels. J. Physiol. 1998;508:647–657. doi: 10.1111/j.1469-7793.1998.647bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Waechter C.J., Schmidt J.W., Catterall W.A. Glycosylation is required for maintenance of functional sodium channels in neuroblastoma cells. J. Biol. Chem. 1983;258:5117–5123. [PubMed] [Google Scholar]
- 58.Ovesný M., Křížek P., Hagen G.M. ThunderSTORM: a comprehensive ImageJ plug-in for PALM and STORM data analysis and super-resolution imaging. Bioinformatics. 2014;30:2389–2390. doi: 10.1093/bioinformatics/btu202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Riedl J., Crevenna A.H., Wedlich-Soldner R. Lifeact: a versatile marker to visualize F-actin. Nat. Methods. 2008;5:605–607. doi: 10.1038/nmeth.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Surface channels were labeled with SA-CF640R and imaged at 10 Hz using TIRF microscopy. The yellow arrows point to static nanoclusters while the white arrow points to a region that transiently accumulates individual channels.
Surface channels labeled with SA-CF640R were imaged at 10 Hz using TIRF microscopy.