Abstract
Purpose
A detailed family history provides an inexpensive alternative to genetic profiling for individual risk assessment. We updated the PCPT Risk Calculator to include detailed family histories.
Materials and Methods
The study included 55,168 prostate cancer cases and 638,218 controls from the Swedish Family Cancer Database who were 55 years old or older in 1999 and had at least 1 male first-degree relative 40 years old or older and 1 female first-degree relative 30 years old or older. Likelihood ratios, calculated as the ratio of risk of observing a specific family history pattern in a prostate cancer case compared to a control, were used to update the PCPT Risk Calculator.
Results
Having at least 1 relative with prostate cancer increased the risk of prostate cancer. The likelihood ratio was 1.63 for 1 first-degree relative 60 years old or older at diagnosis (10.1% of cancer cases vs 6.2% of controls), 2.47 if the relative was younger than 60 years (1.5% vs 0.6%), 3.46 for 2 or more relatives 60 years old or older (1.2% vs 0.3%) and 5.68 for 2 or more relatives younger than 60 years (0.05% vs 0.009%). Among men with no diagnosed first-degree relatives the likelihood ratio was 1.09 for 1 or more second-degree relatives diagnosed with prostate cancer (12.7% vs 11.7%). Additional first-degree relatives with breast cancer, or first-degree or second-degree relatives with prostate cancer compounded these risks.
Conclusions
A detailed family history is an independent predictor of prostate cancer compared to commonly used risk factors. It should be incorporated into decision making for biopsy. Compared with other costly biomarkers it is inexpensive and universally available.
Keywords: prostatic neoplasms, biopsy, family, likelihood functions, breast neoplasms
AS cancer clinical practice moves toward personalized approaches and large-scale data specializing in individual risk factors become increasingly available, the need emerges to synthesize and incorporate this information into existing cancer risk prediction tools. For example, the completion of multiple confirmatory genome-wide association studies identifying common and rare single nucleotide polymorphisms has promoted their incorporation into commonly used cancer risk prediction tools.1–9 To date these markers have had only modest impacts on risk and they are often not widely used due to cost.10
A less expensive and more easily implemented alternative to genetic markers is the collection of a detailed family history of cancer. While the commonly used definition of a family history of disease is dichotomous (ie do you have a FDR with a history of the same disease? Yes or no), a detailed family history assesses for the disease in SDRs, the number of relatives diagnosed, ages at diagnosis and information on related diseases. Although a self-reported family history is easier to obtain and far less costly than genetic measures, it is prone to recall error and large sample sizes are needed to appropriately assess the association between rare family history patterns and disease outcomes.
The SFCD, which includes data on the entire population of Sweden (those born after 1931 plus their biological parents), is the largest comprehensive family cancer registry in the world.11 Data housed in the registry are not self-reported but rather assimilated from a nationwide linked network of death and hospital registries. The latest SFCD update occurred in 2010 and it now includes more than 12.2 million individuals and more than 1.1 million first primary cancers.11 Analogous to the large genome-wide consortiums that maximize sample numbers for clinical outcome predictions based on genetic markers, the SFCD provides the large sample numbers needed to accurately identify the association between a detailed family history and cancer risk prediction.
After a large-scale twin study in Sweden, Denmark and Finland estimated the heritability of prostate cancer at 42%, a SFCD study identified the key detailed family history risk factors associated with the risk of prostate cancer in the next 10 years.12,13 These factors included prostate cancer detected in a FDR younger than 60 vs 60 years old or older, prostate cancer in a SDR, breast cancer in a FDR and esophageal carcinoma in situ in the index man or in a FDR. A comprehensive risk score based only on these factors was proposed for use in prostate cancer screening. This risk assessment score can be easily implemented in clinical practice, requiring that the patient only complete a short questionnaire.
An ideal method to implement a detailed family history into prostate cancer risk assessment would be to use a comprehensive tool that incorporates other validated measures of risk. The PCPTRC (http://myprostatecancerrisk.com/), the most commonly used tool for this assessment, includes PSA, DRE, family history of prostate cancer, prior negative biopsy (if done), age and race/ethnicity.14 Based on these risk factors a simple display of individualized predicted outcomes (negative biopsy and low vs high grade cancer) enables physicians to provide a context to counsel patients on their preference of whether to proceed to biopsy. The PCPTRC was externally validated in dozens of international diverse populations.15–25 Since its development, the calculator has been modified to incorporate newly discovered and FDA (Food and Drug Administration) approved markers for prostate cancer, including PCA3 and percent free PSA, using a Bayesian technique to update a risk tool called the LR.26,27 As these updated PCPTRC calculators became available online, they have also undergone validation studies.28,29 We provide an updated online PCPTRC to incorporate a detailed family history into contemporary clinical prostate cancer risk assessment based on the established risk factors.
MATERIALS AND METHODS
Data on men with at least 1 FDR recorded in the 2010 version of the SFCD were extracted. The men were alive, 55 years old or older and free from prostate cancer at the beginning of the study period (1999 to 2010). The SFCD was collected under approval by the Lund University regional ethics committee in Sweden with an anonymous version used for this analysis. To establish prostate cancer cases and controls the men who met study eligibility requirements were segregated into those in whom prostate cancer did and did not develop, respectively, during the subsequent 11 years until 2010.
Based on the study by Roudgari et al13 FDR and SDR prostate cancer history, and FDR breast cancer history were selected as the detailed family history patterns relevant to prostate cancer risk. FDR prostate cancer history was stratified by whether cancer was diagnosed before vs at or after age 60 years as well as by whether zero, 1, or 2 or more FDRs were diagnosed. SDR prostate cancer and FDR breast cancer history were only stratified by no vs 1 or more respective relatives diagnosed. Roudgari et al used the cutoff point of 60 years as the age most commonly serving as a discriminator for prostate cancer at younger ages, comparable to other published studies. Esophageal carcinoma in a FDR, which was included in the score of Roudgari et al, was not included due to its low incidence.
The LR was defined as the ratio of the proportion of prostate cancer cases with a specific family history pattern vs the corresponding proportion of controls with the pattern. Thus, a LR greater than 1 means that a specific family history pattern was more common in cancer cases, a LR less than 1 means that the pattern was more common in controls and a LR of 1 means that the pattern was equally common in cases and controls.30 No adjustment was made for age or race to preserve sample size and obtain an empirical estimate without relying on a statistical model.
The LRs for FDR family history patterns were calculated for men with at least 1male FDR 40 years old or older and at least 1 female FDR 30 years old or older. Because calculations with the more stringent requirement of at least 2 male FDRs 40 years old or older yielded almost identical results, the larger and less restricted sample was used to maximize statistical power. The LRs for joint FDR and SDR family history were calculated by multiplying the LRs for FDRs by the conditional LRs for SDRs stratified by FDR family history. The latter LRs were calculated for men with at least 1 male FDR 40 years old or older, at least 1 female FDR 30 years old or older and at least 1 male SDR 40 years old or older. We calculated the CIs of LRs using the d method applied to the logarithm and the Bonferroni adjustment using the number of simultaneous intervals calculated to obtain 95% overall confidence.
The PCPTRC was updated in a fashion similar to that in our previous studies of other markers.26,27 Specifically the prior odds of prostate cancer based only on the risk factors PSA, DRE, age, race and biopsy history were calculated using the latest release, PCPTRC 2.0, assuming that the question on a FDR family history of prostate cancer was answered no. This was done so that the influence of family history would not be counted doubly from the PCPT and the SFCD. We used the available PCPTRC 2.0 formulas (http://myprostatecancerrisk.com/). The LRs of the detailed family history trajectories were multiplied by prior odds to obtain the posterior odds according to the Bayes rule. New updated PCPTRC risks incorporating the detailed family history were obtained from the posterior odds.
RESULTS
Of 6,133,364 men with at least 1 FDR recorded in the 2010 SFCD version 4,993,373 were alive and free of prostate cancer at the beginning of the study period (1999 to 2010). About a fifth of these men (1,019,441) were 55 years old or older in 1999, including about 82% (832,110) with at least 1 recorded male FDR 40 years old or older, more than 85% (864,102) with at least 1 reported female FDR 30 years old or older, and more than 27% (272,798) with at least 1 documented male SDR 40 years old or older. The resulting cohort used for analysis comprised 693,386 men, of whom 8.0% were diagnosed with prostate cancer during the subsequent 11-year period.
The table shows the impact of a detailed FDR history of prostate and breast cancer. Family history patterns involving at least 1 FDR diagnosed with prostate cancer were more common among prostate cancer cases than controls. The patterns were statistically significant even after stringent adjustment for multiple testing except for cases with small observed numbers. A FDR diagnosed with prostate cancer at younger than 60 years incurred a higher risk than a FDR diagnosed at age 60 years or greater, and the risk increased as the number of diagnosed relatives increased from 1 to 2 or more. For example, 10.1% of patients with prostate cancer had 1 FDR 60 years old or older who was diagnosed with prostate cancer compared to 6.2% of controls (LR 1.63, CI 1.56 to 1.70). The percent of prostate cancer cases and controls with 1 FDR younger than 60 years decreased (1.5% vs 0.6%) but the LR increased to 2.47 (CI 2.20 to 2.79). Moving from 1 FDR younger than 60 years to 2 or more such FDRs became more rare and was almost 6 times more common in prostate cancer cases than in controls (0.05% vs 0.007%, LR 5.68, CI 2.75 to 11.74).
Patients with prostate cancer and 638,218 controls with 0, 1, or 2 or more FDRs diagnosed with prostate or breast cancer
| No. FDR | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pt No. | Prostate Ca, Less Than 60 |
Prostate Ca, 60 or Greater |
Breast Ca | No. Cases (%) | No. Controls (%) | LR (99.8% CI)* | |||
| 1 | 43,006 | (78.0) | 537,996 | (84.3) | 0.93 | (0.92,0.93) | |||
| 2 | 1 | 3,509 | (6.4) | 45,219 | (7.1) | 0.90 | (0.85,0.95) | ||
| 3 | 2 or More | 221 | (0.4) | 2,556 | (0.4) | 1.00 | (0.81,1.24) | ||
| 4 | 1 | 5,597 | (10.1) | 39,775 | (6.2) | 1.63 | (1.56,1.70) | ||
| 5 | 1 | 1 | 756 | (1.4) | 4,632 | (0.7) | 1.89 | (1.67,2.13) | |
| 6 | 1 | 2 or More | 52 | (0.09) | 378 | (0.06) | 1.59 | (1.01,2.51) | |
| 7 | 2 or More | 662 | (1.2) | 2,215 | (0.3) | 3.46 | (3.02,3.96) | ||
| 8 | 2 or More | 1 | 99 | (0.2) | 309 | (0.05) | 3.71 | (2.60,5.29) | |
| 9 | 2 or More | 2 or More | 8 | (0.01) | 24 | (0.004) | 3.86 | (1.09,13.61) | |
| 10 | 1 | 809 | (1.5) | 3,785 | (0.6) | 2.47 | (2.20,2.79) | ||
| 11 | 1 | 1 | 85 | (0.2) | 419 | (0.07) | 2.35 | (1.63,3.39) | |
| 12 | 1 | 2 or More | 5 | (0.009) | 33 | (0.005) | 1.75 | (0.40,7.72) | |
| 13 | 1 | 1 | 193 | (0.3) | 612 | (0.1) | 3.65 | (2.83,4.71) | |
| 14 | 1 | 1 | 1 | 39 | (0.07) | 65 | (0.01) | 6.94 | (3.71,12.98) |
| 15 | 1 | 1 | 2 or More | 2 | (0.004) | 7 | (0.001) | 3.31 | (0.28,39.37) |
| 16 | 1 | 2 or More | 48 | (0.09) | 79 | (0.01) | 7.03 | (3.99,12.37) | |
| 17 | 1 | 2 or More | 1 or More | 3 | (0.005) | 15 | (0.002) | 2.31 | (0.33,16.33) |
| 18 | 2 or More | 27 | (0.05) | 55 | (0.009) | 5.68 | (2.75,11.74) | ||
| 19 | 2 or More | 1 or More | 4 | (0.007) | 6 | (0.0009) | 7.71 | (1.05,56.68) | |
| 20 | 2 or More | 1 | 18 | (0.03) | 21 | (0.003) | 9.92 | (3.68,26.75) | |
| 21 | 2 or More | 1 | 1 or More | 3 | (0.005) | 3 | (0.0005) | 11.57 | (0.93,144.21) |
| 22 | 2 or More | 2 or More | 11 | (0.02) | 6 | (0.0009) | 21.21 | (4.42,101.76) | |
| 23 | 2 or More | 2 or More | 1 or More | 11 | (0.02) | 8 | (0.001) | 15.91 | (3.79,66.85) |
Percent cancer/percent controls with 99.8% CI reported to account for simultaneous significance of 23 tests at overall 95% confidence.
The supplementary table (http://jurology.com/) shows the impact of a SDR history of prostate cancer. The effects of having 1 or more SDRs with prostate cancer were not statistically significant except in men with no FDR family history of prostate cancer. This observation was ostensibly due to the much smaller population of men with a positive FDR family history. In men with no FDR family history the proportion of prostate cancer cases with a SDR family history was only slightly higher than the respective proportion of controls (12.7% vs 11.7%, LR 1.09, 99.4% CI 1.02 to 1.16).
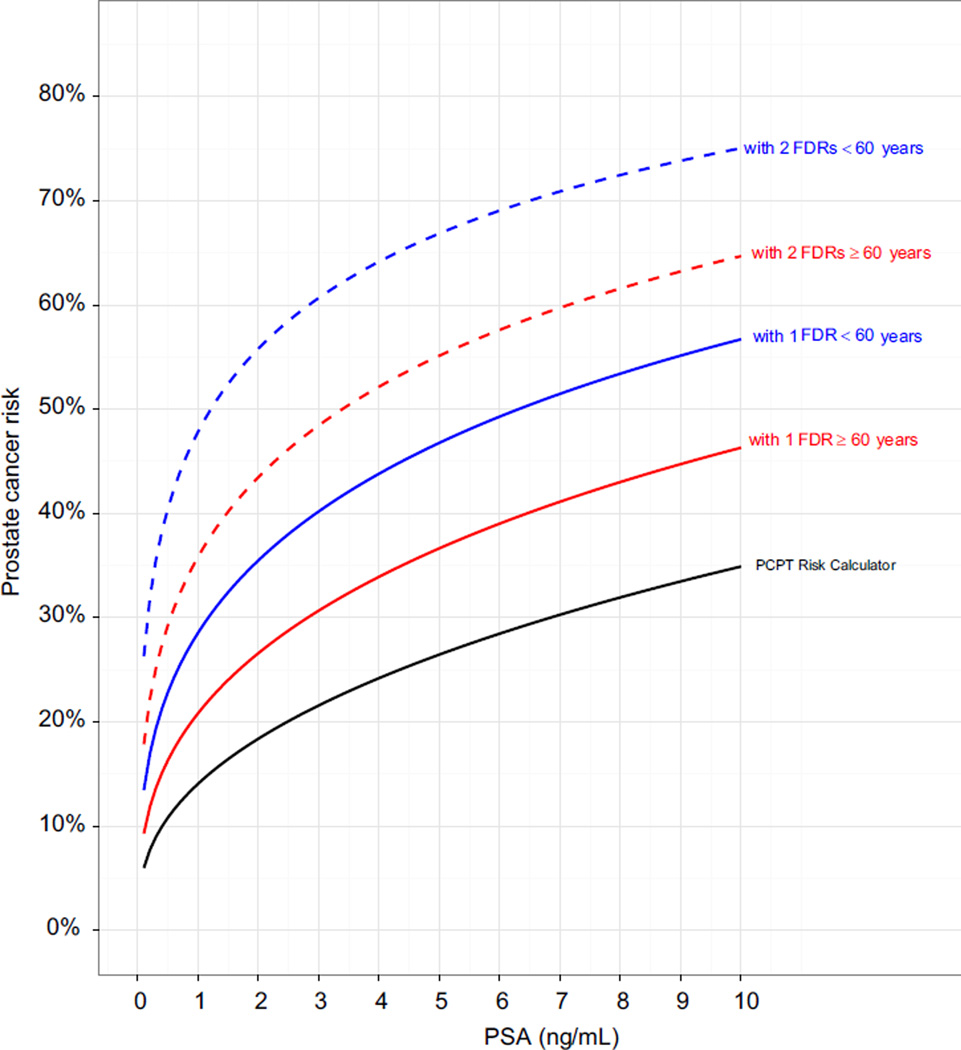
The figure shows the impact of using the calculated FDR and SDR LRs to incorporate a detailed family history of prostate cancer into the PCPTRC. The greatest determinants of risk were FDRs diagnosed with prostate cancer at younger than 60 years, which exerted larger effects than diagnoses at age 60 years or greater. Including the FDR history of breast cancer only slightly moved the curves and a SDR with prostate cancer moved the curves only negligibly.
Estimated risk of prostate cancer on biopsy using PCPTRC without detailed family history and updated PCPTRC with information on FDRs with prostate cancer according to PSA in 65-year-old white man with normal DRE and no biopsy history. Since incorporating SDRs with prostate cancer or FDRs with breast cancer impacted risk only slightly or negligibly, those data are not shown. Dashed blue curve indicates 2 FDRs younger than 60 years. Dashed red curve indicates 2 FDRs 60 years old or older. Solid blue curve indicates 1 FDR younger than 60 years. Solid red curve indicates 1 FDR 60 years old or older. Black curve indicates PCPTRC.
DISCUSSION
A detailed family history is an inexpensive means of using genetic information that adds predictive value to the established clinical risk factors that are routinely used to screen for prostate cancer in the United States, including PSA and DRE. To our knowledge the comprehensive SFCD, which includes the entire population of Sweden (those born after 1931 plus their biological parents), provides the only current resource with sufficient statistical power to determine the influence of a detailed family history on prostate cancer risk.
There are several limitations to this study. The first limitation is that there is currently no internal or external validation of the algorithm because to our knowledge no cohort currently exists with the detailed family history variables recommended by the recent analysis of Roudgari et al13 as well as the PCPT risk factors, including PSA measured within 1 year before biopsy. Thus, the updated PCPTRC to include detailed family history was made available at the PCPTRC website (http://myprostatecancerrisk.com/) to encourage groups at institutions to collect and synthesize the risk factors and pursue validation. This has always been the way that PCPTRC algorithms have been validated with more than 50 published validations of the algorithm and extended algorithms.15–30 Detailed family history is already collected at some clinics in Europe but in the United States this typically requires informed consent. We instigated this process at several clinical sites.
The extended calculator could not be validated because it was built from 2 cohorts for which nonoverlapping risk factors were collected, including the SFCD cohort with detailed family history and the PCPT cohort with PSA, DRE, race, age, FDR prostate cancer history and prior biopsy history. A single large study with all clinical and detailed family history variables recorded on the same individuals would have been preferable. Unfortunately to our knowledge such a large cohort of 693,386 men with detailed family history and PSA recorded within 1 year before biopsy (as required by the PCPT) is not available.
A second study limitation is that the clinically more relevant end point of lethal prostate cancer has not been considered. Mortality data are generally not available and would be the source of a greater degree of error due to bias in assessing cause of death. Gleason grade was unfortunately also not available in the SFCD and it is likely that detection and expectation bias contributed to inflated estimates of low grade, potentially over detected prostate cancers. Expectation bias would have occurred when subjects with a family history were more likely to be screened and, thus, have cancer detected. Detection depends on screening intensity, which was not controlled for in this comprehensive population study of Sweden from 1999 to 2010. We divided the 11-year followup into early (1999 to 2005) and later (2006 to 2010) periods, and recalculated the LRs to see whether they were increased in the later period. We found slight increases in most but not all family history patterns, supporting the hypothesis of an increasing detection and expectation bias role in prostate cancer detection. However, our results importantly held true in each era, indicating that PSA screening did not dramatically affect the ability of a detailed family history to predict prostate cancer risk.
Based on PCPT data we previously found a statistically significant association of a FDR prostate cancer history with the detection of low grade (Gleason score less than 7) but not high grade disease.14 However, the associations were almost of the same magnitude (low vs high grade OR 1.31 vs 1.25). The lack of statistical significance could have been due to a lack of statistical power caused by the smaller number of high grade cases in the screening trial. Results of the updated PCPTRC risk of prostate cancer incorporating detailed family history will be presented with the PCPTRC risk of low vs high grade disease without incorporating detailed family history. An informal approximation of the risk of high grade cancer using the PCPTRC and incorporating detailed family history data could be obtained by multiplying the risk of overall prostate cancer by the fraction of high grade risk from the PCPTRC without incorporating detailed family history. More studies are needed to establish the association of detailed family history with consequential disease.
A third study limitation is the relatively homogeneous Swedish population. While these findings may not be more generalizable to the United States or other populations, to our knowledge the lack of a similar registry that could validate this finding in the United States or other homogeneous populations makes this a unique observation. The online risk calculator will expedite the needed external validation in diverse populations to ascertain its clinical usefulness.
CONCLUSIONS
This analysis demonstrates that risk assessment may potentially be improved using detailed family histories. This is available now via the online PCPTRC for the needed external validation to determine clinical usefulness.
Acknowledgments
Study received Lund University regional ethics committee approval.
Supported by grants UM1 CA182883, U01CA86402 and 5P30 CA0541474, and the EU Transcan Program through the German Ministry of Education and Science (KH).
Abbreviations and Acronyms
- DRE
digital rectal examination
- FDR
first-degree relative
- LR
likelihood ratio
- PCPT
Prostate Cancer Prevention Trial
- PCPTRC
PCPT Risk Calculator
- PSA
prostate specific antigen
- SDR
second-degree relative
- SFCD
Swedish Family Cancer Database
REFERENCES
- 1.Gail MH. Discriminatory accuracy from single-nucleotide polymorphisms in models to predict breast cancer risk. J Natl Cancer Inst. 2008;100:1037. doi: 10.1093/jnci/djn180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gail MH. Value of adding single-nucleotide polymorphism genotypes to a breast cancer risk model. J Natl Cancer Inst. 2009;101:959. doi: 10.1093/jnci/djp130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wacholder S, Hartge P, Prentice R, et al. Performance of common genetic variants in breast-cancer risk models. N Engl J Med. 2010;362:986. doi: 10.1056/NEJMoa0907727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raji OY, Agbaje OF, Duffy SW. Incorporation of a genetic factor into an epidemiologic model for prediction of individual risk of lung cancer: The Liverpool Lung Project. Cancer Prev Res. 2010;3:664. doi: 10.1158/1940-6207.CAPR-09-0141. [DOI] [PubMed] [Google Scholar]
- 5.Akamatsu S, Takahashi A, Takata R, et al. Reproducibility, performance, and clinical utility of a genetic risk prediction model for prostate cancer in Japanese. PLoS One. 2012;7:e46454. doi: 10.1371/journal.pone.0046454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindstroem S, Schumacher FR, Cox D, et al. Common genetic variants in prostate cancer risk prediction—results from the NCI Breast and Prostate Cancer Cohort Consortium (BPC3) Cancer Epidemiol Biomarkers Prev. 2012;21:437. doi: 10.1158/1055-9965.EPI-11-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johansson M, Homstroem B, Hinchliffe SR, et al. Combining 33 genetic variants with prostate-specific antigen for prediction of prostate cancer: longitudinal study. Int J Cancer. 2012;130:129. doi: 10.1002/ijc.25986. [DOI] [PubMed] [Google Scholar]
- 8.Kader AK, Sun J, Reck BH, et al. Potential impact of adding genetic markers to clinical parameters in predicting prostate biopsy outcomes in men following an initial negative biopsy: findings from the REDUCE trial. Eur Urol. 2012;62:953. doi: 10.1016/j.eururo.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newcombe PJ, Reck BH, Sun J, et al. A comparison of Bayesian and frequentist approaches to incorporating external information for the prediction of prostate cancer risk. Genet Epidemiol. 2012;36:71. doi: 10.1002/gepi.21600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park JH, Gail MH, Greene MH. Potential usefulness of single nucleotide polymorphisms to identify persons at high cancer risk: an evaluation of seven common cancers. J Clin Oncol. 2012;30:2157. doi: 10.1200/JCO.2011.40.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hemminki K, Ji JG, Brandt A. The Swedish Family-Cancer Database 2009: prospects for histology-specific and immigrant studies. Int J Cancer. 2010;126:2259. doi: 10.1002/ijc.24795. [DOI] [PubMed] [Google Scholar]
- 12.Lichtenstein P, Holm NV, Verkasalo PK, et al. Environmental and heritable factors in the causation of cancer—analyses of cohorts of twins from Sweden, Denmark and Finland. N Engl J Med. 2000;343:78. doi: 10.1056/NEJM200007133430201. [DOI] [PubMed] [Google Scholar]
- 13.Roudgari H, Hemminki K, Brandt A. Prostate cancer risk assessment model: a scoring model based on the Swedish Family-Cancer Database. J Med Genet. 2012;49:345. doi: 10.1136/jmedgenet-2011-100290. [DOI] [PubMed] [Google Scholar]
- 14.Ankerst DP, Hoefler J, Bock S, et al. The Prostate Cancer Prevention Trial Risk Calculator 2.0 for the prediction of low-versus high-grade prostate cancer. Urology. 2014;83:1362. doi: 10.1016/j.urology.2014.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parekh DJ, Ankerst DP, Higgins BA, et al. External validation of the Prostate Cancer Prevention Trial risk calculator in a screened population. Urology. 2006;68:1152. doi: 10.1016/j.urology.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 16.Eyre SJ, Ankerst DP, Wei JT, et al. Validation in a multiple urology practice cohort of the Prostate Cancer Prevention Trial calculator for predicting prostate cancer detection. J Urol. 2009;182:2653. doi: 10.1016/j.juro.2009.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hernandez DJ, Han M, Humphreys EB, et al. Predicting the outcome of prostate biopsy: Comparison of a novel logistic regression-based model, the prostate cancer risk calculator, and prostate-specific antigen level alone. BJU Int. 2009;103:609. doi: 10.1111/j.1464-410X.2008.08127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cavadas V, Osorio L, Sabell F. Prostate cancer prevention trial and European randomized study of screening for prostate cancer risk calculators: a performance comparison in a contemporary screened cohort. Eur Urol. 2010;58:551. doi: 10.1016/j.eururo.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan DJ, Boorjian SA, Ruth K, et al. Evaluation of the Prostate Cancer Prevention Trial Risk calculator in a high-risk screening population. BJU Int. 2010;105:334. doi: 10.1111/j.1464-410X.2009.08793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nam RK, Kattan MW, Chin JL, et al. Prospective multi-institutional study evaluating the performance of prostate cancer risk calculators. J Clin Oncol. 2011;29:2959. doi: 10.1200/JCO.2010.32.6371. [DOI] [PubMed] [Google Scholar]
- 21.Trottier G, Roobol MJ, Lawrentschuk N, et al. Comparison of risk calculators from the Prostate Cancer Prevention Trial and the European Randomized Study of Screening for Prostate Cancer in a contemporary Canadian cohort. BJU Int. 2011;108:E237. doi: 10.1111/j.1464-410X.2011.10207.x. [DOI] [PubMed] [Google Scholar]
- 22.Oliveira M, Marques V, Carvalho AP. Head-to-head comparison of two online nomograms for prostate biopsy outcome prediction. BJU Int. 2011;107:1780. doi: 10.1111/j.1464-410X.2010.09727.x. [DOI] [PubMed] [Google Scholar]
- 23.Zhu Y, Wang JY, Shen YJ, et al. External validation of the Prostate Cancer Prevention Trial and the European Randomized Study of Screening for Prostate risk calculators in a Chinese cohort. Asian J Androl. 2012;14:738. doi: 10.1038/aja.2012.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ankerst DP, Boeck A, Freedland SJ, et al. Evaluating the PCPT risk calculator in ten international biopsy cohorts: results from the Prostate Biopsy Collaborative Group. World J Urol. 2012;30:181. doi: 10.1007/s00345-011-0818-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee DH, Jung HB, Park JW, et al. Can Western based online prostate cancer risk calculators be used to predict prostate cancer after prostate biopsy for the Korean population? Yonsei Med J. 2013;54:665. doi: 10.3349/ymj.2013.54.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ankerst DP, Groskopf J, Day JR, et al. Predicting prostate cancer risk through incorporation of prostate cancer gene 3. J Urol. 2008;180:1303. doi: 10.1016/j.juro.2008.06.038. [DOI] [PubMed] [Google Scholar]
- 27.Ankerst DP, Koniarski T, Liang Y et al, et al. Updating risk prediction tools: a case study in prostate cancer. Biom J. 2012;54:127. doi: 10.1002/bimj.201100062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perdona S, Cavadas V, Di Lorenzo G, et al. Prostate cancer detection in the “grey area” of prostate-specific antigen below 10 ng/ml: head-to-head comparison of the updated PCPT calculator and Chun’s nomogram, two risk estimators incorporating prostate cancer antigen 3. Eur Urol. 2011;59:81. doi: 10.1016/j.eururo.2010.09.036. [DOI] [PubMed] [Google Scholar]
- 29.Pepe P, Aragona F. Prostate cancer detection rate at repeat saturation biopsy: PCPT risk calculator versus PCA3 score versus case-finding protocol. Can J Urol. 2013;20:6620. [PubMed] [Google Scholar]
- 30.Scales CD, Jr, Zarei M, Dahm P. Evidence-based urology in practice: likelihood ratios. Evidence Based Urology Working Group. BJU Int. 2009;104:892. doi: 10.1111/j.1464-410X.2009.08785.x. [DOI] [PubMed] [Google Scholar]