Abstract
Most dialysis patients are vitamin D deficient, including deficiencies in both activated vitamin D (1, 25-dihydroxyvitamin D) and the less active 25-hydroxyvitamin D. These and other abnormalities associated with chronic kidney disease (CKD), if they remain untreated, lead to secondary hyperparathyroidism and bone changes, such as osteitis fibrosa cystica. Activated vitamin D has been proven to decrease parathyroid hormone (PTH) levels in dialysis patients and is currently used for this indication. There are multiple other potential “pleotrophic” effects associated with vitamin D therapy. These include associations with lower all-cause and cardiovascular mortality, lower rates of infections and improved glycemic indexes. Meta-analyses of multiple observational studies have shown activated vitamin D therapy to be associated with improved survival. Observational data also suggest fewer infections and better glucose control. There have been no randomized clinical trials powered to evaluate mortality or other clinical outcomes. Small trials of nutritional vitamin D (ergocalciferol and cholecalciferol) showed increases in 25-hydroxyvitamin D levels without hypercalcemia or hyperphosphatemia, even when given in addition to activated vitamin D therapy. While activated vitamin D therapy is associated with improved outcomes, it also leads to higher fibroblast growth factor 23 (FGF-23) levels, which may be detrimental in dialysis patients. Further research is needed to evaluate whether activated or nutritional vitamin D therapy are beneficial in dialysis patients for outcomes other than secondary hyperparathyroidism.
Chronic kidney disease – mineral bone disorder (CKD-MBD) is a systemic disorder that involves abnormal biochemical tests, abnormal bones and vascular calcification. The pathophysiology of CKD-MBD is complex and our understanding of it is rapidly evolving. Vitamin D plays a central role in CKD-MBD as the 1-α hydroxylase enzyme is found in abundance in the kidney. Thus, dialysis patients without working kidneys are deficient in activated vitamin D, and are also often deficient in nutritional vitamin D (1). Patients who are on dialysis are treated with activated vitamin D primarily for secondary hyperparathyroidism. Some dialysis patients are also prescribed nutritional vitamin D supplementation. In this review, we will discuss the evidence behind the use of various forms of vitamin D in patients on dialysis.
CKD-MBD and Vitamin D pathophysiology
Vitamin D is obtained either through eating vitamin D rich foods (oily fish, dairy products), supplements or through the skin’s exposure to UVB radiation producing vitamin D. This vitamin D is then converted to 25-hydroxyvitamin D in the liver. 25-hydroxyvitamin D (25(OH)D) circulates in the blood stream and is used to evaluate an individual’s vitamin D nutritional status because of its relatively long half-life (2–3 weeks). Circulating vitamin D is bound to vitamin D binding protein (DBP) (80–90%) and albumin (10–15%), with less than 1% existing in a free, unbound form; free and albumin bound vitamin D constitute the bioavailable 25(OH)D (2). This bioavailable 25(OH)D may correlate better with some clinical outcomes, but is not routinely measured in clinical practice (3, 4). The 1-α hydroxylase enzyme converts 25(OH)D into the more active form of vitamin D, 1,25-dihydroxyvitamin D (1,25(OH)2D), which has a half-life of only 8–12 hours. While 25(OH)D can stimulate the vitamin D receptor (at 100 fold higher concentrations) in vitro, it is unclear whether it has effects in vivo (5, 6). Finally, both 25(OH)D and 1,25(OH)2D are made inactive by the 24-α hydroxylase enzyme, whose activity may be reduced as kidneys fail (7).
Parathyroid hormone (PTH) levels are elevated at lower glomerular filtration rates (GFRs) (8). PTH is made in the parathyroid gland chief cells in response to fluctuations in calcium via the calcium-sensing receptors on the chief cells. PTH promotes release of phosphorus and calcium from bone, increased vitamin D production in the kidney and urinary secretion of phosphorus. The signals for elevation of PTH in CKD are numerous including low calcium levels, high phosphate levels and low 1,25-dihydroxyvitamin D levels. At the same time, or earlier, FGF-23 levels become elevated (9). FGF-23 is a hormone made by the bone that causes phosphaturia (leading to lower serum phosphate levels) and decreased 1-alpha hydroxylase activity in the kidney (leading to lower 1,25-dihydroxyvitamin D levels).
Available Formulations of Vitamin D
Vitamin D is available for supplementation in several formulations. In the United States, nutritional vitamin D (that becomes 25(OH)D in vivo) is available as either ergocalciferol or cholecalciferol. Activated forms of vitamin D (analogs of 1,25(OH)2D) are available as either calcitriol (1,25(OH)2D) or its analogs, paracalcitol and doxercalciferol. Other analogs not available in the United States include alfacalcidol, maxacalcitol, and falecalcitriol. Analogs of calcitriol were developed due to concerns about hypercalcemia. It has been proposed that the mechanism of any lessened calcemic effects of these analogs is due to reduced activation of calcium uptake proteins in the intestines (10). This effect was also found in hemodialysis patients (11). However, more recent studies have shown that hypercalcemia with paricalcitol use may actually be similar in magnitude to that seen with calcitriol use (12, 13). This review will focus on the activated vitamin D formulations because there is more available data about their use.
Activated Vitamin D Use and Bone Parameters
Prior to the development of activated vitamin D therapy, patients with end-stage renal disease frequently had uncontrolled PTH levels with its associated bone changes, osteitis fibrosa cystica, a manifestation of renal osteodystrophy. In patients with CKD these bone changes, a consequence of increased bone formation and resorption, improve after daily administration of calcitriol for one year (14). In a study of dialysis patients with proven osteitis fibrosa, intravenous calcitriol was effective in improving the bone disease and lowering alkaline phosphatase and PTH levels (15). These two studies utilized bone biopsies for their outcomes, a technique that has not been used in large studies of vitamin D therapy. Thus, calcitriol therapy was shown to improve bone turnover parameters, and clinicians currently utilize lowering of PTH levels to guide therapy.
Current guidelines suggest keeping PTH levels in dialysis patients at two to nine times normal levels (16). A meta-analysis of clinical trial data shows that activated vitamin D use in dialysis patients lowers PTH and alkaline phosphatase levels (17). Vitamin D compounds also consistently increase serum phosphate and calcium levels (17). This meta-analysis concluded that there were not enough data on clinical outcomes such as fractures. Interestingly, while activated vitamin D compounds decrease PTH levels, they increase FGF-23 levels (18). The clinical significance of this increase in FGF-23 levels is currently unknown.
Vitamin D and Non-Bone Outcomes in Dialysis
Observational data have suggested that vitamin D levels and/or activated vitamin D use in dialysis patients have significant associations with various health outcomes (1, 19). These include overall (1, 20, 21) and cardiovascular mortality, (22, 23) infections, (24) and insulin resistance (25). Other outcomes that have been hypothesized to be improved with vitamin D therapy including allo-immunity (26), AVF maturation (27), and anemia (28). We will review the literature regarding some of these outcomes.
Bone mineral markers and Mortality
High PTH levels have clearly been associated with adverse outcomes in dialysis patients. One study in over 40,000 dialysis patients showed that levels of PTH >600 pg/mL were associated with a higher risk of mortality (29). Other studies showed similar associations between high PTH levels and mortality (30) and cardiovascular mortality (31). The ideal PTH level in dialysis patients is still not known (32). An analysis of the international Dialysis Outcomes and Practice Patterns Study (DOPPS) showed a higher all-cause mortality risk with PTH levels >300 pg/mL. Further complicating an analysis of this issue is the well-documented association between elevated phosphate levels and all-cause and cardiovascular mortality (21, 29–31). In addition, higher FGF-23 levels have been associated with a higher mortality risk in dialysis patients (33). Thus it is not obvious that the use of activated vitamin D, which lowers PTH levels but increases phosphate and FGF-23, will yield a survival advantage.
Activated Vitamin D Use and All-Cause Mortality: Observational Studies of Vitamin D Use
Low vitamin D levels are associated with mortality in patients on dialysis (1). In addition, numerous observational studies have shown an association between activated vitamin D use and improved survival in dialysis patients (20, 21, 23). One early analysis evaluated 51,037 incident hemodialysis patients that survived for at least 90 days from the initiation of hemodialysis (20). Mortality rates were lower in the group receiving activated vitamin D after adjusting for multiple confounders. This was true even when activated vitamin D was administered to patients with low PTH, high calcium and high phosphorus levels (20). In the few studies comparing different activated vitamin D agents, paricalcitrol and/or doxercalciferol were associated with better survival than calcitriol (34, 35).
In contrast to the many studies showing a benefit with activated vitamin D therapy, some studies suggest there is no association with improved survival (36). It could be that the improved survival found with vitamin D is due to residual confounding, possibly treatment or other biases. Since an adequately powered randomized clinical trial has not been performed we must rely on observational studies and smaller randomized clinical trials that have been combined using meta-analysis techniques.
One such study from 2013 by Zheng et al collected data from 20 studies, of which 11 were prospective cohort studies (37). (Figure 1) Studies included met the following criteria: a cohort study with at least one year follow-up, patients with CKD or on renal replacement therapy, patients treated with active vitamin D but not native vitamin D such as ergocalciferol or cholecalciferol, outcome studied was all-cause mortality or cardiovascular mortality, and quantitative data were available to analyze. Adjusted all-cause mortality in ESRD patients was evaluated in 6 studies totaling 66,639 patients, resulted in a hazard ratio of 0.80 (0.68–0.94) (37). Data from five studies was used to elucidate cardiovascular mortality. Of 4,250 patients in the studies, the hazard ratio was 0.59 (0.41–0.86) (37). (Figure 2) This meta-analysis was limited by the underlying data including multiple different vitamin D agents used and significant heterogeneity in the studies.
Figure 1.
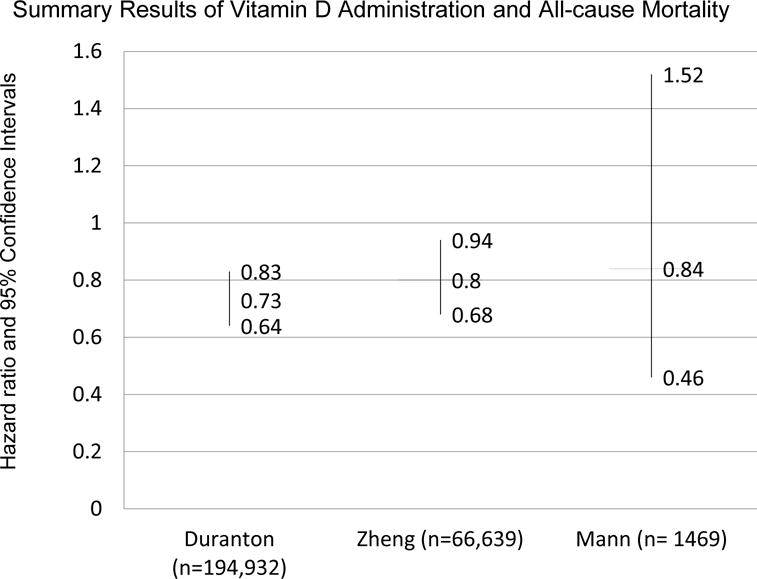
Summary of results of meta-analyses evaluating the administration of vitamin D and all-cause mortality in patients with chronic kidney disease. Duranton et al. and Zheng et al. include observational studies. Mann et al. includes only randomized clinical trials.
Figure 2.
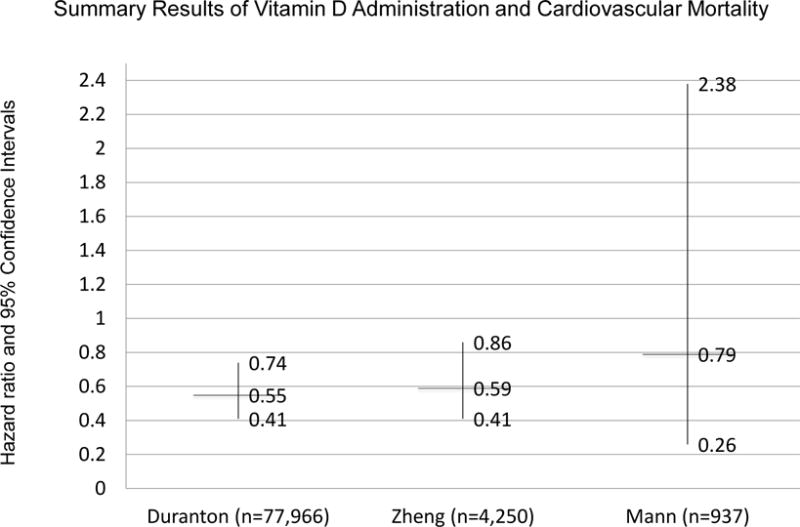
Summary of results of meta-analyses evaluating the administration of vitamin D and cardiovascular mortality in patients with chronic kidney disease. Duranton et al. and Zheng et al. include observational studies. Mann et al. includes only randomized clinical trials.
Another meta-analysis from Spain yielded similar results (22). Inclusion criteria were mostly similar, but studies that had 6 months, rather than at least a year follow-up were included. In addition, studies with outcomes data and at least one death were required. Due to these and other strict inclusion criteria, no randomized controlled trials were included. Fourteen observational studies with 194,932 patients were included, consisting mostly of hemodialysis patients receiving calcitriol or paricalcitol. After 3 years of therapy, the relative risk of death was 0.72 (95% CI 0.65–0.80) and after 5 years, 0.67 (95% CI 0.45–0.98) (22). The risk reduction was greater in patients with higher PTH values. Cardiovascular mortality was also reduced (22). (Figure 2) It is important to remember that these meta-analyses mostly used data from observational studies which are subject to residual confounding. Therefore, these meta-analyses, just like the studies that they combine, do not prove causality.
One meta-analysis combining 13 randomized clinical trials including 1469 participants found no effect of vitamin D compounds on all-cause or cardiovascular mortality (38). This meta-analysis was smaller than the previous ones discussed because it only included randomized clinical trials; only 41 all-cause deaths occurred during the follow-up of the trials (38). Thus, the authors concluded that insufficient patient-level outcome data exist and that larger randomized clinical trials are required.
Activated Vitamin D Use and Cardiovascular Mortality
Accepting that activated vitamin D therapy reduces all-cause mortality, the question becomes which cause of death might be altered by this therapy? Observational data show that lower cardiovascular mortality is associated with activated vitamin D use (22, 23, 37). However, cardiovascular mortality is a broad term for multiple etiologies of death in dialysis patients. Vascular medial calcification, an important underlying cause of cardiovascular disease in CKD and dialysis patients,(39) has a well established link to elevated phosphate levels (40). The link between vitamin D and vascular calcification is more complicated. In a mouse model of kidney disease, low dose activated vitamin D use (paricalcitol or calcitriol) protects against vascular calcification while use of higher doses is associated with more calcification (41). Other studies have shown that calcitriol leads to calcification but paricalcitol does not (42). It is unclear whether currently used doses of vitamin D analogs lead to or protect against vascular calcification.
Another effect of vitamin D in animal models is inhibition of the renin-angiotensin system and associated left ventricular hypertrophy (LVH) (43, 44). These animal models and patient data led to the design of the PRIMO and OPERA trials, which both evaluated the effects of paricalcitol on left ventricular hypertrophy in patients with CKD not on dialysis (45, 46). Neither of these trials showed a difference in LVH between paricalcitol and placebo. There were fewer cardiovascular hospitalizations in the paricalcitol group in both studies (45, 46). A post-hoc analysis of the PRIMO trial revealed that therapy with paricalcitol significantly decreased left atrial volume (47). Thus, while observational data show improvements in cardiovascular outcomes, randomized trial data in CKD suggest no effect on LVH in pre-dialysis CKD patients. In dialysis patients, a meta-analysis of randomized clinical trials showed no improvement in cardiovascular mortality, but there were only 13 cardiovascular deaths (38). Further studies are needed to elucidate whether activated (or nutritional) vitamin D therapy may ameliorate cardiovascular disease in dialysis patients.
Activated Vitamin D Use and Infections
Infection is the second leading cause of death in individuals on dialysis (48). Innate immunity represents a branch of host defense that responds to organisms within minutes to hours of invasion (49). Antimicrobial peptides (AMPs) are key members of the innate immune system; they have been found in many bacteria, plant, fungi, and animal species. The best studied AMPs are defensins and cathelicidins. Cathelicidins are linear structures that are represented on one gene and are expressed on all epithelial cell surfaces, circulating neutrophils, monocytes, natural killer cells, and T cells (50).
Human cathelicidin antimicrobial peptide 18 (hCAP-18) is the only identified cathelicidin in humans; low levels in dialysis patients are associated with a higher risk of infection (51). Patients with hCAP18 levels in the lowest tertile had a 2-fold increased risk of death attributable to infection even after multivariable adjustment (51). This study further showed that hCAP-18 levels had a modest correlation with active vitamin D levels, suggesting an association between the two(51). Interestingly, basic science data shows that the gene for hCAP-18 is transcriptionally regulated by the vitamin D receptor (52). A small trial in 30 ICU patients revealed that a one-time high dose cholecalciferol (200,000 IU or 400,000 IU) treatment at the initiation of sepsis was associated with a rise in 25(OH)D levels and importantly, higher cathelicidin levels (53).
Several studies have looked at the role of supplemented vitamin D in the risk of infections. Tsujimoto et al. found that the incidence of hospitalization because of acute respiratory infection was significantly lower in dialysis patients who had been treated with vitamin D compared to patients who had not (24). Kerschbaum et al., in a retrospective study, reported that oral activated vitamin D was independently associated with a decreased risk for peritonitis in peritoneal dialysis patients (54). Whether this association with a lower risk of infection is mediated by higher cathelicidin levels requires further studies.
Activated Vitamin D and Insulin Resistance
Altered glucose metabolism and insulin resistance are recognized at all stages of CKD and ESRD (55, 56). In ESRD, insulin resistance is an independent non-traditional risk factor for cardiovascular mortality and is associated with protein energy wasting and malnutrition (57). Animal studies have demonstrated improvement in insulin resistance with administration of vitamin D with both increased insulin sensitivity and insulin secretion being affected (58–60). Studies in dialysis patients show some improvements in glucose metabolism with vitamin D supplementation in ESRD patients (61–63) but no improvement in other studies (64). A recent meta-analysis combining data from five randomized clinical trials and 12 non-randomized studies showed significantly lower glucose levels in patients treated with vitamin D (25). Although this meta-analysis used data from some randomized clinical trials, the considerable heterogeneity in the studies precluded a definitive conclusion about causality.
Nutritional Vitamin D Use and Outcomes in Dialysis Patients
A meta-analysis of 22 studies including five randomized clinical trials including patients with all stages of CKD with 25(OH)D levels less than 20–30 ng/ml revealed that supplementation with ergocalciferol or cholecalciferol resulted in higher 25(OH)D and lower PTH levels (65). Since that meta-analysis was performed, two more randomized clinical trials have been published describing the effects of nutritional vitamin D in dialysis patients. A trial of 60 patients on dialysis randomized to either cholecalciferol 50,000 IU weekly for 8 weeks, then monthly, compared to placebo revealed no episodes of hypercalcemia or hyperphosphatemia and an increase in 25(OH)D and 1,25(OH)2D levels after 6 months (66). No differences in PTH levels or muscle function or quality of life measures were noted after 6 months (66). A larger trial similarly showed increases in 25(OH)D levels with weekly or monthly ergocalciferol 50,000 IU (67). No differences were noted in PTH, hemoglobin, or 1,25(OH)2D levels or blood pressure (67). Notably, FGF-23 levels increased in all the groups including the placebo group (67). We can conclude that nutritional vitamin D (ergocalciferol and cholecalciferol) are effective in increasing 25(OH)D levels without increasing the risk of hypercalcemia or hyperphosphatemia but effects on patient outcomes are not currently known.
Conclusions and Future Directions
Dialysis patients are deficient in both nutritional and activated vitamin D. Activated vitamin D therapy with calcitriol, paricalcitol and doxercalciferol controls the secondary hyperparathyroidism associated with CKD. Vitamin D may have “pleotropic” effects in patients on dialysis, effects on organs and outcomes that are not related to bone effects. The most data on these effects are available on associations between vitamin D use and improved survival. However, these associations have only been seen in observational studies. Newer data reveal that nutritional vitamin D supplementation does not cause hypercalcemia or hyperphosphatemia in dialysis patients even when used in combination with activated vitamin D. Future research should concentrate on patient-level outcomes including mortality, cardiovascular events, infections and glucose control.
Acknowledgments
Funding
The writing of this manuscript was supported by the National Institute of Diabetes And Digestive And Kidney Diseases of the National Institutes of Health under Award Number R34DK102174 and an American Society of Nephrology Gottschalk Award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the American Society on Nephrology.
Footnotes
Conflicts of Interest
None of the authors have conflicts of interest to declare.
References
- 1.Wolf M, Shah A, Gutierrez O, Ankers E, Monroy M, Tamez H, et al. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney international. 2007;72:1004–13. doi: 10.1038/sj.ki.5002451. [DOI] [PubMed] [Google Scholar]
- 2.Bikle DD, Gee E, Halloran B, Kowalski MA, Ryzen E, Haddad JG. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J Clin Endocrinol Metab. 1986;63:954–9. doi: 10.1210/jcem-63-4-954. [DOI] [PubMed] [Google Scholar]
- 3.Bhan I, Powe CE, Berg AH, Ankers E, Wenger JB, Karumanchi SA, et al. Bioavailable vitamin D is more tightly linked to mineral metabolism than total vitamin D in incident hemodialysis patients. Kidney international. 2012;82:84–9. doi: 10.1038/ki.2012.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Powe CE, Evans MK, Wenger J, Zonderman AB, Berg AH, Nalls M, et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. The New England journal of medicine. 2013;369:1991–2000. doi: 10.1056/NEJMoa1306357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raisz LG, Trummel CL, Holick MF, DeLuca HF. 1,25-dihydroxycholecalciferol: a potent stimulator of bone resorption in tissue culture. Science. 1972;175:768–9. doi: 10.1126/science.175.4023.768. [DOI] [PubMed] [Google Scholar]
- 6.Brumbaugh PF, Haussler MR. 1 Alpha,25-dihydroxycholecalciferol receptors in intestine. I. Association of 1 alpha,25-dihydroxycholecalciferol with intestinal mucosa chromatin. J Biol Chem. 1974;249:1251–7. [PubMed] [Google Scholar]
- 7.de Boer IH, Sachs MC, Chonchol M, Himmelfarb J, Hoofnagle AN, Ix JH, et al. Estimated GFR and circulating 24,25-dihydroxyvitamin D3 concentration: a participant-level analysis of 5 cohort studies and clinical trials. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2014;64:187–97. doi: 10.1053/j.ajkd.2014.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muntner P, Jones TM, Hyre AD, Melamed ML, Alper A, Raggi P, et al. Association of serum intact parathyroid hormone with lower estimated glomerular filtration rate. Clinical journal of the American Society of Nephrology : CJASN. 2009;4:186–94. doi: 10.2215/CJN.03050608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Isakova T, Wahl P, Vargas GS, Gutierrez OM, Scialla J, Xie H, et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney international. 2011;79:1370–8. doi: 10.1038/ki.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takahashi F, Finch JL, Denda M, Dusso AS, Brown AJ, Slatopolsky E. A new analog of 1,25-(OH)2D3, 19-NOR-1,25-(OH)2D2, suppresses serum PTH and parathyroid gland growth in uremic rats without elevation of intestinal vitamin D receptor content. American journal of kidney diseases : the official journal of the National Kidney Foundation. 1997;30:105–12. doi: 10.1016/s0272-6386(97)90571-0. [DOI] [PubMed] [Google Scholar]
- 11.Coyne DW, Grieff M, Ahya SN, Giles K, Norwood K, Slatopolsky E. Differential effects of acute administration of 19-Nor-1,25-dihydroxy-vitamin D2 and 1,25-dihydroxy-vitamin D3 on serum calcium and phosphorus in hemodialysis patients. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2002;40:1283–8. doi: 10.1053/ajkd.2002.36899. [DOI] [PubMed] [Google Scholar]
- 12.Coyne DW, Goldberg S, Faber M, Ghossein C, Sprague SM. A randomized multicenter trial of paricalcitol versus calcitriol for secondary hyperparathyroidism in stages 3–4 CKD. Clinical journal of the American Society of Nephrology : CJASN. 2014;9:1620–6. doi: 10.2215/CJN.10661013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jamaluddin EJ, Gafor AH, Yean LC, Cader R, Mohd R, Kong NC, et al. Oral paricalcitol versus oral calcitriol in continuous ambulatory peritoneal dialysis patients with secondary hyperparathyroidism. Clinical and experimental nephrology. 2014;18:507–14. doi: 10.1007/s10157-013-0844-2. [DOI] [PubMed] [Google Scholar]
- 14.Baker LR, Abrams L, Roe CJ, Faugere MC, Fanti P, Subayti Y, et al. 1,25(OH)2D3 administration in moderate renal failure: a prospective double-blind trial. Kidney international. 1989;35:661–9. doi: 10.1038/ki.1989.36. [DOI] [PubMed] [Google Scholar]
- 15.Andress DL, Norris KC, Coburn JW, Slatopolsky EA, Sherrard DJ. Intravenous calcitriol in the treatment of refractory osteitis fibrosa of chronic renal failure. The New England journal of medicine. 1989;321:274–9. doi: 10.1056/NEJM198908033210502. [DOI] [PubMed] [Google Scholar]
- 16.Kidney Disease: Improving Global Outcomes CKDMBDWG. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Kidney international Supplement. 2009:S1–130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 17.Palmer SC, McGregor DO, Craig JC, Elder G, Macaskill P, Strippoli GF. Vitamin D compounds for people with chronic kidney disease requiring dialysis. The Cochrane database of systematic reviews. 2009:CD005633. doi: 10.1002/14651858.CD005633.pub2. [DOI] [PubMed] [Google Scholar]
- 18.Cozzolino M, Ketteler M, Martin KJ, Sharma A, Goldsmith D, Khan S. Paricalcitol- or cinacalcet-centred therapy affects markers of bone mineral disease in patients with secondary hyperparathyroidism receiving haemodialysis: results of the IMPACT-SHPT study. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association – European Renal Association. 2014;29:899–905. doi: 10.1093/ndt/gfu011. [DOI] [PubMed] [Google Scholar]
- 19.Melamed ML, Thadhani RI. Vitamin D therapy in chronic kidney disease and end stage renal disease. Clinical journal of the American Society of Nephrology : CJASN. 2012;7:358–65. doi: 10.2215/CJN.04040411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teng M, Wolf M, Ofsthun MN, Lazarus JM, Hernan MA, Camargo CA, Jr, et al. Activated injectable vitamin D and hemodialysis survival: a historical cohort study. Journal of the American Society of Nephrology : JASN. 2005;16:1115–25. doi: 10.1681/ASN.2004070573. [DOI] [PubMed] [Google Scholar]
- 21.Melamed ML, Eustace JA, Plantinga L, Jaar BG, Fink NE, Coresh J, et al. Changes in serum calcium, phosphate, and PTH and the risk of death in incident dialysis patients: a longitudinal study. Kidney international. 2006;70:351–7. doi: 10.1038/sj.ki.5001542. [DOI] [PubMed] [Google Scholar]
- 22.Duranton F, Rodriguez-Ortiz ME, Duny Y, Rodriguez M, Daures JP, Argiles A. Vitamin D treatment and mortality in chronic kidney disease: a systematic review and meta-analysis. American journal of nephrology. 2013;37:239–48. doi: 10.1159/000346846. [DOI] [PubMed] [Google Scholar]
- 23.Shoji T, Shinohara K, Kimoto E, Emoto M, Tahara H, Koyama H, et al. Lower risk for cardiovascular mortality in oral 1alpha-hydroxy vitamin D3 users in a haemodialysis population. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association – European Renal Association. 2004;19:179–84. doi: 10.1093/ndt/gfg513. [DOI] [PubMed] [Google Scholar]
- 24.Tsujimoto Y, Tahara H, Shoji T, Emoto M, Koyama H, Ishimura E, et al. Active vitamin D and acute respiratory infections in dialysis patients. Clinical journal of the American Society of Nephrology : CJASN. 2011;6:1361–7. doi: 10.2215/CJN.08871010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarathy H, Pramanik V, Kahn J, Abramowitz MK, Meier K, Kishore P, et al. The effects of short-term vitamin D supplementation on glucose metabolism in dialysis patients: a systematic review and meta-analysis. International urology and nephrology. 2015;47:537–49. doi: 10.1007/s11255-015-0909-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li L, Lin M, Krassilnikova M, Ostrow K, Bader A, Radbill B, et al. Effect of cholecalciferol supplementation on inflammation and cellular alloimmunity in hemodialysis patients: data from a randomized controlled pilot trial. PloS one. 2014;9:e109998. doi: 10.1371/journal.pone.0109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wasse H, Huang R, Long Q, Zhao Y, Singapuri S, McKinnon W, et al. Very high-dose cholecalciferol and arteriovenous fistula maturation in ESRD: a randomized, double-blind, placebo-controlled pilot study. The journal of vascular access. 2014;15:88–94. doi: 10.5301/jva.5000187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Afsar B, Agca E, Turk S. Comparison of erythropoietin resistance in hemodialysis patients using calcitriol, cinacalcet, or paricalcitol. Journal of clinical pharmacology. 2015 doi: 10.1002/jcph.556. [DOI] [PubMed] [Google Scholar]
- 29.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. Journal of the American Society of Nephrology : JASN. 2004;15:2208–18. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 30.Tentori F, Blayney MJ, Albert JM, Gillespie BW, Kerr PG, Bommer J, et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS) American journal of kidney diseases : the official journal of the National Kidney Foundation. 2008;52:519–30. doi: 10.1053/j.ajkd.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 31.Slinin Y, Foley RN, Collins AJ. Calcium, phosphorus, parathyroid hormone, and cardiovascular disease in hemodialysis patients: the USRDS waves 1, 3, and 4 study. Journal of the American Society of Nephrology : JASN. 2005;16:1788–93. doi: 10.1681/ASN.2004040275. [DOI] [PubMed] [Google Scholar]
- 32.Tentori F, Wang M, Bieber BA, Karaboyas A, Li Y, Jacobson SH, et al. Recent changes in therapeutic approaches and association with outcomes among patients with secondary hyperparathyroidism on chronic hemodialysis: the DOPPS study. Clinical journal of the American Society of Nephrology : CJASN. 2015;10:98–109. doi: 10.2215/CJN.12941213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gutierrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. The New England journal of medicine. 2008;359:584–92. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teng M, Wolf M, Lowrie E, Ofsthun N, Lazarus JM, Thadhani R. Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. The New England journal of medicine. 2003;349:446–56. doi: 10.1056/NEJMoa022536. [DOI] [PubMed] [Google Scholar]
- 35.Tentori F, Hunt WC, Stidley CA, Rohrscheib MR, Bedrick EJ, Meyer KB, et al. Mortality risk among hemodialysis patients receiving different vitamin D analogs. Kidney international. 2006;70:1858–65. doi: 10.1038/sj.ki.5001868. [DOI] [PubMed] [Google Scholar]
- 36.Tentori F, Albert JM, Young EW, Blayney MJ, Robinson BM, Pisoni RL, et al. The survival advantage for haemodialysis patients taking vitamin D is questioned: findings from the Dialysis Outcomes and Practice Patterns Study. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association – European Renal Association. 2009;24:963–72. doi: 10.1093/ndt/gfn592. [DOI] [PubMed] [Google Scholar]
- 37.Zheng Z, Shi H, Jia J, Li D, Lin S. Vitamin D supplementation and mortality risk in chronic kidney disease: a meta-analysis of 20 observational studies. BMC nephrology. 2013;14:199. doi: 10.1186/1471-2369-14-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mann MC, Hobbs AJ, Hemmelgarn BR, Roberts DJ, Ahmed SB, Rabi DM. Effect of oral vitamin D analogs on mortality and cardiovascular outcomes among adults with chronic kidney disease: a meta-analysis. Clinical kidney journal. 2015;8:41–8. doi: 10.1093/ckj/sfu122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gorriz JL, Molina P, Cerveron MJ, Vila R, Bover J, Nieto J, et al. Vascular calcification in patients with nondialysis CKD over 3 years. Clinical journal of the American Society of Nephrology : CJASN. 2015;10:654–66. doi: 10.2215/CJN.07450714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jono S, McKee MD, Murry CE, Shioi A, Nishizawa Y, Mori K, et al. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res. 2000;87:E10–7. doi: 10.1161/01.res.87.7.e10. [DOI] [PubMed] [Google Scholar]
- 41.Mathew S, Lund RJ, Chaudhary LR, Geurs T, Hruska KA. Vitamin D receptor activators can protect against vascular calcification. J Am Soc Nephrol. 2008;19:1509–19. doi: 10.1681/ASN.2007080902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cardus A, Panizo S, Parisi E, Fernandez E, Valdivielso JM. Differential effects of vitamin D analogs on vascular calcification. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2007;22:860–6. doi: 10.1359/jbmr.070305. [DOI] [PubMed] [Google Scholar]
- 43.Xiang W, Kong J, Chen S, Cao LP, Qiao G, Zheng W, et al. Cardiac hypertrophy in vitamin D receptor knockout mice: role of the systemic and cardiac renin-angiotensin systems. Am J Physiol Endocrinol Metab. 2005;288:E125–32. doi: 10.1152/ajpendo.00224.2004. [DOI] [PubMed] [Google Scholar]
- 44.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110:229–38. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thadhani R, Appelbaum E, Pritchett Y, Chang Y, Wenger J, Tamez H, et al. Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: the PRIMO randomized controlled trial. JAMA : the journal of the American Medical Association. 2012;307:674–84. doi: 10.1001/jama.2012.120. [DOI] [PubMed] [Google Scholar]
- 46.Wang AY, Fang F, Chan J, Wen YY, Qing S, Chan IH, et al. Effect of paricalcitol on left ventricular mass and function in CKD–the OPERA trial. Journal of the American Society of Nephrology : JASN. 2014;25:175–86. doi: 10.1681/ASN.2013010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tamez H, Zoccali C, Packham D, Wenger J, Bhan I, Appelbaum E, et al. Vitamin D reduces left atrial volume in patients with left ventricular hypertrophy and chronic kidney disease. American heart journal. 2012;164:902–9 e2. doi: 10.1016/j.ahj.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 48.de Jager DJ, Grootendorst DC, Jager KJ, van Dijk PC, Tomas LM, Ansell D, et al. Cardiovascular and noncardiovascular mortality among patients starting dialysis. Jama. 2009;302:1782–9. doi: 10.1001/jama.2009.1488. [DOI] [PubMed] [Google Scholar]
- 49.Bals R, Weiner DJ, Moscioni AD, Meegalla RL, Wilson JM. Augmentation of innate host defense by expression of a cathelicidin antimicrobial peptide. Infect Immun. 1999;67:6084–9. doi: 10.1128/iai.67.11.6084-6089.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zasloff M. Antimicrobial peptides, innate immunity, and the normally sterile urinary tract. Journal of the American Society of Nephrology : JASN. 2007;18:2810–6. doi: 10.1681/ASN.2007050611. [DOI] [PubMed] [Google Scholar]
- 51.Gombart AF, Bhan I, Borregaard N, Tamez H, Camargo CA, Jr, Koeffler HP, et al. Low plasma level of cathelicidin antimicrobial peptide (hCAP18) predicts increased infectious disease mortality in patients undergoing hemodialysis. Clin Infect Dis. 2009;48:418–24. doi: 10.1086/596314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 53.Quraishi SA, De Pascale G, Needleman JS, Nakazawa H, Kaneki M, Bajwa EK, et al. Effect of Cholecalciferol Supplementation on Vitamin D Status and Cathelicidin Levels in Sepsis: A Randomized, Placebo-Controlled Trial. Crit Care Med. 2015;43:1928–37. doi: 10.1097/CCM.0000000000001148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kerschbaum J, Vychytil A, Lhotta K, Prischl FC, Wiesholzer M, Machhold-Fabrizii V, et al. Treatment with oral active vitamin D is associated with decreased risk of peritonitis and improved survival in patients on peritoneal dialysis. PloS one. 2013;8:e67836. doi: 10.1371/journal.pone.0067836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Boer IH. Vitamin D and glucose metabolism in chronic kidney disease. Curr Opin Nephrol Hypertens. 2008;17:566–72. doi: 10.1097/MNH.0b013e32830fe377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fliser D, Pacini G, Engelleiter R, Kautzky-Willer A, Prager R, Franek E, et al. Insulin resistance and hyperinsulinemia are already present in patients with incipient renal disease. Kidney international. 1998;53:1343–7. doi: 10.1046/j.1523-1755.1998.00898.x. [DOI] [PubMed] [Google Scholar]
- 57.Hung AM, Ikizler TA. Factors determining insulin resistance in chronic hemodialysis patients. Contrib Nephrol. 2011;171:127–34. doi: 10.1159/000327177. [DOI] [PubMed] [Google Scholar]
- 58.Maestro B, Campion J, Davila N, Calle C. Stimulation by 1,25-dihydroxyvitamin D3 of insulin receptor expression and insulin responsiveness for glucose transport in U-937 human promonocytic cells. Endocrine journal. 2000;47:383–91. doi: 10.1507/endocrj.47.383. [DOI] [PubMed] [Google Scholar]
- 59.Norman AW, Frankel JB, Heldt AM, Grodsky GM. Vitamin D deficiency inhibits pancreatic secretion of insulin. Science. 1980;209:823–5. doi: 10.1126/science.6250216. [DOI] [PubMed] [Google Scholar]
- 60.Boquist L, Hagstrom S, Strindlund L. Effect of 1.25-dihydroxycholecalciferol administration on blood glucose and pancreatic islet morphology in mice. Acta pathologica et microbiologica Scandinavica Section A, Pathology. 1977;85:489–500. doi: 10.1111/j.1699-0463.1977.tb03880.x. [DOI] [PubMed] [Google Scholar]
- 61.Mak RH. Intravenous 1,25 dihydroxycholecalciferol corrects glucose intolerance in hemodialysis patients. Kidney international. 1992;41:1049–54. doi: 10.1038/ki.1992.159. [DOI] [PubMed] [Google Scholar]
- 62.Mak RH. Amelioration of hypertension and insulin resistance by 1,25-dihydroxycholecalciferol in hemodialysis patients. Pediatr Nephrol. 1992;6:345–8. doi: 10.1007/BF00869730. [DOI] [PubMed] [Google Scholar]
- 63.Mak RH. 1,25-Dihydroxyvitamin D3 corrects insulin and lipid abnormalities in uremia. Kidney international. 1998;53:1353–7. doi: 10.1046/j.1523-1755.1998.00865.x. [DOI] [PubMed] [Google Scholar]
- 64.Hung AM, Sundell MB, Plotnikova NE, Bian A, Shintani A, Ellis CD, et al. A pilot study of active vitamin D administration and insulin resistance in African American patients undergoing chronic hemodialysis. Journal of renal nutrition : the official journal of the Council on Renal Nutrition of the National Kidney Foundation. 2013;23:185–93. doi: 10.1053/j.jrn.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kandula P, Dobre M, Schold JD, Schreiber MJ, Jr, Mehrotra R, Navaneethan SD. Vitamin D supplementation in chronic kidney disease: a systematic review and meta-analysis of observational studies and randomized controlled trials. Clinical journal of the American Society of Nephrology : CJASN. 2011;6:50–62. doi: 10.2215/CJN.03940510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hewitt NA, O’Connor AA, O’Shaughnessy DV, Elder GJ. Effects of cholecalciferol on functional, biochemical, vascular, and quality of life outcomes in hemodialysis patients. Clinical journal of the American Society of Nephrology : CJASN. 2013;8:1143–9. doi: 10.2215/CJN.02840312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bhan I, Dobens D, Tamez H, Deferio JJ, Li YC, Warren HS, et al. Nutritional vitamin D supplementation in dialysis: a randomized trial. Clinical journal of the American Society of Nephrology : CJASN. 2015;10:611–9. doi: 10.2215/CJN.06910714. [DOI] [PMC free article] [PubMed] [Google Scholar]


