Abstract
Many organic solutes accumulate in ESRD and some are poorly removed removed with urea based prescriptions for hemodialysis. However, their toxicities have been difficult to assess. We have employed an animal model, the zebrafish embryo, to test the toxicity of uremic serum compared to control. Serum was obtained from stable ESRD patients pre-dialysis or from normal subjects. Zebrafish embryos 24 hours post fertilization were exposed to experimental media at a ratio of 3:1 water:human serum. Those exposed to serum from uremic subjects had significantly reduced survival at 8 hours (19% +/− 18% vs. 94% +/− 6%; p < 0.05, uremic serum vs control, respectively). Embryos exposed to serum from ESRD subjects fractionated at 50kD showed significantly greater toxicity with the larger molecular weight fraction (83% +/− 11% vs 7% +/−17% survival, p < 0.05, <50kD vs >50 kD, respectively). Heating serum abrogated its toxicity. EDTA, a potent inhibitor of complement by virtue of calcium chelation, reduced the toxicity of uremic serum compared to untreated uremic serum (96%+/− 5% vs 28%+/− 20% survival, p < 0.016, chelated vs non chelated serum respectively). Anti- factor B, a specific inhibitor of the alternative complement pathway, reduced the toxicity of uremic serum, compared to untreated uremic serum (98% +/− 6% vs. 3% +/− 9% survival, p < 0.016, anti- factor B treated vs non treated, respectively).Uremic serum is thus more toxic to zebrafish embryos than normal serum. Furthermore, this toxicity is associated with a fraction of large size, is inactivated by heat, and is reduced by both specific and non-specific inhibitors of complement activation. Together these data lend support to the hypothesis that at least some uremic toxicities may be mediated by complement.
Introduction
Patients with endstage renal disease (ESRD),including those receiving “adequate” dialysis,are burdened with a complex array of metabolic derangements, clinical symptoms (1) and poor survival compared (2). Compared to normal people, a large number of solutes are elevated in the plasma of people with ESRD, even those receiving hemodialysis (3). However, the relationship between these retained solutes and the residual uremic disabilities remains poorly understood. Among the solutes that have especially poor removal by hemodialysis are those that are protein bound, intracellular or large. Therefore they are especially elevated in dialysis patients (4). Several epidemiologic studies have described a relationship between individual uremic solutes and mortality. (5,6,7). Research into the mechanisms of uremic toxicity has been limited in part by the lack of an animal model. Maintaining these animals on dialysis is technically fraught and has been attempted rarely. Zebrafish have been used extensively to model environmental and pharmacological toxicity (8, 9). Zebrafish exhibit toxicity profiles similar to small mammals; toxic concentrations in ambient fishwater are consistently similar to toxic serum concentrations in experimental mammals (10). Zebrafish produce hundreds of offspring per week, making them a relatively inexpensive and convenient model (11). Experimental toxins can be added to fishwater, obviating complicated delivery systems.
In this study, a zebrafish model was employed to explore a mechanism of uremic toxicity.
Methods
Zebrafish mating pairs were maintained on a lighting schedule of 14 hours light and 10 hours darkness. Mating pairs were paired once weekly, and embryos were collected within 3 hours of spawning. Embryos were maintained at 28.5 °C in standard system water (11). Embryos were manually dechorionated at the initiation of all experiments, with only the most robust normal and apparently healthy embryos used. 9-11 embryos at 24 hours post fertilization (hpf) of the same clutch were transferred into each well of a 6- well plate. Experimental medium was pre-mixed and added to each well, then diluted with three parts fishwater to one part medium. Fishwater is reverse osmosis treated distilled water with 60 mg of Instant Ocean Sea Salt added per liter. Nine to eleven 24-hour-old embryos were added to each well. During toxicity experiments, embryos were observed hourly for survival, defined as presence of a heartbeat, for eight hours. This time interval was chosen from preliminary studies as the point at which approximately 90% of embryos in the uremic solution had died. Results of experiments are expressed as percent survival at eight hours, with the number of wells being the number (n) of observations for an experiment
Blood was obtained from stable maintenance hemodialysis patients before the initiation of treatment, or from volunteers without kidney disease, using standard separator tubes without anticoagulant. Samples were centrifuged for 15 minutes at 3000 RPM. Serum was stored at −30 °C and then thawed in 40 °C water for use. The study was approved by the Institutional Review Board of Albert Einstein College of Medicine.
For separate solutes of large size, serum was centrifuged at 15K RPM using Microcon centrifugal filters with a 50 kD membrane for 20 minutes (Millipore Corp, Bedford, MA). The ultrafiltered volume lost was replaced by saline in the initial serum sample. Heating experiments were performed by heating serum at 60 °C for 30 minutes. EDTA was added to serum at 10 mM (before being diluted 3:1 in fishwater, as above). 500 ug of an anti-factor B antibody mAb 1379 (stock 3.26 mg/ml) and / or a control murine IgG1 clone 171 (stock 23.5 mg/ml) were used per ml of serum, then diluted 3:1 in fishwater as above.
Statistics
Statistical analysis was performed using unpaired two-tailed Student t-tests. Bonferroni correction was used for groups n > 2. Results are reported as means ± standard deviations.
Results
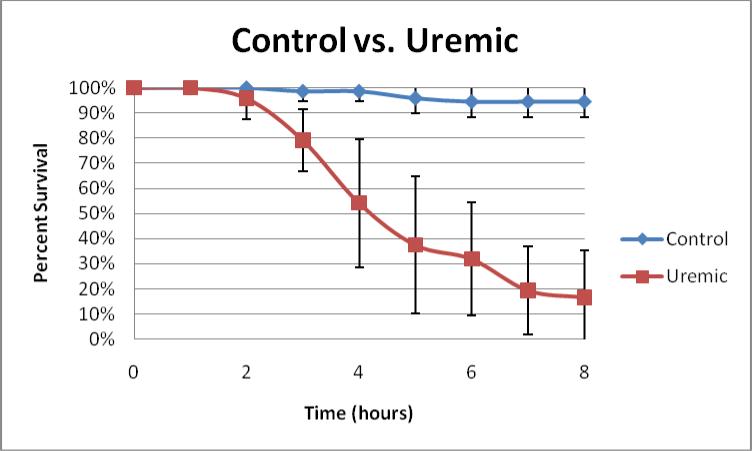
Zebrafish embryos exposed at 24 hpf to uremic serum for 7 hours had diminished survival, as compared to embryos similarly exposed to control serum (19% +/− 18% vs. 94% +/− 6%; p < 0.05, uremic vs. control serum, respectively, with n = 8. See Figure 1). Fractionation of uremic serum demonstrated that toxicity was strongly associated with the >50kD component as opposed to the < 50 kD component, suggesting the toxic molecule(s) was either larger than 50kD or protein bound (83% +/− 11% vs. 7% +/− 17% p < 0.05, <50kD vs. >50 kD, respectively, with n = 5. See Table 1). Heating uremic serum abrogated its toxicity (100% survival) as compared unheated uremic serum (32% +/− 25% survival) and normal serum (96% +/− 6%; p < 0.016 and n ≥ 6), suggesting that the toxicity of uremic serum on zebrafish embryos may be mediated by protein molecule(s) (See Table 2). EDTA, an inhibitor of complement, diminished toxicity when added to uremic serum (96%+/− 5%), compared to uremic serum alone (28%+/− 20%), and yielded survival similar to normal serum (94% +/− 6%; p < 0.016 and n ≥ 4) (See Table 3). To specifically test that complement may be mediating uremic toxicity, a monoclonal antibody to complement factor B was added to uremic serum (12). Uremic serum with anti- factor B was significantly less toxic than uremic serum alone or uremic serum plus a nonspecific murine IgG (98% +/− 6% vs. 3% +/− 9% 1% +/− 4%, respectively; p < 0.016 and n ≥ 12) (See Table 4).
Figure 1.
A time-course plot of percent survival ± standard deviation in Control vs. Uremic serum for 8 hours, n = 8. Note that for this figure and all the tables, n refers to the number of independent experimental observations, each evaluating the survival of approximately 10 embryos.
Table 1.
Percent survival in fractionated uremic serum, <50kD and > 50kD, at 7 hours (p < 0.05), n = 5.
| Percent Survival | |
|---|---|
| Uremic <50kD | 83 ± 11% |
| Uremic >50kD | 7 ± 17% |
Table 2.
Percent survival in Control, Uremic, and Heated Uremic serum at 7 hours (p < 0.0167), n ≥ 6.
| Percent Survival | |
|---|---|
| Control | 96 ± 6% |
| Uremic | 32 ± 25% |
| Heated Uremic | 100 ± 0% |
Table 3.
Percent survival in Control, Uremic, and Uremic + EDTA serum at 7 hours (p < 0.0167), n ≥ 4.
| Percent Survival | |
|---|---|
| Control | 94 ± 6% |
| Uremic | 28 ± 20% |
| Uremic + EDTA | 96 ± 5% |
Table 4.
Percent survival in Uremic, Uremic + anti-factor B Ab (Ab 1379) and Uremic + Control Ab (clone 171) serum at 7hours (p < 0.0167), n ≥ 12.
| Percent Survival | |
|---|---|
| Uremic | 3 ± 9% |
| Uremic + anti-factor B Ab | 98 ± 6% |
| Uremic + Control Ab | 1 ± 4% |
Discussion
Establishing links between individual retained solutes and uremic toxicity has been hindered by several factors, among them the lack of an animal model. Several epidemiologic studies have described a relationship between individual uremic solutes and mortality. (5,6,7). However, with very few exceptions, there is little compelling data linking a specific uremic toxin to its pathologic effect.
Solutes that are relatively poorly removed by hemodialysis include those that are protein bound or of large size. Such solutes are therefore especially elevated in the plasma of dialysis patients (4).
The zebrafish has previously been established as a useful model for the study of environmental and pharmacologic toxicity (10). This study, however, represents the first attempt to use the zebrafish to model uremic toxicity. Zebrafish embryos exposed to uremic serum exhibited in significantly more toxicity than those exposed compared with exposure to the serum of normal subjects. Fractionation experiments demonstrated that the toxicity was associated with solutes in the size fraction, >50 kD, implicating a molecule that is either large or protein bound.
Among the larger molecules likely to be toxic are those of the complement cascade. Given that strong possibility, we tested sequentially maneuvers know to interfere with complement system. First heating rendered the uremic serum innocuous. Next EDTA, a non-specific inhibitor of complement abrogated the toxicity of uremic serum. Monoclonal antibody 1379 is a specific inhibitor of factor B, an essential component of the alternative complement pathway, and has been shown to inhibit complement activity in various animal models, as well as human serum (12). Furthermore it has been demonstrated to protect against the development of complement mediated disease in mouse models of antiphospholipid antibody- induced fetal loss (12). This antibody completely largely abolished the toxicity of uremic serum, strongly suggesting that the demonstrated toxicity is complement- dependent.
A large number of compounds have been identified as elevated in the serum of ESRD patients maintained on hemodialysis. These retained solutes are presumed to cause the uremic condition that persists in ESRD patients, even with adequate maintenance hemodialysis—what Depner termed “residual syndrome” characterized by decreased survival, end organ damage, functional decline, neuro-cognitive deficits, as well as multiple, disparate metabolic derangements (13, 14). Vanholder (15, 16) and others have compiled list of known uremic solutes and described their potential toxic effects. A few studies have demonstrated epidemiologic links between individual retained solutes and mortality in ESRD patients. To date, little work has been done to demonstrate the pathologic mechanism of any a specific retained solute, although recent studies have demonstrated the influence of several uremic toxins on endothelial function (17, 18).
The present study is the first to employ an animal model to link complement activity to uremic toxicity. Complement is a relatively well-described uremic solute. Factor D, a rate-limiting component of the alternative pathway of complement activation, has a molecular weight of 24 kD, is filtered by the glomerulus and catabolized by the proximal tubular epithelium (19, 20).
Clearance is reduced in human subjects with kidney disease, and plasma levels of Factor D correlate well and inversely with GFR (21). Other small components of the complement cascade such as C3a may also accumulate in uremia but have not been explored in the detail of Factor D. We suspect that Factor D probably in the company of other smaller element of the complement system or even unrelated compounds activate the larger elements of the pathway such as C3 in vivo and account for the residual toxicity residing above the molecular weight cutoff of 50kD.In addition, accumulation due to decreased clearance, complement activation or even increased synthesis of some components may occur with uremia treated by dialysis. For example, exposure of blood to artificial surfaces within the hemodialysis membrane may stimulate the formation and activation of complement (22), although this may be ameliorated by more biocompatible dialyzers (23). Furthermore, hemodialysis patients may have increased exposure to endotoxin, either from the dialysis procedure itself or due to reduced immune function, which may be a further stimulant of complement activation (24). Complement has been identified in the intima of atherosclerotic plaques in both normal and uremic patients, and may contribute to the local inflammatory environment thought essential to the development of vascular disease (25, 26, 27).
The present results open several potential lines of inquiry in the treatment of ESRD. Since the advent of hemodialysis, therapy has focused almost exclusively on the clearance of urea. However, urea is relatively non-toxic, and likely plays little role in uremic toxicity (28). Moreover, many potential uremic toxins, some complement components among them, are poorly removed by urea- based dialysis prescriptions. Nevertheless, it must be acknowledged that conventional hemodialysis targeted at urea removal does dramatically reverse the severest symptoms of untreated uremia and can sustain life for years. The residual chronic disabilities that hemodialyzed patients sustain may be related to these poorly dialyzed solutes High flux membranes designed to remove proteins in the size range of beta 2 microglobulin (13kD) have been in use for many years . However, the clearances are still rather modest when compared to the native kidney. Thus, though lower with high flux membranes than with older types of dialyzers, serum levels of “middle molecules” are still much higher than normal. In fact, while high flux hemodialysis does remove factor D, the effect is relatively modest compared to urea, or even b2-microglobulin removal (29). Moreover, there are numerous potential therapies that target complement activation and production, and may prove effective in the treatment of patients with ESRD.
In summary, uremic serum is toxic to zebrafish embryos though a complement mediated pathway. Accumulation of complement components, their activation and /or increases in their synthesis may contribute to uremic toxicity.
Acknowledgements
THH was supported for these studies by a Pilot and Feasibility Grant from O'Brien Center and an R21 NIH R21 DK77326.
References
- 1.Janssen DJ, Spruit MA, Wouters EF, Schols JM. Daily symptom burden in end-stage chronic organ failure: a systematic review. Palliat Med. 2008;22:938–948. doi: 10.1177/0269216308096906. [DOI] [PubMed] [Google Scholar]
- 2.U.S. Renal Data System . USRDS 2010 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2010. [Google Scholar]
- 3.Vanholder R, Baurmeister U, Brunet P, Cohen G, Glorieux G, Jankowski J, European Uremic Toxin Work Group A bench to bedside view of uremic toxins. J Am Soc Nephrol. 2008;19:863–870. doi: 10.1681/ASN.2007121377. [DOI] [PubMed] [Google Scholar]
- 4.Vanholder R, De Smet R, Glorieux G, Argilés A, Baurmeister U, Brunet P, Clark W, Cohen G, De Deyn PP, Deppisch R, Descamps-Latscha B, Henle T, Jörres A, Lemke HD, Massy ZA, Passlick-Deetjen J, Rodriguez M, Stegmayr B, Stenvinkel P, Tetta C, Wanner C, Zidek W, European Uremic Toxin Work Group(EUTox) Review on uremic toxins: classification, concentration, and interindividual variability. Kidney Int. 2003;63:1934–1943. doi: 10.1046/j.1523-1755.2003.00924.x. [DOI] [PubMed] [Google Scholar]
- 5.Meijers BK, Claes K, Bammens B, de Loor H, Viaene L, Verbeke K, Kuypers D, Vanrenterghem Y, Evenepoel P. p-Cresol and cardiovascular risk in mild-to-moderate kidney disease. Clin J Am Soc Nephrol. 2010;5:1182–1189. doi: 10.2215/CJN.07971109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu IW, Hsu KH, Hsu HJ, Lee CC, Sun CY, Tsai CJ, Wu MS. Serum free p-cresyl sulfate levels predict cardiovascular and all-cause mortality in elderly hemodialysis patients--a prospective cohort study. Nephrol Dial Transplant. 2011 Sep 2; doi: 10.1093/ndt/gfr453. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Barreto FC, Barreto DV, Liabeuf S, Meert N, Glorieux G, Temmar M, Choukroun G, Vanholder R, Massy ZA, European Uremic Toxin Work Group (EUTox) Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol. 2009;4:1551–1558. doi: 10.2215/CJN.03980609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubinstein AL. Zebrafish assays for drug toxicity screening. Expert Opinion Drug Metab Toxicol. 2006;2:231–240. doi: 10.1517/17425255.2.2.231. [DOI] [PubMed] [Google Scholar]
- 9.Hill AJ, Teraoka H, Heideman W, Peterson RE. Zebrafish as a Model Vertebrate for Investigating Chemical Toxicity. Toxicological Sci. 2005;86:6–19. doi: 10.1093/toxsci/kfi110. [DOI] [PubMed] [Google Scholar]
- 10.Kari G, Rodeck U, Dicker AP. Zebrafish: an emerging model system for human disease and drug discovery. Clin Pharmacol Ther. 2007;82:70–80. doi: 10.1038/sj.clpt.6100223. [DOI] [PubMed] [Google Scholar]
- 11.Westerfield M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio) 3rd ed. University of Oregon Press; Eugene: 1995. [Google Scholar]
- 12.Thurman JM, Kraus DM, Girardi G, Hourcade D, Kang HJ, Royer PA, Mitchell LM, Giclas PC, Salmon J, Gilkeson G, Holers VM. A novel inhibitor of the alternative complement pathway prevents antiphospholipid antibody-induced pregnancy loss in mice. Mol Immunol. 2005;42:87–97. doi: 10.1016/j.molimm.2004.07.043. [DOI] [PubMed] [Google Scholar]
- 13.Depner TA. Uremic toxicity: urea and beyond. Semin Dia. 2001;14:246–251. doi: 10.1046/j.1525-139x.2001.00072.x. [DOI] [PubMed] [Google Scholar]
- 14.Meyer TW, Hostetter TH. Uremia. N Engl J Med. 2007;357:1316–1325. doi: 10.1056/NEJMra071313. [DOI] [PubMed] [Google Scholar]
- 15.Glorieux G, Vanholder R. New uremic toxins - which solutes should be removed? Contrib Nephrol. 2011;168:117–128. doi: 10.1159/000321750. [DOI] [PubMed] [Google Scholar]
- 16.De Smet R, Van Kaer J, Van Vlem B, De Cubber A, Brunet P, Lameire N, Vanholder R. Toxicity of free p-cresol: a prospective and cross-sectional analysis. Clin Chem. 2003;49:470–478. doi: 10.1373/49.3.470. [DOI] [PubMed] [Google Scholar]
- 17.Schepers E, Barreto DV, Liabeuf S, Glorieux G, Eloot S, Barreto FC, Massy Z, Vanholder R. Symmetric Dimethylarginine as a Proinflammatory Agent in Chronic Kidney Disease. Clin J Am Soc Nephrol. 2011 Aug 4; doi: 10.2215/CJN.01720211. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schepers E, Glorieux G, Dou L, Cerini C, Gayrard N, Louvet L, Maugard C, Preus P, Rodriguez-Ortiz M, Argiles A, Brunet P, Cohen G, Jankowski J, Jankowski V, Massy Z, Rodriguez M, Vanholder R, European Uremic Toxin Work Group (EUTox) Guanidino compounds as cause of cardiovascular damage in chronic kidney disease: an in vitro evaluation. Blood Purif. 2010;30:277–287. doi: 10.1159/000320765. [DOI] [PubMed] [Google Scholar]
- 19.Pascual M, Steiger G, Estreicher J, Macon K, Volanakis JE, Schifferli JA. Metabolism of complement factor D in renal failure. Kidney Int. 1988;34:529–536. doi: 10.1038/ki.1988.214. [DOI] [PubMed] [Google Scholar]
- 20.Sanders PW, Volanakis JE, Rostand SG, Galla JH. Human complement protein D catabolism by the rat kidney. J Clin Invest. 1986;77:1299–1304. doi: 10.1172/JCI112434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Volanakis JE, Barnum SR, Giddens M, Galla JH. Renal filtration and catabolism of complement protein D. N Engl J Med. 1985;312:395–399. doi: 10.1056/NEJM198502143120702. [DOI] [PubMed] [Google Scholar]
- 22.Deppisch R, Schmitt V, Bommer J, Hänsch GM, Ritz E, Rauterberg EW. Fluid phase generation of terminal complement complex as a novel index of bioincompatibility. Kidney Int. 1990;37:696–706. doi: 10.1038/ki.1990.36. [DOI] [PubMed] [Google Scholar]
- 23.Schaefer RM, Huber L, Gilge U, Bausewein K, Vienken J, Heidland A. Clinical evaluation of a new high-flux cellulose acetate membrane. Int J Artif Organs. 1989;12:85–90. [PubMed] [Google Scholar]
- 24.Lonnemann G, Behme TC, Lenzner B, Floege J, Schulze M, Colton CK, Koch KM, Shaldon S. Permeability of dialyzer membranes to TNFα-inducing substances derived from water bacteria. Kidney Int. 1992;42:61–68. doi: 10.1038/ki.1992.261. [DOI] [PubMed] [Google Scholar]
- 25.Deppisch RM, Beck W, Goehl H, Ritz E. Components as uremic toxins and their potential role as mediators of microinflammation. Kidney Int Suppl. 2001;78:S271–S277. doi: 10.1046/j.1523-1755.2001.59780271.x. [DOI] [PubMed] [Google Scholar]
- 26.Speidl WS, Kastl SP, Huber K, Wojta J. Complement in atherosclerosis: friend or foe? J of Thrombosis and Haemostasis. 2011;9:428. doi: 10.1111/j.1538-7836.2010.04172.x. [DOI] [PubMed] [Google Scholar]
- 27.Malik TH, Cortini A, Carassiti D, Boyle JJ, Haskard DO, Botto M. The Alternative Pathway Is Critical for Pathogenic Complement Activation in Endotoxin- and Diet-Induced Atherosclerosis in Low-Density Lipoprotein Receptor–Deficient Mice. Circulation. 2010;122:1948–1956. doi: 10.1161/CIRCULATIONAHA.110.981365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson WJ, Hagge WW, Wagoner RD, Dinapoli RP, Rosevear JW. Effects of urea loading in patients with far-advanced renal failure. Mayo Clin Proc. 1972;47:21–29. [PubMed] [Google Scholar]
- 29.Ward RA, Schmidt B, Hullin J, Hillebrand GF, Samtleben W. A comparison of on-line hemodiafiltration and high-flux hemodialysis: a prospective clinical study. J Am Soc Nephrol. 2000;11:2344–2350. doi: 10.1681/ASN.V11122344. [DOI] [PubMed] [Google Scholar]