Abstract
Background
Progestin‐only contraceptives (POCs) are appropriate for many women who cannot or should not take estrogen. POCs include injectables, intrauterine contraception, implants, and oral contraceptives. Many POCs are long‐acting, cost‐effective methods of preventing pregnancy. However, concern about weight gain can deter the initiation of contraceptives and cause early discontinuation among users.
Objectives
The primary objective was to evaluate the association between progestin‐only contraceptive use and changes in body weight.
Search methods
Until 4 August 2016, we searched MEDLINE, CENTRAL, POPLINE, LILACS, ClinicalTrials.gov, and ICTRP. For the initial review, we contacted investigators to identify other trials.
Selection criteria
We considered comparative studies that examined a POC versus another contraceptive method or no contraceptive. The primary outcome was mean change in body weight or mean change in body composition. We also considered the dichotomous outcome of loss or gain of a specified amount of weight.
Data collection and analysis
Two authors extracted the data. Non‐randomized studies (NRS) need to control for confounding factors. We used adjusted measures for the primary effects in NRS or the results of matched analysis from paired samples. If the report did not provide adjusted measures for the primary analysis, we used unadjusted outcomes. For RCTs and NRS without adjusted measures, we computed the mean difference (MD) with 95% confidence interval (CI) for continuous variables. For dichotomous outcomes, we calculated the Mantel‐Haenszel odds ratio (OR) with 95% CI.
Main results
We found 22 eligible studies that included a total of 11,450 women. With 6 NRS added to this update, the review includes 17 NRS and 5 RCTs. By contraceptive method, the review has 16 studies of depot medroxyprogesterone acetate (DMPA), 4 of levonorgestrel‐releasing intrauterine contraception (LNG‐IUC), 5 for implants, and 2 for progestin‐only pills.
Comparison groups did not differ significantly for weight change or other body composition measure in 15 studies. Five studies with moderate or low quality evidence showed differences between study arms. Two studies of a six‐rod implant also indicated some differences, but the evidence was low quality.
Three studies showed differences for DMPA users compared with women not using a hormonal method. In a retrospective study, weight gain (kg) was greater for DMPA versus copper (Cu) IUC in years one (MD 2.28, 95% CI 1.79 to 2.77), two (MD 2.71, 95% CI 2.12 to 3.30), and three (MD 3.17, 95% CI 2.51 to 3.83). A prospective study showed adolescents using DMPA had a greater increase in body fat (%) compared with a group not using a hormonal method (MD 11.00, 95% CI 2.64 to 19.36). The DMPA group also had a greater decrease in lean body mass (%) (MD ‐4.00, 95% CI ‐6.93 to ‐1.07). A more recent retrospective study reported greater mean increases with use of DMPA versus Cu IUC for weight (kg) at years 1 (1.3 vs 0.2), 4 (3.5 vs 1.9), and 10 (6.6 vs 4.9).
Two studies reported a greater mean increase in body fat mass (%) for POC users versus women not using a hormonal method. The method was LNG‐IUC in two studies (reported means 2.5 versus ‐1.3; P = 0.029); (MD 1.60, 95% CI 0.45 to 2.75). One also studied a desogestrel‐containing pill (MD 3.30, 95% CI 2.08 to 4.52). Both studies showed a greater decrease in lean body mass among POC users.
Authors' conclusions
We considered the overall quality of evidence to be low; more than half of the studies had low quality evidence. The main reasons for downgrading were lack of randomizations (NRS) and high loss to follow‐up or early discontinuation.
These 22 studies showed limited evidence of change in weight or body composition with use of POCs. Mean weight gain at 6 or 12 months was less than 2 kg (4.4 lb) for most studies. Those with multiyear data showed mean weight change was approximately twice as much at two to four years than at one year, but generally the study groups did not differ significantly. Appropriate counseling about typical weight gain may help reduce discontinuation of contraceptives due to perceptions of weight gain.
Plain language summary
Effects of progestin‐only birth control on weight
Progestin‐only contraceptives (POCs) can be used by women who cannot or should not take the hormone estrogen. Many POCs are long acting, cost less than some other methods, and work well to prevent pregnancy. Some people worry that weight gain is a side effect of these birth control methods. Concern about weight gain can keep women from using these methods. Further, some women may stop using birth control early, which can lead to unplanned pregnancy. We looked at studies of POCs and changes in body weight. Until 4 August 2016, we did computer searches for studies of a POC compared with another birth control method or no contraceptive. For the initial review, we wrote to investigators to find other trials. The focus was on change in body weight or other body measure of lean or fat mass. With six new studies in this update, we have 22 studies that included 11,450 women. The groups compared did not differ much for weight change or other body measures in 15 studies. Five studies with moderate or low quality results showed a difference between study groups. Three studies showed differences for users of the injectable ‘depo’ versus no hormonal method. Depo users had a greater weight gain in two studies. In the third study, adolescents had a greater increase in body fat (%) and decrease in lean body mass (%). Two studies showed a greater increase in body fat (%) for users of hormonal intrauterine contraception versus women not using a hormonal method. One also showed a similar difference with a progestin‐only pill. Both studies showed a greater decrease in lean body mass with POC use. We found little evidence of weight gain when using POCs. Mean weight gain at 6 or 12 months was less than 2 kg (4.4 lb) for most studies. The groups using other birth control methods had about the same weight gain. Good counseling about typical weight gain may help women continue using birth control.
Summary of findings
Background
Description of the condition
Many women consider weight gain a side effect of using hormonal contraceptives (Bartz 2011; Raymond 2011). This perception may be based on self‐report of side effects rather than actual weight changes (Paul 1997; Berenson 2008; Nault 2013). Many clinicians and women believe that progestin‐only contraceptives cause weight gain (WebMD 2010; Albright 2015).
Concern about weight gain can deter the initiation of contraceptives and cause early discontinuation among users. In a United States (US) study of bone mineral density, weight gain was reported more often by women using depot medroxyprogesterone acetate (DMPA) than those using a low‐dose oral contraceptive (Berenson 2008). Weight gain was the most common side effect reported with DMPA use, after menstrual disturbances, in a New Zealand study (Paul 1997), and was the most common self‐reported side effect in a study from Iran (Veisi 2013). Reported weight gain has been a major reason for discontinuing DMPA use in the US (Bonny 2004). Some evidence suggests that DMPA is a concern for adolescents who are already obese (Curtis 2009). From a survey of Latin American women across four countries, more women believed levonorgestrel‐releasing intrauterine contraception (LNG‐IUC) led to weight gain, mood swings, and infertility compared with copper IUC (Silva‐Filho 2016). In a US study, more women reported weight gain as a side effect for the etonogestrel implant than for LNG‐IUC (Dickerson 2013). Weight gain was also reported for levonorgestrel implants (Sivin 2003). The gain may have been greater among women in the US than among those in China, and may be partly attributable to differences in dietary habits.
Description of the intervention
Progestin‐only contraceptives (POCs) include injectables, implants, hormonal intrauterine contraception (IUC), and pills. Except for the pills, POCs are longer‐acting and help free women from daily action to prevent unintended pregnancy. Such methods are among the most cost‐effective contraceptives in many areas. Studies of long‐acting methods are often of longer duration than those for pills, making study of weight change over time more feasible.
POCs do not contain estrogen, unlike combined hormonal contraceptives that have both progestin and estrogen. Therefore, POCs are appropriate for women who cannot or should not take estrogen (ACOG 2006). In Medical Eligibility Criteria, POCs are category 1 for women who are obese (body mass index (BMI) ≥ 30 kg/m2) (CDC 2012b; WHO 2015a; WHO 2015b). Category 1 is a condition with no restriction for use of the contraceptive method. For obese adolescents, DMPA is category 2 due to possible effects on bone mineral density. For category 2, method advantages generally outweigh the theoretical or proven risks. POCs are also category 1 for breastfeeding women who are at least six weeks postpartum. Combined hormonal contraceptives are category 3 for such women until six months postpartum (WHO 2015b). In the US, CHCs are considered category 2 by one month postpartum (CDC 2012b).
Worldwide, intrauterine contraception (IUC) is the most commonly used reversible method among women married or in union (UN 2015). In the US in 2012, IUC use was low compared with oral contraceptive use (Guttmacher 2015). However, use of long‐acting reversible methods (LARC) increased to 11.6% from 8.5% in 2009 (Kavanaugh 2015). Nearly three‐fourths of IUC users were using hormonal IUC. Worldwide, the method used most frequently after IUC is oral contraceptives, which include combined oral contraceptives (COCs) and progestin‐only pills (POPs). In the least developed countries, injectables are most commonly used, followed by oral contraceptives (UN 2015). Some injectable contraceptives contain both estrogen and progestin, while others like DMPA are progestin‐only.
How the intervention might work
In general, weight gain is due to an increase in fluid retention, muscle mass, or fat deposition. Research on mechanisms for weight change include investigations related to contraceptive use. Two uncontrolled studies included DMPA initiators, 12 to 21 years old. In a six‐month study with 43 DMPA users, weight increased 1.2 kg among African Americans, as did BMI and total body fat. However, appetite score decreased while on DMPA for both African American and white participants (Bonny 2004). A 12‐month study with 45 young women looked for associations of reported dietary intake with body composition change (Lange 2015). BMI increased significantly over 12 months, i.e. 1.6 kg/m2, but was not associated with total energy intake or macronutrient composition of the diet (carbohydrates, fat, or protein). The study lost 31% of participants.
Other experimental studies included adult women. A nine‐week study of resting metabolic rate (RMR) with DMPA initiation included 13 women with BMI 20 to 35 kg/m2. RMR increased significantly during the first three weeks compared with the next six, especially for those who initiated during the luteal cycle (Steward 2016). An increase in non‐shivering thermogenesis was consistent with the RMR change. A six‐month metabolic study of P‐O methods focused on 25 obese women (BMI ≥ 30) (Bender 2013). Participants chose the levonorgestrel‐releasing intrauterine system (LNG‐IUS), the etonogestrel‐releasing (ETG) implant, or a non‐hormonal method. Fasting glucose increased and insulin sensitivity decreased more with the ETG implant than with the LNG‐IUS when compared to a non‐hormonal method. An eight‐week study examined DMPA effects on food motivation centers in the brain (Basu 2016). Eight of 14 women completed the protocol with data for analysis. All had BMI < 30 and most were Latina. The investigators used functional magnetic resonance imaging (MRI) to assess response to food cues. The blood oxygen level dependent signal was greater after eight weeks of DMPA compared with baseline. Some brain regions had significant activation after DMPA with food versus nonfood images and with high‐calorie versus low‐calorie food cues. Circulating leptin and ghrelin, hormones known to regulate eating behavior, did not change significantly. Such work may help elucidate mechanisms when conducted with larger sample sizes.
During adolescence, some weight gain is developmentally normal and appropriate. Also, people tend to gain weight over time (Flegal 2000). In the US, the prevalence of overweight or obesity is higher for men and women 40 to 59 years of age compared with those aged 20 to 39 years (Ogden 2014). In contraceptive studies, weight change is rarely a primary outcome in contraceptive studies. No consensus exists regarding what is excessive weight gain. Examining contraceptive use and weight gain can be complicated by the initial weight of the users. Recent interest in the effectiveness of hormonal contraceptives among obese women has led to more research with such women, who had been historically excluded from such studies (Bender 2013; Lopez 2013; Edelman 2014).
Concern about contraceptive effectiveness among overweight women (Robinson 2013; Merki‐Feld 2015) has led to questions about contraceptive usage by overweight or obese women. An analysis of medical records from 231 health centers examined contraceptive use among 147,336 US women, age 15 to 44 years (Kohn 2015). The obese women (BMI ≥ 30) were more likely to use LARC than women with a BMI < 30 (13% versus 9%, respectively). Obese women were less likely than women with a lower BMI to use OCs, the injectable, the vaginal ring, or the subdermal patch (76% versus 82%, respectively). An analysis of US survey data examined contraceptive use in the past month (Callegari 2014). The women were sexually active, obese (BMI > 30), and age 20 to 44 years. LARC use among these obese women was nearly 10%, but only about 38% reported using OCs, the patch, the ring, or injectable contraception. While 21% reported not using a contraceptive method in the past month, 31% used a nonprescription method, i.e. condoms or another barrier method, withdrawal, or fertility awareness methods. Those two groups were more likely to be the youngest and oldest (aged 20 to 24 or 40 to 44). Women who used nonprescription methods were less likely to report having discussed contraception with a healthcare provider in the past year.
Why it is important to do this review
Prior to the initial review, no comprehensive systematic review existed on progestin‐only contraceptives and weight change. Concern about weight gain might deter women from using these effective contraceptives and health care providers from recommending them. We did not examine effectiveness nor focus on overweight women. Many reviews have examined effectiveness of specific progestin‐only contraceptives, such as progestin‐only pills (Grimes 2013) and IUC (Grimes 2007). Further, a Cochrane review examined effectiveness of hormonal contraceptives for overweight women versus women who were not overweight (Lopez 2013).
Progestin‐only contraceptives are an attractive option for many women. The longer‐acting POCs, especially IUC and implants, are among the more effective methods with typical use (Trussell 2011). The cost for POCs can be less than that of COCs in some areas, and many postpartum women can use them. Further, POCs are appropriate for women at increased risk for venous thromboembolism such as those who are obese (Merki‐Feld 2015), which is important given the worldwide epidemic of obesity (Prentice 2006; Flegal 2012; Ogden 2014). Being overweight or obese increases also risk for Type 2 diabetes and other diseases and disorders.
Objectives
The primary objective was to evaluate the potential association between progestin‐only contraceptive use and changes in body weight.
Methods
Criteria for considering studies for this review
Types of studies
We considered studies that examined progestin‐only contraceptives used for contraception and their associations with weight change. Reports had to contain information on the specific contraceptive method(s) examined. We searched for studies with comparative data on a progestin‐only contraceptive versus another contraceptive (differing in formulation, dose, regimen, or initiation time) or no hormonal contraceptive. Potential studies included comparisons of a POC with a combination contraceptive as well as comparisons of two different types of progestin‐only contraceptives.
Types of participants
Participants were the women in the studies who used the progestin‐only contraceptive for contraception or who had the comparison intervention or placebo. We did not consider studies focused on women with specific health problems, such as diabetes or HIV.
Types of interventions
We considered any progestin‐only contraceptive, such as an oral contraceptive, an injectable, an implant, or hormonal intrauterine contraception (IUC). Treatment duration must have been at least three cycles or three months.
The progestin method of interest must have been specified and not combined in a group with another method, e.g. a group that used either DMPA or norethisterone enanthate (NET‐EN). The comparison could have been another progestin‐only contraceptive or a group of contraceptives, such as COCs. We did not include comparison groups identified only as oral contraceptive (OC) users, since the oral contraceptives could have been progestin‐only pills or combined oral contraceptives.
The progestin‐only method had to be intended for contraception. We did not consider studies of contraceptives used for treatment for specific disorders, e.g. acne, hirsutism, or polycystic ovary syndrome.
Types of outcome measures
The primary outcome was the continuous outcome of mean change in body weight, BMI, or body composition (e.g. percent body fat) over time with the use of progestin‐only contraceptives. If mean change in body weight or BMI was not available per study arm, we examined the dichotomous outcome of loss or gain of a specified amount of weight in each study arm.
For high quality evidence, the study had to include mean change in body weight, BMI, or body composition. The time frame had to be 12 months.
We used measured weight and not self‐reported weight. We excluded studies that did not report change data but only reported mean weight or BMI at pre‐ and post‐treatment.
Search methods for identification of studies
Electronic searches
Until 4 August 2016, we searched MEDLINE, Cochrane Central Register of Controlled Trials (CENTRAL), POPLINE, Web of Science, and LILACS. We also searched for trials via ClinicalTrials.gov and the search portal of the International Clinical Trials Registry Platform (ICTRP). Appendix 1 shows the 2016 strategies. We listed the previous search strategies in Appendix 2.
Searching other resources
We examined reference lists of relevant articles. For the initial review, we contacted investigators in the field to seek additional unpublished trials or published trials that we may have missed in our search.
Data collection and analysis
Selection of studies
We assessed for inclusion all titles and abstracts identified during the literature searches. One author reviewed the search results and identified reports for inclusion or exclusion. A second author also examined the reports identified for appropriate categorization according to the eligibility criteria above.
We considered all comparative study designs. For example, studies could have been randomized controlled trials (RCTs), other prospective studies (provided intervention; assignment not random), observational studies of users, case‐control studies, or retrospective chart reviews. We also considered post hoc analysis from any of these types of studies. However, the studies had to meet the Criteria for considering studies for this review.
Data extraction and management
Two authors conducted the data extraction. One author entered the data into Review Manager (RevMan 2014), and a second author checked accuracy. These data include the study characteristics, risk of bias, and outcomes. We focused on the primary and secondary outcomes for this review, which do not include all outcomes from each study. The authors resolved discrepancies through discussion.
Assessment of risk of bias in included studies
We examined the RCTs for methodological quality in accordance with recommended principles (Higgins 2011), and entered the information into the Risk of bias tables. Factors considered are randomization method, allocation concealment, blinding, and losses to follow‐up and early discontinuation.
For the NRS, we used the Newcastle‐Ottawa Quality Assessment Scale (NOS) (Higgins 2011; Wells 2014). Of the two NOS versions, i.e. for case‐control and cohort studies, the latter was more pertinent here (Appendix 3). The NOS investigators are examining the criterion validity and construct validity of this scale as well as the inter‐rater reliability and intra‐rater reliability. The scale does not yet have an overall scoring or threshold for a 'good' or 'poor' quality study. The NOS has eight items within three domains: selection (representativeness), comparability (due to design or analysis), and outcomes (assessment and follow‐up). A study can receive one star (✸) for meeting each criterion. The exception is comparability (design or analysis), for which a study can receive two stars (for design and analysis). We adapted the NOS items for this project as suggested by the developers (Wells 2014).
Measures of treatment effect
Outcomes listed in Characteristics of included studies focus on those relevant to this review. We examined weight change in relation to initial body weight or body mass index (BMI) [weight (kg)/height (m)2] when we had the necessary data. Weight change may differ for women who were initially overweight or obese versus those who were not. We preferred BMI over weight alone, as BMI is a better reflection of body fat (CDC 2012a). The measures and cutoffs depended on those used in the included studies. Frequently used BMI categories are 25 to 29.9 (kg/m2) for overweight and 30 or higher for obesity (CDC 2012a).
We examined results by the contraceptive method studied, e.g. injectable or implant, as well as by formulation, dose, or regimen as appropriate. The main comparisons for this review were between users of progestin‐only contraceptives and users of another contraceptive (differing in formulation, dose, or regimen) or no hormonal contraceptive.
For weight change measure with follow‐up of less than one year, we selected the six‐month assessment (if available) and the latest date. If multiple time points were reported up to one year, we used the 6‐ and 12‐month data. If data were available for more than three years, we used one‐year data, the midpoint, and the last measure.
Randomized trials
For continuous variables, we computed the mean difference (MD) with 95% confidence interval (CI). Review Manager uses the inverse variance approach. For the dichotomous outcomes, we calculated the Mantel‐Haenszel odds ratio (OR) with 95% CI. An example is the proportion of women who gained or lost more than 2 kg. Fixed and random effects give the same result if no heterogeneity exists, as when a comparison includes only one study.
Non‐randomized studies
Given the need to control for confounding factors in NRS, we used adjusted measures for the primary effect measures when available or the results of matched analysis from paired samples. Investigators may have used a variety of adjustment strategies. When presenting results, we note the confounding factors considered in the design or analysis. If the report did not provide adjusted measures for the primary analysis, we used unadjusted outcomes with the methods described above for use with RCTs.
Dealing with missing data
We excluded studies with insufficient data on weight or BMI for analysis in this review. Reports sometimes provided results in figures without specific numbers; others presented means without any variance estimate. We contacted investigators for other missing data and for clarifications if the studies were less than 10 years old or had a report within the past five years. Investigators are unlikely to have access to data from older studies. Many studies in the initial review were more than 10 years old.
Assessment of heterogeneity
We expected study populations, designs, and interventions to be heterogeneous. We described the clinical and methodological diversity (or heterogeneity) of the studies. We did not pool data from studies that had different contraceptive methods (e.g. DMPA or implants), different doses of the same method, or different criteria for reporting weight change. Therefore, we were not able to conduct meta‐analysis due to the range of contraceptive methods examined and different reporting for weight change. Heterogeneity is not an issue when a comparison has a single study.
Data synthesis
To assess the quality of evidence and address confidence in the effect estimates, we applied principles from GRADE (Grades of Recommendation, Assessment, Development and Evaluation) (Higgins 2011; GRADE 2013 ). If meta‐analysis is not viable because of varied interventions or outcome measures, a typical 'Summary of findings' table is not feasible. Also, the criteria for quality assessment differ for NRS and RCTs. We provide 'Summary of findings' tables for the main results, although we did not conduct a formal GRADE assessment for all outcomes (GRADE 2013).
We based our assessment of the body of evidence on the quality of evidence from the studies. In 2016, we revised the Risk of bias tables to accommodate RCTs and NRS. For the NRS, we used the Newcastle‐Ottawa Quality Assessment Scale as noted earlier (Appendix 3). Evidence quality included the design, implementation, and reporting of the study. We list the criteria for downgrading below.
Inadequate randomization sequence generation or allocation concealment, or no information provided for either one (RCT), or study was not randomized (NRS)
NRS: high risk of bias in selection (NOS) or retrospective study of selected cases
NRS: no stars for comparability (NOS), i.e. not controlling for relevant confounding
Follow‐up less than 12 months for change in weight or BMI
Losses (by one year or primary endpoint if more than one year): loss to follow‐up greater than 20%, combined loss to follow‐up and discontinuation greater than 50%, or differential losses between groups (greater than 50% difference)
For the initial review in 2010 and the minor update in 2013, we used a basic process to assess evidence quality. For those versions, the initial grade was based on study design: RCTs were considered to provide high quality evidence; prospective non‐randomized studies, moderate quality; and retrospective studies, low quality. Those ratings were then downgraded for high loss to follow‐up and inappropriate exclusions after randomization.
Sensitivity analysis
We examined separately the studies that provided evidence of moderate or high quality.
Results
Description of studies
Results of the search
The 2013 search produced 189 citations: 123 references from the database searches, 63 trials from the clinical trials sites, and 3 references from other sources. After reviewing the full text, we included one new study for a total of 16 studies. We excluded five studies and two secondary articles related to previously excluded studies. The remaining references were discarded after reviewing the titles and abstracts. From the clinical trials sites, we added three new trials to Ongoing studies.
The 2016 search produced 138 unduplicated references from the database searches (Figure 1). With one item from another source, the total was 139. We discarded 122 citations based on title or abstract. After reviewing the full text of 17 articles, we excluded 9 reports (8 primary articles plus a secondary article). We included six new studies that involved six primary reports plus two secondary articles. Searches of recent clinical trials yielded 61 unduplicated listings. Two studies are completed but have not yet produced full reports (Studies awaiting classification). We will assess them for inclusion when full reports are available. Two other trials are Ongoing studies.
1.
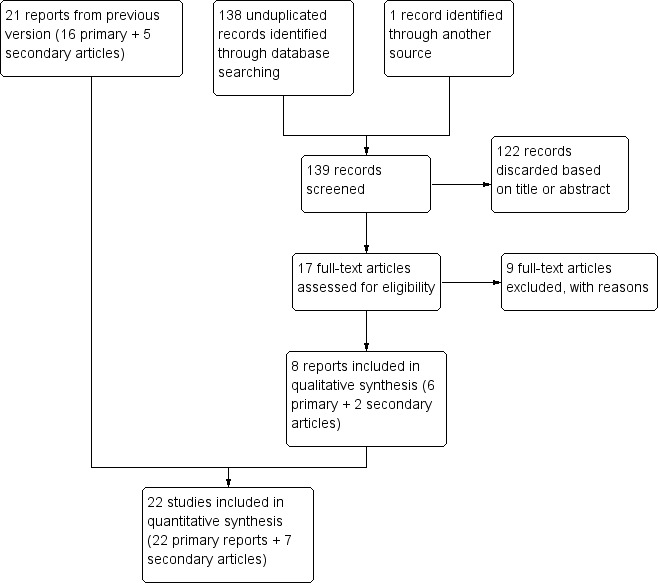
Study flow diagram, 2016
Included studies
With 6 new studies in this update, 22 studies now met our inclusion criteria. Fifteen were prospective and seven were retrospective.
15 prospective studies
5 randomized controlled trials (WHO 1983; Salem 1988; Ball 1991; Sivin 1998; Westhoff 2007)
10 non‐randomized studies (NRS) (Tankeyoon 1976; Castle 1978; Salem 1984; Bonny 2009; Dal'Ava 2012; Nyirati 2013; Vickery 2013; Dal'Ava 2014; Dos Santos 2014; Napolitano 2015)
7 retrospective studies: NRS (Moore 1995; Taneepanichskul 1998; Espey 2000; Sule 2005; Tuchman 2005; Pantoja 2010; Modesto 2015)
The studies examined four categories of progestin‐only contraceptives (Table 4).
2. Overview of interventions, outcome measures, mean changes.
| Study | N | Intervention groups | Time frame | Outcome measures | Mean change |
| Progestin‐only oral contraceptive | |||||
| Ball 1991 | 51 | NET 350 µg vs levonorgestrel 30 µg | 6 months | Weight (kg) | 0 vs 0.6 |
| Napolitano 2015 | 68 | Desogestrel 75 µg vs no hormonal method | 12 months | Weight (kg); BMI (kg/m2); fat mass (%); fat‐free mass (%) |
0.3 vs ‐0.2; 0.5 vs 0.5; 2.8 vs ‐0.5a; ‐2.8 vs 0.5a |
| Depot medroxyprogesterone acetate (DMPA) 150 mg/mL | |||||
| Tuchman 2005 | 222 | DMPA vs MPA + E2C | 6 months; 12 months |
Weight (kg) | 0.6 vs 1.2; 1.7 vs 3.0 |
| DMPA vs COC | 0.6 vs 1.1; 1.7 vs 1.0 | ||||
| Tankeyoon 1976 | 32 | DMPA vs COC | 12 months | Weight (kg) | 1.8 vs 3.1 (estimated) |
| Bonny 2009 | 15 | DMPA + placebo vs DMPA + E2C | 6 months | Total body fat (%); lean body mass (%) |
10.3 vs 2.8; ‐3.4 vs ‐1.2 |
| Castle 1978 | 1000 | DMPA 150 vs DMPA 450 | 6 months | Weight (kg) | 0.33 vs 0.32 |
| Espey 2000 | 172 | DMPA: interval vs postpartum | 1 year; 2 years |
Weight (kg) | 4.2 vs 3.2; 7.2 vs 6.5 |
| Westhoff 2007 | 534 | DMPA‐intramuscular 150 vs DMPA‐subcutaneous 104 | 3 years | Weight (kg) | 5.8 vs 4.5 |
| WHO 1983 | 3172 | DMPA vs NET‐EN (60 days) | 1 year; 2 years |
Weight (kg) | 1.9 vs 1.7; 3.3 vs 3.3 |
| NET‐EN: 60 days vs 84 days | 1.7 vs 1.7; 3.3 vs 3.4 | ||||
| Salem 1988 | 400 | DMPA vs NET‐EN | 1 year | Weight; no unit (kg or lb) | 3.5 vs 2.7 |
| Taneepanichskul 1998 | 100 | DMPA vs Cu IUC | 10 years | Weight (kg) | 10.9 vs 11.2 |
| Pantoja 2010 | 758 | DMPA vs Cu IUC | 1 year; 2 years; 3 years |
Weight (kg) | 1.76 vs ‐0.42;a 3.1 vs 0.4;a 3.9 vs 0.8a |
| Modesto 2015 | 1277 | DMPA 150 vs Cu IUC | 1 year; 4 years; 10 years |
Weight (kg) | 1.3 vs 0.2;a 3.5 vs 1.9;a 6.6 vs 4.9a |
| Vickery 2013 | 167 | DMPA 150 vs Cu IUC | 12 months | Weight (kg) | 2.20 vs 0.16 |
| Dal'Ava 2014 | 110 | DMPA 150 vs Cu IUC | 12 months | Weight (kg); fat mass (kg); lean mass (kg) |
1.9 vs 1.1; 1.6 vs ‐0.9; 0.3 vs 1.2 |
| Dos Santos 2014 | 71 | DMPA 150 vs Cu IUC | 12 months | Weight (kg); body fat (kg); lean mass (kg) |
1.4 vs 0.3; 1.57 vs 0.52; ‐0.31 vs ‐0.26 |
| Bonny 2009 | 26 | DMPA + placebo vs no hormonal method | 6 months | Total body fat (%); lean body mass (%) |
10.3 vs ‐0.1;a ‐3.4 vs 0.6a |
| Nyirati 2013 | 78 | DMPA 150 vs surgical sterilization | 12 months | Weight (lb); BMI (kg/m2); body fat (%) |
2.14 vs 0.6; 0.36 vs 0.04; ‐0.58 vs ‐1.56 |
| Implants | |||||
| Salem 1984 | 150 | Norplant vs Cu IUC | 6 months | Weight (kg) | 1.39 vs 0.92a |
| Norplant vs non‐hormonal method | 6 months | 1.39 vs 0.65a | |||
| Moore 1995 | 100 | Norplant vs DMPA | 12 months | Weight (kg) | ‐0.81 vs 0.06 |
| Sivin 1998 | 1200 | Norplant vs 2‐rod levonorgestrel | 1 year; 3 years; 5 years |
Weight (kg) | 0.99 vs 0.90; 3.12 vs 3.12; 4.14 vs 3.54 |
| Sule 2005 | 516 | Norplant vs non‐hormonal IUC | 1 year; 3 years | Weight (kg) | 2.5 vs 1.4;a 4.8 vs 3.9 |
| Norplant vs COC | 2.5 vs 1.4; 4.8 vs 0.0 | ||||
| Vickery 2013 | 230 | Etonogestrel implant vs Cu IUC | 12 months | Weight (kg) | 2.12 vs 0.16 |
| Levonorgestrel (LNG) intrauterine contraception (IUC) | |||||
| Dal'Ava 2012 | 76 | LNG‐IUC vs non‐hormonal IUC | 12 months | Weight (kg) | 2.9 vs 1.4 |
| Total body fat; lean body mass |
2.5% vs ‐1.3%;a ‐1.4% vs 1.0%a |
||||
| Vickery 2013 | 230 | LNG‐IUC vs Cu IUC | 12 months | Weight (kg) | 1.03 vs 0.16 |
| Modesto 2015 | 1204 | LNG‐IUC vs Cu IUC | 1 year; 4 years; 10 years |
Weight (kg) | 0.7 vs 0.2; 2.7 vs 1.9; 4.0 vs 4.9 |
| Napolitano 2015 | 60 | LNG‐IUC vs no method | 12 months | Weight (kg); BMI (kg/m2); fat mass (%); fat‐free mass (%) |
0.6 vs ‐0.2; 0.2 vs 0.5; 1.1 vs ‐0.5;a ‐1.1 vs 0.5a |
aSignificant difference between comparison groups Cu IUC: copper intrauterine contraception COC: combination oral contraception DMPA: depot medroxyprogesterone acetate E2C: estradiol cypionate LNG‐IUC: levonorgestrel‐releasing intrauterine contraception MPA: medroxyprogesterone acetate NET: norethisterone NET‐EN: norethisterone enanthate
Oral contraceptives (OCs) containing norethisterone 350 µg, levonorgestrel 30 µg, or desogestrel 75 µg
-
Injectables
depot medroxyprogesterone acetate (DMPA): 150 mg/mL versus 450 mg/mL (intramuscular) or versus 104 mg/0.65mL (subcutaneous)
norethisterone enanthate (NET‐EN) 200 mg
Implants: levonorgestrel 6 capsules or 2 rods; etonogestrel 1 rod
Levonorgestrel‐releasing intrauterine contraception (LNG‐IUC)
Comparison groups included no hormonal method or a non‐hormonal contraceptive; a different formulation, regimen, or initiation time of the same POC; another POC; and a combined contraceptive or a supplement containing estrogen.
Studies were conducted in the USA, South America, Europe, Africa, and Asia; some were conducted on multiple continents. Publication dates covered nearly 50 years: five studies from 1976 to 1988; four from 1991 to 1998; five from 2000 to 2009; and eight from 2010 to 2015. Duration of prospective follow‐up or retrospective data collection was six months to two years for 16 studies, while four studies gathered data for three to five years of use, and two studies collected retrospective data for 10 years of use.
The studies included a total of 11,450 women with an average of 520 and a median about 160. Six studies had fewer than 100 participants, seven had 100 to 222 participants, six ranged from 400 to 1000 women, and three had more than 1000 women. A few had comparison groups not used in this review because they did not meet our inclusion criteria.
We were not able to examine weight change in relation to age. Earlier, we identified three studies focused on adolescents and young women. Bonny 2009 analyzed data from a larger study of hormonal contraceptives and bone mineral density. Moore 1995 and Tuchman 2005 were retrospective chart reviews. A certain amount of weight gain is part of normal development for adolescents. None of the newer studies focused on young women. Studies that included both adolescents and adult women did not provide outcome data for age subgroups.
Risk of bias in included studies
Figure 2 summarizes our assessments for the overall review. Table 25 shows how we rated each study, and Figure 3 illustrates our assessment for each study. Because we adapted the Risk of bias tables to accommodate criteria for NRS, some categories are not relevant to an RCT or an NRS. In those cases, we left the cell empty rather than state 'not applicable' to distinguish between 'unclear' and no assessment.
2.
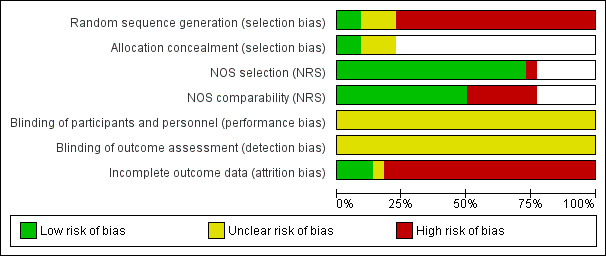
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
1. Evidence quality.
| Study | Randomization methods or NRS | NRS: NOS selection criterion | NRS: NOS comparability | Follow‐up period | Loss or chart review | Evidence qualitya |
| Ball 1991 | ‐1 | NA | NA | ‐1 | ‐1 | Very low |
| Bonny 2009 | ‐1 | _ | _ | ‐1 | _ | Low |
| Castle 1978 | ‐1 | _ | ‐1 | ‐1 | ‐1 | Very low (poor) |
| Dal'Ava 2012 | ‐1 | _ | _ | _ | _ | Moderate |
| Dal'Ava 2014 | ‐1 | _ | _ | _ | ‐1 | Low |
| Dos Santos 2014 | ‐1 | _ | _ | _ | ‐1 | Low |
| Espey 2000 | ‐1 | _ | _ | _ | ‐1 | Low |
| Modesto 2015 | ‐1 | _ | _ | _ | ‐1 | Low |
| Moore 1995 | ‐1 | _ | _ | _ | ‐1 | Low |
| Napolitano 2015 | ‐1 | _ | ‐1 | _ | _ | Low |
| Nyirati 2013 | ‐1 | _ | ‐1 | _ | ‐1 | Very low |
| Pantoja 2010 | ‐1 | _ | _ | _ | ‐1 | Low |
| Salem 1984 | ‐1 | _ | ‐1 | ‐1 | _ | Very low |
| Salem 1988 | _ | NA | NA | _ | ‐1 | Moderate |
| Sivin 1998 | _ | NA | NA | _ | ‐1 | Moderate |
| Sule 2005 | ‐1 | _ | ‐1 | _ | ‐1 | Very low |
| Taneepanichskul 1998 | ‐1 | ‐1 | _ | _ | ‐1 | Very low |
| Tankeyoon 1976 | ‐1 | _ | _ | _ | ‐1 | Low |
| Tuchman 2005 | ‐1 | _ | ‐1 | _ | ‐1 | Very low |
| Vickery 2013 | ‐1 | _ | _ | _ | ‐1 | Low |
| Westhoff 2007 | ‐1 | NA | NA | _ | ‐1 | Low |
| WHO 1983 | ‐1 | NA | NA | _ | ‐1 | Low |
aDowngraded for the following: (1) Risk of bias high for randomization sequence generation or allocation concealment, or no information provided on either, or study not randomized (NRS); (2) NRS: high risk of bias in selection; (3) NRS: no stars for comparability, i.e. not controlling for relevant confounding; (4) follow‐up < 12 months for change in weight or BMI; (6) loss to follow‐up > 20%, combined loss to follow‐up and discontinuation > 50%, major differential losses between groups, or retrospective chart review of selected cases NA = not applicable NOS = Newcastle‐Ottawa Quality Assessessment Scale
3.
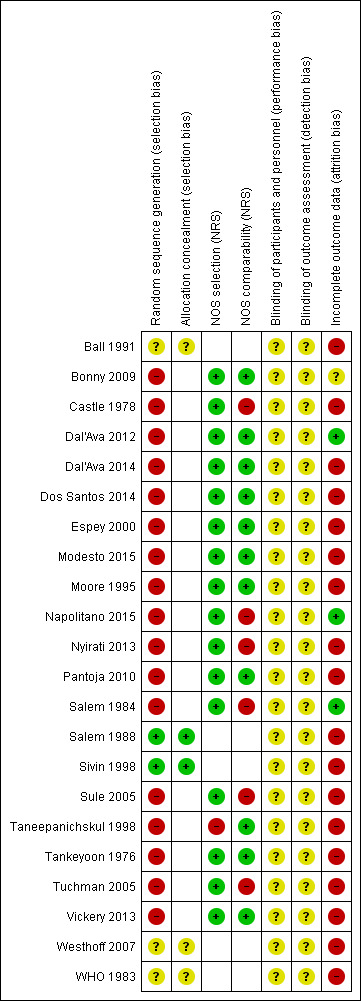
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Of the five RCTs, two reported the method of randomization and allocation concealment (Salem 1988; Sivin 1998). The other three had no information on randomization method or allocation concealment (WHO 1983; Ball 1991; Westhoff 2007). Of the 17 NRS, one did not meet the NOS selection criteria (Taneepanichskul 1998).
Blinding
Two of the five RCTs had information on blinding. Ball 1991 was reportedly "single‐blind" without any specifics. For one trial used in Westhoff 2007, the evaluators were blinded. For most studies, blinding was not feasible due to differences in the contraceptive methods or to women having chosen their contraceptive method in the NRS.
Incomplete outcome data
Of 22 studies, 18 had high risk of bias related to incomplete outcome data. Nine had loss to follow‐up or discontinuation greater than 50% (Castle 1978; WHO 1983; Salem 1988; Ball 1991; Sivin 1998; Westhoff 2007; Nyirati 2013; Dal'Ava 2014; Dos Santos 2014). In Tankeyoon 1976, loss differed substantially between groups. Seven retrospective studies may have selected charts for those with complete data and not accounted for losses (Moore 1995; Taneepanichskul 1998; Espey 2000; Sule 2005; Tuchman 2005; Pantoja 2010; Modesto 2015). Modesto 2015 also had differential losses across group that varied over time. Vickery 2013 recruited women who completed at least 11 months of use.
Selective reporting
After randomization, Taneepanichskul 1998 excluded women who developed a chronic disease or disorder during method use. This exclusion may have biased the results. Weight gain is associated with development of some diseases and disorders.
Other potential sources of bias
NRS: comparability (NOS)
Of 17 non‐randomized studies, eight addressed potential confounding factors. Four considered confounding in the design by matching on age and baseline BMI (Pantoja 2010; Dal'Ava 2012; Dos Santos 2014) or age and weight (Dal'Ava 2014). Four studies conducted analysis that adjusted for potential confounders (Moore 1995; Bonny 2009; Modesto 2015; Vickery 2013). In some cases, a comparison group did not meet our inclusion criteria, so we did not include that group in this review. Details are in Characteristics of included studies.
Effects of interventions
See: Table 1; Table 2; Table 3
for the main comparison.
| DMPA compared with no hormonal contraceptive for effect on weight | ||||
|
Patient or population: women with need for contraception Settings: clinic Intervention: DMPA 150 mg/mL Comparison: no hormonal contraceptive | ||||
| Outcomes | Relative effect (95% CI) | Participants (study) | Quality of the evidence (GRADE) | Comments |
| Change in body fat (%) by 6 months; change in lean body mass (%) by 6 months |
MD 11.00 (2.64 to 19.36); MD ‐4.00 (‐6.93 to ‐1.07) | 26 (Bonny 2009) |
Low | DMPA + placebo vs no hormonal; adolescents 15 to 18 years old |
| Change in weight (kg): 1 year; 2 years; 3 years |
MD 2.28 (1.79 to 2.77); MD 2.71 (2.12 to 3.30); MD 3.17 (2.51 to 3.83); |
758 (Pantoja 2010) |
Low | DMPA vs Cu IUC; women of child‐bearing age |
| Change in weight (kg): 1 year; 4 years; 10 years |
Reported adjusted mean ± SE (reported P):
1.3 ± 0.15 vs 0.2 ± 0.17 (P < 0.0001); 3.5 ± 0.23 vs 1.9 ± 0.23 (P < 0.0001); 6.6 ± 0.61 vs 4.9 ± 0.60 (P < 0.0350) |
1277; 1165; 279 (Modesto 2015) |
Low | DMPA vs Cu IUC; women 18 to 40 years old |
| CI: Confidence interval; MD = mean difference; SD = standard deviation; SE = standard error | ||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||
DMPA: depot medroxyprogesterone acetate Cu IUC: copper intrauterine contraception
2.
| Levonorgestrel‐releasing IUC compared with no hormonal contraceptive for effect on weight | ||||
|
Patient or population: women with need for contraception Settings: clinic Intervention: LNG‐IUC Comparison: no hormonal contraceptive | ||||
| Outcomes by 1 year | Relative effect (95% CI) | Participants (study) | Quality of the evidence (GRADE) | Comments |
| Change in fat mass (%); change in lean mass (%) |
Reported mean ± SD (reported P): 2.5 ± 8.0 vs ‐1.3 ± 6.9 (P = 0.029); ‐1.4 ± 4.7 vs 1.0 ± 3.8 (P = 0.027) |
76 (Dal'Ava 2012) |
Moderate | LNG‐IUC vs non‐hormonal IUC; women 18 to 45 years old |
| Change in fat mass (%); change in fat free mass (%) |
MD 1.60 (0.45 to 2.75); MD ‐1.60 (‐2.75 to ‐0.45) |
60 (Napolitano 2015) |
Low | LNG‐IUC vs no contraceptive; perimenopausal women |
| CI: Confidence interval; MD = mean difference; SD = standard deviation | ||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||
IUC: intrauterine contraception LNG‐IUC: levonorgestrel‐releasing intrauterine contraception
3.
| OC desogestrel 75 µg compared with no hormonal contraceptive for effect on weight | |||
|
Patient or population: perimenopausal women with need for contraception Settings: clinic Intervention: OC containing desogestrel 75 µg Comparison: no hormonal contraceptive | |||
| Outcomes by 1 year | Relative effect (95% CI) | Participants (study) | Quality of the evidence (GRADE) |
| Change in fat mass (%); change in fat free mass (%) |
MD 3.30 (2.08 to 4.52); MD ‐3.30 (‐4.52 to ‐2.08) |
68 (Napolitano 2015) |
Low |
| CI: Confidence interval; MD = mean difference | |||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||
OC: oral contraceptive
We grouped results according to four types of progestin‐only contraceptives studied, though some studies included more than one progestin‐only (P‐O) method. Table 4 summarizes the study interventions and outcomes, along with the mean changes in weight or other body composition measure. Two studies examined progestin‐only pills, 15 addressed DMPA, 5 investigated implants, and four evaluated LNG‐IUC. We subdivided the DMPA studies into those comparing DMPA with a combination contraceptive, another progestin‐only injectable formulation or regimen, or no hormonal contraception.
Progestin‐only oral contraceptives
Two studies examined P‐O oral contraceptives. In the RCT of Ball 1991, weight change at six months did not differ significantly between the norethisterone 350 µg and the levonorgestrel 30 µg groups (Analysis 1.1). Mean changes were small. The NRS of Napolitano 2015 compared body composition changes at 12 months for perimenopausal women receiving desogestrel 75 µg versus a control group with no hormonal treatment. The study also examined LNG‐IUS. Changes in mean weight and BMI did not differ significantly between the OC group and the control group at 12 months (Analysis 2.1; Analysis 2.2). However, the desogestrel group had a greater increase in fat mass (%) (MD 3.30, 95% CI 2.08 to 4.52) (Analysis 2.3).
1.1. Analysis.
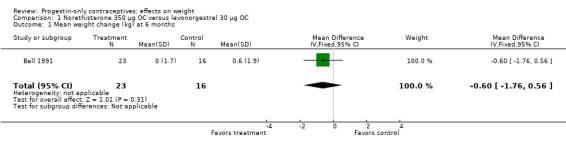
Comparison 1 Norethisterone 350 µg OC versus levonorgestrel 30 µg OC, Outcome 1 Mean weight change (kg) at 6 months.
2.1. Analysis.
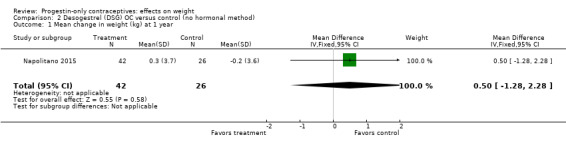
Comparison 2 Desogestrel (DSG) OC versus control (no hormonal method), Outcome 1 Mean change in weight (kg) at 1 year.
2.2. Analysis.
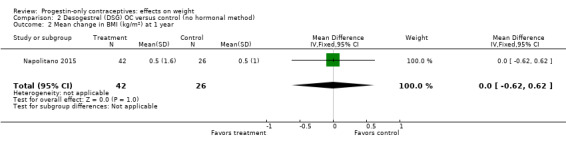
Comparison 2 Desogestrel (DSG) OC versus control (no hormonal method), Outcome 2 Mean change in BMI (kg/m2) at 1 year.
2.3. Analysis.
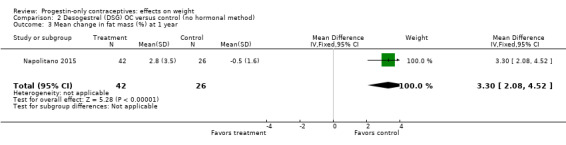
Comparison 2 Desogestrel (DSG) OC versus control (no hormonal method), Outcome 3 Mean change in fat mass (%) at 1 year.
Injectables
DMPA versus a combination contraceptive
Three NRS examined DMPA 150 mg/mL versus a contraceptive or supplement that also contained estrogen.
In the small study of Tankeyoon 1976, the DMPA and COC groups were not significantly different in the proportions that gained (or lost) at least 1 kg by months 6 and 12 (Analysis 3.1 to Analysis 3.4).
The retrospective study of Tuchman 2005 focused on adolescents and young women, age 12 to 21 years. At 6 and 12 months, weight changes were not significantly different between the DMPA group and the COC users (Analysis 3.5 to Analysis 3.8) or the group using medroxyprogesterone acetate (MPA) plus E2C (Analysis 4.1 to Analysis 4.4).
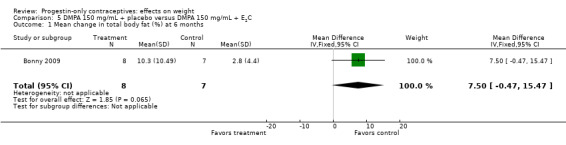
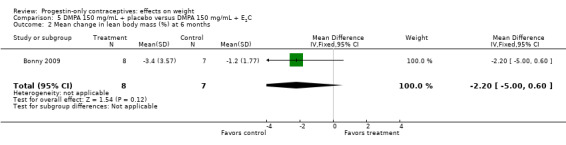
Bonny 2009 compared mean changes in total body fat (%) and lean body mass (%) at six months for DMPA 150 + placebo injection versus DMPA + estradiol cypionate 5 mg (E2C). The study targeted adolescents, age 12 to 18 years. The DMPA group was not significantly different from the DMPA plus E2C group for mean change in percent body fat or percent lean body mass (Analysis 5.1; Analysis 5.2).
3.1. Analysis.
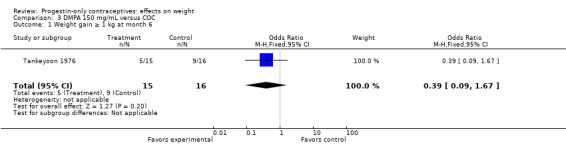
Comparison 3 DMPA 150 mg/mL versus COC, Outcome 1 Weight gain ≥ 1 kg at month 6.
3.4. Analysis.
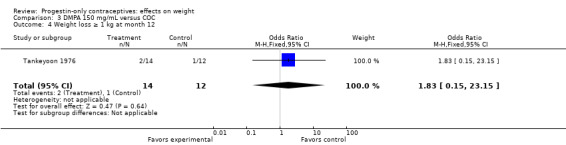
Comparison 3 DMPA 150 mg/mL versus COC, Outcome 4 Weight loss ≥ 1 kg at month 12.
3.5. Analysis.
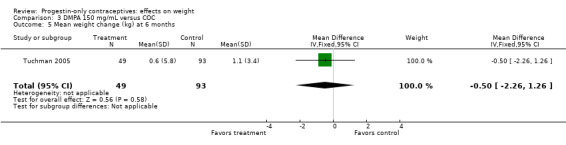
Comparison 3 DMPA 150 mg/mL versus COC, Outcome 5 Mean weight change (kg) at 6 months.
3.8. Analysis.
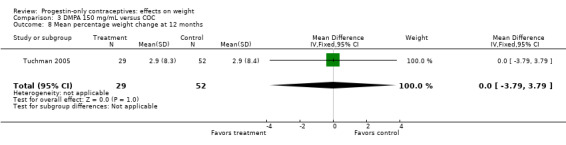
Comparison 3 DMPA 150 mg/mL versus COC, Outcome 8 Mean percentage weight change at 12 months.
4.1. Analysis.
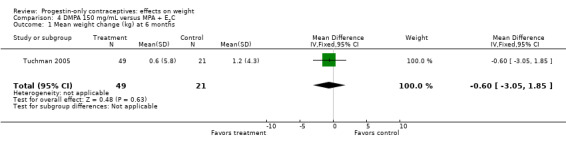
Comparison 4 DMPA 150 mg/mL versus MPA + E2C, Outcome 1 Mean weight change (kg) at 6 months.
4.4. Analysis.
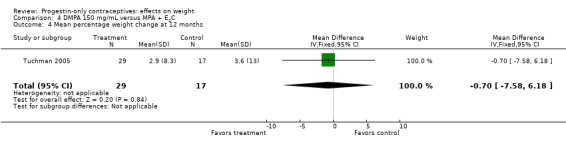
Comparison 4 DMPA 150 mg/mL versus MPA + E2C, Outcome 4 Mean percentage weight change at 12 months.
5.1. Analysis.
Comparison 5 DMPA 150 mg/mL + placebo versus DMPA 150 mg/mL + E2C, Outcome 1 Mean change in total body fat (%) at 6 months.
5.2. Analysis.
Comparison 5 DMPA 150 mg/mL + placebo versus DMPA 150 mg/mL + E2C, Outcome 2 Mean change in lean body mass (%) at 6 months.
DMPA versus another P‐O injectable formulation or regimen
Of five studies in this group, three compared DMPA 150 mg/mL with other DMPA formulations or regimens. In Castle 1978, the mean changes in weight at six months were small and did not differ significantly between the DMPA 150 and DMPA 450 groups (Analysis 6.1). The retrospective study of Espey 2000 did not show a significant difference in weight gain at one or two years for those who initiated DMPA at 20 weeks or more after pregnancy (interval group) compared with those who initiated at 5 to 8 weeks (postpartum group) (Analysis 7.1; Analysis 7.2). In the RCT analyzed in Westhoff 2007, weight change was comparable for the group with intramuscular DMPA 150 and the group with subcutaneous DMPA 104 (Analysis 8.1).
6.1. Analysis.
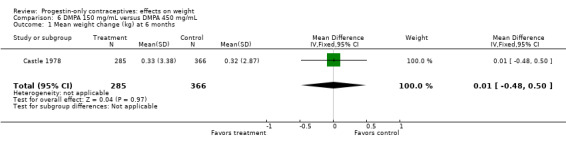
Comparison 6 DMPA 150 mg/mL versus DMPA 450 mg/mL, Outcome 1 Mean weight change (kg) at 6 months.
7.1. Analysis.
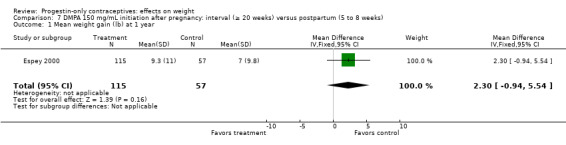
Comparison 7 DMPA 150 mg/mL initiation after pregnancy: interval (≥ 20 weeks) versus postpartum (5 to 8 weeks), Outcome 1 Mean weight gain (lb) at 1 year.
7.2. Analysis.
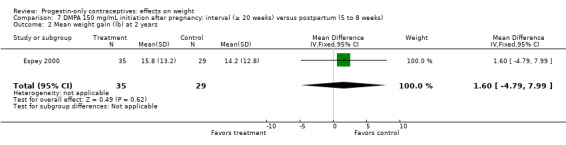
Comparison 7 DMPA 150 mg/mL initiation after pregnancy: interval (≥ 20 weeks) versus postpartum (5 to 8 weeks), Outcome 2 Mean weight gain (lb) at 2 years.
8.1. Analysis.
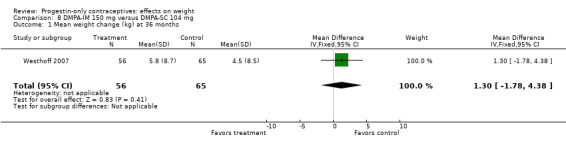
Comparison 8 DMPA‐IM 150 mg versus DMPA‐SC 104 mg, Outcome 1 Mean weight change (kg) at 36 months.
Two RCTs examined DMPA 150 mg/mL versus NET‐EN 200 mg. In WHO 1983, mean weight changes at 12 and 24 months did not differ significantly between the DMPA group and with the group administered NET‐EN at 60‐day intervals (Analysis 9.1; Analysis 9.2). Salem 1988 did not report the units for weight (lb or kg). However, the DMPA and the NET‐EN groups did not differ significantly for mean changes in weight at one year (Analysis 9.3). Also in WHO 1983, two NET‐EN regimens of 60 day‐intervals versus 84‐day intervals did not cause a significant difference in weight change (Analysis 10.1; Analysis 10.2).
9.1. Analysis.
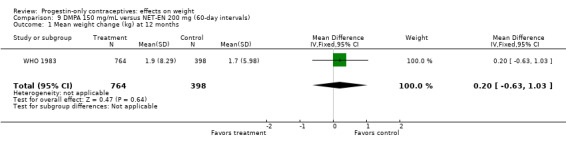
Comparison 9 DMPA 150 mg/mL versus NET‐EN 200 mg (60‐day intervals), Outcome 1 Mean weight change (kg) at 12 months.
9.2. Analysis.
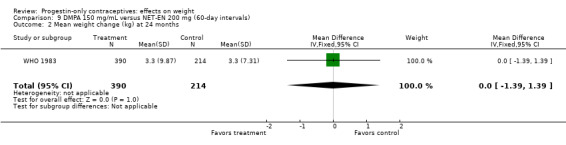
Comparison 9 DMPA 150 mg/mL versus NET‐EN 200 mg (60‐day intervals), Outcome 2 Mean weight change (kg) at 24 months.
9.3. Analysis.
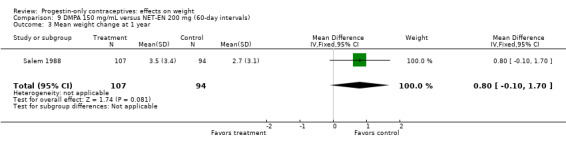
Comparison 9 DMPA 150 mg/mL versus NET‐EN 200 mg (60‐day intervals), Outcome 3 Mean weight change at 1 year.
10.1. Analysis.
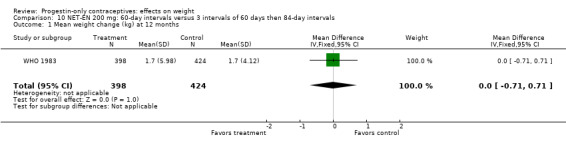
Comparison 10 NET‐EN 200 mg: 60‐day intervals versus 3 intervals of 60 days then 84‐day intervals, Outcome 1 Mean weight change (kg) at 12 months.
10.2. Analysis.
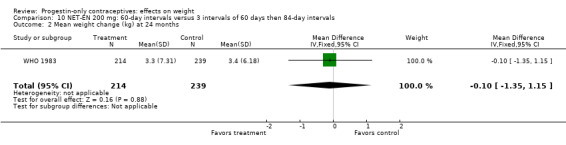
Comparison 10 NET‐EN 200 mg: 60‐day intervals versus 3 intervals of 60 days then 84‐day intervals, Outcome 2 Mean weight change (kg) at 24 months.
DMPA versus no hormonal contraceptive
Eight NRS compared DMPA 150 mg/mL versus no hormonal method. Six had copper (Cu) IUC users as the comparison group; three studies were retrospective (Taneepanichskul 1998; Pantoja 2010; Modesto 2015) and three were prospective (Vickery 2013; Dal'Ava 2014; Dos Santos 2014). The remaining two prospective studies had other comparison groups (Bonny 2009; Nyirati 2013).
DMPA versus Cu IUC
Retrospective studies
Taneepanichskul 1998 did not show a significant difference in weight change between the DMPA and Cu IUC groups at 10 years (Analysis 11.1). Unlike most studies in this review, the participants did not include younger women. All were 37 to 50 years old.
For Pantoja 2010, mean weight gain (kg) was greater for the DMPA group versus the Cu IUC group at years one (MD 2.28, 95% CI 1.79 to 2.77) (Analysis 11.2), two (MD 2.71, 95% CI 2.12 to 3.30) (Analysis 11.3), and three (MD 3.17, 95% CI 2.51 to 3.83) (Analysis 11.4). Per year, the mean weight changes for the DMPA group ranged from 1.76 kg to 3.9 kg, while changes within the IUC group were less than 1 kg (Analysis 11.5). For each year, the difference between contraceptive groups was notable within the normal to lower weight group (BMI < 25) and within the overweight group (BMI 25 to 29.9), but not within the obese group (BMI ≥ 30).
Modesto 2015 examined cumulative weight changes over 10 years of uninterrupted use of DMPA versus the Cu IUC. Using a generalized linear mixed model, the investigators adjusted for years of school and number of children. The adjusted analysis indicated mean weight change was significantly greater for the DMPA group versus the Cu IUC group for the three time points we used. The reported adjusted means were: at 1 year, 1.3 versus 0.2 (P < 0.0001; Analysis 12.1); at 4 years, 3.5 versus 1.9 (P < 0.0001; Analysis 12.2); at 10 years, 6.6 versus 4.9 (P < 0.0350; Analysis 12.3). By four years, the DMPA and LNG‐IUC group lost more than 20%; by 10 years, overall loss was 84%. The groups had differential losses at all three time points.
11.1. Analysis.
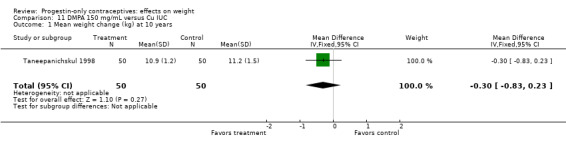
Comparison 11 DMPA 150 mg/mL versus Cu IUC, Outcome 1 Mean weight change (kg) at 10 years.
11.2. Analysis.
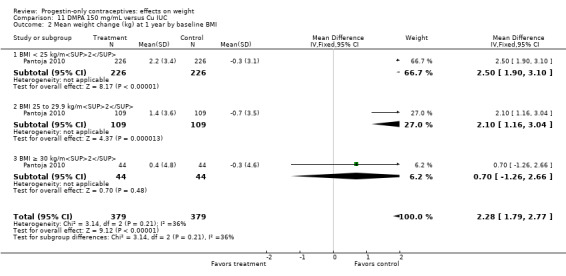
Comparison 11 DMPA 150 mg/mL versus Cu IUC, Outcome 2 Mean weight change (kg) at 1 year by baseline BMI.
11.3. Analysis.
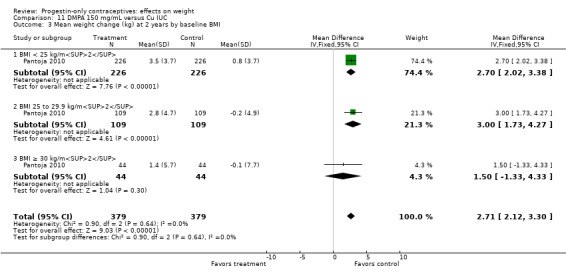
Comparison 11 DMPA 150 mg/mL versus Cu IUC, Outcome 3 Mean weight change (kg) at 2 years by baseline BMI.
11.4. Analysis.
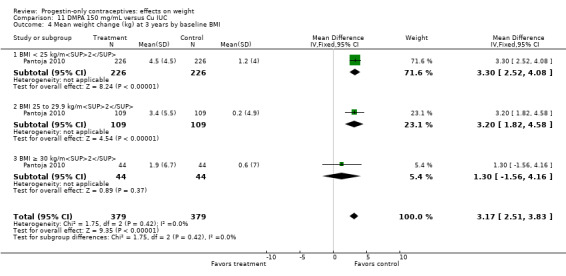
Comparison 11 DMPA 150 mg/mL versus Cu IUC, Outcome 4 Mean weight change (kg) at 3 years by baseline BMI.
11.5. Analysis.
Comparison 11 DMPA 150 mg/mL versus Cu IUC, Outcome 5 Mean weight change (kg) by year.
| Mean weight change (kg) by year | |||
|---|---|---|---|
| Study | Year | Mean change ± SD DMPA | Mean change ± SD IUC |
| Pantoja 2010 | 1 | 1.76 ± 3.6 | ‐0.42 ± 3.4 |
| Pantoja 2010 | 2 | 3.1 ± 4.3 | 0.4 ± 4.7 |
| Pantoja 2010 | 3 | 3.9 ± 5.1 | 0.8 ± 4.7 |
12.1. Analysis.
Comparison 12 DMPA, ENG implant, or LNG‐IUC versus Cu IUC, Outcome 1 Mean weight change (kg) at 1 year.
| Mean weight change (kg) at 1 year | ||||
|---|---|---|---|---|
| Study | Method | Reported adjusted mean ± SE | N | Reported P |
| Modesto 2015 | DMPA | 1.3 ± 0.15 | 675 | < 0.0001 |
| Modesto 2015 | LNG‐IUS | 0.7 ± 0.18 | 602 | 0.1719 |
| Modesto 2015 | Cu IUC | 0.2 ± 0.17 | 602 | Referent |
12.2. Analysis.
Comparison 12 DMPA, ENG implant, or LNG‐IUC versus Cu IUC, Outcome 2 Mean weight change (kg) at 4 years.
| Mean weight change (kg) at 4 years | ||||
|---|---|---|---|---|
| Study | Method | Reported adjusted mean ± SE | N | Reported P |
| Modesto 2015 | DMPA | 3.5 ± 0.23 | 540 | < 0.0001 |
| Modesto 2015 | LNG‐IUS | 2.7 ± 0.27 | 563 | 0.2310 |
| Modesto 2015 | Cu IUC | 1.9 ± 0.23 | 625 | Referent |
12.3. Analysis.
Comparison 12 DMPA, ENG implant, or LNG‐IUC versus Cu IUC, Outcome 3 Mean weight change (kg) at 10 years.
| Mean weight change (kg) at 10 years | ||||
|---|---|---|---|---|
| Study | Method | Reported adjusted mean ± SE | N | Reported P |
| Modesto 2015 | DMPA | 6.6 ± 0.61 | 125 | 0.0350 |
| Modesto 2015 | LNG‐IUS | 4.0 ± 0.97 | 68 | 0.3475 |
| Modesto 2015 | Cu‐IUD | 4.9 ± 0.60 | 154 | Referent |
Prospective studies
Vickery 2013 was a substudy within CHOICE, a prospective study of 9256 women who received contraceptives at no cost. The investigators of the substudy examined weight change among women who had been continuous users of DMPA or the copper IUC for 11 months or longer. With a linear regression model, the investigators adjusted for the potential confounders of age and race. Weight change was not significantly different for use of DMPA compared with the Cu IUC (Analysis 12.4).
Study designs were similar in Dal'Ava 2014 and Dos Santos 2014. While Dal'Ava 2014 paired participants in the DMPA and Cu IUC groups by age (± 2 years) and weight (± 2 kg), Dos Santos 2014 matched by age (± 1 year) and BMI (± 1 kg/m2). The regression model in Dal'Ava 2014 included the potential confounders of physical activity, consumption of coffee and alcohol, and smoking in regression. At 12 months, the study arms did not differ significantly for changes in total body mass (weight), fat mass, or lean mass in either study (Analysis 11.6). A secondary report from Dos Santos 2014 (Modesto 2014) included 29 women using DMPA and 25 using the Cu IUC. Multiple linear regression adjusted for potential confounders such as age, schooling, and pregnancies. DMPA use was significantly associated with change in total fat mass compared with Cu IUC use by 12 months (reported beta 2.09 ± SE 0.58; P < 0.002) but was not associated with change in percent body fat.
12.4. Analysis.
Comparison 12 DMPA, ENG implant, or LNG‐IUC versus Cu IUC, Outcome 4 Weight change (kg) at 12 months.
| Weight change (kg) at 12 months | ||||
|---|---|---|---|---|
| Study | Method | Reported adjusted beta (95% CI) | N | Reported P |
| Vickery 2013 | DMPA | 1.37 (‐0.44 to 3.18) | 67 | 0.14 |
| Vickery 2013 | ENG implant | 1.37 (‐0.16 to 2.91) | 130 | 0.08 |
| Vickery 2013 | LNG‐IUC | 0.46 (‐1.04 to 1.95) | 130 | 0.55 |
| Vickery 2013 | Cu IUC | Referent | 100 | Referent |
11.6. Analysis.
Comparison 11 DMPA 150 mg/mL versus Cu IUC, Outcome 6 Mean changes in body composition by 12 months.
| Mean changes in body composition by 12 months | ||||||
|---|---|---|---|---|---|---|
| Study | Body composition assessment | Reported mean ± SD DMPA | N | Reported mean ± SD Cu T380A IUC | N | Reported P |
| Dal'Ava 2014 | Total body mass (kg) | 1.9 ± 3.5 | 26 | 1.1 ± 3.2 | 26 | 0.38 |
| Dal'Ava 2014 | Total fat mass (kg) | 1.6 ±3.4 | 26 | ‐0.9 ± 7.2 | 26 | 0.14 |
| Dal'Ava 2014 | Total lean mass (kg) | 0.3 ± 1.8 | 26 | 1.2 ± 2.3 | 26 | 0.11 |
| Dos Santos 2014 | Total body mass (kg) | 1.4 ± 3.13 | 20 | 0.3 ± 2.24 | 20 | 0.183 |
| Dos Santos 2014 | Total body fat (kg) | 1.57 ± 3.29 | 20 | 0.52 ± 2.5 | 20 | 0.256 |
| Dos Santos 2014 | Total lean mass (kg) | ‐0.31 ± 1.7 | 20 | ‐0.26 ± 0.94 | 20 | 0.909 |
DMPA versus no hormonal method
Two studies compared women using DMPA to another group using no hormonal method.
Bonny 2009, mentioned above, also compared adolescents using DMPA 150 versus those using no hormonal method. By six months, the DMPA group had a greater increase in percent body fat (MD 11.00, 95% CI 2.64 to 19.36) (Analysis 13.1) and a greater decrease in percent lean body mass (MD ‐4.00, 95% CI ‐6.93 to ‐1.07) (Analysis 13.2).
Nyirati 2013 compared DMPA at six weeks versus surgical sterilization among postpartum women, age 18 or older. By one year postpartum, the study arms did not differ significantly for change in weight, BMI, or percent body fat (Analysis 13.3 to Analysis 13.5). The sample size for the sterilization group was much smaller than that of the DMPA group.
13.1. Analysis.
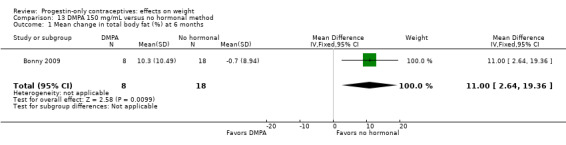
Comparison 13 DMPA 150 mg/mL versus no hormonal method, Outcome 1 Mean change in total body fat (%) at 6 months.
13.2. Analysis.
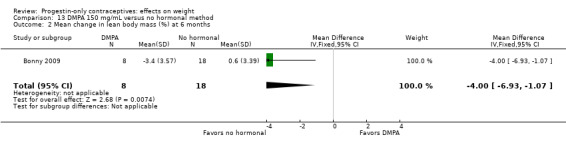
Comparison 13 DMPA 150 mg/mL versus no hormonal method, Outcome 2 Mean change in lean body mass (%) at 6 months.
13.3. Analysis.
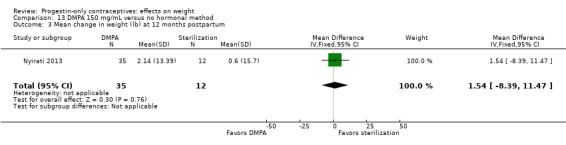
Comparison 13 DMPA 150 mg/mL versus no hormonal method, Outcome 3 Mean change in weight (lb) at 12 months postpartum.
13.5. Analysis.
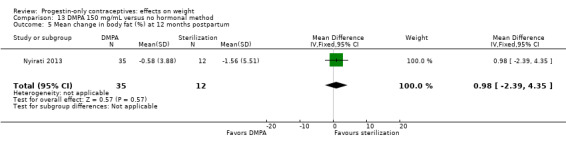
Comparison 13 DMPA 150 mg/mL versus no hormonal method, Outcome 5 Mean change in body fat (%) at 12 months postpartum.
Implants
Five studies examined implants, one RCT and four NRS. A 2013 report compared the single‐rod etonogestrel (ENG) implant versus the copper IUC. Four older studies compared Norplant (six capsules) versus a non‐hormonal IUC or another progestin‐only contraceptive.
Vickery 2013, mentioned above with DMPA, also examined weight change over 12 months for the single‐rod etonogestrel (ENG) implant versus the copper IUC. In the regression model adjusted for age and race, the ENG implant was not significantly associated with weight change compared with the Cu IUC (Analysis 12.4).
-
Two studies utilized a non‐hormonal IUC as the comparison.
In a study with lactating women, Salem 1984 showed a greater weight gain (kg) at six months for the Norplant group versus the Cu IUC group (MD 0.47, 95% CI 0.29 to 0.65) (Analysis 14.1). The Norplant group also had a greater weight gain (kg) than the group that used barrier, 'local,' or no contraceptive method (MD 0.74, 95% CI 0.52 to 0.96) (Analysis 15.1).
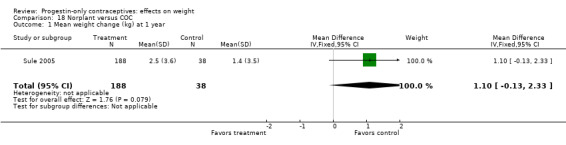
For the retrospective study of Sule 2005, the Norplant group had a significantly greater weight increase (kg) than the group with a non‐hormonal IUC at one year (MD 1.10, 95% CI 0.36 to 1.84) (Analysis 14.2) but not at three years (Analysis 14.3). The same study compared the Norplant group versus a group using COCs. Weight change did not differ significantly between the groups at one year (Analysis 18.1). At three years, the COC group had only two participants.
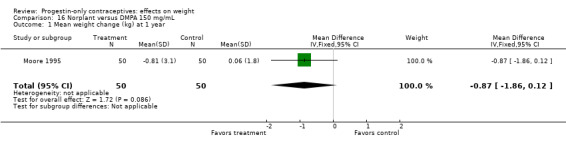
The retrospective study of Moore 1995 targeted adolescents and young women, ages 15 to 30 years. The Norplant and DMPA groups were not significantly different in mean weight change at one year (Analysis 16.1).
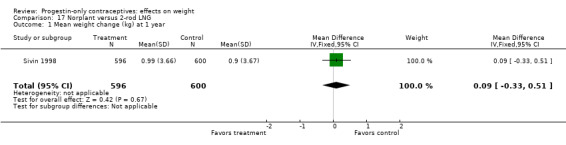
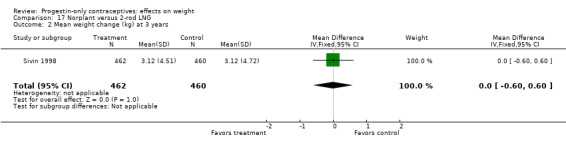
In the RCT of Sivin 1998, mean weight change was not significantly different for the Norplant group versus the two‐rod implant group at one, three, or five years (Analysis 17.1; Analysis 17.2; Analysis 17.3).
14.1. Analysis.
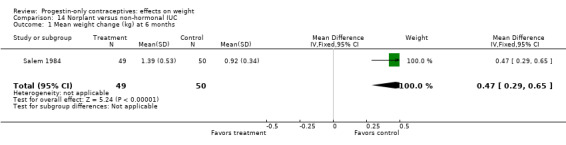
Comparison 14 Norplant versus non‐hormonal IUC, Outcome 1 Mean weight change (kg) at 6 months.
15.1. Analysis.
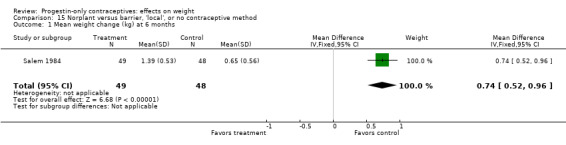
Comparison 15 Norplant versus barrier, 'local', or no contraceptive method, Outcome 1 Mean weight change (kg) at 6 months.
14.2. Analysis.
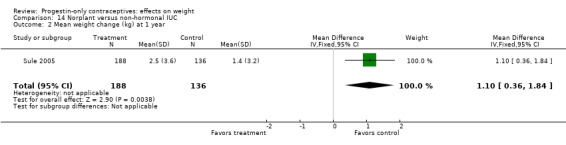
Comparison 14 Norplant versus non‐hormonal IUC, Outcome 2 Mean weight change (kg) at 1 year.
14.3. Analysis.
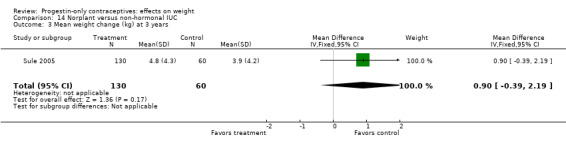
Comparison 14 Norplant versus non‐hormonal IUC, Outcome 3 Mean weight change (kg) at 3 years.
18.1. Analysis.
Comparison 18 Norplant versus COC, Outcome 1 Mean weight change (kg) at 1 year.
16.1. Analysis.
Comparison 16 Norplant versus DMPA 150 mg/mL, Outcome 1 Mean weight change (kg) at 1 year.
17.1. Analysis.
Comparison 17 Norplant versus 2‐rod LNG, Outcome 1 Mean weight change (kg) at 1 year.
17.2. Analysis.
Comparison 17 Norplant versus 2‐rod LNG, Outcome 2 Mean weight change (kg) at 3 years.
17.3. Analysis.
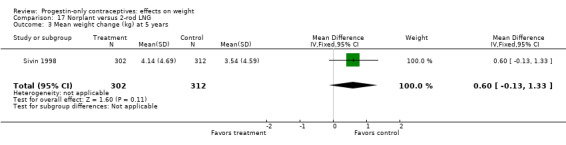
Comparison 17 Norplant versus 2‐rod LNG, Outcome 3 Mean weight change (kg) at 5 years.
Levonorgestrel‐releasing intrauterine contraception (LNG‐IUC)
Four NRS examined the LNG‐IUC versus the non‐hormonal Cu IUC or no treatment.
Dal'Ava 2012 compared body composition changes with LNG‐IUC versus with the Cu IUC. The two groups were paired by age (± 2 years) and BMI (± 2 kg/m2). At 12 months, the LNG‐IUC group differed in percent change in body fat mass compared with Cu IUC group (reported means 2.5% versus ‐1.3%; P = 0.029) (Analysis 19.1). The LNG‐IUC group also differed from the Cu IUC users in percent change in lean body mass (reported means 1.4% versus 1.0%; P = 0.027).
In addition to DMPA and the ENG implant, Vickery 2013 also examined LNG‐IUC versus the Cu IUC. The linear regression model indicated weight change with the LNG‐IUC was not significantly different at 12 months from that with the non‐hormonal IUC (Analysis 12.4).
In addition to examining weight change with DMPA use (above), Modesto 2015 compared weight change with LNG‐IUC versus that with the Cu IUC. The study groups did not differ significantly for mean weight gain (kg) at 1 and 10 years after adjusting for years of school and number of children (Analysis 12.1; Analysis 12.3). As noted above for DMPA, losses were high and differential across groups.
Besides an OC containing desogestrel (above), Napolitano 2015 also compared body composition changes at 12 months for perimenopausal women using LNG‐IUC versus a control group receiving no hormonal treatment. In unadjusted analysis, change in mean weight or BMI did not differ significantly between the LNG‐IUC group and the control group at 12 months (Analysis 19.2; Analysis 19.3). However, compared with the control group, the LNG‐IUC group had a greater mean increase in percent fat mass (MD 1.60, 95% CI 0.45 to 2.75) (Analysis 19.4) and therefore a greater mean decrease in fat free mass (MD ‐1.60, 95% CI ‐2.75 to ‐0.45) (Analysis 19.5).
19.1. Analysis.
Comparison 19 LNG‐IUC versus no hormonal contraceptive, Outcome 1 Mean changes in body composition by 12 months.
| Mean changes in body composition by 12 months | ||||||
|---|---|---|---|---|---|---|
| Study | Body composition assessment | Reported mean ± SD LNG‐IUC | N | Reported mean ± SD Cu T380A IUC | N | Reported P |
| Dal'Ava 2012 | Change in weight (kg) | 2.9 + 5.7 | 38 | 1.4 + 4.4 | 38 | 0.068 |
| Dal'Ava 2012 | Percent change in fat mass | 2.5 + 8.0 | 38 | ‐1.3 + 6.9 | 38 | 0.029 |
| Dal'Ava 2012 | Percent change in lean mass | ‐1.4 + 4.7 | 38 | 1.0 + 3.8 | 38 | 0.027 |
19.2. Analysis.
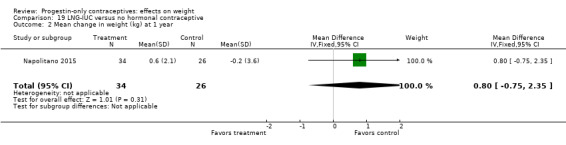
Comparison 19 LNG‐IUC versus no hormonal contraceptive, Outcome 2 Mean change in weight (kg) at 1 year.
19.3. Analysis.
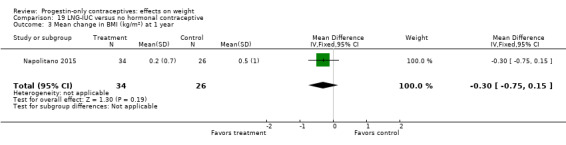
Comparison 19 LNG‐IUC versus no hormonal contraceptive, Outcome 3 Mean change in BMI (kg/m2) at 1 year.
19.4. Analysis.
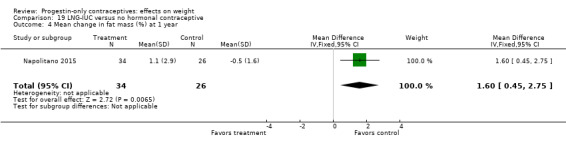
Comparison 19 LNG‐IUC versus no hormonal contraceptive, Outcome 4 Mean change in fat mass (%) at 1 year.
19.5. Analysis.
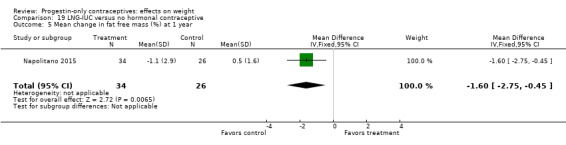
Comparison 19 LNG‐IUC versus no hormonal contraceptive, Outcome 5 Mean change in fat free mass (%) at 1 year.
Discussion
Summary of main results
Actual mean weight gain at 6 or 12 months was limited, i.e. less than 2 kg for most studies up to one year (Table 4). The six studies with multiyear data showed that mean weight change was approximately twice as much at two to four years compared with one year, but generally the study groups did not differ significantly. These studies and their years of data included two RCTs (WHO 1983 [2 years]; Sivin 1998 [5 years]) and four retrospective studies (Espey 2000 [2 years]; Sule 2005 [3 years]; Pantoja 2010 [3 years]; Modesto 2015 [10 years]). Another RCT (Westhoff 2007) and a retrospective study (Taneepanichskul 1998) had data from 3 and 10 years of use, respectively, but not multiyear data.
In Table 5, we synthesized the results for each contraceptive category. Overall, 7 of the 22 included studies indicated a significant difference between groups for change in weight, body fat, or fat free mass (Salem 1984; Sule 2005; Bonny 2009; Pantoja 2010; Dal'Ava 2012; Modesto 2015; Napolitano 2015). Three examined DMPA, two studied the LNG‐IUC, two focused on Norplant (six capsules), and one examined a desogestrel OC. The comparisons were groups using no hormonal method.
3. Results by contraceptive method.
| Study | Comparison groups | Mean difference (95% CI) | Quality of evidencea |
| Progestin‐only oral contraceptive | |||
| Ball 1991 | NET 350 µg vs levonorgestrel 30 µg | NS | Very low |
| Napolitano 2015 | Desogestrel 75 µg vs no hormonal contraceptive | Fat mass (%) 3.30 (2.08 to 4.52); fat‐free mass (%) ‐3.30 (‐4.52 to ‐2.08) | Low |
| Depot medroxyprogesterone acetate (DMPA) 150 mg/mL | |||
| Comparison: combination contraceptive | |||
| Tankeyoon 1976 | DMPA vs COC | NS | Low |
| Tuchman 2005 | DMPA vs COC | NS | Very low |
| DMPA vs MPA + E2C | NS | ||
| Bonny 2009 | DMPA + placebo vs DMPA + E2C | NS | Low |
| Comparison: another progestin‐only injectable formulation or regimen | |||
| Castle 1978 | DMPA 150 vs DMPA 450 | NS | Very low (poor) |
| Espey 2000 | DMPA: interval (≥ 20 weeks) vs postpartum (5 to 8 weeks) | NS | Low |
| Westhoff 2007 | DMPA‐intramuscular 150 vs DMPA‐subcutaneous 104 | NS | Low |
| WHO 1983 | DMPA vs NET‐EN (60 days) | NS | Low |
| NET‐EN: 60 days vs 84 days | NS | ||
| Salem 1988 | DMPA vs NET‐EN | NS | Moderate |
| Comparison: no hormonal contraceptive (retrospective studies) | |||
| Taneepanichskul 1998 | DMPA vs Cu IUC | NS | Very low |
| Pantoja 2010 | DMPA vs Cu IUC | Weight (kg): 2.28 (1.79 to 2.77); 2.71 (2.12 to 3.30); 3.17 (2.51 to 3.83) |
Low |
| Modesto 2015 | DMPA vs Cu IUC | Reported mean weights (kg): 1.3 vs 0.2 (P < 0.0001); 3.5 vs 1.9 (P < 0.0001); 6.6 vs 4.9 (P < 0.0350) |
Low |
| Comparison: no hormonal contraceptive (prospective studies) | |||
| Vickery 2013 | DMPA vs Cu IUC | NS | Low |
| Dal'Ava 2014 | DMPA vs Cu IUC | NS | Low |
| Dos Santos 2014 | DMPA vs Cu IUC | NS | Low |
| Bonny 2009 | DMPA + placebo vs no hormonal | Total body fat (%) 11.00 (2.64 to 19.36); lean body mass (%) ‐4.00 (‐6.93 to ‐1.07) | Low |
| Nyirati 2013 | DMPA: 6 weeks postpartum vs sterilization | NS | Very low |
| Implants | |||
| Vickery 2013 | ENG implant vs Cu IUC | NS | Low |
| Salem 1984 | Norplant vs other non‐hormonal | Weight (kg) 0.74 (0.52 to 0.96) | Very Low |
| Norplant vs Cu IUC | Weight (kg) 0.47 (0.29 to 0.65) | ||
| Sule 2005 | Norplant vs non‐hormonal IUC | Weight (kg) 1.10 (0.36 to 1.84) | Very low |
| Norplant vs COC | NS | ||
| Moore 1995 | Norplant vs DMPA | NS | Low |
| Sivin 1998 | Norplant vs 2‐rod LNG | NS | Moderate |
| Levonorgestrel‐releasing IUC vs no hormonal method | |||
| Dal'Ava 2012 | LNG‐IUC vs non‐hormonal IUC | Reported mean weights (kg): Total body fat (%) 2.5 vs ‐1.3 (P = 0.029); lean body mass (%) ‐1.4 vs 1.0 (P = 0.027) |
Moderate |
| Vickery 2013 | LNG‐IUC vs Cu IUC | NS | Low |
| Modesto 2015 | LNG‐IUC vs Cu IUC | NS | Low |
| Napolitano 2015 | LNG‐IUC vs no contraceptive | Fat mass (%) 1.60 (0.45 to 2.75); fat‐free mass (%) ‐1.60 (‐2.75 to ‐0.45) |
Low |
aFrom Table 25 Cu IUC: copper intrauterine contraception COC: combination oral contraception DMPA: depot medroxyprogesterone acetate LNG‐IUC: levonorgestrel‐releasing intrauterine contraception MPA: medroxyprogesterone acetate NET: norethisterone NET‐EN: norethisterone enanthate NS = no significant difference between groups
Summary of findings tables
This section focuses on the five studies with evidence of moderate or low quality that showed a significant difference between study arms (Table 5). Of 16 studies that examined DMPA (aside from one that also studied Norplant), three indicated an association between DMPA use and greater change in weight or other body composition measure (Table 1). Bonny 2009 was a small study of adolescents from a larger trial. Compared with a group using no hormonal contraceptive, the DMPA group had a greater increase in body fat percentage and a greater decrease in lean body mass. In Pantoja 2010, a retrospective study, mean weight gain was greater for the DMPA group versus the copper IUC group at one, two, and three years. The differences were notable within the normal to lower weight group and the overweight group but not within the obese group. For Modesto 2015, also retrospective, mean weight change was greater for the DMPA group compared with the copper IUC group at 1, 4, and 10 years, the three time points we examined.
Four studies compared the LNG‐IUC with a group not using any hormonal contraceptives. Two showed the study arms differed in body composition change by one year (Table 2), though they did not differ significantly for weight change. Within Dal'Ava 2012, participants using the LNG‐IUC reportedly had a greater increase in fat mass (%) and a decrease in lean mass (%) compared with the non‐hormonal IUC users. Similarly, in Napolitano 2015, the LNG‐IUC group had a greater mean increase in fat mass (%) and a decrease in fat free mass (%) compared with the no‐hormonal group.
One of two studies that examined P‐O oral contraceptives showed an association between the OC and body composition change (Table 3). Napolitano 2015 compared use of an OC containing desogestrel 75 µg versus no hormonal contraceptive. The OC group showed the same pattern as the LNG‐IUC group noted above, i.e. a greater mean increase in fat mass (%) and a decrease in fat free mass (%).
Overall completeness and applicability of evidence
Of the 22 included studies, 18 had data from a year or more of contraceptive use and 8 of those had data from two or more years. Weight gain (or the perception of weight gain) is frequently cited as a reason for discontinuing a contraceptive method. If a contraceptive method is associated with weight gain, a year is long enough to detect some change, though the amount may not be clinically significant. Of the eight studies with data from two or more years of contraceptive use, most showed the study groups did not differ significantly for weight gain, regardless of whether the comparison group used a progestin‐only contraceptive or no hormonal method.
Within contraceptive method group, the studies varied in their comparison groups over time. Earlier DMPA studies generally compared DMPA with a hormonal contraceptive. Of the six studies added in this update, five compared DMPA with a non‐hormonal IUC. For levonorgestrel‐releasing intrauterine contraception, three of the four included studies were new. All four compared LNG‐IUC with no hormonal method; in addition, two studied DMPA and one examined a P‐O oral contraceptive. Overall, two studies of P‐O pills met our inclusion criteria. We did not find any eligible studies of the progesterone‐releasing vaginal ring.
We do not have much evidence regarding weight change with currently marketed implants. Many studies of such implants did not meet our inclusion criteria, mainly due to the lack of comparative data on weight change. Two exceptions were a recent study of the etonogestrel‐releasing implant and one that compared Norplant (six capsules) with a two‐rod implant. Most of the weight change data for implants in this review came from studies of Norplant, which is no longer marketed.
Quality of the evidence
We assessed the quality of evidence as noted earlier (Assessment of risk of bias in included studies). Table 25 has a summary based on the factors used in our assessment. We considered the overall quality of evidence to be low, given that evidence from 12 of the 22 studies was low. Three studies provided moderate quality evidence and seven had very low quality evidence. We downgraded the 17 NRS for lack of randomization and three RCTs for insufficient information on randomization and allocation concealment. Most studies had high loss to follow‐up or were retrospective studies that may not have accounted for losses to follow‐up or early discontinuation.
Potential biases in the review process
We selected studies that had data on mean change in weight or other body composition measure. Several excluded studies did not report the data we needed. For the initial review, many studies were older, which limited our ability to obtain additional information from the investigators.
Agreements and disagreements with other studies or reviews
As noted earlier, many concerns about weight gain with POC use are based on perceptions and discontinuation reasons rather than measures of actual weight change. We found limited evidence of significant change for POC users versus those who did not use hormonal contraceptives. Actual weight gain was less than 2 kg (4.4 lb) up to one year. Another review showed no clear evidence of weight gain with the use of combined hormonal contraceptives (Gallo 2014). People may gain weight over time regardless of contraceptive use.
Two studies compared perceived weight gain with actual weight gain among POC users. In a substudy of the CHOICE project, women who perceived weight gain of 5 lb or more had a mean weight change of 10 lb by 12 months, which is about 8 lb more than those who did not perceive a gain (Nault 2013). Risk of perceived gain was greater for the POC group versus the Cu IUC group. However, Vickery 2013, another substudy of CHOICE, did not show a difference in weight change by 12 months between the POC groups and the Cu IUC group. A secondary analysis of data from an RCT compared users of a two‐rod levonorgestrel implant versus women who did not yet receive the implant (Gallo 2016). Perceived weight gain was more common in the implant arm compared with the control group. Women with perceived weight gain in the implant group were more likely to have a gain of 2 kg by three months. The proportions of women who gained 2 kg did not differ significantly between the two groups nor did median weight gain.
Reviews have suggested that DMPA and weight gain may be a concern for women who are already obese, whether they are adolescents (Curtis 2009) or adults (Merki‐Feld 2015). The review with three studies on adolescents considered the quality of evidence to be fair for two studies. They lacked numbers for those discontinued due to weight gain and did not have a non‐hormonal comparison. The third study lost 37% of DMPA users by 18 months, thus producing high risk of bias. A retrospective study of adult women in this review showed that mean weight gain did not differ significantly between the DMPA and Cu IUC groups within the obese subgroup (Pantoja 2010). Within the normal and overweight subgroups though, weight gain was greater for the DMPA group. We included three studies of young women, but none with adolescents who were obese. One showed an increase in total body fat and a decrease in lean body mass for adolescents in the DMPA group compared with those in a non‐hormonal group (Bonny 2009).
A review of adverse events examined whether early weight among DMPA users was associated with later weight gain (Steenland 2013). The researchers concluded weight change greater than 5% of baseline weight was associated with greater mean change in weight or BMI at follow‐up. Two studies grouped DMPA users by early weight gain in secondary analysis from studies of bone mineral density. One study explored whether a 5% weight change in 6 months predicted weight change by 36 months (Le 2009). The original study lost 60% of DMPA users by 12 months and 76% by 36 months, leading to high risk of bias for the results. Predictors of early weight gain, among the 60% participating at six months, were a BMI < 30 and a reported increase in appetite. The second study was apparently based on a study that lost 24% by 12 months and 43% by 24 months; the report did not include the sample sizes at 6, 12, or 18 months (Bonny 2011). Of participants in the study at 12 or 18 months, baseline characteristics may have differed between those with 5% weight gain at six months and those with less weight gain.
Authors' conclusions
Implications for practice.
We found limited evidence of weight gain when using progestin‐only contraceptives. We identified some significant differences when a P‐O method was compared with no hormonal contraceptive. This includes studies of a P‐O oral contraceptive, DMPA, levonorgestrel IUC, and an older implant.
Overall, actual mean weight gain was low for 6 to 12 months, i.e. less than 2 kg (4.4 lb) in most studies. More weight gain was noted at two and three years, but the comparison groups did not differ much for weight change. People may gain weight over time regardless of contraceptive use. Appropriate and accurate counseling about typical weight gain may help reduce discontinuation of contraceptives due to perceptions of weight gain.
Implications for research.
Five studies with moderate or low quality evidence showed a significant difference between study arms. They examined DMPA, LNG‐IUC, and a P‐O pill versus no hormonal method use. All included a group not using a hormonal method. The outcomes were change in weight, percent body fat, or percent lean body mass. Three were prospective non‐randomized studies and two were retrospective chart reviews. The overall quality of evidence was low, largely due to the lack of randomization and high losses.
Weight change is rarely the focus of prospective contraceptive studies. Some of the newer prospective studies did focus on change in weight or body composition. Well‐designed RCTs assessing weight change over time would better address this issue. Comparisons could be between a P‐O method and a non‐hormonal group. However, careful counseling and follow‐up are needed to avoid the high losses to follow‐up and discontinuation found in many contraceptive studies.
What's new
| Date | Event | Description |
|---|---|---|
| 4 August 2016 | New search has been performed | Search updated |
| 3 March 2016 | New citation required but conclusions have not changed | Included 6 new studies (Dal'Ava 2014; Dos Santos 2014; Modesto 2015; Napolitano 2015; Nyirati 2013; Vickery 2013) |
History
Protocol first published: Issue 11, 2010 Review first published: Issue 4, 2011
| Date | Event | Description |
|---|---|---|
| 11 February 2016 | Amended | Added 'Summary of findings' |
| 17 December 2015 | Amended | Non‐randomized studies (NRS): updated quality assessment using Newcastle‐Ottawa Quality Assessment Scale (Data collection and analysis) Risk of bias tables: incorporated criteria for NRS and expanded for RCTs Revised quality assessment criteria (Table 25) |
| 5 June 2013 | New search has been performed | Search updated. One study was moved from 'ongoing' to Studies awaiting classification (Vickery 2013a). |
| 7 February 2013 | New citation required but conclusions have not changed | One new study included (Dal'Ava 2012). Excluded 5 studies (Bahamondes 2010; Chen 2011; Costa 2012; Kaunitz 2009; Segall‐Gutierrez 2012). Three studies added to Ongoing studies. |
| 23 January 2013 | Amended | External support added |
Acknowledgements
Carol Manion of FHI 360 developed the search strategies for several databases. For the initial review, David Grimes, formerly of FHI 360 and an author of the original review, did part of the second data extraction.
Appendices
Appendix 1. Search 2016
MEDLINE via PubMed (1 January 2013 to 4 August 2016)
(contraceptive agents, female[Mesh] OR contraceptive devices, female[Mesh] OR contracept*) AND (progest* OR "progestin only" OR "progestin only" contracept* OR "progestin only pill" OR progestin* OR progesteron* OR progestational, hormones, synthetic OR progestogen* OR medroxyprogesterone OR DMPA OR (levonorgestrel‐releasing AND intrauterine) OR "intrauterine device" OR "intrauterine contraception" OR IUD OR IUC OR (etonogestrel‐releasing AND implant) OR (ETG AND implant) OR progesterone OR gestagen OR "progestogen only") AND (body mass index OR BMI OR weight) NOT (cancer[ti] OR polycystic [ti] OR exercise [ti] OR physical activity[ti] OR postmenopaus*[ti])
CENTRAL (Cochrane Central Register of Controlled Trials 2016, issue 1 (23 February 2016))
Abstract: weight OR body mass index OR BMI AND Title, Abstract, Keywords: contraception OR contraceptive NOT Record Title: premenstrual OR dysmenor* OR endometr* OR *androgen* OR HIV OR polycystic OR PCOS OR cancer OR exercise OR anorexi* OR bulimi* NOT Record Title: postmenopausal OR post‐menopausal OR hormone therapy OR male hormonal OR male contracept* OR testosterone Publication Year from 2013 to 2015 in Trials
POPLINE (2013 to 2016 (23 February 2016))
Keyword: Body Weight AND Keyword: Contraceptive Agents Progestin
Web of Science (24 March 2016)
TOPIC:(contracept*) AND TOPIC:(overweight OR obese OR obesity OR body mass index OR BMI) NOT TITLE: (cancer OR polycystic OR PCOS OR diabetes OR exercise OR physical activity OR postmenopaus* OR hormone therapy OR replacement) Refined by: RESEARCH AREAS: ( OBSTETRICS GYNECOLOGY OR PHARMACOLOGY PHARMACY OR ENDOCRINOLOGY METABOLISM ) AND DOCUMENT TYPES: ( ARTICLE ) Timespan: 2013‐2016. Search language=Auto
LILACS via VHL Regional Portal (29 March 2016)
tw:((tw:((tw:("contraceptive agents female")) OR (tw:(contracept*)))) AND (tw:(weight OR overweight OR obes* OR "body mass index"))) AND (instance:"regional") AND ( db:("LILACS") AND limit:("female") AND year_cluster:("2012" OR "2013" OR "2014" OR "2015"))
[Note: no listing for 2016]
ClinicalTrials.gov (7 March 2016)
Search terms: overweight OR obese OR obesity OR weight OR body mass index OR BMI Condition: NOT (HIV OR polycystic OR PCOS OR cancer OR anorexia OR pulmonary OR metabolic OR amenorrhea) Intervention: contraceptive OR contraception OR contraceptives Gender: studies with female participants First received: From 1 December 2012 to 16 October 2015
ICTRP (29 March 2016)
Condition: contraceptive OR contraception AND Intervention: progestin OR progestin‐only OR IUD OR implant OR medroxyprogesterone OR norethisterone Recruitment status: All Date of registration is between 1 December 2012 and 29 March 2016
Appendix 2. Previous searches
2013
MEDLINE via PubMed (1 January 2010 to 5 June 2013)
(contraceptive agents, female[Mesh] OR contraceptive devices, female[Mesh] OR contracept*) AND (progest* OR "progestin only" OR "progestin only" contracept* OR "progestin only pill" OR progestin* OR progesteron* OR progestational, hormones, synthetic OR progestogen* OR progesterone OR gestagen OR "progestogen only") AND (body weight changes OR weight gain OR weight loss OR body mass index OR BMI OR weight) NOT (cancer[ti] OR polycystic [ti] OR exercise [ti] OR physical activity[ti] OR postmenopaus*[ti]) limited to human, female
CENTRAL (2010 to 1 June 2013)
weight OR body mass index OR BMI in Abstract AND contraception OR contraceptive in Title, Abstract or Keywords NOT premenstrual OR dysmenor* OR endometr* OR *androgen* OR HIV OR polycystic OR PCOS OR cancer OR exercise OR anorexia OR bulimic in Record Title NOT postmenopausal OR post‐menopausal OR hormone therapy OR male hormonal OR male contracept* OR testosterone in Record Title
POPLINE (2010 to 26 December 2012)
Global: weight OR BMI OR body mass index Keyword: contraceptive agents, progestin OR Low‐Dose Progestins Filter: research report
LILACS (2010 to 26 December 2012)
contraceptive agents or Agentes Anticonceptivos Femeninos or Anticoncepcionais Femininos or contraceptive devices, female or Dispositivos Anticonceptivos Femeninos or Dispositivos Anticoncepcionais Femininos or contraceptives or Anticonceptivos or Anticoncepcionais [Words] AND weight or body weight or Peso Corporal or weight gain or Aumento de Peso or Ganho de Peso or weight reduction or weight loss or Pérdida de Peso or Perda de Peso or body weight changes or Cambios en el Peso Corporal or Alterações do Peso Corporal or body mass index or BMI [Words]
ClinicalTrials.gov (1 January 2010 to 26 December 2012)
Search terms: overweight OR obese OR obesity OR weight OR body mass index OR BMI Condition: NOT (HIV OR polycystic OR PCOS OR cancer OR anorexia OR pulmonary OR metabolic OR amenorrhea) Intervention: contraceptive OR contraception Study type: interventional studies Gender: studies with female participants
ICTRP (2010 to 26 December 2012)
1) Intervention: contraceptive OR contraception
2) Condition: contraceptive OR contraception Intervention: progestin OR progestin‐only OR IUD OR implant OR medroxyprogesterone OR norethisterone
2010
MEDLINE via PubMed (through 11 June 2010)
(contraceptive agents, female[Mesh] OR contraceptive devices, female[Mesh] OR contracept*) AND (progest* OR "progestin only" OR "progestin only" contracept* OR "progestin only pill" OR progestin* OR progesteron* OR progestational, hormones, synthetic OR progestogen* OR progesterone OR gestagen OR "progestogen only") AND (body weight changes OR weight gain OR weight loss OR body mass index OR BMI OR weight) NOT (cancer[ti] OR polycystic [ti] OR exercise [ti] OR physical activity[ti] OR postmenopaus*[ti]) limited to human, female
POPLINE (through 19 May 2010)
(progestin only contracept*/ contraceptive agents, progestin/low‐dose progestins) & (weight/weight gain/weight loss/body weight/BMI/body mass index/ weight change)
CENTRAL (through 19 May 2010)
weight OR body mass index OR BMI in Abstract and contraception OR contraceptive in Title, Abstract or Keywords NOT premenstrual OR dysmenor* OR endometr* OR *androgen* OR HIV OR polycystic OR PCOS OR cancer OR exercise OR anorexia OR bulimic in Record Title NOT postmenopausal OR post‐menopausal OR hormone therapy OR male hormonal OR male contracept* OR testosterone in Record Title
EMBASE (through 15 June 2010)
(contraceptive agent, progestin ‐‐side effects or ((contraceptive device or contraceptives or contracept*) and (gestagen! or progest? or progestin? or progesterone? or progestational, hormones, synthetic or progestogen?or progestin()only or progestin()only()contracept or progestin()only()pill or progestogen()only))) and (body weight! or weight gain or weight reduction or weight()loss or body()mass()index) not (cancer or polycystic or exercise or physical() activity or postmenopaus? or oral contraceptives, combined) and body weight/de limited to human
LILACS (through 1 July 2010)
contraceptive agents or Agentes Anticonceptivos Femeninos or Anticoncepcionais Femininos or contraceptive devices, female or Dispositivos Anticonceptivos Femeninos or Dispositivos Anticoncepcionais Femininos or contraceptives or Anticonceptivos or Anticoncepcionais [Words]
and weight or body weight or Peso Corporal or weight gain or Aumento de Peso or Ganho de Peso or weight reduction or weight loss or Pérdida de Peso or Perda de Peso or body weight changes or Cambios en el Peso Corporal or Alterações do Peso Corporal or body mass index or BMI [Words]
ClinicalTrials.gov (through 27 April 2010)
Search terms: overweight OR obese OR obesity OR weight OR body mass index OR BMI Condition: NOT (HIV OR polycystic OR PCOS OR cancer OR anorexia OR pulmonary OR metabolic OR amenorrhea) Intervention: contraceptive OR contraception Study type: interventional studies Gender: studies with female participants
ICTRP (through 07 September 2010)
1) Intervention: contraceptive OR contraception
2) Condition: contraceptive OR contraception Intervention: progestin OR progestin‐only OR IUD OR implant OR medroxyprogesterone OR norethisterone
Appendix 3. Newcastle‐Ottawa Quality Assessment Scale for cohort studies
Note: A study can be awarded a maximum of one star (✸) for each numbered item within the Selection and Outcome categories. A maximum of two stars can be given for Comparability.
Selection
1) Representativeness of the exposed cohort
a) truly representative of the average _______________ (describe) in the community ✸ b) somewhat representative of the average ______________ in the community ✸ c) selected group of users eg nurses, volunteers d) no description of the derivation of the cohort
2) Selection of the non exposed cohort
a) drawn from the same community as the exposed cohort ✸ b) drawn from a different source c) no description of the derivation of the non exposed cohort
3) Ascertainment of exposure
a) secure record (eg surgical records) ✸ b) structured interview ✸ c) written self report d) no description
4) Demonstration that outcome of interest was not present at start of study
a) yes ✸ b) no
Comparability
1) Comparability of cohorts on the basis of the design or analysis
a) study controls for _____________ (select the most important factor) ✸ b) study controls for any additional factor ✸ (This criteria could be modified to indicate specific control for a second important factor.)
Outcome
1) Assessment of outcome
a) independent blind assessment ✸ b) record linkage ✸ c) self report d) no description
2) Was follow‐up long enough for outcomes to occur
a) yes (select an adequate follow up period for outcome of interest) ✸ b) no
3) Adequacy of follow up of cohorts
a) complete follow up ‐ all subjects accounted for ✸ b) subjects lost to follow up unlikely to introduce bias ‐ small number lost ‐ > ____ % (select an adequate %) follow up, or description provided of those lost) ✸ c) follow up rate < ____% (select an adequate %) and no description of those lost d) no statement
Data and analyses
Comparison 1. Norethisterone 350 µg OC versus levonorgestrel 30 µg OC.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean weight change (kg) at 6 months | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐0.6 [‐1.76, 0.56] |
Comparison 2. Desogestrel (DSG) OC versus control (no hormonal method).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean change in weight (kg) at 1 year | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐1.28, 2.28] |
| 2 Mean change in BMI (kg/m2) at 1 year | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.62, 0.62] |
| 3 Mean change in fat mass (%) at 1 year | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 3.30 [2.08, 4.52] |
| 4 Mean change in fat free mass (%) at 1 year | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | ‐3.30 [‐4.52, ‐2.08] |
2.4. Analysis.
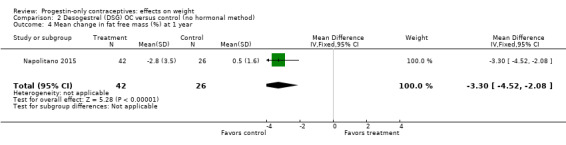
Comparison 2 Desogestrel (DSG) OC versus control (no hormonal method), Outcome 4 Mean change in fat free mass (%) at 1 year.
Comparison 3. DMPA 150 mg/mL versus COC.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight gain ≥ 1 kg at month 6 | 1 | 31 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.09, 1.67] |
| 2 Weight gain ≥ 1 kg at month 12 | 1 | 26 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.08, 2.39] |
| 3 Weight loss ≥ 1 kg at month 6 | 1 | 31 | Odds Ratio (M‐H, Fixed, 95% CI) | 9.24 [0.44, 195.69] |
| 4 Weight loss ≥ 1 kg at month 12 | 1 | 26 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.83 [0.15, 23.15] |
| 5 Mean weight change (kg) at 6 months | 1 | 142 | Mean Difference (IV, Fixed, 95% CI) | ‐0.50 [‐2.26, 1.26] |
| 6 Mean weight change (kg) at 12 months | 1 | 81 | Mean Difference (IV, Fixed, 95% CI) | 0.7 [‐1.92, 3.32] |
| 7 Mean percentage weight change at 6 months | 1 | 142 | Mean Difference (IV, Fixed, 95% CI) | ‐0.7 [‐3.10, 1.70] |
| 8 Mean percentage weight change at 12 months | 1 | 81 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐3.79, 3.79] |
3.2. Analysis.
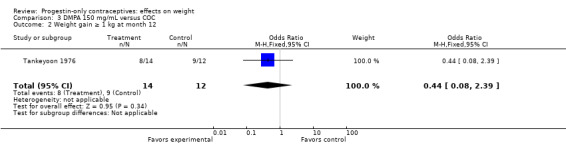
Comparison 3 DMPA 150 mg/mL versus COC, Outcome 2 Weight gain ≥ 1 kg at month 12.
3.3. Analysis.
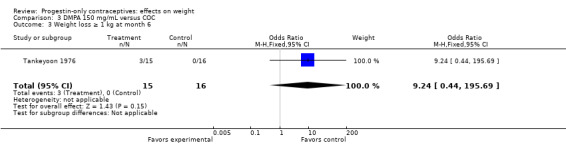
Comparison 3 DMPA 150 mg/mL versus COC, Outcome 3 Weight loss ≥ 1 kg at month 6.
3.6. Analysis.
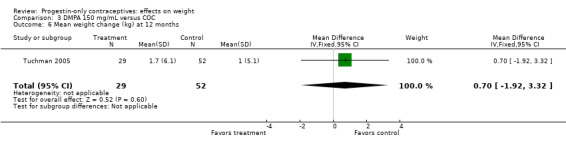
Comparison 3 DMPA 150 mg/mL versus COC, Outcome 6 Mean weight change (kg) at 12 months.
3.7. Analysis.
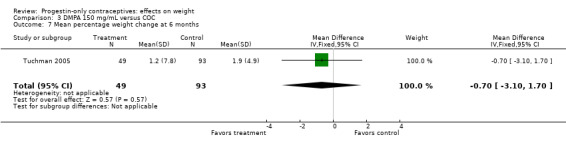
Comparison 3 DMPA 150 mg/mL versus COC, Outcome 7 Mean percentage weight change at 6 months.
Comparison 4. DMPA 150 mg/mL versus MPA + E2C.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean weight change (kg) at 6 months | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐0.6 [‐3.05, 1.85] |
| 2 Mean weight change (kg) at 12 months | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | ‐1.3 [‐6.37, 3.77] |
| 3 Mean percentage weight change at 6 months | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐4.04, 2.84] |
| 4 Mean percentage weight change at 12 months | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐7.58, 6.18] |
4.2. Analysis.
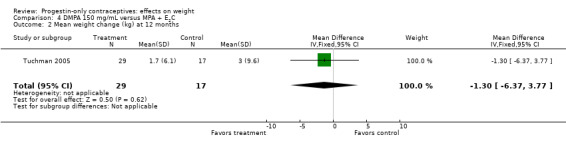
Comparison 4 DMPA 150 mg/mL versus MPA + E2C, Outcome 2 Mean weight change (kg) at 12 months.
4.3. Analysis.
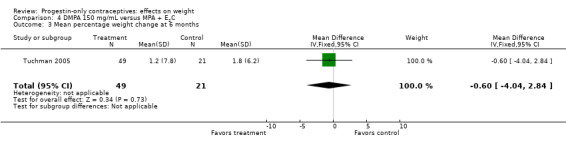
Comparison 4 DMPA 150 mg/mL versus MPA + E2C, Outcome 3 Mean percentage weight change at 6 months.
Comparison 5. DMPA 150 mg/mL + placebo versus DMPA 150 mg/mL + E2C.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean change in total body fat (%) at 6 months | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | 7.50 [‐0.47, 15.47] |
| 2 Mean change in lean body mass (%) at 6 months | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | ‐2.2 [‐3.00, 0.60] |
Comparison 6. DMPA 150 mg/mL versus DMPA 450 mg/mL.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean weight change (kg) at 6 months | 1 | 651 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.48, 0.50] |
Comparison 7. DMPA 150 mg/mL initiation after pregnancy: interval (≥ 20 weeks) versus postpartum (5 to 8 weeks).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean weight gain (lb) at 1 year | 1 | 172 | Mean Difference (IV, Fixed, 95% CI) | 2.30 [‐0.94, 5.54] |
| 2 Mean weight gain (lb) at 2 years | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | 1.60 [‐4.79, 7.99] |
Comparison 8. DMPA‐IM 150 mg versus DMPA‐SC 104 mg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean weight change (kg) at 36 months | 1 | 121 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [‐1.78, 4.38] |
Comparison 9. DMPA 150 mg/mL versus NET‐EN 200 mg (60‐day intervals).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean weight change (kg) at 12 months | 1 | 1162 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.63, 1.03] |
| 2 Mean weight change (kg) at 24 months | 1 | 604 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.39, 1.39] |
| 3 Mean weight change at 1 year | 1 | 201 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐0.10, 1.70] |
Comparison 10. NET‐EN 200 mg: 60‐day intervals versus 3 intervals of 60 days then 84‐day intervals.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean weight change (kg) at 12 months | 1 | 822 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.71, 0.71] |
| 2 Mean weight change (kg) at 24 months | 1 | 453 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐1.35, 1.15] |
Comparison 11. DMPA 150 mg/mL versus Cu IUC.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean weight change (kg) at 10 years | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.83, 0.23] |
| 2 Mean weight change (kg) at 1 year by baseline BMI | 1 | 758 | Mean Difference (IV, Fixed, 95% CI) | 2.28 [1.79, 2.77] |
| 2.1 BMI < 25 kg/m2 | 1 | 452 | Mean Difference (IV, Fixed, 95% CI) | 2.5 [1.90, 3.10] |
| 2.2 BMI 25 to 29.9 kg/m2 | 1 | 218 | Mean Difference (IV, Fixed, 95% CI) | 2.10 [1.16, 3.04] |
| 2.3 BMI ≥ 30 kg/m2 | 1 | 88 | Mean Difference (IV, Fixed, 95% CI) | 0.7 [‐1.26, 2.66] |
| 3 Mean weight change (kg) at 2 years by baseline BMI | 1 | 758 | Mean Difference (IV, Fixed, 95% CI) | 2.71 [2.12, 3.30] |
| 3.1 BMI < 25 kg/m2 | 1 | 452 | Mean Difference (IV, Fixed, 95% CI) | 2.7 [2.02, 3.38] |
| 3.2 BMI 25 to 29.9 kg/m2 | 1 | 218 | Mean Difference (IV, Fixed, 95% CI) | 3.0 [1.73, 4.27] |
| 3.3 BMI ≥ 30 kg/m2 | 1 | 88 | Mean Difference (IV, Fixed, 95% CI) | 1.5 [‐1.33, 4.33] |
| 4 Mean weight change (kg) at 3 years by baseline BMI | 1 | 758 | Mean Difference (IV, Fixed, 95% CI) | 3.17 [2.51, 3.83] |
| 4.1 BMI < 25 kg/m2 | 1 | 452 | Mean Difference (IV, Fixed, 95% CI) | 3.3 [2.52, 4.08] |
| 4.2 BMI 25 to 29.9 kg/m2 | 1 | 218 | Mean Difference (IV, Fixed, 95% CI) | 3.20 [1.82, 4.58] |
| 4.3 BMI ≥ 30 kg/m2 | 1 | 88 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [‐1.56, 4.16] |
| 5 Mean weight change (kg) by year | Other data | No numeric data | ||
| 6 Mean changes in body composition by 12 months | Other data | No numeric data |
Comparison 12. DMPA, ENG implant, or LNG‐IUC versus Cu IUC.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean weight change (kg) at 1 year | Other data | No numeric data | ||
| 2 Mean weight change (kg) at 4 years | Other data | No numeric data | ||
| 3 Mean weight change (kg) at 10 years | Other data | No numeric data | ||
| 4 Weight change (kg) at 12 months | Other data | No numeric data |
Comparison 13. DMPA 150 mg/mL versus no hormonal method.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean change in total body fat (%) at 6 months | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 11.0 [2.64, 19.36] |
| 2 Mean change in lean body mass (%) at 6 months | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐4.0 [‐6.93, ‐1.07] |
| 3 Mean change in weight (lb) at 12 months postpartum | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | 1.54 [‐8.39, 11.47] |
| 4 Mean change in BMI (kg/m2) at 12 months postpartum | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | 0.32 [‐1.40, 2.04] |
| 5 Mean change in body fat (%) at 12 months postpartum | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | 0.98 [‐2.39, 4.35] |
13.4. Analysis.
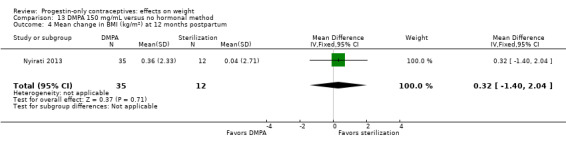
Comparison 13 DMPA 150 mg/mL versus no hormonal method, Outcome 4 Mean change in BMI (kg/m2) at 12 months postpartum.
Comparison 14. Norplant versus non‐hormonal IUC.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean weight change (kg) at 6 months | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | 0.47 [0.29, 0.65] |
| 2 Mean weight change (kg) at 1 year | 1 | 324 | Mean Difference (IV, Fixed, 95% CI) | 1.1 [0.36, 1.84] |
| 3 Mean weight change (kg) at 3 years | 1 | 190 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐0.39, 2.19] |
Comparison 15. Norplant versus barrier, 'local', or no contraceptive method.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean weight change (kg) at 6 months | 1 | 97 | Mean Difference (IV, Fixed, 95% CI) | 0.74 [0.52, 0.96] |
Comparison 16. Norplant versus DMPA 150 mg/mL.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean weight change (kg) at 1 year | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐0.87 [‐1.86, 0.12] |
Comparison 17. Norplant versus 2‐rod LNG.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean weight change (kg) at 1 year | 1 | 1196 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.33, 0.51] |
| 2 Mean weight change (kg) at 3 years | 1 | 922 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.60, 0.60] |
| 3 Mean weight change (kg) at 5 years | 1 | 614 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐0.13, 1.33] |
Comparison 18. Norplant versus COC.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean weight change (kg) at 1 year | 1 | 226 | Mean Difference (IV, Fixed, 95% CI) | 1.1 [‐0.13, 2.33] |
Comparison 19. LNG‐IUC versus no hormonal contraceptive.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean changes in body composition by 12 months | Other data | No numeric data | ||
| 2 Mean change in weight (kg) at 1 year | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.8 [‐0.75, 2.35] |
| 3 Mean change in BMI (kg/m2) at 1 year | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.3 [‐0.75, 0.15] |
| 4 Mean change in fat mass (%) at 1 year | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.6 [0.45, 2.75] |
| 5 Mean change in fat free mass (%) at 1 year | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐1.6 [‐2.75, ‐0.45] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ball 1991.
| Methods | Design: randomized controlled trial Location: likely in Oxford, England Time frame: no information Sample size estimation and outcome of focus: no information |
|
| Participants | 51 women, 17 to 41 years old, requesting oral contraceptives (OC) Inclusion criteria: new OC users had not used OC or hormone therapy for 3 months; switchers were changing from low‐dose combined OC Exclusion criteria: hypertension (diastolic blood pressure (BP) > 100 mm Hg, systolic BP > 140 mm Hg); smoking > 20 cigarettes/day; diabetes |
|
| Interventions | Progestin‐only pills 1) Norethisterone (NET) 350 µg (N = 23) 2) Levonorgestrel (LNG) 30 µg (N = 23) 6 treatment cycles |
|
| Outcomes | Primary: mean change in weight; lipoprotein levels, glucose tolerance, coagulation factors, and blood pressure Time frame: 6 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information other than stratified according to prior OC use |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Single‐blind (unspecified) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information; objective outcome measure |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up: 24% (12/51); analysis for weight included 39 women at 6 months (23 NET; 16 LNG) 5 did not return for follow‐up and were excluded (groups not specified); 9 withdrew after 3 months (1 NET; 8 LNG) |
Bonny 2009.
| Methods | Design: prospective study; part of larger 2‐year study that examined hormonal contraception and bone mineral density Location: 4 urban adolescent health clinics in large metropolitan area, likely Cleveland OH (USA) Time frame: enrollment 2002 to 2003 Sample size estimation and outcome of focus: no information |
|
| Participants | Postmenarchal girls 12 to 18 years of age Inclusion criteria: requesting contraception and selecting DMPA or OC; those who did not want hormonal contraception were eligible for control group Exclusion criteria: pregnancy or DMPA use in past 6 months; OC use in past 3 months; alcohol or drug dependence; medical condition (e.g. renal disease) or medication use (e.g. corticosteroids) associated with outcomes of interest; contraindication to estrogen use; weight > 250 lb (upper limit for dual energy x‐ray absorptiometry [DEXA] scanner); need for confidential contraceptive care |
|
| Interventions | Choice of study group
1) Depot medroxyprogesterone acetate (DMPA) (N = 15): randomized to additional monthly injections of placebo (N = 8) or estradiol cypionate 5 mg (E2C) (N = 7)
2) Control (no hormonal contraception) (N = 18) Third group chose OCs (N = 18); not included here. Type of OC not specified; may have included progestin‐only OCs and combination OCs. |
|
| Outcomes | Percent change in total body fat and in lean body mass Follow‐up: 6 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | NRS: participants chose contraceptive method |
| NOS selection (NRS) | Low risk | Exposed: clinic population; volunteers for study Non‐exposed: same population as exposed but chose different contraceptive Exposure: adherence to DMPA appointments |
| NOS comparability (NRS) | Low risk | Analysis: multivariate models for change in total body fat and change in lean body mass; adjusted for potential confounders, e.g. age, race or ethnicity, caloric intake |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to women having chosen contraceptive method |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information; objective outcome measure |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up: unknown; analysis table does not specify N To remain in study, participants had to adhere to DMPA by appointment. No information on controls. |
Castle 1978.
| Methods | Design: prospective study Location: family planning center in Salisbury, Rhodesia Time frame: enrolment June to December 1976 Sample size estimation and outcome of focus: no information |
|
| Participants | 1000 women; age range: "under 20" to 40 years or older Inclusion criteria: Black women seeking contraception at specific clinic Exclusion criteria: no mention |
|
| Interventions | 1) DMPA 150 mg every 3 months (N = 500) 2) DMPA 450 mg every 6 months (N = 500) | |
| Outcomes | Mean increase in weight Timeframe: 6 months Weight measured at each visit while participant wore only a gown |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | NRS; allocation by participant request |
| NOS selection (NRS) | Low risk | Exposed: clinic population; volunteers for study Non‐exposed: same population as exposed (different DMPA) Exposure: injections given during clinic appointments |
| NOS comparability (NRS) | High risk | Analysis: no adjustment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to women having chosen contraceptive method |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information; objective outcome measure |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up: 39% DMPA 150 mg and 23% DMPA 450 mg; differential losses between groups Withdrawals from study: 21 for DMPA 150 mg and 17 for DMPA 450 mg |
Dal'Ava 2012.
| Methods | Design: prospective study Location: single site in Campinas, Brazil Time frame: enrolled October 2009 to May 2010 Sample size estimation and outcome of focus: based on weight gain in LNG‐IUC users from previous study; 37 needed for each group |
|
| Participants | 76 women Inclusion criteria: 18 to 45 years of age; initiated contraceptive use (either LNG‐IUC or Cu T380A IUC) Exclusion criteria: currently breastfeeding or breastfeeding during 6 months before enrollment; used corticosteroids, thiazide diuretics, or drugs for treatment of thyroid disease; eating disorder; chronic disease |
|
| Interventions | 1) LNG‐IUC (N = 38) 2) Cu T380A IUC (N = 38) |
|
| Outcomes | Change in weight (kg); percent change in total fat mass, total lean mass, central‐peripheral fat ratio Follow‐up: 12 months |
|
| Notes | Limitations: physical activity and daily caloric intake were not monitored during study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Women apparently chose contraceptive method |
| NOS selection (NRS) | Low risk | Exposed: study volunteers; recruitment methods not specified unclear if they were clinic attendees Non‐exposed: same population as exposed but chose non‐hormonal IUC Exposure: clinic inserted IUC |
| NOS comparability (NRS) | Low risk | Design: paired by age (± 2 years) and BMI (± 2 kg/m2) for intervention groups Analysis: no mention of using control variables in analysis; listed sociodemographic variables; obstetric and gynaecologic history; physical activity; consumption of coffee, alcohol, milk; smoking; family history of osteoporosis |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to women having chosen contraceptive method |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information; objective outcome measure |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: no discontinuation at 12 months |
Dal'Ava 2014.
| Methods | Design: prospective matched Location: single site in Campinas, Brazil Time frame: enrolled October 2009 to June 2011 Sample size estimation and outcome of focus: reported change in baseline percentage of body fat 12 months after initiation of contraception (primary outcome), 1.75% increase in DMPA users vs 0.31% reduction in Cu IUC users; required 20 complete cases in each group, using repeated measures ANOVA with 5% significance level and 80% power |
|
| Participants | 97 women Inclusion criteria: 18 to 50 years of age; new DMPA or Cu IUC users Exclusion criteria: breastfeeding; use of DMPA in prior 6 months; history of diabetes, pituitary disorder, liver or kidney disease, cancer; use of corticosteroids, diuretics, hormone therapy; eating disorder |
|
| Interventions | 1) DMPA 150 mg (N = 55) 2) Cu T380A IUC (N = 42) |
|
| Outcomes | Change in weight (kg), fat mass (kg), and lean mass (kg) Follow‐up: 12 months |
|
| Notes | Women using Cu IUC may overlap with those in Dal'Ava 2012; study periods overlap and inclusion criteria are similar. Unable to obtain further information from investigator. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants chose method |
| NOS selection (NRS) | Low risk | Exposed: volunteered for study; unclear if they were clinic attendees Non‐exposed: same population as exposed but chose different contraceptive method Exposure: clinic records (inserted IUC and administered DMPA) |
| NOS comparability (NRS) | Low risk | Design: paired by age (± 2 years) and weight (± 2 kg) for intervention groups Analysis: control variables of physical activity, consumption of coffee and alcohol, smoking in regression; reportedly not significant |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to women having chosen contraceptive method |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention; objective outcome measure |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up and discontinuation: DMPA 53% (29/55); IUC 24% (10/42) From IUC group, only 26 of 32 matched with 26 in DMPA group Major differential losses between groups |
Dos Santos 2014.
| Methods | Design: prospective matched Location: Campinas, Brazil Time frame: enrolled February 2011 to February 2013 Sample size estimation and outcome of focus: none; exploratory study |
|
| Participants | 71 women Inclusion criteria: age 18 to 40 years; BMI < 30; never used DMPA; fasting glucose < 100 mg/dL and glucose level < 140 mg/dL at 120 minutes after 75 g oral glucose load Exclusion criteria: breastfeeding; family history of first‐degree relative with diabetes mellitus; type 1 or 2 diabetes, metabolic syndrome, hypertension, hyper‐ or hypothyroidism, chronic renal failure, hirsutism or hyperandrogenism, polycystic ovary syndrome or acanthosis nigricans; transplant recipient; had undergone bariatric surgery |
|
| Interventions | 1) DMPA 150 mg intramuscular (IM) (N = 44) 2) Cu T380A IUC (N = 27) |
|
| Outcomes | Change in body weight, total fat mass, percent body fat, and total lean mass; change in bone mineral density Follow‐up: 12 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Women chose method |
| NOS selection (NRS) | Low risk | Exposed: clinic population, volunteered for study Non‐exposed: same population as exposed but chose different contraceptive Exposure: clinic records; clinic inserted IUC and administered DMPA |
| NOS comparability (NRS) | Low risk | Design: paired for age (± 1 year) and BMI (± 1 kg/m2) Analysis: multiple linear regression included age, weight, BMI, schooling, skin color, social class, pregnancies, deliveries, smoking, alcohol, coffee (secondary report Modesto 2014) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to women having chosen contraceptive method |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention; objective outcome measures |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up and discontinuation: overall 24% (17/71); DMPA 34% (15/44) and CU IUC 7% (2/27) Major differential losses across groups |
Espey 2000.
| Methods | Design: retrospective chart review Location: 3 Indian Health Service facilities in southwestern USA Time frame: first injection of DMPA from December 1992 to June 1995 Sample size estimation and outcome of focus: no information |
|
| Participants | Female members of Navajo tribe Inclusion criteria: 18 to 40 years old; completed at least 5 consecutive injections of DMPA at 10‐ to 14‐week intervals; had weights recorded at 1‐ or 2‐year intervals Exclusion criteria: history of diabetes or thyroid disease; women in postpartum group who had pre‐eclampsia or multiple gestations within index pregnancy |
|
| Interventions | DMPA initiation 1) Interval (N = 115): first injection ≥ 20 weeks past pregnancy of ≥ 20 weeks gestation 2) Postpartum (N = 57): first injection at 5 to 8 weeks after delivery of singleton pregnancy of ≥ 20 weeks gestation | |
| Outcomes | Mean weight gain (lb) for DMPA by initiation group Time frame: 1 and 2 years |
|
| Notes | For another group, not included in this review, investigators reportedly extracted method of contraception from charts but did not provide specifics. Discussion noted that group "more frequently used" IUC or tubal ligation and included COC users. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | DMPA users by choice |
| NOS selection (NRS) | Low risk | Exposed: clinic population of Navajo women Non‐exposed: same population as exposed Exposure: clinic records of DMPA injections |
| NOS comparability (NRS) | Low risk | Analysis: adjusted for age, parity, and initial weight |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to differences in insertion times |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information; objective outcome measure |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up: for 1 year, only complete records included in retrospective review; does not account for discontinuation and loss; at 2 years, no weight data for 70% of interval group and 49% of postpartum group |
Modesto 2015.
| Methods | Design: retrospective chart review Location: Campinas, Brazil Time frame: 1990 to 2010 Sample size estimation and outcome of focus: based on difference in weight between DMPA users and non‐users; for alpha 5% and beta error 20% using repeated measures ANOVA, 700 required for each group |
|
| Participants | 2138 women Inclusion criteria: 18 to 40 years of age; started using DMPA, Cu IUC or LNG‐IUC in 1990 and used uninterruptedly for 10 years; Cu IUC and LNG‐IUC users monitored annually at clinic and DMPA users attended clinic every 90 days for injection Exclusion criteria: chronic medical disease including dyslipidemia, diabetes, thyroid disease, and renal failure; history of bariatric surgery or organ transplant; LNG‐IUC use for other than contraception |
|
| Interventions | 1) DMPA 150 mg IM (N = 714) 2) Cu IUC (N = 723) 3) LNG‐IUC (N = 701) |
|
| Outcomes | Mean weight gain (kg) Time frame: 1 year and 10 years |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Women chose method; charts reviewed until 700 met eligibility criteria for each group |
| NOS selection (NRS) | Low risk | Exposed: clinic population, using contraceptive method for 10 years, beginning in 1990 Non‐exposed: same population as exposed but chose different contraceptive Exposure: clinic records (clinic inserted IUC and administered DMPA) |
| NOS comparability (NRS) | Low risk | Analysis: generalized linear mixed model adjusted for years of school and number of children |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to women having chosen contraceptive method |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention; objective outcome measure |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up: retrospective review of charts with relevant data; does not account for discontinuation and loss Overall loss: at 1 year 12% (DMPA 5%, LNG‐IUC 14%, Cu IUC 17%); at 4 years 19% (DMPA 24%, LNG‐IUC 20%, Cu IUC 14%); at 10 years 84% (DMPA 82%, LNG‐IUC 90%, Cu IUC 79%) |
Moore 1995.
| Methods | Design: retrospective chart review Location: rural obstetrics and gynecology clinic in Arizona (USA) Time frame: no information Sample size estimation and outcome of focus: none; selected 50 women in each group who met inclusion criteria |
|
| Participants | 150 women, 15 to 30 years old Inclusion criteria: users of OCs, Norplant, or DMPA Exclusion criteria: prior hormonal contraceptive therapy; height < 62 inches (152.4 cm) or > 70 inches (177.8 cm); weight < 100 lb (45.5 kg) or > 180 lb (81.8 kg); presence of diabetes; history of thyroid disease; < 12 months postpartum |
|
| Interventions | 1) Norplant (N = 50)
2) DMPA 150 mg (N = 50) Third group of OC users excluded from this review; type of OC not specified and may have included progestin‐only OCs and combination OCs |
|
| Outcomes | Weight gain (kg) Timeframe: 1 year |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Women presumably chose contraceptive method; charts reviewed until 50 met eligibility criteria for each group |
| NOS selection (NRS) | Low risk | Exposed: rural clinic population Non‐exposed: same population as exposed but used different contraceptive Exposure: clinic records |
| NOS comparability (NRS) | Low risk | Analysis: model adjusted for age, height, weight, and parity at beginning of study period |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible because women presumably chose contraceptive method |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information; objective outcome measure |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up: retrospective review of charts with relevant data; does not account for discontinuation and loss |
Napolitano 2015.
| Methods | Design: prospective study Location: menopause clinic in Italy Time frame: enrolled January 2011 to January 2014 Sample size estimation and outcome of focus: based on prior experience, 11 subjects needed per group to document between‐group difference in fat mass (FM) of 1.2 kg ± 0.6; no power analysis reported |
|
| Participants | 110 women Inclusion criteria: perimenopausal based on serum FSH > 15 IU/ml and irregular menstrual cycles or amenorrhea < 3 months (aged 45 to 55 years) Exclusion criteria: current hormone use; sterilization; BMI < 18 or > 30; vascular disease or coagulation disorder; hypersensitivity to study drug ingredient; thyroid dysfunction; fasting glucose > 110 mg/dl; breast or gynecologic disease |
|
| Interventions | 1) Desogestrel (DSG) 75 µg OC (N = 44) 2) LNG‐IUC (N = 35) 3) Control, no contraception (N = 31) |
|
| Outcomes | Change in weight (kg), BMI, fat mass (%), fat free mass (%), waist (cm), waist to hip ratio, resting metabolic rate (kJ/24 h) Follow‐up: 12 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Women chose method |
| NOS selection (NRS) | Low risk | Exposed: menopause clinic population, volunteered for study Non‐exposed: same population as exposed Exposure: clinic records (clinic inserted IUC) |
| NOS comparability (NRS) | High risk | Analysis: unadjusted |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to women having chosen contraceptive method |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention; objective outcome measure |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up or discontinuation: 7% (8/110); DSG OC 4.5% (2/44), LNG‐IUC 3% (1/35), and control 16% (5/31); control had 2 lost to follow‐up (6%) |
Nyirati 2013.
| Methods | Design: prospective study using convenience sample Location: large Midwestern city, possibly Columbus OH (USA) Time frame: 18‐month period, specific dates not given Sample size estimation and outcome of focus: not reported; body composition changes at 1 year postpartum |
|
| Participants | 78 postpartum women Inclusion criteria: ≥ 18 years of age; elected to use DMPA or surgical sterilization Exclusion criteria: hormone replacement therapy, e.g. OC, thyroid hormone, steroid therapy; significant prenatal and postpartum medical illness including gestational or type II diabetes; BMI > 35 |
|
| Interventions | 1) DMPA 150 mg every 12 weeks, beginning at 6 weeks postpartum (N = 61) 2) Surgical sterilization and no other contraceptive (N = 17) |
|
| Outcomes | Change in weight (lb), BMI, fat %, body dimensions (fat folds and circumferences) from 6 weeks to 1 year postpartum Follow‐up: every 3 months for 1 year |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Women chose method |
| NOS selection (NRS) | Low risk | Exposed: clinic population; postpartum women Non‐exposed: same population as exposed but chose different contraceptive Exposure: clinic records (clinic administered DMPA) |
| NOS comparability (NRS) | High risk | Analysis: no adjustment for potential confounding of outcome measures |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to women having chosen contraceptive method |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention; objective outcome measure |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up or discontinuation: DMPA 43% (26/61); sterilization 29% (5/17) |
Pantoja 2010.
| Methods | Design: retrospective chart review Location: university department of obstetrics and gynecology in Campina, Brazil Time frame: chart data from January 1991 to December 2000 Sample size estimation and outcome of focus: difference in weight between DMPA users and nonusers using analysis of variance for repeat measures; based on mean increase in fat mass in users, 150 DMPA users estimated; significance 5% and power 80% |
|
| Participants | Women who accepted contraceptive method Inclusion criteria: chose DMPA and used continuously ≥ 3 years or who used Cu T380A for similar time period (mean age 29 years) Exclusion criteria: diabetes mellitus; hyperthyroidism or hypothyroidism; chronic renal failure; rheumatic diseases requiring chronic use of corticoids; organ transplant |
|
| Interventions | 1) DMPA (N = 379)
2) Cu T380A IUC (N = 379) After pairing for age and baseline BMI, 379 for each contraceptive group |
|
| Outcomes | Change in weight (kg) by contraceptive group and by baseline BMI (kg/m2) (< 25; 25 to 29.9; ≥ 30) Time frame: 1, 2, and 3 years Weight and height measured at baseline (method initiation) and annually. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Women chose contraceptive method |
| NOS selection (NRS) | Low risk | Exposed: clinic attendees Non‐exposed: same population as controls but chose different contraceptive Exposure: from clinic records |
| NOS comparability (NRS) | Low risk | Design: paired for age and baseline BMI |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to women having chosen contraceptive method |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information; objective outcome measure |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up: chart review of those with 3 years continuous use; does not account for discontinuation and loss |
Salem 1984.
| Methods | Design: prospective study; focused on effect of Norplant use on lactating women and on lactation performance and infant growth Location: postpartum clinic of university hospital in Assiut, Egypt Time frame: no information Sample size estimation and outcome of focus: no information; recruited 50 women for each group |
|
| Participants | 150 lactating women; mean age 29 years Inclusion criteria: normal delivery of normal living baby and exclusively breastfeeding; 1 month after delivery; infant weight ≥ 3500 gm Exclusion criteria: no mention |
|
| Interventions | Acceptors (50 in each group) 1) Norplant 2) Barrier, 'local' or no contraceptive method 3) Cu T380A IUC | |
| Outcomes | Weight gain by study group Follow‐up: 6 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Women apparently chose contraceptive method |
| NOS selection (NRS) | Low risk | Exposed: attendees at postpartum clinic; volunteered to participate Non‐exposed: same population and timeframe as controls but chose different contraceptive Exposure: obtained during monthly follow‐up visits |
| NOS comparability (NRS) | High risk | Analysis: no adjustment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to women having chosen contraceptive method |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information; objective outcome measure |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: only those excluded (1 Norplant group, lost baby and wanted to get pregnant; 2 pregnancies in group with barrier, 'local', or no contraceptive) |
Salem 1988.
| Methods | Design: randomized controlled trial; examined performance of 2 injectables regarding side effects, continuation, and termination. Location: family planning center in Assiut, Egypt Time frame: no information Sample size estimation and outcome of focus: no information |
|
| Participants | 400 women attending family planning clinic Inclusion criteria: 18 to 40 years old; proven fertility and frequent risk of pregnancy; regular menstrual cycles; willing to rely on one method Exclusion criteria: breast‐feeding; cardiovascular disease; liver disease; known or suspected breast malignancy, genital malignancy, uterine fibroids; undiagnosed vaginal bleeding; suspected pregnancy |
|
| Interventions | 200 in each group 1) DMPA 150 mg every 3 months 2) Norethisterone enanthate (NET‐EN) 200 mg every 2 months | |
| Outcomes | Mean change in weight by contraceptive group; units not specified (kg or lb) Follow‐up: 1 year Report had mean change for those with increase, decrease, or no change in weight. We calculated combined weight change means and standard deviations. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table prepared by WHO |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes contained assignments |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to different injection schedules |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information; objective outcome measure |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up: reportedly 19% for DMPA and 13.3% for NET‐EN
One‐year method continuation rates: 68.8% DMPA and 57.1% NET‐EN Finished study: 54% DMPA and 47% NET‐EN |
Sivin 1998.
| Methods | Design: randomized controlled trial; focused on effectiveness of reformulated 2‐rod LNG implant versus 6‐rod implant Location: 7 centers including USA and Finland Time frame: enrollment 1990 to 1994 Sample size estimation and outcome of focus: assumed 50/100 acceptors would continue; pregnancy rate 2.0/100 at 3 years with SE 0.66/100; sample size could distinguish difference in pregnancy of 2/100 between 2 implant types |
|
| Participants | 1200 healthy women, 18 to 40 years old, who sought implant contraception Inclusion criteria: no contraindication to implant use; willing to undergo study procedures Exclusion criteria: cancer; severe cardiovascular problem; hyperlipidemia; diabetes mellitus; mental illness; epilepsy; severe or frequent headaches; undiagnosed genital bleeding; hyperprolactinemia or bloody breast discharge; pelvic inflammatory disease since last pregnancy or ectopic pregnancy |
|
| Interventions | Levonorgestrel implants 1) Norplant: 6 capsules containing levonorgestrel 216 mg (total) 2) LNG rod (Jadelle): 2 rods containing levonorgestrel 150 mg (total); different elastomer in core than earlier implant Follow‐up: 1, 3, 6 months; then semi‐annually to 5 years | |
| Outcomes | Mean weight change by implant group
Weight change for 10th, 50th, and 90th percentiles of body weight (at admission) Follow‐up: 5 years Weighing method not specified |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization by "linear congruential method"; blocks of 50 per clinic |
| Allocation concealment (selection bias) | Low risk | Implants in sealed opaque envelopes numbered sequentially according to randomization lists |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to apparent differences in interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information; objective outcome measures |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up: year 3, 2.7% each group; year 5, 7.2% LNG rod and 10.2% Norplant Discontinuation: end of year 3, LNG rod 31.6% (190/600) and Norplant 31.2% (187/598); end of year 5, LNG rod 54.7% (328/600) and Norplant 60% (359/598) 2 sets of Norplant contaminated and not used (1198/2000 analyzed) |
Sule 2005.
| Methods | Design: retrospective chart review Study examined hormonal contraceptives and weight changes. Location: family planning clinic of university hospital in Zaria, Nigeria Time frame: registered from 01 January 1993 to 31 December 1995 Sample size estimation and outcome of focus: no information |
|
| Participants | 516 new clients Inclusion criteria: used hormonal contraceptive (COC, injectable (DMPA or NET‐EN), Norplant); non‐hormonal IUC users as controls; followed for ≥ 1 year (mean age hormonal users 30.5 years and non‐hormonal IUD 29.1 years) Exclusion criteria: used barrier methods; had bilateral tubal ligation; chose no contraceptive method |
|
| Interventions | Method users 1) Norplant (N = 188) 2) non‐hormonal IUC (N = 136) | |
| Outcomes | Mean weight gain or loss by contraceptive group Time frame: 1 and 3 years Report had mean change for those with increase, decrease, or no change in weight. We calculated combined weight change means and standard deviations. |
|
| Notes | Injectable users not used in this review; DMPA and NET‐EN had been grouped for analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Users of contraceptive method; women presumably chose method |
| NOS selection (NRS) | Low risk | Exposed: clinic attendees Non‐exposed: same population as exposed but chose different contraceptive Exposure: clinic records |
| NOS comparability (NRS) | High risk | Analysis: no adjustment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to women presumably having chosen method |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information; objective outcome measure |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up: investigators selected charts with ≥ 1 year of data, so no loss by 1 year; by 3 years, overall loss by 3 years of 54% (Norplant 31%; COC 95%; IUC 56%) |
Taneepanichskul 1998.
| Methods | Design: retrospective study examined weight change in long‐term users of DMPA versus IUC Location: family planning clinic at a hospital in Bangkok, Thailand Time frame: no information Sample size estimation and outcome of focus: no information |
|
| Participants | 100 women, age 37 to 50 years, attending family planning clinic Inclusion criteria: used DMPA or IUC for 120 months (10 years); followed "regularly"; no history of smoking or alcohol intake IUC users had not used any hormonal contraceptive Exclusion criteria: developed chronic disease or metabolic disorder during DMPA or IUC use |
|
| Interventions | Method chosen 1) DMPA (N = 50) 2) Cu T380A IUC (N = 50) | |
| Outcomes | Mean change in body weight Time frame: 120 months Weight measured in standard manner at 120 months; prior method not specified. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Users of DMPA "recruited randomly"; users of Cu T380A selected as controls |
| NOS selection (NRS) | High risk | Exposed: clinic population, older contraceptive users; used method for 10 years and regularly attended clinic Non‐exposed: same as exposed group but chose different contraceptive Exposure: presumably from clinic records |
| NOS comparability (NRS) | Low risk | Design: matched for age, parity, income, weight |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to women presumably having chosen method |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information; objective outcome measure |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up: retrospective study of women who used method for 10 years and regularly attended clinic; may have recruited women with relevant data in charts Exclusions: developed chronic disease or disorder during method use; may have biased results because weight gain is associated with development of some diseases and disorders |
Tankeyoon 1976.
| Methods | Design: prospective metabolic study; focused on metabolic effects of contraceptive methods Location: Bangkok, Thailand Time frame: no information Sample size estimation and outcome of focus: no sample size calculation; focused on metabolic effects of contraceptive methods |
|
| Participants | 32 healthy women attending the family planning clinic; age 18 to 38 years Inclusion criteria: > 6 weeks postpartum and no other steroid use for past 3 months Exclusion criteria: no information |
|
| Interventions | 1) DMPA 150 mg (3‐month intervals) (N = 16) 2) COC: d‐norgestrel 50 µg + EE 50 µg (N = 16) | |
| Outcomes | Percent of cases with ≥ 1 kg increase or decrease in body weight by contraceptive method Follow‐up: 1, 2, 3, 6, 9, 12 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Women receiving contraceptive method; presumably chose method |
| NOS selection (NRS) | Low risk | Exposed: clinic population, volunteered for study Non‐exposed: same as exposed group but chose different contraceptive Exposure: presumably from clinic records |
| NOS comparability (NRS) | Low risk | Analysis: adjusted for pretreatment value |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to women presumably having chosen method |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information; objective outcome measure |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up: 19% by 12 months; DMPA 2/16 (13%) and COC 4/16 (25%) Differential losses between groups though sample sizes are small Reasons for missing data not specified |
Tuchman 2005.
| Methods | Design: retrospective chart review Location: urban, hospital‐based, teen health center (USA) Time frame: enrollment 01 January 2001 to 31 December 2001 Sample size estimation and outcome of focus: no sample size calculation; emphasis on weight change |
|
| Participants | 222 females, aged 12 to 21 years, attending health center for contraception Inclusion criteria: first‐time use of oral or injectable contraceptive 'New start' defined as no OC in past 3 months or DMPA in past 6 months prior to new method initiation. Exclusion criteria: no other information |
|
| Interventions | Choice of method 1) DMPA every 3 months 2) Medroxyprogesterone acetate + estradiol cypionate 5 mg (MPA + E2C) monthly 3) COC | |
| Outcomes | Mean weight change (kg) and mean percent weight change by contraceptive method Time frame: 3, 6, 9, 12 months Standardized weight and height measures described. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Young women presumably chose contraceptive method |
| NOS selection (NRS) | Low risk | Exposed: clinic population of new users Non‐exposed: same as exposed group but chose different contraceptive Exposure: clinic records |
| NOS comparability (NRS) | High risk | Analysis: no adjustment for weight outcome |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to differences in interventions and women presumably chose method |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information; objective outcome measure |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up or discontinuation: may have selected charts with relevant data for retrospective review; at 12 months, discontinuation 54% to 58% |
Vickery 2013.
| Methods | Design: substudy of CHOICE (see Notes below) Location: St. Louis, MO (USA) Time frame: enrolled between June 2009 and May 2011 Sample size estimation and outcome of focus: for weight change (kg) at 12 months, assumed mean weight gain 0.6 kg over 12 months in Cu IUC users and 2.0 kg in progestin‐only users; assumed alpha 0.05, power 80%, and SD 3.0 kg in all groups; needed 73 women in each arm; for SD of 5.0 kg for Cu IUC and 6.0 kg for progestin‐only users, increased sample size to 100 in Cu IUC group and 130 in progestin‐only groups |
|
| Participants | 427 women enrolled in CHOICE Inclusion criteria: 18 to 45 years old; continuous user for ≥ 11 months of LNG‐IUC, Cu IUC, implant, or DMPA; enrolled at university research site; had height and weight measured at enrollment visit Exclusion criteria: did not speak English; < 18 years old; metabolic disorder known to affect body weight, e.g. hypothyroidism or diabetes |
|
| Interventions | 1) DMPA (N = 67) 2) LNG‐IUC (N = 130) 3) Cu IUC (N = 100) 4) ENG implant (N = 130) |
|
| Outcomes | Weight change (kg) Follow‐up: 12 months |
|
| Notes | CHOICE, prospective cohort study of 9256 women: promote the use of LARC, remove financial barriers by providing contraceptives at no cost, and evaluate method continuation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Women selected method of contraception |
| NOS selection (NRS) | Low risk | Exposed: clinic population; volunteered to participate in substudy Non‐exposed: same population as exposed but chose different contraceptive Exposure: clinic records (inserted IUC or ENG implant and administered DMPA) |
| NOS comparability (NRS) | Low risk | Analysis: stratified by race (associated with weight change); final adjusted linear regression model included age (LNG‐IUC and Cu‐IUC users were slightly older) as well as covariates associated with outcome and exposure and those that altered effect ≥ 10% |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to women having chosen contraceptive method |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention; objective outcome measure |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up: selected women who completed 11 months of use; 35% of women screened met eligibility criteria (749/2145), of which 57% enrolled (427/749) |
Westhoff 2007.
| Methods | Design: analysis of data from 3 RCTs Location: sites in North and South America Time frame: no information Sample size estimation and outcome of focus: no information |
|
| Participants | 534 women, 18 to 49 years old, sexually active and wanting long‐term contraception Inclusion criteria: no OC use for past 2 months; regular menstruation in past 3 months; willing to rely on DMPA for year Exclusion criteria: used OCs, implants, or hormonal IUC in past 2 months or DMPA‐IM in past 10 months; pregnant or infertile; abnormal Pap; undiagnosed genital bleeding; other contraindications to hormonal contraceptives |
|
| Interventions | 1) DMPA‐SC 104 mg (N = 266) 2) DMPA‐IM 150 mg (N = 268) Injections every 3 months for 3 years |
|
| Outcomes | Weight change (as safety endpoint) Follow‐up: 36 months |
|
| Notes | Investigators also analyzed weight change by BMI group: ≤ 25; 25 to 30; > 30 kg/m2. Report notes no consistent differences by BMI groups |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to differences in interventions (intramuscular vs subcutaneous) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information for 2 trials; evaluator blinded in 1 trial, but unclear if relevant to weight outcome |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up or discontinuation: DMPA‐SC 24% (201/266); DMPA‐IM 79% (212/268); reasons for discontinuation unclear |
WHO 1983.
| Methods | Design: phase III randomized controlled trial Location: 13 centers in Africa, Asia, Central and South America, and Europe Time frame: recruitment began 1977; final follow‐up March 1982 Sample size estimation and outcome of focus: no information |
|
| Participants | 3172 women; mean age 27.4 years Inclusion criteria: non‐breastfeeding women who chose injectable contraception Exclusion criteria: contraindication for long‐acting contraceptive methods |
|
| Interventions | 1) DMPA 150 mg at 90‐day intervals (N = 1587) 2) Norethisterone enanthate (NET‐EN) 200 mg at 60‐day intervals (N = 789) 3) NET‐EN 200 mg at 60‐day intervals for 6 months then 84‐day intervals (N = 796) | |
| Outcomes | Mean weight change by contraceptive group Follow‐up: 12 and 24 months |
|
| Notes | Method for measuring weight not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly allocated" |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to differences in injection schedules for interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up: reportedly 10.7% DMPA; 8.9% NET‐EN 60 days; 9.8% NET‐EN 84 days Life‐table rates for total discontinuation: 71% to 74% |
BMI: body mass index DMPA: depot medroxyprogesterone acetate FSH: follicle stimulating hormone LNG‐IUC: levonorgestrel‐releasing intrauterine contraception MPA: medroxyprogesterone acetate NET‐EN: norethisterone enanthate NRS: non‐randomized study OC: oral contraceptive SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agoestina 1978 | Insufficient weight change data: presented in figure without any specific numbers, other than mean gain for DMPA group in text |
| Bahamondes 2010 | Mean weight change not reported. BMI included as control variable for examining bone mineral density. Study examined weight change among participants who had been using the method (LNG‐IUC) for at least 7 years before the study. |
| Barsivala 1974 | Insufficient data: study duration not reported (our criteria was ≥ 3 months); also, investigators did not specify whether the variance reported is standard deviation or standard error. |
| Beksinska 2010 | Analysis combined users of DMPA, NET‐EN, or both. Investigators noted the subgroups were too small to analyze separately and that differences in weight gain were not significant. |
| Berenson 1997 | Insufficient weight change data: means reported without any variance measure |
| Berenson 2009 | Insufficient weight change data: mean change reported without any variance.
Of 240 who chose DMPA, 60% discontinued the method by 12 months and 76% discontinued by 36 months. DMPA users with > 5% weight increase at 6‐month visit were more likely lost to follow‐up by next visit than those who had not gained such weight. Secondary report (Rahman 2012) provided weight gain (by kg categories) over any 6‐month period; categories overlapped. |
| Bonny 2006 | Analysis combined groups that received DMPA + placebo or DMPA + estradiol supplement; reportedly DMPA groups did not differ in weight gain. DMPA discontinuation 37% at 18 months; data from 2000 to 2003 |
| Bonny 2011 | Not comparative |
| Bonny 2015 | Not comparative |
| Casey 2013 | Insufficient data: no weight change reported |
| Chen 2011 | Abstract notes that weight gain was reported as side effect. Wrote to investigator regarding whether weight change was measured. Unable to obtain further information. |
| Clark 2005 | Insufficient weight change data: means (not mean change) presented in a figure. Text mentions mean change for DMPA at 30 months (no variance measure) and that the control group was basically unchanged. Due to discontinuations of DMPA and initiation of hormonal contraception among controls, the samples sizes were 17% (DMPA) and 19% (controls) of baseline by the last visit. |
| Costa 2012 | Mean change in weight was not reported. Participants were 1.5 months postpartum. |
| Dahlberg 1982 | Insufficient weight change data: means reported without any variance measure |
| El Mahgoub 1980 | Insufficient weight change data: mean change reported without any variance measure. Also, percent that lost or gained weight was reported, but no specific amount of weight was provided. |
| Gallo 2016 | Two arms: immediate and delayed insertion of Sino‐Implant II. Secondary analysis of data from RCT that examined condom use. Delayed group was offered OC prescription; no information on how many used it. |
| Hall 1980 | Insufficient weight change data: mean change in 'ideal body weight' shown in figure, except for mean change for progestin‐only group reported in text. |
| Havranek 1972 | Insufficient weight data: mean change for one group reported without any variance measure |
| Hernandez‐Juarez 2014 | Insufficient weight data; weight change not reported |
| Kaunitz 2009 | Insufficient weight data. Mean change was reported within adverse event section without any variance measure. Data from 2001 to 2004. |
| Mangan 2002 | Comparison groups were DMPA users and OC users. Types of OC were not specified and might have included progestin‐only as well as combination OC. |
| Modesto 2014b | Insufficient data: counseling only; no weight change measured |
| Nault 2013 | Insufficient data: weight gain not broken down by contraceptive type |
| Olsson 1988 | Insufficient data for analysis: no N per group for analysis. First‐year continuation rate was 59% for Norplant and 77% for Norplant‐2; methods suggest these were life‐table rates. |
| Ortayli 2001 | Insufficient data: report does not provide sample sizes used for analysis. Outcome data are from a pilot conducted in 1995 and the main study conducted from 1996 to 1998. |
| Risser 1999 | Comparison groups were DMPA users and OC users. Types of OC were not specified and might have included progestin‐only as well as combination OC. |
| Segall‐Gutierrez 2012 | Single‐arm study comparing normal weight and obese women. The one intervention was subcutaneous DMPA. |
| Veisi 2013 | Insufficient weight data: self‐reported weight only |
| WHO 1978 | Insufficient weight data: mean gains reported without any variance measure |
| Yela 2006 | Insufficient weight change data: tables show weight and BMI means (not change) by year; text mentions mean change per group over 5 years without any variance measure. Study began in 1998. |
| Zheng 1999 | Insufficient weight change data: means reported without any variance measure |
BMI: body mass index DMPA: depot medroxyprogesterone acetate LNG‐IUC: levonorgestrel‐releasing intrauterine contraception NET‐EN: norethisterone enanthate OC: oral contraceptive
Characteristics of studies awaiting assessment [ordered by study ID]
Madden 2014.
| Methods | Design: observational prospective cohort; non‐probability sample Purpose: learn if women gain weight using progestin‐only methods of contraception and if so, how much Location: St Louis, MO (USA) Time frame: April 2010 to August 2014 Sample size estimation and outcome of focus: no information |
| Participants | 345 women Inclusion criteria: aged 18 to 45 years; starting copper IUC or implant through provider; first study visit must occur within 14 days of method insertion Exclusion criteria: DMPA in past 16 weeks; POPs, LNG‐IUC, or implant in past 4 weeks; thyroid disease, autoimmune disease, diabetes (excluding gestational); history of eating disorder; currently taking antidepressants for < 6 months, antipsychotics; oral glucocorticoids (steroids, i.e. prednisone) for > 6 months; currently breastfeeding or < 6 months postpartum |
| Interventions | 1) Levonorgestrel‐containing intrauterine contraceptive (LNG‐IUC) 2) Etonogestrel (ENG) subdermal implant 3) Copper IUC |
| Outcomes | Primary: weight change; BMI change Secondary: body composition, including fat mass and percentage and central‐to‐peripheral fat ratio Body composition with dual‐energy x‐ray absorptiometry (DEXA); diet and activity via validated questionnaires Follow‐up: 12 months |
| Notes | Will consider for inclusion when full report is available |
Schreiber 2014.
| Methods | Design: randomized; open label; mixed methods Location: Philadelphia PA (USA) Time frame: April 2011 to October 2012 Sample size estimation and outcome of focus: no information |
| Participants | 100 women Inclusion criteria: English‐speaking; age 18 to 45 years; immediately postpartum of live singleton infant, 37 weeks gestation; desire to delay another pregnancy for next 6 months; willing and able to follow protocol Exclusion criteria: breastfeeding; plans to relocate outside of Philadelphia area in next 6 months; plans for use of weight loss medication or diet pills in next 6 months; wish to use Implanon or DMPA prior to discharge but does not want to be randomized |
| Interventions | 1) DepoProvera (DMPA) 150 mg 2) Implanon; 68 mg etonogestrel 3) Control: select own method of contraception or no contraception |
| Outcomes | Primary: weight change by 6 months postpartum Secondary: pregnancy; contraception satisfaction Follow‐up: 3, 6, and 12 months postpartum |
| Notes | Will consider for inclusion when full report is available |
BMI: body mass index DMPA: depot medroxyprogesterone acetate LNG‐IUC: levonorgestrel‐releasing intrauterine contraception POP: progestin‐only oral pills
Characteristics of ongoing studies [ordered by study ID]
Bonny 2016.
| Trial name or title | Drug Exposure and Depot Medroxyprogesterone Acetate (DMPA) in Adolescent Subjects |
| Methods | Design: randomized, open‐label; pharmacokinetic study Purpose: learn whether DMPA affects weight gain and bone mineral density in teens Location: Columbus, OH (USA) Time frame: September 2011 to April 2015 Sample size estimation and outcome of focus: no information |
| Participants | 45 healthy young women Inclusion criteria: healthy, postmenarchal females; age 12 to 21 years; self‐selected to initiate DMPA; willing to use barrier method of contraception in addition to DMPA Exclusion criteria: chronic disease known to affect weight or bone mineral density (BMD) (e.g. diabetes, kidney); medication known to affect weight or BMD (e.g. corticosteroids); DMPA use in past 12 months; pregnancy in past 6 months; etonogestrel implant, LNG‐IUC or combined contraceptive in past 3 months (OC, transdermal patch, vaginal ring); weight > 450 lb; need for confidential contraceptive care for individuals < 18 years of age |
| Interventions | DMPA, intramuscular injection
|
| Outcomes | Primary: > 5% weight gain at 24 weeks Secondary: > 10% weight gain at 48 weeks Follow‐up: 24 and 48 weeks |
| Starting date | September 2011; planned completion April 2016 |
| Contact information | Andrea Bonny, MD; Nationwide Children's Hospital; Columbus, Ohio (USA) |
| Notes |
Halpern 2017.
| Trial name or title | Pharmacodynamics and Pharmacokinetics Study of Existing DMPA Contraceptive Methods |
| Methods | Design: randomized, multicenter, open label Location: Portland OR (USA); Santo Domingo, Dominican Republic Time frame: September 2015 to August 2017 Sample size estimation and outcome of focus: no information |
| Participants | 48 women Inclusion criteria: in good general health; aged 18 to 40 years; willing to provide consent and follow study requirements; negative urine pregnancy test on day of injection; does not become pregnant in next 24 months; regular menstrual cycle (27 to 35 days); confirmed ovulation in 2 consecutive samples during pre‐treatment phase; low risk of pregnancy; BMI 18 to 35; hemoglobin ≥ 10.5 g/L Exclusion criteria: medical contraindication to DMPA use; use of any investigational drug, prohibited drugs, OCs, LNG‐IUC or implant within 1 month prior to enrollment; use of DMPA in past 12 months; use of combined injectable contraceptive in past 6 months; recent pregnancy (within 3 months); current lactation; ongoing or anticipated use of prohibited drugs; known sensitivity to MPA; plan to move to another location in next 18 months |
| Interventions | 1) 1 subcutaneous injection of 150 mg/mL DMPA in abdomen 2) 1 subcutaneous injection of 300 mg/mL DMPA in abdomen 3) 2 injections of 104 mg/0.65 mL DMPA in abdomen, given at 3‐month intervals |
| Outcomes | Primary: time to ovulation Secondary: weight at follow‐up; Cmax; aggregate of individual Cmax measurements and parameters; adverse events Follow‐up: 18 months |
| Starting date | September 2015; planned completion August 2017 |
| Contact information | Vera Halpern, MD: FHI 360; vhalpern@fhi360.org; 919‐544‐7040 x11390 |
| Notes |
DMPA: depot medroxyprogesterone acetate LNG‐IUC: levonorgestrel‐releasing intrauterine contraception MPA: medroxyprogesterone acetate
Contributions of authors
2010: L Lopez reviewed the search results, extracted and entered the data, and drafted the initial review. A Edelman and F Helmerhorst did part of the second data extraction. M Chen provided guidance on study design, data analysis, and interpretation of results. All authors reviewed and commented on the document.
2013: C Otterness reviewed the search results, did the primary data extraction and entering, and incorporated the new study. L Lopez did the second data extraction and updated the text. M Chen provided guidance on study design, data analysis, and interpretation of results. All authors reviewed and commented on the document.
2016: L Lopez ran the searches, revised the quality assessment for NRS, revised the text and tables to incorporate the new studies, and added the 'Summary of findings tables.' S Ramesh reviewed the search results, extracted and entered study characteristics, and helped update the text. L Lopez and S Ramesh entered and checked the outcome data for new studies. M Chen reviewed and contributed to the revised criteria for quality of evidence for all studies, and consulted on the presentation and interpretation of the outcome data. All authors reviewed and commented on the document.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute of Child Health and Human Development, USA.
2010 to present: Support for conducting the review and updates at FHI 360
-
US Agency for International Development, USA.
2010 through 2013: Support for conducting the review and update at FHI 360
-
National Institute of Child Health and Human Development, USA.
2010: Grant for Infrastructure for Population Research at Princeton University; Grant R24HD047879 (JT, for working on the review)
2015: Infrastructure grant for population research at Princeton University; P2C HD047879 (JT, for working on the review)
Declarations of interest
The authors, Lopez LM, Ramesh S, Chen M, Edelman A, Otterness C, Trussell J, and Helmerhorst FM, have no conflicts of interest to declare regarding this review.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Ball 1991 {published data only}
- Ball MJ, Ashwell E, Gillmer MD. Progestagen‐only oral contraceptives: comparison of the metabolic effects of levonorgestrel and norethisterone. Contraception 1991;44(3):223‐33. [DOI] [PubMed] [Google Scholar]
Bonny 2009 {published data only}
- Bonny AE, Secic M, Cromer BA. A longitudinal comparison of body composition changes in adolescent girls receiving hormonal contraception. Journal of Adolescent Health 2009;45(4):423‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Castle 1978 {published data only}
- Castle WM, Sapire KE, Howard KA. Efficacy and acceptability of injectable medroxyprogesterone: a comparison of 3‐monthly and 6‐monthly regimens. South African Medical Journal 1978; Vol. 53, issue 21:842‐5. [PubMed]
Dal'Ava 2012 {published data only}
- Dal'Ava N, Bahamondes L, Bahamondes MV, Oliveira Santos A, Monteiro I. Body weight and composition in users of levonorgestrel‐releasing intrauterine system. Contraception 2012;86(4):350‐3. [DOI] [PubMed] [Google Scholar]
Dal'Ava 2014 {published data only}
- Dal'Ava N, Bahamondes L, Bahamondes MV, Bottura BF, Monteiro I. Body weight and body composition of depot medroxyprogesterone acetate users. Contraception 2014;90(2):182‐7. [DOI] [PubMed] [Google Scholar]
Dos Santos 2014 {published data only}
- Cursino K, Sider M, Pavin EJ, Dos Santos PN, Bahamondes L, Zantut‐Wittmann DE, et al. Insulin resistance parameters in users of the injectable contraceptive depot medroxyprogesterone acetate during one year of use. European Journal of Contraception and Reproductive Health Care 2015;21(1):1‐8. [DOI] [PubMed] [Google Scholar]
- Dos Santos PdeNS, Modesto WO, Dal'Ava N, Bahamondes M, Pavin E, Fernades A. Body composition and weight gain in new users of the three‐monthly injectable contraceptive, depot‐medroxyprogesterone acetate, after 12 months of follow‐up. European Journal of Contraception and Reproductive Health Care 2014;19(6):432‐38. [DOI] [PubMed] [Google Scholar]
- Modesto W, Bahamondes MV, Silva dos Santos P, Fernandes A, Dal'Ava N, Bahamondes L. Exploratory study of the effect of lifestyle counselling on bone mineral density and body composition in users of the contraceptive depot‐medroxyprogesterone acetate. European Journal of Contraception and Reproductive Health Care 2014;19(4):244‐9. [DOI] [PubMed] [Google Scholar]
Espey 2000 {published data only}
Modesto 2015 {published data only}
- Modesto W, dos Santos PdeNS, Correia VM, Borges L, Bahamondes L. Weight variation in users of depot‐medroxyprogesterone acetate, the levonorgestrel‐releasing intrauterine system and a copper intrauterine device for up to ten years of use. European Journal of Contraception and Reproductive Health Care 2015;20(1):57‐63. [DOI] [PubMed] [Google Scholar]
Moore 1995 {published data only}
Napolitano 2015 {published data only}
Nyirati 2013 {published data only}
- Nyirati CM, Habash DL, Shaffer E. Weight and body fat changes in postpartum depot‐medroxyprogesterone acetate users. Contraception 2013;88(1):169‐76. [DOI] [PubMed] [Google Scholar]
Pantoja 2010 {published data only}
- Pantoja M, Medeiros T, Baccarin MC, Morais S, Fernandes AM. Variation of weight among users of the contraceptive with depot‐medroxyprogesterone acetate according to body mass index in a six‐year follow‐up. Revista Brasileira de Ginecologia e Obstetricia 2009; Vol. 31, issue 8:380‐4. [DOI] [PubMed]
- Pantoja M, Medeiros T, Baccarin MC, Morais SS, Bahamondes L, Fernandes AM. Variations in body mass index of users of depot‐medroxyprogesterone acetate as a contraceptive. Contraception 2010;81(2):107‐11. [DOI] [PubMed] [Google Scholar]
Salem 1984 {published data only}
- Salem HT, Abdullah KA, Shaaban MM. Norplant and lactation. The Norplant subdermal contraceptive system. Proceedings of the Symposium on Longterm Subdermal Contraceptive Implants; 1984 February 23‐24. Assuit, Egypt: Assiut University, 1984:139‐49.
Salem 1988 {published data only}
Sivin 1998 {published data only}
- Sivin I, Campodonico I, Kiriwat O, Holma P, Diaz S, Wan L, et al. The performance of levonorgestrel rod and Norplant contraceptive implants: a 5 year randomized study. Human Reproduction. 1999/01/14 1998; Vol. 13, issue 12:3371‐8. [DOI] [PubMed]
- Sivin I, Viegas O, Campodonico I, Diaz S, Pavez M, Wan L, et al. Clinical performance of a new two‐rod levonorgestrel contraceptive implant: a three‐year randomized study with Norplant implants as controls. Contraception 1997;55(2):73‐80. [DOI] [PubMed] [Google Scholar]
Sule 2005 {published data only}
- Sule S, Shittu O. Weight changes in clients on hormonal contraceptives in Saria, Nigeria. African Journal of Reproductive Health 2005;9(2):92‐100. [PubMed] [Google Scholar]
Taneepanichskul 1998 {published data only}
- Taneepanichskul S, Reinprayoon D, Jaisamrarn U. Effects of DMPA on weight and blood pressure in long term acceptors. Contraception 1999;59(5):301‐3. [DOI] [PubMed] [Google Scholar]
- Taneepanichskul S, Reinprayoon D, Khaosaad P. Comparative study of weight change between long‐term DMPA and IUD acceptors. Contraception 1998;58(3):149‐51. [Google Scholar]
Tankeyoon 1976 {published data only}
Tuchman 2005 {published data only}
Vickery 2013 {published data only}
- Vickery Z, Madden T, Zhao Q, Secura GM, Allsworth JE, Peipert JF. Weight change at 12 months in users of three progestin‐only contraceptive methods. Contraception 2013;88(4):503‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Westhoff 2007 {published data only}
- Jain J, Jakimiuk AJ, Bode FR, Ross D, Kaunitz AM. Contraceptive efficacy and safety of DMPA‐SC. Contraception 2004;70(4):269‐75. [DOI] [PubMed] [Google Scholar]
- Westhoff C, Jain JK, Milsom I, Ray A. Changes in weight with depot medroxyprogesterone acetate subcutaneous injection 104 mg/0.65 mL. Contraception 2007;75(4):261‐7. [DOI] [PubMed] [Google Scholar]
WHO 1983 {published data only}
- World Health Organization. Multinational comparative clinical trial of long‐acting injectable contraceptives: norethisterone enanthate given in two dosage regimens and depot‐medroxyprogesterone acetate. A preliminary report. Contraception. 1982/01/01 1982; Vol. 25, issue 1:1‐11. [DOI] [PubMed]
- World Health Organization. Multinational comparative clinical trial of long‐acting injectable contraceptives: norethisterone enanthate given in two dosage regimens and depot‐medroxyprogesterone acetate. Final report. Contraception. 1983/07/01 1983; Vol. 28, issue 1:1‐20. [DOI] [PubMed]
References to studies excluded from this review
Agoestina 1978 {published data only}
- Agoestina T, Biben A. Use of Depo Provera in immediate post partum and lactating period at Dr Hasan Sadikin Hospital (1978). Report on file 10.
Bahamondes 2010 {published data only}
- Bahamondes MV, Monteiro I, Castro S, Espejo‐Arce X, Bahamondes L. Prospective study of the forearm bone mineral density of long‐term users of the levonorgestrel‐releasing intrauterine system. Human Reproduction 2010;25(5):1158‐64. [DOI] [PubMed] [Google Scholar]
Barsivala 1974 {published data only}
Beksinska 2010 {published data only}
- Beksinska ME, Smit JA, Kleinschmidt I, Milford C, Farley TM. Prospective study of weight change in new adolescent users of DMPA, NET‐EN, COCs, nonusers and discontinuers of hormonal contraception. Contraception 2010;81(1):30‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berenson 1997 {published data only}
- Berenson AB, Wiemann CM, Rickerr VI, McCombs SL. Contraceptive outcomes among adolescents prescribed Norplant implants versus oral contraceptives after one year of use. American Journal of Obstetrics and Gynecology 1997;176(3):586‐92. [DOI] [PubMed] [Google Scholar]
Berenson 2009 {published data only}
- Berenson AB, Rahman M. Changes in weight, total fat, percent body fat, and central‐to‐peripheral fat ratio associated with injectable and oral contraceptive use. American Journal of Obstetrics and Gynecology. 2009/03/04 2009; Vol. 200, issue 3:329 e1‐8. [DOI] [PMC free article] [PubMed]
- Rahman M, Berenson AB. Self‐perception of weight gain among multiethnic reproductive‐age women. Journal of women's health (2002) 2012;21(3):340‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bonny 2006 {published data only}
- Bonny A, Camlin K, Harvey R, Islam KM, Debanne S. Prospective analysis of weight changes in adolescent females initiating depot medroxyprogesterone acetate (DMPA), oral contraceptive pills (OC), or no hormonal contraceptive method. Journal of Adolescent Health 2003;32(2):134. [Google Scholar]
- Bonny AE, Secic M, Cromer BA. Relationship between weight and bone mineral density in adolescents on hormonal contraception. Journal of Pediatric and Adolescent Gynecology 2011;24(1):35‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonny AE, Ziegler J, Harvey R, Debanne SM, Secic M, Cromer BA. Weight gain in obese and nonobese adolescent girls initiating depot medroxyprogesterone, oral contraceptive pills, or no hormonal contraceptive method. Archives of Pediatrics & Adolescent Medicine. 2006/01/04 2006; Vol. 160, issue 1:40‐5. [DOI] [PubMed]
Bonny 2011 {published data only}
- Bonny AE, Secic M, Cromer B. Early weight gain related to later weight gain in adolescents on depot medroxyprogesterone acetate. Obstetrics and Gynecology 2011;117:793‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bonny 2015 {published data only}
- Bonny AE, Lange HL, Hade EM, Kaufman B, Reed MD, Mesiano S. Serum adipocytokines and adipose weight gain: a pilot study in adolescent females initiating depot medroxyprogesterone acetate. Contraception 2015;92(4):298‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Casey 2013 {published data only}
- Casey PM, Long ME, Marnach ML, Fleming‐Harvey J, Drozdowicz LB, Weaver AL. Association of body mass index with removal of etonogestrel subdermal implant. Contraception 2013;87(3):370‐4. [DOI] [PubMed] [Google Scholar]
Chen 2011 {published data only (unpublished sought but not used)}
- Chen J, Wang XJ, Liang Y, Jin Q. [Clinic observation of a levonorgestrel‐releasing intrauterine system inserted immediately after artificial abortion]. Zhonghua yi xue za zhi 2011;91(45):3176‐8. [PubMed] [Google Scholar]
Clark 2005 {published data only}
- Clark MK, Dillon JS, Sowers M, Nichols S. Weight, fat mass, and central distribution of fat increase when women use depot‐medroxyprogesterone acetate for contraception. International Journal of Obesity 2005; Vol. 29, issue 10:1252‐8. [DOI] [PubMed]
- Clark MK, Sowers MR, Nichols S, Levy B. Bone mineral density changes over two years in first‐time users of depot medroxyprogesterone acetate. Fertility and Sterility. 2004/12/14 2004; Vol. 82, issue 6:1580‐6. [DOI] [PubMed]
Costa 2012 {published data only}
- Costa ML, Cecatti JG, Krupa FG, Rehder PM, Sousa MH, Costa‐Paiva L. Progestin‐only contraception prevents bone loss in postpartum breastfeeding women. Contraception 2012;85(4):374‐80. [DOI] [PubMed] [Google Scholar]
Dahlberg 1982 {published data only}
El Mahgoub 1980 {published data only}
- Mahgoub S. Body weight and cycle control of injectable contraceptives. Journal of Reproductive Medicine 1980;24(3):119‐26. [PubMed] [Google Scholar]
Gallo 2016 {published data only}
- Gallo MF, Legardy‐Williams J, Hylton‐Kong T, Rattray C, Kourtis AP, Jamieson DJ, et al. Association of Progestin Contraceptive Implant and Weight Gain. Obstetrics and Gynecology 2016;127(3):573‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattray C, Wiener J, Legardy‐Williams J, Costenbader E, Pazol K, Medley‐Singh N, et al. Effects of initiating a contraceptive implant on subsequent condom use: A randomized controlled trial. Contraception 2015;92(6):560‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hall 1980 {published data only}
- Hall WD, Douglas MB, Blumenstein BA, Hatcher RA. Blood pressure and oral progestational agents. A prospective study of 119 black women. American Journal of Obstetrics and Gynecology 1980;136(3):344‐8. [DOI] [PubMed] [Google Scholar]
Havranek 1972 {published data only}
- Havranek F, Tichy M, Divila F, Hejda V. Experience with the contraceptive effect of Depo‐Provera, 300mg and 450mg administered at 6 month intervals [Zkusenosti s antikoncepcni ucinnosti Depo‐Provery v davce 300mg a 450mg podanych v intervalech 6 mesicu]. Ceskoslovenska Gynekologie 1972; Vol. 37, issue 2:95‐6. [PubMed]
Hernandez‐Juarez 2014 {published data only}
- Hernandez‐Juarez J, Garcia‐Latorre EA, Moreno‐Hernandez M, Moran‐Perez JF, Rodriguez‐Escobedo MA, Cogque‐Hernandez G, et al. Metabolic effects of the contraceptive skin patch and subdermal contraceptive implant in Mexican women: a prospective study. Reproductive Health 2014;11:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaunitz 2009 {published data only}
- Kaunitz AM, Darney PD, Ross D, Wolter KD, Speroff L. Subcutaneous DMPA vs. intramuscular DMPA: a 2‐year randomized study of contraceptive efficacy and bone mineral density. Contraception 2009;80(1):7‐17. [DOI] [PubMed] [Google Scholar]
Mangan 2002 {published data only}
Modesto 2014b {published data only}
- Modesto W, Bahamondes MV, Bahamondes L. A randomized clinical trial of the effect of intensive versus non‐intensive counselling on discontinuation rates due to bleeding disturbances of three long‐acting reversible contraceptives. Human Reproduction 2014;29(7):1393‐9. [DOI] [PubMed] [Google Scholar]
Nault 2013 {published data only}
- Nault AM, Peipert JF, Zhao Q, Madden T, Secura GM. Validity of perceived weight gain in women using long‐acting reversible contraception and depot medroxyprogesterone acetate. American Journal of Obstetrics and Gynecology 2013;208(1):48 e1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Olsson 1988 {published data only}
Ortayli 2001 {published data only}
- Ortayli N, Bulut A, Sahin T, Sivin I. Immediate postabortal contraception with the levonorgestrel intrauterine device, Norplant, and traditional methods. Contraception 2001;63(6):309‐14. [DOI] [PubMed] [Google Scholar]
Risser 1999 {published data only}
Segall‐Gutierrez 2012 {published data only}
- Segall‐Gutierrez P, Xiang A H, Watanabe R M, Trigo E, Stanczyk F Z, Liu X, et al. Deterioration in cardiometabolic risk markers in obese women during depot medroxyprogesterone acetate use. Contraception 2012;85(1):36‐41. [DOI] [PubMed] [Google Scholar]
Veisi 2013 {published data only}
- Veisi F, Zangeneh M. Comparison of two different injectable contraceptive methods: depo‐medroxy progesterone acetate (DMPA) and Cyclofem. Journal of Family and Reproductive Health 2013;7(3):109‐13. [PMC free article] [PubMed] [Google Scholar]
WHO 1978 {published data only}
- World Health Organization. Multinational comparative clinical evaluation of two long‐acting injectable contraceptive steroids: norethisterone oenanthate and medroxyprogesterone acetate. 2. Bleeding patterns and side effects. Contraception. 1978/05/01 1978; Vol. 17, issue 5:395‐406. [PubMed]
Yela 2006 {published data only}
Zheng 1999 {published data only}
References to studies awaiting assessment
Madden 2014 {published data only}
- Madden TE. Comparison of Body Composition & Weight Change in Users of Progestin‐only Contraception During the First Year of Use (DEXA). clinicaltrials.gov/ct2/show/NCT01579773 (accessed 29 September 2015).
Schreiber 2014 {published data only}
- Schreiber CA. The Impact of Contraception on Postpartum Weight Loss (PPWL). clinicaltrials.gov/ct2/show/NCT01579773 (accessed 29 September 2015).
References to ongoing studies
Bonny 2016 {published data only}
- Bonny A. Drug Exposure and Depot Medroxyprogesterone Acetate (DMPA) in Adolescent Subjects. clinicaltrials.gov/ct2/show/NCT01461824 (accessed 29 September 2015).
Halpern 2017 {published data only}
- Halpern V. Pharmacodynamics and Pharmacokinetics Study of Existing DMPA Contraceptive Methods. clinicaltrials.gov/ct2/show/NCT02456584 (accessed 16 October 2016).
Additional references
ACOG 2006
- American College of Obstetricians and Gynecologists. ACOG technical bulletin. Use of hormonal contraception in women with coexisting medical conditions. Washington, D.C.: American College of Obstetricians and Gynecologists, 2006. [Google Scholar]
Albright 2015
- Albright M, Rani S, Gavagan T. HelpDesk answers: do hormonal contraceptives lead to weight gain?. Journal of Family Practice 2015;64(6):371‐2. [PubMed] [Google Scholar]
Bartz 2011
- Bartz D, Goldberg AB. Injectable contraceptives. In: Hatcher RA, Trussell J, Nelson AL, Cates W, Kowal D, Policar MS editor(s). Contraceptive Technology. 20th Edition. New York: Ardent Media, Inc., 2011:209‐36. [Google Scholar]
Basu 2016
Bender 2013
- Bender NM, Segall‐Gutierrez P, Najera SO, Stanczyk FZ, Montoro M, Mishell DR Jr. Effects of progestin‐only long‐acting contraception on metabolic markers in obese women. Contraception 2013;88(3):418‐25. [DOI] [PubMed] [Google Scholar]
Berenson 2008
- Berenson AB, Odom SD, Breitkopf CR, Rahman M. Physiologic and psychologic symptoms associated with use of injectable contraception and 20 µg oral contraceptive pills. American Journal of Obstetrics and Gynecology. 2008/07/05 2008; Vol. 199, issue 4:351e1‐12. [DOI] [PMC free article] [PubMed]
Bonny 2004
- Bonny AE, Britto MT, Huang B, Succop P, Slap GB. Weight gain, adiposity, and eating behaviors among adolescent females on depot medroxyprogesterone acetate (DMPA). Journal of Pediatric and Adolescent Gynecology 2004;17(2):109‐15. [DOI] [PubMed] [Google Scholar]
Callegari 2014
- Callegari LS, Nelson KM, Arterburn DE, Prager SW, Schiff MA, Schwarz EB. Factors associated with lack of effective contraception among obese women in the United States. Contraception 2014;90(3):265‐71. [DOI] [PubMed] [Google Scholar]
CDC 2012a
- Centers for Disease Control and Prevention. Defining Overweight and Obesity. http://www.cdc.gov/obesity/adult/defining.html (accessed 19 December 2012).
CDC 2012b
- Centers for Disease Control and Prevention. Summary Chart of U.S. Medical Eligibility Criteria for Contraceptive Use. www.cdc.gov/reproductivehealth/unintendedpregnancy/usmec.htm (accessed 1 March 2016).
Curtis 2009
Dickerson 2013
- Dickerson LM, Diaz VA, Jordon J, Davis E, Chirina S, Goddard JA, et al. Satisfaction, early removal, and side effects associated with long‐acting reversible contraception. Family Medicine 2013;45(10):701‐7. [PubMed] [Google Scholar]
Edelman 2014
- Edelman AB, Cherala G, Munar MY, McInnis M, Stanczyk FZ, Jensen JT. Correcting oral contraceptive pharmacokinetic alterations due to obesity: a randomized controlled trial. Contraception 2014;90(5):550‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Flegal 2000
- Flegal KM, Troiano RP. Changes in the distribution of body mass index of adults and children in the US population. International Journal of Obesity and Related Metabolic Disorders 2000;24(7):807‐18. [DOI] [PubMed] [Google Scholar]
Flegal 2012
- Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999‐2010. JAMA 2012;307(5):491‐7. [DOI] [PubMed] [Google Scholar]
Gallo 2014
- Gallo MF, Lopez LM, Grimes DA, Schulz KF, Helmerhorst FM. Combination contraceptives: effects on weight. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD003987.pub5] [DOI] [PubMed] [Google Scholar]
GRADE 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A. GRADE Handbook [updated October 2013]. http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html (accessed 29 December 2015).
Grimes 2007
- Grimes DA, Lopez LM, Manion C, Schulz KF. Cochrane systematic reviews of IUD trials: lessons learned. Contraception 2007;75(6 Suppl):S55‐9. [DOI] [PubMed] [Google Scholar]
Grimes 2013
- Grimes DA, Lopez LM, O'Brien PA, Raymond EG. Progestin‐only pills for contraception. Cochrane Database of Systematic Reviews 2013, Issue 11. [DOI: 10.1002/14651858.CD007541.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guttmacher 2015
- Guttmacher Institute. Contraceptive Use in the United States. October 2015. www.guttmacher.org/pubs/fb_contr_use.html (accessed 2 March 2016).
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. John Wiley & Sons, Ltd, (accessed 1 October 2015).
Kavanaugh 2015
- Kavanaugh ML, Jerman J, Finer LB. Changes in use of long‐acting reversible contraceptive methods among U.S. women, 2009‐2012. Obstetrics and Gynecology 2015;126(5):917‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kohn 2015
- Kohn JE, Lopez PM, Simons HR. Weight and body mass index among female contraceptive clients. Contraception 2015;91(6):470‐3. [DOI] [PubMed] [Google Scholar]
Lange 2015
- Lange HL, Belury MA, Secic M, Thomas A, Bonny AE. Dietary intake and weight gain among adolescents on depot medroxyprogesterone acetate. Journal of Pediatric and Adolescent Gynecology 2015;28(3):139‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Le 2009
- Le YC, Rahman M, Berenson AB. Early weight gain predicting later weight gain among depot medroxyprogesterone acetate users. Obstetrics and Gynecology 2009;114(2 Pt 1):279‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lopez 2013
- Lopez LM, Grimes DA, Chen‐Mok M, Westhoff C, Edelman A, Helmerhorst FM. Hormonal contraceptives for contraception in overweight or obese women. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD008452.pub3] [DOI] [PubMed] [Google Scholar]
Merki‐Feld 2015
- Merki‐Feld GS, Skouby S, Serfaty D, Lech M, Bitzer J, Crosignani PG, et al. European society of contraception statement on contraception in obese women. European Journal of Contraception and Reproductive Health Care 2015;20(1):19‐28. [DOI] [PubMed] [Google Scholar]
Ogden 2014
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the united states, 2011‐2012. JAMA 2014;311(8):806‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Paul 1997
Prentice 2006
- Prentice AM. The emerging epidemic of obesity in developing countries. International Journal of Epidemiology 2006;35(1):93‐9. [DOI] [PubMed] [Google Scholar]
Raymond 2011
- Raymond EG. Progestin‐only pills. In: Hatcher RA, Trussell J, Nelson AL, Cates W Jr, Kowal D, Policar MS editor(s). Contraceptive technology. 20th Edition. New York: Ardent Media, Inc., 2011:237‐47. [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Robinson 2013
- Robinson JA, Burke AE. Obesity and hormonal contraceptive efficacy. Womens Health 2013;9:453‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Silva‐Filho 2016
- Silva‐Filho AL, Lira J, Rocha AL, Ferreira MC, Lamaita RM, Candido EB, et al. Non‐hormonal and hormonal intrauterine contraception: survey of patients' perceptions in four Latin American countries. European Journal of Contraception and Reproductive Health Care 2016 Feb 5 [Epub ahead of print]:1‐7. [DOI] [PubMed]
Sivin 2003
Steenland 2013
- Steenland MW, Zapata LB, Brahmi D, Marchbanks PA, Curtis KM. Appropriate follow up to detect potential adverse events after initiation of select contraceptive methods: a systematic review. Contraception 2013;87(5):611‐24. [DOI] [PubMed] [Google Scholar]
Steward 2016
- Steward RG, Bateman LA, Slentz C, Stanczyk FZ, Price TM. The impact of short‐term depot‐medroxyprogesterone acetate treatment on resting metabolic rate. Contraception 2016;93(4):317‐22. [DOI] [PubMed] [Google Scholar]
Trussell 2011
- Trussell J. Contraceptive failure in the United States. Contraception 2011;83(5):397‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
UN 2015
- United Nations, Department of Economic and Social Affairs. World Contraceptive Patterns 2015. www.un.org/en/development/desa/population/publications/ (accessed 23 February 2015).
WebMD 2010
- WebMD. Progestin‐only hormonal methods (mini‐pills, implants, and shots). http://www.webmd.com/sex/birth‐control/progestin‐only‐hormonal‐methods‐mini‐pills‐shots (accessed 15 Jun 2010).
Wells 2014
- GA Wells, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed 6 October 2015).
WHO 2015a
- World Health Organization, Department of Reproductive Healh. Medical eligibility criteria for contraceptive use. Fifth edition ‐ Executive Summary, 2015. http://www.who.int/reproductivehealth/publications/family_planning/Ex‐Summ‐MEC‐5/en/ (accessed 2 July 2015).
WHO 2015b
- World Health Organization, Department of Reproductive Health. Medical eligibility criteria wheel for contraceptive use. 2015. http://www.who.int/reproductivehealth/publications/family_planning/Ex‐Summ‐MEC‐5/en/ (accessed 2 July 2015).


