Abstract
Aims
To assess resource utilization associated with severe hypoglycaemia across three insulin regimens in a large phase 3a clinical programme involving people with Type 1 diabetes treated with basal–bolus insulin, people with Type 2 diabetes treated with multiple daily injections and people with Type 2 diabetes treated with basal–oral therapy.
Methods
Data relating to severe hypoglycaemia events (defined as episodes requiring external assistance) from the insulin degludec and insulin degludec/insulin aspart programme (15 trials) were analysed using descriptive statistics. Comparators included insulin glargine, biphasic insulin aspart, insulin detemir and sitagliptin. Mealtime insulin aspart was used in some regimens. This analysis used the serious adverse events records, which documented the use of ambulance/emergency teams, a hospital/emergency room visit ≤ 24 h, or a hospital visit > 24 h.
Results
In total, 536 severe hypoglycaemia events were analysed, of which 157 (29.3%) involved an ambulance/emergency team, 64 (11.9%) led to hospital/emergency room attendance of ≤ 24 h and 36 (6.7%) required hospital admission (> 24 h). Although there were fewer events in people with Type 2 diabetes compared with Type 1 diabetes, once a severe episode occurred, the tendency to utilize healthcare resources was higher in Type 2 diabetes vs. Type 1 diabetes. A higher proportion (47.6%) in the basal–oral therapy group required hospital treatment for > 24 h versus the Type 1 diabetes (5.0%) and Type 2 diabetes multiple daily injections (5.3%) groups.
Conclusion
This analysis suggests that severe hypoglycaemia events often result in emergency/ambulance calls and hospital treatment, incurring a substantial health economic burden, and were associated with all insulin regimens.
What's new?
The study is unique in reporting resource use associated with severe hypoglycaemia from a randomized controlled trial setting, because resource use has previously been analysed by using registers in which only the events with resource use have been registered, or by patient questionnaires.
Severe hypoglycaemia events with and without resource use have been collected from a large‐scale clinical trial programme.
The findings suggest that severe hypoglycaemia often necessitates the use of emergency/ambulance teams and hospital treatment, incurring a substantial healthcare burden, regardless of the type of insulin regimen used.
What's new?
The study is unique in reporting resource use associated with severe hypoglycaemia from a randomized controlled trial setting, because resource use has previously been analysed by using registers in which only the events with resource use have been registered, or by patient questionnaires.
Severe hypoglycaemia events with and without resource use have been collected from a large‐scale clinical trial programme.
The findings suggest that severe hypoglycaemia often necessitates the use of emergency/ambulance teams and hospital treatment, incurring a substantial healthcare burden, regardless of the type of insulin regimen used.
Introduction
Hypoglycaemia is a major side effect of some glucose‐lowering therapies, in particular, insulin and the insulin secretagogues (sulphonylureas and glinides). It is a frequent occurrence in people treated with insulin and is more common in Type 1 than in Type 2 diabetes 1, 2, 3. Hypoglycaemia increases in frequency and severity with the duration of insulin treatment 3. Impaired awareness of hypoglycaemia is common in people treated with insulin, particularly those with Type 1 diabetes 4.
Hypoglycaemia has a significant burden as it can engender fear and anxiety, disrupt sleep and adversely affect domestic and social life 5, 6. Interestingly, rates of severe hypoglycaemia have not generally diminished over the years, despite the introduction of insulin analogues and advances in glucose monitoring 7, 8. This may be a consequence of the drive to tighter glucose targets, which have diminished the benefits of technological advances. Attempting to reduce this burden can encourage the maintenance of a suboptimal glycaemic control 5, 9, 10, and both the severity and frequency of hypoglycaemia unequivocally reduce health‐related quality of life 11, 12, 13, 14. Hypoglycaemia is also a burden on healthcare resources and on society as a consequence of the direct costs of its treatment and the indirect costs associated with lost productivity 9, 15, 16. These and other factors can negatively influence physicians and people with diabetes, promoting reluctance to initiate or intensify therapy — insulin in particular — because of the perceived burden of hypoglycaemia 10, 17.
The benefits of stricter glycaemic control are counterbalanced by the enhanced risk of concomitant hypoglycaemia. The most expensive aspect of treating severe hypoglycaemia is hospital admission and inpatient care 15, 16. Severe hypoglycaemia is a common cause of hospitalization in elderly people with diabetes 18, 19, 20, 21. To our knowledge, no large‐scale studies of the resource use associated with severe hypoglycaemia have been performed that have examined this resource use according to type of diabetes and insulin regimen. The aim of this analysis was to estimate the resource use attributable to severe hypoglycaemia events in a large cohort of people with insulin‐treated diabetes, using data from a large‐scale clinical trial programme.
Materials and methods
This analysis used data from the insulin degludec (IDeg) and insulin degludec/insulin aspart (IDegAsp) phase 3a clinical trial programme, including 15 phase 3a therapeutic confirmatory trials, which involved more than 8000 participants. All trials were registered on ClinicalTrials.gov, the trial protocols were approved by independent ethics committees or institutional review boards, and written informed consent was obtained from participants before enrolment. Trials were undertaken in accordance with the Declaration of Helsinki and good clinical practice guidelines 22, 23. The trials were categorized into three groups, depending on the patient population and the type of insulin regimen: Type 1 diabetes receiving basal–bolus therapy, Type 2 diabetes receiving multiple daily injections and Type 2 diabetes receiving basal–oral therapy.
Severe hypoglycaemia events were identified using information relating to adverse‐event case reports in the clinical trial safety database. Only clearly stated resource use was included in the analysis, and all events were analysed independently. The data were analysed descriptively, taking into consideration three variables (that are not necessarily mutually exclusive):
Non‐medical assistance only, with the response categories: Yes, No.
Ambulance or (onsite) emergency team, with the response categories: Yes, No.
Hospital or emergency room visit, with the response categories: No, Yes ≤ 24 h, Yes > 24 h.
Given the multiplicity of countries in the trial programme, it was decided not to attempt to distinguish between hospital inpatient treatment and emergency room treatment (with subsequent discharge), because the local procedures varied. Hospital visits were therefore distinguished solely on duration (≤ 24 h or > 24 h).
Results
Details of the trials and characteristics of the patient populations are summarized in Table 1 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36. In general, people with Type 1 diabetes had a lower mean age, longer disease duration and lower BMI compared with those with Type 2 diabetes. Across the 15 open‐label, randomized, treat‐to‐target clinical trials, severe hypoglycaemia was an infrequent occurrence: 536 severe hypoglycaemia events were recorded and analysed from a total of 8364 patient records. Most occurred in people with Type 1 diabetes receiving basal–bolus therapy, fewer occurred in those with Type 2 diabetes on multiple daily injections, and a small number occurred in people with Type 2 diabetes on basal–oral therapy (Table 1). One reported event of severe hypoglycaemia had a fatal outcome.
Table 1.
Severe hypoglycaemia events by trial
| Group | Trial type | Trial | Number of subjects randomized | Duration of trial (weeks) | Number of events | Proportion of patients with eventsb | Rate per 100 patient years of exposure | Age, years mean (sd) | Duration of diabetes, years, mean (sd) | BMI, kg/m2, mean (sd) |
|---|---|---|---|---|---|---|---|---|---|---|
| Type 1 diabetes basal–bolus | IDeg vs. IGlar trials | BEGIN Basal–bolus Type 1 (3583) 24 | 629 | 52 | 113 | 11.8% | 20 | 43.0 (13.6) | 18.9 (12.0) | 26.3 (3.8) |
| BEGIN Flex T1 (3770) 25 | 493 | 26 | 90 | 11.0% | 40 | 43.7 (13.1) | 18.5 (12.2) | 26.7 (3.9) | ||
| IDeg vs. IDet trial | BEGIN BB T1 (3585) 26 | 456 | 26 | 73 | 10.6% | 34 | 41.3 (14.7) | 13.9 (10.3) | 23.9 (3.5) | |
| IDegAsp vs. IDet trial | BOOST T1 (3594, inc. extension 3645) 27 | 548 | 52 | 144 | 14.9% | 33 | 41.3 (13.2) | 17.4 (11.6) | 26.4 (4.0) | |
| Type 2 diabetes multiple daily injections | IDeg vs. IGlar trial | BEGIN Basal–bolus Type 2 (3582) 28 | 1006 | 52 | 53 | 4.5% | 6 | 58.9 (9.3) | 13.5 (7.3) | 32.2 (4.6) |
| IDegAsp BID vs. BIAsp BID trials | BOOST Intensify Premix I (3592) 29 | 447 | 26 | 34 | 5.2% | 17 | 58.7 (9.8) | 13.0 (7.1) | 29.3 (4.8) | |
| BOOST Intensify All (3597) 30 | 424 | 26 | 8 | 1.4% | 4 | 59.8 (10.0) | 16.3 (8.0) | 25.4 (3.5) | ||
| Type 2 diabetes basal–oral therapy | IDeg vs. IGlar trials | BEGIN Low Volume (3672) 31 | 460 | 26 | 0 | 0.0% | 0 | 57.5 (9.2) | 8.2 (6.2) | 32.4 (5.4) |
| BEGIN Once Long (3579) 32 | 1030 | 52 | 7 | 0.7% | 1 | 59.1 (9.8) | 9.2 (6.2) | 31.1 (4.7) | ||
| BEGIN Once Asia (3586) 33 | 435 | 26 | 1 | 0.2% | 0 | 58.6 (9.9) | 11.6 (6.5) | 25.0 (3.6) | ||
| BEGIN Flex (3668) 34 | 687 | 26 | 6 | 0.7% | 2 | 56.4 (9.6) | 10.6 (6.7) | 29.6 (4.7) | ||
| IDeg vs. sitagliptin trial | BEGIN Early (3580) (in IDeg arm) 35 | 458 | 26 | 1 | 0.2 | 1 | 55.7 (10.9) | 7.7 (6.0) | 30.4 (5.1) | |
| IDegAsp vs. IGlar | BOOST Start 1 (3590)a , c | 530 | 26 | 2 | 0.4% | 1 | 56.9 (9.1) | 9.2 (6.1) | 30.7 (5.1) | |
| BOOST Intensify Basal (3593)a , c | 465 | 26 | 4 | 0.6% | 2 | 58.1 (9.8) | 11.5 (7.0) | 30.1 (5.2) | ||
| BOOST Japan (3896) 36 | 296 | 26 | 0 | 0.0% | 0 | 60.5 (9.8) | 11.7 (8.0) | 25.1 (3.8) |
NB. The three‐times‐weekly trials were not included in this analysis.
All but one event occurred in the IGlar arm in the trials 3590 and 3593.
As a proportion of the number of randomized patients included in the safety analysis set in each trial. All trial products were administered once daily unless otherwise stated.
Study not published.
BIAsp 30, biphasic insulin aspart; BID, twice daily; BMI, body mass index; IDeg, insulin degludec; IDegAsp, insulin degludec/insulin aspart; IDet, insulin detemir; IGlar, insulin glargine.
Overall, 157/536 (29.3%) of severe hypoglycaemia events required an ambulance/emergency team (mainly involving paramedics, but also including physicians, although the analysis did not distinguish between them) (Table 2). Additionally, following 100/536 (18.7%) of the severe hypoglycaemia events, hospital or emergency room treatment was required, with 36/536 (6.7% points) of these requiring a hospital stay of > 24 h (Table 2).
Table 2.
Proportion of severe hypoglycaemia events leading to ambulance, emergency room or hospital visits
| Group | Total number of events | Resource use | Number of events (%) |
|---|---|---|---|
| Type 1 diabetes basal–bolus | 420 | Non‐medical assistance only | 261 (62.1) |
| Ambulance/emergency team | 130 (31.0) | ||
| Hospital or emergency room ≤ 24 h | 40 (9.5) | ||
| Hospital > 24 h | 21 (5.0) | ||
| Type 2 diabetes multiple daily injections | 95 | Non‐medical assistance only | 52 (54.7) |
| Ambulance/emergency team | 24 (25.3) | ||
| Hospital or emergency room ≤ 24 h | 22 (23.2) | ||
| Hospital > 24 h | 5 (5.3) | ||
| Type 2 diabetes basal–oral therapy | 21 | Non‐medical assistance only | 9 (42.9) |
| Ambulance/emergency team | 3 (14.3) | ||
| Hospital or emergency room ≤ 24 h | 2 (9.5) | ||
| Hospital > 24 h | 10 (47.6) | ||
| All | 536 | Non‐medical assistance only | 322 (60.1) |
| Ambulance/emergency team | 157 (29.3) | ||
| Hospital or emergency room ≤ 24 h | 64 (11.9) | ||
| Hospital > 24 h | 36 (6.7) |
Although fewer events were recorded in people with Type 2 compared with Type 1 diabetes, once an event occurred, the proportion resulting in hospital or emergency room treatment was greatest in the basal–oral therapy treatment group. Resources were used in relation to 57.1%, 45.3% and 37.9% of severe hypoglycaemia events in the Type 2 diabetes basal–oral therapy, Type 2 diabetes multiple daily injections and Type 1 diabetes basal–bolus groups, respectively (Fig. 1). The greatest difference was in the proportion of events requiring a hospital stay of > 24 h: 47.6% in the Type 2 diabetes basal–oral therapy, 5.3% for Type 2 diabetes multiple daily injections, and 5.0% for the Type 1 diabetes basal–bolus group (Table 2).
Figure 1.
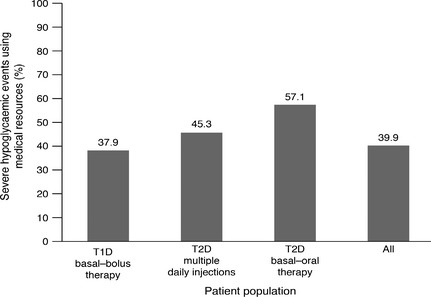
The proportion of severe hypoglycaemia events utilizing medical resources. Medical resource use includes use of ambulance/emergency team, hospital visit < 24 h and > 24 h. The variables measured were not necessarily mutually exclusive. T1D, Type 1 diabetes; T2D, Type 2 diabetes.
Discussion
Poor glycaemic control in diabetes is associated with serious complications such as sight‐threatening retinopathy and renal failure, and optimizing glycaemic control is fundamental to minimize this morbidity 37, 38, 39. However, severe hypoglycaemia, a serious adverse effect of some glucose‐lowering therapies — particularly insulin — can also cause significant morbidity and have quality of life and economic consequences.
This analysis utilized data from a large‐scale clinical trial programme and subdivided records by type of diabetes and also by insulin regimen. This has shown that in the controlled clinical trial setting, severe hypoglycaemia seldom occurs, but when it does, it leads to direct healthcare resource use in ~ 40% of cases.
A large difference was observed between the different regimens in the proportion of people experiencing severe hypoglycaemia who required hospitalization for > 24 h. The proportion of participants using resources following a severe hypoglycaemia event was highest in the people with Type 2 diabetes on basal–oral therapy, despite the overall lower number of events compared with the patients on multiple daily injections. This may be because many of the participants receiving basal–oral therapy (and their family/carers) were much less experienced in treating severe hypoglycaemia. They may be more inclined to seek medical assistance, in contrast to people with Type 1 diabetes and those with insulin‐treated Type 2 diabetes taking multiple daily injections who probably have greater experience of exposure to severe hypoglycaemia. People with diabetes and their family members in these groups are less likely to seek assistance because they have learned to cope effectively with this emergency and the immediate requirements of treating an episode of severe hypoglycaemia. Furthermore, the people with Type 2 diabetes included in the trials had a higher mean age than those with Type 1 diabetes, and this may mean that more of them had more comorbidities, were frailer and were more vulnerable to morbidity associated with severe hypoglycaemia, so contributing to the longer duration of hospital admissions. Several studies have shown that hypoglycaemia requiring hospital treatment is more frequent in the elderly compared with younger people with diabetes 19, 20, 21.
It should also be noted that, in some trials, people with Type 2 diabetes were taking concomitant oral anti‐diabetes agents, including sulphonylureas, and it is not clear whether these contributed to any of the severe hypoglycaemia events. Some specialists believe that hospital admission is necessary for people who experience severe hypoglycaemia as a consequence of sulphonylurea therapy, which might account for some of the hospital attendances by the participants receiving basal–oral therapy 40, 41. A previous study observed that the proportion of people for whom emergency medical assistance was sought was higher in those with Type 2 diabetes than with Type 1 diabetes (33% vs. 10%) 2. A population‐based study analysing resource use associated with severe hypoglycaemia treated by emergency medical services in Tayside, Scotland, recorded 260 episodes over a 12‐month period. Of these, 34% involved the ambulance service alone, 7% were treated by emergency/primary care services alone and 52% used both. Some 28% of cases required hospital admission, resulting in hospital occupancy of 230 bed days 16. Although this is higher than the 18.6% who visited hospital in our study, it should be noted that this Scottish study analysed only those events that had required emergency medical treatment, thus representing a more severe end of the spectrum.
The use of medical resources following severe hypoglycaemia is costly, considering the large number of people with insulin‐treated diabetes and the extensive use of sulphonyl‐ureas. For example, an estimate of this resource utilization and associated financial burden based on the 2013–2014 UK National Health Service tariffs for ‘Admitted Patient Care & Outpatient Procedures — Diabetes with Hypoglycaemic Disorders’ equates to £1269 for people aged ≤ 69 years and £2187 for people aged ≥ 70 years, in addition to £235 for an ambulance transfer. This yields an average cost per event across treatment regimens of £305 [(11.9% + 6.7%) * £1269 + 29.3% * £235] for people aged ≤ 69 years and £476 [(11.9% + 6.7%) * £2187 + 29.3% * £235] for people aged ≥ 70 years 42. Hypoglycaemia is more costly in elderly populations with diabetes, who may be much more susceptible to severe hypoglycaemia, perhaps in relation to co‐existing comorbidities 18, 19, 20, 21.
Several other studies using different methodologies have provided results that are in broad agreement with our study. Hospitalization is the major cost associated with treating hypoglycaemia, but costs vary depending on the countries involved and local practices and procedures. For example, the costs associated with treating hypoglycaemia in Germany are higher than elsewhere because people with diabetes are often admitted to hospital for several days to receive additional education 43. The study based in Tayside estimated the direct costs of treating severe hypoglycaemia — based on £127 for an ambulance, £89 for emergency room treatment and £218 for each patient admitted to a ward — to be over £13 million per year for the UK in 2003 (equivalent to £17 million in 2014 prices) 16. Based on these values, the cost of a single severe hypoglycaemia event, which required some medical assistance, has been estimated at £335 44. Data obtained using a questionnaire regarding a participant's most recent severe hypoglycaemia event performed in Germany, Spain and the UK revealed that the cost of treating a person with Type 2 diabetes (Germany, €533; Spain, €691; UK, €537) was higher than for a person with Type 1 diabetes (€441, €577 and €236, respectively) 15. A US‐based study investigating the cost of hypoglycaemia in people with insulin‐treated Type 2 diabetes estimated the direct costs of severe events requiring assistance from a healthcare professional as $1729, whereas those events requiring non‐medical assistance were $242 per event 45.
Most previous studies have been retrospective assessments of all people with severe hypoglycaemia, and often exclusively those who had utilized additional healthcare resources. Therefore, they have not consistently stratified people according to their treatment regimen. Although some of these studies included cost analyses, they did not include all severe hypoglycaemia events, so it is difficult to determine what proportion of events required ambulance and hospital treatment. This study benefits from its size and the more accurate and reliable recording associated with controlled clinical trials, by contrast with data that have been collected in real‐world observational or retrospective studies.
This study has the limitation that the rates of hypoglycaemia may be lower than in a real‐world setting, because people at high risk of severe hypoglycaemia or who were judged by the investigator to have impaired hypoglycaemia awareness were excluded from the clinical trials. However, because the examination was not primarily aimed at assessing the risk of events, but rather exploring the consequences of such an event, it is unclear to what extent such a limitation would influence the results. Additionally, because of the global nature of the clinical trials, a huge variation in healthcare utilization and local procedures are to be expected, making estimates of resource use difficult to determine with accuracy. Healthcare utilization in trials is often protocol driven, but this has not been assumed in this analysis because of the acute nature of severe hypoglycaemia. Potential exists for the under‐reporting of ambulance use, or inconsistent reporting of resource use. Although it is known when people had attended hospital, information about how they arrived there was not consistently recorded, so only clearly stated resource use was included in the analysis. This study did not attempt to capture length of stay or what medication was received because this would be healthcare‐sector specific and could be confounded by other comorbidities. The study has reported only the direct resource use relating to the involvement of emergency services and hospital treatment and does not include additional blood glucose measurements, costs of glucagon or intravenous dextrose, or any subsequent medical consultations that were necessary following a severe hypoglycaemia episode. These costs were estimated in a Canadian study 46. Productivity loss or the costs of informal caregivers were not measured.
Conclusion
This analysis suggests that severe hypoglycaemia events often result in emergency/ambulance calls and treatment in hospital, thereby incurring a substantial economic burden. A high rate of resource utilization was observed with all insulin regimens, and although the incidence of severe hypoglycaemia events was low, the greatest level of resource use following an event occurred in people with Type 2 diabetes on a regimen of basal insulin combined with oral anti‐diabetes drugs.
Funding sources
The clinical trials were sponsored by Novo Nordisk. Novo Nordisk contributed to the study design and conduct, data collection, analysis and interpretation of the trials.
Competing interests
SH has served on advisory panels and speaker bureaus for Eli Lilly, Novo Nordisk and Takeda, and received research support from Medtronic. BMF has served on advisory panels and speaker bureaus for Eli Lilly, Novo Nordisk, MSD, Sanofi, Boehringer Ingelheim and Janssen. MH and JG are employees of Novo Nordisk. SG has served on advisory boards for Novo Nordisk, Sanofi, Takeda and Eli Lilly and has received research support from Novo Nordisk, Sanofi and Takeda.
Authors’ contributions
All authors contributed to the design, interpretation, writing, evaluation and approval of this manuscript. JG and MH were responsible for the data collection and analysis. SH takes responsibility for the integrity of this article.
Acknowledgements
The authors thank Adele Norman and Daria Renshaw of Watermeadow Medical, UK, for assistance with the drafting and editing of this article: this work was funded by Novo Nordisk.
Diabet. Med. 33, 471–477 (2016)
References
- 1. Akram K, Pedersen‐Bjergaard U, Carstensen B, Borch‐Johnsen K, Thorsteinsson B. Frequency and risk factors of severe hypoglycaemia in insulin‐treated Type 2 diabetes: a cross‐sectional survey. Diabet Med 2006; 23: 750–756. [DOI] [PubMed] [Google Scholar]
- 2. Donnelly LA, Morris AD, Frier BM, Ellis JD, Donnan PT, Durrant R et al Frequency and predictors of hypoglycaemia in type 1 and insulin‐treated type 2 diabetes: a population‐based study. Diabet Med 2005; 22: 749–755. [DOI] [PubMed] [Google Scholar]
- 3. UK Hypoglycaemia Study Group . Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia 2007; 50: 1140–1147. [DOI] [PubMed] [Google Scholar]
- 4. Graveling AJ, Frier BM. Impaired awareness of hypoglycaemia: a review. Diabetes Metab 2010; 36(Suppl 3): S64–S74. [DOI] [PubMed] [Google Scholar]
- 5. Frier BM. How hypoglycaemia can affect the life of a person with diabetes. Diabetes Metab Res Rev 2008; 24: 87–92. [DOI] [PubMed] [Google Scholar]
- 6. Harris SB, Khunti K, Landin‐Olsson M, Galbo‐Jørgensen CB, Bøgelund M, Chubb B et al Descriptions of health states associated with increasing severity and frequency of hypoglycemia: a patient‐level perspective. Patient Prefer Adherence 2013; 7: 925–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. MacLeod KM, Hepburn DA, Frier BM. Frequency and morbidity of severe hypoglycaemia in insulin‐treated diabetic patients. Diabet Med 1993; 10: 238–245. [DOI] [PubMed] [Google Scholar]
- 8. Kristensen PL, Hansen LS, Jespersen MJ, Pedersen‐Bjergaard U, Beck‐Nielsen H, Christiansen JS et al Insulin analogues and severe hypoglycaemia in type 1 diabetes. Diabetes Res Clin Pract 2012; 96: 17–23. [DOI] [PubMed] [Google Scholar]
- 9. Brod M, Christensen T, Thomsen TL, Bushnell DM. The impact of non‐severe hypoglycemic events on work productivity and diabetes management. Value Health 2011; 14: 665–671. [DOI] [PubMed] [Google Scholar]
- 10. Leiter LA, Yale JF, Chiasson JL, Harris SB, Kleinstiver P, Sauriol L. Assessment of the impact of fear of hypoglycemic episodes on glycemic and hypoglycemic management. Can J Diabetes 2005; 29: 186–192. [Google Scholar]
- 11. Levy AR, Christensen TL, Johnson JA. Utility values for symptomatic non‐severe hypoglycaemia elicited from persons with and without diabetes in Canada and the United Kingdom. Health Qual Life Outcomes 2008; 6: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lundkvist J, Berne C, Bolinder B, Jonsson L. The economic and quality of life impact of hypoglycemia. Eur J Health Econ 2005; 6: 197–202. [DOI] [PubMed] [Google Scholar]
- 13. Vexiau P, Mavros P, Krishnarajah G, Lyu R, Yin D. Hypoglycaemia in patients with type 2 diabetes treated with a combination of metformin and sulphonylurea therapy in France. Diabetes Obes Metab 2008; 10(Suppl 1): 16–24. [DOI] [PubMed] [Google Scholar]
- 14. Evans M, Khunti K, Mamdani M, Galbo‐Jørgensen CB, Gundgaard J, Bøgelund M et al Health‐related quality of life associated with daytime and nocturnal hypoglycaemic events: a time trade‐off survey in five countries. Health Qual Life Outcomes 2013; 11: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hammer M, Lammert M, Mejias SM, Kern W, Frier BM. Costs of managing severe hypoglycaemia in three European countries. J Med Econ 2009; 12: 281–290. [DOI] [PubMed] [Google Scholar]
- 16. Leese GP, Wang J, Broomhall J, Kelly P, Marsden A, Morrison W et al Frequency of severe hypoglycemia requiring emergency treatment in type 1 and type 2 diabetes: a population‐based study of health service resource use. Diabetes Care 2003; 26: 1176–1180. [DOI] [PubMed] [Google Scholar]
- 17. Dailey GE III. Early insulin: an important therapeutic strategy. Diabetes Care 2005; 28: 220–221. [DOI] [PubMed] [Google Scholar]
- 18. Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med 2011; 365: 2002–2012. [DOI] [PubMed] [Google Scholar]
- 19. Geller AI, Shehab N, Lovegrove MC, Kegler SR, Weidenbach KN, Ryan GJ et al National estimates of insulin‐related hypoglycemia and errors leading to emergency department visits and hospitalizations. JAMA Intern Med 2014; 174: 678–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Greco D, Pisciotta M, Gambina F, Maggio F. Severe hypoglycaemia leading to hospital admission in type 2 diabetic patients aged 80 years or older. Exp Clin Endocrinol Diabetes 2010; 118: 215–219. [DOI] [PubMed] [Google Scholar]
- 21. Lipska KJ, Ross JS, Wang Y, Inzucchi SE, Minges K, Karter AJ et al National trends in US hospital admissions for hyperglycemia and hypoglycemia among Medicare beneficiaries, 1999 to 2011. JAMA Intern Med 2014; 174: 1116–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. International Conference on Harmonisation . ICH Harmonised Tripartite Guideline: guideline for good clinical practice. J Postgrad Med 2001; 47: 199–203. [PubMed] [Google Scholar]
- 23. World Medical Association . Declaration of Helsinki. Ethical principles for medical research involving human subjects. J Indian Med Assoc 2009; 107: 403–405. [PubMed] [Google Scholar]
- 24. Heller S, Buse J, Fisher M, Garg S, Marre M, Merker L et al Insulin degludec, an ultra‐longacting basal insulin, versus insulin glargine in basal–bolus treatment with mealtime insulin aspart in type 1 diabetes (BEGIN Basal‐Bolus Type 1): a phase 3, randomised, open‐label, treat‐to‐target non‐inferiority trial. Lancet 2012; 379: 1489–1497. [DOI] [PubMed] [Google Scholar]
- 25. Mathieu C, Hollander P, Miranda‐Palma B, Cooper J, Franek E, Russell‐Jones D et al Efficacy and safety of insulin degludec in a flexible dosing regimen vs insulin glargine in patients with type 1 diabetes (BEGIN: Flex T1): a 26‐week randomized, treat‐to‐target trial with a 26‐week extension. J Clin Endocrinol Metab 2013; 98: 1154–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Davies MJ, Gross JL, Ono Y, Sasaki T, Bantwal G, Gall MA et al Efficacy and safety of insulin degludec given as part of basal–bolus treatment with mealtime insulin aspart in type 1 diabetes: a 26‐week randomized, open‐label, treat‐to‐target non‐inferiority trial. Diabetes Obes Metab 2014; 16: 922–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hirsch IB, Bode B, Courreges JP, Dykiel P, Franek E, Hermansen K et al Insulin degludec/insulin aspart administered once daily at any meal, with insulin aspart at other meals versus a standard basal‐bolus regimen in patients with type 1 diabetes: a 26‐week, phase 3, randomized, open‐label, treat‐to‐target trial. Diabetes Care 2012; 35: 2174–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Garber AJ, King AB, Del Prato S, Sreenan S, Balci MK, Muñoz‐Torres M et al Insulin degludec, an ultra‐longacting basal insulin, versus insulin glargine in basal–bolus treatment with mealtime insulin aspart in type 2 diabetes (BEGIN Basal‐Bolus Type 2): a phase 3, randomised, open label, treat‐to‐target non‐inferiority trial. Lancet 2012; 379: 1498–1507. [DOI] [PubMed] [Google Scholar]
- 29. Fulcher GR, Christiansen JS, Bantwal G, Polaszewska‐Muszynska M, Mersebach H, Andersen TH et al Comparison of insulin degludec/insulin aspart and biphasic insulin aspart 30 in uncontrolled, insulin‐treated type 2 diabetes: a phase 3a, randomized, treat‐to‐target trial. Diabetes Care 2014; 37: 2084–2090. [DOI] [PubMed] [Google Scholar]
- 30. Kaneko S, Chow F, Choi DS, Taneda S, Hirao K, Park Y et al Insulin degludec/insulin aspart versus biphasic insulin aspart 30 in Asian patients with type 2 diabetes inadequately controlled on basal or pre‐/self‐mixed insulin: a 26‐week, randomised, treat‐to‐target trial. Diabetes Res Clin Pract 2015; 107: 139–147. [DOI] [PubMed] [Google Scholar]
- 31. Gough SC, Bhargava A, Jain R, Mersebach H, Rasmussen S, Bergenstal RM. Low‐volume insulin degludec 200 Units/mL once daily improves glycemic control similarly to insulin glargine with a low risk of hypoglycemia in insulin‐naive patients with type 2 diabetes: a 26‐week, randomized, controlled, multinational, treat‐to‐target trial: the BEGIN Low Volume trial. Diabetes Care 2013; 36: 2536–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zinman B, Philis‐Tsimikas A, Cariou B, Handelsman Y, Rodbard HW, Johansen T et al Insulin degludec versus insulin glargine in insulin‐naive patients with type 2 diabetes: a 1‐year, randomized, treat‐to‐target trial (BEGIN Once Long). Diabetes Care 2012; 35: 2464–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Onishi Y, Iwamoto Y, Yoo SJ, Clauson P, Tamer SC, Park S. Insulin degludec compared with insulin glargine in insulin‐naïve patients with type 2 diabetes: a 26‐week, randomized, controlled, Pan‐Asian, treat‐to‐target trial. J Diabetes Invest 2013; 4: 605–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Meneghini L, Atkin SL, Gough SC, Raz I, Blonde L, Shestakova M et al The efficacy and safety of insulin degludec given in variable once‐daily dosing intervals compared with insulin glargine and insulin degludec dosed at the same time daily: a 26‐week, randomized, open label, parallel‐group, treat‐to‐target trial in people with type 2 diabetes. Diabetes Care 2013; 36: 858–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Philis‐Tsimikas A, Del Prato S, Satman I, Bhargava A, Dharmalingam M, Skjøth TV et al Effect of insulin degludec versus sitagliptin in patients with type 2 diabetes uncontrolled on oral antidiabetic agents. Diabetes Obes Metab 2013; 15: 760–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Onishi Y, Ono Y, Rabol R, Endahl L, Nakamura S. Superior glycaemic control with once‐daily insulin degludec/insulin aspart versus insulin glargine in Japanese adults with type 2 diabetes inadequately controlled with oral drugs: a randomized, controlled phase 3 trial. Diabetes Obes Metab 2013; 15: 826–832. [DOI] [PubMed] [Google Scholar]
- 37. The Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group . Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA 2002; 287: 2563–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA et al Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000; 321: 405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. UK Prospective Diabetes Study (UKPDS) Group . Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837–853. [PubMed] [Google Scholar]
- 40. Frier BM, Heller S, McCrimmon R eds. Hypoglycaemia in Clinical Diabetes, 3rd ed Chichester: Wiley‐Blackwell, 2014. [Google Scholar]
- 41. Heller SR. Hypoglycaemia and diabetes In Pickup JE, Williams G. (Eds), Textbook of Diabetes, 3rd ed Oxford: Blackwell Science Ltd, 2003; 33.1–33.9. [Google Scholar]
- 42. UK National Health Service . UK Tariffs for Admitted Patient Care & Outpatient Procedures ‐ Diabetes with Hypoglycaemia Disorder, 2014. Available at https://www.gov.uk/government/publications/payment-by-results-pbr-operational-guidance-and-tariffs Last accessed June 2014.
- 43. Lammert M, Hammer M, Frier BM. Management of severe hypoglycaemia: cultural similarities, differences and resource consumption in three European countries. J Med Econ 2009; 12: 269–280. [DOI] [PubMed] [Google Scholar]
- 44. Waugh N, Cummins E, Royle P, Clar C, Marien M, Richter B et al Newer agents for blood glucose control in type 2 diabetes: systematic review and economic evaluation. Health Technol Assess 2010; 14: 1–248. [DOI] [PubMed] [Google Scholar]
- 45. Foos V, Grant D, Palmer JL, Varol N, Curtis B, Boye KS et al Quantifying the direct and indirect costs associated with severe and non‐severe hypoglycaemia in subjects with type–2 diabetes who are treated with insulin. Value Health 2013; 16: A436–A437. [Google Scholar]
- 46. Harris SB, Leiter LA, Yale JF, Chiasson JL, Kleinstiver P, Sauriol L. Out‐of‐pocket costs of managing hyperglycemia and hypoglycemia in patients with type 1 diabetes and insulin‐treated type 2 diabetes. Can J Diabetes 2007; 31: 25–33. [Google Scholar]


