Abstract
This randomized, parallel‐group study in patients inadequately controlled on olmesartan medoxomil/amlodipine (OLM/AML) 40/10 mg assessed the effects of adding hydrochlorothiazide (HCTZ) 12.5 mg and 25 mg, using seated blood pressure (SeBP) measurements and ambulatory blood pressure (BP) monitoring. Enrolled patients were screened and tapered off of therapy if required. All patients received OLM/AML 40/10 mg and those with mean seated BP (SeBP) ≥140/90 mm Hg after 8 weeks (n=808) were randomized (1:1:1) to continue with OLM/AML 40/10 mg or receive OLM/AML/HCTZ 40/10/12.5 or 40/10/25 mg for a further 8 weeks. The primary endpoint was the change in seated diastolic BP (SeDBP) from the start to the end of the randomized treatment period. The addition of HCTZ 25 mg significantly reduced SeDBP (−2.8 mm Hg; P<.0001), lowered seated systolic BP (SeSBP) and ambulatory DBP and SBP, and improved BP goal rates. In patients uncontrolled on OLM/AML 40/10 mg, adding HCTZ led to further BP reductions, particularly in ambulatory BP.
For the majority of patients with elevated blood pressure (BP), antihypertensive therapy with a single agent is not sufficiently effective. To lower BP to levels recommended by treatment guidelines, the majority of patients require combination therapy.1 Clinical trials have shown that combination therapy is associated with increased BP reductions and high levels of BP control.2, 3, 4 However, a recent European survey found that fewer than 40% of treated hypertensive patients had BP <140/90 mm Hg.5 This issue is addressed in the 2013 European Society of Hypertension/European Society of Cardiology (ESH/ESC) guidelines for the management of arterial hypertension in a section dedicated to the improvement of BP control.1 This notes that a key contributor to poor BP control is poor adherence to treatment, a common and widespread issue associated with elevated BP and increased risk of cardiovascular (CV) events.1
Since hypertension is common and increases in prevalence with advancing age, many patients may need to take medications for other conditions. Many patients need two or even three antihypertensive drugs to achieve BP control, and so pill intake can easily become a burden. To address this issue, single‐pill fixed‐dose combinations (FDCs) have been developed. The 2013 ESH/ESC guidelines favor the use of single‐pill combinations of two antihypertensive drugs at fixed doses because reducing pill burden improves adherence. The guidelines also note that the same guidance is true for FDCs of three drugs, which usually comprise a blocker of the renin‐angiotensin system (RAS), a calcium channel blocker (CCB), and a diuretic.1 Combinations based on agents that block the RAS, especially angiotensin II receptor blockers (ARBs), are associated with good BP‐lowering efficacy and excellent tolerability.6 Combining ARBs with agents such as CCBs and diuretics offers increased BP‐lowering with little or no effect on tolerability.6
A single‐pill fixed‐dose triple combination of the ARB olmesartan medoxomil (OLM) plus the CCB amlodipine (AML) and the diuretic hydrochlorothiazide (HCTZ) has been developed for patients with hypertension. The efficacy and safety of fixed‐dose triple‐combination therapy with these three agents has previously been established.7, 8 In this report, we describe a study that assessed OLM/AML/HCTZ as second‐line add‐on therapy in patients whose BP was inadequately controlled on dual‐combination therapy. The study used conventional seated diastolic BP (SeDBP) as the primary efficacy parameter, with seated systolic BP (SeSBP) and 24‐hour ambulatory BP monitoring (ABPM) as secondary measures.
Patients and Methods
Study Population
Eligible patients, aged 18 years and older, were included based on their history of antihypertensive therapy. Patients who were not taking antihypertensive therapy at screening, and patients receiving combination antihypertensive therapy prior to the study that did not include OLM, AML, or HCTZ (who were tapered off their medication within 5 weeks of screening), were required to have a mean SeSBP ≥160 mm Hg and a mean SeDBP ≥100 mm Hg. Criteria for patients receiving a stable dose of antihypertensive monotherapy for 4 weeks prior to screening were a mean SeSBP ≥150 mm Hg and mean SeDBP ≥95 mm Hg. Also, patients receiving a stable dose of any combination of OLM, AML, or HCTZ therapy for 4 weeks prior to screening were required to have a mean SeSBP ≥140 mm Hg and mean SeDBP ≥90 mm Hg.
Key exclusion criteria included uncontrolled type 1 or 2 diabetes, clinical evidence of renal disease, secondary hypertension, or severe heart failure (New York Heart Association class III–IV). Patients with myocardial infarction, unstable angina pectoris, percutaneous coronary intervention, heart failure, hypertensive encephalopathy, cerebrovascular accident (stroke), or transient ischemic attack within the previous 6 months were also excluded. In addition, patients already taking four or more antihypertensive agents were excluded. In addition, patients with a mean SeSBP ≥200 mm Hg, mean SeDBP ≥115 mm Hg, 24‐hour ambulatory diastolic BP (DBP) >104 mm Hg, or bradycardia (heart rate <50 beats per minute at rest) were excluded or immediately discontinued if such observations were made during the study.
Study Design
This was a phase III, randomized, parallel‐group, multicenter, multinational, add‐on study (ClinicalTrials.gov identifier: NCT00902538). The study design (Figure 1) included a screening/tapering‐off period (1–5 weeks), a single‐blind run‐in, and a randomized double‐blind add‐on period.
Figure 1.
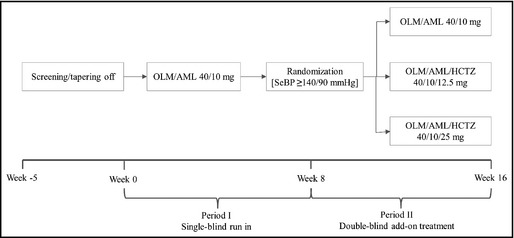
Study design. AML indicates amlodipine; HCTZ, hydrochlorothiazide; OLM, olmesartan; SeBP, seated blood pressure.
The protocol, amendments, and informed consent document were approved by the independent ethics committee for each center prior to study initiation. This study was designed and monitored in accordance with the Declaration of Helsinki. Written informed consent was received from all patients prior to admission.
Schedule of Interventions
Patients receiving antihypertensive medication at screening entered a 1‐ to 5‐week tapering‐off period. Period I was a single‐blind run‐in (weeks 0–8) during which all patients received OLM/AML 40/10 mg. Patients who experienced symptomatic hypotension were discontinued. Patients who had a mean SeBP ≥140/90 mm Hg at week 8 continued into period II, an 8‐week, double‐blind, add‐on period (weeks 8–16) during which patients who satisfied the BP criteria were randomized (1:1:1) to either continue with OLM/AML 40/10 mg or receive OLM/AML/HCTZ 40/10/12.5 mg or OLM/AML/HCTZ 40/10/25 mg. During randomization, patients were stratified by age group (<65 years, ≥65 years), diabetic status, and study site to provide a balanced distribution of these attributes in each treatment group.
Schedule of Assessments
Patients visited the clinic at screening and at weeks 0, 4, 8, 12, and 16. If required, patients began a washout period within 3 weeks of screening and were tapered off their medication over the course of 1 to 2 weeks. SeBP was measured at screening and each subsequent study visit. The mean of three measurements was recorded as the SeBP value for that visit. ABPM was performed at weeks 0, 8, and 16 and began 24 hours prior to the visit after study medication intake. The 24‐hour profiles were recorded with standard measurement devices and readings taken at intervals of at least 15 minutes during the daytime (6 am–9:59 pm) and at least 30 minutes during the nighttime (10:00 pm–5:59 am). Baseline BP was the last measurement prior to the first dose of double‐blind medication in period II.
Patients were instructed to take their study medication at the same approximate time every morning. Compliance was assessed by counting tablets in study medication kits that were returned at each visit.
Efficacy Assessments
The primary efficacy endpoint was the change in mean SeDBP from baseline to week 16 after 8 weeks of double‐blind treatment with the last‐observation‐carried‐forward (LOCF) method. Secondary efficacy variables included the change in mean SeDBP from baseline to week 12 and the change in mean SeSBP from baseline to weeks 12 and 16 with the LOCF method. In addition, achievement of SeBP goal (SeBP <140/90 mm Hg, or <130/80 mm Hg for patients with diabetes, chronic renal disease, or chronic CV disease) and SeBP thresholds (SeBP <140/90 mm Hg, SeSBP <140 mm Hg, and SeDBP <90 mm Hg) were assessed from baseline to week 16 with LOCF; note that the study was designed before the ESH called into question the validity of the <130/80 mm Hg goal for patients with diabetes.9 In addition, least‐squares (LS) mean changes in 24‐hour, daytime, and nighttime ambulatory BP from baseline to week 16 with LOCF were measured. The achievement of 24‐hour ambulatory BP goal (BP <140/90 mm Hg, or <130/80 mm Hg for patients with diabetes, chronic renal disease, or chronic CV disease) and ambulatory BP thresholds (BP <130/85 mm Hg, <130/80 mm Hg, and <120/80 mm Hg) were also assessed from baseline to week 16 with LOCF.
Safety Assessments
Safety and tolerability were assessed at each clinic visit. This included adverse events (AEs), vital signs, electrocardiogram tests, physical examinations, and clinical laboratory evaluations. Investigators assessed the severity of AEs and whether they were treatment‐related. Serious AEs were defined as any untoward medical occurrence that at any dose resulted in death, was life‐threatening, required in‐patient hospitalization or prolonged an existing hospitalization, resulted in persistent or significant disability/incapacity, was a congenital anomaly/birth defect, or any other medically important condition. In addition, important medical events that did not result in death, were not life‐threatening, or did not require hospitalization could be considered a serious adverse experience when, based on appropriate medical judgment, they jeopardized the patient and required medical or surgical intervention to prevent one of these outcomes.
Laboratory variables were measured at screening and at weeks 0, 8, and 16, and electrocardiogram tests were performed at screening and week 16. Vital signs (SeBP, weight, and heart rate) were taken at each visit prior to taking blood for laboratory evaluations. SeBP was measured at trough, after the patient had been sitting for at least 5 minutes. For safety reasons, patients were given home BP monitors and diaries at week 0 and instructed to record their BP before taking their daily study medication. Investigators reviewed the diaries and patients were asked to contact investigators if their home SBP was ≥160 mm Hg or home DBP was ≥100 mm Hg. The home BP recordings by patients were not collected or entered into the study database.
Statistical Methods
The hypothesis was that 8 weeks of treatment with OLM/AML/HCTZ 40/10/12.5 mg or 40/10/25 mg would reduce SeDBP by ≥2.5 mm Hg than OLM/AML 40/10 mg therapy. By setting the two‐sided significance level at .05, it was estimated that 666 randomized patients (222 per arm) would be required to complete period II to detect a difference of 2.5 mm Hg between OLM/AML/HCTZ and OLM/AML with 80% power. Assuming a possible dropout rate of 15%, a total of 786 patients (262 patients per arm) were estimated to be required in period II. It was also estimated that 60% of the patients would reach goal at the end of period I and subsequently planned that a total of 1965 patients would be enrolled.
The randomized analysis set included all patients who were randomized to treatment during period II and had a randomization date. The full analysis set included all randomized patients who received at least one dose of double‐blind study medication and provided at least one SeDBP measurement after randomization. The primary efficacy analysis was performed on the full analysis set. Safety analyses were performed on the safety analysis set, which included all randomized patients who received at least one dose of double‐blind study medication in period II. Treatment comparisons were performed using an analysis of covariance model. The model included baseline BP as a covariate and treatment, age group (<65 years, ≥65 years), and diabetic status as fixed effects. Dunnett's test was also used to adjust the P values in order to control the overall two‐sided type I error rate at a .05 level of significance.
Results
Study Population
Of 3420 patients screened, 1216 (35.6%) discontinued before period I, the most common reason being failure to satisfy inclusion/exclusion criteria (1039 patients [30.4%]). Of the 2204 patients who entered period I, 2086 (94.6%) completed this part of the study. The main reasons for discontinuation were AEs (49 patients [2.2%]), patient withdrawal from the study (30 patients [1.4%]), and protocol violation (22 patients [1.0%]). Of the remaining patients, 1235 (56.0%) met BP criteria for randomization into period II; there were 427 patients (19.4%) who did not continue to period II. A total of 808 patients (randomized analysis set) were randomized to double‐blind treatment during period II, 785 (97.2%) of whom completed this part of the study; nine (1.1%) patients discontinued because of an AE, four (0.5%) patients withdrew from the study, four (0.5%) patients discontinued because of protocol violations, two (0.2%) patients were lost to follow‐up, and four (0.5%) patients discontinued for other reasons. Both the full analysis set and the safety analysis set each comprised 806 patients (99.8%). Randomized patients included 469 men (58.0%) and 339 women (42.0%) and 99.9% were Caucasian; the mean age was 55.8 years and the mean weight was 88.2 kg. Of the randomized patients, 12.9% were diabetic, 36.0% had CV disease, and 2.5% had chronic renal disease. The population had a mean body mass index of 30.4 kg/m2. The mean duration of hypertension was 9.9 years, mean baseline SeSBP/SeDBP was 148.3/93.6 mm Hg, and mean baseline 24‐hour ambulatory BP was 130.4/80.0 mm Hg (Table 1). Overall, there were no significant differences among treatment groups in terms of baseline patient characteristics and BP measurements. Mean compliance with treatment, which ranged from 99.0% to 99.9%, and the use of concomitant medications was similar among treatment groups.
Table 1.
Patient Demographics and Baseline Characteristics (Randomized Analysis Set)
| Characteristics | OLM/AML 40/10 mg (n=269) | OLM/AML/HCTZ 40/10/12.5 mg (n=269) | OLM/AML/HCTZ 40/10/25 mg (n=270) | All Groups (N=808) |
|---|---|---|---|---|
| Age, y | 55.9 (10.6) | 56.5 (10.3) | 54.9 (10.4) | 55.8 |
| <65 | 213 (79.2) | 214 (79.6) | 216 (80.0) | 643 (79.6) |
| ≥65 | 56 (20.8) | 55 (20.4) | 54 (20.0) | 165 (20.4) |
| Men | 150 (55.8) | 165 (61.3) | 154 (57.0) | 469 (58.0) |
| Diabetic | 37 (13.8) | 34 (12.6) | 33 (12.2) | 104 (12.9) |
| Mean BMI, kg/m2 | 30.3 (4.9) | 30.3 (4.7) | 30.5 (4.9) | 30.4 |
| BMI ≥30 kg/m2 | 131 (48.7) | 125 (46.5) | 138 (51.1) | 394 (48.8) |
| Cardiovascular disease | 97 (36.1) | 95 (35.3) | 99 (36.7) | 291 (36.0) |
| Chronic renal disease | 9 (3.3) | 5 (1.9) | 6 (2.2) | 20 (2.5) |
| Ex‐smoker | 29 (10.8) | 27 (10.0) | 27 (10.0) | 83 (10.3) |
| Current smoker | 48 (17.8) | 47 (17.5) | 48 (17.8) | 143 (17.7) |
| Duration of HTN, y | 9.8 (8.7) | 10.1 (8.8) | 9.7 (8.8) | 9.9 |
| SeSBP/SeDBP, mm Hga | 147.9/93.6 | 148.8/93.6 | 148.3/93.7 | 148.3/93.6 |
| 24‐h ambulatory SBP/DBP, mm Hga | 130.4/79.8 | 130.3/80.5 | 130.4/79.8 | 130.4/80.0 |
| Daytime ambulatory SBP/DBP, mm Hga | 134.9/83.7 | 134.7/84.4 | 134.8/83.7 | 134.8/83.9 |
| Nighttime ambulatory SBP/DBP, mm Hga | 121.3/72.0 | 121.5/72.7 | 121.6/71.9 | 121.5/72.2 |
| Mild or moderate HTNb , c | 248 (92.2) | 255 (95.1) | 243 (90.3) | 746 (92.3) |
| Severe HTNb , c | 21 (7.8) | 13 (4.9) | 26 (9.7) | 60 (7.4) |
Abbreviations: AML, amlodipine; BMI, body mass index; DBP, diastolic blood pressure; HCTZ, hydrochlorothiazide; HTN, hypertension; OLM, olmesartan; SBP, systolic blood pressure; SeDBP, seated diastolic blood pressure; SeSBP, seated systolic blood pressure. Continuous variables are expressed as mean (standard deviation [SD]) and categorical variables as number (percentage). aBaseline for blood pressure was defined as the last measurement prior to the first dose of randomized study medication in period II. bMild: SeSBP ≥140 mm Hg and ≤159 mm Hg; SeDBP ≥90 mm Hg and ≤99 mm Hg; moderate: SeSBP >159 mm Hg and ≤179 mm Hg; SeDBP >99 mm Hg and ≤109 mm Hg; severe: SeSBP >179 mm Hg; SeDBP >109 mm Hg. cHypertension class data were based on values from the full analysis set.
Efficacy
Changes in Seated BP
The mean change in SeDBP levels from baseline to week 16 (primary efficacy variable) after 8 weeks of double‐blind treatment was larger in the two triple‐combination groups than in the OLM/AML group (Table 2). The between‐group difference was statistically significant for the OLM/AML/HCTZ 40/10/25 mg group (−2.8 mm Hg, P<.0001 for comparison of LS mean changes; Table 2) but not the OLM/AML/HCTZ 40/10/12.5 mg group (−1.0 mm Hg, P<.21). In contrast, after 4 weeks of treatment at week 12, both the OLM/AML/HCTZ 40/10/12.5 and 25 mg groups showed significantly larger SeDBP reductions than the OLM/AML group (−1.5 mm Hg, P=.015 and −2.0 mm Hg, P=.0006, respectively, for comparison of LS mean changes; Table 2).
Table 2.
Mean SeDBP and SeSBP Values at Baseline and LS Mean Changes by Weeks 16 and 12
| Measurement | OLM/AML 40/10 mg | OLM/AML/HCTZ 40/10/12.5 mg | OLM/AML/HCTZ 40/10/25 mg | OLM/AML 40/10 mg | OLM/AML/HCTZ 40/10/12.5 mg | OLM/AML/HCTZ 40/10/25 mg |
|---|---|---|---|---|---|---|
| Week 16 | SeDBP | SeSBP | ||||
| No.a | 269 | 268 | 269 | 269 | 268 | 269 |
| Baseline mean (SD), mm Hgb | 93.6 (3.4) | 93.7 (3.7) | 93.7 (3.3) | 147.9 (6.67) | 148.8 (7.53) | 148.3 (7.12) |
| Week 16 with LOCF mean (SD), mm Hg | 87.3 (7.3) | 86.4 (7.2) | 84.6 (8.0) | 139.7 (9.99) | 138.6 (11.21) | 136.3 (12.33) |
| LS mean (SE) change, mm Hg | −6.1 (0.55) | −7.1 (0.55) | −8.9 (0.55) | −6.9 (0.76) | −8.6 (0.77) | −10.5 (0.77) |
| P value vs baseline | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 |
| Comparison with dual therapy | ||||||
| LS mean (95% CI) difference, mm Hg | −1.0 (−2.2 to 0.2) | −2.8 (−4.0 to −1.6) | −1.8 (−3.5 to −0.1) | −3.6 (−5.3 to −1.9) | ||
| P valuec | .2062 | <.0001 | .0777 | <.0001 | ||
| Week 12 | SeDBP | SeSBP | ||||
|---|---|---|---|---|---|---|
| No.a | 265 | 266 | 264 | 265 | 266 | 264 |
| Baseline mean (SD), mm Hgb | 93.5 (3.33) | 93.7 (3.68) | 93.6 (3.27) | 147.9 (6.60) | 148.8 (7.56) | 148.2 (7.05) |
| Week 12 with LOCF mean (SD), mm Hg | 89.0 (5.56) | 87.6 (6.93) | 87.1 (7.58) | 142.4 (9.07) | 140.4 (11.36) | 139.2 (11.21) |
| LS mean (SE) change, mm Hg | −4.5 (0.48) | −6.0 (0.49) | −6.5 (0.49) | −4.9 (0.71) | −7.6 (0.72) | −8.4 (0.72) |
| P value vs baseline | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 |
| Comparison with dual therapy | ||||||
| LS mean (95% CI) difference, mm Hg | −1.5 (−2.5 to −0.4) | −2.0 (−3.1 to −0.9) | −2.0 (−3.6 to −0.3) | −3.4 (−5.0 to −1.7) | ||
| P valuec | .0145 | .0006 | .0016 | <.0001 | ||
Abbreviations: AML, amlodipine; CI, confidence interval; HCTZ, hydrochlorothiazide; LOCF, last‐observation‐carried‐forward; LS, least‐squares; OLM, olmesartan; SD, standard deviation; SE, standard error; SeDBP, seated diastolic blood pressure; SeSBP, seated systolic blood pressure. aNo. is the number of patients with values at baseline and time point analyzed. bBaseline for blood pressure was defined as the last measurement prior to the first dose of randomized study medication in period II. cAdjusted P values were obtained from Dunnett's test to control the overall type 1 error at 0.05 level.
Changes in mean SeSBP showed a similar pattern to SeDBP. At week 16, reductions in SeSBP were larger in the two triple groups than in the OLM/AML group (Table 2). The between‐group difference was statistically significant for the OLM/AML/HCTZ 40/10/25 mg group (−3.6 mm Hg, P<.0001 for comparison of LS mean changes; Table 2) but not the OLM/AML/HCTZ 40/10/12.5 mg (−1.8, P=.08). At week 12, both the OLM/AML/HCTZ 40/10/12.5 and 40/10/25 mg groups showed significantly larger SeSBP reductions than the OLM/AML group (−2.0 mm Hg, P=.0016 and −3.4, P<.0001, respectively; Table 2). In each of the three groups, mean SeDBP and SeSBP levels were significantly reduced at weeks 12 and 16 relative to baseline (P<.0001 for each LS mean difference).
Achievement of SeBP Goal and Thresholds
At week 16, the addition of HCTZ to OLM/AML 40/10 mg enabled a greater proportion of patients to reach SeBP goal (using LOCF) compared with those who continued on OLM/AML 40/10 mg. The increase in the proportion of patients at goal was statistically significant for the OLM/AML/HCTZ 40/10/25 mg group (41.3% vs 24.2%; P<.0001) but not for the OLM/AML/HCTZ 40/10/12.5 mg group (29.5% vs 24.2%). Achievement of SeBP thresholds (SeBP <140/90 mm Hg, SeSBP <140 mm Hg, and SeDBP <90 mm Hg using LOCF) at week 16 is shown in Table 3. Treatment with triple‐combination therapy resulted in higher proportions of patients achieving all BP thresholds compared with OLM/AML 40/10 mg, but this was not statistically significant. Compared with the OLM/AML 40/10 mg group, patients who received OLM/AML/HCTZ 40/10/25 mg had significantly higher levels of SeBP <140/90 mm Hg, SeSBP <140 mm Hg, and SeDBP <90 mm Hg threshold achievement (P≤.0014 for each). For the OLM/AML/HCTZ 40/10/12.5 mg group, the levels achieved were numerically but not statistically significantly higher.
Table 3.
Proportion of Patients at Week 16 Achieving Seated and 24‐Hour Ambulatory BP Thresholds
| Treatment Comparison | Patients Achieving Threshold | P Valuea | |
|---|---|---|---|
| Triple Group | Dual Group | ||
| Threshold: SeBP <140/90 mm Hg | |||
| OLM/AML/HCTZ 40/10/12.5 mg vs OLM/AML 40/10 mg | 114 (42.5) | 102 (37.9) | .3001 |
| OLM/AML/HCTZ 40/10/25 mg vs OLM/AML 40/10 mg | 146 (54.3) | 102 (37.9) | .0001 |
| Threshold: SeSBP <140 mm Hg | |||
| OLM/AML/HCTZ 40/10/12.5 mg vs OLM/AML 40/10 mg | 130 (48.5) | 118 (43.9) | .3063 |
| OLM/AML/HCTZ 40/10/25 mg vs OLM/AML 40/10 mg | 155 (57.6) | 118 (43.9) | .0014 |
| Threshold: SeDBP <90 mm Hg | |||
| OLM/AML/HCTZ 40/10/12.5 mg vs OLM/AML 40/10 mg | 158 (59.0) | 147 (54.6) | .3493 |
| OLM/AML/HCTZ 40/10/25 mg vs OLM/AML 40/10 mg | 188 (69.9) | 147 (54.6) | .0003 |
| Threshold: ambulatory BP <130/85 mm Hg | |||
| OLM/AML/HCTZ 40/10/12.5 mg vs OLM/AML 40/10 mg | 174 (70.4) | 144 (61.5) | .0434 |
| OLM/AML/HCTZ 40/10/25 mg vs OLM/AML 40/10 mg | 179 (73.7) | 144 (61.5) | .0034 |
| Threshold: ambulatory BP <130/80 mm Hg | |||
| OLM/AML/HCTZ 40/10/12.5 mg vs OLM/AML 40/10 mg | 153 (61.9) | 110 (47.0) | .0011 |
| OLM/AML/HCTZ 40/10/25 mg vs OLM/AML 40/10 mg | 158 (65.0) | 110 (47.0) | <.0001 |
| Threshold: ambulatory BP <120/80 mm Hg | |||
| OLM/AML/HCTZ 40/10/12.5 mg vs OLM/AML 40/10 mg | 91 (36.8) | 52 (22.2) | .0005 |
| OLM/AML/HCTZ 40/10/25 mg vs OLM/AML 40/10 mg | 101 (41.6) | 52 (22.2) | <.0001 |
Abbreviations: AML, amlodipine; BP, blood pressure; HCTZ, hydrochlorothiazide; OLM, olmesartan; SeBP, seated blood pressure; SeDBP, seated diastolic blood pressure; SeSBP, seated systolic blood pressure. aEach P value was obtained from individual Cochran‐Mantel‐Haenszel tests stratified by age group and diabetic status comparing triple‐ with dual‐combination therapy.
Changes in Ambulatory BP
At week 16, each triple‐combination group showed lower levels of mean ambulatory BP than the dual‐combination group (Figure 2). Compared with the dual group, mean 24‐hour ambulatory DBP was significantly lower in patients who received OLM/AML/HCTZ 40/10/12.5 mg (−1.9 mm Hg, 95% confidence interval [CI], −3.0 to −0.8; P=.0018) and in those who received OLM/AML/HCTZ 40/10/25 mg (−3.2 mm Hg, 95% CI, −4.3 to −2.0; P<.0001). Mean daytime ambulatory DBP was also significantly lower in patients who received OLM/AML/HCTZ 40/10/12.5 mg (−2.0 mm Hg, 95% CI, −3.2 to −0.8; P=.0024) and OLM/AML/HCTZ 40/10/25 mg (−3.6 mm Hg, 95% CI, −4.8 to −2.3; P<.0001) compared with the OLM/AML group. Mean nighttime ambulatory DBP showed a significant reduction in patients who received OLM/AML/HCTZ 40/10/12.5 mg (−1.6 mm Hg, 95% CI, −2.9 to −0.3; P=.0300) and OLM/AML/HCTZ 40/10/25 mg (−2.3 mm Hg, 95% CI, −3.6 to −1.0; P=.0012) compared with OLM/AML recipients.
Figure 2.
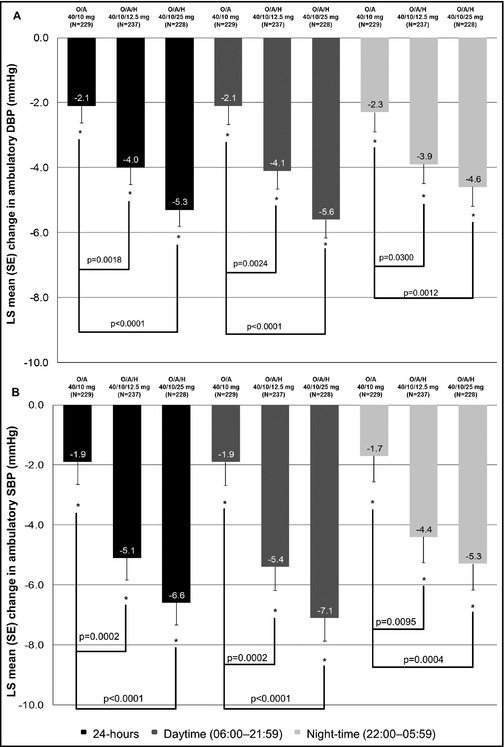
Least‐squares (LS) mean changes in ambulatory 24‐hour, daytime, and nighttime (a) diastolic blood pressure (DBP) and (b) systolic blood pressure (SBP) values from baseline (week 8) to week 16. A indicates amlodipine; H, hydrochlorothiazide; O, olmesartan; SE, standard error.
The pattern of changes in ambulatory SBP reflected those seen in ambulatory DBP. Compared with the dual OLM/AML group, mean 24‐hour ambulatory SBP was significantly lower in patients who received OLM/AML/HCTZ 40/10/12.5 mg (−3.2 mm Hg, 95% CI, −4.8 to −1.6; P=.0002) and in recipients of OLM/AML/HCTZ 40/10/25 mg (−4.6 mm Hg, 95% CI, −6.3 to −3.0; P<.0001). Mean daytime ambulatory SBP was significantly reduced in patients who received OLM/AML/HCTZ 40/10/12.5 mg (−3.4 mm Hg, 95% CI, −5.1 to −1.7; P=.0002) and OLM/AML/HCTZ 40/10/25 mg (−5.1 mm Hg, 95% CI, −6.9 to −3.4; P<.0001) compared with OLM/AML recipients. For mean nighttime ambulatory SBP, a significant reduction was seen in patients who received OLM/AML/HCTZ 40/10/12.5 mg (−2.7 mm Hg, 95% CI, −4.5 to −0.8; P=.0095) and OLM/AML/HCTZ 40/10/25 mg (−3.6 mm Hg, 95% CI, −5.4 to −1.7; P=.0004) compared with the dual OLM/AML group.
Achievement of Ambulatory BP Goal and Thresholds
The addition of HCTZ to OLM/AML 40/10 mg resulted in higher rates of 24‐hour ambulatory BP goal achievement (<140/90 mm Hg, or <130/80 mm Hg in patients with diabetes, chronic renal disease, or chronic CV disease) than continuing with OLM/AML 40/10 mg treatment. The achievement rate in the OLM/AML 40/10 mg group was 68.8%, in the OLM/AML/HCTZ 40/10/12.5 mg group it was significantly higher at 77.7% (P<.05), and in the OLM/AML/HCTZ 40/10/25 mg group it was 74.9%. Achievement of ambulatory BP thresholds using LOCF (ambulatory BP <130/85 mm Hg, <130/80 mm Hg, and <120/80 mm Hg) at week 16 are shown in Table 3. For each ambulatory BP threshold, both triple‐combination groups showed significantly higher achievement rates than the OLM/AML 40/10 mg group.
Tolerability and Safety
Treatment was well tolerated in the triple‐ and dual‐combination groups and no new safety concerns were identified for either treatment. An overview of treatment‐emergent AEs (TEAEs) and a selection of the most clinically relevant by preferred term are listed in Table 4. The proportion of patients with TEAEs was low in all three treatment groups and ranged from 13.4% to 15.0%. The frequency of drug‐related TEAEs was also similar in the triple‐ and dual‐treatment groups, ranging from 5.2% to 5.6%. Most TEAEs and drug‐related TEAEs were considered mild or moderate in severity. In addition, no patients had a serious AE that was considered drug‐related and there were no patterns of TEAE incidence that signified there might be a safety or tolerability issue in any particular treatment group. A total of nine patients (1.1%) discontinued because of any AE and seven patients (0.9%) discontinued because of any TEAE or any drug‐related TEAE (Table 4). There was a similar frequency of discontinuation because of an AE, TEAE, or drug‐related TEAE in the triple‐ and dual‐combination groups.
Table 4.
Overview of Adverse Events (Safety Analysis Set)
| Characteristic | OLM/AML 40/10 mg (n=269) | OLM/AML/HCTZ 40/10/12.5 mg (n=267) | OLM/AML/HCTZ 40/10/25 mg (n=270) | Total (N=806) |
|---|---|---|---|---|
| Total no. of TEAEs | 36 (13.4) | 40 (15.0) | 39 (14.4) | 115 (14.3) |
| Drug‐relateda TEAEs | 14 (5.2) | 14 (5.2) | 15 (5.6) | 43 (5.3) |
| Common drug‐relateda TEAEs of interest | ||||
| Peripheral edema | 8 (3.0) | 4 (1.5) | 5 (1.9) | 17 (2.1) |
| Upper respiratory tract infection | 6 (2.2) | 4 (1.5) | 2 (0.7) | 12 (1.5) |
| Bronchitis | 3 (1.1) | 3 (1.1) | 1 (0.4) | 7 (0.9) |
| Hypotension | 1 (0.4) | 0 (0.0) | 3 (1.1) | 4 (0.5) |
| Headache | 0 (0.0) | 3 (1.1) | 0 (0.0) | 3 (0.4) |
| Mild TEAE | 26 (9.7) | 28 (10.5) | 31 (11.5) | 85 (10.5) |
| Moderate TEAE | 10 (3.7) | 12 (4.5) | 7 (2.6) | 29 (3.6) |
| Severe TEAE | 0 (0.0) | 0 (0.0) | 1 (0.4) | 1 (0.1) |
| Drug‐relateda mild TEAE | 11 (4.1) | 12 (4.5) | 13 (4.8) | 36 (4.5) |
| Drug‐relateda moderate TEAE | 3 (1.1) | 2 (0.7) | 2 (0.7) | 7 (0.9) |
| Drug‐relateda severe TEAE | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| SAE | 2 (0.7) | 1 (0.4) | 2 (0.7) | 5 (0.6) |
| Drug‐relateda SAE | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Reason for discontinuation | ||||
| AE | 4 (1.5) | 2 (0.7) | 3 (1.1) | 9 (1.1) |
| TEAE | 3 (1.1) | 1 (0.4) | 3 (1.1) | 7 (0.9) |
| Drug‐relateda TEAE | 3 (1.1) | 1 (0.4) | 3 (1.1) | 7 (0.9) |
Abbreviations: AML, amlodipine; HCTZ, hydrochlorothiazide; OLM, olmesartan; SAE, serious adverse event; TEAE, treatment‐emergent adverse event. Values are expressed as number (percentage). aDrug‐related is defined as definitely, probably, or possibly related to randomized study medication.
Discussion
This add‐on study demonstrated that OLM/AML/HCTZ is effective as second‐line therapy in patients whose BP was inadequately controlled on the dual combination of OLM/AML 40/10 mg. The majority of the differences in SeBP changes between the triple and dual groups were statistically significant. The differences between the groups in ambulatory BP provided further evidence of the benefits of adding HCTZ to dual‐combination therapy with OLM/AML 40/10 mg.
The significance of this study is that it demonstrates, in a large population, the benefit of add‐on HCTZ in patients whose BP is uncontrolled on the recommended, full dose of dual‐combination therapy (OLM/AML 40/10 mg) and who need additional treatment to reach BP goal. Since all patients who were randomized were uncontrolled on dual therapy, this approach is reflective of real‐life clinical practice. The superiority of triple‐ vs dual‐combination therapy based on OLM 40 mg was established in the Triple Therapy with Olmesartan Medoxomil, Amlodipine, and Hydrochlorothiazide in Hypertensive Patients Study (TRINITY).7 The design of TRINITY compared patients who had been randomly allocated to treatment with dual‐ or triple‐combination therapy and did not investigate the benefits of adding HCTZ as second‐line therapy.7 Furthermore, the present findings are supported by analyses of changes in ambulatory BP, a more accurate and robust method of BP measurement than BP recordings made in the clinic setting.9, 10, 11 Changes in ambulatory BP were assessed in the TRINITY study, but only in a relatively small subset of patients,12 whereas in the present study, ABPM was carried out in all patients. The ABPM data presented here support the findings from the TRINITY ABPM substudy and show that OLM/AML/HCTZ triple‐combination therapy effectively reduces BP over the 24‐hour dosing period.
In the present study, each dose of triple‐combination therapy produced a significantly larger reduction in ambulatory SBP and DBP compared with patients who continued on the dual combination, and there was evidence of a dose effect. Achievement of ambulatory BP thresholds was also improved by the addition of HCTZ. This difference between seated and ambulatory BP measurements highlights the advantages of ABPM, which includes absence of “white‐coat” hypertension.9, 10, 11
The availability of single‐pill fixed‐dose combinations containing three antihypertensive agents offers major benefits for the management of hypertension. For patients who require several medications to control their BP, single‐pill FDCs have been recommended by treatment guidelines for some time, as these reduce pill burden and favor compliance.1, 13, 14 The TRINITY study compared high doses of dual‐ and triple‐combination therapy and demonstrated that triple‐combination therapy provides significantly larger BP reductions.7 A study reported by Volpe and colleagues8 showed increased BP‐lowering with triple‐ vs dual‐combination therapy across a range of doses. After completing the double‐blind study described by Volpe and colleagues, patients entered a long‐term open‐label extension in which they were treated, according to investigators' preferences, with the aim of maintaining BP control. This extension, designed to reflect general clinical practice, showed that BP reductions were maintained over the long‐term and associated with a high BP control rate (78.1%).15 The present results add to these findings by demonstrating that adding HCTZ can increase BP‐lowering in patients with inadequate BP control on a dual combination.
The availability of a treatment option that allows a patient's treatment to be initiated as monotherapy and then intensified to dual‐ and even triple‐combination therapy whilst using a single once‐daily pill throughout offers advantages for hypertension management. Some authors have suggested a treatment platform that enables the matching of appropriate dual or triple FDCs with patient types, which is based on clinical evidence, guidelines, and best practice.6 Considering the gap between BP control achieved in clinical trials and the general hypertensive population, the availability of such a platform may offer significant clinical benefits.6
In this study, relatively large changes in SeBP were seen in the group that continued with dual‐combination treatment and received a placebo instead of HCTZ. Similar results have been observed in other antihypertensive trials with a “nonresponder” design.16, 17, 18, 19, 20, 21, 22, 23, 24, 25 For fixed‐combination medicinal products, the European Medicines Agency (EMA) prefers that confirmatory clinical trials use a parallel‐group design.26 Furthermore, the use of a randomized study in a population of patients whose BP cannot be controlled at the prior stage is recommended for the development of second‐line antihypertensive therapies.27 Thus, the regulatory preference for nonresponder studies is likely to have contributed to the smaller‐than‐expected between‐group differences in SeBP reductions. Patients who were randomized to continue with OLM/AML 40/10 mg during weeks 8 to 16 showed a form of placebo effect, which undoubtedly resulted from the change from single‐blind to double‐blind treatment. When the effects of treatment on BP were assessed using ABPM, this placebo effect was less pronounced.
The present study also showed that OLM/AML/HCTZ triple‐combination therapy is well tolerated, with a favorable tolerability profile that is comparable with that of OLM.28 The incidence of AEs was low in all three treatment groups during the study, and no new safety concerns were identified. The frequency and severity of TEAEs were similar among the three treatment groups, as were TEAEs leading to discontinuation.
Conclusions
In patients with inadequately controlled hypertension on the high‐dose dual combination of OLM/AML, the addition of both HCTZ 12.5 mg and 25 mg increased reductions in seated and ambulatory BP goals. Furthermore, triple‐combination therapy improved achievement of seated and ambulatory BP targets. Larger‐than‐expected changes in SeBP were seen in the group that continued with dual‐combination therapy. This finding was undoubtedly related to the nonresponder parallel‐group design of the trial, which was based on regulatory criteria. When changes in BP were assessed using ambulatory BP, the between‐group differences were larger and more significantly in favor of the triple‐combination group. All treatments were generally well tolerated.
Disclosures
LCR has received honoraria from Daiichi Sankyo for talks and consultancy. JS has received honoraria for talks and consultancy from Daiichi Sankyo Deutschland GmbH, Berlin‐Chemie AG, Bayer Vital GmbH, and Merckle Recordati, all of which manufacture ARBs and/or antihypertensive FDCs. PL is an employee of Daiichi Sankyo Deutschland GmbH and BA was an employee of Daiichi Sankyo Deutschland GmbH.
Acknowledgments
Funding for this study was provided by Daiichi Sankyo Europe GmbH. Editorial support for the preparation of this manuscript was funded by Daiichi Sankyo Europe GmbH and provided by Faysal Riaz, PhD, of inScience Communications, Springer Healthcare. The authors would like to thank Dieter Magometschnigg of the Institut für Hypertoniker, Wien, Austria, who provided input and suggestions on earlier drafts of this manuscript.
J Clin Hypertens (Greenwich). 2016;18:60–69. DOI: 10.1111/jch.12621. © 2015 The Authors. The Journal of Clinical Hypertension Published by Wiley Periodicals, Inc.
The copyright line for this article was changed on October 6, 2015 after original online publication.
References
- 1. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281–1357. [DOI] [PubMed] [Google Scholar]
- 2. Jamerson K, Weber MA, Bakris GL, et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high‐risk patients. N Engl J Med. 2008;359:2417–2428. [DOI] [PubMed] [Google Scholar]
- 3. Pepine CJ, Handberg EM, Cooper‐DeHoff RM, et al. A calcium antagonist vs a non‐calcium antagonist hypertension treatment strategy for patients with coronary artery disease. The International Verapamil‐Trandolapril Study (INVEST): a randomized controlled trial. JAMA. 2003;290:2805–2816. [DOI] [PubMed] [Google Scholar]
- 4. Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood‐pressure lowering and low‐dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998;351:1755–1762. [DOI] [PubMed] [Google Scholar]
- 5. Banegas JR, Lopez‐Garcia E, Dallongeville J, et al. Achievement of treatment goals for primary prevention of cardiovascular disease in clinical practice across Europe: the EURIKA study. Eur Heart J. 2011;32:2143–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Volpe M, de la Sierra A, Kreutz R, et al. ARB‐based single‐pill platform to guide a practical therapeutic approach to hypertensive patients. High Blood Press Cardiovasc Prev. 2014;21:137–147. [DOI] [PubMed] [Google Scholar]
- 7. Oparil S, Melino M, Lee J, et al. Triple therapy with olmesartan medoxomil, amlodipine besylate, and hydrochlorothiazide in adult patients with hypertension: the TRINITY multicenter, randomized, double‐blind, 12‐week, parallel‐group study. Clin Ther. 2010;32:1252–1269. [DOI] [PubMed] [Google Scholar]
- 8. Volpe M, Rump LC, Ammentorp B, Laeis P. Efficacy and safety of triple antihypertensive therapy with the olmesartan/amlodipine/hydrochlorothiazide combination. Clin Drug Investig. 2012;32:649–664. [DOI] [PubMed] [Google Scholar]
- 9. Clement DL, De Buyzere ML, De Bacquer DA, et al. Prognostic value of ambulatory blood‐pressure recordings in patients with treated hypertension. N Engl J Med. 2003;348:2407–2415. [DOI] [PubMed] [Google Scholar]
- 10. White WB, Giles T, Bakris GL, et al. Measuring the efficacy of antihypertensive therapy by ambulatory blood pressure monitoring in the primary care setting. Am Heart J. 2006;151:176–184. [DOI] [PubMed] [Google Scholar]
- 11. Lovibond K, Jowett S, Barton P, et al. Cost‐effectiveness of options for the diagnosis of high blood pressure in primary care: a modelling study. Lancet. 2011;378:1219–1230. [DOI] [PubMed] [Google Scholar]
- 12. Izzo JL Jr, Chrysant SG, Kereiakes D, et al. 24‐hour efficacy and safety of Triple‐Combination Therapy With Olmesartan, Amlodipine, and Hydrochlorothiazide: the TRINITY ambulatory blood pressure substudy. J Clin Hypertens (Greenwich). 2011;13:873–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mancia G, Laurent S, Agabiti‐Rosei E, et al. Reappraisal of European guidelines on hypertension management: a European Society of Hypertension Task Force document. J Hypertens. 2009;27:2121–2158. [DOI] [PubMed] [Google Scholar]
- 14. Mancia G, De Backer G, Dominiczak A, et al. 2007 Guidelines for the Management of Arterial Hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25:1105–1187. [DOI] [PubMed] [Google Scholar]
- 15. Volpe M, de la Sierra A, Ammentorp B, Laeis P. Open‐label study assessing the long‐term efficacy and safety of triple olmesartan/amlodipine/hydrochlorothiazide combination therapy for hypertension. Adv Ther. 2014;31:561–574. [DOI] [PubMed] [Google Scholar]
- 16. Rump LC, Girerd X, Sellin L, Stegbauer J. Effects of high dose olmesartan medoxomil plus hydrochlorothiazide on blood pressure control in patients with grade 2 and grade 3 hypertension. J Hum Hypertens. 2010;25:565–574. [DOI] [PubMed] [Google Scholar]
- 17. Volpe M, Brommer P, Haag U, Miele C. Efficacy and tolerability of olmesartan medoxomil combined with amlodipine in patients with moderate to severe hypertension after amlodipine monotherapy: a randomized, double‐blind, parallel‐group, multicentre study. Clin Drug Investig. 2009;29:11–25. [DOI] [PubMed] [Google Scholar]
- 18. Lacourciere Y, Tytus R, O'Keefe D, et al. Efficacy and tolerability of a fixed‐dose combination of telmisartan plus hydrochlorothiazide in patients uncontrolled with telmisartan monotherapy. J Hum Hypertens. 2001;15:763–770. [DOI] [PubMed] [Google Scholar]
- 19. Hall WD, Montoro R, Littlejohn T, et al. Efficacy and tolerability of valsartan in combination with hydrochlorothiazide in essential hypertension. Clin Drug Investig. 1998;16:203–210. [DOI] [PubMed] [Google Scholar]
- 20. Chrysant SG, Bakris GL. Amlodipine/benazepril combination therapy for hypertensive patients nonresponsive to benazepril monotherapy. Am J Hypertens. 2004;17:590–596. [DOI] [PubMed] [Google Scholar]
- 21. Schunkert H, Glazer RD, Wernsing M, et al. Efficacy and tolerability of amlodipine/valsartan combination therapy in hypertensive patients not adequately controlled on amlodipine monotherapy. Curr Med Res Opin. 2009;25:2655–2662. [DOI] [PubMed] [Google Scholar]
- 22. Blumenstein M, Romaszko J, Calderon A, et al. Antihypertensive efficacy and tolerability of aliskiren/hydrochlorothiazide (HCT) single‐pill combinations in patients who are non‐responsive to HCT 25 mg alone. Curr Med Res Opin. 2009;25:903–910. [DOI] [PubMed] [Google Scholar]
- 23. Ke Y, Zhu D, Hong H, et al. Efficacy and safety of a single‐pill combination of amlodipine/valsartan in Asian hypertensive patients inadequately controlled with amlodipine monotherapy. Curr Med Res Opin. 2010;26:1705–1713. [DOI] [PubMed] [Google Scholar]
- 24. Sinkiewicz W, Glazer RD, Kavoliuniene A, et al. Efficacy and tolerability of amlodipine/valsartan combination therapy in hypertensive patients not adequately controlled on valsartan monotherapy. Curr Med Res Opin. 2009;25:315–324. [DOI] [PubMed] [Google Scholar]
- 25. Mallion JM, Carretta R, Trenkwalder P, et al. Valsartan/hydrochlorothiazide is effective in hypertensive patients inadequately controlled by valsartan monotherapy. Blood Press. 2003;12:36–43. [DOI] [PubMed] [Google Scholar]
- 26. Committee for Medicinal Products for Human Use . Guideline on clinical development of fixed combination medicinal products. European Medicines Agency 2008; CPMP/EWP/240/95 Rev. 1: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003686.pdf. Accessed June 30, 2015.
- 27. Committee for Medicinal Products for Human Use . Guideline on clinical investigation of medicinal products in the treatment of hypertension. European Medicines Agency 2009; CPMP/EWP/238/95 Rev. 3: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/12/WC500100191.pdf. Accessed June 30, 2015.
- 28. Scholze J, Schaefer A, Kreutz R. Safety and efficacy of olmesartan: an observational pooled‐analysis of 156,682 hypertensive patients. Expert Opin Drug Saf. 2011;10:185–196. [DOI] [PubMed] [Google Scholar]


