Abstract
Setting: The three government tertiary care hospitals providing care for people living with the human immunodeficiency virus (PLHIV) in Kathmandu, Nepal.
Objectives: To assess 1) the screening cascades for intensified case finding for tuberculosis (TB), 2) isoniazid preventive therapy (IPT), including demographic and clinical factors associated with treatment interruption, and 3) TB infection control (IC) in the health facilities.
Design: A cross-sectional study of new PLHIV enrolled from January 2012 to December 2014.
Results: Among 572 registered PLHIV, 91% were on antiretroviral therapy. Of those registered, 561 (98%) were screened for TB and 73 (13%) were diagnosed with TB (17 [25%] sputum smear-positive, 17 [25%] smear-negative and 35 [51%] extra-pulmonary). Among the 488 (87%) PLHIV without active TB, 157 (32%) were initiated on IPT, of whom 136 (87%) completed treatment and 17 (11%) interrupted treatment. Those who experienced adverse events were 12 times more likely to interrupt IPT. TB IC showed gaps in personal control measures and supporting structures and policies.
Conclusion: The implementation of the Three I's for collaborative TB-HIV activities in pilot sites in Nepal was successful and should be scaled up.
Keywords: tuberculosis, human immunodeficiency virus, isoniazid preventive therapy, intensified case finding, infection control, Nepal
Abstract
Contexte : Les trois hôpitaux d'état de niveau tertiaire offrant des soins aux personnes vivant avec le virus de l'immunodéficience humaine (PVVIH) à Katmandou, Népal.
Objectifs : Evaluer 1) les étapes du dépistage pour une recherche intensifiée de cas (ICF) de tuberculose (TB) ; 2) le traitement préventif par isoniazide (TPI), y compris les facteurs démographiques et cliniques associés à l'interruption du traitement ; et 3) la lutte contre l'infection tuberculeuse (IC) dans les structures de santé.
Schéma : Etude transversale auprès des nouveaux PVVIH enrôlés entre janvier 2012 et décembre 2014.
Résultats : Parmi 572 PVVIH enregistrés, 91% étaient sous traitement antirétroviral. Parmi les inscrits, 561 (98%) ont eu un dépistage de TB et 73 (13%) ont eu un diagnostic de TB (17 [25%] TB à frottis positif, 17 [25%] TB à frottis négatif et 35 [51%] TB extra-pulmonaire). Parmi les 488 (87%) PVVIH sans TB active, 157 (32%) ont été mis sous TPI, 136 (87%) d'entre eux l'ont achevé et 17 (11%) ont interrompu le traitement. Ceux qui ont eu des effets secondaires ont été 12 fois plus susceptibles d'interrompre le TPI. Les mesures de lutte contre l'infection TB connaissent des lacunes en termes de mesures de protection individuelle et des structures et des politiques de soutien.
Conclusion : La mise en œuvre des Trois I pour des activités collaborative TB-VIH dans des sites pilotes au Népal s'est faite avec succès et devrait être étendue.
Abstract
Marco de referencia: Los tres hospitales públicos de atención terciaria que prestan servicios a las personas aquejadas de infección por el virus de la inmunodeficiencia humana (PVVIH) en Katmandú, Nepal.
Objetivos: Evaluar 1) el algoritmo de detección en la búsqueda intensiva de casos de tuberculosis (TB); 2) el tratamiento preventivo con isoniazida (TPI), incluidos los factores demográficos y clínicos que se asocian con su interrupción; y 3) el control de la infección (IC) tuberculosa en los establecimientos de salud.
Método: Fue este un estudio transversal de los PVVIH recién inscritos en el programa de enero del 2012 a diciembre del 2014.
Resultados: De 572 PVVIH inscritos, el 91% recibía tratamiento antirretrovírico. De los pacientes registrados, en 561 se practicó la detección sistemática de la TB (98%) y se diagnosticaron 73 casos de enfermedad activa (13%) (17 obtuvieron un resultado positivo de la baciloscopia del esputo [25%], 17 un resultado negativo [25%] y ocurrieron 35 casos de TB extrapulmonar [51%]). De los 488 PVVIH sin TB activa (87%), 157 iniciaron el TPI (32%), 136 de ellos lo completaron (87%) y 17 lo interrumpieron (11%). La probabilidad de interrumpir el TPI fue 12 veces mayor en los pacientes que presentaron reacciones adversas. Al evaluar las medidas de control de las infecciones se observaron deficiencias en las medidas personales, las estructuras auxiliares y en las normas.
Conclusión: La ejecución de actividades conjuntas, los Tres I's, de atención de la TB-VIH en tres centros piloto en Nepal fue eficaz y sería muy útil ampliar su escala de aplicación.
Globally, tuberculosis (TB) is the most common opportunistic infection and leading cause of mortality in people living with the human immunodeficiency virus (PLHIV), contributing to at least one in four of such deaths.1 In Nepal, TB is a public health problem, with an estimated incidence of 158 per 100000 population.1 The estimated prevalence of HIV among people aged 15–49 years was 0.2% in 2013, with the epidemic concentrated in key populations, notably among people who inject drugs (4.6–8.1%), men who have sex with men (3.8%), female sex workers (1–1.7%) and male labour migrants (1.1–1.4%) and their families (0.8%).2 The 2012 national sentinel surveillance revealed an 11.5% prevalence of TB among PLHIV.3
PLHIV are at least 26 times more likely to develop TB disease than people without HIV.1 The World Health Organization (WHO) recommended a package of collaborative TB-HIV activities in 2004.4 One of the pillars—to reduce the burden of TB among PLHIV—involves the Three I's approach of intensified case finding (ICF), isoniazid preventive therapy (IPT) and TB infection control (IC), in addition to early antiretroviral therapy (ART).5,6 ICF involves regular screening of all PLHIV for active TB disease and the provision of anti-tuberculosis treatment for active disease. IPT treats tuberculous infection, and can reduce progression to active TB by 32–62%.7 Nosocomial transmission of TB in health facilities has been recognised as a significant issue in high TB burden settings.8 Each health care and congregate setting should have a TB IC plan that includes administrative, environmental and personal protection measures to reduce the transmission of TB and for the surveillance of TB disease among workers.9
The Nepal National Centre for AIDS and Sexually Transmitted Disease Control (NCASC) implemented TB ICF and IC in its HIV programme in 2010. IPT for PLHIV was piloted country-wide in December 2012 in all five ART centres. This study aimed to assess the implementation of the Three I's in Kathmandu, Nepal, where three of the ART centres are located. Specific objectives were to assess, among newly registered PLHIV, 1) the screening cascade for TB ICF, 2) the IPT care cascade and factors associated with interruption, and 3) TB IC implementation at health facilities.
METHODS
Study design, setting and population
We conducted a cross-sectional study using routine programme data from three government tertiary ART centres in Kathmandu, Nepal, where the Three I's package was piloted: Sukraraj Tropical and Infectious Diseases Hospital (STIDH), Trivuwan University Teaching Hospital (TUTH) and Bir Hospital (BH). All new PLHIV patients registered for care from January 2012 to December 2014 in the three pilot sites were included in the study.
TB-HIV collaborative activities in Nepal
All PLHIV presenting at ART centres are registered in the pre-ART register and assigned a unique pre-ART registration number. During the study period, the Nepal NCASC guidelines criteria for ART were PLHIV aged >5 years with a CD4 count of ⩽350 cells/mm3, regardless of WHO clinical stage. This was changed to ⩽500 cells/mm3 in line with the WHO guidelines in December 2014, after the study period.10 Those eligible for ART initiation are recorded in the ART register. Cotrimoxazole preventive therapy (CPT) is initiated for HIV-infected adults with a CD4 count of <350 cells/mm3, while both CPT and ART are started for all PLHIV with active TB disease, regardless of CD4 cell count.11
As part of the ICF strategy, TB screening is performed using the WHO symptom screen (cough, excessive night sweats and fever >2 weeks and weight loss of >3 kg within 4 weeks) and a clinical assessment by a medical officer.12 Presence of any of the four symptoms is considered to indicate presumptive TB, prompting further investigations.11 Patients who present with none of the symptoms are considered free of active TB and are eligible for IPT. Patients with contra-indications such as liver disease, active alcohol use, jaundice, adherence barriers, previous isoniazid resistance, peripheral neuropathy or unexplained illness are not offered IPT. While it is recommended that ICF for TB be performed at each visit to the ART centre, we defined ‘screened’ as being performed once for each patient during the study period. If patients on IPT are identified as having presumptive TB, IPT is discontinued, investigations are performed and anti-tuberculosis treatment is initiated among those confirmed as having TB according to the national TB guidelines.13
Data collection and analysis
Data were collected using a structured questionnaire between August and September 2015 from ART and pre-ART registers and PLHIV personal profiles (electronic and paper-based medical records) maintained at the ART centres for the first two objectives (ICF and IPT). Data variables collected were sociodemographic characteristics, HIV risk group, ART status, WHO clinical stage, screening and diagnosis for TB and IPT initiation and outcome. Interruption of IPT was defined as failure to complete 24 weeks of treatment for any reason, except developing TB. Data on adverse drug reactions (ADRs) related to IPT were collected from the medical records. An IPT ADR, as defined per national guidelines, included skin rash, gastrointestinal symptoms, hepatotoxicity, neurological symptoms and arthralgia.9 Infection control was assessed at each faculty using a structured observational checklist14 that provides a score based on four components: supporting structures and activities (such as a TB IC committee, TB IC plan, training activities and education), environmental controls, administrative controls and personal controls. The principal investigator of the study completed the TB IC checklist through site visits, observation and interviewing key personnel in the selected centres.
EpiData software was used for data entry and analysis (version 3.1 for entry and version 2.2.2.183 for analysis, EpiData Association, Odense, Denmark). Data were double-entered and validated before undertaking analysis. The primary outcomes of interest, such as TB screening status, eligibility, IPT initiation and outcome, were expressed as proportions. The magnitude of the associations of demographic and clinical characteristics with IPT interruption was assessed using relative risk (RR) with 95% confidence intervals (CI).
Ethics approval
Ethical approval was obtained from the Ethics Advisory Group of the International Union Against Tuberculosis and Lung Disease, Paris, France, and the National Health Research Council, Kathmandu, Nepal. The ethics committees waived the need for informed consent from individual patients as the study involved analysis of existing programme data.
RESULTS
Of 572 PLHIV registered, 561 (98%) were screened for TB at least once during the study period. Of these, 73 (13%) were diagnosed with TB, resulting in a number needed to screen (NNS) of seven (Figure). Among the 488 (87%) PLHIV without active TB, 157 (32%) were initiated on IPT; 136 (87%) of these completed treatment, and 17 (11%) interrupted treatment (Figure). Four PLHIV were excluded from the analysis: one developed active TB and three were already on IPT at the time of the study.
FIGURE.
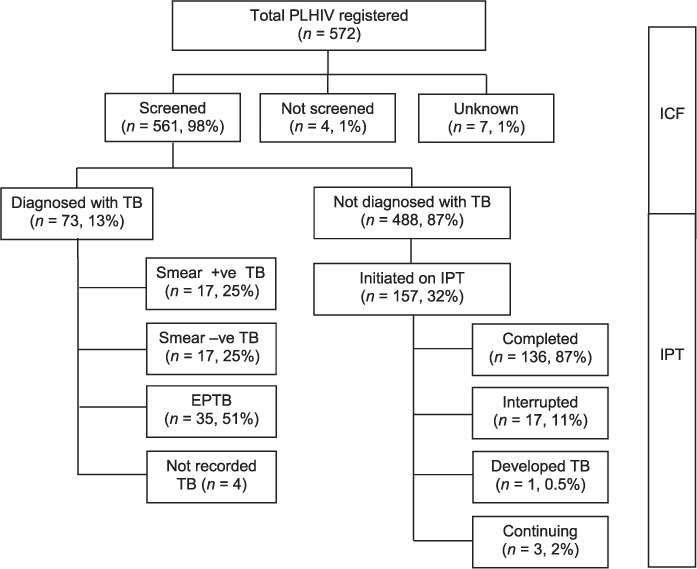
ICF for TB screening cascade and IPT care cascade in three ART centres in Kathmandu, Nepal, 2012–2014. ICF = intensified case finding; TB = tuberculosis; IPT = isoniazid preventive therapy; ART = antiretroviral therapy; PLHIV = people living with the human immunodeficiency virus; +ve = positive; −ve = negative; EPTB = extra-pulmonary TB.
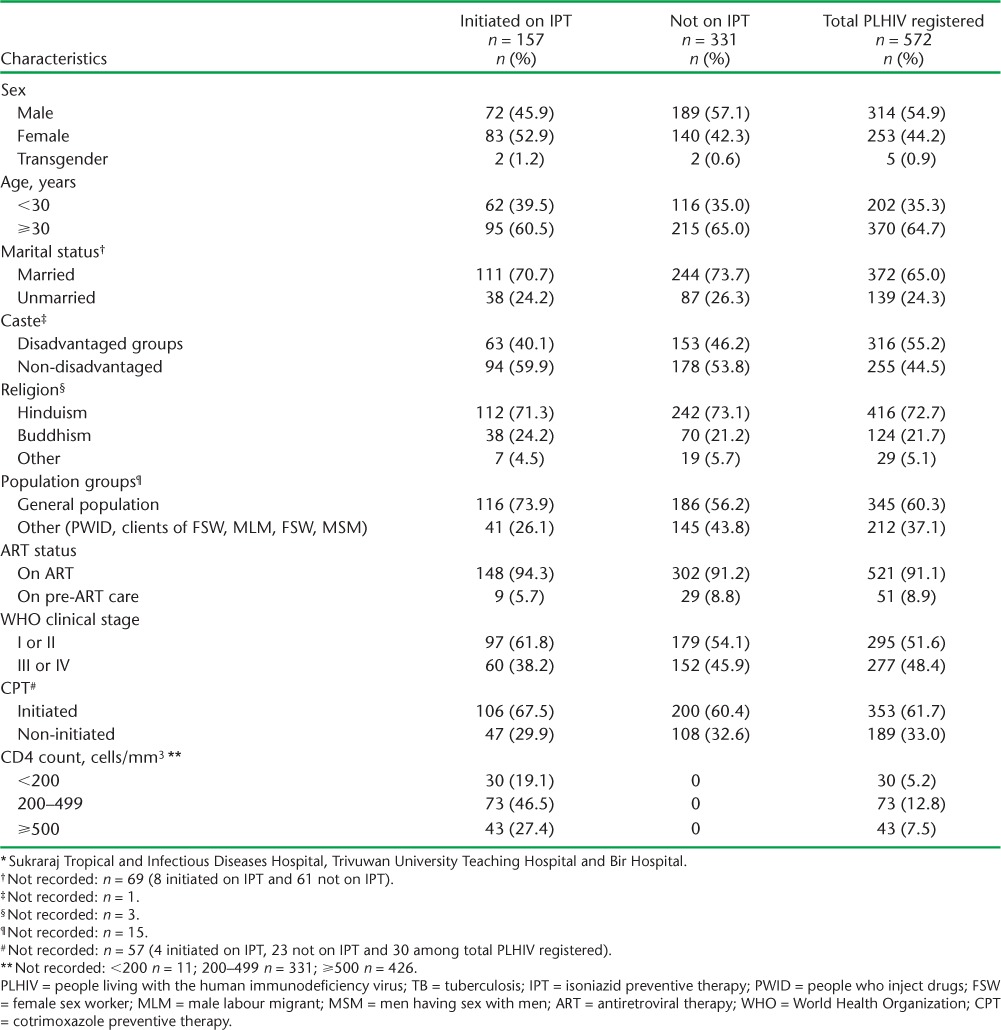
The majority of the PLHIV registered were on ART (521, 91%), and 62% were on CPT. Only 5.2% had severe immunosuppression, with a CD4 count <200 cells/mm3, and half were in the early WHO clinical stages (I and II). More than one third (37%) of the PLHIV were high-risk groups. The sociodemographic and clinical characteristics of the PLHIV screened but not diagnosed with TB (n = 488) are shown in Table 1.
TABLE 1.
Sociodemographic and clinical characteristics of PLHIV registered (n = 572) and eligible for IPT (n = 488) according to IPT initiation status in three pilot sites * in Kathmandu, Nepal, from January 2012 to December 2014
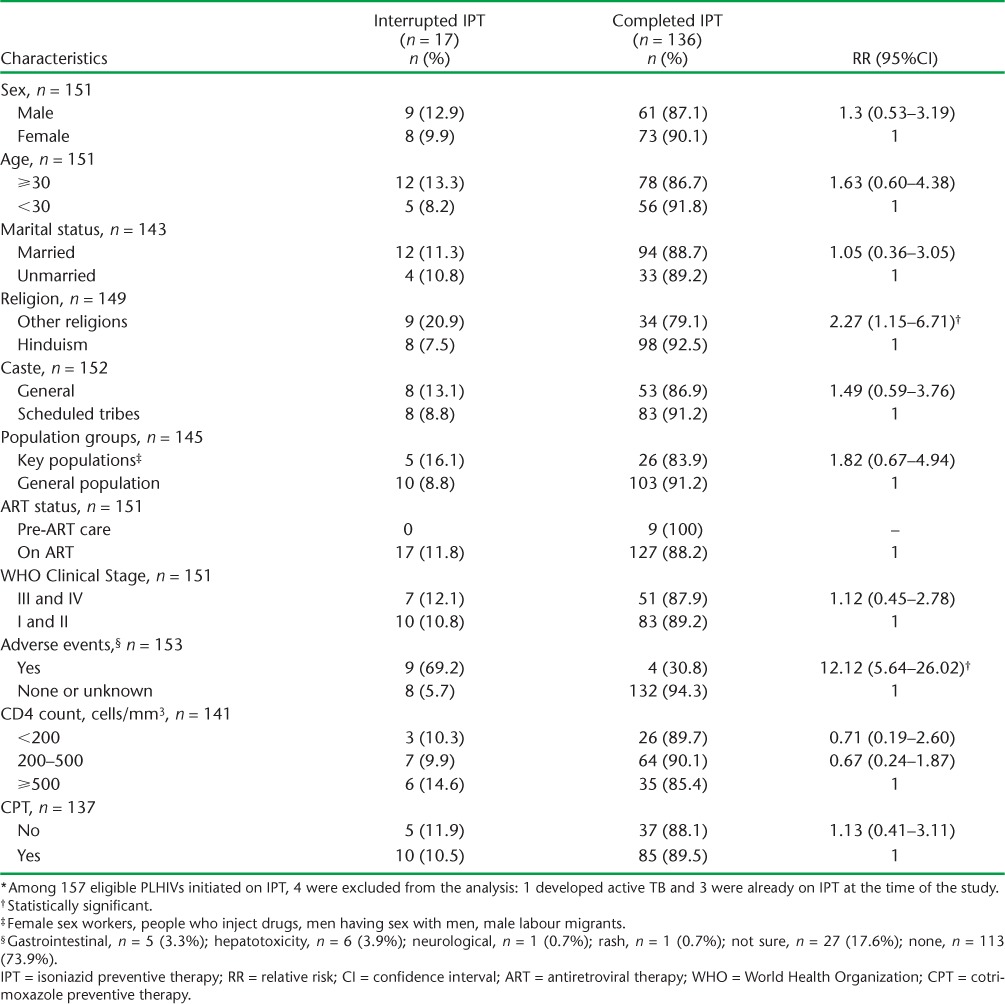
The IPT interruption rate among PLHIV was 11%. Table 2 illustrates the sociodemographic and clinical characteristics associated with interruption of IPT. Around 8.5% of the PLHIV on IPT experienced an adverse event; this group had a 12-fold higher risk of interrupting IPT (RR 12.2, 95%CI 5.64–26.02). The adverse events reported were hepatotoxicity (3.9%), gastrointestinal effects (3.3%), neurological effects (0.7%) and rash (0.7%).
TABLE 2.
Sociodemographic and clinical characteristics associated with interruption of IPT at three pilot sites in Kathmandu, Nepal, 2012–2014 (N = 153 * )
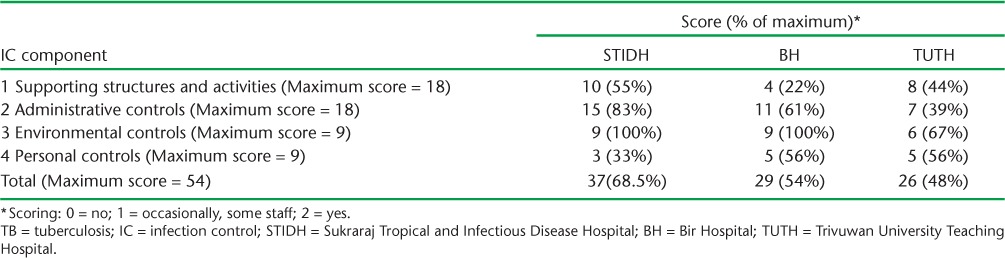
In all three IPT pilot sites, although guidelines and plans for TB IC had been established, implementation was inadequate. The results of the TB IC assessment, which were determined using a standardised tool,14 are summarised in Table 3. Major gaps were found in the equipment and supply chains, especially for the N95 respirator masks. Training and capacity building on TB IC for health workers was not conducted regularly. Aspects related to administrative controls, such as separating infectious patients from others, separate ventilated facilities for sputum collection, or fast-tracking presumptive TB cases for sputum collection and examination, were not adequately implemented. Although the implementation of environmental controls was better (particularly in the STIDH and BH sites), staff knowledge of the direction of airflow and seating arrangements in each consultation room and the installation of ultraviolet germicidal irradiation facilities in high-risk areas still needs improvement. Personal control measures had gaps at all sites, most importantly in the use of N95 respirators and regular staff screening for TB and HIV infection.
TABLE 3.
TB IC assessment at the three sites of Kathmandu, Nepal, using a standardised tool12
DISCUSSION
This is the first study from Nepal to assess the implementation of TB-HIV collaborative activities in the country and one of the few studies from the SouthEast Asia region. While many operational research studies have assessed the implementation of and barriers to the uptake of IPT, few studies have assessed all the components of the Three I's as a package.
The key findings are that 91% of the study participants were on ART and almost all PLHIV were screened for TB (ICF), with a yield of 13% for active TB disease. The IPT initiation rate was low, while the completion rate was high among those who initiated IPT. Adverse effects during IPT were associated with discontinuation of IPT, and the implementation of TB IC had gaps mainly in the areas of personal control measures and supporting structures and policies.
The strengths of this study are that it assessed all three ART centres in Kathmandu (of a total of five ART sites in Nepal), which account for over 75% of PLHIV registered in Nepal, and it included all newly diagnosed PLHIV over a 2-year period. The study results are therefore generalisable and can inform national policy. The overall limitations of the study were that only routine data collected under programme conditions were analysed, and therefore gaps exist in terms of a comprehensive assessment of the Three I's, i.e., the study did not assess whether ICF was conducted at each clinical encounter. Data variable gaps include the number of PLHIV eligible for IPT and reasons for not commencing IPT. While the IC checklist provided a general indication of the status of implementation, a more thorough assessment could be conducted.
The implementation of ICF was excellent, with almost all registered PLHIV (98%) screened for TB. Training on ICF for health workers was conducted prior to initiating the pilot, and standardised tools were developed. The 13% yield of active TB in PLHIV was consistent with the 2012 national sentinel surveillance result of 11.5%,3 and is similar to other settings.15–17 The diagnostic algorithm for TB in PLHIV at the sites was assumed to be similar at all sites and aligned with national guidelines, although this was not specifically assessed. This algorithm includes sputum smear microscopy, X-ray and Xpert® MTB/RIF (Cepheid, Sunnyvale, CA, USA), which was introduced in Nepal in 2010 and will significantly increase the yield of diagnosed TB cases among PLHIV. Sputum smear-positive cases accounted for 25% of all TB cases detected in the study, which is within expected norms,18 although it is not known how many cases were bacteriologically confirmed by Xpert in the study population.
The implementation of IPT had similar challenges to those previously reported in the literature, with low rates of uptake despite longstanding accepted guidelines.19,20 In the present study, one third of PLHIV who did not have active TB were initiated on IPT. This proportion likely underestimates the true uptake of IPT, as we were not able to assess the number of PLHIV determined to be eligible for IPT. Of the 488 PLHIV who did not have active TB, some may have had contraindications for IPT, such as active hepatitis or peripheral neuropathy, or may not have been offered IPT by clinicians; this would be expected to be a small proportion, however. Furthermore, some patients may not have consented to IPT. Barriers to health care, such as drug stock-outs or the breakdown of the referral and follow-up pathways, may also have contributed.21 To strengthen this component of the programme and prevent further cases of TB it would be valuable to explore and understand the true proportion of eligible patients and why over two thirds of PLHIV without active TB did not receive IPT.
The completion rate of IPT in this study was excellent, at 87%. The non-completion rate of 11%, although on the lower end of the spectrum, is similar to other settings.22 Most of the sociodemographic and clinical characteristics studied that were associated with the interruption of IPT were not statistically significant. A possible explanation for this could be the small sample size of those who interrupted treatment (n = 17) and missing data for many characteristics. The presence of adverse events on IPT was the strongest risk factor for non-completion (RR = 12.12, 95%CI 5.64–26.02), and the wide CI was due to this small absolute number of interruptions. It is unclear if the association of being non-Hindu with non-completion has clinical/programmatic significance, although IPT completion rates were lower among religious minorities.
A substantial percentage of patients in the study (8.5%) reported adverse events while on IPT, which is similar to other studies.23,24 The majority of events were due to hepatotoxicity and gastrointestinal effects. The attribution of adverse events to IPT was made by the clinician; the exact cause, however, was not assessed in the study. For example, adverse events may have been due to concurrent ART, other opportunistic infections or medical conditions, rather than to IPT itself. The rates of hepatotoxicity resulting in IPT discontinuation are higher than other studies that have reported rates of between 0.07% and 1%.25,26 It would be interesting to explore the rates of alcohol use, other hepatotoxic medication (herbal or prescription) and viral hepatitis in the study population. Clinician decision-making and the threshold for discontinuing IPT when liver function tests are abnormal may be reasons for the higher discontinuation rates.
TB IC measures in all three sites need strengthening, especially as all the sites are TB-HIV centres where the risk of nosocomial transmission of TB remains high. The study demonstrated that particular attention needs to be given to personal control measures and supporting structures and activities, such as N95 respirator procurement. A simplified approach to TB IC, known as the F-A-S-T strategy, has been implemented in several other settings.8 Dedicated TB IC officers or focal points may enhance the implementation of guidelines, the monitoring of supply chains and regular staff screening.
CONCLUSION
This study demonstrates that the pilot of the Three I's for TB-HIV in Nepal has been largely successful and is suitable for scale-up. Screening for TB among PLHIV and completion of IPT for those who initiated were satisfactory. Areas for further improvement include increasing IPT initiation rates and the implementation of IC practices. More operational research is needed to further quantify and explore the low rate of IPT initiation. Ongoing training and capacity building among health workers and strengthening of patient care systems are likely to improve programme performance. Nepal has a strong civil society and non-governmental organisations for TB-HIV that collaborate with the Ministry of Health, and their ongoing engagement is needed to move forward to decentralise HIV care to the community, including ICF and IPT. Nepal has established a sound platform of TB-HIV collaborative activities and must now accelerate the response as the world moves toward the ambitious goals of eliminating TB by the year 2030 through expanding programmes, exploring patient-centred care models and intensifying research.
Acknowledgments
This research was conducted through the Structured Operational Research and Training Initiative (SORT IT), a global partnership led by the Special Programme for Research and Training in Tropical Diseases at the World Health Organization (WHO/TDR). The model is based on a course developed jointly by the International Union Against Tuberculosis and Lung Disease (The Union) and Médecins Sans Frontières (MSF). The specific SORT IT programme that resulted in this publication was jointly developed and implemented by The Union South-East Asia Regional Office, New Delhi, India; the Centre for Operational Research, The Union, Paris, France; the Operational Research Unit (LUXOR), MSF, Brussels Operational Centre, Luxembourg; the School of Public Health, Postgraduate Institute of Medical Education and Research, Chandigarh, India; the Department of Preventive and Social Medicine, Jawaharlal Institute of Postgraduate Medical Education & Research, Puducherry, India; and the Burnet Institute, Melbourne, VIC, Australia.
The programme was funded by The Union and the Department for International Development, London, UK. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflicts of interest: none declared.
In accordance with WHO's open-access publication policy for all work funded by WHO or authored/co-authored by WHO staff members, the WHO retains the copyright of this publication through a Creative Commons Attribution IGO licence (http://creativecommons.org/licenses/by/3.0/igo/legalcode), which permits unrestricted use, distribution and reproduction in any medium provided the original work is properly cited.
References
- 1.World Health Organization. Global tuberculosis report 2015. Geneva, Switzerland: WHO; 2015. WHO/HTM/TB/2015.22. www.who.int/tb/publications/global_report/en Accessed June 2016. [Google Scholar]
- 2.Nepal National Centre for AIDS and STD Control. Country progress report on HIV/AIDS response Nepal. Kathmandu, Nepal: Ministry of Health and Population; 2014. [Google Scholar]
- 3.National Tuberculosis Centre. Annual TB progress report of Nepal. Thimi, Bhaktapur, Nepal: Ministry of Health and Population; 2013. http://www.nepalntp.gov.np Accessed June 2016. [Google Scholar]
- 4.World Health Organization. Interim policy on collaborative TB/HIV activities. Geneva, Switzerland: WHO; 2004. WHO/HTM/TB/2004.330. WHO/HTM/HIV/2004.1. [Google Scholar]
- 5.World Health Organization. WHO Three I's Meeting Report. Intensified case finding (ICF), isoniazid preventive therapy (IPT) and TB infection control (IC) for people living with HIV. Geneva, Switzerland: WHO; 2008. http://www.who.int/hiv/pub/meetingreports/WHO_3Is_meeting_report.pdf?ua=1 Accessed June 2016. [Google Scholar]
- 6.Badri M, Wilson D, Wood R. Effect of highly active antiretroviral therapy on incidence of tuberculosis in South Africa: a cohort study. Lancet. 2002;359:2059–2064. doi: 10.1016/S0140-6736(02)08904-3. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings. Geneva, Switzerland: WHO; 2011. http://apps.who.int/iris/bitstream/10665/44472/1/9789241500708_eng.pdf Accessed June 2016. [Google Scholar]
- 8.Barrera E, Livchits V, Nardell E. F-A-S-T: a refocused, intensified, administrative tuberculosis transmission control strategy. Int J Tuberc Lung Dis. 2015;19:381–384. doi: 10.5588/ijtld.14.0680. [DOI] [PubMed] [Google Scholar]
- 9.Nepal National Centre for AIDS and STD Control. TB-HIV co-infection management training manual for health care providers in Nepal. 5th ed. Kathmandu, Nepal: Ministry of Health and Population; 2014. [Google Scholar]
- 10.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommendations for a public health approach. Geneva, Switzerland: WHO; 2013. http://www.who.int/hiv/pub/guidelines/arv2013/download/en/ Accessed September 2015. [PubMed] [Google Scholar]
- 11.Nepal National Centre for AIDS and STD Control. National consolidated guideline for treating and preventing HIV. Kathmandu, Nepal: Ministry of Health and Population; 2014. [Google Scholar]
- 12.World Health Organization. Systematic screening for active tuberculosis: principles and recommendations. Geneva, Switzerland: WHO; 2013. WHO/HTM/TB/2013.04. http://www.who.int/tb/tbscreening/en/ Accessed September 2015. [PubMed] [Google Scholar]
- 13.National Tuberculosis Centre. National Tuberculosis Program General Manual. Thimi, Bhaktapur, Nepal: Ministry of Health and Population; 2012. [Google Scholar]
- 14.Reproductive Health & HIV Research Unit, University of the Witwatersrand. Implementing TB infection control in health facilities. Witwatersrand, South Africa: Reproductive Health & HIV Research Unit, University of the Witwatersrand; 2009. http://rhru.witshealth.co.za/Documents/TBinfectioncontrolManual_11-11-09.pdf Accessed June 2016. [Google Scholar]
- 15.Denegetu A W, Dolamo B L. Tuberculosis case finding and isoniazid preventive therapy among people living with HIV at public health facilities of Addis Ababa, Ethiopia: a cross-sectional facility based study. BMC Public Health. 2014;14:52. doi: 10.1186/1471-2458-14-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howard A A, El-Sadr W M. Integration of tuberculosis and HIV services in sub-Saharan Africa: lessons learned. Clin Infect Dis. 2010;50(Suppl 3):S238–S244. doi: 10.1086/651497. [DOI] [PubMed] [Google Scholar]
- 17.Balcha T T, Sturegård E, Winqvist N et al. Intensified tuberculosis case-finding in HIV-positive adults managed at Ethiopian health centers: diagnostic yield of Xpert MTB/RIF compared with smear microscopy and liquid culture. PLOS ONE. 2014;9:e85478. doi: 10.1371/journal.pone.0085478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Getahun H, Harrington M, O'Brien R, Nunn P. Diagnosis of smear-negative pulmonary tuberculosis in people with HIV infection or AIDS in resource-constrained settings: informing urgent policy changes. Lancet. 2007;369:2042–2049. doi: 10.1016/S0140-6736(07)60284-0. [DOI] [PubMed] [Google Scholar]
- 19.Wesen A, Mitke G. Screening and case detection for tuberculosis among people living with HIV in Addis Ababa, Ethiopia. Ethiop Med J. 2009;47:109–115. [PubMed] [Google Scholar]
- 20.Yirdaw K D, Jerene D, Gashu Z et al. Beneficial effect of isoniazid preventive therapy and antiretroviral therapy on the incidence of tuberculosis in people living with HIV in Ethiopia. PLOS ONE. 2014;9:e104557. doi: 10.1371/journal.pone.0104557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Getahun H, Granich R, Sculier D et al. Implementation of isoniazid preventive therapy for people living with HIV worldwide: barriers and solutions. AIDS. 2010;24(Suppl 5):S57–S65. doi: 10.1097/01.aids.0000391023.03037.1f. [DOI] [PubMed] [Google Scholar]
- 22.Zaeh S, Kempker R, Stenehjem E et al. Improving tuberculosis screening and isoniazid preventive therapy in an HIV clinic in Addis Ababa, Ethiopia. Int J Tuberc Lung Dis. 2013;17:1396–1401. doi: 10.5588/ijtld.13.0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mindachew M, Deribew A, Tessema F, Biadgilign S. Predictors of adherence to isoniazid preventive therapy among HIV positive adults in Addis Ababa, Ethiopia. BMC Public Health. 2011;11:916. doi: 10.1186/1471-2458-11-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Getachew Y, Mekonnen W. Correlates of adherence and utilization of isoniazid preventive therapy in HIV patients. J Microbiol Infect Dis. 2015;5:45–50. [Google Scholar]
- 25.Grant A D, Mngadi K T, van Halsema C L, Luttig M M, Fielding K L, Churchyard G J. Adverse events with isoniazid preventive therapy: experience from a large trial. AIDS. 2010;24(Suppl 5):S29–S36. doi: 10.1097/01.aids.0000391019.10661.66. [DOI] [PubMed] [Google Scholar]
- 26.Hiransuthikul N, Nelson K E, Hiransuthikul P, Vorayingyong A, Paewplot R. INH preventive therapy among adult HIV-infected patients in Thailand. Int J Tuberc Lung Dis. 2005;9:270–275. [PubMed] [Google Scholar]


