Abstract
Background: Chronic respiratory disease (CRD) causes substantial morbidity and mortality. Although the global CRD epidemic collides with the tuberculosis (TB) epidemic in many low- and middle-income country settings, the risk of TB-associated CRD is not well described in countries with a high burden of TB.
Methods: We recruited 136 patients with a history of sputum smear-positive pulmonary TB (PTB) from the TB clinic at Omdurman Teaching Hospital in Khartoum, Sudan, and 136 age- and sex-matched community controls, between 28 July 2013 and 30 December 2013. Data were collected using standardised questionnaires and spirometry was performed before and after bronchodilator.
Results: The mean age of the subjects with previous PTB and controls was respectively 44.0 years (SD 8.5) and 44.5 years (SD 8.6), with 27.2% females in both groups. Chronic respiratory symptoms such as chronic cough (OR 6.67, 95%CI 2.98–14.90, P < 0.001) and the presence of chronic airflow obstruction (OR 12.4, 95%CI 1.56–98.40, P = 0.02) were both strongly associated with a past history of PTB after adjusting for potential confounders.
Conclusion: The clinical features of CRDs are strongly associated with past history of PTB. An integrated approach to improve the management of these common conditions should be considered.
Contexte : Les maladies respiratoires chroniques (MRC) sont à l'origine d'une morbidité et d'une mortalité considérables dans le monde. Bien que l'épidémie mondiale des MRC entre en conflit avec l'épidémie de tuberculose (TB) dans de nombreux pays à revenu faible ou moyen, le risque de MRC associée à la TB n'est pas bien décrit dans les pays durement frappés par la TB.
Méthodes : Nous avons recruté 136 patients ayant des antécédents de tuberculose pulmonaire (TBP) à frottis positif dans le service de pneumologie du Centre Hospitalier Universitaire Omdurman à Khartoum, Soudan, et 136 témoins de la communauté, appariés sur l'âge et le sexe, entre le 28 juillet 2013 et le 30 décembre 2013. Les données ont été recueillies grâce à des questionnaires standardisés ainsi qu'à une spirométrie avant et après bronchodilatateur.
Résultats : L'âge moyen des cas et des témoins a été de 44,0 ans (DS 8,5) et 44,5 ans (DS 8,61), respectivement, avec 27,2% de femmes dans les deux groupes. Des symptômes respiratoires chroniques comme une toux chronique (OR 6,67 ; IC95% 2,98–14,90 ; P < 0,001) et la présence d'une obstruction chronique des voies aériennes (OR 12,39 ; IC%95 1,56–98,40 ; P = 0,02) ont été tous deux fortement associés à des antécédents de TBP après ajustement sur les facteurs de confusion potentiels.
Conclusion : Les caractéristiques cliniques des MRC sont fortement associées à des antécédents de TBP. Une approche intégrée visant à améliorer la prise en charge de ces pathologies fréquentes devrait être envisagée.
Marco de referencia: La enfermedad pulmonar crónica (EPC) es una causa importante de morbilidad y mortalidad. Aunque la epidemia mundial de EPC rivaliza con la epidemia de tuberculosis (TB) en muchos entornos de países con bajos y medianos recursos, el riesgo de aparición de EPC asociado con la TB se ha descrito cabalmente en los países con una alta carga de morbilidad por TB.
Métodos: Entre el 28 de julio y el 30 de diciembre del 2013, participaron en el estudio 136 pacientes con antecedente de tuberculosis pulmonar (TBP) y baciloscopia positiva del esputo que habían recibido tratamiento en el consultorio de neumología del Hospital Universitario Omdurman de Jartún, en Sudán, y 136 testigos sanos de la comunidad, emparejados en función de la edad y el sexo. Se recogieron datos mediante cuestionarios normalizados y se practicó una espirometría antes y despuès una prueba de broncodilatación.
Resultados: El promedio de la edad en el grupo de los casos fue 44,0 (desviación estándar 8,5 años) y en el grupo de testigos fue 44,5 años (8,6 años); la proporción de mujeres en ambos grupos fue 27,2%. Se observó una fuerte asociación entre la presencia de síntomas respiratorios crónicos como la tos (OR 6,67; IC95% 2,98–14,90; P < 0,001) y la presencia de obstrucción crónica al flujo en las vías respiratorias (OR 12,39; IC95% 1,56–98,40; P = 0,02) en las personas con antecedente de TBP, una vez corregidos los posibles factores de confusión.
Conclusión: La presencia de rasgos clínicos de EPC exhibe una fuerte correlación con el antecedente de TBP. Es preciso considerar la posibilidad de aplicar un enfoque integrado con el fin de mejorar la atención de ambas enfermedades tan frecuentes.
Keywords: pulmonary tuberculosis, COPD, spirometry, airflow obstruction, low- and middle-income countries
There is a worldwide epidemic of chronic non-communicable diseases (NCDs), which have been the most common cause of death and disability globally for at least the last three decades.1,2 Chronic respiratory diseases (CRDs) are amongst the highest burden NCDs, accounting for 4.7% of disability adjusted life years (DALYs) lost each year.3 Most people with CRDs live and die in low- and middle-income countries, and the majority of deaths from CRDs are attributed to chronic obstructive pulmonary disease (COPD).4 The same is true for tuberculosis (TB), which remains a leading cause of global morbidity and mortality, responsible for a million deaths every year.5 There are associations between infectious and non-communicable diseases.6 The association between pulmonary TB (PTB) and chronic airflow obstruction, a distinctive feature of COPD,7 may represent a good example here.
Smoking is recognised as the most significant risk factor for COPD in high-, middle- and low-income countries.8 More than 25% of patients with chronic airflow obstruction that fulfils the criteria for COPD are, however, non-smokers.9 In low- and middle-income countries, where the impact of COPD is expected to be greatest,10 other risk factors have not been determined, but TB is suggested to play an important role.11 Evidence from a small number of studies has shown that COPD and bronchiectasis are more common in people with a past history of PTB.12–16 The risk of TB-associated CRDs has not been well described in countries with a high burden of TB, such as in the North Africa and Middle-East regions.
We conducted a study to explore the association between CRD and TB among subjects with a history of previous PTB compared to a matched control group in Khartoum, Sudan.
METHODS
Setting
The present study was conducted in the TB clinic at Omdurman Teaching Hospital in Khartoum, Sudan. The clinic is part of the National Tuberculosis Programme (NTP) under the auspices of the Ministry of Health.
Design and aims
To explore the association between CRDs and their risk factors, including a previous history of PTB, we conducted a study among previous PTB patients (subjects) matched by age and sex with non-previous PTB patients (controls).
Participants
Potential subjects were identified using a patient register in the TB service at Omdurman Hospital. We worked through the list in the registers from 2008 and 2009, contacting potential subjects by telephone and inviting them to participate. To be included, subjects had to be aged 30–70 years, to have been treated for smear-positive PTB at least 5 years previously, to be currently well without a prior diagnosis of asthma, to have no contraindication to spirometry and to have a cell phone number on record. Potential subjects were excluded if they were currently unwell, had a prior history of asthma or a recent history of pneumothorax. Recruitment continued in this way until the required sample size was met. Potential age- and sex-matched controls were identified from the immediate relatives and neighbours of the subjects.
Data collection
We used an investigator-administered standardised paper questionnaire to collect data from consenting participants and their medical records on socio-economic status, risk factors for CRDs and anti-tuberculosis treatment, if relevant. All questionnaires were cross-checked for completeness and corrected on site if needed. Height and weight were measured using scales with a built-in stadiometer. Spirometry was conducted using the One Flow FVC #7880 spirometer (Clement Clarke International, Harlow, UK) by a single investigator trained to American Thoracic Society standards. The best of three readings before and 15 minutes after administration of 200 μg inhaled salbutamol, device generated, were selected.
Data entry and quality control
The data from the questionnaires were double-entered into a database at the study office and cross-checked. The spirometry data were transferred to a computer and opened using the One Flow software programme (Clement Clarke). Spirograms were evaluated for quality and interpreted by a single investigator, and were then cross-checked by an independent chest specialist.
Data interpretation
A diagnosis of CRD was considered in any patient with symptoms of cough, sputum production, or shortness of breath. Fixed airflow obstruction in keeping with COPD was defined as a post-bronchodilator forced expiratory volume in 1 second (FEV1) < 80% of the predicted value (European Respiratory Society 93 predicted values) in combination with a FEV1/forced vital capacity (FVC) <0.7. The severity of airflow obstruction was graded according to the GOLD criteria for classification of COPD severity from grade 1 to 4.7 Restriction was defined as FVC <80%, FEV1 < 80% and FEV1/FVC >0.7.
Study size
With 136 participants in each group, the study had 80% power to detect a difference in the proportion of previous PTB subjects and controls with CRD from expected proportions of respectively 10% and 2% at the 5% significance level.
Data analysis
The χ2 test was used for categorical variables and the t-test was used to compare between the means in numerical variables. Linear regression was used to assess the spirometric indices (FEV1, FVC and FEV1/FVC ratio) pre- and post-bronchodilator. Binary logistic regression was used to calculate the odds of having CRD after past exposure to PTB. Univariate analysis was conducted for every variable alone, and statistically significant results (P < 0.05) were then included together in the multivariate model. All analyses were performed using the Statistical Package for the Social Sciences software (SPSS, version 20, IBM, Armonk, NY, USA).
Ethical considerations
The study was approved by the Regional Committees for Medical and Health Research Ethics, Oslo, Norway, and the National Research Ethics Review Committee at the Federal Ministry of Health, Khartoum, Sudan. Written informed consent was obtained from all participants.
RESULTS
Participants
Of 500 potential subjects identified from the 2008–2009 TB service records, only 190 were contactable by telephone. Of these, five declined to participate and 31 had moved out of Khartoum state. Two were excluded due to a previous history of asthma and one was excluded because the spirometric curves were technically unsatisfactory. Complete questionnaire and spirometry data were obtained from 136 subjects; 136 control participants were also identified (Figure).
FIGURE.
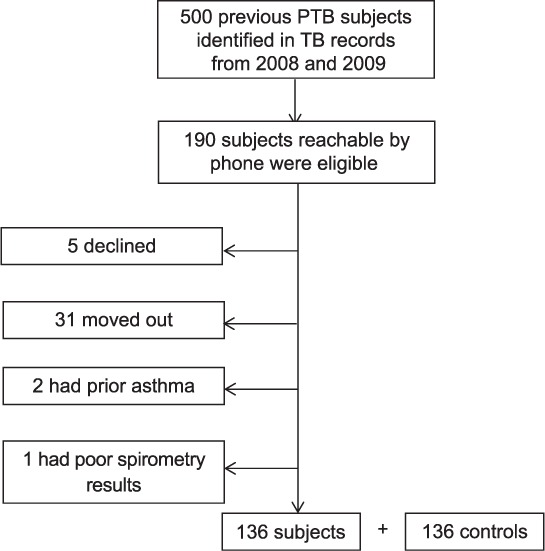
Flow chart of the study sample. PTB = pulmonary tuberculosis.
All study participants completed the pre-bronchodilator tests. Two subjects and eight controls refused to perform the post-bronchodilator tests as they did not wish to use the bronchodilator. None had airflow obstruction pre-bronchodilator.
Descriptive data
The mean age of the subjects and controls was respectively 44.0 years (± 8.5) and 44.5 years (± 8.6). The sex distribution was the same for the subjects and controls, with 27.2% females in each group.
Respiratory symptoms
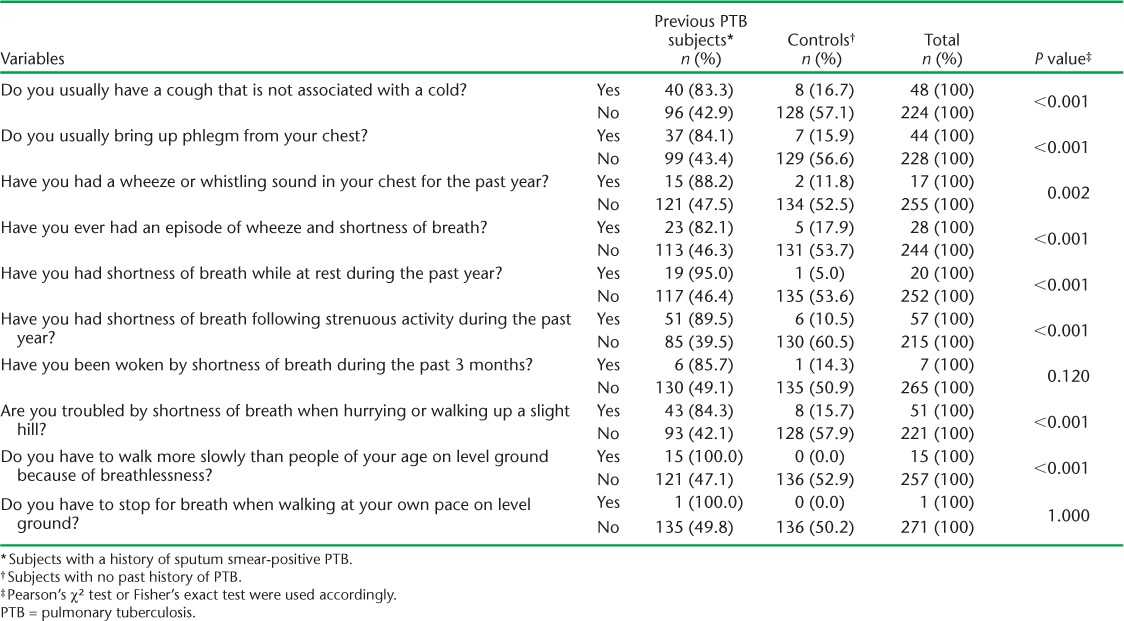
Chronic cough, phlegm, wheeze and breathlessness at rest were reported by respectively 17.6%, 16.2%, 6.3% and 7.4% of subjects. The odds of having these chronic respiratory symptoms in the subjects compared to the controls were respectively 6.67 (95% confidence interval [CI] 2.98–14.90, P < 0.001), 6.89 (95%CI 2.95–16.10, P < 0.001), 8.31 (95%CI 1.86–37.07, P = 0.002) and 21.92 (95%CI 2.89–166.27, P < 0.001) (Table 1).
TABLE 1.
Association between chronic respiratory symptoms and previous PTB
Respiratory disease risk factors
The presence of any chronic co-morbidity (diabetes mellitus, hypertension, human immunodeficiency virus [HIV] and ischaemic heart disease) (odd ratio [OR] 1.98, 95%CI 1.03–3.80, P = 0.037), exposure to dust at work (OR 4.09, 95%CI 1.32–12.67, P = 0.015) and smoke from burning biomass for cooking or heating in the home (OR 1.97, 95%CI 1.08–3.60, P = 0.025) were associated with previous PTB. Neither active nor passive tobacco smoke, nor body mass index (BMI), were associated with previous PTB.
Spirometry
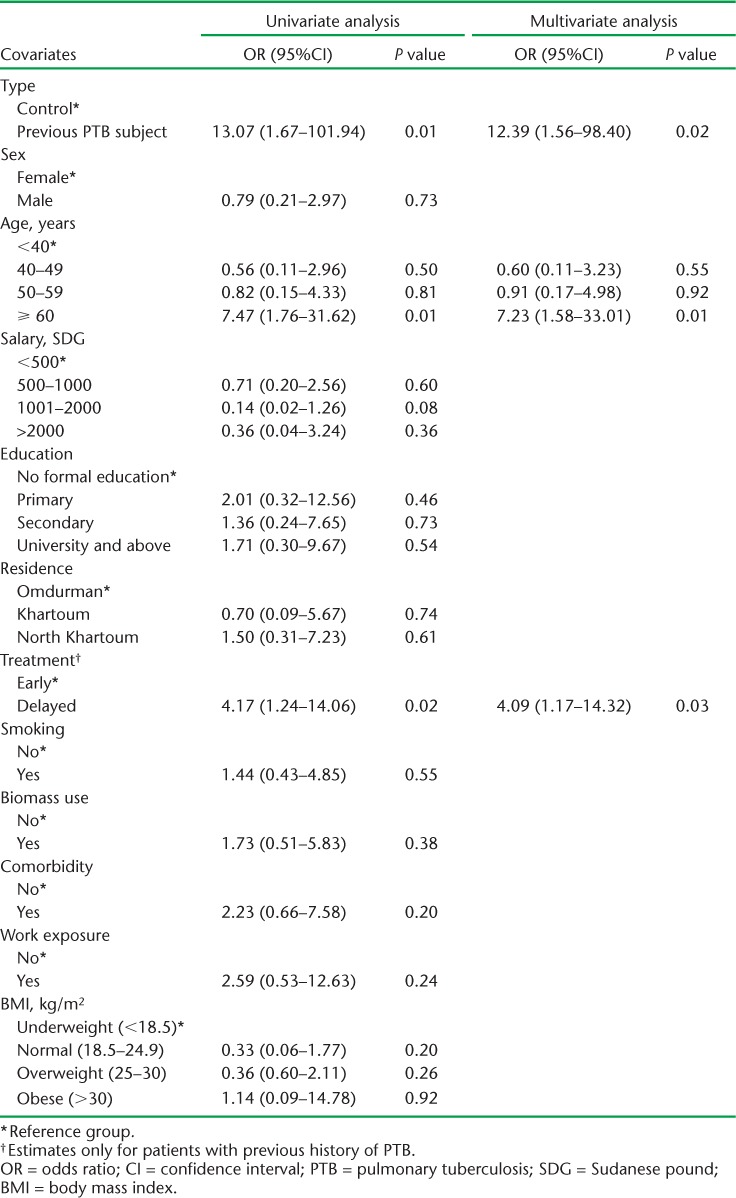
Spirometric indices were on the whole more impaired among subjects than controls (Table 2). Post-bronchodilator airflow obstruction was diagnosed in 12 (8.8%) subjects and 1 (0.7%) control, giving an OR of 12.4 (95%CI 1.6–98.4, P = 0.02) for the association with previous PTB after adjusting for age and delay in treatment, which were the only two statistically significant factors in the univariate model (Table 3). Eight of the subjects with airflow obstruction had a combined obstructive restrictive pattern. The distribution in severity of airflow obstruction by GOLD criteria among subjects was as follows: mild (8.3%), moderate (58.3%), severe (16.7%) and very severe (16.7%). A restrictive pattern alone was found in seven subjects, while none was found among controls.
TABLE 2.
Differences in spirometric results of previous PTB patients compared to controls
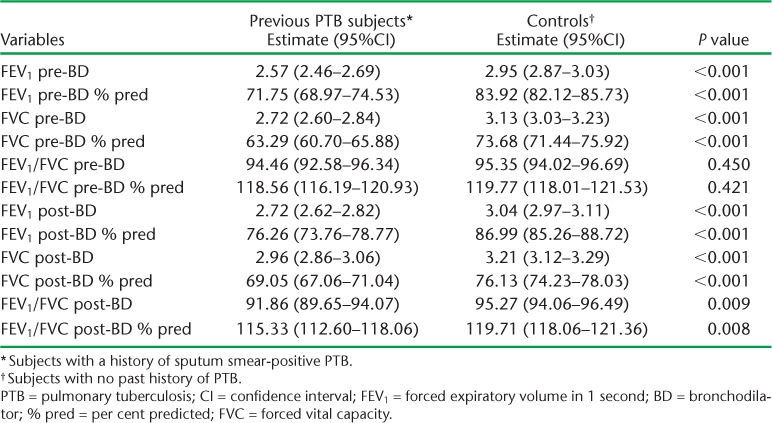
TABLE 3.
Logistic regression model for the risk of chronic airflow obstruction
DISCUSSION
In this study from the North Africa and Middle-East region, we found that chronic respiratory symptoms are common amongst people previously treated for PTB, that the presence of a chronic disease, such as diabetes or HIV, dusty work environments and exposure to biomass smoke in the home are associated with an increased risk of PTB and that irreversible airflow obstruction is strongly and significantly associated with previous PTB, as reported earlier.12
The high burden of chronic respiratory symptoms in people previously treated for PTB in our study setting is an important finding, reflecting an under-recognised burden of CRD and an unmet need for care. These findings are consistent with studies conducted in other areas of the world.13 While it is not surprising that chronic comorbidities14,15 and exposure to dust at work14 are associated with previous PTB, our findings in relation to exposure to smoke from tobacco and burning biomass in the home are notable. Exposure to smoke from cigarettes was more commonly seen among men than women (32.3% vs. 1.4%), with the opposite sex bias seen in relation to exposure to biomass smoke (11.6% vs. 45.9%). No association was seen in our study between previous PTB and tobacco smoke exposure, while an association was seen with biomass smoke exposure. Although studies from other areas of the world have found an association between TB and exposure to biomass smoke, the literature is not sufficiently consistent to draw definitive conclusions.17
The strong association observed between previous PTB and the presence of post-bronchodilator airflow obstruction illustrates the importance of the interrelationship between non-communicable and communicable respiratory diseases in settings such as Sudan. In our study, previous PTB was the strongest predictor for irreversible airflow obstruction, with an unadjusted OR of 13.07 (95%CI 1.67–101.94) and 12.39 (95%CI 1.56–98.40) after adjustment for potential confounders. This finding is consistent with the findings of studies conducted in diverse settings.12–16,18 The BOLD study, for example, a large multi-centre population-based study including 21 962 participants aged ⩾40 years from 27 sites in several countries around the world, found an OR of 2.51 (95%CI 1.83–3.42) for the association between previous PTB and airflow obstruction.19 Delay in receiving treatment for TB was associated with irreversible airflow obstruction, providing some support for causal inference. As expected, we saw an increased risk of irreversible airflow obstruction with increasing age and in ever smokers, although the latter was not statistically significant, probably reflecting study power.
One of the strengths of our study is that this is the first report of the association between CRD and TB from Sudan and the wider North Africa and Middle East region, a challenging setting in which to conduct research. We paid careful attention to quality control during the data collection and in the conduct of post-bronchodilator spirometry in particular. The study design brings with it limitations, including the direction of the association. Selection bias may have occurred in the selection of subjects; as this was based on a convenience sample from paper-based records, potential participants without a cell phone (generally of lower socio-economic status) were not eligible, and those we could not contact or who had died may have introduced other biases. Reporting bias may have increased the likelihood of people with previous PTB reporting chronic respiratory symptoms compared to controls, and recall bias may have affected the reporting of duration of delay in diagnosis and treatment. The number of participants with irreversible airflow obstruction was small, resulting in wide confidence intervals, and made some associations difficult to establish.
In conclusion, the clinical features of CRD are common in people with previous PTB and strongly associated after adjusting for potential confounders. While TB services are generally available in low- and middle-income countries, the provision of care for people with CRDs is often patchy or lacking. Our study adds important new data on the intersection of these communicable and non-communicable diseases. An integrated approach to their management is likely to be needed as countries around the world experience the global epidemiological transition towards higher rates of NCDs.
Acknowledgments
RKO and KM are joint first authors of this article. We would like to thank respiratory medicine specialist N Bakri for her contribution to the interpretation of the spirometric results and University of Oslo (Oslo, Norway) statistician I Madala for his assistance in the statistical analysis. We would like to thank the staff at the TB clinic at Omdurman Teaching Hospital for making this survey possible. We also thank all the participants. The Ivar Helles Foundation, through the University of Oslo, contributed NOK 8650 (EUR 923 as of June 2016). Epi-Lab, Khartoum, Sudan, provided stationery and space.
Footnotes
Conflicts of interest: none declared.
References
- 1.Lozano R, Naghavi M, Foreman K et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vos T, Flaxman A D, Naghavi M et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray C J L, Vos T, Lozano R et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 4.Burney P, Jarvis D, Perez-Padilla R. The global burden of chronic respiratory disease in adults. Int J Tuberc Lung Dis. 2015;19:10–20. doi: 10.5588/ijtld.14.0446. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Global tuberculosis report 2013. Geneva, Switzerland: WHO; 2013. WHO/HTM/TB/2013.11. [Google Scholar]
- 6.Bousquet J, Dahl R, Khaltaev N. Global alliance against chronic respiratory diseases. Allergy. 2007;62:216–223. doi: 10.1111/j.1398-9995.2007.01307.x. [DOI] [PubMed] [Google Scholar]
- 7.Vestbo J, Hurd S S, Agusti A G et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 8.Lopez A D, Mathers C D, Ezzati M, Jamison D T, Murray C J, editors. Global burden of disease and risk factors. Washington, DC, USA: The International Bank for Reconstruction and Development/World Bank Group; 2006. [PubMed] [Google Scholar]
- 9.Salvi S S, Barnes P J. Chronic obstructive pulmonary disease in non-smokers. Lancet. 2009;374:733–743. doi: 10.1016/S0140-6736(09)61303-9. [DOI] [PubMed] [Google Scholar]
- 10.Hooper R, Burney P, Vollmer W M et al. Risk factors for COPD spirometrically defined from the lower limit of normal in the BOLD project. Eur Respir J. 2012;39:1343–1353. doi: 10.1183/09031936.00002711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buist A, Vollmer W, McBurnie M. Worldwide burden of COPD in high- and low-income countries. Part I. The Burden of Obstructive Lung Disease (BOLD) Initiative. Int J Tuberc Lung Dis. 2008;12:703–708. [PubMed] [Google Scholar]
- 12.Byrne A L, Marais B J, Mitnick C D, Lecca L, Marks G B. Tuberculosis and chronic respiratory disease: a systematic review. Int J Infect Dis. 2015;32:138–146. doi: 10.1016/j.ijid.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 13.Ehrlich R I, Adams S, Baatjies R, Jeebhay M F. Chronic airflow obstruction and respiratory symptoms following tuberculosis: a review of South African studies. Int J Tuberc Lung Dis. 2011;15:886–891. doi: 10.5588/ijtld.10.0526. [DOI] [PubMed] [Google Scholar]
- 14.Menezes A M, Hallal P C, Perez-Padilla R et al. Tuberculosis and airflow obstruction: evidence from the PLATINO study in Latin America. Eur Respir J. 2007;30:1180–1185. doi: 10.1183/09031936.00083507. [DOI] [PubMed] [Google Scholar]
- 15.Lee C H, Lee M C, Lin H H et al. Pulmonary tuberculosis and delay in anti-tuberculous treatment are important risk factors for chronic obstructive pulmonary disease. PLOS ONE. 2012;7:e37978. doi: 10.1371/journal.pone.0037978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lam K B, Jiang C Q, Jordan R E et al. Prior TB, smoking, and airflow obstruction: a cross-sectional analysis of the Guangzhou Biobank Cohort Study. Chest. 2010;137:593–600. doi: 10.1378/chest.09-1435. [DOI] [PubMed] [Google Scholar]
- 17.Enarson D A, Aït-Khaled N, Chiang C-Y Lung health consequences of exposure to smoke from domestic use of solid fuels: a guide for low-income countries on what it is and what to do about it. Paris, France: International Union Against Tuberculosis and Lung Disease; 2009. [Google Scholar]
- 18.Jordan T S, Spencer E M, Davies P. Tuberculosis, bronchiectasis and chronic airflow obstruction. Respirology. 2010;15:623–628. doi: 10.1111/j.1440-1843.2010.01749.x. [DOI] [PubMed] [Google Scholar]
- 19.Amaral A F, Coton S, Kato B et al. Tuberculosis associates with both airflow obstruction and low lung function: BOLD results. Eur Respir J. 2015;46:1104–1112. doi: 10.1183/13993003.02325-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]


