Abstract
Background
Patients with newly diagnosed dilated cardiomyopathy (DCM) and advanced heart failure have a very high morbidity and mortality with an unpredictable clinical course. We investigated the role of cardiovascular magnetic resonance (CMR) imaging using late gadolinium enhancement (LGE) in this cohort of high‐risk patients. We hypothesized that LGE has high prognostic value in primary DCM patients referred for possible transplantation/left ventricular assist device (LVAD) consideration.
Methods
Over 49 consecutive months, 61 consecutives DCM patients were referred for standard CMR(1.5T, GE) to interrogate the LV pattern, distribution, and extent of LGE (MultiHance, Princeton, NJ). Inclusion criteria for a primary non‐ischaemic DCM and EF <45% were met in 31 patients. DCM patients were categorized into: (i) presence of midwall LV stripe (+Stripe) and (ii) absence of midwall stripe (−Stripe) groups. Primary outcome was defined by the composite of death, need for LV assist device (LVAD), and urgent orthotopic cardiac transplantation (Tx) during a 12‐month follow‐up period. Kaplan–Meier survival analysis was conducted grouping patients by +Stripe and −Stripe.
Results
There were no differences between groups for demographics, blood pressure, labs, baseline LVEF, NYHA class, or invasive haemodynamics. There were 18 patients (58%) with +Stripe. Nine events occurred: seven patients required urgent Tx and/or LVAD implantation and two patients died. The +Stripe categorization strongly predicted the need for LVAD, urgent Tx surgery, and death (log‐rank = 9, P = 0.002). All the events occurred in the +Stripe patients with no MACE experienced in the −Stripe group. The −Stripe group experienced marked signs of improvement in LVEF (P = 0.01) at follow‐up. LVEDD was predictive of need for LVAD/Tx and death by univariate analysis. Otherwise, no common clinical metric such as LVEF, LVEDV, RVEF, RVEDV, or any invasive haemodynamic parameter predicted MACE.
Conclusions
The presence of +Stripe on CMR is strongly predictive of LVAD, transplant need, and death during a 12‐month follow‐up period in DCM patients in this proof of concept study. All −Stripe patients survived without experiencing any events. Incorporating CMR imaging into routine clinical practice may have prognostic value in DCM patients; indicating conservative management in low‐risk patients while expectantly managing high‐risk patients.
Keywords: Heart transplantation, Midwall fibrosis, Cardiac MRI
Introduction
Dilated cardiomyopathies (CM) are characterized by ventricular chamber enlargement and systolic dysfunction with normal wall thickness leading to progressive heart failure (HF), tachyarrhythmias, conduction abnormalities, and sudden cardiac or HF‐related death.1 Cardiovascular magnetic resonance (CMR) is a robust tool to differentiate cardiomyopathy (CM) and ischaemic cardiomyopathy (ICM), particularly idiopathic cardiomyopathy, and has been recognized as the ‘gold standard’ for idiopathic and hypertrophic cardiomyopathies.1, 2, 3 Patients with ICM and primary dilated cardiomyopathy (DCM) have prognostic models that have been validated helping to identify those at risk of adverse outcomes, including those in whom cardiac transplantation or mechanical device therapy may be considered.4, 5 However, in DCM patients, the models have been validated mainly in established chronic heart failure ambulatory patients. Conversely, patients with ‘de novo’ presentations of DCM lacks a clear algorithm for prognosis and frequently rely on subsequent follow‐up visits and changes in clinical metrics such as the left ventricle (LV) ejection fraction (EF), serum sodium, New York Heart Association (NYHA) functional status, VO2 consumption, haematocrit, or QRS widened to determine prognosis acutely or over time,5 or acute deterioration of their clinical status. In general, risk stratification in DCM patients with advanced HF remains a clinical challenge. Therefore, a simple means to non‐invasively risk stratify this cohort (newly diagnosed DCM) would have obvious advantages. CMR late gadolinium enhancement (LGE) has been routinely used for assessment of myocardial viability and revascularization potential.4, 5, 6, 7 The same principle has been recently used to identify fibrosis in patients with non‐ischaemic cardiomyopathy, as manifested by midwall fibrosis or patchy or subepicardial fibrosis which generally spare the endocardium and demonstrate patterns following a non‐coronary distribution.2, 8, 9, 10, 11 We investigated the role of CMR LGE in newly diagnosed DCM patients referred to our Advanced Heart Failure/Transplant Center in Pittsburgh, PA. We hypothesized that the finding of a positive mid‐wall stripe pattern (+Stripe) within the myocardium portends an adverse prognosis as defined by need for urgent orthotopic cardiac transplantation (TX), LV assist device (LVAD) insertion, or death. Further, we sought to relate LGE to additional MACE (heart failure hospital readmission requiring intravenous diuretics and/or inotropes use).
Methods
Patients
We performed an IRB approved retrospective review with a prospective sub‐arm analysis of a total of 61 consecutive patients (April 2006 until April 2010) with newly diagnosed non‐ischaemic cardiomyopathy who were referred to our cardiac magnetic resonance (CMR) department, as part of the initial work‐up for a ‘de novo’ diagnosis of heart failure as directed by the heart failure/transplant team. Following an initial observation of the markedly adverse outcome in one patient who presented with the index CMR feature, a retrospective review of 13 patients was then performed. Then, prospectively, a separate cohort (18 patients) was followed for 12 months after receiving their CMR exam. Patients were excluded (30 patients), if CMR LVEF was >45% at presentation, CAD by left heart catheterization (>50% stenosis), primary valvular heart disease, hypertrophic cardiomyopathy, infiltrative heart disease, pacemakers/AICD, or GFR < 30 mL/min/1.73 m2 or those who could not undergo CMR imaging. This report incorporates the findings of the 31 patients who were classified as pure non‐ischaemic cardiomyopathy.
Demographic characteristics, medication usage, laboratory values, echocardiography, CMR, and haemodynamic findings at baseline were compared. Follow‐up data at 6 and 12 months from study enrolment were collected, and major adverse cardiovascular events (MACE) were recorded. Pathology samples were acquired from endomyocardial biopsy (n = 1), in explanted hearts (n = 6) or apical core at time of LVAD implantation (n = 2). Haematoxylin–eosin and Masson's trichrome stains were used to identify the extent of fibrosis (Figure 1). A careful system to ensure follow‐up was designed such that throughout the entire study, no patient was lost to follow‐up.
Figure 1.
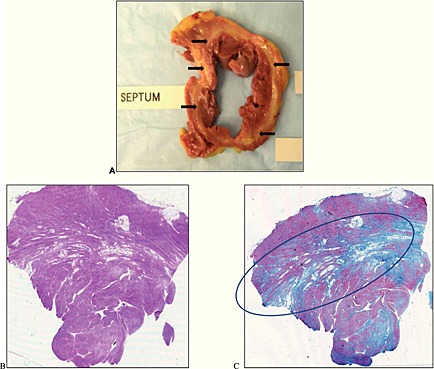
Cross‐section of explanted heart, H&E, and Masson's trichrome stain. Panel A shows a cross‐sectional of the native heart with a near circumferential midwall fibro‐lipomatous degeneration of the left ventricle (arrows). Panel B represents the microscopic examination of the cross‐section depicted in panel A with haematoxylin and eosin stain. This shows hypertrophic myocytes with areas of fibrosis with predilection in the midwall (circle) as evidenced by Masson's trichrome staining in Panel C.
The index CMR findings were jointly interpreted by two specialized CMR readers (RWWB, VKR). In no instance was there any discrepancy in adjudicating the presence of a midwall stripe. The CMR exam results, including a description of the general LGE findings, were transmitted to the referring physician. All patients were managed with guideline‐based optimal pharmacological therapy, including the use of defibrillators and cardiac resynchronization as guided by the ACC/AHA guidelines. Importantly, LGE ‘stripe’ data, by their very embryonic nature was not used for clinical decision making.
Changes in LVEF from baseline and follow‐up at 4 months were assessed by echocardiography parameters as a majority of patients subsequently required automatic implantable cardioverter defibrillator (AICD), and hence, precluded the use of CMR.
The investigation conforms with the principles outlined in the Declaration of Helsinki.
Study endpoints
The primary endpoint was the need for urgent orthotopic heart transplantation (defined by UNOS status 1A/1B listing), left ventricular assist device (LVAD) insertion, and/or death. The secondary endpoint was additional defined as a composite of rehospitalisation for acute HF decompensation requiring intravenous diuretics and/or inotropes, death, or heart transplantation or LVAD insertion.
Cardiac magnetic resonance imaging
Standard 3D‐CMR (1.5T; GE, Milwaukee, WI) was performed at initial presentation to interrogate LV volumetrics and pattern, distribution, and extent of LGE (MultiHance, Princeton, NJ). A single intravenous contrast dose of 0.15 mmol/kg was given. Steady‐state free precession (SSFP) breath‐hold cines were performed for 3D volumetric quantitation (TE [echo time]/TR [repetition time] 1.6/3.2 ms, flip angle 60°) via contiguous 8‐mm short‐axis slices from the atrioventricular ring to apex, and in the two‐, three,‐ and four‐chamber long‐axis views. LGE images were acquired 10–12 min after gadolinium was administered using a manually optimized inversion‐recovery gradient‐echo preparation. Inversion times were adjusted to null normal myocardium (typically 150–250 ms; pixel size 1.4 mm × 1.9 mm). Ventricular volumes and function were measured for both ventricles using standard techniques, and analysed using semi‐automated software (MEDIS, GE). Patients were classified in a binary manner according to the presence or absence of a mid‐wall stripe defined as linear mid myocardial signal, generally basal septal, of post‐contrast enhancement (Figure 2). As the analysis was a proof of concept, no specific need for LGE quantitation was undertaken (or has it been shown either in our hands or others to invalidate the gross findings in this population).
Figure 2.
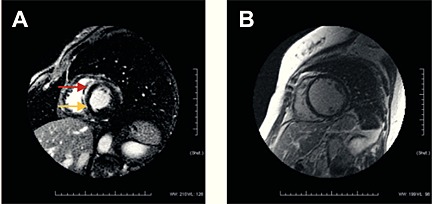
CMR with late gadolinium enhancement midwall stripe. Panel A shows midwall stripe (arrows) in the antero‐septal wall with late gadolinium enhancement (+midwall stripe). Panel B showed an extensive remodelling of left ventricle of a separate patient without enhancement (−Stripe).
Statistical analysis
Continuous data were reported as mean + SD and compared between the +Stripe and −Stripe groups using the two‐sample t‐test. Categorical data were reported as frequency and percentage and were compared between groups using the Fisher exact test. Cox proportional hazards regression proportional hazards regression analysis was performed. Because there was no variation in the comparator group, classic univariate or multivariate analysis was not permissible to identify risk between the +Stripe group and −Stripe group.12 Therefore Kaplan–Meier survival analysis was used to plot days from CMR diagnosis of +Stripe vs. −Stripe to MACE with the log‐rank test being used to compare groups. The Kolmogorov–Smirnov test was used to evaluate the normality of the data. Nonparametric tests were used to evaluate data that broke parametric assumptions. All statistical tests were two‐tailed and a P value <0.05 was considered statistically significance. Statistical analysis was performed using SPSS version 18.0 (SPSS Inc., Chicago, IL). Data were stored for analysis in password protected MS Access 2003.
Results
All 61 patients completed the CMR exam in 49 ± 9 min without complication. When excluding those patients with inflammatory disease, concomitant CAD, and a LVEF ≥ 45%, 31 patients had a pure non‐ischaemic dilated cardiomyopathy and were included in the primary analysis with 100% follow‐up at all time points. Among these patients, 67% (21/31) had LGE findings; 18/21 (86%) patients with LGE demonstrated a +Stripe, while 3/21 (14%) demonstrated a non‐midwall fibrosis pattern (patchy and/or subepicardial LGE). In the 31 patients with primary non‐ischaemic cardiomyopathy, 18 (58%) had +Stripe; the other 13 patients (42%) had −Stripe. Table 1 depicts demographic and clinical characteristics for +Stripe and −Stripe patients. Average age of +Stripe and −Stripe patients was 42 ± 16 years and 50 ± 12 years, respectively, (P = 0.14). The +Stripe group was predominantly male while there was a more even distribution in the −Stripe group (P = 0.006). All other demographic characteristics were similar between the two groups. Percentages prescribed for pharmacotherapeutics for: ACEi and/or ARB, beta‐blockers, aldosterone antagonist, statins, combination of hydralazine/nitrates, or digoxin were 100%, 72%, 61%, 17%, 6%, and 31%, respectively in the +Stripe group. There were no statistical difference for the percentage prescribed of the mentioned medications in the −Stripe group (P = NS).
Table 1.
Demographic characteristics and clinical data of subjects
| Variable | +Stripe (n = 18) (%) | −Stripe (n = 13) (%) | P value |
|---|---|---|---|
| Female | 2 (11) | 8 (62) | 0.006 |
| Age | 42 + 16 | 50 + 12 | 0.14 |
| CAD | 0 | 0 | 1.0 |
| Tobacco | 5 (27.8) | 5 (38.5) | 0.7 |
| HTN | 6 (33.3) | 7 (53.8) | 0.44 |
| Diabetes | 2 (11.1) | 1 (7.7) | 1.0 |
| CVA | 0 | 1 (7.7) | 0.42 |
| Family history of CMP | 1 (5.6) | 2 (15.4) | 0.56 |
| COPD/asthma | 4 (22.2) | 3 (23.1) | 1.0 |
| PE/hypercoagulable | 3 (16.7) | 0 | 0.25 |
| OSA | 2 (11.1) | 0 | 0.497 |
| GERD | 3 (16.7) | 2 (15.4) | 1.0 |
| Alcohol | 4 (22.2) | 2 (15.4) | 1.0 |
| Hypothyroidism | 2 (11.1) | 2 (15.4) | 1.0 |
| Medications | |||
| Beta blocker | 13 (72.2) | 12 (92.3) | 0.36 |
| ACE inhibitor | 18 (100) | 12(92.3) | 0.42 |
| ARB | 0 | 2 (15.4) | 0.17 |
| Aldosterone antagonist | 11 (61.1) | 6 (46.2) | 0.41 |
| Digoxin | 6 (33.3) | 4 (30.8) | 1.0 |
| Furosemide | 14 (77.8) | 7 (53.8) | 0.25 |
| Statin | 3 (16.7) | 4 (30.8) | 0.41 |
| Hydralazine‐nitrate | 1 (5.6) | 2 (15.4) | 0.56 |
| Allopurinol | 0 | 1 (7.7) | 0.42 |
| Amiodarone | 3 (16.7) | 0 | 0.25 |
Data presented are means ± SD. ACE inhibitors: angiotensin converting enzyme inhibitors; ARB: angiotensin receptor blockers; CAD denotes: coronary artery disease; CMP: cardiomyopathy; COPD: chronic obstructive pulmonary disease; CVA: cerebro‐vascular disease; ETOH: alcohol; GERD: gastro‐esophageal reflux disease; OSA: obstructive sleep apnoea; PE: pulmonary embolism; HTN: hypertension.
All patients were alive at 6 months, and two patients died by 12 months. Complete data for all patients were available for 360‐day follow‐up. There were no significant differences between the groups in terms of baseline LVEF, baseline RVEF, RVEDV, NYHA classification, or degree of mitral regurgitation (Table 2). Also, there was no difference between groups with respect to haemodynamics including: pulmonary capillary wedge pressure (PCWP), PA mean pressure, cardiac output (CO), cardiac index (CI), and PA O2 saturation at baseline (Table 2). Those patients with +Stripe had significantly higher LVEDD, LVEDV, and RVEDV (Table 2). The median time of diagnosis to CMR was not significantly different between groups nor was the median time for appearance of symptoms to diagnosis (Table 3). Regarding hospitalization status at the time of enrolment, 17/18 in the +Stripe group (94%) were inpatients, while 10/13 (77%) in the −Stripe group were inpatients (P = NS) (Table 3). There was no difference in systolic or diastolic blood pressure between both groups or among the serum sodium (Na+), BUN, creatinine (Cr), haemoglobin (Hb), lymphocyte count, total cholesterol (Tc), LDL‐cholesterol, and HDL‐cholesterol (P = NS) (Table 3). The primary endpoint was met in 9/18 (50%) at 12 months; urgent transplant in nine patients, LVAD insertion in two patients, and two deaths (two patients had LVAD followed by transplant). Importantly, all patients who reached the primary endpoint possessed a +Stripe on CMR (P = 0.002) (Table 4). Moreover, no patients with a −Stripe on CMR reached either a primary or secondary outcome. The median time from CMR to LVAD/heart transplant was 2 ± 0.4 weeks and to death was 29 ± 8 weeks (Figure 3). Only the presence of +Stripe predicted outcome (P = 0.002). Because no events occurred in the index comparator group (−Stripe) no Cox proportional hazards regression analysis for univariate or multivariate analysis was permitted by statistical convention. Specifically, when the null hypothesis is rejected for the covariate ‘Stripe cohort’, the dataset violates the assumption of proportional hazards for Cox regression because it is a dichotomous variable without events. When the hazards are not proportional, Cox regression is not applicable.12 However, failure of Cox modelling does negate a time‐varying analysis. Thus, Kaplan–Meier survival curves demonstrate the rapid and sharp separation between those patients with and without a midwall stripe as related to requirement for LVAD/Tx or death (χ2 = 9, P = 0.002) (Figure 3).
Table 2.
Baseline CMR parameters, NYHA class, and right heart haemodynamics
| Variable | +Stripe (N = 18) | −Stripe (N = 13) | P value |
|---|---|---|---|
| CMR | |||
| LVEF (%) | 15.4 + 7.5 | 20.6 + 10.15 | 0.11 |
| LVEDV (mL/m2) | 370.7 + 98.2 | 289.1 + 59.2 | 0.013 |
| LVEDD (mL) | 74.6 + 10 | 67.2 + 8 | 0.037 |
| MR | 1.61 + 0.6 (Median=2.0) | 1.38 + 0.5 (Median=1.0) | 0.38 |
| RVEDV (mL) | 217.7 + 65 | 156.1 + 57 | 0.02 |
| RVEF (%) | 26.4 + 12 | 31.4 + 15 | 0.32 |
| NYHA class | Median = 3 | Median = 3 | 0.27 |
| Right heart haemodynamics | |||
| RA (mmHg) | 12.75 + 8 | 13.22 + 12 | 0.91 |
| PAS (mmHg) | 46.19 + 10 | 47.56 + 20 | 0.82 |
| PAD (mmHg) | 19.13 + 8 | 17.8 + 11 | 0.71 |
| PA Mean (mmHg) | 29.44 + 8 | 29.78 + 14 | 0.94 |
| P Wedge (mmHg) | 25.93 + 8 | 20.9 + 13 | 0.23 |
| CO (L/min) | 4.76 + 1.6 | 4.06 + 1.3 | 0.29 |
| CI (L/min/m2) | 2.22 + 0.7 | 2.34 + 0.6 | 0.67 |
| PA SAT (sat%) | 55.74 + 12 | 56.67 + 10 | 0.84 |
Data presented are means ± SD.
CI: cardiac index; CO: cardiac output; LVEDD: left ventricular end‐diastolic diameter; LVEDV: left ventricular end‐diastolic volume; LVEF denotes: left ventricular ejection fraction; MR: mitral regurgitation (0–4+; absent to severe); NYHA: New York heart association class. PAD: pulmonary diastolic pressure; PA mean: pulmonary arterial mean; PAS: pulmonary systolic pressure; P wedge: pulmonary capillary wedge; PA SAT: pulmonary arterial saturation; RA: right atrium; RVEDV: right ventricular end‐diastolic volume; RVEF: right ventricular ejection fraction.
Table 3.
Baseline weight, blood pressure, laboratory parameters, time of symptoms to diagnosis, and QRS widening
| Variable | +Stripe (N = 18) | −Stripe (N = 13) | P value |
|---|---|---|---|
| Weight (pounds) | 216.6 + 39 | 166.4 + 37.5 | 0.001 |
| Systolic blood pressure | 112.1 + 14.9 | 116.5 + 19.9 | 0.49 |
| Diastolic blood pressure | 72.17 + 10.9 | 72.15 + 14.1 | 0.99 |
| Time of symptoms to diagnosis (weeks) | 7 ± 7.5 (n = 18) | 10.58 ± 8.6 (n = 13) | 0.3 |
| Hospitalization status | |||
| Inpatients | 17 (94%) | 10 (77%) | 0.48 |
| Labs | |||
| Sodium | 137.6 + 3.61 | 138.5 + 2.88 | 0.49 |
| BUN | 19.06 + 7.25 | 16.91 + 5.43 | 0.41 |
| Creatinine | 1.02 + 0.22 | 0.93 + 0.25 | 0.32 |
| Haemoglobin | 13.7 + 1.83 | 13.71 + 12.8 | 0.99 |
| Lymphocytes | 21.36 + 4.7 | 28.18 + 3.25 | 0.43 |
| Total cholesterol | 144.3 + 35.5 | 154.2 + 35.2 | 0.52 |
| LDL‐cholesterol | 87.2 + 22.8 | 95.78 + 27.3 | 0.42 |
| HDL‐cholesterol | 35.7 + 12.1 | 35.3 + 12.1 | 0.94 |
| EKG (QRS) ms | 116 ± 28.5 | 103.1 ± 23.5 | 0.19 |
Data presented are means ± SD.
Table 4.
Need for LVAD, urgent orthotopic cardiac transplantation, death, and MACE over a 12‐month period after diagnosis
| Outcome | +Stripe (18) | −Stripe (13) | P value |
|---|---|---|---|
| LVAD/transplant/death (primary outcome) | 9 | 0 | 0.002 |
| LVAD/heart transplant | 7 | 0 | 0.025 |
| Death | 2 | 0 | 0.497 |
| MACE (secondary outcome) | 10 | 0 | 0.001 |
Data presented as numbers of patients. LVAD denotes: left ventricular assist device. MACE: major adverse clinical event.
Figure 3.
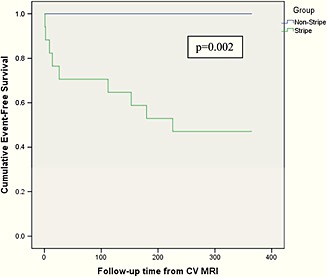
Kaplan–Meier survival function. Kaplan–Meier curves compare patients with and without stripe from time (days) of CMR diagnosis to orthotopic heart transplantation/LVAD or death. During the follow‐up period, event rates for patients with and without +Stripe were 50% vs. 0, respectively (P = 0.002).
By univariate analysis the LVEDD was the only parameter able to predict the need for LVAD/Tx or death (P = 0.032) while the LV EF was nearly significant (P = 0.052). There was a statistically significant difference between the changes in LVEF in the +Stripe vs. the −Stripe groups as assessed by echocardiography from admission to follow‐up at a mean of 4 months ± 58 days of follow‐up (Figure 4). The LVEF improved from a mean of 18% at time of diagnosis to 21% at follow‐up for an absolute increase of 3% in the +Stripe group as compared to 16% at baseline vs. 36% at follow‐up, representing a 20% improvement (>six‐fold) experienced in the −Stripe group (Figure 4) (P = 0.002).
Figure 4.
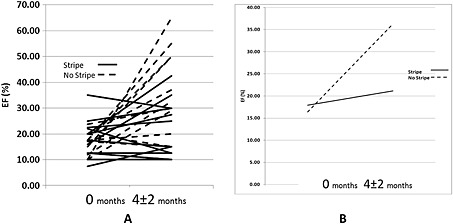
Left ventricle EF assessed by echocardiogram on admission and follow‐up. A) Bold lines represent LV EF in the +Stripe patients. Dashed lines represent LV EF in the −Stripe patients. LV EF assessed by echocardiography at time of admission and at 4‐months ± 2‐month follow‐up. B) Mean baseline LV EF in +Stripe (bold line) group is 18% and follow‐up at 4 months ± 2 months is 21%. Mean baseline LVEF in −Stripe (dashed line) group is 16% and follow‐up is 36%.
All tissue samples from LVAD (core) or heart transplantation (native heart) underwent conventional analysis in the Pathology Laboratory via H&E and Masson's trichrome stains confirming the presence of myocardial fibrosis without acute inflammation findings. Occasionally, lipomatous changes along with midwall fibrosis were reported.
Discussion
Dilated cardiomyopathy is the third most common cause of heart failure and one of the most common cause of death in many heart transplantation centres.13, 14 In this study, incorporating patients with ‘newly’ diagnosed dilated cardiomyopathy referred to a busy Advanced Heart Failure/Transplant service, we demonstrated that the presence of LV late gadolinium enhancement in the form of a midwall myocardial Stripe on CMR, predicted the need for urgent LVAD, cardiac transplantation, or death at 12 months independent of standard 3D CMR LVEF, RVEF, LVEDV, RVEDV, QRS widening, haemodynamics (CO/CI, PCWP, and PA saturation), conventional laboratory values (Na+, BUN, Cr, Hb, lymphocytes, TC, Ldl‐c, and Hdl‐c), BNPq, or NYHA functional class. Moreover, the acute temporal nature of the observation was critical; the median time from CMR to LVAD/Tx was just 2 weeks in the +Stripe patients (range 3–153 days).
Our cardinal finding in this proof of concept study is the prediction of LVAD/Tx requirement or death by CMR depiction of the presence or absence of a mid‐wall stripe. All (100%) of those requiring LVAD/Tx and experiencing deaths by 12 months possessed a midwall stripe. Equally important, 100% of those patients not possessing a midwall stripe did not require LVAD/Tx nor die during the 12‐month follow‐up period; indeed, all −Stripe patients had a very favourable clinical course over that same time period. Importantly, the −Stripe group had relevant clinical improvement of their LVEF by an absolute increase of 20% vs. 3% in the +Stripe group (P = 0.002). It is important to note that serial follow‐up testing by CMR was not possible because of newly implanted AICDs in many of the patients; hence, we compared baseline (performed at same time of CMR) and subsequent follow‐up LVEF by echocardiography alone to avoid introducing bias by analysing data with different methodologies.
To date, prognostic models have been developed to help identify those at risk of adverse outcomes, and those in whom cardiac transplantation or mechanical device therapy may be considered in ‘chronic’ established DCM patients.4, 5 Conventional risk factors, in general, are less helpful in determining shorter‐term prognosis in ‘newly’ diagnosed DCM patients. CMR promises a new feature for the evaluation of these patients. The Kaplan–Meier survival plots illustrate the clinical hazard predicated solely via stripe morphology. No other measurable metric whether it be derived non‐invasively from CMR, invasively from the catheterization lab or from the blood laboratory provided predictive dichotomization (although a slightly smaller heart was noted in the −Stripe group failing to be predictive by Kaplan–Meier curves). Our population was in extremis; we note that the need for urgent orthotopic heart transplantation defined by the United Network for Organ Sharing (UNOS) status 1A or 1B was present in all seven cases that required LVAD and/or heart transplantation. The mean time from diagnosis until patients were listed in the UNOS system was 30 days. Two of the transplanted patients needed a LVAD insertion prior to transplant suggesting the more acute and fulminant decline in these patients (inserted at day 2 and 7 post CMR).
Is there any precedence for this novel finding? Recently, Assomull et al. studied the significance of the CMR LGE pattern of midwall fibrosis (synonymous with our +Stripe) in 101 patients with chronic (>12 months) symptomatic DCM with LVEF < 55%.15 They concluded that in patients with DCM, the presence of myocardial fibrosis had a higher incidence of all cause‐mortality, hospitalization, sudden cardiac death, and ventricular arrhythmias over the ensuing 2 ± 1 years. Interestingly, in their studied population, orthotopic heart transplantation was performed in three patients with all of them demonstrating myocardial fibrosis. Also, there were six deaths (17%) in the +LGE group and four deaths (6%) in the −LGE patients. By Kaplan–Meier survival analysis, they concluded there were ‘no’ significant differences in all‐cause mortality; however, if they had considered incorporating orthotopic heart transplantation as a surrogate of death, the incidence of all‐cause mortality and need for heart transplantation would have been statistically different with a 26% incidence in the +LGE group against 6% in the −LGE group.
Similarly, Wu et al. described a relationship between LGE and appropriate ICD firings, heart failure hospitalization, and cardiac death via quantifying LGE extent over 1.5 years in 65 non‐ischaemic cardiomyopathy patients with LVEF < 35%.16 In contrast to the previous two studies, Hombach et al. analysed 141 patients with chronic idiopathic dilated cardiomyopathy and found by univariate analysis, the presence of diabetes mellitus, QRS >110 ms, right ventricular end diastolic volume index, CI, LVEF, and the presence of LGE (without discriminating stripe vs. non‐stripe) as significant predictors for cardiac or sudden death. In their multivariate analysis only CI, diabetes mellitus, RVEDVI, and QRS > 110 ms were found to be predictors of cardiac or sudden death.17
More recently, Levya and colleagues demonstrated that the presence of a midwall stripe conveyed an independent risk to non‐ischaemic but not ischaemic patients in those contemplating cardiac resynchronization therapy (CRT) over the ensuing 3 years. Scar burden was not an independent predictor of total or cardiovascular mortality.18
Our study markedly expands these earlier findings. Here, a shorter follow‐up time was needed to reach statistical significance, predicted a much more acute deterioration in this selected group who were specifically referred for advanced heart failure diagnosis/treatment, did not require quantitative LGE assessment (we used a binary assessment), and was more powerful in acutely predicting LVAD/transplantation, often within days to weeks, not months to years. Specifically, Wu et al. with a much larger, but more inclusive non‐ischaemic population, was unable to detect a simple common denominator of myocardial enhancement pattern to prognosticate, although they suggested that a pattern might well exist. In contrast to Hombach et al., in the present study it was shown that the presence of diabetes mellitus, CI, or QRS > 110 ms was not predictive of LVAD/Tx or death. In our population, clinical important risk factor such as diabetes mellitus that generally predisposes to acute coronary syndrome and sudden cardiac death was relatively absent. Also, the QRS was not statistically different between +Stripe and −Stripe groups. We believe that one of the key distinguishing elements of the present study is the inclusion of ‘newly diagnosed’ DCM presenting with heart failure symptoms referred for consideration of transplant; all other studies have looked at the ‘chronic‐established’ DCM patients. It should be noted that recently, Dweck et al. demonstrated that midwall fibrosis was an independent predictor of mortality in patients with moderate and severe aortic stenosis,19 pointing to the primacy of fibrosis as an end‐stage marker.
The mechanism for our observation remains speculative. As others have suggested,12, 14, 15, 16, 17, 18, 19, 20 this most likely represents intramyocardial fibrosis as a pathologic response to injury mediated by inflammation, cytokine activation, adrenergic tone, myocyte hypertrophy, and apoptosis with eventual collagen deposition. Notably, this finding is distinctly absent in ischaemic cardiomyopathy albeit the somewhat similar pathologic cascade suggesting the mechanism of initial injury dictates the eventual myocardial response pattern. That all patients underwent pathological confirmation corroborating fibrosis in either the stripe (exact co‐localization with MRI) and/or the apical core lends support to this theory.
Originally, the +Stripe was first described in acute and chronic myocarditis20, 21, 22 and was believed to be the source of the +Stripe seen in DCM patients. However, currently, there is more evidence that this phenomenon is also seen in different pathologies such aortic stenosis, chemotherapy induced cardiomyopathy, muscular dystrophy, and systemic sclerosis,19, 23, 24, 25, 26 suggesting that the finding of +Stripe is perhaps a common final pathway in the process of remodelling of the LV independently of the aetiology. It should also be made clear that the presence (or absence) of this Stripe helps to nearly completely exclude an infiltrative or hypertrophic cardiomyopathy and always completely excludes an ischaemic aetiology independent of the manner in which a patient may clinically present. Thus, beyond the singular finding of the ‘Impact Factor’ of the +Stripe, it provides additive information to the diagnosis that potentially may translate into improved therapeutic options.
In summary, we were able to define a clear influence of a ‘pattern driven prognosis’ based on the initial CMR post‐contrast LGE pattern. Importantly, predictive power was possible entirely based upon the finding of late gadolinium enhancement manifested in the form of +Stripe and was independent of stripe length, thickness, or need to quantify its volume. The unique characteristics of this study are: (i) the ability to risk stratify patients when a de novo diagnosis of dilated cardiomyopathy is made in patients who are presenting with a NYHA functional class II–IV; (ii) we believe that this study is the first to identify the prognostic value of a +Stripe in predicting the urgent (within 6 months, remaining robust at 1 year) requirement for LVAD and/or orthotopic heart transplantation in a DCM population that has been referred to a heart failure/transplantation centre; and (iii) this approach may provide a paradigm change in favour of the anticipation for LVAD and/or transplantation need as opposed to the current more traditional ‘non‐stripe’ approach.
Limitations
A limitation of this study is the sample of patients was limited to newly diagnosed, non‐ischaemic cardiomyopathy patients most requiring hospitalization and referral to a heart failure/transplant service. Even though we included outpatients and inpatients referred to our heart failure/transplant service, the majority of patients were hospitalized at time of referral; hence, the finding of +Stripe and its prognostic value needs further investigation in the non‐hospitalized patients. Other limitations are the limited number of dilated cardiomyopathy patients despite our relatively large national transplant centre (top 10% in USA), limited pathological correlates via biopsy (as per standard clinical practice), and the lack of MVO2 in all patients. However, the striking results in correlating CMR findings and prognosis in DCM patients demand investigation in this preliminary but provocative finding. We also recognize that a univariate and multivariate analysis could not be performed for LGE because of the lack of variation in the −Stripe group, i.e. if the comparator arm has no events these statistical tools are, by definition, not statistically permissible by rule as previously described.12 To be sure, this is an interesting paradox. If, for example, the perfect test could be designed that could ‘binarily’ predict an event, univariate or multivariate analysis would indicate an imperfect test.
Finally, we recognize that the study, per protocol, truncated follow‐up at 12 months mostly to obtain the number of patients that were in the study. The primacy of the CMR findings dovetailing with the immediacy of LVAD/transplantation suggests, albeit imperfectly, a lower likelihood of long‐term adverse outcomes. By definition, long‐term follow‐up of this cohort is crucial to confirm our preliminary findings and may further underscore the importance of this early‐term observation. This observation should be confirmed in a prospective multicentre study and is the focus of our ongoing efforts.
Conclusions
Dilated cardiomyopathy patients with advanced heart failure require an improved risk stratification policy. Using standard CMR, the presence of +Stripe was highly predictive of LVAD and transplantation need over the ensuing 12 months. No other classical clinical, biologic, or haemodynamic metric strongly predicted the need for transplantation. Also, the presence of a midwall stripe, signifying myocardial fibrosis, strongly predicted MACE. Further, those −Stripe patients had a far better prognosis along with a clinical important improvement in LVEF where there was neither need for LVAD/transplantation nor death at 12‐month follow‐up. Incorporating this approach into routine clinical practice may help conservatively manage low risk patients while expectantly manage high‐risk patients. Data at 2‐year follow‐up will further place more long‐term outcomes in perspective.
Acknowledgements
We wish to thank the Pathology Lab, OR staff, and IRB for assistance in this work.
Funding
Internal funding.
Conflict of interest
All authors declare that they have no conflict of interest.
Venero, J. V. , Doyle, M. , Shah, M. , Rathi, V. K. , Yamrozik, J. A. , Williams, R. B. , Vido, D. A. , Rayarao, G. , Benza, R. , Murali, S. , Glass, J. , Olson, P. , Sokos, G. , and Biederman, R. W. W. (2015) Mid wall fibrosis on CMR with late gadolinium enhancement may predict prognosis for LVAD and transplantation risk in patients with newly diagnosed dilated cardiomyopathy—preliminary observations from a high‐volume transplant centre. ESC Heart Failure, 2: 150–159. doi: 10.1002/ehf2.12041.
References
- 1. Shehata ML, Turkbey EB, Vogel‐Claussen J, Bluemke DA. Role of cardiac magnetic resonance imaging in assessment on nonischemic cardiomyopathy. Top Magn Reson Imaging 2008; 19: 43–57. [DOI] [PubMed] [Google Scholar]
- 2. McCrohon JA, Moon JC, Prasad SK, McKenna WJ, Lorenz CH, Coats AJ, Pennell DJ. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium‐enhanced cardiovascular magnetic resonance. Circulation 2003; 108: 54–59. [DOI] [PubMed] [Google Scholar]
- 3. Wu E, Judd RM, Vargas JD, Klocke FJ, Bonow RO, Kim RJ. Visualization of presence, location, and transmural extent of healed Q‐wave and non‐Q‐wave myocardial infarction. Lancet 2001; 357: 21–28. [DOI] [PubMed] [Google Scholar]
- 4. Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, Anand I, Maggioni A, Burton P, Sullivan MD, Pitt B, Poole‐Wilson PA, Mann DL, Packer M. The Seattle heart failure model: prediction of survival in heart failure. Circulation 2006; 113: 1424–1433. [DOI] [PubMed] [Google Scholar]
- 5. Aaronson KD, Schwartz JS, Chen TM, Wong KL, Goin JE, Mancini DM. Development and prospective validation of a clinical index to predict survival in ambulatory patients referred for cardiac transplantation. Circulation 1997; 95: 2660–2667. [DOI] [PubMed] [Google Scholar]
- 6. Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, Klocke FJ, Bonow RO, Judd RM. The use of contrast‐enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med 2000; 343: 1445–1453. [DOI] [PubMed] [Google Scholar]
- 7. Jessup M, Abraham WT, Casey DE, Feldman AM, Francis GS, Ganiats TG, Konstam MA, Mancini DM, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 Focused Update: ACCF/AHA Guidelines for the Diagnosis and Management Of Heart Failure in Adults: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: Developed in Collaboration With The International Society for Heart and Lung Transplantation. Circulation 2009; 119: 1977–2016. [DOI] [PubMed] [Google Scholar]
- 8. Bello D, Shah DJ, Farah GM, Di Luzio S, Parker M, Johnson MR, Cotts WG, Klocke FJ, Bonow RO, Judd RM, Gheorghiade M, Kim RJ. Gadolinium cardiovascular magnetic resonance predicts reversible myocardial dysfunction and remodeling in patients with heart failure undergoing beta‐blocker therapy. Circulation 2003; 108: 1945–1953. [DOI] [PubMed] [Google Scholar]
- 9. Kanderian AS, Renapurkar R, Flamm SD. Myocardial viability and revascularization. Heart Fail Clin 2009; 5: 333–348,vi. [DOI] [PubMed] [Google Scholar]
- 10. Biederman RW, Doyle M, Yamrozik J. Cardiovascular MRI Tutorial. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. p133–160. [Google Scholar]
- 11. Cooper LT Jr. Myocarditis. N Engl J Med 2009; 360: 1526–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Collett D. Modelling Survival Data in Medical Research. New York: Chapman & Hall; 1994. p253–265. [Google Scholar]
- 13. Gottlieb I, Macedo R, Bluemke DA, Lima JA. Magnetic resonance imaging in the evaluation of non‐ischemic cardiomyopathies: current and future perspectives. Heart Fail Rev 2006; 11: 313–323. [DOI] [PubMed] [Google Scholar]
- 14. Calore C, Cacciavillani L, Boffa GM, Silva C, Tiso E, Marra MP, Bacchiega E, Corbetti F, Iliceto S. Contrast‐enhanced cardiovascular magnetic resonance in primary and ischemic dilated cardiomyopathy. J Cardiovasc Med 2007; 8: 821–829. [DOI] [PubMed] [Google Scholar]
- 15. Assomull RG, Prasad SK, Lyne J, Smith G, Burman ED, Khan M, Sheppard MN, Poole‐Wilson PA, Pennell DJ. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol 2006; 48: 1977–1985. [DOI] [PubMed] [Google Scholar]
- 16. Wu KC, Weiss RG, Thiemann DR, Kitagawa K, Schmidt A, Dalal D, Lai S, Bluemke DA, Gerstenblith G, Marbán E, Tomaselli GF, Lima JA. Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in nonischemic cardiomyopathy. J Am Coll Cardiol 2008; 51: 2414–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hombach V, Merkle N, Torzewski J, Kraus JM, Kunze M, Zimmermann O, Kestler HA, Wöhrle J. Electrocardiographic and cardiac magnetic resonance imaging parameters as predictor of a worst outcome in patients with idiopathic dilated cardiomyopathy. Eur Heart J 2009; 30: 2011–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leyva F, Taylor RJ, Foley PW, Umar F, Mulligan LJ, Patel K, Stegemann B, Haddad T, Smith RE, Prasad SK. Left ventricular midwall fibrosis as a predictor of mortality and morbidity after cardiac resynchronization therapy in patients with nonischemic cardiomyopathy. J Am Coll Cardiol 2012; 60: 1659–1667. [DOI] [PubMed] [Google Scholar]
- 19. Dweck M, Joshi S, Murigu T, Alpendurada F, Jabbour A, Melina G, Banya W, Gulati A, Roussin I, Raza S, Prasad NA, Wage R, Quarto C, Angeloni E, Refice S, Sheppard M, Cook SA, Kilner PJ, Pennell DJ, Newby DE, Mohiaddin RH, Pepper J, Prasad SK. Midwall fibrosis is an independent predictor of mortality in patients with aortic stenosis. JACC 2011; 58: 1271–1279. [DOI] [PubMed] [Google Scholar]
- 20. Felker GM, Thompson RE, Hare JM, Hruban RH, Clemetson DE, Howard DL, Baughman KL, Kasper EK. Underlying causes and long‐term survival in patients with initially unexplained cardiomyopathy. N Engl J Med 2000; 342: 1077–1084. [DOI] [PubMed] [Google Scholar]
- 21. Gutberlet M, Spors B, Thoma T, Bertram H, Denecke T, Felix R, Noutsias M, Schultheiss HP, Kühl U. Suspected chronic myocarditis at cardiac MR: diagnostic accuracy and association with immunohistologically detected inflammation and viral persistence. Radiology 2008; 246: 401–409. [DOI] [PubMed] [Google Scholar]
- 22. Mahrholdt H, Goedecke C, Wagner A, Meinhardt G, Athanasiadis A, Vogelsberg H, Fritz P, Klingel K, Kandolf R, Sechtem U. Cardiovascular magnetic resonance assessment of human myocarditis: a comparison to histology and molecular pathology. Circulation 2004; 109: 1250–1258. [DOI] [PubMed] [Google Scholar]
- 23. Foley P, Hamilton M, Leyva F. Myocardial scarring following chemotherapy for multiple myeloma detected using late gadolinium hyperenhancement cardiovascular magnetic resonance. J Cardiovasc Med (Hagerstown) 2010; 11: 386–388. [DOI] [PubMed] [Google Scholar]
- 24. Catalano O, Antonaci S, Moro G, Baldi M, Cobelli F, Opasich C. Contrast‐enhanced cardiac magnetic resonance in a patient with chemotoxic cardiomyopathy. J Cardiovasc Med 2007; 8: 214–215. [DOI] [PubMed] [Google Scholar]
- 25. Silva M, Meira Z, Gurgel Giannetti J, da Silva MM, Campos AF, Barbosa Mde M, Starling Filho GM, Ferreira Rde A, Zatz M, Rochitte CE. Myocardial delayed enhancement by magnetic resonance imaging in patients with muscular dystrophy. J Am Coll Cardiol 2007; 49: 1874–1879. Epub 2007 Apr 23. [DOI] [PubMed] [Google Scholar]
- 26. Tzelepis G, Kelekis N, Plastiras S, Mitseas P, Economopoulos N, Kampolis C, Gialafos EJ, Moyssakis I, Moutsopoulos HM. Pattern and distribution of myocardial fibrosis in systemic sclerosis: a delayed enhanced magnetic resonance imaging study. Arthritis Rheum 2007; 56: 3827–3836. [DOI] [PubMed] [Google Scholar]


