Abstract
Objectives
The study hypothesis was that a target‐specific anticoagulant would allow successful home treatment of selected patients with deep vein thrombosis (DVT) and pulmonary embolism (PE) diagnosed in two urban emergency departments (EDs).
Methods
A protocol was established for treating low‐risk DVT or PE patients with rivaroxaban and clinic, follow‐up at both 2 to 5 weeks, and 3 to 6 months. Patients were determined to be low‐risk by using a modified version of the Hestia criteria, supplemented by additional criteria for patients with active cancer. Acceptable outcome rates were defined as venous thromboembolism (VTE) recurrence ≤ 2.1% or bleeding ≤ 9.4% during treatment. VTE recurrence required positive imaging of any VTE. The International Society of Thrombosis and Hemostasis definition of major or clinically relevant nonmajor bleeding was used.
Results
From March 2013 through April 2014, a total of 106 patients were treated. Seventy‐one (68%) had DVT, 30 (28%) had PE, and five (3%) had both, representing 51% of all DVTs and 27% of all PEs diagnosed in both EDs during the period of study. The 106 patients have been followed for a mean (±SD) of 389 (±111) days (range = 213 to 594 days). No patient had VTE recurrence, and no patient had a major or clinically relevant bleeding event while on therapy (none of the 106, 0%, 95% confidence interval [CI] = 0% to 3.4%). However, three patients 2.8% (95% CI = 1% to 8%) had recurrent DVT after cessation of therapy.
Conclusions
Patients diagnosed with VTE and immediately discharged from the ED while treated with rivaroxaban had a low rate of VTE recurrence and bleeding.
Continuing Medical Education
Continuing Medical Education Activity in Academic Emergency Medicine
CME Editor: Corey Heitz, MD
Authors: Daren M. Beam, MD, MS, Zachary P. Kahler, MD, and Jeffrey A. Kline, MD
Article Title: Immediate Discharge and Home Treatment With Rivaroxaban of Low‐risk Venous Thromboembolism Diagnosed in Two U.S. Emergency Departments: A One‐year Preplanned Analysis
If you wish to receive free CME credit for this activity, please refer to the website: http://www.wileyhealthlearning.com/aem.
Accreditation and Designation Statement:
Blackwell Futura Media Services designates this journal‐based CME activity for a maximum of 1 AMA PRA Category 1 Credit tm . Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
After completing this exercise the participant will be better able to discuss the outcomes of patients with venous thromboembolism (VTE) discharged from the ED and treated with rivaroxaban.
Activity Disclosures
This study was funded in part by the Lilly Physician Scientist Award.
Faculty Disclosures:
CME Editor: Corey Heitz, MD has no relevant financial relationships to disclose.
Authors: Daren M. Beam, MD, MS, and Zachary P. Kahler, MD have no relevant financial relationships to disclose. Jeffrey A. Kline, MD has received research grant funding from AHRQ, PCORI, and NIH within the past 3 years and has been a consultant to Stago Diagnostica, Janssen, and Pfizer.
This manuscript underwent peer review in line with the standards of editorial integrity and publication ethics maintained by Academic Emergency Medicine. The peer reviewers have no relevant financial relationships. The peer review process for Academic Emergency Medicine is double‐blinded. As such, the identities of the reviewers are not disclosed in line with the standard accepted practices of medical journal peer review.
Conflicts of interest have been identified and resolved in accordance with Blackwell Futura Media Services's Policy on Activity Disclosure and Conflict of Interest.
Instructions on Receiving Free CME Credit
For information on applicability and acceptance of CME credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within an hour; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period, which is up to two years from initial publication.
Follow these steps to earn credit:
Log on to to http://www.wileyhealthlearning.com
Read the target audience, educational objectives, and activity disclosures.
Read the article in print or online format.
Reflect on the article.
Access the CME Exam, and choose the best answer to each question.
Complete the required evaluation component of the activity.
This activity will be available for CME credit for twelve months following its publication date. At that time, it will be reviewed and potentially updated and extended for an additional twelve months.
The medical care of patients with venous thromboembolism (VTE; collectively deep venous thrombosis [DVT] or pulmonary embolism [PE]) is undergoing a shift in both process and delivery. Systematic reviews and meta‐analyses of literature have suggested a very low failure rate associated with home treatment of patients with low‐risk PE as determined by validated prognostic scores.1, 2 In real practice, home treatment of VTE is stymied by several problems associated with the U.S. health care delivery system, lack of precedent literature, inability to arrange follow‐up, and medicolegal concerns.1, 2, 3, 4, 5, 6
We report the initial results of a rivaroxaban‐based treatment protocol to allow early home treatment of emergency department (ED) patients with DVT and PE deemed to be low risk using the validated Hestia criteria, supplemented with additional criteria for patients with active cancer.7, 8, 9, 10 A pooled analysis of EINSTEIN DVT and PE found a 2.1% rate of VTE recurrence and a 9.4% rate of clinically relevant major and nonmajor bleeding within 208 days (SD ± 96 days) while taking rivaroxaban.11 We therefore aimed for a ≤ 2.1% rate of image‐proven VTE recurrence and a ≤ 9.4% rate of bleeding that required an unscheduled physician visit while on therapy.
Methods
Study Design
This was a prospective observational study to test the safety and use effectiveness of a clinical pathway that allowed immediate discharge of low‐risk VTE from the ED using a novel target‐specific anticoagulant. The protocol was approved as a research study by the Indiana University Institutional Review Board and is registered (NCT02079584). The authors constructed a two‐page written summary of the protocol, which in sequence was presented to the pharmacy and therapeutics committees, emergency medicine (EM) faculty and residents, and then medical executive committees at two hospitals prior to initiation of the study.
Study Setting and Population
The protocol was instituted at two academic EDs in March 2013. One site is an urban teaching hospital that saw 92,000 ED patients in 2014, with approximately 80% of its patient population below the federal poverty line and 60% self‐pay. The other site is an urban teaching hospital that is the flagship of a large hospital system, with 102,000 ED patients in 2014, with less than 30% prevalence of low‐income patients. Both are staffed by board‐certified emergency physicians and EM residents.
Study Protocol
This program includes a standard care protocol divided into two phases: the ED phase with VTE diagnosis and the follow‐up clinic phase. DVT was diagnosed on the basis of compression venous ultrasound, interpreted by either hospital‐credentialed vascular medicine physicians or board‐certified radiologists. The diagnosis of PE was by either computerized tomographic pulmonary angiography or high‐probability ventilation‐perfusion lung scanning, interpreted by a board certified radiologist. The protocol used a slightly modified version of the Hestia criteria (Table 1) to identify low‐risk patients who may be discharged with new diagnoses of DVT, PE, or both.8 Patients with active malignancy were further risk stratified using the POMPE‐C tool (Table 2).12
Table 1.
Modified Hestia Exclusion Criteria
| 1. Systolic hypotension (<100 mm Hg in absence of a history of low blood pressure at baseline). |
| 2. Contraindication to low‐molecular‐weight heparin or warfarin treatment (active bleeding or high‐risk postoperative status, creatinine clearance < 30 mL/min, history of heparin‐induced thrombocytopenia, or warfarin skin necrosis), |
| 3. Other medical condition requiring hospital treatment (sepsis, new or decompensated existing organ failure, intractable pain). |
| 4. Social condition requiring hospital treatment (homelessness with history of nonadherence to treatment, suspected neglect or abuse, untreated psychosis, severe alcohol or drug dependency). |
| 5. Coagulopathy, any INR > 1.7, or thrombocytopenia (platelet count < 50 × 109/L). |
| 6. Pregnancy. |
| 7. Incarceration. |
Table 2.
Variables Required for the Online Prediction of Mortality From Pulmonary Embolism in Cancer (POMPE‐C) Logistic Regression Equation (http://www.studymaker.com/projects/pompe/index.php)
| Independent Variable |
|---|
| Patient weight (lb) |
| Highest respiratory rate (breaths/min) |
| SaO2 (%) |
| Heart rate > 99 beats/min |
| Altered mental status |
| Respiratory distress |
| Do not resuscitate status |
| Unilateral limb swelling |
Clinicians completed a survey (REDCap; see Data Supplement S1, available as supporting information in the online version of this paper) in real time that required all components of Hestia to be satisfied and initiated a standardized order set including complete blood count, a basic metabolic panel, optionally 1 mg/kg enoxaparin, and one 15‐mg rivaroxaban dose by mouth, prior to discharge. We had two reasons for administering the single dose of enoxaparin in addition to rivaroxaban at diagnosis. First, 73% of patients in the EINSTEIN trials received heparin prior to receiving rivaroxaban. Second, prior to initiation of our protocol, rivaroxaban had to be ordered from pharmacy, while enoxaparin was stocked in the ED. Thus, waiting on rivaroxaban had the potential to impart lengthy delays in time to anticoagulation.
Eligible patients were prescribed 15 mg of rivaroxaban twice per day for 21 days and then a 1‐month prescription for 20 mg once per day. Subsequent prescriptions for rivaroxaban were written during clinic follow‐up. Access to drug was enhanced by the fact that Indiana Medicaid lists rivaroxaban as a preferred drug. Low‐income, uninsured patients were offered a national foundation to provide rivaroxaban free of charge or at a deeply discounted rate for up to 1 year (www.jjpaf.org). Patients with private insurance could take advantage of a manufacturer‐sponsored supplemental coverage plan to reduce the copay. In the minority of patients who did not fall in any of the above categories, local, county, and statewide assistance programs were investigated to obtain drug. All patients receive discharge instructions that include the mobile telephone numbers of the physician running the clinic.
Follow‐up
One to 2 days after discharge, a member of the care team called the patient to confirm that the patient was able to fill the rivaroxaban prescription and to answer other questions about his or her diagnosis. The first follow‐up visit was approximately 3 weeks after diagnosis and the second follow‐up was 3 to 6 months later. The authors supervised each clinic, and each patient received a standardized history and physical (Data Supplement S2, available as supporting information in the online version of this paper). The history and physical form was designed to assess bleeding risk, recurrence, and presence or absence of postthrombotic syndrome. This form included four questions about compliance: 1) where the medicine was filled, 2) if any doses were missed, 3) what time of day pills were taken, and 4) pill count verification.13 Additionally, all patients who followed up were asked to provide written informed consent for participation in a research registry. For patients who did not follow up in the scheduled clinic, we used a sequential approach as previously described to determine any adverse events.14 For patients contacted by telephone, we asked the same questions as in clinic and examined the medical record for evidence of image‐proven VTE recurrence or bleeding that required unscheduled physician visits. For patients who could not be contacted by telephone, we examined a comprehensive electronic medical record system (the Indiana Network for Patient Care [INPC], which includes statewide clinical data from over 90 hospitals, public health departments, local laboratories and imaging centers, and a few large group practices) for any outcome measure. Each patient's medical record was evaluated by one of the authors for 1) any imaging study mentioning recurrent VTE, 2) any documentation by a physician indicating hemorrhage, or 3) any discharge summary indicating new VTE or hemorrhage using methods previously described.14 We also queried the Social Security Death Index (SSDI) for death 6 months or more after diagnosis. Patients with evidence of VTE recurrence, bleeding, or death were reviewed by all three authors to generate consensus.
Stopping Criteria
We used a combination of published criteria, evidence, clinician judgment, and shared decision‐making to decide the duration of anticoagulation for each patient.15, 16, 17, 18, 19, 20, 21 Factors that caused us to recommend longer duration of anticoagulation were prior VTE history, high‐risk clot location (e.g., proximal, recurrent PE), male sex, obesity, postthrombotic symptoms, and D‐dimer concentration on therapy. Factors that caused us to recommend shorter duration included risks for bleeding (e.g., alcoholic with fall risk), distal DVT or PE, and provoked VTE.15, 16, 19, 20, 21, 22 We measured the prothrombin time and D‐dimer during treatment to assist with decision to stop anticoagulation for patients with proximal DVT or PE. We did not routinely measure the D‐dimer for calf, saphenous, or brachial vein thrombosis, nor did we routinely test for thrombophilia.23, 24 All patients with proximal DVT or any PE and no contraindications are instructed to take 81 mg of aspirin daily for life after completing their anticoagulation, as this has been shown to decrease recurrence rates by half when compared with no aspirin treatment.25
Data Analysis
We planned in advance to report the outcomes of this protocol after 1 year, assuming that we would enroll approximately 100 patients, with treatment success defined as a low rate of recurrent VTE or significant bleeding event while on therapy. Our definition of recurrent VTE required repeat imaging that showed acute DVT or PE. The definition of a significant bleeding event was either major bleeding (using the International Society for Thrombosis and Hemostasis definition of >2 g/dL acute drop in hemoglobin [Hb] or >2‐unit blood transfusion, bleeding in a critical area, or bleeding that contributing to death) or clinically relevant nonmajor bleeding (bleeding that required the patient to make an unscheduled visit to any health care provider for evaluation, permanently discontinue rivaroxaban, or significantly alter activities of daily living for more than a few days). Outcomes were adjudicated by at least two board‐certified emergency physicians who ran the clinic (JAK, DMB, ZPK), requiring agreement by two of the physicians. We defined success as a rate of VTE recurrence not more than 2.1% while on treatment, and a bleeding rate not more than 9.4%, based upon the pooled data from the EINSTEIN DVT and PE.11 We report confidence intervals (CIs) for point estimates of proportions from the exact Clopper‐Pearson method. For D‐dimer and prothrombin time measurements we report medians and interquartile ranges (IQR). Outcomes were determined through patient follow‐up visits, telephone conversations, and chart extraction. Charts were reviewed by two study authors using preplanned criteria, with discordances resolved by a third author. During each follow‐up visit or telephone conversation, the patients were queried about any excessive bleeding such as epistaxis, menorrhagia, or bruising. The authors then evaluated if such an event met criterion for major bleed or clinically relevant nonmajor bleeding event.
Results
The protocol (see Data Supplement S3, available as supporting information in the online version of this paper) was implemented in March 2013. From March 25, 2013, until April 30, 2014, a total of 106 unique patients were discharged from one of our two EDs, including 71 (67%) with DVT, 30 (28%) with PE, and five (5%) with both DVT and PE. Figure 1 shows a flow diagram of patients identified and discharged home. During the same time period, 131 patients were diagnosed with PE, and 140 patients with DVT. Thus, this protocol captured 35 of 131 (27%, 95% CI = 19% to 35%) patients with PE and 71 of 140 (51%, 95% CI = 42% to 59%) patients with DVT. No ED patients were discharged with VTE outside of this protocol. Table 3 shows the demographic and clinical variables. Most were 50 years or younger and VTE was unprovoked in 101 of the 106 (95%). Significant comorbidities include hypertension (n = 11), active cancer (n = 5), diabetes mellitus (n = 3), dementia (n = 2), alcoholism (n = 1), heart failure (n = 1), and acquired immunodeficiency syndrome (n = 1). Approximately one‐third of patients related some degree of chronic pain, most unrelated to their thrombosis. Table 4 shows the clot locations. Among patients with isolated DVT, approximately one‐half were proximal. Most (28 of 37, 76%) PEs were segmental or larger. The source of medical payment was commercial insurance in 22, Medicare in 20, Medicaid in 11, and self‐pay in the remainder (53, 50%).
Figure 1.
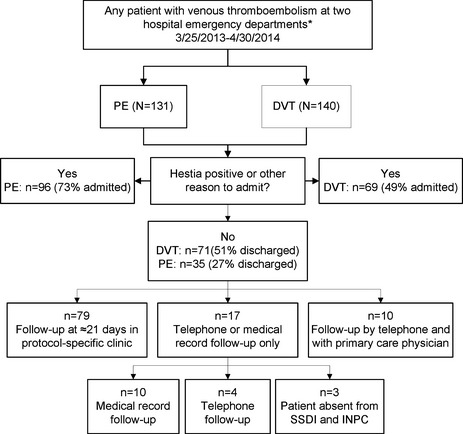
Flow diagram of patient enrollment and outcomes. *Hospitals include Wishard (now Eskenazi) and Methodist hospitals, both in Indianapolis, IN. DVT = deep vein thrombosis; INPC = Indiana Network for Patient Care; PE = pulmonary embolism; SSDI = Social Security Death Index.
Table 3.
Demographic Features
| Characteristic | Number | Percent or ± SD |
|---|---|---|
| Sex | ||
| Male | 58 | 55 |
| Female | 48 | 45 |
| Age (yr) | ||
| Mean | 47 | ± 16 |
| 18–30 | 16 | 15 |
| 31–40 | 24 | 23 |
| 41–50 | 26 | 25 |
| 51–60 | 18 | 17 |
| 61–70 | 11 | 10 |
| 71–80 | 5 | 5 |
| >80 | 6 | 6 |
| Race | ||
| White | 47 | 44 |
| Black | 50 | 47 |
| Other | 9 | 8 |
| Ethnicity | ||
| Hispanic | 7 | 7 |
| Hospital | ||
| Methodist | 55 | 52 |
| Eskenazi | 51 | 48 |
Table 4.
Clot Locationsa
| Diagnosis | n (%) |
|---|---|
| DVT | |
| Upper extremityb | 3 (4) |
| Axillary | 2 (3) |
| Subclavian | 1 (1) |
| Lower extremity | 68 (96) |
| Femoral | 34 (48) |
| Popliteal | 25 (35) |
| Calf veins | 33 (46) |
| PEc | |
| Right | 18 (51) |
| Left | 27 (77) |
| Lobar or larger | 6 (17) |
| Segmental or multiple segmental | 20 (57) |
| Subsegmental only | 8 (23) |
DVT = deep vein thrombosis; PE = pulmonary embolism.
Representation of all the clot locations, with patients able to have multiple locations.
All upper‐extremity DVT were in the left extremity.
Twenty‐eight patients had more than one filling defect.
Outcomes
During the course of treatment, no patient (0%, 95% CI = 0 to 3.4%) developed a recurrent or new VTE while on therapy. As of October 31, 2014 (6 months after the 106th patient), three of the 106 (2.8%, 95% CI = 0.6% to 8%) patients have had a VTE recurrence. All three recurrences occurred in compliant patients who had completed their prescribed courses of anticoagulants. One patient had an unprovoked proximal DVT, was treated for 6 months, discharged from clinic, and two months later developed recurrent same‐side proximal DVT. The second was discharged after a 3‐month treatment for a provoked proximal DVT then developed a same‐side proximal DVT 8 months later. The third patient had a below‐the‐knee DVT, was treated successfully for 3 months, and 3 months later developed another acute below‐the‐knee DVT.
Table 5 delineates outpatient compliance and distribution of follow‐up. Eighty‐eight patients (82%) had at least one clinic follow‐up in person. Seventy‐nine were seen in the ED clinic run by the authors per protocol. Ten patients elected to be followed by either their primary care providers or their specialty providers, and all of these patients were contacted by telephone by the investigator team. All 89 patients who physically visited our clinic or their primary care providers filled their prescriptions within 36 hours after ED discharge. In clinic, pill counts were within one tablet in all but one of 79 patients. Forty‐nine of these patients returned for their second visits (63%), of which five (6%) elected to have their primary care physicians or hematologists manage their anticoagulation, two (3%) were discharged from the clinic after completing their treatment courses, and two (3%) were discharged from clinic without being seen but having some form of testing. The remaining patients chose to not return, ostensibly discharging themselves.
Table 5.
Follow‐up Demographics
| Follow Up | n (%) |
|---|---|
| Followed up in clinic | 89 (82) |
| ED clinic | 79 (75) |
| Primary or specialty provider | 10 (9) |
| Overall compliance | 72 (92) |
| Completed therapy | 56 (71) |
| Ongoing | 28 (31) |
| No clinic follow‐up | 18 (17) |
| Self‐selected discharge | 4 (4) |
| Returns to EDa | 10 (9) |
| Lost to follow‐upb | 4 (4) |
VTE = venous thromboembolism; SSDI = Social Security Death Index.
Returns to the ED that were not associated with new VTE or bleeding events.
No interval return to any clinic or ED in the capture area but with a negative SSDI.
Of the remaining 17 patients who did not have clinic or primary care physician follow‐up, four were contacted by telephone and had elected to stop taking rivaroxaban against medical advice. Ten others had histories and physical examinations documented in INPC that confirmed they were alive, with no written evidence of current or prior bleeding or recurrent VTE. Three patients had no concrete follow‐up. All three had Indianapolis addresses, but could not be contacted by telephone, had no visits documented in the INPC, and were absent from the SSDI.
Two patients died from causes unrelated to VTE or rivaroxaban treatment. One patient had end‐stage metastatic cancer and the other had end‐stage liver disease. Both elected to receive comfort care measures because of the severity and futility of their disease processes, one during the treatment period (who subsequently stopped all medications, including rivaroxaban) and the other post treatment.
No patient had a major bleeding event. Two patients called study physicians about bleeding: one with menorrhagia and the second with jaw swelling after elective dental extraction. Both patients were instructed to hold one dose of rivaroxaban and were encouraged to seek medical care if needed, but neither required additional follow‐up or an unscheduled physician visit. Two additional patients noted menorrhagia on standard questioning during a clinic visit. Both had normal Hb concentrations and prothrombin times of <15 seconds. Both were given instructions to hold one dose of rivaroxaban for worsened menorrhagia. None of these events met the definition of clinically relevant nonmajor bleeding.
D‐dimer and Prothrombin Time Values
At presentation to the ED, the median D‐dimer concentration was 1,320 ng/mL (IQR = 584 to 2,948 ng/mL) and prothrombin time was 11.3 seconds (IQR = 10.7 to 12.1 seconds). At the first visit, representing 2 to 5 weeks of therapy, the median D‐dimer concentration was 266 ng/mL (IQR = 143 to 467 ng/mL) and the median prothrombin time was 12.7 seconds (IQR = 11.4 to 14.4 seconds). At the second follow‐up, the median D‐dimer concentration was 200 ng/mL (IQR = 185 to 275 ng/mL), with the highest D‐dimer of 1,100 ng/mL. The median prothrombin time at the second visit was 12.1 seconds (IQR = 10.6 to 14.6 seconds).
Analysis of Outcomes
We have followed 106 patients for a mean (±SD) duration of 389 (± 111) days (range = 213 to 594 days). None of 106 patients (0%, 95% CI = 0 to 3.4%) developed VTE recurrence on therapy. While taking anticoagulation, none of the 106 (0%, 95% CI = 0 to 3.4%) patients experienced clinically significant bleeding (major or clinically relevant nonmajor bleeding). However, three of 106 patients (2.8%, 95% CI = 0.6% to 8.0%) experienced VTE recurrence within 1 year after discontinuation of anticoagulation. This analysis includes outcomes of all patients and assumes that the three patients who were lost to follow‐up with no visits in the INPC system had no VTE and no bleeding.
Discussion
We report an ED‐initiated protocol of that allows the immediate home treatment of patients with VTE using a target‐specific oral anticoagulant that requires minimal laboratory monitoring. The rate of VTE recurrence and bleeding was 0% during the period of anticoagulation. We consider this work to be early, but our initial outcome rates for VTE recurrence (0%) and bleeding (0%) while on therapy align favorably with the respective rates of 2.1 and 9.4% observed in the pooled EINSTEIN data.11 We believe that this to be the first study to report the use of a target‐specific anticoagulant for home treatment of newly diagnosed VTE patients.1, 2, 7, 26, 27, 28 Our findings add to those of Jara‐Palomares et al.,29 who reported outcomes on 103 patients with DVT and PE treated with rivaroxaban in Spain. These authors found that eight of the 103 patients had bleeding (one major), and one had a DVT recurrence on therapy. The majority (71%) of the patients in the study by Jara‐Palomares et al.29 were already on systemic anticoagulation for a median duration of 16 months prior to starting rivaroxaban, and almost half had evidence of chronic kidney failure. In contrast, none of our patients were systemically anticoagulated at enrollment, most had been diagnosed acutely with their first VTE, and abnormal renal function was an exclusion criterion. The present protocol was implemented in two urban EDs, and most of our patients were economically disadvantaged. The observed lower rates of adverse bleeding in this study emphasize that careful risk stratification using the Hestia criteria (and POMPE‐C for five patients with active cancer) can select patients at low risk for VTE and bleeding complications using rivaroxaban.
The main strength of this work is that these data are taken from a functioning, real‐world referral clinic. Our standard care protocol governed both ED and clinic best practices and was designed to allow secondary research. We prospectively gathered research data at all stages of the protocol, including the ED, clinic, telephone calls, and medical record data. We wrote this report with the intent to help other sites implement outpatient treatment protocols for VTE. Relevant to this point, in the opinion of the investigators, adoption by our ED faculty and housestaff was rapid and enthusiastic. We also have preliminary data indicating that patients preferred this approach over conventional treatment with low‐molecular‐weight heparin‐warfarin. In future work we will present qualitative feedback from focus groups and the results of quality‐of‐life surveys from our clinic. These will demonstrate patient perceptions of their health and treatment preferences. Because cost plays such an important role in generalizing this work, a companion study in this same issue of Academic Emergency Medicine examines charges and costs of medical care for our rivaroxaban case patients, compared with historical warfarin‐treated control group patients.30
Limitations
By design, the patient sample was selected with bias such that only 27% of the PEs and 51% of DVTs diagnosed in the ED were discharged from the ED on rivaroxaban over the study time frame. It is possible that these are overestimates of our true capture rate (we were surprised at the low estimates for the total number of DVT and PE cases for both hospitals). Clinicians may have used implicit criteria to decide to admit some patients who would have been Hestia negative; we have no outcomes for this hypothetical patient subset. One‐quarter of our patients did not physically follow up in our clinic. However, this proportion does not differ from prior literature with warfarin where noncompliance has been studied.31, 32 Three patients were lost to follow‐up and were classified as VTE negative—if the truth is that one or more had VTE, the results would be worse. Although the INPC has a wide reach, and we believe includes virtually all hospital admissions in our region, it does not encompass all outpatient imaging studies, nor primary care offices where a small recurrent VTE or clinically relevant nonmajor bleeding event could have been diagnosed and treated without hospital admission. As with all prospective studies, outcomes data were determined in part from retrospective analysis of patient charts
Conclusions
This preliminary report provides data to support the initial outpatient treatment of low‐risk ED patients with deep vein thrombosis and pulmonary embolism. These patients can be treated at home with an orally available target‐specific anticoagulant with a low rate of venous thromboembolism recurrence and low rate of bleeding.
Supporting information
Data Supplement S1. Mandatory survey completed by clinicians for emergency patients with venous thromboembolism to be eligible for home treatment with rivaroxaban.
Data Supplement S2. History and physical for clinic follow‐up.
Data Supplement S3. Introduction of protocol to ED staff at Wishard and Methodist hospitals in Indianapolis, IN, issued in February 2013.
Academic Emergency Medicine 2015;22:789–795 © 2015 The Authors. Academic Emergency Medicine published by Wiley Periodicals, Inc. on behalf of Society for Academic Emergency Medicine.
Presented at the Society for Academic Emergency Medicine Annual Meeting, Dallas, TX, May 2014.
JAK has received research grant funding from AHRQ, PCORI, and NIH within the past 3 years and has been a consultant to Stago Diagnostica, Janssen, and Pfizer. The other authors have no potential conflicts to disclose.
This study was funded in part by the Lilly Physician Scientist Award.
References
- 1. Vinson DR, Zehtabchi S, Yealy DM. Can selected patients with newly diagnosed pulmonary embolism be safely treated without hospitalization? A systematic review. Ann Emerg Med 2012;60:651–62. [DOI] [PubMed] [Google Scholar]
- 2. Piran S, Le Gal G, Wells PS, et al. Outpatient treatment of symptomatic pulmonary embolism: a systematic review and meta‐analysis. Thromb Res 2013;132:515–9. [DOI] [PubMed] [Google Scholar]
- 3. Zondag W, Kooiman J, Klok FA, Dekkers OM, Huisman MV. Outpatient versus inpatient treatment in patients with pulmonary embolism: a meta‐analysis. Eur Respir J 2013;42:134–44. [DOI] [PubMed] [Google Scholar]
- 4. Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010;363:2499–510. [DOI] [PubMed] [Google Scholar]
- 5. Buller HR, Prins MH, Lensin AW, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012;366:1287–97. [DOI] [PubMed] [Google Scholar]
- 6. Wasserlauf G, Grandi SM, Filion KB, Eisenberg MJ. Meta‐analysis of rivaroxaban and bleeding risk. Am J Cardiol 2013;112:454–60. [DOI] [PubMed] [Google Scholar]
- 7. Zondag W, Hiddinga BI, Crobach MJ, et al. Hestia criteria can discriminate high‐ from low‐risk patients with pulmonary embolism. Eur Respir J 2013;41:588–92. [DOI] [PubMed] [Google Scholar]
- 8. Zondag W, Mos IC, Creemers‐Schild D, et al. Outpatient treatment in patients with acute pulmonary embolism: the Hestia Study. J Thromb Haemost 2011;9:1500–7. [DOI] [PubMed] [Google Scholar]
- 9. Kline JA, Yealy DM. Venous thromboembolism: EINSTEIN transforms anticoagulant therapy in acute PE. Nat Rev Cardiol 2012;9:378–80. [DOI] [PubMed] [Google Scholar]
- 10. Zondag W, den Exter PL, Crobach MJ, et al. Comparison of two methods for selection of out of hospital treatment in patients with acute pulmonary embolism. Thromb Haemost 2013;109:47–52. [DOI] [PubMed] [Google Scholar]
- 11. Prins MH, Lensing AW, Bauersachs R, et al. Oral rivaroxaban versus standard therapy for the treatment of symptomatic venous thromboembolism: a pooled analysis of the EINSTEIN‐DVT and PE randomized studies. Thromb J 2013;11:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kline JA, Roy PM, Than MP, et al. Derivation and validation of a multivariate model to predict mortality from pulmonary embolism with cancer: The POMPE‐C tool. Thromb Res 2012;129:e194–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kahn SR, Shapiro S, Wells PS, et al. Compression stockings to prevent post‐thrombotic syndrome: a randomised placebo‐controlled trial. Lancet 2014;383:880–8. [DOI] [PubMed] [Google Scholar]
- 14. Kline JA, Mitchell AM, Runyon MS, Jones AE, Webb WB. Electronic medical record review as a surrogate to telephone follow‐up to establish outcome for diagnostic research studies in the emergency department. Acad Emerg Med 2005;12:1127–33. [DOI] [PubMed] [Google Scholar]
- 15. Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest 2012;141:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ageno W, Dentali F, Donadini MP, Squizzato A. Optimal treatment duration of venous thrombosis. J Thromb Haemost 2013;11(Suppl 1):151–60. [DOI] [PubMed] [Google Scholar]
- 17. Poli D, Palareti G. Assessing recurrence risk following acute venous thromboembolism: use of algorithms. Curr Opin Pulm Med 2013;19:407–12. [DOI] [PubMed] [Google Scholar]
- 18. Rodger MA, Kahn SR, Wells PS, et al. Identifying unprovoked thromboembolism patients at low risk for recurrence who can discontinue anticoagulant therapy. CMAJ 2008;179:417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eichinger S, Heinze G, Kyrle PA. D‐dimer levels over time and the risk of recurrent venous thromboembolism: an update of the Vienna prediction model. J Am Heart Assoc 2014;3:e000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tosetto A, Iorio A, Marcucci M, et al. Predicting disease recurrence in patients with previous unprovoked venous thromboembolism: a proposed prediction score (DASH). J Thromb Haemost 2012;10:1019–25. [DOI] [PubMed] [Google Scholar]
- 21. MacLean S, Mulla S, Akl EA, et al. Patient values and preferences in decision making for antithrombotic therapy: a systematic review: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest 2012;141:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Douketis J, Tosetto A, Marcucci M, et al. Patient‐level meta‐analysis: effect of measurement timing, threshold, and patient age on ability of D‐dimer testing to assess recurrence risk after unprovoked venous thromboembolism. Ann Intern Med 2010;153:523–31. [DOI] [PubMed] [Google Scholar]
- 23. Cohn DM, Vansenne F, de Borgie CA, Middeldorp S. Thrombophilia testing for prevention of recurrent venous thromboembolism. Cochrane Database Syst Rev 2012;(12):CD007069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. De Stefano V, Rossi E. Testing for inherited thrombophilia and consequences for antithrombotic prophylaxis in patients with venous thromboembolism and their relatives. A review of the Guidelines from Scientific Societies and Working Groups. Thromb Haemost 2013;110:697–705. [DOI] [PubMed] [Google Scholar]
- 25. Becattini C, Agnelli G, Schenone A, et al. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med 2012;366:1959–67. [DOI] [PubMed] [Google Scholar]
- 26. Rymes NL, Lester W, Connor C, Chakrabarti S, Fegan CD. Outpatient management of DVT using low molecular weight heparin and a hospital outreach service. Clin Lab Haematol 2002;24:165–70. [DOI] [PubMed] [Google Scholar]
- 27. Boccalon H, Elias A, Chale JJ, Cadene A, Gabriel S. Clinical outcome and cost of hospital vs home treatment of proximal deep vein thrombosis with a low‐molecular‐weight heparin: the Vascular Midi‐Pyrenees study. Arch Intern Med 2000;160:1769–73. [DOI] [PubMed] [Google Scholar]
- 28. Othieno R, Abu Affan M, Okpo E. Home versus in‐patient treatment for deep vein thrombosis. Cochrane Database Syst Rev 2007;(3):CD003076. [DOI] [PubMed] [Google Scholar]
- 29. Jara‐Palomares L, Sanchez‐Oro‐Gomez R, Elias‐Hernandez T, et al. Rivaroxaban for the treatment of venous thromboembolism. A “real‐life” perspective in 103 patients. Thromb Res 2014;134:617–21. [DOI] [PubMed] [Google Scholar]
- 30. Kahler ZP, Beam DM, Kline JA. Cost of treating venous thromboembolism with heparin and warfarin versus home treatment with rivaroxaban. Acad Emerg Med. 2015;22:796–802. [DOI] [PubMed] [Google Scholar]
- 31. Chen SY, Wu N, Gulseth M, et al. One‐year adherence to warfarin treatment for venous thromboembolism in high‐risk patients and its association with long‐term risk of recurrent events. J Manag Care Pharm 2013;19:291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kimmel SE, Chen Z, Price M, et al. The influence of patient adherence on anticoagulation control with warfarin: results from the International Normalized Ratio Adherence and Genetics (IN‐RANGE) Study. Arch Intern Med 2007;167:229–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Supplement S1. Mandatory survey completed by clinicians for emergency patients with venous thromboembolism to be eligible for home treatment with rivaroxaban.
Data Supplement S2. History and physical for clinic follow‐up.
Data Supplement S3. Introduction of protocol to ED staff at Wishard and Methodist hospitals in Indianapolis, IN, issued in February 2013.


