Abstract
Objective
To investigate familial aggregation of Sjögren's syndrome (SS) and the relative risks (RRs) of other autoimmune disease in relatives of patients with SS.
Methods
We identified 23,658,577 beneficiaries enrolled in the Taiwan National Health Insurance system in 2010, of whom 12,754 had SS. We identified 21,009,551 parent–child relationships and 17,168,340 pairs of full siblings. The familial risks of SS and other autoimmune diseases, tetrachoric correlation, and familial transmission were estimated.
Results
We identified 105 patients with SS who had an affected first‐degree relative. The RR of SS was 18.99 (95% confidence interval [95% CI] 9.76–36.93) in siblings of patients with SS, 11.31 (95% CI 8.34–15.33) in offspring, and 12.46 (95% CI 9.34–16.62) in parents. Tetrachoric correlation coefficients were 0.53 (95% CI 0.41–0.65) for cotwins of affected individuals and 0.21 (95% CI 0.16–0.26) for full siblings. The familial transmission (heritability plus shared environmental contribution) was 0.54 (95% CI 0.44–0.77). In first‐degree relatives of patients with SS, the RRs were 2.95 (95% CI 2.33–3.73) for rheumatoid arthritis, 6.25 (95% CI 5.15–7.58) for systemic lupus erythematosus, 2.39 (95% CI 0.77–7.41) for systemic sclerosis, 0.71 (95% CI 0.10–5.07) for idiopathic inflammatory myopathy, 1.97 (95% CI 1.29–3.02) for type 1 diabetes mellitus, 3.38 (95% CI 1.26–9.05) for multiple sclerosis, 1.67 (95% CI 0.83–3.33) for myasthenia gravis, 1.25 (95% CI 1.04–1.50) for psoriasis, 1.21 (95% CI 0.39–3.76) for inflammatory bowel disease, and 2.29 (95% CI 1.19–4.40) for vasculitis.
Conclusion
The risk of SS and other autoimmune diseases is increased in relatives of patients with SS, and more than one‐half of phenotypic variance in SS can be explained by familial factors.
Sjögren's syndrome (SS) is an autoimmune disease characterized by dry eyes and dry mouth and pathologic features such as lymphocytic infiltration and destruction of the lacrimal and salivary glands 1. In addition to exocrinopathy, SS may involve many organ systems and can cause heterogeneous clinical presentations, including arthritis 2 and renal disease 3. The prevalence of SS varies widely depending on the study design and the population studied. A recent report summarizing 3 population‐based studies in Greece, Norway, and France estimated the prevalence of SS in Europe to be 0.04% 4. Using the National Health Insurance Research Database (NHIRD), which contains health information for almost all inhabitants of Taiwan, our group recently estimated that the prevalence of SS in Taiwan was 0.06% in 2005 5.
Familial clustering of SS 6, 7, 8, 9, 10, 11, 12, 13, 14 as well as its co‐aggregation with other autoimmune diseases 15, 16 have been suggested, but solid evidence for both notions is sparse. Several case reports have described concordance of SS in twins 6, 7, 8, 9 and in families with ≥2 cases of SS 10, 11, 12, 13, 14. The tendency of SS to cluster within families suggests a role for familial factors such as genes and shared environment in the pathogenesis of the disease. Consequently, efforts to define the pathogenesis of SS have focused on genetic factors, and recent studies successfully identified susceptibility loci for SS 17, 18, 19, 20, 21. Environmental factors (such as viruses) and hormonal factors are also thought to participate in disease pathogenesis 22. Although these reports support the contribution of both genetic and shared environmental factors in the susceptibility to SS, they provide no quantitative estimates of the measures of familial risks of SS and the proportion of phenotypic variance that can be explained by familial factors (familial transmission). In addition, estimates of the respective risks of other autoimmune diseases in relatives of patients with SS have not been reported previously.
Therefore, using genealogy and linked health information derived from the NHIRD, we conducted this nationwide study comprising essentially the entire population of Taiwan in 2010 to determine familial aggregation of SS and to assess the relative contribution of familial factors to susceptibility to the disease. In addition, we also estimated the relative risks (RRs) of other autoimmune diseases associated with a family history of SS.
PATIENTS AND METHODS
Study population
This study was approved by the Institutional Review Board of Chang Gung Memorial Hospital (approval no. 101‐2178B) and by the National Health Research Institutes, which compiles data for the NHIRD. We constructed a cohort containing all beneficiaries enrolled in the Taiwan National Health Insurance (NHI) system in 2010, using data from the registry for beneficiaries, the registry for patients with catastrophic illness, and data sets of ambulatory care expenditures and details of ambulatory case orders, all of which are components of the NHIRD. Enrollment in the NHI system is mandatory by law in Taiwan. In 2010, >99.5% of the general population in Taiwan was covered by the insurance 23. The NHIRD contains registration information and data for original claims for reimbursement, including comprehensive information on personal details, socioeconomic status, family relationships, dates of clinical visits, medical diagnoses, medical expenditures, prescription details, examinations, and procedures. Data are updated biannually. All data for a given individual are linked by a unique national identification number that is associated with every record for that individual in the database. To ensure confidentiality, identification numbers were encrypted before being released for research, but the uniqueness of the encrypted identification is retained to ensure valid internal linkage.
Methods of identifying first‐degree relatives and family ascertainment have been previously reported (see Supplementary material, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39127/abstract) 24. Briefly, lineal blood relatives and spouses can be directly identified using the indicators of relationships and unique national identification numbers. Full siblings of an individual are identified if they shared the same parents. Twins are full siblings with the same date of birth (±1 day), but twin zygosity cannot be derived from the database. To consider the correlation among individuals from the same family, we grouped individuals into families according to their relationships. In total, 21,009,551 parent–child relationships, 17,168,340 pairs of full siblings, and 342,066 twin pairs were identified, and these relationships were used to assemble 4,229,301 families, with a mean family size of 4.8 persons, spanning up to 5 generations.
Case definition of SS and other autoimmune diseases
The case definition for SS was a person with a catastrophic illness certificate for SS (International Classification of Diseases, Ninth Revision code 7102). The holders of a catastrophic illness certificate are entitled to a waiver of medical copayments. In order for a patient to receive a certificate for SS, the diagnosis must be supported by comprehensive clinical and laboratory assessments, and this information is required by the insurance administration for a review by commissioned expert panels to confirm the diagnosis before approval of waivers. The panel reviews the diagnosis, in compliance with the updated classification criteria. For instance, the preliminary European classification criteria for SS 25 and classification criteria by the American–European Consensus Group 26 were used in recent years to assist the review of certificate applications for SS. To eliminate patients with secondary SS, only those without a catastrophic illness certificate for rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), systemic sclerosis, or idiopathic inflammatory myopathy were included. We also identified patients with other autoimmune diseases including RA, SLE, systemic sclerosis, idiopathic inflammatory myopathy, type 1 diabetes mellitus, multiple sclerosis, myasthenia gravis, psoriasis, inflammatory bowel disease, and vasculitis (for full code lists, see Supplementary material, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39127/abstract).
Covariates
We considered age, sex, socioeconomic factors (place of residence, occupation, and income level), and family size as factors that might confound or modify the familial associations. A place of residence for each individual was categorized according to the level of urbanization 27. Occupations were classified into 5 categories, and income levels were categorized into sex‐specific income quintiles (for additional details, see Supplementary material, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39127/abstract).
Statistical analysis
The prevalence of SS among individuals with affected first‐degree relatives and the general population was calculated. Any individual with valid insurance registration in 2010 who met the case definition of SS between January 1, 1996 and December 31, 2010 was defined as a prevalent case. The number of individuals in the general population in Taiwan was used as the denominator for the prevalence of SS in 2010. The recurrence risk for SS was defined as the likelihood of a diagnosis of SS in an individual with an affected first‐degree relative with a diagnosis of SS. We calculated the recurrence risk for a specific type of relative (parents, offspring, sibling, and twins) of patients with SS as the prevalence of SS in individuals with a specific type of affected relative 28. The adjusted prevalence ratio was used as a measure for the RR of SS 29 and was calculated as the prevalence of SS among individuals with affected relatives divided by the prevalence of SS in the general population. The RR estimated in this study is equivalent to the relative recurrence ratio, but for simplicity we refer to it as RR throughout. The marginal Cox proportional hazards model with an equal followup time for all subjects 30, adjusted for age, sex, socioeconomic factors, and family size, was used to estimate the RRs and 95% confidence intervals (95% CIs). We used the robust sandwich estimator to calculate corrected 95% CIs to account for possible case clustering within families 31. This approach has been applied previously in other diseases and validated 32. We also estimated tetrachoric correlation coefficients to measure the degree of similarity in different types of relatives. We assumed that a continuous normally distributed liability underlies the diagnosis of SS.
Heritability was defined as the proportion of phenotypic variance that is attributable to genetic factors, and familial transmission was defined as the sum of heritability and the proportion attributable to shared environmental factors. Each of these values can be calculated using a polygenic liability model 33, 34, 35. The familial transmission in this study was estimated as the function of the difference of normal deviation of the threshold from the mean liability between individuals with affected siblings and the normal population (for details, see Supplementary material, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39127/abstract).
The original model assumes zero common environmental variance, and therefore familial transmission equals heritability. We consider this assumption not to be applicable in the case of SS, however, considering known environmental factors that may predispose to the disease and be shared among family members 22. In a previous study by our group, we further used spouses as a control to separate shared environmental component and heritability 24. However, because SS predominantly affects females and its prevalence is low, it was not possible to identify enough affected spouse pairs to produce reliable estimates. Therefore, we reported only familial transmission. Next, for patients with SS, we calculated the probability of not having a family history of SS (sporadic cases) according to the formula based on the polygenic liability model developed by Yang et al 36. We restricted family history to first‐degree relatives and assumed an average of 2 siblings in a family.
We further estimated the extent of familial co‐aggregation of other autoimmune diseases in affected families. RRs and 95% CIs for RA, SLE, systemic sclerosis, idiopathic inflammatory myopathy, type 1 diabetes mellitus, multiple sclerosis, myasthenia gravis, inflammatory bowel diseases, psoriasis, and vasculitis were estimated as the adjusted prevalence ratio of specified autoimmune diseases between individuals with a first‐degree relative with SS and the general population. We estimated the RR for other autoimmune diseases using a marginal Cox proportional hazards regression model with an equal followup time for all subjects. The RRs were adjusted for age, sex, and family size, and were considered case clustering within families by using the robust sandwich estimate. Two‐tailed P values less than or equal to 0.05 were considered significant. All analyses were performed using SAS version 9.3.
RESULTS
Prevalence of SS in individuals with an affected first‐degree family member versus the general population
The study population comprised 23,658,577 individuals enrolled in the NHI system in Taiwan in 2010. There were 12,754 individuals (11,462 women and 1,292 men) with SS in the general population of Taiwan in 2010, which is equivalent to a prevalence of 0.05% (0.10% in women and 0.01% in men). In the general population of Taiwan in 2010, 21,985 individuals (0.09%) had at least one first‐degree relative with SS. Among these individuals, 105 had SS (prevalence 0.48%). The mean ± SD age of the patients with SS was lower in those with a family history (46.6 ± 16.2 years) than in those without a family history (57.0 ± 14.2 years; P < 0.001, by Student's t‐test). For individuals with an affected first‐degree relative, the age‐specific prevalence of SS was significantly higher than that in the general population (Figure 1). Other characteristics of the study population are shown in Table 1.
Figure 1.
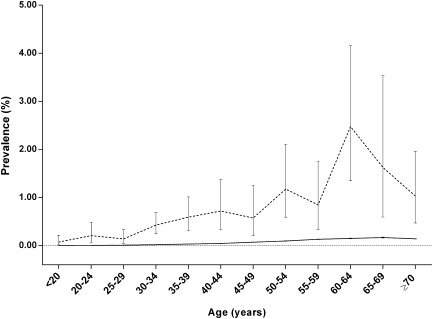
Age‐specific prevalence of Sjögren's syndrome in individuals with a first‐degree relative with Sjögren's syndrome (broken line) and in the general population (solid line) in Taiwan in 2010. Bars show 95% confidence intervals.
Table 1.
Baseline characteristics of the individuals with relatives affected by SS and the general populationa
| Variable | Women | Men | ||||
|---|---|---|---|---|---|---|
| At least 1 affected relative | General population | P | At least 1 affected relative | General population | P | |
| (n = 10,416) | (n = 11,926,513) | (n = 11,569) | (n = 11,732,064) | |||
| Age, mean ± SD years | 33.2 ± 16.9 | 37.9 ± 20.4 | <0.001 | 33.4 ± 16.3 | 37.1 ± 20.6 | <0.001 |
| Patients with SS | 95 (0.91) | 11,462 (0.10) | <0.001 | 10 (0.09) | 1,292 (0.01) | <0.001 |
| Place of residence | <0.001 | <0.001 | ||||
| Urban | 6,901 (66.25) | 7,197,968 (60.35) | 7,166 (61.94) | 6,737,087 (57.42) | ||
| Suburban | 2,664 (25.58) | 3,209,020 (26.91) | 3,051 (26.37) | 3,372,637 (28.75) | ||
| Rural | 707 (6.79) | 1,087,991 (9.12) | 793 (6.85) | 1,098,656 (9.36) | ||
| Unknown | 144 (1.38) | 431,534 (3.62) | 559 (4.83) | 523,684 (4.46) | ||
| Income level | <0.001 | <0.001 | ||||
| Quintile 1 | 1,499 (14.39) | 1,960,003 (16.43) | 1,690 (14.61) | 2,117,136 (18.05) | ||
| Quintile 2 | 1,549 (14.87) | 1,839,576 (15.42) | 1,389 (12.01) | 1,495,341 (12.75) | ||
| Quintile 3 | 2,138 (20.53) | 3,161,293 (26.51) | 2,398 (20.73) | 3,135,633 (26.73) | ||
| Quintile 4 | 2,196 (21.08) | 2,252,173 (18.88) | 2,534 (29.86) | 2,294,886 (19.56) | ||
| Quintile 5 | 2,875 (27.60) | 2,274,656 (19.07) | 2,986 (25.81) | 2,163,222 (18.44) | ||
| Unknown | 159 (1.53) | 438,812 (3.68) | 572 (4.94) | 525,846 (4.48) | ||
| Occupation | <0.001 | <0.001 | ||||
| Dependent of insured individual | 3,835 (36.82) | 4,924,319 (41.29) | 3,952 (34.16) | 4,285,015 (36.52) | ||
| Civil servant, teacher, military personnel, and veterans | 706 (6.78) | 401,734 (3.37) | 660 (5.70) | 582,717 (4.97) | ||
| Non–manual labor worker and professional | 3,469 (33.30) | 3,031,660 (25.42) | 4,323 (37.37) | 3,325,548 (28.35) | ||
| Manual labor worker | 1,534 (14.73) | 2,612,534 (21.91) | 1,498 (12.95) | 2,272,550 (19.37) | ||
| Other | 872 (8.37) | 956,266 (8.02) | 1,136 (9.82) | 12,66234 (10.79) | ||
Except where indicated otherwise, values are the number (%). SS = Sjögren's syndrome.
Relative risks of SS in individuals with affected first‐degree relatives
Table 2 shows the prevalence (recurrence risk) of SS in individuals with an affected first‐degree relative, according to different relationships and sexes of the affected individuals and their families. Overall, having an affected first‐degree relative with SS was associated with an adjusted RR of 12.37 (95% CI 9.54–16.05) for the disease. Individuals with female and male affected relatives had respective RRs for SS of 13.24 (95% CI 10.15–17.27) and 10.06 (95% CI 4.91–20.61), suggesting that the sex of the affected relative did not influence the RR.
Table 2.
Relative risks (RRs) and tetrachoric correlation for Sjögren's syndrome in different kinships*
| Type of affected relative, sex of affected relative, sex of individual | No. of cases | Prevalence, % | RR (95% CI)a | Tetrachoric correlation coefficient (95% CI) |
|---|---|---|---|---|
| Any relative | ||||
| Female | ||||
| Female | 88 | 0.94 | 13.75 (10.36–18.26) | 0.24 (0.21–0.26) |
| Male | 8 | 0.08 | 10.37 (5.21–20.62) | 0.17 (0.10–0.24) |
| All | 96 | 0.48 | 13.24 (10.15–17.27) | 0.22 (0.19–0.24) |
| Male | ||||
| Female | 7 | 0.67 | 8.92 (4.28–18.61) | 0.17 (0.09–0.24) |
| Male | 2 | 0.17 | 21.15 (2.97–150.36) | 0.21 (0.08–0.33) |
| All | 9 | 0.41 | 10.06 (4.91–20.61) | 0.17 (0.10–0.23) |
| All | ||||
| Female | 95 | 0.91 | 13.24 (10.13–17.31) | 0.24 (0.21–0.26) |
| Male | 10 | 0.09 | 11.57 (5.90–22.67) | 0.18 (0.12–0.24) |
| All | 105 | 0.48 | 12.37 (9.54–16.05) | 0.22 (0.19–0.24) |
| Parent | ||||
| Female, mother | ||||
| Female | 39 | 0.55 | 13.12 (9.55–18.02) | 0.17 (0.13–0.20) |
| Male | 5 | 0.06 | 14.26 (5.97–34.07) | 0.14 (0.06–0.22) |
| All | 44 | 0.29 | 13.04 (9.68–17.57) | 0.15 (0.12–0.18) |
| Male, father | ||||
| Female | 3 | 0.38 | 8.47 (2.72–26.36) | 0.11 (0.01–0.21) |
| Male | 0 | 0 | NA | NA |
| All | 3 | 0.17 | 7.37 (2.37–22.92) | 0.09 (0.01–0.18) |
| All | ||||
| Female | 42 | 0.53 | 12.65 (9. 32–17.17) | 0.17 (0.13–0.20) |
| Male | 5 | 0.05 | 12.71 (5.32–30.36) | 0.13 (0.05–0.21) |
| All | 47 | 0.28 | 12.46 (9.34–16.62) | 0.15 (0.12–0.18) |
| Offspring | ||||
| Female, daughter | ||||
| Female | 33 | 2.65 | 11.79 (8.43–16.50) | 0.32 (0.28–0.36) |
| Male | 3 | 0.29 | 8.94 (2.90–27.54) | 0.26 (0.15–0.37) |
| All | 36 | 1.59 | 11.41 (8.28–15.74) | 0.31 (0.27–0.34) |
| Male, son | ||||
| Female | 4 | 2.74 | 11.79 (4.49–30.99) | 0.28 (0.18–0.39) |
| Male | 0 | 0 | NA | NA |
| All | 4 | 1.58 | 10.44 (3.98–27.41) | 0.27 (0.17–0.36) |
| All | ||||
| Female | 37 | 2.66 | 11.79 (8.59–16.19) | 0.33 (0.29–0.37) |
| Male | 3 | 0.27 | 8.04 (2.61–24.78) | 0.25 (0.14–0.36) |
| All | 40 | 0.31 | 11.31 (8.34–15.33) | 0.25 (0.14–0.36) |
| Sibling | ||||
| Female, sister | ||||
| Female | 14 | 1.36 | 21.78 (10.77–44.12) | 0.24 (0.18–0.30) |
| Male | 0 | 0 | NA | NA |
| All | 14 | 0.63 | 18.92 (9.37–38.40) | 0.21 (0.16–0.26) |
| Male, brother | ||||
| Female | 0 | 0 | NA | NA |
| Male | 2 | 1.77 | 153.48 (20.90–1,127.20) | 0.39 (0.25–0.53) |
| All | 2 | 0.93 | 19.72 (2.89–1,34.54) | 0.21 (0.09–0.34) |
| All | ||||
| Female | 14 | 1.23 | 19.09 (9.42–38.68) | 0.23 (0.17–0.29) |
| Male | 2 | 0.15 | 20.13 (2.81–144.30) | 0.20 (0.08–0.32) |
| All | 16 | 0.65 | 18.99 (9.76–36.93) | 0.21 (0.16–0.26) |
| Twin | ||||
| Female, twin sister | ||||
| Female | 4 | 36.36 | 756.32 (332.32–1,721.27) | 0.57 (0.43–0.70) |
| Male | 0 | 0 | NA | NA |
| All | 4 | 28.57 | 723.05 (314.11–1,664.39) | 0.55 (0.43–0.68) |
| Male, twin brother | ||||
| Female | 0 | 0 | NA | NA |
| Male | 0 | 0 | NA | NA |
| All | 0 | 0 | NA | NA |
| All | ||||
| Female | 4 | 33.33 | 695.68 (296.16–1,634.17) | 0.55 (0.42–0.69) |
| Male | 0 | 0 | NA | NA |
| All | 4 | 23.53 | 661.75 (278.09–1,574.70) | 0.53 (0.41–0.65) |
95% CI = 95% confidence interval; NA = not applicable.
Adjusted for age, sex, place of residence, quintile of income level, occupation, and family size.
In SS, the degree of genetic distance between family relatives is associated with the RRs. The RRs of SS were 661.75 (95% CI 278.09–1,574.70) in cotwins of patients with SS, 18.99 (95% CI 9.76–36.93) in siblings, 11.31 (95% CI 8.34–15.33) in offspring, and 12.46 (95% CI 9.34–16.62) in parents. In addition, the RRs increased with the number of types of affected first‐degree relatives. Compared with the general population, individuals with 1 type of affected first‐degree relative had an RR of 12.71 (95% CI 9.80–16.49), and those with 2 or more affected first‐degree relatives had an RR of SS of 70.36 (95% CI 10.28–481.60).
Familial transmission and tetrachoric correlation of SS
Overall, the tetrachoric correlation coefficient for first‐degree relatives was 0.22 (95% CI 0.19–0.24) (Table 2). The tetrachoric correlation coefficients were estimated to be 0.53 (95% CI 0.41–0.65) for twins and 0.21 (95% CI 0.16–0.26) for full siblings. Using a polygenic liability model, we estimated that the familial transmission for SS was 0.54 (95% CI 0.44–0.77). Given the parameters estimated previously, 84.0% of SS patients were expected to be sporadic cases.
Co‐aggregation of other autoimmune diseases
Table 3 shows the adjusted RRs (95% CIs) for other autoimmune diseases in individuals with affected first‐degree relatives compared with the general population. The RR in individuals with a first‐degree relative with SS was 2.95 (95% CI 2.33–3.73) for RA, 6.25 (95% CI 5.15–7.58) for SLE, 2.39 (95% CI 0.77–7.41) for systemic sclerosis, 0.71 (95% CI 0.10–5.07) for idiopathic inflammatory myopathy, 1.97 (95% CI 1.29–3.02) for type 1 diabetes mellitus, 3.38 (95% CI 1.26–9.05) for multiple sclerosis, 1.67 (95% CI 0.83–3.33) for myasthenia gravis, 1.25 (95% CI 1.04–1.50) for psoriasis, 1.21 (95% CI 0.39–3.76) for inflammatory bowel disease, and 2.29 (95% CI 1.19–4.40) for vasculitis.
Table 3.
Relative risks (RRs) of other autoimmune diseases in individuals with first‐degree relatives with Sjögren's syndromea
| With affected relatives | General population | RR (95% CI)† | |||
|---|---|---|---|---|---|
| No. of cases | Prevalence, % | No. of cases | Prevalence, % | ||
| Rheumatoid arthritis | |||||
| Female | 50 | 0.48 | 29,527 | 0.25 | 2.87 (2.18–3.78) |
| Male | 18 | 0.16 | 7,887 | 0.07 | 3.21 (2.02–5.09) |
| All | 68 | 0.31 | 37,414 | 0.16 | 2.95 (2.33–3.73) |
| Systemic lupus erythematosus | |||||
| Female | 96 | 0.92 | 16,822 | 0.14 | 6.24 (5.10–7.65) |
| Male | 13 | 0.11 | 1,984 | 0.02 | 6.31 (3.65–10.88) |
| All | 109 | 0.50 | 18,806 | 0.08 | 6.25 (5.15–7.58) |
| Systemic sclerosis | |||||
| Female | 2 | 0.02 | 1,493 | 0.01 | 2.12 (0.53–8.50) |
| Male | 1 | 0.01 | 395 | 0.003 | 3.24 (0.46–22.91) |
| All | 3 | 0.01 | 1,888 | 0.01 | 2.39 (0.77–7.41) |
| Idiopathic inflammatory myopathy | |||||
| Female | 1 | 0.01 | 1,259 | 0.01 | 1.10 (0.16–7.86) |
| Male | 0 | 0 | 548 | 0.005 | NA |
| All | 1 | 0.005 | 1,807 | 0.01 | 0.71 (0.10–5.07) |
| Type 1 diabetes mellitus | |||||
| Female | 10 | 0.10 | 5,406 | 0.05 | 1.69 (0.91–3.14) |
| Male | 13 | 0.11 | 4,852 | 0.04 | 2.26 (1.31–3.89) |
| All | 23 | 0.10 | 10,258 | 0.04 | 1.97 (1.29–3.02) |
| Multiple sclerosis | |||||
| Female | 4 | 0.04 | 961 | 0.01 | 4.86 (1.81–13.00) |
| Male | 0 | 0 | 287 | 0.002 | NA |
| All | 4 | 0.02 | 1,248 | 0.01 | 3.38 (1.26–9.05) |
| Myasthenia gravis | |||||
| Female | 6 | 0.06 | 3,466 | 0.03 | 2.12 (0.95–4.73) |
| Male | 2 | 0.02 | 2,248 | 0.02 | 1.03 (0.26–4.12) |
| All | 8 | 0.04 | 5,714 | 0.02 | 1.67 (0.83–3.33) |
| Psoriasis | |||||
| Female | 51 | 0.64 | 67,857 | 0.58 | 1.29 (0.98–1.70) |
| Male | 74 | 0.49 | 45,447 | 0.38 | 1.22 (0.97–1.54) |
| All | 125 | 0.57 | 113,304 | 0.48 | 1.25 (1.04–1.50) |
| Inflammatory bowel disease | |||||
| Female | 1 | 0.01 | 1,026 | 0.01 | 1.26 (0.18–8.97) |
| Male | 2 | 0.02 | 1,686 | 0.01 | 1.18 (0.29–4.72) |
| All | 3 | 0.01 | 2,712 | 0.01 | 1.21 (0.39–3.76) |
| Vasculitis | |||||
| Female | 6 | 0.06 | 1,837 | 0.02 | 4.07 (1.83–9.07) |
| Male | 3 | 0.03 | 2,907 | 0.02 | 1.22 (0.39–3.79) |
| All | 9 | 0.04 | 4,744 | 0.02 | 2.29 (1.19–4.40) |
95% CI = 95% confidence interval; NA = not applicable.
Adjusted for age, sex, place of residence, quintile of income level, occupation, and family size.
DISCUSSION
This study is the first to investigate the risk of SS in individuals with affected first‐degree relatives and to estimate the familial transmission of SS in a general population. We observed that the prevalence of SS in relatives of patients with the disease is 12‐fold higher than that in the general population, and that genetic distance is associated with the magnitude of risk. The familial transmission of SS was 0.54. Despite this, most cases of SS are expected to be sporadic, based on the polygenic liability model. Furthermore, the prevalence of other autoimmune diseases is higher among individuals with an affected first‐degree relative.
Our results have several implications. First, the study provides quantitative estimates for absolute risks and RRs, familial transmission, and the proportion of sporadic cases of SS, which are valuable for clinical counseling. Second, these data may help in the planning of future genetic studies to determine candidate susceptibility genes. Third, the co‐aggregation of SS and certain other autoimmune diseases suggests an overlapping pathogenesis that deserves further elucidation.
Formal evidence for familial aggregation and the magnitude of any familial or genetic contribution are rarely reported. Concordance of SS in several twin pairs has been reported previously 6, 7, 8, 9, generally with very similar phenotypes (e.g., pathologic findings and serologic and clinical presentations). For example, a pair of monozygotic twins with SS exhibited nearly identical and clonally restricted anti‐Ro/SSA autoantibodies 9. In addition, several studies in families that include a member with SS have been reported 10, 11, 13, 14, 37, 38. However, previous studies have not included twin concordance rates, tetrachoric correlation coefficients, and familial transmission.
Our study provides several lines of evidence supporting the importance of familial factors, including both genes and environment, in susceptibility to SS. First, the age‐specific prevalence of SS was significantly higher in first‐degree relatives of SS patients in all age bands compared with the general population, and the adjusted RR associated with a family history was high (12‐fold higher than that in the general population). Second, the RR and tetrachoric correlation coefficient varied according to genetic distance, and sibling RRs are higher than parental and offspring RRs despite the same genetic distance, suggesting contributions of both genes and shared environmental factors to disease susceptibility. Finally, using a polygenic liability model, we estimated that 54% of phenotypic variance can be explained by familial factors.
Under the polygenic liability model, however, most cases of SS are expected to be sporadic rather than familial. This phenomenon seems counterintuitive but appears to be the norm for many common complex diseases 36. For example, a theoretical estimate showed that the probability of sporadic cases of RA was 78–84%, depending on the parameters used 36. Real‐world epidemiologic data also support this notion. A prospective inception cohort study recruited 204 RA patients with complete family histories, and 162 of the patients (79.4%) were identified as having sporadic RA 39. A similar phenomenon exists in SLE 36, despite one study showing no phenotypic differences between sporadic and familial cases 40.
Collectively, our results provide useful information for counseling patients and their family members. Full information on familial absolute risks and RRs, familial transmission, and the probability of sporadic cases should be provided and fully explained to prevent misconception and undue distress. Given both a low absolute risk (<1%) and a high probability of sporadic cases, decisions based solely on family history to screen for the disease in asymptomatic family members of the affected patients do not seem to be justified. Nonetheless, further studies should be undertaken to test the utility of family history as a tool to identify at‐risk individuals.
Recent genome‐wide association studies (GWAS) identified an expanding array of candidate genes that are associated with an increased risk of SS 41. Recently, 2 GWAS in SS were undertaken 20, 21. One of these studies analyzed 395 patients with SS and 1,975 population controls of European descent and showed strong associations with genes within the HLA region 20. Several candidate genes outside the regions that were previously identified, such as STAT4 and IFR5 18, 19, did not exceed the significance limit. Another study genotyped 597 patients with primary SS and 1,090 healthy controls of Han Chinese ethnic origin and demonstrated a new susceptibility locus and also confirmed previously reported loci 21.
GWAS have also been very successful in identifying candidate genes for other autoimmune disorders, such as RA, SLE, type 1 diabetes mellitus, and multiple sclerosis. The similarity of the magnitude of the sibling risk between SS (15‐fold) and type 1 diabetes mellitus (15‐fold), RA (8‐fold), SLE (30‐fold), and multiple sclerosis (20‐fold) 42 suggests that it should be possible to identify specific genetic risk factors for SS both within and outside the HLA region, in a relatively small GWAS (∼2,000 cases), as was done for the aforementioned autoimmune diseases 43, 44, 45. Our results therefore may be useful for planning further GWAS to identify specific susceptibility genes for SS.
Apart from genetic factors, shared environmental factors may also contribute to familial clustering of SS. For example, Epstein‐Barr virus (EBV) infection, with subsequent activation of epithelial cells and the immune system, has been proposed as a plausible environmental factor contributing to the development of SS 46. EBV most commonly spreads via bodily fluids (primarily saliva), and family members of infected patients are potentially at higher risk of infection. Therefore, both genetic factors and shared environmental factors may contribute to the phenotypic variance in SS.
First‐degree relatives of patients with SS have an increased risk of other autoimmune diseases, and the magnitude of the risk was particularly high for SLE. This tendency for familial co‐aggregation of autoimmune diseases with SS was also suggested previously, but the magnitude of the risk associated with specific diseases has not been reported. A multicenter hospital‐based study in Italy comprising 140 patients with SS and 109 in‐patient controls without a history of autoimmune disease showed that patients with SS had a 5‐fold risk of having a first‐degree relative with autoimmune diseases (including 1 with SS) 16. Our results suggest that some autoimmune diseases share part of the pathogenesis of SS, but the magnitude of overlapping factors contributing to disease manifestation is different. Sjögren's syndrome appears to share many of these factors with SLE and RA, which is expected, because SLE and RA often coexist with SS. A high prevalence of sicca syndrome in patients with type 1 diabetes mellitus was reported previously 47, but a link between type 1 diabetes mellitus and SS was demonstrated only recently 48. The coexistence of multiple sclerosis 49 and vasculitis 50 with SS has also been reported. Currently, however, there are no data on familial co‐aggregation of these diseases in families in which a member is affected by SS. Our data could be of value when counseling families of patients with SS.
The present study has some limitations. First, the classification of cases was based purely on the diagnosis recorded in the registry of patients with catastrophic illnesses or based on records in primary care. The NHIRD is primarily a health insurance database and lacks full information on clinical findings, laboratory testing, and examinations; therefore, formal classification criteria for SS could not be applied. Nevertheless, issuance of a catastrophic illness certificate requires strong medical evidence for a diagnosis of SS that is agreed upon by an expert panel, and applications for these certificates are submitted almost exclusively by rheumatologists. Therefore, any misclassification is unlikely to unduly affect our conclusion. Second, patients with less severe disease may not have received a certificate and thus will not have been identified as cases. Third, the estimate for probability of sporadic cases was based on data derived from first‐degree relatives. In this study, therefore, sporadic cases are limited to patients with no first‐degree relative. Fourth, our model cannot effectively separate contributions from genetic and shared environmental factors. Finally, whether these results apply to different populations and settings outside of Taiwan requires further study.
In conclusion, this population‐wide study confirms that in Taiwan, SS clusters within families, and that both genetic and environmental factors contribute to susceptibility to the disease. Relatives of patients with SS tend to have an increased risk of other autoimmune diseases. These findings may also be useful when counseling families of patients with SS. In addition, these results may help inform the design of future studies of familial and genetic risks of SS.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Yu had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Kuo, Zhang, Doherty.
Acquisition of data
Kuo, See, Luo, Yu.
Analysis and interpretation of data
Kuo, Grainge, Valdes, See, Zhang, Doherty.
Supporting information
Supporting Information
This study is based in part on National Health Insurance Research Database data provided by the Administration of National Health Insurance, Department of Health, and managed by the National Health Research Institutes. The interpretation and conclusions contained herein do not represent positions of the National Health Insurance Administration or the National Health Research Institutes.
REFERENCES
- 1. Fox RI. Sjogren's syndrome. Lancet 2005;366:321–31. [DOI] [PubMed] [Google Scholar]
- 2. Castro‐Poltronieri A, Alarcon‐Segovia D. Articular manifestations of primary Sjogren's syndrome. J Rheumatol 1983;10:485–8. [PubMed] [Google Scholar]
- 3. Goules A, Masouridi S, Tzioufas AG, Ioannidis JP, Skopouli FN, Moutsopoulos HM. Clinically significant and biopsy‐documented renal involvement in primary Sjogren syndrome. Medicine (Baltimore) 2000;79:241–9. [DOI] [PubMed] [Google Scholar]
- 4. Cornec D, Chiche L. Is primary Sjogren's syndrome an orphan disease? A critical appraisal of prevalence studies in Europe. Ann Rheum Dis 2015;74:e25. [DOI] [PubMed] [Google Scholar]
- 5. See LC, Kuo CF, Chou MJ, Yu KH. Sex- and age-specific incidence of autoimmune rheumatic diseases in the Chinese population: a Taiwan population-based study. Semin Arthritis Rheum 2013;43:381–6. [DOI] [PubMed] [Google Scholar]
- 6. Besana C, Salmaggi C, Pellegrino C, Pierro L, Vergani S, Faravelli A, et al. Chronic bilateral dacryo‐adenitis in identical twins: a possible incomplete form of Sjogren syndrome. Eur J Pediatr 1991;150:652–5. [DOI] [PubMed] [Google Scholar]
- 7. Bolstad AI, Haga HJ, Wassmuth R, Jonsson R. Monozygotic twins with primary Sjogren's syndrome. J Rheumatol 2000;27:2264–6. [PubMed] [Google Scholar]
- 8. Houghton KM, Cabral DA, Petty RE, Tucker LB. Primary Sjogren's syndrome in dizygotic adolescent twins: one case with lymphocytic interstitial pneumonia. J Rheumatol 2005;32:1603–6. [PubMed] [Google Scholar]
- 9. Scofield RH, Kurien BT, Reichlin M. Immunologically restricted and inhibitory anti‐Ro/SSA in monozygotic twins. Lupus 1997;6:395–8. [DOI] [PubMed] [Google Scholar]
- 10. Reveille JD, Wilson RW, Provost TT, Bias WB, Arnett FC. Primary Sjogren's syndrome and other autoimmune diseases in families: prevalence and immunogenetic studies in six kindreds. Ann Intern Med 1984;101:748–56. [DOI] [PubMed] [Google Scholar]
- 11. Lichtenfeld JL, Kirschner RH, Wiernik PH. Familial Sjogren's syndrome with associated primary salivary gland lymphoma. Am J Med 1976;60:286–92. [DOI] [PubMed] [Google Scholar]
- 12. Koivukangas T, Simila S, Heikkinen E, Rasanen O, Wasz‐Hockert O. Sjogren's syndrome and achalasia of the cardia in two siblings. Pediatrics 1973;51:943–5. [PubMed] [Google Scholar]
- 13. Boling EP, Wen J, Reveille JD, Bias WB, Chused TM, Arnett FC. Primary Sjogren's syndrome and autoimmune hemolytic anemia in sisters: a family study. Am J Med 1983;74:1066–71. [DOI] [PubMed] [Google Scholar]
- 14. Sabio JM, Milla E, Jimenez‐Alonso J. A multicase family with primary Sjogren's syndrome. J Rheumatol 2001;28:1932–4. [PubMed] [Google Scholar]
- 15. Alarcon‐Segovia D, Alarcon‐Riquelme ME, Cardiel MH, Caeiro F, Massardo L, Villa AR, et al. Familial aggregation of systemic lupus erythematosus, rheumatoid arthritis, and other autoimmune diseases in 1,177 lupus patients from the GLADEL cohort. Arthritis Rheum 2005;52:1138–47. [DOI] [PubMed] [Google Scholar]
- 16. Priori R, Medda E, Conti F, Cassara EA, Sabbadini MG, Antonioli CM, et al. Risk factors for Sjogren's syndrome: a case‐control study. Clin Exp Rheumatol 2007;25:378–84. [PubMed] [Google Scholar]
- 17. Cruz‐Tapias P, Rojas‐Villarraga A, Maier‐Moore S, Anaya JM. HLA and Sjogren's syndrome susceptibility: a meta‐analysis of worldwide studies. Autoimmun Rev 2012;11:281–7. [DOI] [PubMed] [Google Scholar]
- 18. Miceli‐Richard C, Comets E, Loiseau P, Puechal X, Hachulla E, Mariette X. Association of an IRF5 gene functional polymorphism with Sjogren's syndrome. Arthritis Rheum 2007;56:3989–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nordmark G, Kristjansdottir G, Theander E, Eriksson P, Brun JG, Wang C, et al. Additive effects of the major risk alleles of IRF5 and STAT4 in primary Sjogren's syndrome. Genes Immun 2009;10:68–76. [DOI] [PubMed] [Google Scholar]
- 20. Lessard CJ, Li H, Adrianto I, Ice JA, Rasmussen A, Grundahl KM, et al. Variants at multiple loci implicated in both innate and adaptive immune responses are associated with Sjogren's syndrome. Nat Genet 2013;45:1284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li Y, Zhang K, Chen H, Sun F, Xu J, Wu Z, et al. A genome‐wide association study in Han Chinese identifies a susceptibility locus for primary Sjogren's syndrome at 7q11.23. Nat Genet 2013;45:1361–5. [DOI] [PubMed] [Google Scholar]
- 22. Tzioufas AG, Kapsogeorgou EK, Moutsopoulos HM. Pathogenesis of Sjogren's syndrome: what we know and what we should learn. J Autoimmun 2012;39:4–8. [DOI] [PubMed] [Google Scholar]
- 23.National Health Insurance Administration. Ministry of Health and Welfare. The National Health Insurance Statistics, 2010. URL: http://www.nhi.gov.tw/English/webdata/webdata.aspx?menu=11&menu_id=296&webdata_id=1942&WD_ID=296.
- 24. Kuo CF, Grainge MJ, See LC, Yu KH, Luo SF, Valdes AM, et al. Familial aggregation of gout and relative genetic and environmental contributions: a nationwide population study in Taiwan. Ann Rheum Dis 2015;74:369–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vitali C, Bombardieri S, Moutsopoulos HM, Balestrieri G, Bencivelli W, Bernstein RM, et al, the European Study Group on Diagnostic Criteria for Sjögren's Syndrome. Preliminary criteria for the classification of Sjögren's syndrome: results of a prospective concerted action supported by the European Community. Arthritis Rheum 1993;36:340–7. [DOI] [PubMed] [Google Scholar]
- 26. Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, et al. Classification criteria for Sjogren's syndrome: a revised version of the European criteria proposed by the American‐European Consensus Group. Ann Rheum Dis 2002;61:554–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu CY, Huang YT, Chuang YL, Chen YJ, Weng WS, Liu JS, et al. Incorporating development stratification of Taiwan townships into sampling design of large scale health interview survey. J Health Manag 2006;14:1–22. [Google Scholar]
- 28. Risch N. Linkage strategies for genetically complex traits. I. Multilocus models. Am J Hum Genet 1990;46:222–8. [PMC free article] [PubMed] [Google Scholar]
- 29. Chou CT, Pei L, Chang DM, Lee CF, Schumacher HR, Liang MH. Prevalence of rheumatic diseases in Taiwan: a population study of urban, suburban, rural differences. J Rheumatol 1994;21:302–6. [PubMed] [Google Scholar]
- 30. Lee J, Chia KS. Estimation of prevalence rate ratios for cross sectional data: an example in occupational epidemiology. Br J Ind Med 1993;50:861–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lin DY. Cox regression analysis of multivariate failure time data: the marginal approach. Stat Med 1994;13:2233–47. [DOI] [PubMed] [Google Scholar]
- 32. Lichtenstein P, Yip BH, Bjork C, Pawitan Y, Cannon TD, Sullivan PF, et al. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population‐based study. Lancet 2009;373:234–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Falconer DS. The inheritance of liability to diseases with variable age of onset, with particular reference to diabetes mellitus. Ann Hum Genet 1967;31:1–20. [DOI] [PubMed] [Google Scholar]
- 34. Reich T, James JW, Morris CA. The use of multiple thresholds in determining the mode of transmission of semi‐continuous traits. Ann Hum Genet 1972;36:163–84. [DOI] [PubMed] [Google Scholar]
- 35. Reich T, Rice J, Cloninger CR, Wette R, James J. The use of multiple thresholds and segregation analysis in analyzing the phenotypic heterogeneity of multifactorial traits. Ann Hum Genet 1979;42:371–90. [DOI] [PubMed] [Google Scholar]
- 36. Yang J, Visscher PM, Wray NR. Sporadic cases are the norm for complex disease. Eur J Hum Genet 2010;18:1039–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mason AM, Golding PL. Multiple immunological abnormalities in a family. J Clin Pathol 1971;24:732–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sestak AL, Shaver TS, Moser KL, Neas BR, Harley JB. Familial aggregation of lupus and autoimmunity in an unusual multiplex pedigree. J Rheumatol 1999;26:1495–9. [PubMed] [Google Scholar]
- 39. Radstake TR, Barrera P, Albers JM, Swinkels HL, van de Putte LB, van Riel PL. Familial vs sporadic rheumatoid arthritis (RA): a prospective study in an early RA inception cohort. Rheumatology (Oxford) 2000;39:267–73. [DOI] [PubMed] [Google Scholar]
- 40. Sestak AL, Nath SK, Kelly JA, Bruner GR, James JA, Harley JB. Patients with familial and sporadic onset SLE have similar clinical profiles but vary profoundly by race. Lupus 2008;17:1004–9. [DOI] [PubMed] [Google Scholar]
- 41. Burbelo PD, Ambatipudi K, Alevizos I. Genome‐wide association studies in Sjogren's syndrome: what do the genes tell us about disease pathogenesis? Autoimmun Rev 2014;13:756–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vyse TJ, Todd JA. Genetic analysis of autoimmune disease. Cell 1996;85:311–8. [DOI] [PubMed] [Google Scholar]
- 43. Smyth DJ, Cooper JD, Bailey R, Field S, Burren O, Smink LJ, et al. A genome‐wide association study of nonsynonymous SNPs identifies a type 1 diabetes locus in the interferon‐induced helicase (IFIH1) region. Nat Genet 2006;38:617–9. [DOI] [PubMed] [Google Scholar]
- 44. Stahl EA, Raychaudhuri S, Remmers EF, Xie G, Eyre S, Thomson BP, et al. Genome‐wide association study meta‐analysis identifies seven new rheumatoid arthritis risk loci. Nat Genet 2010;42:508–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hom G, Graham RR, Modrek B, Taylor KE, Ortmann W, Garnier S, et al. Association of systemic lupus erythematosus with C8orf13‐BLK and ITGAM‐ITGAX. N Engl J Med 2008;358:900–9. [DOI] [PubMed] [Google Scholar]
- 46. Inoue N, Harada S, Miyasaka N, Oya A, Yanagi K. Analysis of antibody titers to Epstein‐Barr virus nuclear antigens in sera of patients with Sjogren's syndrome and with rheumatoid arthritis. J Infect Dis 1991;164:22–8. [DOI] [PubMed] [Google Scholar]
- 47. Binder A, Maddison PJ, Skinner P, Kurtz A, Isenberg DA. Sjogren's syndrome: association with type‐1 diabetes mellitus. Br J Rheumatol 1989;28:518–20. [DOI] [PubMed] [Google Scholar]
- 48. McGuire HM, Vogelzang A, Ma CS, Hughes WE, Silveira PA, Tangye SG, et al. A subset of interleukin‐21+ chemokine receptor CCR9+ T helper cells target accessory organs of the digestive system in autoimmunity. Immunity 2011;34:602–15. [DOI] [PubMed] [Google Scholar]
- 49. De Seze J, Devos D, Castelnovo G, Labauge P, Dubucquoi S, Stojkovic T, et al. The prevalence of Sjogren syndrome in patients with primary progressive multiple sclerosis. Neurology 2001;57:1359–63. [DOI] [PubMed] [Google Scholar]
- 50. Tsokos M, Lazarou SA, Moutsopoulos HM. Vasculitis in primary Sjogren's syndrome: histologic classification and clinical presentation. Am J Clin Pathol 1987;88:26–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


