Abstract
Background
Determining treatment response for patients with hidradenitis suppurativa (HS) can be challenging due to limitations of current disease activity evaluations.
Objective
Evaluate the novel, validated endpoint, Hidradenitis Suppurativa Clinical Response (HiSCR) and its utility as an outcome measure.
Methods
Patients with baseline total abscess and inflammatory nodule count (AN count) of at least three and draining fistula count of 20 or fewer comprised the post hoc subpopulation analysed. HiSCR (at least a 50% reduction in total AN count, with no increase in abscess count, and no increase in draining fistula count relative to baseline) and HS‐PGA Response [Hidradenitis Suppurativa‐Physician's Global Assessment score of clear, minimal, or mild, with at least a 2‐grade improvement from baseline] were used to evaluate patient response after adalimumab treatment weekly, every other week, or placebo (1 : 1 : 1).
Results
The subpopulation included 132 (85.7%) patients; 70.5% women and 73.5% white. At week 16, HiSCR was achieved by 54.5% receiving weekly adalimumab, 33.3% every other week, and 25.6% placebo and HS‐PGA Response was achieved by 20.5% receiving weekly adalimumab, 6.7% every other week and 2.3% placebo.
Conclusion
HiSCR was more responsive to change than HS‐PGA Response in this subpopulation.
Introduction
Hidradenitis suppurativa (HS), also known as acne inversa, is a painful, chronic, recurrent, inflammatory, debilitating skin disease1, 2 that can be difficult to diagnose and to treat.1, 3 Determination of response to treatment can also be challenging due to limitations of currently available methods for evaluating disease activity.
Despite the recognized need for more sensitive, accurate, efficient and less complicated measures of disease severity and treatment outcomes in clinical trials,4 dynamic evaluation of HS severity has developed slowly over the past decade. The severity of HS is frequently evaluated by means of Hurley Staging,5 although it was not designed to be a dynamic evaluation of a treatment outcome. Of the few existing measures of HS disease severity that can also detect treatment response, the most common dynamic measures are the Modified Sartorius Score6 and the Physician's Global Assessment (PGA).7
The Hidradenitis Suppurativa‐PGA (HS‐PGA) 6‐point scale was developed and used in a recent phase 2 clinical trial.8 Although HS‐PGA simplified point calculation by means of an objective total count of HS lesions, the stringent response threshold of the study's primary efficacy endpoint (achievement of HS‐PGA of clear, minimal, or mild with at least a 2‐grade improvement relative to baseline at Week 16) may have contributed to a reduced sensitivity to identify changes related to treatment effect, reflected by the achievement of the endpoint by only 20.5% of patients.
To address issues with HS‐PGA and other HS scoring systems, we developed a novel, validated, endpoint, Hidradenitis Suppurativa Clinical Response (HiSCR)9 that is responsive to improvement in disease activity, simplifies the scoring process and increases the sensitivity to detect HS‐specific lesions during clinical evaluation. The objective of the current post hoc analysis was to report the treatment response utilizing the HiSCR endpoint, evaluate the utility of HiSCR as a tool for clinical investigation, and to provide context for clinicians on how to interpret this endpoint.
Methods
The subpopulation in this analysis of the placebo‐controlled, first 12 weeks of the phase 2 trial,8 included all randomized patients who had a baseline abscess and inflammatory nodule count (AN count) of 3 or more and draining fistula count of 20 or fewer. The primary analysis was the proportion of the subpopulation who achieved HiSCR (defined as at least a 50% reduction in the total AN count with no increase in abscess count and no increase in draining fistula count relative to baseline) at week 12. The threshold of 50% reduction in AN count is the defined level that is clinically appropriate and meaningful to the patient regarding improvement in quality of life and pain level.9, 10 We compared HiSCR against the phase‐2 trial's pre‐specified, primary efficacy endpoint, the proportion of patients who achieved treatment response using HS‐PGA of clear, minimal, or mild, with at least a 2‐grade improvement relative to baseline, henceforth referred to as HS‐PGA Response, which indicated a positive treatment effect with adalimumab in HS patients.8 The time to achieve HiSCR and AN count reduction of at least 50%, 75% and 100% relative to baseline (AN50, AN75, AN100, respectively) were also evaluated.
Statistical methods
The Cochran–Mantel–Haenszel (CMH) test with factors of treatment and baseline Hurley staging (I/II vs. III, the study stratification factor) were used to analyse categorical variables, and non‐responder imputation was used to impute missing data. The stratified log‐rank test was used to analyse time to reach HiSCR, where patients who completed or discontinued the first 16 weeks of the study without achieving HiSCR were censored at their last evaluation. All statistical tests were 2‐sided with the significance level of 0.05. Safety was also analysed.
Results
Of the 154 patients randomized in the phase 2 study, the majority (92.4%) completed, and 132 (85.7%) were included in the current post hoc subpopulation. Of the 22 excluded patients, 16 had AN counts of <3 and 6 had draining fistula counts of >20. Overall baseline demographics and clinical characteristics (Table 1) were similar to the primary ITT study population,8 as well as across the subpopulation treatment arms.
Table 1.
Baseline demographics and clinical characteristics of the subpopulation
| PBO (n = 43) | ADA eow (n = 45) | ADA ew (n = 44) | |
|---|---|---|---|
| Age, years; mean (SD) | 37.7 (12.01) | 36.1 (12.77) | 36.6 (10.68) |
| Sex, n (%) | |||
| Female | 29 (67.4) | 33 (73.3) | 31 (70.5) |
| Male | 14 (32.6) | 12 (26.7) | 13 (29.5) |
| Race, n (%) | |||
| White | 31 (72.1) | 34 (75.6) | 32 (72.7) |
| Black | 7 (16.3) | 7 (15.6) | 8 (18.2) |
| Othera | 5 (11.6) | 4 (8.9) | 4 (9.1) |
| Weight, kg; mean (SD) | 98.9 (24.30) | 99.8 (28.15) | 91.9 (21.66) |
| BMI, kg/m2; mean (SD) | 34.7 (7.55) | 35.2 (9.71) | 31.9 (7.79) |
| ≥30 (obese)b | 33 (76.7) | 30 (66.7) | 24 (54.5) |
| ≥40 (morbidly obese)b | 13 (30.2) | 14 (31.1) | 8 (18.2) |
| Smokers (current or ever), n (%) | 35 (81.4) | 28 (62.2) | 32 (72.7) |
| Disease duration, years; mean (SD) | 13.3 (9.53) | 11.4 (8.48) | 12.1 (9.34) |
| HS‐PGA, n (%)c | |||
| Moderate | 28 (65.1) | 30 (66.7) | 31 (70.5) |
| Severe/very severe | 15 (34.9) | 14 (31.1) | 13 (29.5) |
| VAS skin pain (0–100); mean (SD) | 60.2 (28.26) | 53.3 (26.11) | 51.7 (25.70) |
| Hurley stage, n (%) | |||
| I | 6 (14.0) | 7 (15.6) | 8 (18.2) |
| II | 24 (55.8) | 25 (55.6) | 25 (56.8) |
| III | 13 (30.2) | 13 (28.9) | 11 (25.0) |
| Modified Sartorius Score; median | 78.0 | 63.5 (n = 44)d | 65.5 |
Includes Asian, American Indian/Alaska native, native Hawaiian or other Pacific islander, other and multi‐race.
Obesity and morbid obesity levels defined in Sturm, et al.11
One patient at baseline had mild HS according to HS‐PGA.
One patient had a missing value.
PBO, placebo; ADA, adalimumab; eow, every other week; ew, every week; SD, standard deviation; BMI, body mass index; HS‐PGA, Hidradenitis Suppurativa‐Physician's Global Assessment; VAS, visual analog scale.
At each dose and time point, a greater percentage of patients achieved treatment response by HiSCR compared with HS‐PGA Response (Fig. 1). Similar to HS‐PGA Response, a greater percentage of patients randomized to weekly adalimumab achieved HiSCR compared with patients randomized to adalimumab every other week, and to placebo (Fig. 1). Differences between weekly adalimumab and placebo treatment for HiSCR were significant at more time points than seen for HS‐PGA Response; P < 0.001 at weeks 2, 4 and 12, and P = 0.007 at week 16. Improvement in AN count overall and at different percentages of improvement (AN50, AN75 and AN100) was observed at weeks 12 and 16 in both adalimumab groups (Fig. 1).
Figure 1.
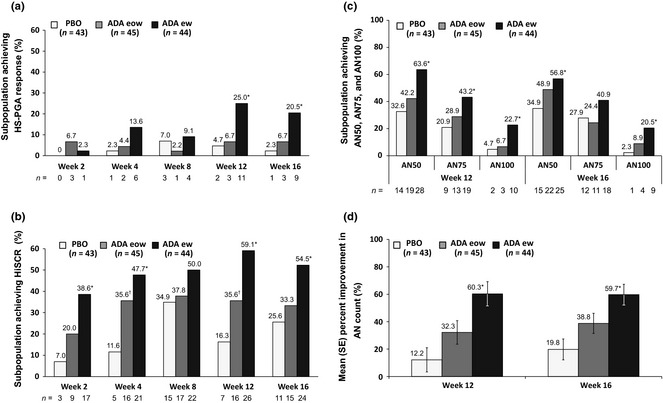
Achievement of HS‐PGA response, HiSCR and AN count improvement for the subpopulation during Period 1: (a) proportion of subpopulation achieving HS‐PGA response. For ADA ew vs. PBO: *P = 0.010 at weeks 12 and 16. (b) Proportion of subpopulation achieving response by HiSCR. For ADA ew vs. PBO: *P < 0.001 at weeks 2, 4 and 12; *P = 0.007 at week 16; For ADA eow vs. PBO: †P = 0.009 at week 4 and †P = 0.042 at week 12. (c) proportion of subpopulation achieving AN50, AN75 and AN100. For ADA ew vs. PBO: AN50, *P = 0.005 at week 12 and *P = 0.050 at week 16; AN75, *P = 0.033 at week 12; AN100, *P = 0.018 at week 12 and *P = 0.010 at week 16. (d) Mean (SE) percent improvement in AN count; *P ≤ 0.001 for ADA ew vs. PBO at weeks 12 and 16. Non‐responder imputation. HS‐PGA Response, Hidradenitis Suppurativa‐Physician Global Assessment score of clear, minimal, or mild, with at least a 2‐grade improvement from baseline; HiSCR, Hidradenitis Suppurativa Clinical Response; AN, abscesses and inflammatory nodules; PBO, placebo; ADA, adalimumab; eow, every other week; ew, every week.
As shown in Table 2, a significantly greater proportion of patients with Hurley Stage II at baseline who were treated with weekly adalimumab achieved HiSCR at week 16 compared with patients receiving placebo (P = 0.004). A significant difference was also seen in HS‐PGA response for Hurley Stage II patients at baseline who were treated with weekly adalimumab (P = 0.004). Response by HiSCR at Week 16 was more evident than response by HS‐PGA, across all three baseline Hurley Stages and dose groups.
Table 2.
Proportion of the subpopulation achieving treatment response at week 16 by baseline hurley stage
| Hurley stage | PBO n = 43 n/N (%) | ADA eow n = 45 n/N (%) | ADA ew n = 44 n/N (%) | P‐value ew vs. PBO |
|---|---|---|---|---|
| Response by HiSCR | ||||
| I | 3/6 (50.0) | 3/7 (42.9) | 4/8 (50.0) | >0.999 |
| II | 7/24 (29.2) | 8/25 (32.0) | 18/25 (72.0) | 0.004a |
| III | 1/13 (7.7) | 4/13 (30.8) | 2/11 (18.2) | 0.576 |
| HS‐PGA response | ||||
| I | 1/6 (16.7) | 1/7 (14.3) | 0/8 (0) | 0.429 |
| II | 0/24 (0) | 2/25 (8.0) | 8/25 (32.0) | 0.004a |
| III | 0/13 (0) | 0/13 (0) | 1/11 (9.1) | 0.458 |
Statistically significant difference.
Non‐responder imputation.
PBO, placebo; ADA, adalimumab; eow, every other week; ew, every week; HiSCR, Hidradenitis Suppurativa Clinical Response; HS‐PGA, Hidradenitis Suppurativa‐Physician's Global Assessment.
As shown in Fig. 2, a shorter time to achieving HiSCR was observed for patients treated with weekly adalimumab compared with every other week, regardless of their baseline Hurley stage. A difference in the time to achieving HiSCR was also observed for patients treated with weekly adalimumab compared with placebo (significant for all patients, P = 0.0001, and patients with Hurley Stage I/II at baseline, P = 0.0002).
Figure 2.
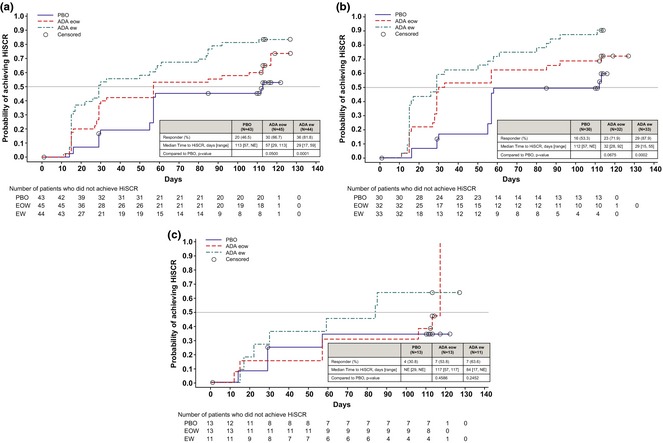
Time to HiSCR for the subpopulation during Period 1 in (a) all patients, (b) patients at Hurley Stage I/II and (c) patients at Hurley Stage III. P‐values from stratified log‐rank test. Patients who did not achieve HiSCR during Period 1 were censored at the last Period 1 assessment. PBO, placebo; eow, every other week; ew, every week; NE, not estimable.
Response was also evaluated by Sartorius Score.6 Median (25th, 75th quartiles) change from baseline was −9.0 (−33.0, 10.0) for patients receiving placebo, and −16.5 (−48.0, −0.5) and −32.0 (−49.5, −8.0) for patients receiving adalimumab every other week and weekly, respectively.
Safety findings have been previously presented.8
Discussion
Evaluation of treatment response for patients with HS can be challenging due to limitations of currently available outcome measures. This study explored the utility of the HiSCR, a novel, validated, endpoint of treatment response for patients with HS, by analysing a large, comprehensive data set of treatment outcome measures in a post hoc assessment.
While other outcome measures appear to focus on disease chronicity manifested by fistulas, scars and sinus tracts, the HiSCR captures the more acute phase of HS activity that involves inflammatory changes, as identified by inflammatory nodule and abscess counts. The parameter of this study's subpopulation (all randomized patients with baseline AN count of 3 or more and draining fistula count of 20 or fewer) are consistent with the population studied in the adalimumab HS phase 3 clinical development program, which excludes mild disease that may not be severe enough to warrant treatment with a biologic agent.
The primary component of HiSCR evaluation is the objective and uncomplicated counting of HS lesions. Following adalimumab treatment, AN count showed marked improvement. Patients receiving weekly adalimumab achieved significantly greater improvement in AN counts (AN50, AN75 and AN100) and greater mean improvement in AN count compared with placebo, which are supportive of HiSCR as better able than HS‐PGA Response to differentiate treatment effect.
Response by HiSCR in the current analysis does not contradict response by HS‐PGA in the primary phase 2 population or by Modified Sartorius Score, but rather represents a more sensitive measure of change in disease activity, resulting in a more accurate representation of patient response and treatment evaluation.
Although our analysis found that patients at Hurley Stage II (moderate HS) appeared to benefit the most from treatment as evaluated by both HiSCR and by HS‐PGA, this result may have been negatively influenced by the small number of patients at Hurley Stages I and III. In addition, response of patients may have been hidden by disease fluctuation or by disease chronicity. We were unable to compare our results with response based on Sartorius scoring, which lacks a defined upper limit or accepted outcome for success. As with previously reported outcome measures of HS, the HiSCR does not take into account the size or severity of individual lesions and does not measure how treatment response affects a patient's level of pain or quality of life.
Despite the limited number of patients in this study, HiSCR demonstrated a meaningful difference, due to its enhanced sensitivity to detect treatment effects. The execution of the HiSCR relies on objective measurements, and, therefore, provides an effective, practical and easy‐to‐use outcome measure for HS that can be utilized in clinical trials as well as in clinical practice. Efficacy analyses using HiSCR have been further examined in the larger, phase 3, PIONEER I and PIONEER II trials of adalimumab treatment for patients with moderate to severe HS (clinicaltrials.gov, NCT01468207 and NCT01468233, respectively).
Conclusions
This post hoc analysis demonstrated that HiSCR was more responsive in detecting changes in response to treatment than HS‐PGA Response in this subpopulation of patients, and may represent a useful tool in clinical practice and research trials when assessing the efficacy of HS therapy.
Acknowledgements
The authors thank Dr. Noah Scheinfeld, MD, of Dermatology Midtown West, New York, NY, for contributing to the interpretation of AN count data, and Jody Bennett, DC, employee of AbbVie, for medical writing assistance.
Conflicts of Interest
A Kimball has received honoraria and payments for serving as a consultant and investigator for AbbVie, Janssen and Amgen, and has received fellowship funding from Janssen. J Sobell has received honoraria from AbbVie, Amgen and Janssen, for serving on advisory boards and as a speaker, and has received grants from Amgen, Janssen, Eli Lilly, Celgene, Novartis and Merck for serving as an investigator. C Zouboulis has received honoraria from AbbVie and Stiefel/GSK for his participation on advisory boards and as an investigator and speaker, from Dermira and Galderma for his participation on advisory boards, from Leo for his participation as a consultant, and from Almirall, Bayer Health Care, Bioderma, Biogen‐Idec, General Topics, Glenmark and Pierre Fabre for his participation as a speaker; his department received grants from AbbVie, Astra‐Zeneca, Biogen‐Idec, BMS, Immundiagnostik AG, Leo Pharma, LVMH, Merz, Novartis, Pierre Fabre and UCB for his participation as an investigator, and from Intendis for his participation on an advisory board. G Jemec has received honoraria from AbbVie, Astra‐Zeneca, Pfizer, Novartis and MSD for serving on ad boards, and has received grants from AbbVie, Leo Pharma, Acetlion and Janssen‐Cilag for serving as an investigator. Y Gu, D Williams, M Sundaram and H Teixeira are employed by AbbVie and own AbbVie stock or receive AbbVie stock options.
Funding/Support
AbbVie Inc. funded this study and participated in the design and conduct of the study, in the collection, management, analysis and interpretation of data, in the preparation, review and approval of the manuscript, and in the decision to submit the manuscript for publication. All authors were involved in the decision to submit the manuscript for publication, and had the right to accept or reject comments or suggestions. AbbVie also sponsored two phase 3 trials of adalimumab treatment for patients with hidradenitis suppurativa.
References
- 1. Menter A, Tyring SK, Gordon K et al Adalimumab therapy for moderate to severe psoriasis: a randomized, controlled phase III trial. J Am Acad Dermatol 2008; 58: 106–115. [DOI] [PubMed] [Google Scholar]
- 2. Fimmel S, Zouboulis CC. Comorbidities of hidradenitis suppurativa (acne inversa). Dermato‐Endocrinology 2010; 2: 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nazary M, van der Zee HH, Prens EP et al Pathogenesis and pharmacotherapy of Hidradenitis suppurativa. Eur J Pharmacol 2011; 672: 1–8. [DOI] [PubMed] [Google Scholar]
- 4. Adams DR, Gordon KB, Devenyi AG, Ioffreda MD. Severe hidradenitis suppurativa treated with infliximab infusion. Arch Dermatol 2003; 139: 1540–1542. [DOI] [PubMed] [Google Scholar]
- 5. Hurley HJ. Hidradenitis Suppurativa In Roenigk RK, Roenigk HH, Jr, eds. Dermatologic Surgery: Principles and Practice, 2nd edn Marcel Dekker, New York, 1996: 623–645. [Google Scholar]
- 6. Sartorius K, Emtestam L, Jemec GB, Lapins J. Objective scoring of hidradenitis suppurativa reflecting the role of tobacco smoking and obesity. Br J Dermatol 2009; 161: 831–839. [DOI] [PubMed] [Google Scholar]
- 7. Robinson A, Kardos M, Kimball AB. Physician Global Assessment (PGA) and Psoriasis Area and Severity Index (PASI): why do both? A systematic analysis of randomized controlled trials of biologic agents for moderate to severe plaque psoriasis. J Am Acad Dermatol 2012; 66: 369–375. [DOI] [PubMed] [Google Scholar]
- 8. Kimball AB, Kerdel F, Adams D et al Adalimumab for the treatment of moderate to severe hidradenitis suppurativa: a parallel randomized trial. Ann Intern Med 2012; 157: 846–855. [DOI] [PubMed] [Google Scholar]
- 9. Kimball AB, Jemec GB, Yang M et al Assessing the validity, responsiveness and meaningfulness of the Hidradenitis Suppurativa Clinical Response (HiSCR) as the clinical endpoint for hidradenitis suppurativa treatment. Br J Dermatol 2014; 171: 1434–1442. [DOI] [PubMed] [Google Scholar]
- 10. Giamarellos‐Bourboulis EJ, Pelekanou E, Antonopoulou A et al An open‐label phase II study of the safety and efficacy of etanercept for the therapy of hidradenitis suppurativa. Br J Dermatol 2008; 158: 567–572. [DOI] [PubMed] [Google Scholar]
- 11. Sturm R. Increases in morbid obesity in the USA: 2000‐2005. Public Health 2007; 121: 492–496. [DOI] [PMC free article] [PubMed] [Google Scholar]


