Summary
Background
The prevalence of hepatitis C virus (HCV) infection in Egypt is the highest in the world, yet the total economic burden has not been quantified. Improved understanding of costs and the impact of treatment strategies will provide for better allocation of resources to reduce HCV disease and economic burden.
Aim
A modelling approach was used to quantify the current HCV‐infected population, future disease progression and associated costs in Egypt.
Methods
Direct healthcare costs were calculated from a nationally representative hospital and a disability adjusted life year (DALY) template was used with monetary value assigned to lost life years. Three scenarios were considered: (i) Historical treatment scenario: 50% SVR; 65 000 treated annually, (ii) Current treatment scenario: 90% sustained virologic response (SVR); 65 000 treated annually, (iii) Increased treatment scenario: 90% SVR; 325 000 treated annually by 2018.
Results
Cumulative DALYs (2015–2030) under Scenario 1 were estimated at 7.88 million and cumulative costs estimated at $89.07 billion. Annual DALYs increased 16% during 2015–2030 while annual costs more than doubled. Scenario 2 reduced cumulative DALYs and costs by 7% and 4%, respectively. Under Scenario 3, total costs declined 73% to $1047 million during 2015–2030. As compared to Scenario 1, cumulative DALYs and costs decreased 37% and 35%, respectively.
Conclusions
This is the first estimate of the total economic burden of HCV in Egypt. Extraordinary measures are necessary to substantially reduce HCV disease and cost burden. With newer therapies, strategies to reduce disease burden are feasible and cost‐effective.
Introduction
The prevalence of hepatitis C virus (HCV) infection in Egypt is the highest in the world,1, 2 yet the total cost and burden of chronic HCV infection on the Egyptian economy, including healthcare sector costs (direct costs), the value of lost productivity among chronically infected individuals (indirect costs), and the burden of disability associated with HCV infection have not been evaluated. Thus, the total burden on the economy remains unknown.
The total financial burden needs to be evaluated as more effective therapies with direct acting antivirals (DAAs) are introduced.3, 4 Improved understanding of the economic cost of HCV and its major determinants helps inform policymakers to allow better allocation of resources to reduce HCV prevalence and burden.
In this study, the national economic burden of hepatitis C infection in 2015 is estimated, as well as future costs, based on modelling of three scenarios, comparing to the historical case had DAAs not been adopted by the national treatment programme, to evaluate the impact of adopting highly effective therapy for HCV.
Methods
Direct healthcare costs for each disease state were calculated using data from the National Liver Institute (NLI), a nationally representative government hospital. Direct costs were applied to HCV‐infected individuals who were diagnosed and under care. To calculate indirect costs, years of life lost to disability (YLD) and years of life lost due to premature death (YLL) were estimated5 and applied to determine cumulative disability adjusted life years (DALYs) from 2015 to 2030 and the value of lost productivity using Egyptian estimates for the value of a statistical life year (VSLY). Future disease progression was based on previously published work,6 and costs were projected from 2015 to 2030 based on historical therapy of pegylated interferon and ribavirin (Scenario 1). The impact of changing treatment to the currently used therapies with greater efficacy (Scenario 2), as well as the additive effect of increasing the treated population and reducing new infections (Scenario 3), were considered in terms of future economic burden and compared with the historical therapeutic regimen (Scenario 1).
Population
Population data came from the Egyptian Central Agency for Public Mobilization and Statistics (CAPMAS),7 and future estimates came from the United Nations' database.8
Prevalence
Prevalence estimates are based upon the 2008 Egypt Demographic and Health Survey (EDHS),9 where seroprevalence and viraemia estimates were reported by 5‐year age group and gender for individuals aged 15–59 years. Total anti‐HCV prevalence was 14.7%, and viraemic prevalence was 9.8%. To estimate the 2008 prevalence in the population younger than 15 years, an exponential decline in viraemic prevalence was trended. Prevalence in those aged >59 years was set equal to prevalence in those aged 55–59 years.
Disease progression
The number of patients in different disease stages, including chronic hepatitis (METAVIR stages F0–F3), compensated cirrhosis (F4), decompensated cirrhosis, hepatocellular carcinoma (HCC) and liver transplant was calculated based on the disease progression model as described earlier.6
The number of patients with non‐Hodgkin's lymphoma (NHL) attributed to HCV was based on published data and age‐standardised incidence rates (ASR) of NHL in Egypt.10, 11, 12, 13 The age and gender distribution of NHL was obtained from the Gharbia cancer registry.14 The ASR of NHL in Egypt is 14.2/100 000,13 representing approximately 12 500 new cases annually. The proportion caused by HCV infection is 42%,13 or an estimated 4930 incident NHL cases annually. The incremental prevalence of diabetes attributable to HCV came from previously published literature.15, 16 There were an estimated 580 240 prevalent cases of HCV‐attributable diabetes in 2013 (9.7% of the viraemic population).
While HCV viraemia is associated with numerous other extrahepatic manifestations (e.g. arthralgia, cryoglobulinemia, skin manifestations, sicca syndrome, thyroid disorders) and increases the risk for circulatory diseases, kidney diseases, renal failure, cancers of the oesophagus, prostate and thyroid,17 and all cause mortality,18 these were not included in this analysis. Only diabetes and NHL have been quantified with known cost for therapy and disability in Egypt, and are included in this analysis.
Diagnosed, under care and treated
According to the EDHS, 1.4% of the Egyptian population had received a positive HCV diagnosis as of 2008,9 equivalent to approximately 15% of the viraemic population. After 2008, it was estimated that 125 000 viraemic individuals were newly diagnosed annually. Data from the NLI and expert opinion were used to estimate the proportion diagnosed by stage: 10% of those with chronic hepatitis, 30% of those with compensated cirrhosis and 70% of those with decompensated cirrhosis or HCC.
It was estimated that 25% of diagnosed chronic hepatitis cases were under care and incurring direct costs, compared with 50% of those with diagnosed compensated cirrhosis and 85% of those with decompensated cirrhosis and HCC. Previous analysis estimated approximately 65 000 HCV patients were treated with pegylated interferon in 2014.6, 19
For Scenarios 1 and 2 it was assumed that 125 000 new cases were diagnosed and 65 000 patients were treated annually from 2015 to 2030, and incident cases were assumed to remain constant at 168 620 new cases annually. Scenario 2 included an increase in SVR to 90% beginning in 2015. For Scenario 3, it was assumed that the annual number diagnosed would increase sufficiently to accommodate increases in the treated population, and the number treated gradually increased up to 325 000 in 2018, based on previous analysis that showed that this is the number needed for disease control of HCV in Egypt by 203019. In addition, incident cases were assumed to gradually decline to 39 930 by 2030. For all scenarios, the proportion of diagnosed patients under care was assumed to gradually reach 100% by 2030.
Direct costs
Costs were analysed for the previous year's out‐patients and admitted patients at the NLI hospital. Each patient file was analysed for disease stage, medications and procedures. A more exhaustive explanation of direct costs calculations is described in Data S1. A summary of direct costs used in the analysis is shown in Table 1.
Table 1.
Direct cost per patient
| Average Cost | Cost per Patient | Cost per Patient |
|---|---|---|
| (One time) | (Annual) | |
| Chronic hepatitis C virus infection | $571 | |
| Compensated cirrhosis | $685 | |
| Decompensated cirrhosis | $2930 | |
| HCV treatment (pegylated interferon/ribavirin) | $1836 | |
| Hepatocellular carcinoma | $1225 | |
| Liver transplantation (first year) | $42 500 | |
| Liver transplantation (subsequent years) | $3500 | |
| Non‐Hodgkin's lymphoma (CD20 negative) | $6000 | |
| Non‐Hodgkin's lymphoma (CD20 positive) | $686 | |
| Diabetes | $176 |
Indirect costs
The DALY metric from the global burden of disease (GBD) study20 was used to estimate indirect costs. It is a measure of the impact of disease on population health, combining YLLs and YLDs, weighted according to the severity of the disability. DALYs were assigned a monetary value to indicate the economic burden of disease due to loss of productivity.
To calculate YLLs and YLDs, the GBD DALY template was used as described in Data S1.21 A separate template was used for chronic hepatitis, compensated cirrhosis, decompensated cirrhosis, HCC, NHL and diabetes.
Life expectancy by age and gender for the Egyptian population were sourced from World Health Organization (WHO) data.22 The age‐adjusted prevalence in 2015 for each stage of the disease for males and females came from the output of the model, and the age at death was estimated based on NLI admissions data. For HCC, mortality inputs came from HCC data based on 2000 cases between 2007 and 2012.23
This study assessed the burden of disease from 2015 to 2030. When calculating YLD, the prevalent population in each year was considered with duration of disability set to 1, as the model calculated disease prevalence by stage of the disease by year. YLDs incurred before 2015 and after 2030 were not considered as part of this analysis, except for lifetime cost calculations.
The disability weights for chronic hepatitis, compensated and decompensated cirrhosis and HCC were derived from an Australian disease burden study.24 YLLs and YLDs were calculated for individuals aged <70 years. Mortality and disability for individuals aged ≥70 years were not included in DALY and indirect cost calculations.
Chronic hepatitis that is undiagnosed was assigned a weight of 0.0. Diagnosed symptomatic cases with chronic hepatitis were assigned a weight of 0.1 and compensated cirrhosis a weight of 0.3. Disability weights were set to 0.0 for cases aged <30 years for these two conditions.
For decompensated cirrhosis, the weighted average was set to 0.5 (0.4 for patients with bleeding varices being managed by repeated endoscopic management and beta blockers, 0.4 for patients with ascites on diuretic therapy, 0.8 for patients with encephalopathy or spontaneous bacterial peritonitis).
The disability weight for HCC varied according to the stage and extent of the disease. The presenting features of 770 consecutive patients in the last 3 months of 2013 were analysed: 22% stage A (weight 0.2), 32% stage B (weight 0.5), 32.5% stage C (weight 0.83), 13.5% stage D (weight 0.93) and an average disability weight of 0.6 was calculated for HCC.
The disability weight of NHL in its different stages and for diabetes was obtained from previous research.25 It is estimated that 90% of the patients with diabetes have no major complications, and their disability weight is set to 0.07; 10% have major complications, and their disability weight is set at 0.4. Severe complications including total blindness, amputations and end stage kidney failure were rare and not considered.
Monetary value of a DALY
Several methods were employed to reach an average value for a DALY: lost production represented by actual per capita annual productivity of the labour force, lost earnings based on the average national salary and VSLY (Table S1).
Lost production: annual productivity was calculated by dividing the gross domestic product (GDP) by the labour force (ages 20–65). GDP in 2012 came from the World Bank,26 and the labour force came from CAPMAS.7
Lost earning: average annual salary was calculated from CAPMAS for the year 2012, and adjusted by the annual salary increase.27
- VSLY: The value of a statistical life (VSL) in Egypt was calculated from the average of the following methods:
- Examining the ratio between GDP per capita in Egypt to countries with published GDP and VSL values28, 29
A value of 1.5 for VSL elasticity was used in this study32 where r is the discount rate (3%) and L is the life expectancy at age × (set to be 31 years, the life expectancy at a mean age of 46 years,33 the mean age for patients with HCV). - Per capita GDP multiple: The WHO recommended in 2001 to value a statistical life year or a DALY at one to three times per capita GDP.30 A value of two times the per capita GDP was applied.
The mean of the output of the three methods was used to set a monetary value for a DALY. The monetary value of a DALY was $6137 by comparing the GDP/capita in Egypt to US VSL values, $6830 based on the cost of productivity of a person in the labour force in Egypt, and $6367 using the CMH2 method. Based on lost earnings (the weekly salary was $92 in 2012,27 with estimated 20% annual inflation to reach $110 in 2013), the average annual income was $5720 in 2013 (Table S1). The mean value of a DALY was $6265. The value of a DALY was assumed to increase 3% annually during 2015–2030.
Lifetime cost
To estimate lifetime costs and DALYs, a model simulation was completed where a set of 30‐ to 34‐year‐old males in a single disease stage entered the model in 2015, and disease progression and mortality were tracked for 85 years. Future lifetime costs (direct and indirect) along with DALYs were calculated by disease stage. In addition, the cost per DALY averted was calculated based on an assumed treatment cost of $2000 per cured case.
Cost‐effectiveness analysis
The primary endpoint in the incremental cost‐effectiveness ratio (ICER) analysis was the additional cost per DALY averted.34 Scenario 3 is compared to Scenarios 1 and 2 (Table 2). The economic feasibility of the ICER was assessed in relation to the annual per capita GDP35 and was considered to be highly cost‐effective if the ICER/DALY averted was <$3184 per DALY (1 times per capita GDP) and cost‐effective if the ICER was <$9553 per DALY (three times per capita GDP).36
Table 2.
Disability adjusted life years averted and incremental costs by Scenario – Egypt, 2015–2030
| 1. Historical treatment scenario: 50% sustained viral response rate; 65 000 treated annually | 2. Current treatment scenario: 90% sustained viral response rate; 65 000 treated annually | 3. Increased treatment scenario: 90% sustained viral response rate; 325 000 treated annually by 2018 | |
|---|---|---|---|
| Cumulative disability adjusted life years 2013–2030 | 7 875 440 | 7 343 640 | 4 923 210 |
| Disability adjusted life years averted vs. Scenario 1 | – | 531 800 | 2 952 230 |
| Disability adjusted life years averted vs. Scenario 2 | – | – | 2 420 430 |
| Cumulative direct costs 2013–2030 (US Dollars) | 23 244 377 860 | 24 192 586 440 | 18 632 607 710 |
| Incremental cost vs. Scenario 1 (US Dollars) | – | 948 208 580 | −4 611 770 150 |
| Incremental cost effectiveness ratio vs. Scenario 1 (US Dollars) | – | 1780 | −1560 |
| Incremental cost vs. Scenario 2 (US Dollars) | – | – | −5 559 978 730 |
| Incremental cost effectiveness ratio vs. Scenario 2 (US Dollars) | – | – | −2300 |
| Cumulative indirect costs 2013–2030 (US Dollars) | 65 822 552 110 | 61 547 348 360 | 38 929 874 750 |
| Incremental cost vs. Scenario 1 (US Dollars) | – | −4 275 203 750 | −26 892 677 360 |
| Incremental cost effectiveness ratio vs. Scenario 1 (US Dollars) | – | −8040 | −9110 |
| Incremental cost vs. Scenario 2 (US Dollars) | – | – | −22 617 473 610 |
| Incremental cost effectiveness ratio vs. Scenario 2 (US Dollars) | – | – | −9340 |
| Cumulative total costs 2013–2030 (US Dollars) | 89 066 929 970 | 85 739 934 800 | 57 562 482 460 |
| Incremental cost vs. Scenario 1 (US Dollars) | – | −3 326 995 170 | −31 504 447 510 |
| Incremental cost effectiveness ratio vs. Scenario 1 (US Dollars) | – | −6260 | −10 670 |
| Incremental cost vs. Scenario 2 (US Dollars) | – | – | −28 177 452 340 |
| Incremental cost effectiveness ratio vs. Scenario 2 (US Dollars) | – | – | −11 640 |
Results
Disease burden
Under Scenario 1, the total prevalence of HCV antibody positivity among all ages in 2008 was estimated at 12.5% as reported earlier,37 resulting in overall viraemic prevalence of 8.5% in 2008. After taking into consideration mortality, new infections and cured patients, the 2015 viraemic prevalence was estimated at 6.8% or 5 896 060 individuals. The majority of patients with HCV were born prior to 1965, a result of the widespread use of anti‐schistosomal therapy in the 1960s and 1970s. The total number of diagnosed cases in 2015 is estimated at 1 312 420 cases, corresponding to approximately 22% of the viraemic population. In 2015, 5 011 260 individuals were estimated to have chronic hepatitis without cirrhosis, 635 180 compensated cirrhosis, 85 410 decompensated cirrhosis and 17 070 HCC.
Had DAAs not been adopted, under Scenario 1, the viraemic population would have decreased 31% to 4 437 180 cases by 2030. Prevalent cases of HCC in 2030 are estimated at 17 980 cases (5% increase from 2013) after peaking in 2024 at 18 570 annual cases. Similarly, liver‐related mortality would have peaked in 2017 at 36 480 deaths, and there would have been a projected 35 470 deaths in 2030, a 1% increase from 2015 (35 000 deaths).
With the use of effective DAAs under Scenario 2, there will be 8% fewer viraemic individuals and 14% fewer HCC cases in 2030 as compared to Scenario 1. The number of liver‐related deaths will decrease by 15% as compared to Scenario 1 to 30 120 and decompensated cirrhosis will decrease by 20% to 104 730 prevalent cases in 2030.
Adopting Scenario 3 will result in 4 157 180 fewer viraemic individuals in 2030, a 94% reduction as compared to Scenario 1. By 2025, overall viraemic prevalence drops to 1.9%, and by 2030 viraemic prevalence is estimated at 0.3%. The number of prevalent HCC cases in 2030 is estimated to decrease by 86% to 2440 cases, and the number of liver‐related deaths will decrease by 75% to 8890 deaths. Decompensated and compensated cirrhosis will decrease by 86% to 17 850 and 87 970 cases respectively.
DALYs
Under Scenario 1, annual DALYs increased 16%, from 463 310 in 2015 to 535 660 in 2030 (Figure 1). Total cumulative DALYs from 2015 to 2030 are estimated at 7.88 million with 66% attributable to YLD (Figure 2). With the current treatment in Scenario 2, annual DALYs increased 16% to 526 540 in 2030. Annual YLDs increased 87% to 442 550 in 2030, while YLLs decreased 61% to 83 990. Total cumulative DALYs were estimated at 7.34 million, a 7% reduction as compared to Scenario 1. Cumulative YLLs decreased by 21% while cumulative YLDs increased by 0.5%, as compared to Scenario 1 (Figure 2).
Figure 1.
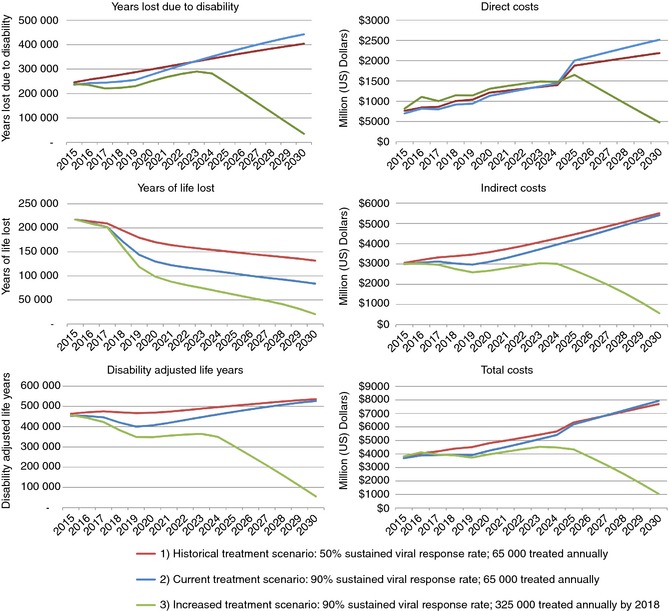
Disability adjusted life year and cost outputs by Scenario – Egypt, 2015–2030.
Figure 2.
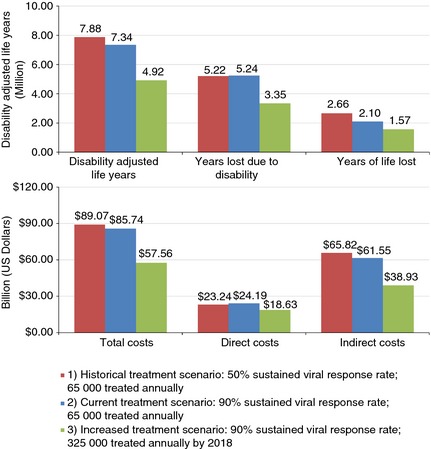
Cumulative disability adjusted life years and costs by Scenario – Egypt, 2015–2030.
Under Scenario 3, annual DALYs decreased to 55 650 from 2014 to 2030, an 88% decrease. From 2014 to 2030, YLDs decreased 85% to 35 340 while YLLs decreased 91% to 20 320. Under this scenario, total cumulative DALYs are estimated at 4.92 million from 2014 to 2030, a 37% decrease from Scenario 1 (Figure 2). Both cumulative YLDs and YLLs decreased by over 35% as compared to Scenario 1.
Future costs
Under Scenario 1, total costs associated with HCV infection in 2015 would have amounted to $3.82 billion in both direct healthcare costs ($763 million) and indirect costs of premature mortality and cost of disability ($3.05 billion).
Total costs associated with chronic HCV infection increased 101% under Scenario 1 to $7.69 billion during 2015–2030 (Figure 1). Direct costs increased more (187%) relative to indirect costs (80%). Total costs from 2015 to 2030 were estimated at $89.07 billion (Figure 2).
Under the current treatment programme in Scenario 2, total costs increased 115% from 2015 values to $7.93 billion in 2030. Direct costs in 2030 were estimated at $2.52 billion, an increase of 260% from 2015. Indirect costs in 2030 ($5.41 billion) were 81% higher than 2015. Total costs from 2015 to 2030 were decreased by 4% as compared to Scenario 1 (Figure 2).
Under Scenario 3, total costs declined 73% to $1.05 billion by 2030. Annual direct costs decreased by 42% to $475 million in 2030, while annual indirect costs decreased 81% to $572 million in 2030. Annual total costs in 2030 were 86% lower than for Scenario 1, and cumulative costs during 2015–2030 were over 35% lower, with a reduction of $31.50 billion (Figure 2). Cumulative direct costs from 2015 to 2030 were 20% lower as compared to Scenario 1, while 2030 annual costs were 78% lower. Cumulative indirect costs were 41% lower as compared to Scenario 1, with a reduction of 90% in 2030 annual costs.
Lifetime cost
Model simulation estimated the lifetime cost of a diagnosed male aged 30–34 years under care by disease state in 2015 (Figure 3). Estimated direct lifetime costs ranged from $2150 (HCC) to $66 610 (liver transplant). Costs generally increased with disease severity; an exception is HCC, where a high mortality rate resulted in relatively low direct cost. For most disease stages, indirect costs accounted for at least 80% of total costs, ranging from $83 840 (F0) to $183 960 (decompensated cirrhosis).
Figure 3.
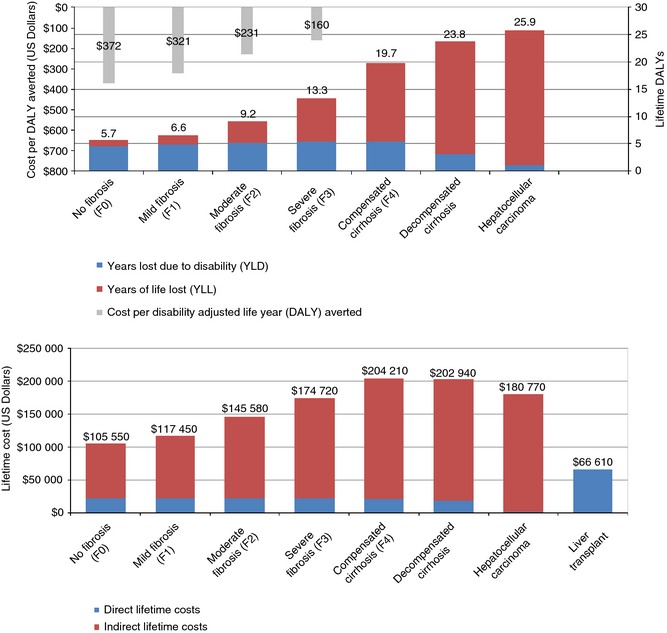
Estimated future lifetime cost and disability adjusted life years for 30–34 year old male (diagnosed and under care) by disease state in 2015.
Estimated lifetime DALYs ranged from 5.7 (F0) to 25.9 (HCC) (Figure 3). Estimated YLLs ranged from 1.1 for F0 to 24.7 for HCC. Based on lifetime DALYs and assumed costs of $2000 to achieve SVR, it is estimated that the cost per DALY averted ranges from $160 (F3) up to $372 (F0). Costs per DALY averted were less than $100 for patients with decompensated cirrhosis and HCC, although these patients may be ineligible for treatment regimens.
ICERs
Adopting Scenario 3 will result in averting 2.95 million DALYs between 2015 and 2030 at a savings of $4.61 billion in direct costs and $26.89 billion in indirect costs as compared to Scenario 1, and averting 2.42 million DALYs at a savings of $5.56 billion in direct costs and $22.62 billion in indirect costs as compared to Scenario 2. For total costs, the ICER/DALY averted for Scenario 3 is −$10 670 and −$11 640, as compared to Scenarios 1 and 2 respectively (Table 2).
Discussion
Although HCV infection is a major health problem in Egypt, this is the first analysis of its total economic burden, including a projection of its future cost and the impact of different control strategies. Current HCV‐related healthcare costs were analysed along with indirect costs due to disability and loss of life. The cost of a DALY for Egypt was calculated for the first time using the VSLY. The value used for the VSL is low (at 36–41 multiples of per capita GDP) compared with other work comparing VSL values between countries (130–195 multiples).38 Yet this analysis showed that HCV and its related complications form a substantial health and economic burden in Egypt. The total economic burden of HCV in Egypt is $3.81 billion, equivalent to 1.4% of GDP, relatively as large as the cost of diabetes in the US (1.45% of GDP).39 Direct healthcare costs of HCV‐related disease exceed $700 million annually and consume approximately 4.0% of the total health expenditure in Egypt, exemplifying the magnitude of the HCV epidemic in Egypt.40
This analysis shows that while overall HCV prevalence in Egypt is declining, disease burden related to HCV and associated costs will continue to grow as the number of individuals experiencing advanced liver disease and liver‐related deaths increases. Similar trends have been modelled for other countries,41 where peak costs lag peak prevalence by approximately three decades. US studies examining costs have found dramatic increases as patients progress to advanced liver disease,42, 43 reiterating the importance of initiating treatment among earlier stage patients. In addition, a US study found that two‐thirds of total costs associated with HCV were indirect costs. In the scenarios presented in this analysis, the cumulative direct costs during 2015–2030 as a proportion of total ranges from 26% (Scenario 1) to 33% (Scenario 3).
With the current treatment strategy under Scenario 2, increased treatment efficacy alone resulted in smaller reductions in future disease burden and costs. Under Scenario 3, increases in diagnosis and treatment levels, coupled with a reduction in new cases, resulted in much larger impacts in disease and cost burden. The scenarios presented have the potential to greatly reduce the burden of HCV‐related morbidity and mortality in Egypt, including a reduction in prevalence to 2.0% by 2025 under Scenario 3. Adoption of new therapies also reduces negative patient‐reported outcomes associated with earlier treatment regimens,44 but will depend upon a number of constraints. The number of diagnosed individuals needs to increase dramatically, with a similar increase in treatment. In addition, substantial reductions in prevalence are dependent upon decreased incidence. Implementation of strategies will depend upon the capacity of the healthcare system to diagnose and treat new patients, as well as executing effective measures to reduce incidence.
It is anticipated that newly infected individuals may present an increased burden on the healthcare system due to long‐life expectancy, highlighting the continued importance of prevention. However, if the number of diagnosed and treated patients and the average SVR are increased, prevalence can drop below 1% before 2030. As a result, it is possible that the costs averted are greater than the costs of the strategy.
A limitation of the cost analysis is uncertainty surrounding costs for extrahepatic conditions associated with chronic HCV infection, which impact up to 74% of infected individuals.45 While incremental prevalence and costs for diabetes and NHL among HCV cases in Egypt have been well quantified, numerous other extrahepatic conditions could contribute towards the HCV‐related economic burden. Remission of some extrahepatic conditions such as cryoglobulinemia is common after SVR46; however, the prevalence and associated costs for numerous other conditions after SVR have not been studied. Costs and remission rates after SVR for extrahepatic conditions could substantially impact the results of this analysis.
The Egyptian National Control Strategy for Viral Hepatitis notes the importance of reducing prevalence of HCV in Egypt, as well as increasing awareness, diagnosis and treatment.47 In addition, the national strategy highlights the importance of preventing transmission in medical settings and improving the safety of injections given in nonmedical settings. As the majority of infected individuals in Egypt are unaware of their infection, the national control strategy also emphasises efforts to increase awareness and testing for HCV. Based on our analysis, it is estimated that 70% of the prevalent population in 2015 was born between 1945 and 1975. Targeted screening efforts based on birth cohort can be efficacious for maximising diagnosis and treatment resources.48
In conclusion, our analysis demonstrated that HCV prevalence in Egypt is declining. However, the prevalence of advanced liver disease and associated costs will continue to increase. It is possible to substantially reduce HCV disease and economic burden through increased treatment and diagnosis, along with efforts to reduce transmission.
Authorship
Guarantor of the article: Chris Estes.
Author contributions: IW conceptualised study objectives and design, created tables and figures and contributed to manuscript writing. CE, MAK, WAR, EAS, MA, AG, WO, HR and HZ performed literature searches, extracted data, created tables and figures and contributed to manuscript writing. All authors approved the final version of this manuscript.
Supporting information
Appendix S1. Direct cost estimation.
Table S1. Value of statistical life year (VSLY) inputs.
Table S2. Hepatitis C virus related outcomes by scenario – 2015–2030.
Acknowledgements
Expert opinion used in previous published work from Professors Wahid Doss, Manal H. El‐Sayed, Gamal Esmat, Gamal E. Shiha and Ayman Yosry was valuable in the projection and modelling of the disease control strategy upon which the cost modelling was based.
Declaration of personal interests: C Estes and H Razavi are employees of Center for Disease Analysis (CDA). CDA receives funding from public and private (Gilead Sciences, AbbVie, Boehringer Ingelheim) sources. I Waked has served as a speaker for Hoffman La Roche, Merck, BMS, GSK, Gilead, AbbVie; has served on advisory boards for Janssen, Hoffman La Roche, Merck and Abbott; and has acted as a principal investigator in clinical trials for Hoffman La Roche, BMS, GSK, Bayer, Janssen and AbbVie. M Abdel Kareem, W Adel Razek, E Abdelsamea, A Gomaa, W Osman, M Abuzeid and H Zaghla have no conflicts of interest to declare.
Declaration of funding interests: This study was funded by Center for Disease Analysis (CDA) and National Liver Institute (NLI).
This article was accepted for publication after full peer‐review.
References
- 1. Sievert W, Altraif I, Razavi HA, et al A systematic review of hepatitis C virus epidemiology in Asia, Australia and Egypt. Liver Int 2011; 31(Suppl. 2): 61–80. [DOI] [PubMed] [Google Scholar]
- 2. Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect 2011; 17: 107–15. [DOI] [PubMed] [Google Scholar]
- 3. Asselah T, Marcellin P. Direct acting antivirals for the treatment of chronic hepatitis C: one pill a day for tomorrow. Liver Int 2012; 32(Suppl. 1): 88–102. [DOI] [PubMed] [Google Scholar]
- 4. Schinazi R, Halfon P, Marcellin P, Asselah T. HCV direct‐acting antiviral agents: the best interferon‐free combinations. Liver Int 2014; 34(Suppl. 1): 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization . Disability adjusted life years (DALY). Available at: http://www.who.int/healthinfo/global_burden_disease/metrics_daly/en (accessed 14 November 2014).
- 6. Razavi H, Waked I, Sarrazin C, et al The present and future disease burden of hepatitis C virus (HCV) infection with today's treatment paradigm. J Viral Hepat 2014; 21(Suppl. 1): 34–59. [DOI] [PubMed] [Google Scholar]
- 7. Central Agency for Public Mobilization and Statistics (CAPMAS) . Estimates of midyear population by age groups, 2012. [cited: November 21 2013]. Available at: http://www.capmas.gov.eg/pdf/Electronic%20Static%20Book2013/english/population/untitled1/pop.aspx (accessed 14 November 2014).
- 8. United Nations; Department of Economic and Social Affairs; Population Division . World Population Prospects: The 2010 Revision, Volume I: Comprehensive Tables. ST/ESA/SER.A/313, 2011.
- 9. El‐Zanaty F, Way A. Egypt Demographic and Health Survey, 2008. Cairo, Egypt: Ministry of Health and Population, Demographic and Health Survey (EDHS), 2009. [Google Scholar]
- 10. Cowgill KD, Loffredo CA, Eissa SA, et al Case‐control study of non‐Hodgkin's lymphoma and hepatitis C virus infection in Egypt. Int J Epidemiol 2004; 33: 1034–9. [DOI] [PubMed] [Google Scholar]
- 11. Goldman L, Ezzat S, Mokhtar N, et al Viral and non‐viral risk factors for non‐Hodgkin's lymphoma in Egypt: heterogeneity by histological and immunological subtypes. Cancer Causes Control 2009; 20: 981–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gouda I, Nada O, Ezzat S, et al Immunohistochemical detection of hepatitis C virus (genotype 4) in B‐cell NHL in an Egyptian population: correlation with serum HCV‐RNA. Appl Immunohistochem Mol Morphol 2010; 18: 29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Soliman A, Boffetta P. Lymphoma and leukemia In: Freedman LS, Edwards BK, Ries LAG, Young JL, eds. Cancer Incidence in Four Member Countries (Cyprus, Egypt, Israel, and Jordan) of the Middle East Cancer Consortium (MECC) Compared With US SEER, Chapter 14. Bethesda, MD: National Cancer Institute; NIH Pub. No. 06‐5873, 2006; 131–40. Available at: http://seer.cancer.gov/archive/publications/mecc/mecc_monograph.pdf (accessed 14 November 2014). [Google Scholar]
- 14. Herzog CM, Dey S, Hablas A, et al Geographic distribution of hematopoietic cancers in the Nile delta of Egypt. Ann Oncol 2012; 23: 2748–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Elhawary EI, Mahmoud GF, El‐Daly MA, Mekky FA, Esmat GG, Abdel‐Hamid M. Association of HCV with diabetes mellitus: an Egyptian case‐control study. Virol J 2011; 8: 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. White DL, Ratziu V, El‐Serag HB. Hepatitis C infection and risk of diabetes: a systematic review and meta‐analysis. J Hepatol 2008; 49: 831–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee MH, Yang HI, Lu SN, et al ; R.E.V.E.A.L.‐HCV Study Group . Chronic hepatitis C virus infection increases mortality from hepatic and extrahepatic diseases: a long‐term prospective study. J Infect Dis 2012; 206: 469–77. [DOI] [PubMed] [Google Scholar]
- 18. van der Meer AJ, Veldt BJ, Feld JJ, et al Association between sustained virological response and all‐cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA 2012; 308: 2584–93. [DOI] [PubMed] [Google Scholar]
- 19. Waked I, Doss W, El‐Sayed MH, et al The current and future disease burden of chronic hepatitis C virus infection in Egypt. Arab J Gastroenterol 2014; 15: 45–52. [DOI] [PubMed] [Google Scholar]
- 20. Murray CJL, Lopez AD. The Global Burden of Disease. Geneva: WHO, Harvard School of Public Health, World Bank, 1996. [Google Scholar]
- 21. WHO Health Statistics and Information Systems , 2014. Available at: www.who.int/healthinfo/bodreferencedalycalculationtemplate.xls (accessed 14 November 2014).
- 22. WHO Global Health Observatory Data Repository , 2014. Available at: http://apps.who.int/gho/data/node.main (accessed 14 November 2014).
- 23. Gomaa AI, Hashim MS, Waked I. Comparing staging systems for predicting prognosis and survival in patients with hepatocellular carcinoma in Egypt. PLoS ONE 2014; 9(3): e90929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mathers C, Vos T, Stevenson C. The burden of disease and injury in Australia. AIHW cat. no. PHE 17. Canberra: AIHW, 1999. Available at: http://www.aihw.gov.au/WorkArea/DownloadAsset.aspx?id=6442459196 (accessed 14 November 2014).
- 25. Stouthard M, Essink‐Bot ML, Bonsel GJ, et al Disability Weights for Diseases in the Netherlands. Rotterdam, Netherlands: Erasmus University Press, 1997. [Google Scholar]
- 26. World Bank . 2014. Egypt, Arab Rep. Available at: http://data.worldbank.org/country/egypt-arab-republic March 13, 2014 (accessed 14 November 2014).
- 27. CAPMAS: Annual statistics of jobs, salaries, and work hours for 2012. Available at: http://www.capmas.gov.eg/pdf/news/Twzf2012.pdf (accessed 14 November 2014).
- 28. US Department of Transportation . Revised Departmental Guidance 2013: Treatment of the Value of Preventing Fatalities and Injuries in Preparing Economic Analyses, 2013. Available at: http://www.dot.gov/sites/dot.gov/files/docs/VSL%20Guidance_2013.pdf (accessed 14 November 2014).
- 29. The White House, US Government . 2013 Draft Report to Congress On the Benefits and Costs of Federal Regulations and Agency Compliance with the Unfunded Mandates Reform Act. Available at: http://www.whitehouse.gov/sites/default/files/omb/inforeg/2013_cb/draft_2013_cost_benefit_report.pdf (accessed 14 November 2014).
- 30. WHO: Commission on Macroeconomics and Health . Macroeconomics and Health: Investing in Health for Economic Development, Sachs J, ed. Geneva: WHO, 2001. [Google Scholar]
- 31. Viscusi WK. Policy challenges of the heterogeneity of the value of statistical life. Foundations Trends Microecon 2010; 6: 99–172. [Google Scholar]
- 32. Suhrcke M. Chronic disease: alternative perspective Chapter 3.2. In: Lomborg B, ed. Global Problems: Smart Solutions, Costs and Benefits. Cambridge: Cambridge University Press, 2013; 180–5. [Google Scholar]
- 33. WHO Global Health Observatory Data Repository . Life tables by country: Egypt, 2014. Available at: http://apps.who.int/gho/data/view.main.60500 (accessed 14 November 2014).
- 34. Musgrove P, Fox‐Rushby J. Cost‐Effectiveness Analysis for Priority Setting. Chapter 15, 271‐286 In: Jamison DT, Breman J, Measham A, Alleyne G, Claeson M, Evans D, Jha P, Mills A, Musgrove P, eds. Disease Control Priorities in Developing Countries. Second Edition: Oxford University Press and The World Bank, 2006. [Google Scholar]
- 35. Goldie SJ, Yazdanpanah Y, Losina E, et al Cost‐effectiveness of HIV treatment in resource‐poor settings—the case of Cote d'Ivoire. N Engl J Med 2006; 355: 1141–53. [DOI] [PubMed] [Google Scholar]
- 36. WHO: Cost‐effectiveness thresholds. Available at: http://www.who.int/choice/costs/CER_thresholds/en/(accessed 14 November 2014).
- 37. Bruggmann P, Berg T, Ovrehus AL, et al Historical epidemiology of hepatitis C virus (HCV) in selected countries. J Viral Hepat 2014; 21(Suppl. 1): 5–33. [DOI] [PubMed] [Google Scholar]
- 38. Miller TR. Variations between countries in values of statistical life. J Transport Econ Policy 2000; 34: 169–88. [Google Scholar]
- 39. American Diabetes Association . Economic costs of diabetes in the U.S. in 2012. Diabetes Care 2013; 36: 1033–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. World Bank: Health expenditure, total (% of GDP). Available at: http://data.worldbank.org/indicator/SH.XPD.TOTL.ZS (accessed 14 November 2014).
- 41. Myers RP, Krajden M, Bilodeau M, et al Burden of disease and cost of chronic hepatitis C infection in Canada. Can J Gastroenterol Hepatol 2014; 28: 243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gordon SC, Pockros PJ, Terrault NA, et al Impact of disease severity on healthcare costs in patients with chronic hepatitis C (CHC) virus infection. Hepatology 2012; 56: 1651–60. [DOI] [PubMed] [Google Scholar]
- 43. Davis KL, Mitra D, Medjedovic J, Beam C, Rustgi V. Direct economic burden of chronic hepatitis C virus in a United States managed care population. J Clin Gastroenterol 2011; 45: e17–24. [DOI] [PubMed] [Google Scholar]
- 44. Younossi Z, Henry L. Systematic review: patient‐reported outcomes in chronic hepatitis C–the impact of liver disease and new treatment regimens. Aliment Pharmacol Ther 2015; 41: 497–520. [DOI] [PubMed] [Google Scholar]
- 45. Cacoub P, Poynard T, Ghillani P, et al Extrahepatic manifestations of chronic hepatitis C. MULTIVIRC Group. Multidepartment Virus C. Arthritis Rheum 1999; 42: 2204–12. [DOI] [PubMed] [Google Scholar]
- 46. Charles ED, Dustin LB. Hepatitis C virus‐induced cryoglobulinemia. Kidney Int 2009; 76: 818–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Doss W. Egyptian National Control Strategy for Viral Hepatitis 2008–2012. Arab Republic of Egypt: Ministry of Health and Population, National Committee for the Control of Viral Hepatitis, 2008. [Google Scholar]
- 48. Smith BD, Morgan RL, Beckett GA, et al Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945‐1965. MMWR Recomm Rep 2012; 61: 1–32. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Direct cost estimation.
Table S1. Value of statistical life year (VSLY) inputs.
Table S2. Hepatitis C virus related outcomes by scenario – 2015–2030.


