Abstract
Background and Objectives
: The purpose of this study was to retrospectively analyze the features of patients with papillary thyroid carcinoma (PTC) presenting with neck lymph node (LN) metastasis.
Methods
: The study enrolled 909 patients with PTC who had undergone total thyroidectomy. After a median follow‐up of 14.6 years, 73 (8.0%) patients died of thyroid cancer. A total of 536 patients had the tumor confined to the thyroid (intra‐thyroid), 111 had lymph node (LN) metastasis, 225 showed soft tissue invasion, and 37 had distant metastasis.
Results
: Compared with the intra‐thyroid group, the group with LN metastases showed larger tumor size, higher postoperative thyroglobulin levels, advanced TNM stage, higher recurrence rates (5.2% vs. 31.5%), and higher disease‐specific mortality (1.3% vs. 12.6%). Of the 111 patients with PTC and LN metastases, 35 (31.5%) were diagnosed with recurrence during a mean follow‐up period of 16.9 ± 0.6 years. Among the 35 patients with recurrent PTC, 14 (40.0%) died of thyroid cancer. The mortality group was characterized by older, mostly male patients who presented with larger initial tumor size compared with survivors.
Conclusions
: In patients with PTC, the rates of recurrence and cancer mortality were higher in the group with LN metastasis than that in the intra‐thyroid tumor group. J. Surg. Oncol. 2015 111:149–154. © 2015 The Authors. Journal of Surgical Oncology Published by Wiley Periodicals, Inc.
Keywords: lymph node dissection, total thyroidectomy, thyroglobulin, radioactive iodine, cancer specific survival
INTRODUCTION
Locoregional lymph node (LN) metastasis is typically one of the first steps in the progression of papillary thyroid carcinoma (PTC) to distant metastasis from the thyroid 1, 2. There have been reports that the incidence of well‐differentiated thyroid cancers is increasing; most cases follow a relatively benign clinical course, which may cause the physician and patient to neglect the importance of long‐term follow‐up. Previous studies have shown that 67.9% of classic PTC cases present with LN metastasis; however, disease‐specific survival of patients with PTC was not affected by LN metastasis, nor was it only influenced in older patients with LN metastasis of the lateral neck 3, 4. Most of these studies were conducted with a follow‐up period of less than 10 years. There is, however, a lack of information concerning recurrence after initial thyroidectomy with LN dissection in patients with PTC, and locoregional relapse or distant metastasis may occur after initial LN dissection. Additional surgery, long‐term radioactive iodine (131I) therapy, and external radiotherapy are used to treat recurrence in patients with PTC. The aim of this study was to evaluate long‐term therapeutic outcomes in patients with PTC presenting with neck LN metastasis, and to identify risk factors associated with distant metastasis and disease‐specific mortality.
MATERIALS AND METHODS
Patients with PTC were included in the present study if they had histologically proven PTC and had undergone thyroidectomy before 2004. Patients were excluded if they were lost to follow‐up for >1 year, they had undergone primary thyroidectomy at another institution, and the tumor size was ≤10 mm. Patients with papillary microcarcinoma were excluded due to the high ratio of incidental findings and because they had undergone partial thyroidectomy without LN dissection 5. From 1977 to 2004, a total of 909 patients with PTC (728 women and 181 men) with a mean age of 40.7 ± 13.8 years (range: 11−83 years) underwent total or near‐total thyroidectomy with or without LN dissection and were followed‐up at Chang Gung Medical Center in Linkou, Taiwan. In our center, LN dissection is performed for PTC patients with grossly enlarged LNs found intra‐operatively. Prophylactic LN dissection is not routinely performed. However, surgeons do occasionally perform prophylactic LN dissection as the patient need dictates. All patients were staged using the International Union Against Cancer Tumor‐Node‐Metastasis (UICC‐TNM) criteria (6th edition) 6. Intra‐thyroid PTC was defined as macroscopic and microscopic findings without evidence for extra‐thyroid extension of PTC and no involved LN detected intra‐operatively or in the final histopathological report. Among 909 patients, 536 patients (59.0%) had intra‐thyroid PTC and the remaining 373 patients (41.0%) with PTC showed LN metastasis (n = 111, 12.2%), soft tissue invasion (n = 225, 24.8%), and distant metastasis (n = 37, 4.1%). A total of 225 patients with soft tissue invasion and 37 with distant metastasis were not enrolled in the risk factor analysis. There were 656 patents (72.2%) categorized as having TNM stage I disease. Among these, 120 TNM stage I patients with lymph node or soft tissue extension were <45 years old.
All thyroid carcinoma tissues were pathologically classified according to the criteria of the World Health Organization 7. Depending on clinical indications, noninvasive examinations included the following: chest radiography, computed tomography, magnetic resonance imaging, bone scintigraphy, 201Tl scintigraphy, and fluoro‐18‐deoxyglucose positron emission tomography. Thyroid remnant ablation was recommended for PTC with histologically demonstrated LN metastasis at 4 to 6 weeks after surgery. The 131I ablation dose for most patients was 1.1–3.7 GBq (30–100 mCi). Whole‐body scintigraphy (WBS) was performed at 1 week after 131I administration using a dual‐head gamma camera (Dual Genesys, ADAC, GE Infinia Hawkeye 4, Haifa, Israel) equipped with a high‐energy collimator. The whole‐body image was acquired using continuous mode scanning at a speed of 5 cm/min. Furthermore, thyroid scintigraphy was performed using a pinhole collimator with a 4‐mm aperture placed 7 cm above the neck, for a total of 50 K counts or 30 min. Subsequent treatment with L‐T4 was initiated in order to decrease thyroid stimulating hormone (TSH) levels without inducing clinical thyrotoxicosis. Cases in which foci of 131I uptake extended beyond the thyroid bed were classified as persistent disease or metastatic. Such patients received increased therapeutic doses at 3.7–7.4 GBq (100–200 mCi). Hospital isolation was arranged at doses exceeding 1.1 GBq. WBS was performed 2 weeks after administration of the higher therapeutic dose of 131I. As described in our previous report, high‐risk patients with PTC and distant metastasis or locoregional recurrence received a higher dose of 131I therapy (3.7–7.4 GBq). In contrast, the low risk group received a lower dose of 131I therapy (1.1–3.7 GBq) 8. According to the radiation regulations in Taiwan, patients receiving <1.1 GBq were treated as outpatients.
In patients without detectable 131I uptake beyond the thyroid bed during post‐ablation WBS, thyroid hormone treatment was withdrawn after 6–12 months, and the levels of thyroglobulin (Tg), TSH, and anti‐Tg antibodies were measured. Serum Tg levels were measured using an IRMA assay (CIS Bio International, Gif Sur Yvette, France) and the level of Tg was considered accurate only if the recovery test (performed for all serum samples) was less than 80%.
The following data were collected from admission records: age, sex, primary tumor size, ultrasonographic findings, results of fine needle aspiration cytology, preoperative thyroid function, surgical methods, histopathological findings, TNM staging, 1‐month postoperative serum Tg levels, presence of Tg antibodies, diagnostic results, results of therapeutic 131I scan, 131I accumulated dose, postoperative chest radiography findings, analysis of distant metastases assessed by noninvasive radiological and nuclear medical studies, treatment outcomes, cause of death, and survival status.
At the end of 2013, patients were categorized into the following groups: recurrence, nonrecurrence, and disease‐free. Patients in the nonrecurrence group included those with no recurrent thyroid cancer detected with 131I scintigraphy or other noninvasive examinations. The disease‐free group comprised patients with negative 131I WBS results, undetectable Tg in the absence of thyroxine treatment, undetectable anti‐Tg antibodies at final follow‐up, and no local or distant metastasis identifiable using noninvasive examinations 9. All data are expressed as mean ± standard error. Univariate and multivariate statistical analyses were performed to determine the significance of various factors, using the Kaplan–Meier method and log‐rank test 10. P values of <0.05 were considered statistically significant. In addition, survival rates were calculated using the Kaplan–Meier method and were compared using the Breslow and Mantel–Cox tests.
RESULTS
After a median follow‐up of 14.6 years, of the 909 patients with PTC, there were 187 (20.6%) patients with recurrent thyroid cancer, while 73 (8.0%) patients had died of thyroid cancer. Figure 1 shows the number of cases (left) and percentages (right) of patients with intra‐thyroid PTC, LN metastasis, soft tissue invasion, and distant metastasis, categorized by age. For the groups representing ages <21 years and >64 years, 17.8% and 16.3% of patients presented with LN metastasis, respectively.
Figure 1.
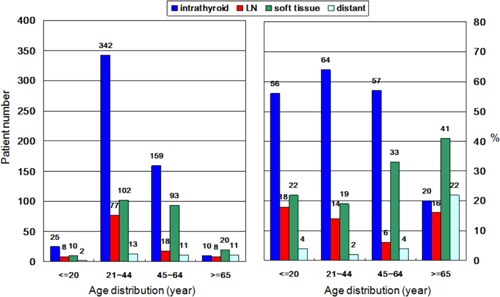
Number of cases (left) and percentages (right) of 909 patients with papillary thyroid carcinoma (PTC), stratified according to presence of intra‐thyroid PTC, lymph node metastasis, soft tissue invasion, or distant metastasis.
Comparing the 111 patients with PTC and LN metastasis with the 536 patients with intra‐thyroid PTC, the former group showed larger tumor size, higher postoperative Tg levels, advanced TNM stage, higher recurrence rates, and higher disease‐specific mortality (12.6% vs. 1.3%) (Table I). There were five patients with TNM stage IVA disease due to bilateral or contralateral cervical LN metastasis. Of the 111 patients with pathologically proven LN metastases, 93 (83.8%) patients underwent lateral LN dissection. There were nine cases without specified central or lateral dissection. There was no significant difference in age or sex between the two groups. Figure 2 illustrates the recurrence‐free survival curves of 909 patients with PTC stratified according to intra‐thyroid PTC, LN metastasis, soft tissue invasion, and distant metastasis. The recurrence‐free survival rates for the intra‐thyroid PTC, LN metastasis, soft tissue invasion, and distant metastasis groups were as follows: 96.8%, 76.6%, 80.7%, and 0.10% at 5 years; 95.7%, 71.2%, 72.6%, and 0% at 10 years; and 94.3%, 66.7%, 60.1%, and 0% at 20 years, respectively (Fig. 2).
Table I.
Clinical Features of Papillary Thyroid Cancer Groups Confined in Thyroid and Lymph Node Metastasis
| Clinical characteristic | Intrathyroid | Lymph node metastases | P‐value |
|---|---|---|---|
| Total patient | 536 | 111 | |
| Gender (Female) | 448 (83.9%) | 86 (77.5%) | 0.1231 |
| Age at diagnosis (year) | 38.9 ± 12.2 | 37.5 ± 15.5 | 0.3845 |
| Mean tumor size (cm) | 2.5 ± 0.1 | 2.9 ± 0.2 | 0.0108 |
| Thyroglobulin level postoperative 1 month (ng/ml) | 24.9 ± 11.2 | 68.6 ± 18.1 | 0.0001 |
| Multifocality | |||
| Single | 458 (85.5%) | 95 (85.6%) | 0.9701 |
| Multiple (≥2) | 78 (14.5%) | 16 (14.4%) | |
| Histological variant | – | ||
| Classical or FVPTC | 527 (98.3%) | 108 (97.3%) | 0.4420 |
| Tall cell variants | 9 (1.7%) | 3 (2.7%) | |
| Thyroidectomy (Total) | – | ||
| Total thyroidctomy only | 352 (65.7%) | – | |
| Lymph node dissectiona | 184 (34.3%) | 111 b | |
| Central | – | 9 (8.1%) | |
| Lateral | 93 (83.8%) | ||
| Nonspecified | 9 (8.1%) | ||
| TNM stage a | – | ||
| Stage I | 458 (85.4%) | 86 (77.5%) | |
| Stage II | 65 (12.1%) | – | |
| Stage III | 13 (2.4%) | 17 (15.3%) | |
| Stage IVA | 0 | 5 (4.5%) | |
| Follow‐up period (year) | 16.9 ± 0.2 | 16.9 ± 0.6 | 0.9288 |
| Postoperative 131I accumulative dose (mCi) | 130 ± 6.2 | 224 ± 26 | 0.0001 |
| Recurrence | 28 (5.2%) | 35 (31.5%) | 0.0001 |
| Mortality due to thyroid cancer | 7 (1.3%) | 14 (12.6%) | 0.0001 |
Three cases age over 45‐year‐old uncertained central or lateral dissection.
Including 12 cases with modified lateral neck dissection.
Figure 2.
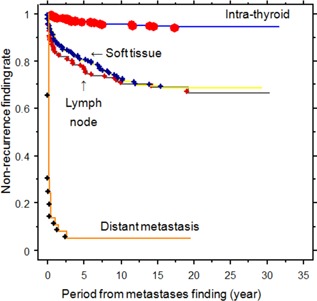
Recurrence‐free survival curves of 909 patients with PTC in patients with intra‐thyroid PTC, lymph node metastasis, soft tissue invasion, or distant metastasis (P = 0.0001 between intra‐thyroid vs. lymph node metastasis, soft tissue invasion, and distant metastasis; lymph node metastasis vs. distant metastasis; soft tissue invasion vs. distant metastasis; P = 0.6673 between lymph node metastasis vs. soft tissue invasion).
Of the 111 patients with PTC with LN metastases, 35 (31.5%) were diagnosed with recurrence during the mean follow‐up period of 16.9 ± 0.6 years. Of these, 23 (20.7%) patients with recurrent PTC underwent 1–4 repeat neck LN dissections. Among these 23 patients, 9 were diagnosed with co‐existent distant metastases; 7 of these patients died of thyroid cancer. Of the remaining 12 patients with recurrent PTC who did not undergo additional neck dissections, 11 were diagnosed with distant metastases. The mean period of recurrence after primary thyroidectomy with LN dissection was 3.4 ± 1.7 years, and ranged from 0.2 years to 19.2 years. Of the 35 patients with recurrent PTC, 14 (40.0%) died of thyroid cancer after a follow‐up of 15.0 ± 1.2 years. In contrast, five patients (14.3%) achieved disease‐free status. Considering the clinical features of patients with PTC with LN metastasis in the postoperative recurrence and nonrecurrence groups, the former showed older age, higher number of male patients, larger initial tumor size, and higher postoperative Tg levels.
At the end of the follow‐up period, 14 patients died from their thyroid cancer. The mortality group comprised older, mostly male patients who had presented with larger initial tumor sizes than the survival group (Table II). The mean follow‐up period from the time of first thyroidectomy to disease‐induced mortality in the mortality group was 9.4 ± 1.6 years (range: 16 months to 19.0 years). Of these 14 patients who died of thyroid cancer, 13 had developed distant metastases after total thyroidectomy with LN dissection. Figure 3 illustrates the disease‐specific survival rates in the PTC groups with intra‐thyroid occurrence, LN metastasis, soft tissue invasion, and distant metastases. The survival rates for the four thyroid cancer groups were as follows: 99.8%, 96.4%, 93.3%, and 51.4% at 5 years; 99.8%, 92.8%, 90.2%, and 45.9% at 10 years; and 99.1%, 83.7%, 84.3%, and 41.8% at 20 years, respectively. Further multivariate analysis for factors associated with recurrence and disease‐specific mortality revealed that age, male sex, and tumor size were significant independent factors (Tables III and IV).
Table II.
Survival and Mortality Patients of Papillary Thyroid Carcinoma With Lymph Node Metastases at Diagnosis
| Mortality | Survival | P‐value | |
|---|---|---|---|
| Number | 14 | 97 | |
| Age (year) | 63.3 ± 14.0 | 34.1 ± 11.8 | 0.0001 |
| Age (≥45, %) | 12 (85.7%) | 14 (14.4%) | 0.0001 |
| Gender (F, %) | 5 (35.7%) | 81 (85.5%) | 0.0001 |
| Tumor size | 4.6 ± 0.8 | 2.6 ± 0.1 | 0.0001 |
| Postoperative 1 month thyroglobulin (ng/ml) | 113 ± 59.0 | 62.5 ± 19.5 | 0.3636 |
| TNM stage (Stage I/II/III/IVA) a | 2/0/8/2 | 84/0/8/4 | – |
| Postoperative 131I accumulative dose (mCi) | 555 ± 141 | 183 ± 20 | 0.0001 |
| Disease‐free (%) | 0 | 44 (45.4%) | 0.0007 |
| Follow‐up period (year) | 9.4 ± 1.6 | 18.0 ± 0.5 | 0.0001 |
Three cases age over 45‐year‐old uncertained central or lateral dissection.
Figure 3.
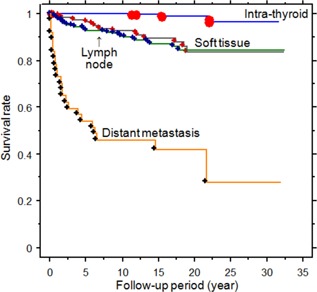
Disease‐specific survival curves in the groups with intra‐thyroid, lymph node metastasis, soft tissue invasion, or distant metastasis of PTC (P = 0.0005 between intra‐thyroid vs. lymph node metastasis; P = 0.0001 between intra‐thyroid vs. soft tissue invasion, and intra‐thyroid vs. distant metastasis; lymph node metastasis vs. distant metastasis; soft tissue invasion vs. distant metastasis; P = 0.6261 between lymph node metastasis vs. soft tissue invasion).
Table III.
Multivariate Analysis for Factors of Recurrence of Papillary Thyroid Carcinoma With Lymph Node Metastases at Diagnosis
| Coefficient | Standard error | Standard coefficient | P‐value | |
|---|---|---|---|---|
| Intercept | 0.6234 | 0.1558 | 0.6234 | 0.0001 |
| Age at diagnosis (year) | 0.0060 | 0.0029 | 0.1925 | 0.0443 |
| Sex (female/male) | 0.2134 | 0.1008 | 0.1928 | 0.0366 |
| Tumor size (cm) | 0.0632 | 0.0283 | 0.2134 | 0.0275 |
| Postoperative 1 month Tg* (ng/ml) | 0.0003 | 0.0002 | 0.1286 | 0.1638 |
Tg*, serum thyroglobulin.
Table IV.
Multivariate Analysis for Factors of Survival of Papillary Thyroid Carcinoma With Lymph Node Metastases at Diagnosis
| Coefficient | Standard error | Standard coefficient | P‐value | |
|---|---|---|---|---|
| Intercept | 0.2900 | 0.0845 | 0.2900 | 0.0009 |
| Age at diagnosis (year) | 0.0109 | 0.0016 | 0.4995 | 0.0001 |
| Sex (female/male) | 0.2375 | 0.0547 | 0.3033 | 0.0001 |
| Tumor size (cm) | 0.0476 | 0.0153 | 0.2273 | 0.0025 |
| Postoperative 1 month Tg* (ng/ml) | −0.0003 | 0.0001 | −0.0160 | 0.8201 |
Tg*, serum thyroglobulin.
DISCUSSION
Total or near‐total thyroidectomy is the standard surgical treatment for PTC with tumors >1 cm in size unless there are contraindications 11, 12. The value of prophylactic central LN dissection for cancer‐specific survival and tumor recurrence is a controversial issue 13, 14. The guidelines of the American Thyroid Association recommend the use of preoperative ultrasonography to clinically detect relevant nodal disease and support the conservative management of central LN dissection 11, 15. Furthermore, LN metastases are associated with increased post‐ablative Tg levels in PTC. Complete lymphadenectomy is associated with lowering of Tg levels 16. No prophylactic LN dissections were performed at our center. However, in a recent prospective randomized controlled study, micrometastatic lymph nodes were found during prophylactic central compartment dissection in 50% of PTC patients, but prophylactic LN dissection was not associated with differences in outcomes 17. The therapeutic outcomes of the PTC cohort evaluated herein are similar to those reported by a recent study from Chicago, with recurrence rates of 28% and cancer‐specific mortality of 8% after a median follow‐up of 27 years 2. All diagnoses of LN metastasis without soft tissue invasion in our study were pathologically confirmed, either during or after initial thyroidectomy and LN dissection.
Previous studies have indicated that LN metastasis of PTC may increase the incidence of recurrence; however, cancer‐related mortality was not increased 18, 19. Herein, patients with PTC and LN metastasis showed increased rates of recurrence and cancer mortality when compared to patients with intra‐thyroid PTC at 10‐year follow‐up. Aggressive surgical procedures, as well as postoperative 131I therapy and close monitoring by imaging, were indicated. Controversies remain concerning whether or not the number, size, LN compartments, and extra‐LN involvement influence the rates of recurrence and mortality in patients with PTC 20, 21, 22, 23. Compared with a recent investigation, our study showed lower rates of PTC recurrence with LN metastasis after LN dissection (31.5% vs. 34.7%), and longer follow‐up times 24. For cases with a clinically negative central compartment LN of PTC, low rates of recurrence (5.2%) and cancer mortality (1.3%) were observed after a mean follow‐up of 16.9 years. These values are marginally higher than those reported in a recent study with a median follow‐up period of 70 months at Memorial Sloan Kettering Cancer Center 25. In our disease‐specific survival and recurrence analyses, there were no differences between groups with LN metastasis or soft tissue invasion. Unlike a previous study, we did not separate the soft tissue invasion group into massive and minimal extension 26. In that study, massive extra‐thyroid extension was recognized as an independent prognostic factor for progressive‐free survival.
In the group showing recurrence after initial LN dissection, 15 of 35 patients (42.9%) developed distant metastases. By the end of 2013, 14 patients had died of thyroid cancer in our cohort. Of these, 12 patients presented with distant metastases. As reported in previous studies, older age, distant metastasis, or advanced locoregional extension are associated with poor prognosis in patients with PTC 27, 28. Bone metastases and radioactive iodine avidity have previously been implicated as independent prognostic factors 29. In our study, tumor size was an important factor for both LN metastasis and mortality outcomes. In contrast, according to a recent study, the postoperative serum Tg level was identified as an important factor of LN metastasis or poor outcome 30. In the present study, in patients with PTC and LN metastasis, Tg was higher in those experiencing cancer‐associated mortality than in survivors, although this difference was not statistically significant. In contrast, male sex was as an independent prognostic factor for mortality in the recurrent PTC group in our study. There is controversy surrounding the implication of male sex as a poor prognostic factor of recurrence and mortality in patients with PTC 31, 32. Considering the entire group of PTC patients, gender did not show a statistically significant difference in recurrence or disease‐specific mortality. In contrast, for specific high‐risk PTC patients such as those with LN metastasis, male sex correlated with poor prognosis 33. Further study is needed in order to resolve this issue. Reoperation has been suggested as a risk factor for disease‐specific mortality 34. As most reoperations were performed in cases of cancer recurrence, the reoperation group did not show increased mortality rates considering the disease‐specific mortality already present in the recurrent cases. In addition, the co‐existence of locoregional and distant metastases with older age may reduce the need for reoperation. A potential limitation in our study is the inclusion of patients with intra‐thyroid tumors who did not undergo prophylactic LN dissection. Thus, the presence of microscopic LN metastasis without enlarged LNs cannot be excluded. In addition, during the early period of this study, the compartments of LN dissection were not well recorded.
In conclusion, there was no significant difference in age or sex between intra‐thyroid tumors and those with LN metastasis among patients with PTC undergoing total thyroidectomy; however, patients with LN metastasis showed higher rates of recurrence and cancer‐related mortality after a follow‐up of 16.1 years. Cancer‐related mortality in patients with recurrent PTC was 40.0%. Furthermore, age and male sex were found to influence thyroid cancer‐related mortality, and a majority of the patients who died (13 of 14) had developed distant metastases.
SYNOPSIS
Of the 111 papillary thyroid carcinoma (PTC) cases with lymph node metastases, 35 were recurrent. Among the patients with recurrent PTC, 14 died of thyroid cancer. The mortality group was characterized by older, mostly male patients and with larger initial tumor size compared with survivors.
REFERENCES
- 1. Schneider DF, Chen H, Sippel RS: Impact of lymph node ratio on survival in papillary thyroid cancer. Ann Surg Oncol 2013; 20:1906–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grogan RH, Kaplan SP, Cao H, et al.: A study of recurrence and death from papillary thyroid cancer with 27 years of median follow‐up. Surgery 2013; 154:1436–1446. [DOI] [PubMed] [Google Scholar]
- 3. Ito Y, Kudo T, Kihara M, et al.: Improvement of lymph node recurrence rate, but not distant recurrence and carcinoma death rates, in patients with papillary thyroid carcinoma after disease‐free survival for 5 years. Endocr J 2012; 59:895–901. [DOI] [PubMed] [Google Scholar]
- 4. Smith VA, Sessions RB, Lentsch EJ: Cervical lymph node metastasis and papillary thyroid carcinoma: Does the compartment involved affect survival? Experience from the SEER database. J Surg Oncol 2012; 106:357–362. [DOI] [PubMed] [Google Scholar]
- 5. Lin JD, Kuo SF, Chao TC, et al.: Incidental and non‐incidental papillary thyroid microcarcinoma. Ann Surg Oncol 2008; 15:2287–2292. [DOI] [PubMed] [Google Scholar]
- 6. Sobin L.H, UICC: Thyroid gland—rules for classification In: Wittekind Ch, editor. TNM Classification of malignant tumors, 6th edition New York, Wiley‐Liss; 2002. pp. 52–56. [Google Scholar]
- 7. Delellis RA, Lloyd RV, Heitx PU, et al.: Pathology and genetics of tumors of endocrine organs. In World Health Organization of Tumours. IARC , Lyon 2004. pp. 73–76.
- 8. Huang BY, Lin JD, Chao TC, et al.: Therapeutic outcomes of papillary thyroid cancer patients in different risk groups. Oncology 2011; 80:123–129. [DOI] [PubMed] [Google Scholar]
- 9. Lin JD, Lin KL, Chao TC, et al.: Therapeutic outcome of papillary thyroid carcinoma advance than T1N0M0. Radiother Oncol 2008; 89:97–104. [DOI] [PubMed] [Google Scholar]
- 10. Zhang DD, Zhou XH, Freeman DH, et al.: A non‐parametric method for the comparison of partial areas under ROC curves and its application to large health care data sets. Stat Med 2002; 21:701–715. [DOI] [PubMed] [Google Scholar]
- 11. Cooper DS, Doherty GM, Haugen BR, et al.: American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009; 19:1167–1214. [DOI] [PubMed] [Google Scholar]
- 12. Pacini F, Schlumberger M, Dralle H, et al.: European Thyroid Cancer Taskforce. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur J Endocrinol 2006; 154:787–803. [DOI] [PubMed] [Google Scholar]
- 13. Zetoune T, Keutgen X, Buitrago D, et al.: Prophylactic central neck dissection and local recurrence in papillary thyroid cancer: a meta‐analysis. Ann Surg Oncol 2010; 17:3287–3293. [DOI] [PubMed] [Google Scholar]
- 14. Barczyński M, Konturek A, Stopa M, et al.: Prophylactic central neck dissection for papillary thyroid cancer. Br J Surg 2013; 100:410–418. [DOI] [PubMed] [Google Scholar]
- 15. Moreno MA, Edeiken‐Monroe BS, Siegel ER, et al.: In papillary thyroid cancer, preoperative central neck ultrasound detects only macroscopic surgical disease, but negative findings predict excellent long‐term regional control and survival. Thyroid 2012; 22:347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Low TH, Delbridge L, Sidhu S, et al.: Lymph node status influences follow‐up thyroglobulin levels in papillary thyroid cancer. Ann Surg Oncol 2008; 15:2827–2832. [DOI] [PubMed] [Google Scholar]
- 17. Viola D, Materazzi G, Valerio L, et al.: Prophylactic central compartment lymph node dissection in papillary thyroid carcinoma: Clinical implications derived from the first prospective randomized controlled single institution study. J Clin Endocrinol Metab 2015; 100:1316–1324. [DOI] [PubMed] [Google Scholar]
- 18. Lin JD, Liou MJ, Chao TC, et al.: Prognostic variable of papillary and follicular thyroid carcinomas with lymph node metastases and no distant metastases. Endocr‐Relat Cancer 1999; 6:109–115. [DOI] [PubMed] [Google Scholar]
- 19. Nixon IJ, Wang LY, Palmer FL, et al.: The impact of nodal status on outcome in older patients with papillary thyroid cancer. Surgery 2014; 156:137–146. [DOI] [PubMed] [Google Scholar]
- 20. Ryu IS, Song CI, Choi SH, et al.: Lymph node ratio of the central compartment is a significant predictor for locoregional recurrence after prophylactic central neck dissection in patients with thyroid papillary carcinoma. Ann Surg Oncol 2014; 21:277–283. [DOI] [PubMed] [Google Scholar]
- 21. Vas Nunes JH, Clark JR, Gao K, et al.: Prognostic implications of lymph node yield and lymph node ratio in papillary thyroid carcinoma. Thyroid 2013; 23:811–816. [DOI] [PubMed] [Google Scholar]
- 22. Randolph GW1, Duh QY, Heller KS, et al.: American Thyroid Association Surgical Affairs Committee's Taskforce on Thyroid Cancer Nodal Surgery. The prognostic significance of nodal metastases from papillary thyroid carcinoma can be stratified based on the size and number of metastatic lymph nodes, as well as the presence of extranodal extension. Thyroid 2012; 22:1144–1152. [DOI] [PubMed] [Google Scholar]
- 23. Merdad M, Eskander A, Kroeker T, et al.: Metastatic papillary thyroid cancer with lateral neck disease: pattern of spread by level. Head Neck 2013; 35:1439–1442. [DOI] [PubMed] [Google Scholar]
- 24. Conzo G, Docimo G, Pasquali D, et al.: Predictive value of nodal metastases on local recurrence in the management of differentiated thyroid cancer. Retrospective clinical study. BMC Surg 2013; 13:S3. doi: 10.1186/1471‐2482‐13‐S2‐S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nixon IJ, Ganly I, Patel SG, et al.: Observation of clinically negative central compartment lymph nodes in papillary thyroid carcinoma. Surgery 2013; 154:1166–1172. [DOI] [PubMed] [Google Scholar]
- 26. Ito Y, Tomoda C, Uruno T, et al.: Prognostic significance of extrathyroid extension of papillary thyroid carcinoma: massive but not minimal extension affects the relapse‐free survival. World J Surg 2006; 30:780–786. [DOI] [PubMed] [Google Scholar]
- 27. Verburg FA, Mäder U, Tanase K, et al.: Life expectancy is reduced in differentiated thyroid cancer patients ≥ 45 years old with extensive local tumor invasion, lateral lymph node, or distant metastases at diagnosis and normal in all other DTC patients. J Clin Endocrinol Metab 2013; 98:172–180. [DOI] [PubMed] [Google Scholar]
- 28. Lin JD, Chao TC, Chou SC, et al.: Papillary thyroid carcinomas with lung metastases. Thyroid 2004; 14:1091–1096. [DOI] [PubMed] [Google Scholar]
- 29. Lang BH, Wong KP, Cheung CY, et al.: Evaluating the prognostic factors associated with cancer‐specific survival of differentiated thyroid carcinoma presenting with distant metastasis. Ann Surg Oncol 2013; 20:1329–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee CW, Roh JL, Gong G, et al.: Risk factors for recurrence of papillary thyroid carcinoma with clinically node‐positive lateral neck. Ann Surg Oncol 2015; 22:117–124. [DOI] [PubMed] [Google Scholar]
- 31. Pathak KA, Mazurat A, Lambert P, et al.: Prognostic nomograms to predict oncological outcome of thyroid cancers. J Clin Endocrinol Metab 2013; 98:4768–4775. [DOI] [PubMed] [Google Scholar]
- 32. Karatzas T, Vasileiadis I, Kapetanakis S, et al.: Risk factors contributing to the difference in prognosis for papillary versus micropapillary thyroid carcinoma. Am J Surg 2013; 206:586–593. [DOI] [PubMed] [Google Scholar]
- 33. Toniato A, Boschin I, Casara D, et al.: Papillary thyroid carcinoma: Factors influencing recurrence and survival. Ann Surg Oncol 2008; 15:1518–1522. [DOI] [PubMed] [Google Scholar]
- 34. Young S, Harari A, Smooke‐Praw S, et al.: Effect of reoperation on outcomes in papillary thyroid cancer. Surgery 2013; 154:1354–1361. [DOI] [PubMed] [Google Scholar]


