Abstract
BACKGROUND
Metrafenone has been used in Europe in integrated pest management programmes since 2006 to control powdery mildews, including Erysiphe necator. Its exact mode of action is not known, but it is unique among fungicide classes used in powdery mildew management. Recently, resistance to metrafenone was reported in Blumeria graminis f. sp. tritici. In this study we investigated metrafenone resistance in Erysiphe necator in northern Italy.
RESULTS
Metrafenone efficacy to control grapevine powdery mildew was monitored in three consecutive years in the field, and its reduced activity was observed in 2013. Out of 13 monoconidial isolates, two sensitive strains were identified, which did not grow at the fungicide concentration recommended for field application. The remaining strains showed variable response to metrafenone, and five of them grew and sporulated similarly to the control, even at 1250 mg L−1 of metrafenone. Moreover, the resistant strains showed cross‐resistance to pyriofenone, which belongs to the same FRAC group as metrafenone.
CONCLUSION
The results indicate the emergence of metrafenone resistance in an Italian population of Erysiphe necator. Further studies are needed to gain insight into the metrafenone's mode of action and to understand the impact of resistance on changes in the pathogen population structure, fitness and spread of resistant strains, which will be indicative for designing appropriate antiresistance measures. © 2015 The Authors. Pest Management Science published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
Keywords: Uncinula necator, Oidium tuckeri, fungicide resistance, benzophenone, pyriofenone, Vitis vinifera
1. INTRODUCTION
Grapevine powdery mildew, caused by the obligate biotrophic fungus Erysiphe necator Schwein. (previously Uncinula necator (Schwein.) Burrill; anamorph Oidium tuckeri Berk.), is one of the most important fungal pathogens of cultivated grapevine worldwide.1, 2 It infects all green parts of the plant, such as leaves, shoots, flowers and fruit clusters.2, 3
The control of E. necator in Italian vineyards follows principles of integrated pest management (IPM), which combines the use of chemicals with agronomical practices.4 However, the use of fungicides remains the main and the most effective means to control powdery mildew epidemics. They are usually applied in preventive programmes. The treatments start as early as the 3–5‐leaf stage of shoot development (BBCH stage 13–15) if the epidemic risk is high and associated with the pathogen overwintering in dormant buds or conditions favourable for ascospore infections, and continue until grape veraison (BBCH stage 81–83), which implies a minimum of 4–8 fungicide treatments during the growing season.5
The Fungicide Resistance Action Committee (FRAC) classifies commercial fungicides into different groups based on their mode of action.6 The majority of modern fungicides have a single‐site mode of action, which greatly increases the risk of resistance in diverse pathogen populations. Therefore, their use is limited to 2–3 applications per season, which implies the availability of a large number of different fungicide groups. In Italy, fungicides belonging to ten different FRAC groups are currently on the market to control grapevine powdery mildew in conventional agriculture.
E. necator is listed as a medium‐risk pathogen by FRAC, but as a high‐risk pathogen by the European and Mediterranean Plant Protection Organisation (EPPO).7 It has developed resistance to several fungicide groups, including methyl benzimidazole carbamates (MBCs, FRAC group 1), demethylation inhibitors (DMIs, FRAC group 3), azanaphthalenes (FRAC group 13) and quinone outside inhibitors (QoIs, or strobilurins, FRAC group 11).8, 9, 10, 11, 12, 13 In the case of QoIs and DMIs, the main molecular mechanisms of their modes of action are known, and diverse mutations responsible for the resistance have already been described.10, 14, 15, 16
However, identification of resistant isolates does not always lead to field resistance.17 To prevent or delay the selection and spreading of resistant isolates in the pathogen population, implementation of appropriate antiresistance management strategies is of utmost importance. One of the key strategies is the rotation of fungicides with different modes of action.18
Metrafenone (3‐bromo‐2′,3′,4′,6‐tetramethoxy‐2,6′‐dimethylbenzophenone) is a fungicide active against diverse powdery mildews, including grapevine powdery mildew. It is representative of the chemical class benzophenones, and together with a benzoylpyridine pyriofenone belongs to the U8 FRAC group.6 Its exact mode of action is not known; however, early studies on barley powdery mildew (Blumeria graminis Speer f. sp. hordei Marchal) and wheat powdery mildew (Blumeria graminis Speer f. sp. tritici Marchal) suggest that it interferes with hyphal morphogenesis, polarised hyphal growth and the establishment and maintenance of cell polarity.19, 20 Metrafenone has a unique mode of action, different from other fungicides used in powdery mildew management, as demonstrated by the absence of cross‐resistance with other known chemical classes, and therefore it represents a valuable choice in fungicide rotation programmes.14, 19, 20 Recently, metrafenone‐resistant isolates of B. graminis f. sp. tritici were detected in an extensive monitoring study of cereal powdery mildews; however, its performance in the field was not reduced.21 Until now, this is the only report of reduced efficacy of metrafenone in the field.
Our objective was to verify the efficacy of metrafenone to control grapevine powdery mildew pathogen, E. necator, after 8 years of its extensive use in Italian vineyards. For this purpose we monitored the efficacy of metrafenone to contain powdery mildew epidemics in a vineyard in Timoline, Brescia, in northern Italy, in three consecutive years. Furthermore, we determined the response of E. necator isolates, collected subsequently from the experimental vineyard, to elevated concentrations of metrafenone in terms of mycelium growth and sporulation. Finally, we monitored the presence of metrafenone‐resistant isolates in the Franciacorta area in 2013 and 2014.
2. MATERIALS AND METHODS
2.1. Disease epidemics and metrafenone activity in the field
Biological activity of metrafenone was assessed in a vineyard in Timoline (Franciacorta area, Italy) during the years 2011–2013. The vineyard was planted with Vitis vinifera cultivar ‘Chardonnay’, cordon trained and spur pruned, with a planting density of 6250 plants ha−1.
The experimental scheme consisted of randomised blocks with four replicas, and each plot included 12 plants grown on a surface of approximately 18 m2 (8.8 × 2 m). Downy mildew was managed using Forum MZ (9.0 g ha−1 of dimethomorf + 60.0 g ha−1 of mancozeb; BASF, Ludwigshafen, Germany) and Pergado MZ (50 g L−1 of mandipropamid + 600 g L−1 of mancozeb; Syngenta, Basel, Switzerland), applied at rates of 220 and 250 g ha−1 respectively.
To assess the biological activity of metrafenone, Vivando (500 g AI L−1; BASF) was applied preventively, before the appearance of powdery mildew symptoms, starting from BBCH stage 13–15 (3–5 fully unfolded leaves), at a rate of 250 mL ha−1 with an interval of 10–12 days. The disease progress in the untreated control and in metrafenone‐treated plots was monitored periodically from bud‐break to veraison by inspecting 100 leaves or grape clusters per plot.22 The disease incidence (DI) was expressed as the percentage of infected leaves or grape clusters. The disease severity (DS) was calculated according to the Townsend–Heuberger formula,23 using eight classes of severity index based on the surface of the organ colonised by the pathogen. Subsequently, protection indices for disease incidence and severity were calculated.24
2.2. Sample collection and maintenance of E. necator strains in the laboratory
In 2013, infected leaves and grape clusters were collected from the vineyard in Timoline from the untreated control and metrafenone‐treated plots, and ten monoconidial isolates were obtained in the laboratory (Table 1). Three additional monoconidial isolates of E. necator were obtained from a commercial vineyard in the Franciacorta area, 2.5 km from the Timoline vineyard, to verify whether the metrafenone resistance was more widely spread in the area. The infected tissues were observed under a dissecting microscope, and single conidial chains were transferred from the infected leaf to a newly formed leaf of V. vinifera cv. ‘Chardonnay’ grown in the laboratory as described previously.25
Table 1.
Erysiphe necator strains collected from the experimental vineyard in Timoline from control or metrafenone‐treated plots, and from commercial vineyards in the Franciacorta area in 2013
| Strain | Locality | Treatment | Organ infected |
|---|---|---|---|
| 1C | Timoline | Control | Leaves |
| 2C | Timoline | Control | Leaves |
| 3C | Timoline | Control | Grape clusters |
| 4C | Timoline | Control | Grape clusters |
| 1M | Timoline | Metrafenone | Grape clusters |
| 2M | Timoline | Metrafenone | Leaves |
| 3M | Timoline | Metrafenone | Grape clusters |
| 4M | Timoline | Metrafenone | Grape clusters |
| 5M | Timoline | Metrafenone | Leaves |
| 6M | Timoline | Metrafenone | Leaves |
| 1F | Franciacorta | Unknown | Grape clusters |
| 2F | Franciacorta | Unknown | Leaves |
| 3F | Franciacorta | Unknown | Grape clusters |
In 2014, the vineyard in Timoline changed from IPM to biological management, and the use of synthetic fungicides was discontinued. Powdery‐mildew‐infected leaves were collected, and single conidial chains from sporulating colonies of E. necator were directly transferred onto leaves preventively treated with metrafenone (125 mg L−1) or onto untreated leaves, and incubated in Petri plates containing water–agar medium (8 g L−1 of agar bacteriology grade; Applichem, Darmstadt, Germany) added with 5 mg L−1 of tetracycline. The growth of resistant isolates was verified after 2 weeks of incubation in a growth chamber with a 16 h day cycle at 24 °C.
The strains were routinely maintained on leaves of V. vinifera plants, cv. ‘Chardonnay’, grown from seeds in laboratory conditions. Leaves from 4–5‐week‐old plants were surface sterilised for 15 s in 70% ethanol and for 1 min in 0.5% hypochlorite and rinsed 3 times in sterile water. Leaves were dried with paper tissues and placed in Petri plates containing water–agar medium. The plates were cultivated in a growth chamber with a 16 h day cycle at 24 °C. Strains were transferred to fresh leaves every 2 weeks.
2.3. Mycelium growth and sporulation of E. necator exposed and not exposed to metrafenone in the laboratory
The strains of E. necator, collected in 2013, were grown for 2 weeks as described previously to sporulate abundantly. Four‐week‐old plantlets of Vitis vinifera cv. ‘Chardonnay’ were sprayed with two different concentrations of metrafenone: 125 mg L−1 (field treatment concentration) or 1250 mg L−1 (10 times the field treatment concentration) and were left to air dry for 2 h. Control plants were sprayed with water. The plants were subsequently inoculated by depositing spores of E. necator on the two youngest leaves at 4 points per leaf. The inoculated plants were incubated for 2 weeks, and the mycelium growth was assessed 14 days after inoculation. The colony growth was estimated by transferring the dimensions of the colony observed under the dissecting microscope to millimetre graph paper and then calculating the colony area using the program ImageJ.26, 27 Sporulation was evaluated 14 days after inoculation: the entire colony was cut out and put in an Eppendorf tube containing 100 µL of 0.9% NaCl + 0.02% Tween 80, and the number of conidia µL−1 was counted with a haemocytometer after vortexing. The sporulation (Sp) was expressed as conidia cm−2: Sp = N *100/A 14, where N is the number of conidia µL−1, and A 14 is the area of the colony 14 days after inoculation. The experiments were repeated 2 times.
2.4. Cross‐resistance
We studied the cross‐resistance of E. necator metrafenone‐resistant strains to pyriofenone (90 mg L−1, Kusabi; Belchim, Londerzeel, Belgium), a fungicide belonging to the same U8 FRAC group as metrafenone. Moreover, we tested two additional fungicides widely used in powdery mildew management, belonging to the QoI and DMI groups: azoxystrobin (175 mg L−1, Quadris; Syngenta) and myclobutanil (60 mg L−1, Thiocur Forte; Dow AgroSciences, Indianapolis, IN), respectively. All fungicides were tested at recommended concentrations for field application. One metrafenone‐sensitive strain (2C) and two resistant strains (1M and 2M) of E. necator were grown for 2 weeks to sporulate abundantly as described previously. Four‐week‐old plantlets of Vitis vinifera cv. ‘Chardonnay’ were treated with the recommended field concentrations of the above‐listed fungicides, or metrafenone (125 mg L−1), and were left to air dry for 2 h. Control plants were treated with water. The plants were subsequently inoculated with spores of E. necator using an inoculation tower and air current. Inoculated plants were incubated in a growth chamber for 12–14 days, and disease severity was determined by estimating the percentage of the leaf surface colonised by sporulating colonies of E. necator. The experiments were repeated 2 times.
2.5. Statistical analyses
The statistical analyses were performed using R software, v.R3.0.2.28 The percentage data of disease incidence and disease severity in control and metrafenone‐treated plots were square root arcsine transformed and submitted to ANOVA. Similarly, ANOVA was performed for mycelium growth and sporulation data, followed by Tukey's post hoc test for multiple comparison (P = 0.05), using the TukeyC package.29
3. RESULTS
3.1. Powdery mildew epidemics in 2011–2013
During the 3 years of the study in the Timoline field, the dynamics of powdery mildew epidemics was variable. The disease dynamics was monitored in the control plots from bud‐break (mid‐April) to veraison (mid‐July) (Fig. 1). The 2011 season was characterised by conditions unsuitable for disease development. The disease progress was slow, resulting in 8% of grape clusters infected in mid‐July, while the disease was almost absent on leaves. In 2012, the first symptoms appeared at the end of May, and afterwards the disease incidence increased constantly. In mid‐July, almost 100% of grape clusters and ca 60% of leaves were infected, with a disease severity index of ca 50%. In 2013, the disease incidence had the fastest progress, and in mid‐July almost 90% of leaves and 100% of grape clusters were infected.
Figure 1.
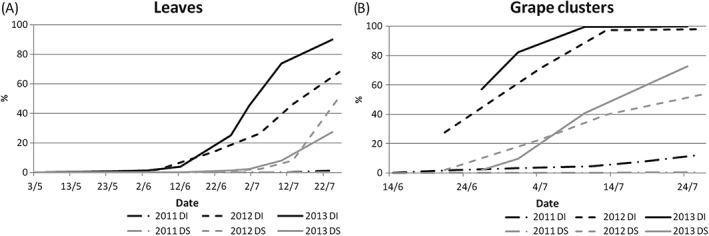
Epidemics of Erysiphe necator during 2011–2013 in Timoline, Brescia, Italy. The dynamics of disease incidence (DI, black) and disease severity (DS, grey) on leaves (A) and grape clusters (B) was recorded each year from the beginning of May to mid‐July.
3.2. Biological activity of metrafenone in the field
Powdery mildew incidence and severity were evaluated on leaves and grape clusters of control plots and plots treated with metrafenone in mid‐July in 2011–2013, and the respective protection indices were calculated (Fig. 2). Control plots were heavily infected in 2012 and 2013, with the disease particularly serious on grape clusters, while it was sporadic in 2011.
Figure 2.
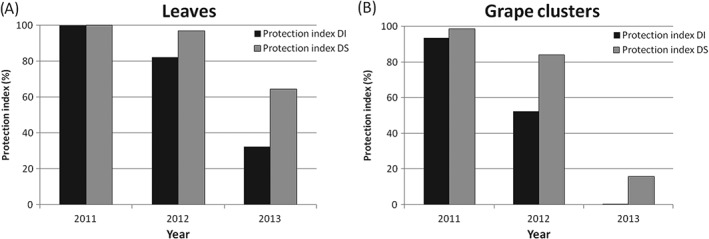
Metrafenone protection indices for disease incidence (DI, black) and disease severity (DS, grey) on leaves (A) and grape clusters (B) in mid‐July, in years 2011–2013, in Timoline, Brescia, Italy.
In 2011, metrafenone showed 100% efficacy on leaves and 93.5 and 98.7% protection of grape clusters in terms of disease incidence and severity, respectively. In 2012, metrafenone protected grape clusters by 52.2% (DI) and 84.0% (DS) compared with the control, and showed even better protection on leaves. Finally, the 2013 season was even more favourable for disease epidemic development, with approximately 90% of leaves and 100% of grape clusters infected by E. necator in control plots by mid‐July. Surprisingly, metrafenone did not prevent disease development on grape clusters, with results similar to those of the control (0.5 and 15.8% protection for disease incidence and severity, respectively), and only partially protected leaves (32.3 and 64.5% protection for disease incidence and severity, respectively). In 2011 and 2012, metrafenone treatment significantly reduced disease incidence and severity on leaves and grape clusters by comparison with the control. On the other hand, metrafenone treatment did not significantly reduce disease incidence and severity in 2013, which were similar to the control (data not shown).
3.3. Identification of metrafenone‐resistant strains in the laboratory
Owing to the surprisingly low efficacy of metrafenone observed in the Timoline field in 2013, infected leaves and grape clusters were collected from the experimental vineyard, and ten monoconidial isolates of E. necator were obtained (Table 1). Three additional monoconidial strains were isolated from a commercial vineyard in the Franciacorta area. We previously determined that the sensitivity of strains collected before 2013 to metrafenone in terms of mycelium growth and sporulation was lower than 12.5 mg L−1, the concentration that inhibited mycelium growth by 95% and completely inhibited sporulation (data not shown).
To assess the sensitivity of the 13 putatively resistant strains collected in 2013, mycelium growth and sporulation of strains grown on plants treated with metrafenone at the concentration used in the field (125 mg L−1) and at a 10 times higher concentration (1250 mg L−1) were compared with the untreated control. All isolates grew abundantly on control plants; after 14 days of growth, their average colony area was 89.4 mm2 (standard deviation SD = 39.9), and they produced on average 2212.1 spores cm−2 (SD = 1668.8). Two out of 13 strains were metrafenone sensitive (2C and 3C) and did not grow on metrafenone‐treated plants (Tables 2 and 3). Four strains (1M, 3M, 4M and 3F) grew and sporulated equally to the control at both metrafenone concentrations. The remaining tested strains also grew and sporulated on metrafenone‐treated plants, but to a lower extent than on control plants, especially at a concentration of 1250 mg L−1.
Table 2.
Mycelium growth of Erysiphe necator strains on control and metrafenone‐treated plants
| Strain | Mycelium growth (mm2) | ||
|---|---|---|---|
| Control | Metrafenone | ||
| 125 mg L−1 | 1250 mg L−1 | ||
| 1C | 87.04 (38.65)a nsb | 93.98 (26.02) ns | 72.05 (33.98) ns |
| 2C | 69.13 (19.57) a | 0 (0) b | 0 (0) b |
| 3C | 42.64 (16.6) a | 0 (0) b | 0 (0) b |
| 4C | 67.01 (17.68) ns | 76.92 (23.60) ns | 69.4 (13.22) ns |
| 1M | 72.88 (31.79) ns | 73.15 (21.81) ns | 57.58 (15.83) ns |
| 2M | 111.11 (40.55) a | 110.63 (38.89) a | 72.95 (16.95) b |
| 3M | 99.93 (26.55) ns | 109.83 (42.89) ns | 97.57 (28.34) ns |
| 4M | 80.24 (12.74) ns | 76.03 (4.99) ns | 76.8 (19.47) ns |
| 5M | 79.12 (35.59) b | 117.31 (14.13) a | 97.03 (8.77) ab |
| 6M | 154.62 (59.36) a | 140.03 (26.61) ab | 98.85 (22.91) b |
| 1F | 84.52 (33.77) a | 43.91 (12.18) b | 32.4 (6.73) b |
| 2F | 90.05 (33.8) a | 52.98 (21.78) b | 54.27 (19.97) b |
| 3F | 107.88 (42.62) ns | 101.06 (35.75) ns | 82.74 (17.49) ns |
The mean value followed by SD.
Tukey's post hoc test; means in a row with the same letters are not significantly different (P = 0.05); ns = not significant.
Table 3.
Sporulation of Erysiphe necator strains on control and metrafenone‐treated plants
| Strain | Sporulation (conidia cm−2) | ||
|---|---|---|---|
| Control | Metrafenone | ||
| 125 mg L−1 | 1250 mg L−1 | ||
| 1C | 2694.05 (1341.83)a nsb | 1954.94 (1215) a | 758.64 (66.05) b |
| 2C | 5112.21 (1622.79) a | 0 (0) b | 0 (0) b |
| 3C | 1227.32 (839.33) a | 0 (0) b | 0 (0) b |
| 4C | 3736.81 (2374.32) a | 2677.56 (1864.2) ab | 1106.15 (336.59) b |
| 1M | 1687.92 (1303.42) ns | 1346.67 (1059.37) ns | 1236.17 (1057.67) ns |
| 2M | 2696.87 (1303.33) a | 2992.07 (1507.14) a | 931.78 (735.28) b |
| 3M | 1460.06 (1029.66) ns | 1966.89 (1325.06) ns | 2773.22 (2529.84) ns |
| 4M | 1523.18 (497.11) ns | 1272.06 (468.82) ns | 1039.67 (721.71) ns |
| 5M | 1325.15 (1436.85) a | 2416.44 (726.21) ab | 973.17 (429.13) b |
| 6M | 2725.8 (1070.9) ns | 3488.3 (1337.86) ns | 2337.69 (1129.79) ns |
| 1F | 1465.04 (1105.83) a | 375.95 (210.03) b | 239.25 (302.26) b |
| 2F | 1357.28 (1287.46) ns | 683.75 8 (583.86) ns | 940.36 (1014.41) ns |
| 3F | 960.11 (563.63) ns | 517.3 (264.18) ns | 778.37 (543.71) ns |
The mean value followed by SD.
Tukey's post hoc test; means in a row with the same letters are not significantly different (P = 0.05); ns = not significant.
In 2014, single conidial chains from sporulating colonies collected in the field were placed directly to germinate on metrafenone‐treated and untreated leaves. Two out of ten conidia developed colonies on control leaves, indicating a 20% infection rate. In contrast, from 120 conidia transferred to metrafenone‐treated (125 mg L−1) leaves, only two potentially resistant colonies were obtained (8.3% infection rate).
3.4. Cross‐resistance
The metrafenone‐sensitive strain (2C) grew and sporulated abundantly on control plants, while it was efficiently controlled by metrafenone and the other tested fungicides. On the other hand, the two resistant strains (1M and 2M) grew abundantly on control and metrafenone‐treated plants, with 100% of the leaf surface covered by sporulating colonies (Figs 3A and B). Complete colonisation of the leaf surface was also observed for pyriofenone‐treated plants, which confirmed our expectations of cross‐resistance between metrafenone and pyriofenone (Fig. 3C). However, the growth of metrafenone‐resistant strains was fully controlled by the other two fungicides representative of the QoI and DMI groups (azoxystrobin and myclobutanil, respectively), which indicates the absence of cross‐resistance with these groups (Figs 3D and E).
Figure 3.
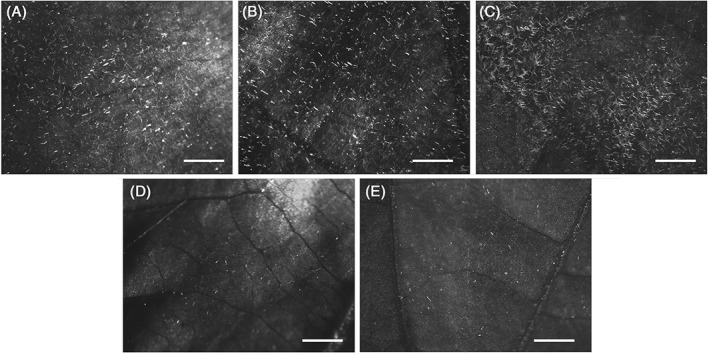
Cross‐resistance of E. necator metrafenone‐resistant strain 1M sporulating on leaves treated with water (A), metrafenone (B) and pyriofenone (C), and inhibition of growth of E. necator 1M on leaves treated with azoxystrobin (D) and myclobutanil (E). Leaves were observed under a dissecting microscope at a magnification of 20×. The bar represents 1 mm.
4. DISCUSSION
Powdery mildew is one of the major diseases of grapevine worldwide. Although cultural practices and biological control help to reduce the severity of epidemics, its management relies on the use of fungicides.1 However, resistant strains of Erysiphe necator to several major fungicide classes, including QoIs and DMIs, have been described.10, 12, 13 QoIs are typical fungicides with a single‐site mode of action, and resistance often leads to suboptimal control or complete loss of activity in the field.14, 18 On the other hand, resistance to DMIs is thought to be oligogenic and quantitative, and resistance levels in the field are often low and only rarely result in control failure.30 Moreover, the use of synthetic pesticides is becoming severely limited owing to the new European Union strategy, which heavily restricts the registration and use of chemicals and favours alternative methods.31, 32 In this situation, reduced efficacy of a fungicide to control powdery mildew epidemics represents a serious problem, as it may imply resistance in the pathogen population, which could ultimately lead to loss of efficacy of the entire fungicide group.
Metrafenone was registered in Europe in 2006 to manage powdery mildews and eye spot disease (Oculimacula spp.) epidemics in cereals, and since then it has been used extensively, especially on cereals, cucurbits and grapevine. The first report of resistance to metrafenone in Blumeria graminis f. sp. tritici appeared in the literature only after 3 years of its use, and 3.9% of tested isolates were classified as moderately or highly resistant, while no resistance was observed in B. graminis f. sp. hordei.21
In our study we investigated the sensitivity of E. necator to metrafenone in the Franciacorta area in northern Italy. We demonstrated the reduced efficacy of metrafenone to control grapevine powdery mildew in the field in 2013, as there were no differences observed in disease incidence and severity between the untreated control and the metrafenone‐treated plots. Based on this evidence, 13 putatively resistant samples were collected in 2013 from the experimental vineyard and an additional commercial vineyard in the Franciacorta area, to determine their response to metrafenone in the laboratory. Metrafenone does not inhibit the germination of E. necator spores, but blocks further development beyond the formation of appressoria (data not shown);20 therefore, its activity was assessed in terms of pathogen mycelium growth and sporulation. Examples from other fungicide‐resistant pathogens indicate that strains with lower resistance factors are still well controlled by the recommended field doses of the fungicides;10, 33, 34 however, 85% of the strains obtained in this study showed moderate to high resistance to metrafenone, with the estimated resistance factor higher than 100. In fact, all resistant strains also grew and sporulated at 1250 mg metrafenone L−1, a concentration 10 times higher than the recommended field dose. The detection of two additional resistant isolates in 2014, even after metrafenone treatments were discontinued, indicates that metrafenone‐resistant strains persisted in the experimental vineyard also in the absence of selection. We hypothesise that the low frequency of their recovery might have been caused by a fitness penalty in comparison with metrafenone‐sensitive strains, or by an influx of sensitive isolates from surrounding vineyards.
The cross‐resistance studies indicate that metrafenone‐resistant strains are still controlled by fungicides representative of two groups widely used in powdery mildew management: strobilurins (azoxystrobin) and DMIs (myclobutanil). However, our data indicate cross‐resistance with pyriofenone, a new fungicide belonging to the same U8 FRAC group as metrafenone.35 These results further confirm that the two fungicides most likely act in the same molecular pathway, hypothesised to be involved in actin localisation or hyphal morphogenesis.19
In contrast to QoIs or DMIs, where mutations causing the resistance are known, the monitoring for metrafenone‐resistant strains remains difficult, as no quick PCR‐based methods are available.14, 18, 36 It has been hypothesised that metrafenone compromises hyphal tip organisation via disruption of signal transduction, involving Rho or Ras GTP‐ases.17, 19 Our identification of metrafenone‐resistant strains provides a valuable tool for studying the possible mechanism of action also by transcriptome analysis and using specific microsatellite markers.37, 38
To our knowledge, this is the first paper to report the loss of metrafenone efficacy in the field owing to resistance in E. necator. Previously, one E. necator metrafenone‐resistant strain was detected in a monitoring study in 2010; however, resistance of this strain decreased rapidly after several transfers, indicating that adaptation was not stable or that the strain was a mix of sensitive and adapted isolates, and that the sensitive isolates increasingly dominated the population during propagation steps in the following year.39 Further studies are needed to understand the implications for metrafenone use in IPM programmes. Reduced fitness has been observed in fungicide‐resistant strains of some pathogens;40, 41 however, in most cases mutations conferring fungicide resistance do not cause any obvious fitness penalty.42, 43 Study of the fitness of metrafenone‐resistant strains will be indicative of possible re‐evaluation and decisions about the use of metrafenone in grape‐growing areas. E. necator populations can vary from clonal to highly genetically diverse and randomly mating.44, 45, 46 Populations with mixed mating (sexual and asexual), high genetic flow, large effective population size and high mutation rates have higher potential for breaking down the resistance.47 The analysis of E. necator population structure and the genetic diversity of resistant strains could highlight the association of certain haplotypes with resistance traits, and contribute to understanding whether the resistant strains have the potential to spread in the pathogen population.
ACKNOWLEDGEMENTS
The authors would like to thank Dr Erica Debenedetti for her help with laboratory‐based experiments.
REFERENCES
- 1. Gadoury DM, Cadle‐Davidson L, Wilcox WF, Dry IB, Seem RC and Milgroom MG, Grapevine powdery mildew (Erysiphe necator): a fascinating system for the study of the biology, ecology and epidemiology of an obligate biotroph. Mol Plant Pathol 13:1–16 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Glawe DA, The powdery mildews: a review of the world's most familiar (yet poorly known) plant pathogens. Annu Rev Phytopathol 46:27–51 (2008). [DOI] [PubMed] [Google Scholar]
- 3. Reuveni M, Activity of trifloxystrobin against powdery and downy mildew diseases of grapevines. Can J Plant Pathol 23:52–59 (2001). [Google Scholar]
- 4. Fernandez‐Cornejo J, Environmental and economic consequences of technology adoption: IPM in viticulture. Agric Econ 18:145–155 (1998). [Google Scholar]
- 5. Caffi T, Rossi V, Legler SE and Bugiani R, A mechanistic model simulating ascosporic infections by Erysiphe necator, the powdery mildew fungus of grapevine. Plant Pathol 60:522–531 (2011). [Google Scholar]
- 6. FRAC Code List 2014: Fungicides Sorted by Mode of Action (Including FRAC Code Numbering) [Online]. FRAC (2014). Available: http://www.frac.info [1 February 2015].
- 7. FRAC Pathogen Risk List [Online]. FRAC (2014). Available: http://www.frac.info [1 February 2015].
- 8. Pearson RC, Benomyl‐resistant strains of Uncinula necator on grapes. Plant Dis 64:677 (1980). [Google Scholar]
- 9. Garibaldi A, McKenzie LI and Gullino ML, Comparsa in Italia di una popolazione di Uncinula Necatrix (Schw.) Burr. che presenta ridotta sensibilità verso alcuni inibitori della biosintesi degli steroli. Giornate Fitopatologiche: Protezione delle Piante, Qualita, Ambiente, Pisa, Italy, pp. 143–150 (1990).
- 10. Délye C, Laigret F and Corio‐Costet MF, A mutation in the 14 alpha‐demethylase gene of Uncinula necator that correlates with resistance to a sterol biosynthesis inhibitor. Appl Environ Microbiol 63:2966–2970 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Genet J‐L and Jaworska G, Baseline sensitivity to proquinazid in Blumeria graminis f. sp. tritici and Erysiphe necator and cross‐resistance with other fungicides. Pest Manag Sci 65:878–84 (2009). [DOI] [PubMed] [Google Scholar]
- 12. Wilcox W, Burr J, Riegel D and Wong F, Practical resistance to QoI fungicides in New York populations of Uncinula necator associated with quantitative shifts in pathogen sensitivities. Phytopathology 93:S90 (2003). [Google Scholar]
- 13. Green EA and Gustafson GD, Sensitivity of Uncinula necator to quinoxyfen: evaluation of isolates selected using a discriminatory dose screen. Pest Manag Sci 62:492–497 (2006). [DOI] [PubMed] [Google Scholar]
- 14. Miles LA, Miles TD, Kirk WW and Schilder AMC, Strobilurin (QoI) resistance in populations of Erysiphe necator on grapes in Michigan. Plant Dis 96:1621–1628 (2012). [DOI] [PubMed] [Google Scholar]
- 15. Frenkel O, Cadle‐Davidson L, Wilcox WF and Milgroom MG, Mechanisms of resistance to an azole fungicide in the grapevine powdery mildew fungus, Erysiphe necator. Phytopathology 105:370–377 (2015). [DOI] [PubMed] [Google Scholar]
- 16. Leroux P and Walker A‐S, Multiple mechanisms account for resistance to sterol 14α‐demethylation inhibitors in field isolates of Mycosphaerella graminicola . Pest Manag Sci 67:44–59 (2011). [DOI] [PubMed] [Google Scholar]
- 17. Hollomon DW, New modes of action contribute to resistance management, in Fungicide Resistance in Crop Protection: Risk and Management, ed. by Thind TS. CABI, Wallingford, Oxon, UK, pp. 104–115 (2011). [Google Scholar]
- 18. Brent KJ and Hollomon DW, Fungicide Resistance in Crop Pathogens: How Can it be Managed? Fungicide Resistance Action Committee (Croplife International), Brussels, Belgium: (2007). [Google Scholar]
- 19. Opalski KS, Tresch S, Kogel K‐H, Grossmann K, Köhle H and Hückelhoven R, Metrafenone: studies on the mode of action of a novel cereal powdery mildew fungicide. Pest Manag Sci 62:393–401 (2006). [DOI] [PubMed] [Google Scholar]
- 20. Schmitt MR, Carzaniga R, Cotter HVT, O'Connell R and Hollomon D, Microscopy reveals disease control through novel effects on fungal development: a case study with an early‐generation benzophenone fungicide. Pest Manag Sci 62:383–392 (2006). [DOI] [PubMed] [Google Scholar]
- 21. Felsenstein F, Semar M and Stammler G, Sensitivity of wheat powdery mildew (Blumeria graminis f. sp. tritici) towards metrafenone. Gesunde Pfl 62:29–33 (2010). [Google Scholar]
- 22. Cortesi P, Bisiach M, Ricciolini M and Gadoury DM, Cleistothecia of Uncinula necator – an additional source of inoculum in Italian vineyards. Plant Dis 81:922–926 (1997). [DOI] [PubMed] [Google Scholar]
- 23. Townsend G and Heuberger J, Methods for estimating losses caused by diseases in fungicide experiments. Plant Dis Rep 27: 340–343 (1943). [Google Scholar]
- 24. Abbott WS, A Method of computing the effectiveness of an insecticide. J Econ Entomol 18:265–267 (1925). [Google Scholar]
- 25. Cortesi P, Ottaviani MP and Milgroom MG, Spatial and genetic analysis of a flag shoot subpopulation of Erysiphe necator in Italy. Phytopathology 94:544–550 (2004). [DOI] [PubMed] [Google Scholar]
- 26. Rasband W, ImageJ 1997–2012. [Online]. US National Institutes of Health, Bethesda, MD (2012). Available: http://imagej.nih.gov/ij/ [1 February 2015]. [Google Scholar]
- 27. Schneider CA, Rasband WS and Eliceiri KW, NIH Image to ImageJ: 25 years of image analysis. Nat Meth 9:671–675 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. R: A Language and Environment for Statistical Computing R Core Team, The R Foundation for Statistical Computing, Vienna, Austria: (2013). [Google Scholar]
- 29. Faria JC, Jelihovschi EG and Allaman IB, Conventional Tukey Test. Universidade Estadual de Santa Cruz – UESC, Ilheus, Bahia, Brazil: (2014). [Google Scholar]
- 30. Cools HJ, Hawkins NJ and Fraaije BA, Constraints on the evolution of azole resistance in plant pathogenic fungi. Plant Pathol 62:36–42 (2013). [Google Scholar]
- 31. Steel CC, Blackman JW and Schmidtke LM, Grapevine bunch rots: impacts on wine composition, quality, and potential procedures for the removal of wine faults. J Agric Food Chem 61:5189–5206 (2013). [DOI] [PubMed] [Google Scholar]
- 32. Jess S, Kildea S, Moody A, Rennick G, Murchie AK and Cooke LR, European Union policy on pesticides: implications for agriculture in Ireland. Pest Manag Sci 70:1646–1654 (2014). [DOI] [PubMed] [Google Scholar]
- 33. Mutations Associated with QoI‐Resistance [Online]. FRAC (2006). Available: http://www.frac.info [1 February 2015].
- 34. Kunova A, Pizzatti C, Bonaldi M and Cortesi P, Sensitivity of nonexposed and exposed populations of Magnaporthe oryzae from rice to tricyclazole and azoxystrobin. Plant Dis 98:512–518 (2014). [DOI] [PubMed] [Google Scholar]
- 35. Ogawa M, Nieto J and Ruggiero P, Pyriofenone: nuovo fungicida antioidico per la difesa della vite Giornate Fitopatologiche: Protezione delle Colture, Qualità, Ambiente, Milano Marittima, Ravenna, Italy, pp. 201–206 (2012). [Google Scholar]
- 36. Dufour MC, Fontaine S, Montarry J and Corio‐Costet MF, Assessment of fungicide resistance and pathogen diversity in Erysiphe necator using quantitative real‐time PCR assays. Pest Manag Sci 67:60–69 (2011). [DOI] [PubMed] [Google Scholar]
- 37. Wakefield L, Gadoury DM, Seem RC, Milgroom MG, Sun Q and Cadle‐Davidson L, Differential gene expression during conidiation in the grape powdery mildew pathogen, Erysiphe necator. Phytopathology 101:839–846 (2011). [DOI] [PubMed] [Google Scholar]
- 38. Frenkel O, Portillo I, Brewer MT, Péros JP, Cadle‐Davidson L and Milgroom MG, Development of microsatellite markers from the transcriptome of Erysiphe necator for analysing population structure in North America and Europe. Plant Pathol 61:106–119 (2012). [Google Scholar]
- 39. Stammler G, Semar M, Strobel D, Resistance management of metrafenone in powdery mildews, in Modern Fungicides and Antifungal Compounds, Vol. VII, ed. by Dehne HW, Deising HB, Fraaije B, Gisi U, Hermann D, Mehl A. et al. Deutsche Phytomedizinische Gesellschaft, Braunschweig, Germany, pp. 179–184, ISBN: 978‐3‐941261‐13‐6 (2014). [Google Scholar]
- 40. Karaoglanidis GS, Thanassoulopoulos CC and Ioannidis PM, Fitness of Cercospora beticola field isolates resistant and sensitive to demethylation inhibitor fungicides. Eur J Plant Pathol 107:337–347 (2001). [Google Scholar]
- 41. Saito S, Cadle‐Davidson L and Wilcox WF, Selection, fitness, and control of grape isolates of Botrytis cinerea variably sensitive to fenhexamid. Plant Dis 98:233–240 (2014). [DOI] [PubMed] [Google Scholar]
- 42. Rallos LEE, Johnson NG, Schmale DG, Prussin AJ and Baudoin AB, Fitness of Erysiphe necator with G143A‐based resistance to quinone outside inhibitors. Plant Dis 98:1494–1502 (2014). [DOI] [PubMed] [Google Scholar]
- 43. Petit A‐N, Vaillant‐Gaveau N, Walker A‐S, Leroux P, Baillieul F, Panon M‐L et al., Effects of fudioxonil on Botrytis cinerea and on grapevine defence response. Phytopathol Mediterr 50:130–138 (2011). [Google Scholar]
- 44. Cortesi P, Mazzoleni A, Pizzatti C and Milgroom MG, Genetic similarity of flag shoot and ascospore subpopulations of Erysiphe necator in Italy. Appl Environ Microbiol 71:7788–7791 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Brewer MT, Cadle‐Davidson L, Cortesi P, Spanu PD and Milgroom MG, Identification and structure of the mating‐type locus and development of PCR‐based markers for mating type in powdery mildew fungi. Fungal Genet Biol 48:704–713 (2011). [DOI] [PubMed] [Google Scholar]
- 46. Cortesi P, Pizzatti C, Bertocchi D and Milgroom MG, Persistence and spatial autocorrelation of clones of Erysiphe necator overwintering as mycelium in dormant buds in an isolated vineyard in northern Italy. Phytopathology 98:148–152 (2008). [DOI] [PubMed] [Google Scholar]
- 47. McDonald BA and Linde C, Pathogen population genetics, evolutionary potential, and durable resistance. Annu Rev Phytopathol 40:349–379 (2002). [DOI] [PubMed] [Google Scholar]


