Abstract
Western-style high fat, high sugar diets are associated with non-alcoholic fatty liver disease (NAFLD) and increased liver cancer risk. Sulforaphane from broccoli may protect against these. Previously we initiated broccoli feeding to mice prior to exposure to the hepatocarcinogen diethylnitrosamine (DEN), and saw protection against NAFLD and liver cancer. Here we administered DEN to unweaned mice, initiating broccoli feeding two weeks later, to determine if broccoli protects against cancer progression. Specifically, male 15-day-old C57BL/6J mice were given DEN and placed on a Western or Western+10%Broccoli diet from the age of 4 weeks through 7 months. Dietary broccoli decreased hepatic triacylglycerols, NAFLD, liver damage and tumour necrosis factor by month 5 without changing body weight or relative liver weight, but did not slow carcinogenesis, seen in 100% of mice. We conclude that broccoli, a good source of sulforaphane, slows progression of hepatic lipidosis, but not tumourigenesis in this robust model.
Keywords: Fatty liver disease, liver cancer, Western diet, broccoli, diethylnitrosamine
1. Introduction
In the United States, more than one-third of the adult population is obese (Ogden, Carroll, Kit, & Flegal, 2014). Excess intake of energy (typically from saturated fats and sugars) favours the development of obesity because of the loss of energy balance (Spiegelman & Flier, 2001). Liver is the major metabolic centre for both carbohydrate and lipid, thereby maintaining glucose homeostasis (Postic, Dentin, & Girard, 2004). An overload of dietary carbohydrate and fat disturbs the metabolic function of the liver and may lead to non-alcoholic fatty liver disease (NAFLD), a cluster of related liver diseases that begins with simple steatosis, developing into non-alcoholic steatohepatitis (NASH), cirrhosis, and possibly hepatocellular carcinoma (HCC) (Angulo, 2002; Cohen, Horton, & Hobbs, 2011). Compared to non-obese people, the risk for hepatic steatosis is almost 5-fold higher in obese people (Bellentani et al., 2000). For obese males, their mortality risks from liver cancer are 4.5-fold higher than men with a normal BMI (Calle, Rodriguez, Walker-Thurmond, & Thun, 2003).
Broccoli, like other brassica vegetables, is rich in many bioactive compounds, including flavonoids, ascorbate, and especially the glucosinolate glucoraphanin (Jeffery et al., 2003). Glucosinolates are stable, sulphur-rich glycosides, until they are hydrolyzed by plant- or microbiome-derived myrosinase to form bioactive aglycones (Angelino et al., 2015). Sulforaphane, the aglycone of the broccoli glucosinolate glucoraphanin, activates nuclear factor erythroid-2-related factor (Nrf2), which in turn upregulates detoxification, ameliorates inflammation, and induces apoptosis (Jeffery & Araya, 2009).
To evaluate the relation between diet and liver diseases, including NAFLD and liver cancer, several animal models are available. We recently fed broccoli to mice receiving either a control or Western diet (WD), giving the hepatocarcinogen diethylnitrosamine (DEN) one week after initiating dietary treatments (Chen, Wallig, & Jeffery, 2016). At six months, hepatic lipidosis was lessened and that there was a small but significant protective effect against liver cancer in those mice fed broccoli. These findings are in agreement with those of Kay and colleagues who found that Nrf2 upregulated by sulforaphane, inhibits LXRα-dependent hepatic lipogenesis (Kay et al., 2011). Multiple mechanisms have been proposed for protection against cancer by sulforaphane, with Nrf2 playing a key role. Our recent study is the first to show protection against liver cancer (Chen, Wallig, & Jeffery, 2016). However, most studies, including ours, do not identify whether the impact of dietary broccoli was on the initiation or progression of these liver diseases. The present study, a longitudinal, diet-facilitated (WD) and chemical-induced (DEN) liver cancer model, in which DEN was administered before dietary intervention, was used to evaluate progression of hepatic lipidosis and cancer through 7 months, separate from initiation. Our aim was to evaluate the role of broccoli in protection against progression of NAFLD and liver cancer.
2. Material and Methods
2.1. Animal and study design
Six to eight week-old dams and male C57BL/6J mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA) for breeding. All mice used for breeding were maintained on rodent chow diet; except during gestation and lactation where the dams were fed AIN-93G. Fifteen-day-old male pups were used in this longitudinal study, given 25 mg DEN/kg body weight intraperitoneally in normal saline. The DEN-treated mice were housed individually, and randomly assigned to WD or Western+Broccoli diet (WBD) groups at 4 weeks of age, and given ad libitum access to water and feed. Feed was replaced daily. Animals were maintained under a 12-hour light/dark cycle at 22 and 60% humidity. Animal care was in compliance with the approved protocol by the Institutional Animal Care and Use Committee and the Division of Animal Resources at the University of Illinois, Urbana-Champaign, according to the National Institutes of Health guidelines. Six animals from each dietary group were sacrificed at 3, 5, and 7 months after starting the dietary treatments, for evaluating the progression of hepatic lipidosis and tumorigenesis. Body weight and feed intake were monitored each week.
2.2. Reagents and diets
Diethylnitrosamine was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Western diet was formulated by modifying AIN-93G to increase the sucrose and saturated fat content (Supplemental Table 1). Freeze dried broccoli powder (Brassica oleracea L. var. Green Magic), was kindly provided by Dr. John A. Juvik, which contained 4 mmol sulforaphane/kg broccoli powder, determined by hydrolysis of endogenous glucoraphanin, as previously reported (Dosz & Jeffery, 2013). Other diet ingredients were purchased from the Harlan Laboratory (Indianapolis, IN, USA). Broccoli powder (10%, w/w) was incorporated into the WBD, which was balanced nutritionally by partly replacing the corn starch and cellulose with the fiber and carbohydrate from broccoli (Supplemental Table 1).
2.3. Hepatic triglyceride content
Liver lipid was extracted by the method of Folch (Folch, Lees, & Stanley, 1957) with some modifications. Liver tissue was homogenized with chloroform/methanol (2:1, v/v) with 0.01% butylated hydroxytoluene (BHT). Homogenates were washed with ultrapure water and centrifuged for the separation of organic and water phases. The chloroform phase was heated to 50 under a stream of nitrogen gas, for evaporation. The remaining lipid extract was stored at −20°C for determining hepatic triacylglycerol concentrations by the glyceride phosphate oxidase method using a reagent set (Pointe Scientific, Canton, MI, USA).
2.4. Hepatic lesion detection and NAFLD score
At sacrifice, livers were weighed and examined for macroscopic lesions. Visible nodules (diameter ≥ 1 mm) in each liver lobe were counted and the maximum diameter was measured using calipers. Mouse liver (median lobe) was fixed in 10% neutral buffered formalin for later paraffin section. Sections (3 μm) were stained with hematoxylin and eosin (H&E) for histological examination. All histology work was done at the Veterinary Diagnostic Laboratory (University of Illinois, Urbana, IL, USA). Liver sections with H&E staining were examined for microscopic lesions, including altered hepatic foci (AHF), hepatic adenomas (HA), and HCC, and NAFLD scores. All pathology was carried out by a board-certified veterinary pathologist; the scoring criteria are shown in Supplemental Table 2.
2.5. Serum alanine aminotransferase (ALT)
Serum ALT levels were determined using the Liquid ALT Reagent Kit (Pointe Scientific, Canton, MI, USA), according to manufacturer's instructions.
2.6. Quinone oxidoreductase activity
Hepatic cytosolic fractions were prepared and NAD(P)H: quinone oxidoreductase 1 (NQO1) activity was performed as previously reported (Lai, Keck, Walling, West, & Jeffery, 2008).
2.7. Real time quantitative PCR
Total hepatic RNA was extracted from liver with Trizol Reagent (Life Technologies, Carlsbad, CA, USA), following the manufacturer's instructions. Complementary DNA was synthesized from RNA samples for probe-based (6-FAM/ZEN/IBFQ) real time quantitative PCR. PrimeTime qPCR 5’Nuclease primer and probe sets (Integrated DNA Technologies, Coralville, IA, USA) and TaqMan Universal PCR Master Mix (Life Technologies, Carlsbad, CA, USA) were used for PCR procedures. Murine Cd68, interferon-γ (Ifng), tumor necrosis factor-α (Tnf), Nqo1, and glyceraldehyde 3-phosphate dehydrogenase (Gapdh; as a housekeeping control) expression were quantified. Primer sets and probe sequences are shown in Supplemental Table 3. Thermal cycler procedures were run using the 7900HT Fast Real-Time PCR System (Life Technologies, Carlsbad, CA, USA).
2.8. Statistical analysis
Results are presented as mean ± standard deviation (SD). Time and diet (dietary broccoli) effects were evaluated by two-way analysis of variance (ANOVA) with Statistical Analysis System 9.4 Software (SAS Institute Inc., Cary, NC, USA), followed by Fisher's least significant difference test when differences were indicated. A P value of less than 0.05 was considered significant in this study.
3. Results
3.1. Body weight was not changed by long-term broccoli consumption
There was no difference in body weight or relative liver weight, between WD and WBD groups, throughout the study (Table 1). Although body weights increased with time, the effect of dietary broccoli on body weight was not significant (Table 1). Relative liver weight showed an increase with time and there was a time x diet interaction, indicating the WBD increased relative liver weight by month 7 (Table 1).
Table 1.
Body Weight and Hepatic Biomarker1
| Objective outcomes | Month 3 |
Month5 |
Month 7 |
P (2-way ANOVA) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| WD | WBD | WD | WBD | WD | WBD | Time | Diet | Time*Diet | |
| Body weight [g] | 39.5 ± 3.7b | 39.9 ± 4.6b | 47.0 ± 3.7a | 46.0 ± 2.6a | 48.9 ± 3.4a | 50.5 ± 3.1a | < 0.0001 | NS | NS |
| Liver [%]1 | 5.2 ± 1.3c | 5.4 ± 1.1c | 7.2 ± 0.9ab | 6.4 ± 1.2bc | 6.4 ± 1.4bc | 8.5 ± 1.7a | 0.0013 | NS | 0.0326 |
| Hepatic TG [mg] | 132 ± 37c | 112 ± 51c | 261 ± 60a | 149 ± 65bc | 231 ± 120ab | 306 ± 92a | 0.0006 | NS | 0.0296 |
| NAFLD score | 1.5 ± 0.5b | 0.5 ± 0.5c | 2.0 ± 0.0ab | 0.5 ± 0.5c | 2.5 ± 0.5a | 1.5 ± 0.5b | < 0.0001 | < 0.0001 | NS |
| ALT [IU/L] | 16.8 ± 3.1c | 16.1 ± 3.7c | 48.3 ± 9.2a | 30.3 ± 6.3b | 42.1 ± 9.8a | 50.7 ± 14.2a | < 0.0001 | NS | 0.0080 |
| QR activity [nmol MTT]2 | 28.9 ± 7.2e | 33.3 ± 7.1de | 44.0 ± 5.9bc | 40.7 ± 8.7cd | 52.2 ± 12.8b | 65.4 ± 6.1a | < 0.0001 | NS | NS |
Data are presented as mean±SD, n=6. Values on the same row not sharing a common letter are significantly different. P<0.05 is considered statistically significant. ALT, alanine aminotransferase; NAFLD, non-alcoholic fatty liver disease; NS, not significant; QR, quinone reductase; TG, triglycerides; WBD, Western+Broccoli diet; WD, Western diet.
Percent body weight.
Specific activity, nmol thiazolyl blue tetrazolium bromide (MTT)/min per mg protein.
3.2. Dietary broccoli decreased hepatic triglyceride accumulation and NAFLD scores
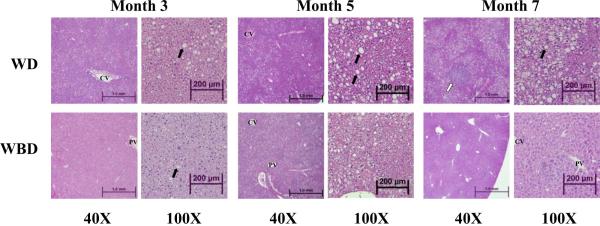
Hepatic triglycerides were increased from month 3 to month 5 and reached a plateau between months 5 and 7 with WD treatment (Table 1). In mice receiving the WBD, hepatic triglycerides were lower than in those mice receiving the WD at 5 months, but this effect was no longer evident by month 7 (Table 1). Histological evaluation showed changes over time in liver morphology associated with hepatic lipidosis, first impacting livers of WD-fed mice and later WBD-fed mice, with lower NAFLD scores in the WBD mice at all times evaluated (Table 1). Nevertheless, NAFLD scores increased with time for both dietary groups. Representative images are shown in Figure 1.
Fig. 1.
Effect of dietary broccoli on hepatic lipodosis in Western diet-fed mice. Liver sections were stained with hematoxylin and eosin. Representative images from each group at medium and high magnification are shown 40X and 100X magnifications. Clear, round vacuoles (closed arrows) indicate triglyceride accumulation. Basophilic cell focus (open arrow) is an altered hepatic focus. CV, central vein; PV, portal vein; WBD, Western+Broccoli diet; WD, Western diet.
3.3. Dietary broccoli ameliorated hepatocyte damage
At month 3, serum ALT levels were low and were not altered by broccoli, suggesting no significant liver damage (Table 1). Although all mice exhibited elevated serum ALT by 5 months, mice receiving dietary broccoli had lower mean serum ALT, indicating a protective effect (Table 1). However, the positive effect from broccoli was lost by 7 months, as indicated by the time x diet interaction in serum ALT (Table 1). Unexpectedly, hepatic NQO1, a downstream product of Nrf2 activation, increased with time in both groups. However, this increase was not significantly greater in the WBD group compared to the WD group until month 7, estimated as both enzyme activity and gene expression (Tables 1 and 2).
Table 2.
Hepatic Gene Expression
| Hepatic gene expression | Month 3 |
Month5 |
Month 7 |
P (2-way ANOVA) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| WD | WBD | WD | WBD | WD | WBD | Time | Diet | Time*Diet | |
| Nqo1 | 1.0 ± 0.2b | 1.1 ± 0.2b | 1.4 ± 0.1b | 1.4 ± 0.5b | 1.3 ± 0.2b | 2.2 ± 0.8a | 0.0008 | NS | 0.0136 |
| Ifng | 1.0 ± 0.3ab | 0.7 ± 0.1b | 1.1 ± 0.5ab | 0.9 ± 0.3ab | 1.1 ± 0.4ab | 1.6 ± 0.6a | 0.0407 | NS | NS |
| Cd68 | 1.0 ± 0.1bc | 0.9 ± 0.2c | 1.4 ± 0.2ab | 1.1 ± 0.3bc | 1.5 ± 0.4a | 1.5 ± 0.4a | 0.0006 | NS | NS |
| Tnf | 1.0 ± 0.2b | 0.9 ± 0.1b | 1.9 ± 0.7a | 0.9 ± 0.2b | 1.8 ± 0.6a | 1.2 ± 0.5b | 0.0125 | 0.0017 | NS |
Data are shown as fold change from the Month 3-WD group and presented as mean ± SD, n=6. Values on the same row not sharing a common letter are significantly different. P<0.05 is considered statistically significant. NS, not significant; WBD, Western+Broccoli diet; WD, Western diet.
3.4. Long-term broccoli consumption exerted a mild protective effect on inflammation but not on hepatic tumorigenesis
There were no macroscopic or microscopic lesions in livers of mice at month 3, but grossly, hepatic nodules were visible with maximum hepatic nodule size associated with dietary broccoli treatment (Table 3). However, by 5 months, the mice receiving the WD exhibited HA, whereas there were no HA in the WBD-fed mice (Table 3). Although there were HA in the WD-fed group and not in the WBD-fed group at 5 months, HCC occurred in both groups by 7 months (Table 3). All animals exhibited visible hepatic nodules of all types by month 7, with increased numbers and sizes compared to month 5 (Table 3).
Table 3.
Macroscopic and Microscopic Hepatic Neoplasm-Related Lesions
| Hepatic neoplasm-related lesions | Month 3 |
Month5 |
Month 7 |
P (2-way ANOVA) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| WD | WBD | WD | WBD | WD | WBD | Time | Diet | Time*Diet | |
| Macroscopic lesions | |||||||||
| Nodule incidence1 | 0/6 | 0/6 | 5/6 | 3/6 | 6/6 | 6/6 | -- | -- | -- |
| Max nodule diameter [mm]2 | -- | -- | 1.8 ± 1.1c | 4.8 ± 2.0b | 5.4 ± 1.8b | 11.1 ± 1.9a | < 0.0001 | 0.0002 | NS |
| Average nodule number1 | 0.0 ± 0.0c | 0.0 ± 0.0c | 4.0 ± 3.9ab | 1.2 ± 1.6bc | 20.2 ± 10.0bc | 34.8 ± 18.0a | 0.0013 | NS | 0.0326 |
| Microscopic lesions | |||||||||
| AHF incidence | 0/6 | 0/6 | 4/6 | 4/6 | 5/6 | 5/6 | -- | -- | -- |
| HA incidence | 0/6 | 0/6 | 1/6 | 0/6 | 3/6 | 0/6 | -- | -- | -- |
| HCC incidence | 0/6 | 0/6 | 0/6 | 0/6 | 1/6 | 2/6 | -- | -- | -- |
Data are presented as mean±SD, n=6. Values on the same row not sharing a common letter are significantly different. P<0.05 is considered statistically significant. NS, not significant; WBD, Western+Broccoli diet; WD, Western diet.
Visible nodules (diameter ≥ 1 mm).
Mean maximum nodule diameter.
With regards to markers of inflammation, evaluation of hepatic macrophage activation showed that expression of the macrophage activator, Infg, and activated macrophage marker, Cd68, were not influenced by dietary broccoli. However, the increase seen over time in hepatic Tnf expression in the WD-fed mice was not evident in the WBD-fed mice (Table 2).
4. Discussion
Steatosis, also known as lipidosis, is an early change in the progression of NAFLD (Cohen et al., 2011). Mice receiving dietary broccoli exhibited less severe hepatic lipidosis (Table 1). However, an increase in hepatic NQO1 activity and expression was not evident until month 7, suggesting broccoli may influence lipid metabolism and antioxidant enzymes via different mechanisms. Alternatively, there may be an interactive effect among dietary broccoli, high dietary fat, and age to produce change in the Nrf2 pathway. It has been reported that a high fat diet decreases hepatic Nqo1 expression after 4 weeks of feeding, with the opposite effect reported after 25 weeks of feeding (Chartoumpekis et al., 2013; Tanaka et al., 2008). Moreover, hepatic NQO1 activity decreases with age in rodents (Shih & Yen, 2007). However, this study design could not distinctly differentiate among the impact of diet and age.
The mechanism by which broccoli regulates lipid metabolism is not yet established. Recently it was shown that a broccoli diet could lower plasma LDL cholesterol in humans, suggesting that hepatic trafficking of lipids may be altered by broccoli (Armah et al., 2015). Our previous study using mice support this finding, showing decreased lipidosis by supplemental dietary broccoli in mice consuming a Westernized diet (Chen, Wallig, & Jeffery, 2016).
Studies using purified sulforaphane, a key bioactive component of broccoli, suggest that sulforaphane regulates lipid metabolism via the 5′ AMP-activated protein kinase (AMPK) signaling pathway. Sulforaphane has been observed to enhance hormone-sensitive lipase activity by inhibiting the phosphorylation of AMPK at Thr-172 in 3T3-L1 cells (Lee et al., 2012). However, in a whole animal study, sulforaphane inhibited lipogenesis in adipose tissue by enhancing the phosphorylation of AMPK and acetyl CoA carboxylase (Choi et al., 2014). Even though in vitro and in vivo study results are inconsistent, these studies do suggest that dietary broccoli may alter lipogenesis and lipolysis pathways, consistent with our observation. Clearly more studies are needed to determine the impact of broccoli and broccoli components on lipid metabolism.
The dose of sulforaphane may influence pathway involvement, leading to different outcomes for different doses. In the in vitro study that resulted in inhibition of AMPK phosphorylation, relatively high concentrations of sulforaphane (2.5-10 μM) were used (Lee et al., 2012), whereas in the in vivo study where there was enhanced phosphorylation, the mice were provided approximately 20 μmole sulforaphane/day in the diet, which likely resulted in less than 2 μM in circulation based on a similar study (Choi et al., 2014; Hu et al., 2004). In our study, mice only received approximately 2 μmole sulforaphane/day, which might be much closer to a low dose-long term treatment that favors the activation of AMPK for improved hepatic lipid control.
In this study we used a WD-facilitated and DEN-induced model to evaluate the impact of broccoli on the progression of both hepatic lipidosis and carcinogenesis. However, because here tumorigenesis was initiated by the administration of carcinogen, this model does not fully represent the slow development of NAFLD-associated diseases that start with lipidosis, continue with development of NASH, and finally progress to liver cancer.
Bioactivation of DEN is mainly carried out by cytochrome P450 2E1, of which there is an abundance in mouse liver and which is up-regulated by high dietary fat (Kang, Wanibuchi, Morimura, Gonzalez, & Fukushima, 2007; Matsuzawa-Nagata et al., 2008). However in this study, bioactivation was completed prior to initiating the WD, which therefore only served as a promoter.
Our result shows that post-DEN dietary broccoli treatment during the progression phase was not able to reverse the carcinogenic process initiated by DEN treatment in infant mice, which is robust and has been reported to cause more than 80% liver tumor incidence in mice received a normal diet (Park et al., 2010). Also unexpectedly, the WBD-fed mice had larger mean maximum hepatic nodule size compared to the WD-fed mice (Table 3). Similar results were observed in our previous in vitro study showing that low doses of sulforaphane (0.5-1 μM) may stimulate the proliferation of the human HCC cell line HepG2, even though larger doses (5–10 μM) were found to inhibit proliferation (Harris & Jeffery, 2008). The actual mechanism of this dual effect on proliferation is still unclear. Overall, this specific DEN mouse model of hepatocarcinogenesis, in which the impact of broccoli was separated from any impact on bioactivation of the carcinogen, shows that dietary broccoli temporarily slowed, but could not overcome the effects of DEN and a WD on tumor progression.
5. Conclusion
Dietary broccoli suppressed development of lipidosis in animals receiving a Western-style diet rich in refined carbohydrate and saturated fat. After initiation of hepatic tumorigenesis by DEN, consumption of broccoli temporarily slowed liver damage and inflammation, but was not able to impede the progression of liver tumor development in mice. Further studies are needed to show if these effects are due to sulforaphane upregulation of Nrf2.
Supplementary Material
Highlights.
Broccoli decreases the accumulation of hepatic triglycerides.
Liver inflammation and damage are partially ameliorated by dietary broccoli.
Post-carcinogen dietary broccoli is not able to impede liver cancer in mice.
Acknowledgements
This work was supported by National Cancer Institute (E.H.J., grant number 5RO3CA162539-02). The authors thank Dr. Edward B. Dosz for his assistance with the quantification of sulforaphane content in broccoli samples.
Abbreviations
- AHF
Altered hepatic foci
- ALT
alanine aminotransferase
- AMPK
5′ AMP-activated protein kinase
- Cyp
cytochrome P450
- DEN
diethylnitrosamine
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- HA
hepatic adenomas
- HCC
hepatocellular carcinomas
- IFNg
interferon-γ
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- Nrf2
Nuclear factor erythroid-2-related factor
- NQO1
NAD(P)H-quinone oxidoreductase 1
- TNF
tumor necrosis factor
- WBD
Western+Broccoli diet
- WD
Western diet
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
None.
References
- Angelino D, Dosz EB, Sun J, Hoeflinger JL, Van Tassell ML, Chen P, Harnly JM, Miller MJ, Jeffery EH. Myrosinase-dependent and -independent formation and control of isothiocyanate products of glucosinolate hydrolysis. Frontiers in Plant Science. 2015;6:831. doi: 10.3389/fpls.2015.00831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angulo P. Nonalcoholic fatty liver disease. The New England Journal of Medicine. 2002;346(16):1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- Armah CN, Derdemezis C, Traka MH, Dainty JR, Doleman JF, Saha S, Leung W, Potter JF, Lovegrove JA, Mithen RF. Diet rich in high glucoraphanin broccoli reduces plasma LDL cholesterol: Evidence from randomised controlled trials. Molecular Nutrition and Food Research. 2015;59(5):918–926. doi: 10.1002/mnfr.201400863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellentani S, Saccoccio G, Masutti F, Croce LS, Brandi G, Sasso F, Cristanini G, Tiribelli C. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Annals of Internal Medicine. 2000;132(2):112–117. doi: 10.7326/0003-4819-132-2-200001180-00004. [DOI] [PubMed] [Google Scholar]
- Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. The New England Journal of Medicine. 2003;348(17):1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- Chartoumpekis DV, Ziros PG, Zaravinos A, Iskrenova RP, Psyrogiannis AI, Kyriazopoulou VE, Sykiotis GP, Habeos IG. Hepatic gene expression profiling in Nrf2 knockout mice after long-term high-fat diet-induced obesity. Oxidative Medicine and Cellular Longevity. 2013;2013:340731. doi: 10.1155/2013/340731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Wallig MA, Jeffery EH. Dietary broccoli ameliorates development of fatty liver and cancer in mice given diethylnitrosamine with or without a Western diet. Journal of Nutrition. 2016 doi: 10.3945/jn.115.228148. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KM, Lee YS, Kim W, Kim SJ, Shin KO, Yu JY, Lee MK, Lee YM, Hong JT, Yun YP, Yoo HS. Sulforaphane attenuates obesity by inhibiting adipogenesis and activating the AMPK pathway in obese mice. Journal of Nutritional Biochemistry. 2014;25(2):201–207. doi: 10.1016/j.jnutbio.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332(6037):1519–1523. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosz EB, Jeffery EH. Commercially produced frozen broccoli lacks the ability to form sulforaphane. Journal of Functional Foods. 2013;5(2):987–990. [Google Scholar]
- Folch J, Lees M, Stanley GHS. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. Journal of Biological Chemistry. 1957;226(1):497–509. [PubMed] [Google Scholar]
- Harris KE, Jeffery EH. Sulforaphane and erucin increase MRP1 and MRP2 in human carcinoma cell lines. Journal of Nutritional Biochemistry. 2008;19(4):246–254. doi: 10.1016/j.jnutbio.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Hu R, Hebbar V, Kim BR, Chen C, Winnik B, Buckley B, Soteropoulos P, Tolias P, Hart RP, Kong AN. In vivo pharmacokinetics and regulation of gene expression profiles by isothiocyanate sulforaphane in the rat. Journal of Pharmacology and Experimental Therapeutics. 2004;310(1):263–271. doi: 10.1124/jpet.103.064261. [DOI] [PubMed] [Google Scholar]
- Jeffery EH, Araya M. Physiological effects of broccoli consumption. Phytochemistry Reviews. 2009;8(1):283–298. [Google Scholar]
- Jeffery EH, Brown AF, Kurilich AC, Keck AS, Matusheski N, Klein BP, Juvik JA. Variation in content of bioactive components in broccoli. Journal of Food Composition and Analysis. 2003;16(3):323–330. [Google Scholar]
- Kang JS, Wanibuchi H, Morimura K, Gonzalez FJ, Fukushima S. Role of CYP2E1 in diethylnitrosamine-induced hepatocarcinogenesis in vivo. Cancer Ressearch. 2007;67(23):11141–11146. doi: 10.1158/0008-5472.CAN-07-1369. [DOI] [PubMed] [Google Scholar]
- Kay HY, Kim WD, Hwang SJ, Choi HS, Gilroy RK, Wan YJ, Kim SG. Nrf2 inhibits LXRalpha-dependent hepatic lipogenesis by competing with FXR for acetylase binding. Antioxidants & Redox Signaling. 2011;15(8):2135–2146. doi: 10.1089/ars.2010.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai RH, Keck AS, Walling MA, West LG, Jeffery EH. Evaluation of the safety and bioactivity of purified and semi-purified glucoraphanin. Food and Chemical Toxicology. 2008;46(1):195–202. doi: 10.1016/j.fct.2007.07.015. [DOI] [PubMed] [Google Scholar]
- Lee JH, Moon MH, Jeong JK, Park YG, Lee YJ, Seol JW, Park SY. Sulforaphane induced adipolysis via hormone sensitive lipase activation, regulated by AMPK signaling pathway. Biochemical and Biophysical Research Communications. 2012;426(4):492–497. doi: 10.1016/j.bbrc.2012.08.107. [DOI] [PubMed] [Google Scholar]
- Matsuzawa-Nagata N, Takamura T, Ando H, Nakamura S, Kurita S, Misu H, Ota T, Yokayama M, Honda M, Miyamoto K, Kaneko S. Increased oxidative stress precedes the onset of high-fat diet-induced insulin resistance and obesity. Metabolism. 2008;57(8):1071–1077. doi: 10.1016/j.metabol.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. Journal of the American Medical Association. 2014;311(8):806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EJ, Lee JH, Yu GY, He GB, Ali SR, Holzer RG, Osterreicher CH, Takahashi H, Karin M. Dietary and Genetic Obesity Promote Liver Inflammation and Tumorigenesis by Enhancing IL-6 and TNF Expression. Cell. 2010;140(2):197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postic C, Dentin R, Girard J. Role of the liver in the control of carbohydrate and lipid homeostasis. Diabetes and Metababolism. 2004;30(5):398–408. doi: 10.1016/s1262-3636(07)70133-7. [DOI] [PubMed] [Google Scholar]
- Shih PH, Yen GC. Differential expressions of antioxidant status in aging rats: the role of transcriptional factor Nrf2 and MAPK signaling pathway. Biogerontology. 2007;8(2):71–80. doi: 10.1007/s10522-006-9033-y. [DOI] [PubMed] [Google Scholar]
- Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104(4):531–543. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Aleksunes LM, Yeager RL, Gyamfi MA, Esterly N, Guo GL, Klaassen CD. NF-E2-related factor 2 inhibits lipid accumulation and oxidative stress in mice fed a high-fat diet. Journal of Pharmacology and Experimental Therapeutics. 2008;325(2):655–664. doi: 10.1124/jpet.107.135822. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.