Summary
c-di-GMP is a bacterial second messenger that is enzymatically synthesized and degraded in response to environmental signals. Cellular processes are affected when c-di-GMP binds to receptors which include proteins containing the PilZ domain. Although each c-di-GMP synthesis or degradation enzyme metabolizes the same molecule, many of these enzymes can be linked to specific downstream processes. Here we present evidence that c-di-GMP signaling specificity is achieved through differences in affinities of receptor macromolecules. We show that the PilZ domain proteins of Salmonella Typhimurium, YcgR and BcsA, demonstrate a 43-fold difference in their affinity for c-di-GMP. Modulation of the affinities of these proteins altered their activities in a predictable manner in vivo. Inactivation of yhjH, which encodes a predicted c-di-GMP degrading enzyme, increased the fraction of the population that demonstrated c-di-GMP levels high enough to bind to the higher-affinity YcgR protein and inhibit motility, but not high enough to bind to the lower-affinity BcsA protein and stimulate cellulose production. Finally, seven PilZ domain proteins of Pseudomonas aeruginosa demonstrated a 145-fold difference in binding affinities, suggesting that regulation of c-di-GMP signaling by binding affinity may be a conserved mechanism that allows organisms with many c-di-GMP-binding macromolecules to rapidly integrate multiple environmental signals into one output.
Keywords: c-di-GMP, PilZ, YcgR, BcsA, c-di-GMP biosensor, Salmonella
Introduction
c-di-GMP is a second messenger utilized by many bacteria to integrate environmental information from different inputs in order to direct cellular behavior. c-di-GMP has been implicated in the regulation of many cellular processes, including production of adherence factors and exopolysaccharide, flagellar motility, the cell cycle, virulence for mammals, and antibiotic sensing and resistance (for reviews see (Hengge, 2009, Schirmer & Jenal, 2009, Mills et al., 2011)). Diguanylate cyclases (DGCs) generate c-di-GMP by cyclizing two molecules of GTP via GGDEF domains, while phosphodiesterases (PDEs) degrade c-di-GMP by the hydrolytic actions of their EAL or HD-GYP domains. Because many GGDEF and EAL domains are physically linked to putative environmental sensing domains, the precise concentration of c-di-GMP is likely tightly controlled by multiple environmental signals (Mills et al., 2011). Signal transduction is accomplished by c-di-GMP binding to specific downstream receptors including proteins containing the PilZ domain, which makes direct contacts with c-di-GMP (Amikam & Galperin, 2006, Ryjenkov et al., 2006, Christen et al., 2007). Many bacterial species encode dozens of c-di-GMP components in their genome (Galperin et al., 2001).
Studies in the last decade have provided insight into the c-di-GMP networks of Salmonella Typhimurium. S. Typhimurium has three defined processes known to be regulated by c-di-GMP: flagellar-based motility, the generation of curli fimbriae, and cellulose production (Zogaj et al., 2001, Solano et al., 2002, Kader et al., 2006, Ryjenkov et al., 2006, Weber et al., 2006). The genes encoding curli fimbriae are directly up-regulated by the transcription factor CsgD, whose expression is induced by c-di-GMP at temperatures of 30°C or lower through an as-yet unidentified receptor (Ogasawara et al., 2011, Zakikhany et al., 2010, Romling et al., 1998a, Romling et al., 1998b). Like most Enterobacteria, S. Typhimurium has two PilZ domain proteins: YcgR, which controls flagellar-based motility, and BcsA, the bacterial cellulose synthase. Escherichia coli YcgR has been shown to bind specifically to the flagellar complex to inhibit torque generation and thus decrease motility when it binds to c-di-GMP (Ryjenkov et al., 2006, Boehm et al., 2010, Paul et al., 2010, Fang & Gomelsky, 2010). c-di-GMP stimulates cellulose production presumably by binding to the PilZ domain of BcsA (Zogaj et al., 2001, Solano et al., 2002). Thus, a rise in c-di-GMP concentration results in a decrease in motility (through YcgR) and an increase in cellulose production (through BcsA). Several of the 20 GGDEF/EAL domain proteins of S. Typhimurium have been linked to one of these processes. AdrA, a DGC that is dependent on CsgD for its expression, is required for the production of cellulose on LB-agar plates. Under this condition, its loss cannot be complemented by the native expression of any other DGC, even though these are also expressed in cellulose-producing conditions (Romling et al., 2000, Zogaj et al., 2001, Garcia et al., 2004, Solano et al., 2009). Several of these other DGCs have instead been implicated in motility inhibition or CsgD expression, based on studies in S. Typhimurium or in E. coli (Kader et al., 2006, Pesavento et al., 2008, Boehm et al., 2010, Sanchez-Torres et al., 2011). The PDE that appears to be the most important for maintaining c-di-GMP levels low enough to allow flagellar motility is YhjH (Ko & Park, 2000, Ryjenkov et al., 2006, Paul et al., 2010, Boehm et al., 2010). Like YcgR, YhjH is a member of the flagellar regulon and is expressed from a flagellar class III promoter (Ko & Park, 2000, Frye et al., 2006, Wang et al., 2006).
Although they metabolize the same small, diffusible second messenger molecule, many c-di- GMP synthesizing and degrading enzymes, such as AdrA, can be specifically linked to a particular downstream c-di-GMP-regulated process. These observations have led to the conclusion that c-di-GMP signaling may be functionally compartmentalized (Weber et al., 2006, Pesavento et al., 2008, Hengge, 2009). There are several potential mechanisms for how this compartmentalization could occur. One of these mechanisms involves coordinate regulation of the expression of c-di-GMP components, in which c- di-GMP metabolizing enzymes would be expressed under the same conditions as their downstream receptors. Indeed, some c-di-GMP components are known to be under the transcriptional control of various signals (Pesavento et al., 2008, Weber et al., 2006); however, others appear to be constitutively expressed, so this mechanism does not adequately account for all observed signaling specificity (Solano et al., 2009). Another proposed model for signaling specificity involves the spatial sequestration of individual c-di-GMP components, such that the generation and degradation of c-di-GMP would act locally on a particular target. Although there is some evidence to support local activity of c-di-GMP metabolizing enzymes in some organisms (Massie et al., 2012, Merritt et al., 2010), the rapid diffusion of c-di-GMP in the bacterial cytoplasm would make it difficult to establish such local pools inside of the bacterial cell in the absence of some kind of compartmentalizing structure (Mills et al., 2011), and there is no firm evidence that such microdomains exist in bacteria. An alternative, though not mutually exclusive, mechanism by which c-di-GMP signaling specificity could be achieved is through the binding affinities of downstream receptors (Hengge, 2009, Ko et al., 2010, Mills et al., 2011), wherein downstream receptor affinity for c-di-GMP would determine which receptors are activated at particular cytoplasmic c-di-GMP levels. Such a mechanism would allow for rapid responses when coupled with the enzymatic ability to alter the concentration of c-di-GMP in response to environmental signals. This hypothesis is supported by the measured affinities of c-di-GMP receptors from a variety of organisms, which have been found to vary considerably (Christen et al., 2006, Benach et al., 2007, Christen et al., 2007, Merighi et al., 2007, Pratt et al., 2007, Hickman & Harwood, 2008, Sudarsan et al., 2008, Duerig et al., 2009, Navarro et al., 2009, Christen et al., 2010, Krasteva et al., 2010, Srivastava et al., 2011, Duvel et al., 2012, Whitney et al., 2012). Differences in binding affinities of receptor proteins could potentially represent a method for c-di-GMP to affect some PilZ domain proteins but not others, thereby selectively activating certain PilZ domain proteins depending on its concentration within the cell.
Based on these observations, we performed a systematic study to measure and compare the c- di-GMP binding affinities of all PilZ domain proteins within two organisms: Salmonella Typhimurium, which has two PilZ domain proteins, and P. aeruginosa, which has eight (Amikam & Galperin, 2006). We found that the binding affinities of these proteins vary considerably even within the same organism. We then underwent a strategy of site-directed mutagenesis to generate S. Typhimurium PilZ domain proteins that have altered affinities but otherwise retain the functions of the native proteins. Assessing the activities of these mutant proteins in phenotypic assays showed that binding affinity is an important determinant for activity. Finally, we demonstrated that inactivation of the EAL domain protein YhjH results in an increase in the percentage of cells that demonstrate a level of c-di-GMP high enough to bind to YcgR but not to BcsA. This work supports the hypothesis that regulation by binding affinity of downstream receptors is a mechanism for the selective activation of c-di-GMP controlled processes.
Results
The binding affinities of S. Typhimurium PilZ domain proteins differ by 43-fold
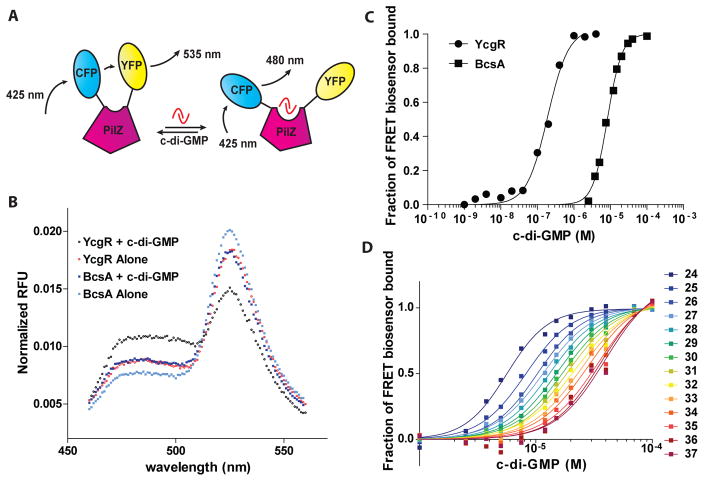
We measured the c-di-GMP binding affinities of PilZ domain proteins by constructing FRET- based c-di-GMP biosensors using these proteins (Fig 1A). Intramolecular FRET is a very sensitive method for detecting changes in protein conformation, such as those that occur upon binding to small molecules (Jares-Erijman & Jovin, 2003, Roy et al., 2008). This method has previously been used to measure the c- di-GMP binding affinity of YcgR in a YPet-YcgR-CyPet fusion protein (Christen et al., 2010, Nguyen & Daugherty, 2005), and to measure affinities of proteins for other second messengers (Miyawaki et al., 1997, Nikolaev et al., 2006, Ohashi et al., 2007, Russwurm et al., 2007, Jager et al., 2012). In this FRET- based method, the conformational change experienced by a PilZ domain protein upon its binding to c-di- GMP manifests as a change in the FRET output emission spectrum. Using this method, we generated a FRET-based biosensor from the BcsA protein in order to compare its c-di-GMP binding properties to those of YcgR. BcsA is predicted to include nine transmembrane helices, a glycosyltransferase domain, and a PilZ domain (InterPro - http://www.ebi.ac.uk/interpro/). Making a FRET fusion protein with the entire BcsA protein would not have been possible due to the transmembrane domains, so we used the BcsA PilZ domain as the c-di-GMP-detecting sensor. This biosensor, and the YcgR-based biosensor described above, were purified from E. coli BL21(DE3) cells, and their FRET emission spectrum profiles were analyzed in the presence or absence of 40 μM c-di-GMP using a fluorescence plate reader. For both FRET constructs, the presence of c-di-GMP results in a corresponding decrease in FRET (Fig 1B), suggesting that binding results in an increase in the distance between the amino- and carboxyl-termini, or a change in their dipole-dipole orientation, of these fusion proteins. Thus, c-di-GMP binding to each FRET-based biosensor can be detected by a change in the ratio of YFP emission (535 nm) to CFP emission (480 nm) (Fig 1B).
Fig. 1. The binding properties of the BcsA FRET construct for c-di-GMP.
A. PilZ domain FRET proteins were generated by the fusion of the fluorophores mYPet (YFP) and mCyPet (CFP) to the termini of a PilZ domain protein. These FRET fusion proteins demonstrate a characteristic FRET signal when exposed to the excitation wavelength of mCyPet (425 nm). Binding of c-di-GMP to the PilZ domain changes the conformation of the PilZ domain protein, which results in a shift in the emission ratio of mYPet (535 nm) to mCyPet (480 nm). In this figure, binding of c-di-GMP results in a decrease in FRET, though c-di-GMP binding can also result in a FRET increase depending on the PilZ domain protein used. B. Normalized fluorescence emission spectra of YcgR and BcsA based FRET biosensors upon excitation of mCyPet at 425 nm in vitro. Shown are the emission spectra of the YcgR biosensor in the presence (black circles) and absence (red circles) of 40 μM c-di-GMP, and the BcsA biosensor in the presence (dark blue squares) and absence (light blue squares) of 40 μM c-di-GMPat 25°C. For both biosensors, the addition of c-di- GMP results in a decrease in the amount of FRET. C. c-di-GMP binding curves for YcgR (circles) and BcsA (squares) biosensors at 25°C as measured by FRET. The fraction of FRET biosensor bound to c-di-GMP was calculated as follows: Fraction of FRET biosensor bound = (FRET ratio(x) - FRET ratio free) / (FRET ratio bound - FRET ratio free). A number of 0 indicates that the FRET biosensor is completely unbound to c-di-GMP, and a number of 1 indicates that the FRET biosensor is completely bound to c-di-GMP. D. Binding curves of the BcsA biosensor at temperatures ranging from 24°C to 37°C at 1°C intervals as indicated by the legend. Binding affinity decreases as temperature increases.
We used this construct to explore the c-di-GMP binding properties of BcsA. The c-di-GMP binding affinities for both PilZ domain protein based c-di-GMP biosensors were measured by analyzing their FRET profiles in the presence of increasing concentrations of c-di-GMP. This yielded a concentration-responsive curve for c-di-GMP (Fig 1C). We measured the Hill coefficient and the equilibrium binding constant Kd by fitting a line to the concentration-responsive curve (Fig 1C). The Hill coefficient was approximately two for the BcsA PilZ domain, similar to Hill coefficients measured for other PilZ domains (Ko et al., 2010, Ramelot et al., 2007), suggesting binding to two molecules of c-di- GMP and indicating positive cooperativity in c-di-GMP binding (Table S1). Unexpectedly, we observed a Hill coefficient closer to 1.5 for the YcgR biosensor at 25°C, compared to the Hill coefficient previously published of approximately 2 for this protein (Christen et al., 2010); however, as temperature increased, the Hill coefficient approximated 2 (Table S1). The Kd of YcgR for c-di-GMP at 25°C was 191 ± 18 nM, similar to the Kd of 195 nM previously published for this FRET-based biosensor (Christen et al., 2010), and to the Kd of 182 nM of YcgR for c-di-GMP that was determined by UV cross-linking to radiolabeled c- di-GMP (Christen et al., 2007). For BcsA, the binding affinity was 8240 ± 238 nM at 25°C (Table 1). This represents an approximately 43-fold difference in affinity for c-di-GMP between these two proteins. The c-di-GMP binding affinity of each PilZ domain protein measured was highly dependent on temperature, since the affinity dropped as temperature increased (Fig 1D, Table S1). This indicates that binding is entropically unfavorable, as was also determined for the binding of the Vibrio cholerae PilZ domain protein VCA0042 to c-di-GMP (Benach et al., 2007). The disparity in binding affinities between YcgR and BcsA was maintained at every temperature measured (Table S1). Comparison of the binding curves of these two proteins indicates that there exists a range of cellular c-di-GMP concentration that would stimulate binding to YcgR but would not be high enough to stimulate BcsA binding (Fig 1C), and implies that their specific binding affinities may thus be important for their biological functions.
Table 1.
Affinities of c-di-GMP-binding molecules in selected bacteria
| Protein | Binding Affinity (nM)
|
Binding Motif | Temp (°C) | Measurement Method | Reference | |
|---|---|---|---|---|---|---|
| This Work | Published | |||||
| Salmonella Typhimurium | ||||||
| YcgR (STM1798) | 191 ± 18 | 182 ± 29 | PilZ | 25 | FRET, 33P-Labeled c-di-GMP | This work, Christen 2007 |
| BcsA (STM3619) | 8240 ± 238 | PilZ | 25 | FRET | This work | |
|
| ||||||
| Pseudomonas aeruginosa | ||||||
| PA3353 site 1a | 88 ± 17 | PilZ | 25 | FRET | This work | |
| PA3353 site 2a | 732 ± 122 | 262 ± 66b | PilZ | 25, 20 | FRET, surface plasmon res | This work, Duvel 2012 |
| FimX (PA4959) | 125 | EAL | 20 | ITC | Navarro 2009 | |
| PA2989 | 288 | PilZ | 25 | FRET | Christen 2010 | |
| PA0012 | 402 ± 39 | PilZ | 25 | FRET | Christen 2010 | |
| PA4324 | 735 ± 474c | PilZ | 25 | FRET | This work | |
| PelD (PA3061) | 1900 | RXXD I-Site | 20 | ITC | Whitney 2012 | |
| PA2799 | 1998 ± 448 | PilZ | 25 | FRET | Christen 2010 | |
| PA4608 | 6126 ± 525 | PilZ | 25 | FRET | Christen 2010 | |
| Alg44 (PA3542) | 12739 ± 1680 | 8400 | PilZ | 25 | FRET, ITC | This work, Merighi 2007 |
| FleQ (PA1097) | 15000–25000 | Unknown | 37 | 32P-Labeled c-di-GMP | Hickman 2008 | |
| PA2960 | DNBd | DNBd | Not Applicable | 25 | FRET, ITC | This work, Merighi 2007 |
PA3353 binding curves indicate two binding sites
The published affinity of this protein was measured at approximately 20°C, corresponding to ~530 nM at 25°C.
The dynamic range of FRET for PA4324 between bound and unbound states was small, resulting in a high error for the Kd of this protein
Does not bind to c-di-GMP
The binding affinities of P. aeruginosa PilZ domain proteins span a 145-fold range
Next we compared the binding affinities of PilZ domain proteins of P. aeruginosa (Amikam & Galperin, 2006) using the same sensitive FRET-based method. This method has previously been used to measure the affinities of four P. aeruginosa PilZ domain proteins (PA0012, PA2799, PA2989, and PA4608) (Christen et al., 2010) (Table 1). We generated FRET-based biosensors to measure the c-di- GMP binding affinities of the remaining PilZ domain proteins (PA2960, PA3353, PA3542, and PA4324). As for the PilZ domain proteins of S. Typhimurium, the c-di-GMP-binding affinities of P. aeruginosa PilZ domain proteins spanned a sizable range of c-di-GMP concentrations. The highest and lowest c-di-GMP binding affinities measured for P. aeruginosa PilZ domain proteins differed by 145-fold (Table 1). The Hill coefficient approximated two for every PilZ domain protein measured, and all measured binding affinities decreased as temperatures increased (Fig S1 and data not shown).
Interestingly, the binding curve for PA3353 suggested two separate binding sites, each binding two molecules of c-di-GMP, that differed by approximately eight-fold, at c-di-GMP concentrations of 88 nM and 732 nM at 25°C (Table 1 and Fig S1). PA3353 has also been measured by another group using surface plasmon resonance to be 262 ± 66 nM (Table 1), although this binding affinity was measured at 20°C (Duvel et al., 2012). We have found that for every °C increase in temperature, the binding affinity decreases by approximately 15% for this protein (Fig S1). Therefore this measurement of 262 ± 66 nM at 20°C would be estimated to be approximately 530 ± 100 nM at 25°C, which is comparable to the lower-affinity site. The published affinity for PA3542, measured using isothermal titration calorimetry, is 8.4 μM (Merighi et al., 2007), which is slightly higher than the affinity of PA3542 measured in this study of 12.7 ± 1.7 μM (Table 1). This difference could be due to differences in measurement methods, since isothermal titration calorimetry is known to be fairly inaccurate in measuring low binding affinities, and cannot accurately resolve affinities of less than approximately 10 μM (Rehm, 2006). No FRET shift was observed for PA2960, suggesting that it does not bind c-di-GMP. This is consistent with a previous study in which PA2960 was shown by UV cross-linking to not bind to c-di-GMP (Merighi et al., 2007), likely because it lacks the RxxxR motif required for binding (Amikam & Galperin, 2006, Christen et al., 2007, Ryjenkov et al., 2006).
To determine how binding affinities of PilZ domain proteins affected their biological output functions, we chose to pursue this study further in S. Typhimurium, which has two PilZ domain proteins, both with published phenotypes.
Mutation of the amino acid residue at Position-X alters the affinities of S. Typhimurium PilZ proteins for c-di-GMP
The striking variability in c-di-GMP-binding affinities of PilZ domain proteins suggests that binding affinities may be important for the biological phenotypes of these proteins. To test this hypothesis, we employed a strategy of rational, site-directed mutagenesis to alter c-di-GMP binding affinity of the PilZ domain proteins of S. Typhimurium. Crystal and NMR studies of PilZ domain proteins in complex with c-di-GMP have elucidated the roles of residues of the binding pocket, especially those of the so-called switch region including the RxxxR motif, in binding to c-di-GMP (Benach et al., 2007, Ko et al., 2010, Shin et al., 2011). Specifically, the presence of an arginine at the position immediately N- terminal of the RxxxR motif, dubbed “Position-X”, has been shown for the Pseudomonas putida YcgR homologue, PP4397, to be an important determinant of the affinity for c-di-GMP-binding (Ko et al., 2010). S. Typhimurium YcgR also has an arginine at Position-X (arginine-113) (Fig 2A), so we reasoned that mutation of this arginine might decrease binding affinity as it does in PP4397. Indeed, mutation of arginine-113 to alanine resulted in a YcgR variant that bound c-di-GMP with an affinity of 3.2 μM, which was approximately 17-fold lower than the affinity measured for the wild-type YcgR protein (Fig 2B).
Fig. 2. Mutation of the residue at Position-X alters the c-di-GMP binding affinities of S. Typhimurium PilZ domain proteins.
A. Table showing the c-di-GMP switch region for YcgR and BcsA. Shown in white are mutated residues. Small arrows show the locations of the invariant arginines of the RxxxR motif. The affinities shown are for proteins measured at 25°C. B. c-di-GMP binding curves for YcgR wild-type (open circles) and YcgR R113A (closed circles) FRET biosensors at 25°C. C. c-di-GMP binding curves for BcsA PilZ wild-type (open squares) and BcsA PilZ V695R (closed squares) FRET biosensors at 25°C.
The BcsA PilZ domain has a low binding affinity for c-di-GMP compared to YcgR (8.2 μM compared to 191 nM), so our goal for BcsA was to increase its affinity. BcsA has a valine at Position-X (Fig 2A). The V. cholerae YcgR homologue, VCA0042, also has a large hydrophobic residue, a leucine, in this position. Mutation of this leucine to arginine has been shown to increase the binding affinity of VCA0042 for c-di-GMP by approximately three-fold (Ko et al., 2010). Although BcsA is not similar in sequence to VCA0042 outside of the conserved residues of the binding pocket, we reasoned that mutation of the valine in Position-X of BcsA to an arginine might increase binding affinity in a similar manner as mutation of leucine to arginine in Position-X of VCA0042. Mutating this valine to an arginine increased the affinity of BcsA for c-di-GMP to 4.2 μM at 25°C, which is roughly two-fold greater than that of the WT protein (Fig 2C). Both the BcsA and YcgR mutant proteins maintained similar fold differences compared to the wild-type proteins from 24°C to 37°C (data not shown). These mutations did not change the wild-type binding stoichiometries of c-di-GMP to the PilZ domain, indicating that the change in affinity was the major determinant of change in downstream function (data not shown). Therefore we were able by site directed mutagenesis to generate PilZ domain proteins with altered binding affinities for c-di-GMP.
A S. Typhimurium strain harboring a BcsA protein with increased affinity for c-di-GMP demonstrates an increase in cellulose production at low levels of c-di-GMP
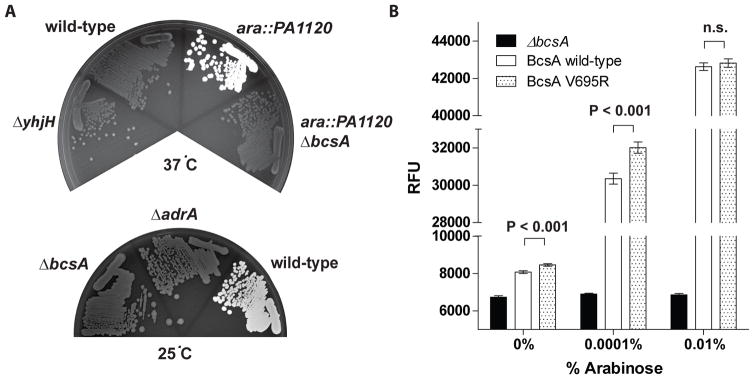
Once we had generated binding affinity mutants of PilZ domain proteins, we needed functional assays to determine the effects of these mutations on phenotype. For BcsA, the bacterial cellulose synthase, activity can be assessed by determining cellulose production, which is detected by its binding to the fluorescent dye calcofluor. The production of cellulose on LB medium is dependent upon the DGC AdrA, which is expressed at 30°C or lower, but not at 37°C (Zogaj et al., 2001, Solano et al., 2002, Garcia et al., 2004) (Fig 3A). The ability of AdrA-produced c-di-GMP to stimulate cellulose production is not through transcriptional or translational effects, since protein extracts from strains grown at different temperatures demonstrated similar amounts of BcsA protein, and cell lysates prepared from strains grown at 37°C are capable of producing cellulose (Fig S2). Thus, c-di-GMP affects cellulose production on a post-translational level, most likely through its binding to the BcsA PilZ domain. In order to compare cellulose production between strains harboring BcsA variants, we constructed a strain of S. Typhimurium in which the level of c-di-GMP can be controlled by the addition of arabinose. This is due to the presence of a DGC, PA1120 from P. aeruginosa (Kulasakara et al., 2006), located on the chromosome under the control of the native arabinose-inducible pBAD promoter. In this strain, addition of arabinose to the growth media results in induction of the DGC and cellulose production even at 37°C (Fig 3A, Fig S3).
Fig. 3. c-di-GMP binding affinity of S. Typhimurium BcsA affects cellulose production in vivo.
A. S. Typhimurium makes cellulose at 24°C, but not at 37°C, in a manner that is dependent on both AdrA and BcsA. Calcofluor agar plates incubated at either 37°C (top) or 24°C (bottom) with wild-type, ΔyhjH, ara::DGC, ara::DGC ΔbcsA, ΔbcsA, or ΔadrA strains of S. Typhimurium. ara::DGC is a strain that harbors an arabinose-inducible DGC on the chromosome. The plate incubated at 37°C was supplemented with 0.001% arabinose. Plates were exposed to UV light with an excitation wavelength of 365 nm, which is a wavelength that stimulates fluorescence of calcofluor when it is bound to cellulose. White coloring indicates calcofluor binding to cellulose. B. Calcofluor fluorescence at different concentrations of arabinose at 37°C for ara::DGC with wild-type bcsA, or ara::PA1120 in which the bcsA gene has either been deleted (ΔbcsA) or mutated (BcsA V695R). Shown is the average fluorescence emission intensity at 420 nm, after excitation at 365 nm, of cells growing on 200 μl agar in individual wells of a 96-well plate in a representative experiment. RFU, relative fluorescence units. n.s. = not significant.
To examine whether the increased-affinity BcsA V695R mutation affects cellulose production, we generated a strain of S. Typhimurium that contains the bcsA-V695R mutation on the chromosome in the background of the arabinose-inducible DGC strain. We then analyzed differences in cellulose production, as determined by cellulose binding to calcofluor, between the ara::DGC, ara::DGC ΔbcsA, and ara::DGC bcsA-V695R strains at different expression levels of the DGC by varying the arabinose concentration. At low arabinose levels, the bcsA-V695R mutant generated reproducibly higher calcofluor fluorescence levels compared to the BcsA wild-type strain (Fig 3B), implying that this strain produces more cellulose at low levels of arabinose than the wild-type strain. Upon addition of higher amounts of arabinose, the amount of cellulose synthesis between the wild-type and bcsA-V695R strains was not significantly different, suggesting that the higher DGC levels produce a cellular c-di-GMP concentration above the Kd values for both BcsA V695R mutant and BcsA wild-type enzymes, resulting in saturation and cellulose production by both enzymes (Fig 3C). These data show that the binding affinity of BcsA for c-di-GMP affects its biological function.
A S. Typhimurium strain harboring YcgR with a lower binding affinity for c-di-GMP requires higher levels of c-di-GMP to inhibit flagellar motility
Next we needed a functional assay to assess the effect of the YcgR R113A mutation on phenotype. S. Typhimurium YcgR has been shown to decrease the swimming halo diameter of colonies on motility agar plates when intracellular levels of c-di-GMP are artificially raised (Ryjenkov et al., 2006). Since the c-di-GMP binding affinity of the YcgR R113A mutant protein is 17-fold less than that of the wild-type protein, we hypothesized that the YcgR R113A protein would require a higher concentration of c-di-GMP than the wild-type YcgR protein in order to inhibit swimming. To test this hypothesis, S. Typhimurium strains harboring the DGC PA1120 under the control of an IPTG-inducible tac promoter were generated and evaluated. The addition of IPTG results in a decrease in swimming motility for this strain compared to cells that do not harbor the IPTG-inducible DGC, as visualized by a decrease in this strain in the halo diameter on motility agar plates (data not shown). A ΔycgR deletion mutant does not demonstrate as severe of a decrease in swimming diameter compared to the wild-type strain in the presence of the DGC, indicating a partial relief of c-di-GMP-induced swimming inhibition in this strain (Fig 4A). Expressing YcgR from its native promoter off of a plasmid complemented the ycgR mutation (Fig 4A).
Fig. 4. c-di-GMP binding affinity of S. Typhimurium YcgR affects motility inhibition.
A. Swimming halos on motility agar plates with 25 μM IPTG of S. Typhimurium strains harboring pDGC or pDGC-YcgR at 37°C for 12 hours. A wild-type strain without exogenous DGC expression was not shown since the swimming diameter of this strain is too large to include and adequately show the differences between strains harboring pDGC. B. Swimming diameters of ΔycgR harboring either pDGC-YcgR or pDGC-YcgR R113A relative to the swimming diameter of the uncomplemented ΔycgR pDGC strain, at indicated levels of IPTG at 37°C. Both YcgR and YcgR R113A effect a decrease in motility at increasing IPTG levels, but the YcgR R113A mutant protein requires a higher concentration of IPTG to inhibit motility than the wild-type YcgR protein.
To determine whether the YcgR R113A mutation affected swimming, we complemented the ΔycgR mutant strain with either the wild-type YcgR protein, or the YcgR R113A mutant protein, and analyzed swimming halo diameter on motility agar plates with different concentrations of IPTG. Complementation with wild-type YcgR partially inhibited swimming even in the absence of IPTG, which could be an overexpression effect of YcgR as has been observed previously (Paul et al., 2010) (Fig 4B). Increasing the IPTG concentration decreased the ability of S. Typhimurium complemented with YcgR to swim, indicating that c-di-GMP-bound YcgR was inhibiting motility (Fig 4B). At 20 μM IPTG, the YcgR wild-type protein completely inhibited swimming. Unlike the wild-type YcgR protein, complementation with YcgR R113A had no effect on swimming in the absence of IPTG. Increasing IPTG also decreased motility in the YcgR R113A complemented strain, but 50 μM IPTG was required to completely inhibit swimming by the YcgR R113A mutant protein (Fig 4B). This indicates that the YcgR R113A protein requires a higher concentration of c-di-GMP than the wild-type YcgR protein to inhibit swimming, which is consistent with its lower binding affinity.
Inactivation of yhjH results in an increase in the percentage of S. Typhimurium cells that demonstrate c- di-GMP levels high enough to bind YcgR
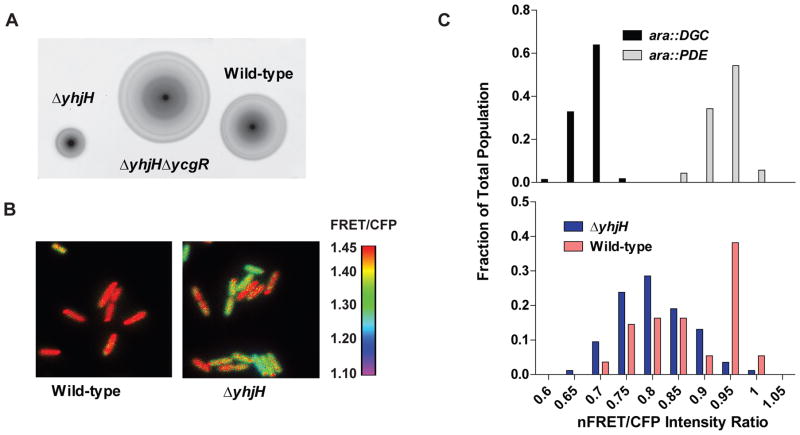
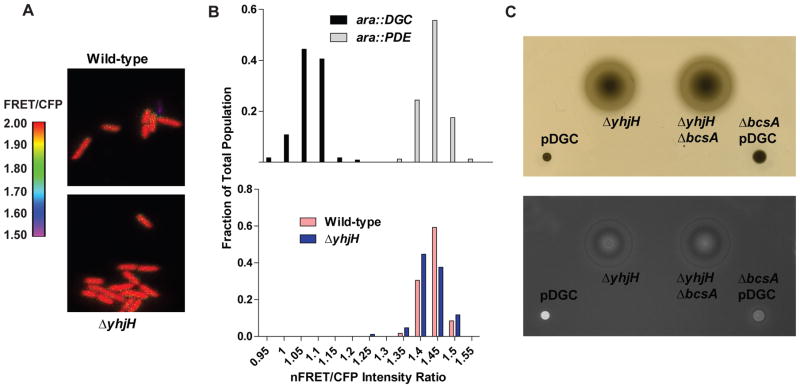
Another way to explore the affinity hypothesis is in the context of inactivation of the PDE YhjH. YcgR is most frequently studied in the context of a yhjH mutant background, in which a second mutation in ycgR partially relieves the swimming defect that is caused by inactivation of YhjH (Boehm et al., 2010, Paul et al., 2010, Fang & Gomelsky, 2010, Ryjenkov et al., 2006) (Fig 5A). In E. coli, cells that harbor a mutation in yhjH demonstrate increased c-di-GMP levels compared to wild-type cells (Boehm et al., 2010). However, mutation in S. Typhimurium yhjH does not appear to result in a significant difference in total c-di-GMP levels in this strain compared to a wild-type strain (Simm et al., 2007), so it is unknown how yhjH inactivation results in a motility inhibition in this organism. In order to determine whether the yhjH mutation results in a rise in c-di-GMP levels that are high enough to stimulate binding to, and motility inhibition by, YcgR, we analyzed the binding to c-di-GMP of a synthetic YcgR-based FRET biosensor in vivo, encoded on the plasmid pYcgR-Spy (Christen et al., 2010) (Fig 5B). Binding of this construct to c-di-GMP can be visualized in live individual cells by determining the FRET/CFP ratios using fluorescence microscopy (Christen et al., 2010). Since this construct is based on YcgR, it allows for an accurate determination of the bound state of YcgR when expressed inside of live cells.
Fig. 5. A S. Typhimurium strain harboring a yhjH mutation demonstrates a larger proportion of cells with c-di-GMP bound to YcgR than the wild-type strain.
A. Swimming halos on motility agar plates of wild-type, ΔyhjH, and ΔyhjHΔycgR at 37°C. B. Dual-emission ratio microscopic images (FRET/CFP) of S. Typhimurium (above) or a ΔyhjH mutant (below) expressing pYcgR-Spy. Pseudocolors represent emission ratios (527/480 nm) of the FRET-based biosensor as indicated by the figure legend to the right. C. Histogram showing the fraction of cells that demonstrate the indicated average nFRET/CFP ratios for either control strains expressing a DGC or a PDE off of an arabinose-inducible promoter (top), or for wild-type or yhjH mutant strains (bottom). nFRET/CFP ratios differ from FRET/CFP ratios since nFRET intensities have been corrected for bleedthrough and fluorescence in the YFP channel. The difference between the wild-type and ΔyhjH strains is highly significant (P<0.0001). nFRET: net FRET intensity, calculated by subtracting bleedthrough coefficients and intensity of the YFP channel as detailed in Materials and Methods. Over 100 cells were analyzed for each strain.
Wild-type S. Typhimurium, or an isogenic ΔyhjH mutant strain, both harboring pYcgR-Spy, were grown in liquid LB media at 37°C to an OD600 of 0.20. At this point, cells were collected and imaged using fluorescence microscopy, and their net FRET (nFRET)/CFP intensity ratios were calculated. Control strains expressing either the DGC PA1120 (ara::DGC) or the PDE PA2133 (ara::PDE), and pYcgR-Spy, were used to determine the bound and unbound nFRET/CFP ratios for the YcgR FRET construct in this in vivo assay. As expected, these control strains defined homogeneous populations of cells that demonstrated distinct nFRET/CFP ratios (Fig 5C upper panel, Fig S4). Interestingly, in the wild-type strain expressing pYcgR-Spy, a heterogeneous population of c-di-GMP concentrations was observed (Fig 5C, lower panel). This heterogeneity in c-di-GMP concentrations has also been observed previously for other bacterial species, and it is hypothesized that this phenomenon is due to the production of progeny cells after cell division that harbor different c-di-GMP concentrations, and therefore demonstrate different cellular behaviors, in order to promote a diversification strategy that may enhance survival of the bacterial species (Christen et al., 2010). In the wild-type strain, 43.7% of cells demonstrated a nFRET/CFP ratio higher than 0.9, indicative that YcgR was completely unbound to c-di-GMP in these cells. In contrast, in the ΔyhjH mutant, only 4.8% of cells demonstrated a nFRET/CFP ratio higher than 0.9, a 10-fold decrease compared to wild-type cells (Fig 5C). The remainder of cells demonstrated a nFRET/CFP ratio of 0.9 or lower, indicating that at least some of the YcgR FRET constructs were bound to c-di-GMP in these cells. As demonstrated by Boehm et al, binding of c-di-GMP to only a fraction of the YcgR molecules present in the cell can lead to a decrease in swimming motility, with swimming inhibition increasing as the number of YcgR molecules bound to c-di-GMP in the cell increases (Boehm et al., 2010). Thus it is possible that the ΔyhjH mutant strain is inhibited for motility because a higher fraction of the YcgR proteins in these cells is bound to c-di-GMP. This experiment demonstrates that mutation of yhjH does result in an increase in the percentage of cells with an average level of c-di-GMP that is high enough to bind to the YcgR protein, which is then able to inhibit swimming. However, the change in intracellular c-di-GMP concentration does not appear to be large, which is consistent with previous studies (Simm et al., 2007).
A S. Typhimurium strain harboring the lower-affinity ycgR-R113A mutation does not demonstrate YcgR- dependent motility inhibition in a ΔyhjH mutant
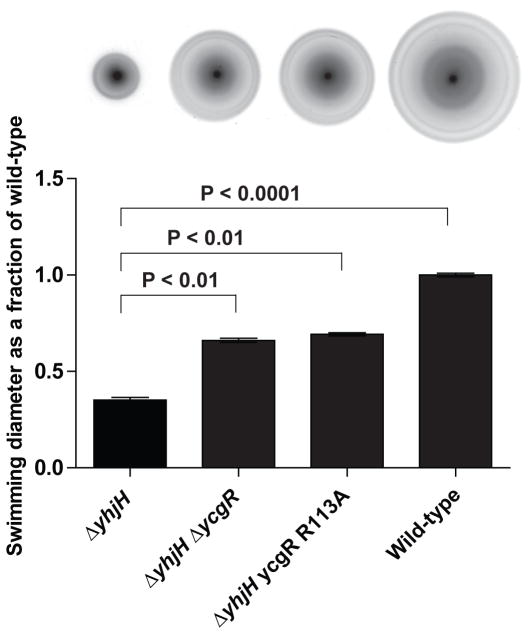
Once we had established that mutation in yhjH results does affect intracellular c-di-GMP levels enough to be relevant to YcgR, we wanted to assess the effect of the ycgR-R113A mutation in the context of a yhjH mutant background. To examine this, a ΔyhjH deletion mutant was generated on a ycgR wild-type, ycgR-R113A or ΔycgR deletion mutant background of S. Typhimurium. In this assay, the swimming diameter of the ΔyhjH mutant was roughly 35% of that observed for the wild-type strain (Fig 6). Swimming was partially restored in the ΔyhjH mutant by a second deletion in ycgR (66% swimming diameter of the wild-type strain). Remarkably, chromosomal replacement of the ycgR codon encoding arginine-113 with a codon that encodes alanine resulted in a phenotype practically indistinguishable from the deletion mutant (69% swimming diameter of the wild-type strain) (Fig 6). Thus the YcgR R113A mutant cannot functionally replace YcgR in this experiment (Fig 6). This suggests that the ΔyhjH mutation causes the intracellular concentration of c-di-GMP to rise high enough to bind to YcgR, but not high enough to bind to the YcgR R113A mutant protein.
Fig. 6. A S. Typhimurium strain harboring the lower-affinity ycgR-R113A mutation does not demonstrate YcgR-dependent motility inhibition in a ΔyhjH mutant.
Above: swimming diameters (from left to right) of ΔyhjH, ΔyhjHΔycgR, ΔyhjH ycgR-R113A, and wild-type S. Typhimurium, on motility agar plates incubated at 37°C for 9 hours. Below: quantitation of the swimming diameters of indicated S. Typhimurium strains compared to the wild-type strain. Statistical significance is indicated.
yhjH inactivation does not result in c-di-GMP levels that are high enough to bind to BcsA and stimulate cellulose production
Our data indicate that mutation in yhjH results in an increase in the fraction of cells that contain c-di-GMP levels that are high enough to bind to the wild-type YcgR protein, but not high enough to bind to the R113A mutant YcgR protein. Since the affinity of the BcsA PilZ domain for c-di-GMP is lower than that of the YcgR R113A protein, we reasoned that ΔyhjH mutation would not result in c-di-GMP levels high enough to bind to the BcsA PilZ domain. To test this hypothesis, we expressed a BcsA PilZ FRET- based construct inside of live cells and monitored their nFRET/CFP ratios in a similar manner to that of the YcgR FRET construct (Fig 7A). As for the YcgR FRET construct, ara::DGC and ara::PDE strains harboring the BcsA PilZ FRET construct were separated into homogeneous populations based on their nFRET/CFP ratios (Fig 7B upper panel, Fig S5). Unlike cells expressing the YcgR FRET construct, wild-type cells expressing the BcsA PilZ construct were homogeneous in their nFRET/CFP ratios (Fig 7A, 7B). The ΔyhjH mutant strain did not demonstrate nFRET/CFP ratios that were significantly different than that of the wild-type strain, and in both strains, these ratios matched that of the ara::PDE strain, indicating that the BcsA PilZ domain was unbound to c-di-GMP in this assay (Fig 7B). Thus, the ΔyhjH mutation does not result in a change in c-di-GMP levels that are large enough to affect the BcsA PilZ construct.
Fig. 7. A S. Typhimurium ΔyhjH strain does not demonstrate c-di-GMP binding to the BcsA PilZ domain or cellulose production.
A. Dual-emission ratio microscopic images (FRET/CFP) of wild-type (top) or ΔyhjH mutant (bottom) S. Typhimurium expressing pBcsA-Spy. Pseudocolors represent emission ratios (527/480 nm) of the FRET-based biosensor as indicated by the figure legend to the left. B. Histogram showing the fraction of cells that demonstrate the indicated nFRET/CFP ratios for control strains expressing a DGC or a PDE off of an arabinose-inducible promoter (top) and either wild-type or ΔyhjH mutant strains (bottom). nFRET/CFP ratios are different than FRET/CFP ratios since nFRET intensities have been corrected for bleedthrough and fluorescence in the YFP channel. nFRET: net FRET intensity, calculated by subtracting bleedthrough coefficients and intensity of the YFP channel as detailed in Materials and Methods. The difference in nFRET/CFP ratios between the wild-type and the ΔyhjH is not significant (P > 0.05). C. Visible light (top) and UV (bottom) images of strains of S. Typhimurium on soft- agar motility plates with calcofluor, incubated at 37°C for 2 hours. This plate was supplemented with 100 mM IPTG.
Since no binding of c-di-GMP to the BcsA PilZ domain was observed in the ΔyhjH mutant strain, we hypothesized that even though ΔyhjH mutation stimulates motility inhibition, it does not result in the production of cellulose. This was observed during growth on solid agar plates (Fig 3A), implying that the increased c-di-GMP concentration that results from ΔyhjH deletion does not induce BcsA activity; however, bacterial detection of solid surfaces may result in different levels of c-di-GMP on solid agar plates than in the low-agar motility plate assay (Guvener & Harwood, 2007). Therefore, we analyzed the production of cellulose on motility agar plates under the same conditions at which ΔyhjH-dependent motility inhibition is observed. As a control, the S. Typhimurium pDGC and ΔbcsA pDGC strains were included. Induction of the DGC results in the production of a level of c-di-GMP that completely inhibits motility (Fig 7C upper panel). In the soft-agar motility plate assay, cellulose production was observed in the pDGC strain by its binding to the fluorescent dye calcofluor (Fig 7C lower panel). In contrast, no calcofluor binding was observed, as expected, to the pDGC strain lacking bcsA. Although the ΔyhjH deletion strain demonstrated an inhibition in swimming motility in this assay, no calcofluor binding in this strain was observed, as the calcofluor fluorescence of this strain was indistinguishable from that of a ΔyhjHΔbcsA double mutant (Fig 7C). This demonstrates that the ΔyhjH mutation results in motility inhibition but does not stimulate the production of cellulose.
Discussion
In this work, we sought to understand an important question in the field of c-di-GMP signaling: how is specificity between c-di-GMP metabolizing enzymes and their downstream receptors achieved, despite the fact that c-di-GMP is a small, freely diffusible second messenger molecule? To this end, we have explored the validity of the hypothesis that signaling specificity is achieved through different binding affinities of these receptors for c-di-GMP.
Using the same FRET-based method, we compared the affinities of PilZ domain proteins, which are c-di-GMP downstream receptors, within the same organism and found that they span a large range. For P. aeruginosa, this difference is 145-fold (Table 1). This is supported by published binding affinities of three non-PilZ c-di-GMP-binding proteins in P. aeruginosa, which also span a large range (Table 1), although it should be noted that the binding affinities of these proteins were not measured at 25°C. In addition, c-di-GMP-binding molecules of Caulobacter crescentus and Vibrio cholerae also exhibit large differences in binding affinities from the nM to the μM range (Christen et al., 2006, Benach et al., 2007, Christen et al., 2007, Pratt et al., 2007, Sudarsan et al., 2008, Duerig et al., 2009, Krasteva et al., 2010, Srivastava et al., 2011). This suggests that intracellular c-di-GMP concentrations could selectively target certain receptors based on their binding affinities in these organisms. Indeed, measurements of c-di- GMP concentrations in wild-type P. aeruginosa under different conditions have resulted in very different c-di-GMP levels (Christen et al., 2010, Malone et al., 2010, Ha et al., 2011, Roy et al., 2012). This is particularly interesting in light of the fact that many DGCs are negatively feedback-inhibited via binding of c-di-GMP to an allosteric I-site, allowing them to maintain homeostasis of c-di-GMP concentrations (Christen et al., 2006). It is attractive to speculate that DGC feedback inhibition may work synergistically with downstream receptor binding affinities, such that some DGCs would produce enough c-di-GMP to “communicate” with receptors that have low c-di-GMP-binding affinity, while other DGCs that bind c-di- GMP with high affinity at the I-site would be limited to activating only receptors with high c-di-GMP binding affinities. Finally, the P. aeruginosa PilZ domain protein PA3353 demonstrated the presence of two binding sites in this study. PA3353 might thus act as a bandpass filter, active or inactive only within a narrow range of c-di-GMP levels. Further work is needed to determine the structural basis of the second c-di-GMP binding site in this protein.
For S. Typhimurium, the difference in binding affinities of the two PilZ domain proteins is 43-fold (Table 1). Using site-directed mutagenesis of Position-X residues, we generated S. Typhimurium PilZ domain proteins that have altered c-di-GMP binding affinities. For YcgR, mutation of arginine-113 to alanine in Position-X likely led to the loss of important H-bond and/or π-stacking interactions with c-di- GMP, thereby reducing binding affinity. For BcsA, the effect of mutation of valine-695 to arginine is less clear. It was hypothesized that the reason why mutation of leucine to arginine in Position-X of VCA0042 caused only a minor increase in binding affinity was because the mutation did not generate any new bonds to c-di-GMP (Ko et al., 2010). If this is also true for the BcsA V695R mutation, this could explain why the effect of Position-X mutation on binding affinity was smaller in BcsA than for YcgR. By using phenotypic assays, these mutations were shown to affect the phenotypes of the proteins in a predictable manner, suggesting that binding affinity is important for the biological functions of these proteins.
This hypothesis is supported by the observation that mutation of yhjH results in c-di-GMP levels that are high enough, in most cells, to bind YcgR, but not high enough to bind BcsA, and that this correlates with the activities of these proteins in phenotypic assays. The ΔyhjH mutation results in a considerable increase in the fraction of the population that contains c-di-GMP levels that are high enough to demonstrate at least partial binding to a YcgR FRET construct (Fig 5). However, a BcsA PilZ FRET construct, which demonstrates a lower affinity for c-di-GMP than YcgR, remained entirely unbound to c-di-GMP in both wild-type and ΔyhjH mutant strains (Fig 7). This, combined with the observation that the ΔyhjH mutation does not appear to affect motility inhibition by the lower-affinity YcgR R113A mutant protein (Fig 6), provides evidence that the rise in c-di-GMP levels in the ΔyhjH mutant is small. This explains why a previous comparison of the total c-di-GMP concentrations between wild-type and ΔyhjH mutant strains of S. Typhimurium did not find a significant difference in c-di-GMP concentration (Simm et al., 2007). Gross measurement of total c-di-GMP concentrations as an average amount from a cell culture would fail to detect a small but significant increase in the free level of c-di-GMP in a fraction of cells. Additionally, the hypothesis that the increase in c-di-GMP levels in a yhjH mutant is small is consistent with our data demonstrating that the ΔyhjH mutation fails to stimulate cellulose production at 37°C despite the presence of a functional cellulose synthase complex (Fig 3A, Fig 7C, Fig S2). It is possible that in wild-type S. Typhimurium, YhjH maintains the level of c-di-GMP close to the Kd for binding YcgR. This is supported by the observed heterogeneity in populations of wild-type cells in which some cells have c-di-GMP levels lower than the Kd of YcgR, while others demonstrate slightly higher c-di- GMP levels (Fig 5). Environmental changes that result in a decrease in YhjH activity could then very quickly result in an increase of c-di-GMP levels that are just above the Kd of YcgR, and are therefore just large enough to be biologically relevant to the YcgR protein, but not to affect BcsA, thus allowing S. Typhimurium to modulate its swimming behavior without also investing energy in building an extracellular matrix.
Based on this evidence, we propose the following model to illustrate c-di-GMP signaling regulation in S. Typhimurium in rich media based on binding affinity (Fig 8). This model involves the sequential activation of c-di-GMP receptors in S. Typhimurium through increasing c-di-GMP levels, which is consistent with the progression of biofilm formation. While the c-di-GMP concentration is maintained at a low level by YhjH, it does not bind to either YcgR or BcsA, resulting in a cell that is motile and does not produce cellulose. Upon sensing appropriate environmental conditions, levels of c-di-GMP rise (perhaps through inactivation of YhjH), which have the immediate impact of decreasing swimming motility through YcgR. This is an important early step in microbial adherence to surfaces since cellular motility must be tightly regulated for appropriate colonization (Sanchez-Torres et al., 2011). Increased c-di-GMP levels induce the expression of CsgD, which would then directly activate the transcription of curli fimbriae, an important attachment and aggregative factor of S. Typhimurium (Romling, 2005). CsgD expression also results in the production of AdrA, which, upon sensing appropriate conditions through its environmental sensing domain, raises the intracellular level of c-di-GMP even higher, resulting in c-di-GMP binding to BcsA and stimulating cellulose production, further protecting organisms after attachment. The requirement for a higher level of c-di-GMP to induce cellulose production, but not to activate the production of curli fimbriae, is potentially due the fact that cells that produce cellulose before generating curli fimbriae appear to be deficient in initial attachment to surfaces (Gualdi et al., 2008). There are several lines of evidence that suggest that AdrA produces a large amount of c-di- GMP inside of the cell. During cellulose-producing conditions, AdrA has been shown to be responsible for producing most of the c-di-GMP present inside of the cell (Kader et al., 2006). AdrA is capable of producing more c-di-GMP than other Salmonella DGCs tested. Its overexpression has been found to raise the cellular c-di-GMP level by 10,000-fold, compared to two other Salmonella DGCs that have been shown to affect motility and CsgD expression, which raise the c-di-GMP level by no more than 6-fold (Kader et al., 2006).
Fig. 8. The binding affinities of YcgR and BcsA for c-di-GMP determines cellular phenotypes.
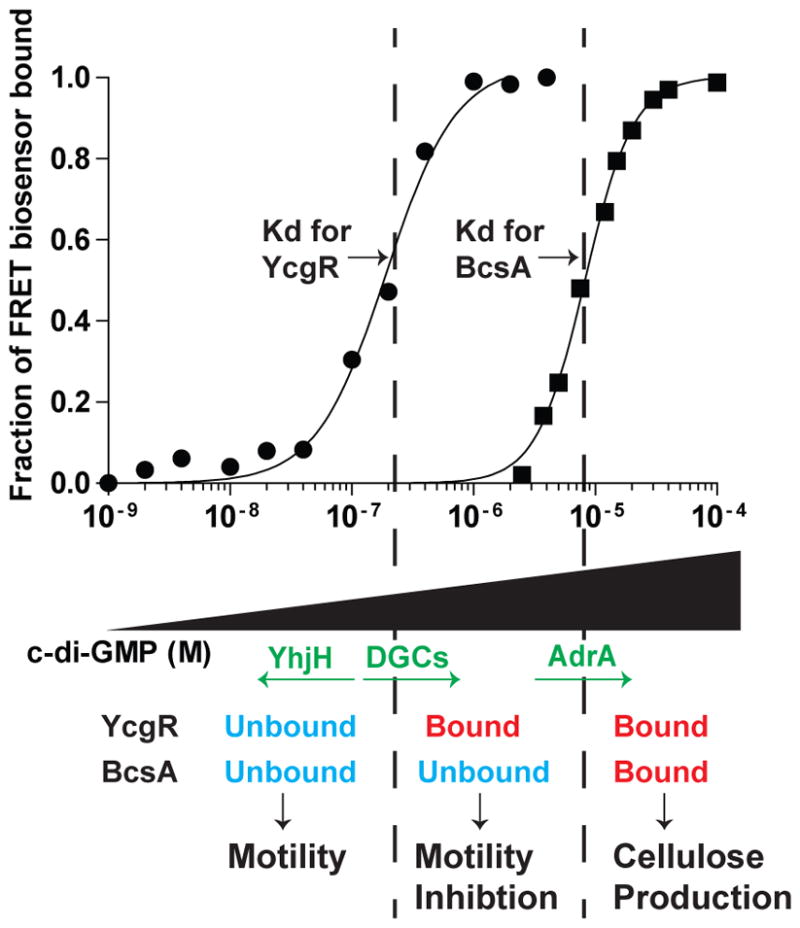
When cellular c-di-GMP levels are kept low by PDEs such as YhjH, neither PilZ domain protein is bound to c-di- GMP, resulting in a motile cell that does not produce cellulose. As cellular DGCs increase the concentration of c-di-GMP past the Kd for YcgR, YcgR becomes bound to c-di-GMP and thus inhibits motility, even at the levels of c-di-GMP that are not high enough to bind BcsA. Activation of AdrA expression results in enough c-di-GMP being produced to bind to BcsA, and cellulose synthesis occurs.
In conclusion, our data provides evidence to the hypothesis that specificity in c-di-GMP signaling can be achieved through the affinities of downstream receptors. This theory is consistent with environmental signal integration by DGCs and PDEs resulting in the generation of a specific level of intracellular c-di-GMP. The complex regulation of c-di-GMP likely involves various strategies which will be unraveled by future studies into the c-di-GMP signaling networks of many different bacteria.
Experimental Procedures
Bacterial strains
All Salmonella enterica serovar Typhimurium strains used in this study were S.Typhimurium ATCC 14028s, except for the arabinose-inducible PA1120 strain used to generate Fig 3B, which was constructed on a S. Typhimurium LT2 background. For cloning purposes, Escherichia coli DH5α or DH10β were used (Invitrogen). Chromosomal mutations, insertions, and deletions were generated using the method outlined by Datsenko and Wanner (Datsenko & Wanner, 2000). Antibiotic resistance markers inserted into the genome for the purpose of generating chromosomal mutations were transferred to a genetically unmanipulated strain by P22 transduction, to ensure that the phenotype was due to the intended mutation. Chromosomal deletion mutants were constructed by the substitution of genetic antibiotic resistance cassettes encoding kanamycin, chloramphenicol, or tetracycline resistance, in such a way that deletion did not affect surrounding genes. To insert PA1120 or PA2133 into the arabinose locus, a genetic cassette encoding resistance to tetracycline was first inserted into the chromosome replacing the araBAD genes. Then, primers were used to amplify PA1120 or PA2133 (Kulasakara et al., 2006) from P. aeruginosa PAO1 genomic DNA with flanking regions of homology to the araBAD locus such that, upon transformation of the PCR product, PA1120 or PA2133 replaced the tetracycline resistance cassette. Strains in which the PA1120 or PA2133 genes had replaced the tetracycline resistance cassette were then selected by their growth on agar plates containing chlortetracycline, which selects for tetracycline sensitivity (Bochner et al., 1980). S. Typhimurium 14028s expressing FLAG-tagged BcsA protein was generated by chromosomal insertion, using the tetracycline selection method described above, of a nucleotide sequence encoding the FLAG tag (DYKDDDDK) immediately 5′ of the STOP codon of the bcsA gene. This strain was streaked onto calcofluor agar plates along with the wild-type and bcsA mutant strains, and it was verified that there was no difference in production of cellulose between the wild-type and BcsA-FLAG strains (Fig S2). ycgR-R113A and bcsA-V695R chromosomal mutations were generated using the tetracycline selection method described above with oligonucleotides that encoded the single point mutation. All gene manipulations were designed using the software program Geneious Pro™, and were verified by PCR amplification and sequencing.
Plasmids
The plasmids used for protein expression for in vivo phenotypic analysis in this work were generated in a pMMB67EH background encoding ampicillin resistance (Furste et al., 1986). To make pDGC, PA1120 from P. aeruginosa PAO1 was inserted into pMMB67EH under the control of the tac promoter, and the pET15B RBS (ACTTTAAGAAGGAGATATCAT) was inserted immediately upstream of the PA1120 start codon. To make pDGC-YcgR, ycgR and its corresponding promoter region (118 bp taken upstream of the ycgR start codon) were cloned downstream of PA1120 in pDGC. To prevent read-through of the ycgR gene whenever PA1120 was induced with IPTG, the transcriptional terminator from phage T4 gene 32 was inserted between the end of PA1120 and the beginning of the ycgR promoter region (Frey & Krisch, 1985). The ycgR-R113A mutation was generated in this plasmid background using the Quikchange XL™ kit (Agilent) with primers containing the mutation. To generate plasmids encoding PilZ-based FRET constructs for in vitro protein purification, a pET15B plasmid containing the N-terminal His-tagged yfp- cfp FRET fusion pair under the control of a tac promoter was used as previously described (Christen et al., 2010). PilZ domain proteins were cloned into the SpeI and KpnI sites between the yfp and cfp genes to generate plasmids that would result in the production of His-tagged, FRET fusion proteins when their expression was induced with IPTG. BcsA of S. Typhimurium and PA3542 of P. aeruginosa are membrane proteins, so the regions of the genes comprising the PilZ domains were cloned into the FRET construct (encoding amino acid residues 691–791 for BcsA and 299–389 for PA3542). A truncated version of PA3353 was generated lacking the residues corresponding to the first alpha helix (amino acid residues 1–21), since the full-length protein did not demonstrate a change in FRET between the bound and unbound states that was large enough to accurately measure binding affinity. The BcsA V695R and YcgR R113A point mutations were made on the corresponding pET15B fusion construct backgrounds using the Quikchange XL™ kit (Agilent) with primers containing the mutation. pYcgR-Spy, encoding gentamicin resistance and the YcgR FRET-based in vivo biosensor, was obtained from Christen et al (Christen et al., 2010). This plasmid contains the gene mYPet_synthYcgR_mCYPet under the control of an IPTG-inducible promoter. In this plasmid, the ycgR gene, which had been codon-optimized to remove rare codons during expression in S. Typhimurium, was cloned between the mYPet and mCYPet genes in SpeI and ACC651 sites, resulting in mYPet-YcgR-mCYPet fusion protein expression in the presence of IPTG. pBcsA- Spy was constructed by digesting pET15B-BcsAPilZSpy with the restriction enzymes SpeI and ACC651 (FastDigest®, Fermentas) and ligating the released bcsA PilZ region into pYcgR-Spy (Rapid DNA Ligation Kit, Fermentas) that had also been digested with SpeI and ACC651, releasing the ycgR fragment. All plasmids were designed using the software program Geneious Pro™, and were verified by PCR amplification and sequencing.
Growth Conditions
Unless otherwise stated, cells were grown in Luria Broth at 37°C with shaking, in the presence of antibiotics, arabinose, IPTG, and/or calcofluor (Fluorescence Brightener 28, Spectrum Chemical MFG) when appropriate. Antibiotic concentrations were: ampicillin, 100 μg ml−1; chloramphenicol, 20 μg ml−1; gentamicin, 15 μg ml−1; kanamycin, 45 μg ml−1; tetracycline, 10 μg ml−1.
Fluorescence spectra and binding affinity measurements
YPet-PilZ-CyPet fusion proteins were expressed in Escherichia coli BL21(DE3) cells and underwent purification through Ni-NTA resin as previously described (Christen et al., 2010). For the affinity measurements of BcsA and YcgR, and their respective affinity mutants, the FRET constructs were further purified by size exclusion chromatography using a Superdex™ 200 10/300 GL column (GE Healthcare). Affinity measurements of PilZ-based FRET constructs were calculated using methods developed by Christen et al (Christen et al., 2010). Briefly, c-di-GMP was added to purified FRET fusion protein in 96- well black plates (Greiner), and the amount of FRET was quantified on an Envision multilabel plate reader (PerkinElmer). Fluorescence spectra were recorded at 24°C using the quad monochromator (excitation at 425 nm, emission scan from 460 to 560 nm in 2 nm intervals) using 50–500 nM biosensor in PBS pH 7.4 with 10% glycerol, 200 mM NaCl, and 5 mM β-mercaptoethanol. Emission spectra were normalized to the total amount of fluorescence emission over the entire scan. Binding affinity was determined by adding increasing concentrations of c-di-GMP and measuring the resulting steady-state FRET/CFP ratio using a filter-based measurement method (CFP excitation filter CWL 430 nm, BW 24 nm; CFP emission filter CWL 485 nm, BW 14 nm; YFP emission filter CWL 535 nm, BW 30 nm, dual dichroic mirror D450/D505 nm) to determine FRET ratios from 24°C to 37°C in 1°C intervals. In the filter-based method, the fraction of FRET biosensor bound to c-di-GMP was calculated for each biosensor by measuring the FRET/CFP ratio at each indicated c-di-GMP concentration, subtracting the unbound FRET/CFP ratio, and dividing this number by the difference between the bound and unbound FRET/CFP ratios. A number of 0 indicates that the FRET biosensor is completely unbound to c-di-GMP, and a number of 1 indicates that the FRET biosensor is completely bound to c-di-GMP. Binding affinities and Hill coefficients were calculated by fitting a curve to the plot of FRET ratio over c-di-GMP concentration in GraphPad Prism™.
Soft-agar motility plate assays
Strains were inoculated as overnight cultures in LB at 37°C with shaking with the appropriate antibiotic (if applicable). The following morning, strains were diluted to OD600 of 0.1. 1–1.5 μl were then spotted onto 0.3–0.35% agar LB plates. Plates were incubated at 37°C for 9–13 hours, then scanned using an Epson® Perfection 4990 scanner and imported as TIFF files. The diameter of swimming of each strain was measured by using the magic wand tool in Adobe™ Photoshop™ CS5. The data was analyzed using a Student’s two-tailed T test.
Cellulose production
The calcofluor plates used in this work were LB-agar plates containing 50 μg ml−1 calcofluor (Fluorescence Brightener 28, Spectrum Chemical MFG). Strains were streaked on calcofluor plates and incubated overnight (37°C) or for 2–3 days (24–30°C). Plates were then analyzed by illumination with an excitation wavelength for of 365 nm unless otherwise specified using a UVP Bioimaging System© equipped with a digital camera for capturing images. For the quantitative calcofluor measurements shown in Fig. 3B, S. Typhimurium ara::DGC, ara::DGC bcsA-V695R, or ara::DGC ΔbcsA were incubated overnight at 37°C. The next day, cells were diluted to an OD600 of 0.02, incubated at 37°C with shaking until the OD reached 3.3, then diluted to an OD600 of 0.0625. 8 μl of each strain were spotted using a multichannel pipettor into each well of a 96-well black plate (Greiner) with 200 μl of calcofluor agar (3 μg ml−1) and the indicated amount of arabinose. After incubation at 37°C for 20 hours, the intensity of calcofluor fluorescence was then quantified by reading the fluorescence values in an ENVISION multilabel reader (PerkinElmer). The data was analyzed using a Student’s two-tailed T test.
Measurement of in vivo FRET/CFP ratios in individual live cells
FRET analyses in individual cells were performed using the protocols developed by Christen et. al. (Christen et al., 2010). To determine FRET/CFP ratios in S. Typhimurium 14028s and an isogenic yhjH mutant, cells were inoculated into one ml LB with gentamicin and 75 μM IPTG, and incubated at 30°C overnight with shaking. The next day, cells were diluted into 25 ml fresh LB with 15 μg ml−1 gentamicin and 500 μM IPTG at an OD600 of 0.02. Cells were incubated at 37°C with shaking for approximately 2 hours until the OD600 reached 0.2. To prepare samples for fluorescence microscopy, cells were centrifuged, washed in M63 media (11 mM KH2PO4, 20 mM K2HPO4, 7.5 mM (NH4)2SO4, 100 mM glycerol, 5 μM Fe2+NH4SO4, 2 mM MgCl2, 10 mM NaCl, 15 μg ml−1 gentamicin, 500 μM IPTG), and resuspended in M63 media to an OD of 2.0. M63 media was used in these experiments since LB media demonstrates autofluorescence at the wavelengths used for imaging. 1.5 μl of resuspended cells were then spotted onto 0.75 mm thick agar pads (M63 media with 1% agarose) on prewarmed, prewashed 1 mm microscope slides (Thermo Fisher Scientific) and allowed to dry. A prewarmed number 1.5 glass coverslip (Corning Life Sciences) was applied to the agar pads, and the coverslip was sealed to the slide using a glue gun to prevent liquid evaporation. Fluorescence microscopy images were acquired using a Nikon Eclipse TIE inverted, wide-field microscope prewarmed to 37°C using the same hardware and software as previously published (Christen et al., 2010). Exposure times for the determination of intensities in the FRET, CFP, and YFP channels were 1000, 1300, and 200 ms, respectively, with a conversion gain of 2x and 2×2 binning. For each image, all channels were exported in a TIFF file format by Nikon Elements analytical software and analyzed in ImageJ software as described previously (Christen et al., 2010). The average intensity values for each region of interest were then exported to Microsoft® Excel software. Regions of aggregated FRET protein, represented by localized, unphysiologically high intensity values in either the CFP or YFP channels near one pole of the cell were deselected when observed and were not counted in the analysis. Net FRET (nFRET) was calculated by subtracting spectral bleedthrough and direct YFP excitation from the FRET intensity value (Christen et al., 2010, Xia & Liu, 2001). To control for brightness, nFRET was normalized to the intensity of the CFP channel (Vanderklish et al., 2000).
Measurement of cellulose production in swimming agar plates
For determination of cellulose production in swimming agar plates, S. Typhimurium strains ΔyhjH, ΔyhjH ΔbcsA, pDGC, and pDGC ΔbcsA were inoculated as described above onto 0.3% agar swimming plates containing 50 μg ml−1 calcofluor. To induce production of the DGC PA1120, IPTG had been added to the plate at a concentration of 100 μM. Plates were incubated at 37°C for 12 hours, then scanned using an Epson® Perfection 4990 scanner and imaged using a UVP Bioimaging System© with the excitation wavelength at 254 nm.
Supplementary Material
Acknowledgments
We thank Richard Pfuetzner for his biochemical expertise during in vitro protein purification. Erez Mills provided helpful discussion throughout this work. This work was supported by an NSF Graduate Research Fellowship, an ARCS Foundation Fellowship, and the National Institute for Allergy and Infectious Diseases.
References
- Amikam D, Galperin MY. PilZ domain is part of the bacterial c-di-GMP binding protein. Bioinformatics. 2006;22:3–6. doi: 10.1093/bioinformatics/bti739. [DOI] [PubMed] [Google Scholar]
- Benach J, Swaminathan SS, Tamayo R, Handelman SK, Folta-Stogniew E, Ramos JE, Forouhar F, Neely H, Seetharaman J, Camilli A, Hunt JF. The structural basis of cyclic diguanylate signal transduction by PilZ domains. EMBO J. 2007;26:5153–5166. doi: 10.1038/sj.emboj.7601918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner BR, Huang HC, Schieven GL, Ames BN. Positive selection for loss of tetracycline resistance. J Bacteriol. 1980;143:926–933. doi: 10.1128/jb.143.2.926-933.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm A, Kaiser M, Li H, Spangler C, Kasper CA, Ackermann M, Kaever V, Sourjik V, Roth V, Jenal U. Second messenger-mediated adjustment of bacterial swimming velocity. Cell. 2010;141:107–116. doi: 10.1016/j.cell.2010.01.018. [DOI] [PubMed] [Google Scholar]
- Christen B, Christen M, Paul R, Schmid F, Folcher M, Jenoe P, Meuwly M, Jenal U. Allosteric control of cyclic di-GMP signaling. J Biol Chem. 2006;281:32015–32024. doi: 10.1074/jbc.M603589200. [DOI] [PubMed] [Google Scholar]
- Christen M, Christen B, Allan MG, Folcher M, Jeno P, Grzesiek S, Jenal U. DgrA is a member of a new family of cyclic diguanosine monophosphate receptors and controls flagellar motor function in Caulobacter crescentus. Proc Natl Acad Sci U S A. 2007;104:4112–4117. doi: 10.1073/pnas.0607738104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christen M, Kulasekara HD, Christen B, Kulasekara BR, Hoffman LR, Miller SI. Asymmetrical distribution of the second messenger c-di-GMP upon bacterial cell division. Science. 2010;328:1295–1297. doi: 10.1126/science.1188658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K- 12 using PCR products. Proc Natl Acad Sci U S A. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerig A, Abel S, Folcher M, Nicollier M, Schwede T, Amiot N, Giese B, Jenal U. Second messenger-mediated spatiotemporal control of protein degradation regulates bacterial cell cycle progression. Genes Dev. 2009;23:93–104. doi: 10.1101/gad.502409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvel J, Bertinetti D, Moller S, Schwede F, Morr M, Wissing J, Radamm L, Zimmermann B, Genieser HG, Jansch L, Herberg FW, Haussler S. A chemical proteomics approach to identify c-di-GMP binding proteins in Pseudomonas aeruginosa. J Microbiol Methods. 2012;88:229–236. doi: 10.1016/j.mimet.2011.11.015. [DOI] [PubMed] [Google Scholar]
- Fang X, Gomelsky M. A post-translational, c-di-GMP-dependent mechanism regulating flagellar motility. Mol Microbiol. 2010;76:1295–1305. doi: 10.1111/j.1365-2958.2010.07179.x. [DOI] [PubMed] [Google Scholar]
- Frey J, Krisch HM. Omega mutagenesis in gram-negative bacteria: a selectable interposon which is strongly polar in a wide range of bacterial species. Gene. 1985;36:143–150. doi: 10.1016/0378-1119(85)90078-2. [DOI] [PubMed] [Google Scholar]
- Frye J, Karlinsey JE, Felise HR, Marzolf B, Dowidar N, McClelland M, Hughes KT. Identification of new flagellar genes of Salmonella enterica serovar Typhimurium. J Bacteriol. 2006;188:2233–2243. doi: 10.1128/JB.188.6.2233-2243.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furste JP, Pansegrau W, Frank R, Blocker H, Scholz P, Bagdasarian M, Lanka E. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene. 1986;48:119–131. doi: 10.1016/0378-1119(86)90358-6. [DOI] [PubMed] [Google Scholar]
- Galperin MY, Nikolskaya AN, Koonin EV. Novel domains of the prokaryotic two- component signal transduction systems. FEMS Microbiol Lett. 2001;203:11–21. doi: 10.1111/j.1574-6968.2001.tb10814.x. [DOI] [PubMed] [Google Scholar]
- Garcia B, Latasa C, Solano C, Garcia-del Portillo F, Gamazo C, Lasa I. Role of the GGDEF protein family in Salmonella cellulose biosynthesis and biofilm formation. Mol Microbiol. 2004;54:264–277. doi: 10.1111/j.1365-2958.2004.04269.x. [DOI] [PubMed] [Google Scholar]
- Gualdi L, Tagliabue L, Bertagnoli S, Ierano T, De Castro C, Landini P. Cellulose modulates biofilm formation by counteracting curli-mediated colonization of solid surfaces in Escherichia coli. Microbiology. 2008;154:2017–2024. doi: 10.1099/mic.0.2008/018093-0. [DOI] [PubMed] [Google Scholar]
- Guvener ZT, Harwood CS. Subcellular location characteristics of the Pseudomonas aeruginosa GGDEF protein, WspR, indicate that it produces cyclic-di-GMP in response to growth on surfaces. Mol Microbiol. 2007;66:1459–1473. doi: 10.1111/j.1365-2958.2007.06008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha DG, Merritt JH, Hampton TH, Hodgkinson JT, Janecek M, Spring DR, Welch M, O’Toole GA. 2-Heptyl-4-quinolone, a precursor of the Pseudomonas quinolone signal molecule, modulates swarming motility in Pseudomonas aeruginosa. J Bacteriol. 2011;193:6770–6780. doi: 10.1128/JB.05929-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengge R. Principles of c-di-GMP signalling in bacteria. Nat Rev Microbiol. 2009;7:263–273. doi: 10.1038/nrmicro2109. [DOI] [PubMed] [Google Scholar]
- Hickman JW, Harwood CS. Identification of FleQ from Pseudomonas aeruginosa as a c-di- GMP-responsive transcription factor. Mol Microbiol. 2008;69:376–389. doi: 10.1111/j.1365-2958.2008.06281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager R, Russwurm C, Schwede F, Genieser HG, Koesling D, Russwurm M. Activation of PDE10 and PDE11 Phosphodiesterases. J Biol Chem. 2012;287:1210–1219. doi: 10.1074/jbc.M111.263806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jares-Erijman EA, Jovin TM. FRET imaging. Nat Biotechnol. 2003;21:1387–1395. doi: 10.1038/nbt896. [DOI] [PubMed] [Google Scholar]
- Kader A, Simm R, Gerstel U, Morr M, Romling U. Hierarchical involvement of various GGDEF domain proteins in rdar morphotype development of Salmonella enterica serovar Typhimurium. Mol Microbiol. 2006;60:602–616. doi: 10.1111/j.1365-2958.2006.05123.x. [DOI] [PubMed] [Google Scholar]
- Ko J, Ryu KS, Kim H, Shin JS, Lee JO, Cheong C, Choi BS. Structure of PP4397 reveals the molecular basis for different c-di-GMP binding modes by Pilz domain proteins. J Mol Biol. 2010;398:97–110. doi: 10.1016/j.jmb.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Ko M, Park C. Two novel flagellar components and H-NS are involved in the motor function of Escherichia coli. J Mol Biol. 2000;303:371–382. doi: 10.1006/jmbi.2000.4147. [DOI] [PubMed] [Google Scholar]
- Krasteva PV, Fong JC, Shikuma NJ, Beyhan S, Navarro MV, Yildiz FH, Sondermann H. Vibrio cholerae VpsT regulates matrix production and motility by directly sensing cyclic di-GMP. Science. 2010;327:866–868. doi: 10.1126/science.1181185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulasakara H, Lee V, Brencic A, Liberati N, Urbach J, Miyata S, Lee DG, Neely AN, Hyodo M, Hayakawa Y, Ausubel FM, Lory S. Analysis of Pseudomonas aeruginosa diguanylate cyclases and phosphodiesterases reveals a role for bis-(3′-5′)-cyclic-GMP in virulence. Proc Natl Acad Sci U S A. 2006;103:2839–2844. doi: 10.1073/pnas.0511090103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone JG, Jaeger T, Spangler C, Ritz D, Spang A, Arrieumerlou C, Kaever V, Landmann R, Jenal U. YfiBNR mediates cyclic di-GMP dependent small colony variant formation and persistence in Pseudomonas aeruginosa. PLoS Pathog. 2010;6:e1000804. doi: 10.1371/journal.ppat.1000804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massie JP, Reynolds EL, Koestler BJ, Cong JP, Agostoni M, Waters CM. Quantification of high-specificity cyclic diguanylate signaling. Proc Natl Acad Sci U S A. 2012 doi: 10.1073/pnas.1115663109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merighi M, V, Lee T, Hyodo M, Hayakawa Y, Lory S. The second messenger bis-(3′-5′)-cyclic- GMP and its PilZ domain-containing receptor Alg44 are required for alginate biosynthesis in Pseudomonas aeruginosa. Mol Microbiol. 2007;65:876–895. doi: 10.1111/j.1365-2958.2007.05817.x. [DOI] [PubMed] [Google Scholar]
- Merritt JH, Ha DG, Cowles KN, Lu W, Morales DK, Rabinowitz J, Gitai Z, O’Toole GA. Specific control of Pseudomonas aeruginosa surface-associated behaviors by two c-di-GMP diguanylate cyclases. MBio. 2010;1 doi: 10.1128/mBio.00183-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills E, I, Pultz S, Kulasekara HD, Miller SI. The bacterial second messenger c-di-GMP: mechanisms of signalling. Cell Microbiol. 2011;13:1122–1129. doi: 10.1111/j.1462-5822.2011.01619.x. [DOI] [PubMed] [Google Scholar]
- Miyawaki A, Llopis J, Heim R, McCaffery JM, Adams JA, Ikura M, Tsien RY. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature. 1997;388:882–887. doi: 10.1038/42264. [DOI] [PubMed] [Google Scholar]
- Navarro MV, De N, Bae N, Wang Q, Sondermann H. Structural analysis of the GGDEF-EAL domain-containing c-di-GMP receptor FimX. Structure. 2009;17:1104–1116. doi: 10.1016/j.str.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen AW, Daugherty PS. Evolutionary optimization of fluorescent proteins for intracellular FRET. Nat Biotechnol. 2005;23:355–360. doi: 10.1038/nbt1066. [DOI] [PubMed] [Google Scholar]
- Nikolaev VO, Gambaryan S, Lohse MJ. Fluorescent sensors for rapid monitoring of intracellular cGMP. Nat Methods. 2006;3:23–25. doi: 10.1038/nmeth816. [DOI] [PubMed] [Google Scholar]
- Ogasawara H, Yamamoto K, Ishihama A. Role of the biofilm master regulator CsgD in cross- regulation between biofilm formation and flagellar synthesis. J Bacteriol. 2011;193:2587–2597. doi: 10.1128/JB.01468-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi T, Galiacy SD, Briscoe G, Erickson HP. An experimental study of GFP-based FRET, with application to intrinsically unstructured proteins. Protein Sci. 2007;16:1429–1438. doi: 10.1110/ps.072845607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul K, Nieto V, Carlquist WC, Blair DF, Harshey RM. The c-di-GMP binding protein YcgR controls flagellar motor direction and speed to affect chemotaxis by a “backstop brake” mechanism. Mol Cell. 2010;38:128–139. doi: 10.1016/j.molcel.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesavento C, Becker G, Sommerfeldt N, Possling A, Tschowri N, Mehlis A, Hengge R. Inverse regulatory coordination of motility and curli-mediated adhesion in Escherichia coli. Genes Dev. 2008;22:2434–2446. doi: 10.1101/gad.475808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt JT, Tamayo R, Tischler AD, Camilli A. PilZ domain proteins bind cyclic diguanylate and regulate diverse processes in Vibrio cholerae. J Biol Chem. 2007;282:12860–12870. doi: 10.1074/jbc.M611593200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramelot TA, Yee A, Cort JR, Semesi A, Arrowsmith CH, Kennedy MA. NMR structure and binding studies confirm that PA4608 from Pseudomonas aeruginosa is a PilZ domain and a c-di-GMP binding protein. Proteins. 2007;66:266–271. doi: 10.1002/prot.21199. [DOI] [PubMed] [Google Scholar]
- Rehm H. Protein Biochemistry and Proteomics. Elsevier; 2006. [Google Scholar]
- Romling U. Characterization of the rdar morphotype, a multicellular behaviour in Enterobacteriaceae. Cell Mol Life Sci. 2005;62:1234–1246. doi: 10.1007/s00018-005-4557-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romling U, Bian Z, Hammar M, Sierralta WD, Normark S. Curli fibers are highly conserved between Salmonella typhimurium and Escherichia coli with respect to operon structure and regulation. J Bacteriol. 1998a;180:722–731. doi: 10.1128/jb.180.3.722-731.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romling U, Rohde M, Olsen A, Normark S, Reinkoster J. AgfD, the checkpoint of multicellular and aggregative behaviour in Salmonella typhimurium regulates at least two independent pathways. Mol Microbiol. 2000;36:10–23. doi: 10.1046/j.1365-2958.2000.01822.x. [DOI] [PubMed] [Google Scholar]
- Romling U, Sierralta WD, Eriksson K, Normark S. Multicellular and aggregative behaviour of Salmonella typhimurium strains is controlled by mutations in the agfD promoter. Mol Microbiol. 1998b;28:249–264. doi: 10.1046/j.1365-2958.1998.00791.x. [DOI] [PubMed] [Google Scholar]
- Roy AB, Petrova OE, Sauer K. The phosphodiesterase DipA (PA5017) is essential for Pseudomonas aeruginosa biofilm dispersion. J Bacteriol. 2012;194:2904–2915. doi: 10.1128/JB.05346-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy R, Hohng S, Ha T. A practical guide to single-molecule FRET. Nat Methods. 2008;5:507–516. doi: 10.1038/nmeth.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russwurm M, Mullershausen F, Friebe A, Jager R, Russwurm C, Koesling D. Design of fluorescence resonance energy transfer (FRET)-based cGMP indicators: a systematic approach. Biochem J. 2007;407:69–77. doi: 10.1042/BJ20070348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryjenkov DA, Simm R, Romling U, Gomelsky M. The PilZ domain is a receptor for the second messenger c-di-GMP: the PilZ domain protein YcgR controls motility in enterobacteria. J Biol Chem. 2006;281:30310–30314. doi: 10.1074/jbc.C600179200. [DOI] [PubMed] [Google Scholar]
- Sanchez-Torres V, Hu H, Wood TK. GGDEF proteins YeaI, YedQ, and YfiN reduce early biofilm formation and swimming motility in Escherichia coli. Appl Microbiol Biotechnol. 2011;90:651–658. doi: 10.1007/s00253-010-3074-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmer T, Jenal U. Structural and mechanistic determinants of c-di-GMP signalling. Nat Rev Microbiol. 2009;7:724–735. doi: 10.1038/nrmicro2203. [DOI] [PubMed] [Google Scholar]
- Shin JS, Ryu KS, Ko J, Lee A, Choi BS. Structural characterization reveals that a PilZ domain protein undergoes substantial conformational change upon binding to cyclic dimeric guanosine monophosphate. Protein Sci. 2011;20:270–277. doi: 10.1002/pro.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simm R, Lusch A, Kader A, Andersson M, Romling U. Role of EAL-containing proteins in multicellular behavior of Salmonella enterica serovar Typhimurium. J Bacteriol. 2007;189:3613–3623. doi: 10.1128/JB.01719-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano C, Garcia B, Latasa C, Toledo-Arana A, Zorraquino V, Valle J, Casals J, Pedroso E, Lasa I. Genetic reductionist approach for dissecting individual roles of GGDEF proteins within the c-di-GMP signaling network in Salmonella. Proc Natl Acad Sci U S A. 2009;106:7997–8002. doi: 10.1073/pnas.0812573106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano C, Garcia B, Valle J, Berasain C, Ghigo JM, Gamazo C, Lasa I. Genetic analysis of Salmonella enteritidis biofilm formation: critical role of cellulose. Mol Microbiol. 2002;43:793–808. doi: 10.1046/j.1365-2958.2002.02802.x. [DOI] [PubMed] [Google Scholar]
- Srivastava D, Harris RC, Waters CM. Integration of cyclic di-GMP and quorum sensing in the control of vpsT and aphA in Vibrio cholerae. J Bacteriol. 2011;193:6331–6341. doi: 10.1128/JB.05167-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudarsan N, Lee ER, Weinberg Z, Moy RH, Kim JN, Link KH, Breaker RR. Riboswitches in eubacteria sense the second messenger cyclic di-GMP. Science. 2008;321:411–413. doi: 10.1126/science.1159519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderklish PW, Krushel LA, Holst BH, Gally JA, Crossin KL, Edelman GM. Marking synaptic activity in dendritic spines with a calpain substrate exhibiting fluorescence resonance energy transfer. Proc Natl Acad Sci U S A. 2000;97:2253–2258. doi: 10.1073/pnas.040565597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Fleming RT, Westbrook EM, Matsumura P, McKay DB. Structure of the Escherichia coli FlhDC complex, a prokaryotic heteromeric regulator of transcription. J Mol Biol. 2006;355:798–808. doi: 10.1016/j.jmb.2005.11.020. [DOI] [PubMed] [Google Scholar]
- Weber H, Pesavento C, Possling A, Tischendorf G, Hengge R. Cyclic-di-GMP-mediated signalling within the sigma network of Escherichia coli. Mol Microbiol. 2006;62:1014–1034. doi: 10.1111/j.1365-2958.2006.05440.x. [DOI] [PubMed] [Google Scholar]
- Whitney JC, Colvin KM, Marmont LS, Robinson H, Parsek MR, Howell PL. Structure of the cytoplasmic region of PelD, a degenerate diguanylate cyclase receptor that regulates exopolysaccharide production in Pseudomonas aeruginosa. J Biol Chem. 2012 doi: 10.1074/jbc.M112.375378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z, Liu Y. Reliable and global measurement of fluorescence resonance energy transfer using fluorescence microscopes. Biophys J. 2001;81:2395–2402. doi: 10.1016/S0006-3495(01)75886-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakikhany K, Harrington CR, Nimtz M, Hinton JC, Romling U. Unphosphorylated CsgD controls biofilm formation in Salmonella enterica serovar Typhimurium. Mol Microbiol. 2010;77:771–786. doi: 10.1111/j.1365-2958.2010.07247.x. [DOI] [PubMed] [Google Scholar]
- Zogaj X, Nimtz M, Rohde M, Bokranz W, Romling U. The multicellular morphotypes of Salmonella typhimurium and Escherichia coli produce cellulose as the second component of the extracellular matrix. Mol Microbiol. 2001;39:1452–1463. doi: 10.1046/j.1365-2958.2001.02337.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.