Abstract
Hemophilic arthropathy is a form of joint disease that develops in secondary to joint bleeding and presents with synovial hypertrophy, cartilage and bony destruction. The arthropathy can develop despite clotting factor replacement and is especially disabling in the aging population. Pathobiological tissue changes are triggered by release of hemoglobin and iron deposition in the joint, but the sequence of events and the molecular mechanisms resulting in joint deterioration are incompletely understood. Treatment options other than clotting factor replacement are limited. Improvements in the treatment of hemophilia necessitate a better understanding of the processes that lead to this disabling condition and better diagnostic tools. Towards that end, studies of the molecular mechanisms leading to the arthropathy, as well as the development of sensitive imaging techniques and biomarkers are needed. These will pave the way to identify the cause of acute pain such as joint bleeding or synovitis, detect early, potentially reversible structural changes, and predict progression of disease. This review describes current imaging techniques and the development of high resolution musculoskeletal ultrasound with power Doppler to afford point-of-care diagnosis and management, the potential utility of diagnostic biomarkers, and summarizes our current knowledge of the pathobiology of hemophilic arthropathy.
Introduction
Patients with severe Factor (F)VIII or FIX deficiency (Hemophilia A or B) suffer from spontaneous joint bleeding in childhood1 that results in “target joints”, defined as joints with several consecutive bleeds within a 6 month period.2 Target joints often progress to hemophilic arthropathy (HA)1, 2 that is characterized by joint deformities, synovial hypertrophy, and cartilage and bone destruction. Compared to episodic treatment of joint bleeds, prophylactic clotting factor treatment can reduce joint bleeding and the development of HA dramatically.3 There is compelling evidence that initiation of prophylaxis in early childhood (< age 2)4 and higher intensity dosing regimens have beneficial effects on joint outcomes.5 However, HA cannot be entirely avoided with clotting factor replacement, as shown by a high percentage of adults with hemophilia from industrialized countries (~30–50% of patients) presenting with clinical arthropathy despite access to prophylaxis since childhood.6–8 These recent studies do not always provide detailed information regarding number of patients on uninterrupted prophylaxis, start or intensity of prophylaxis and adherence, all of which may influence joint outcomes. However, these studies do provide evidence that HA is highly prevalent in the aging population of patients with hemophilia. This is important since HA is a disabling condition that negatively impacts physical activity and quality of life.9, 10 In the last century patients died at a relatively early age and age-related comorbidities were therefore of minor interest. This has changed with the advent of virally safe clotting factor. The life span of hemophilia patients has become comparable to that of the general population11, making diagnosis, prevention and treatment of HA a critical focus of hemophilia care. However, few treatment options are currently available outside of clotting factor replacement, and management chiefly comprises various synovial ablation techniques12, 13 and surgical correction including joint replacement.14, 15 Preventing or slowing the arthropathy will require targeted management, which explains the growing interest in this field. Current efforts mainly focus on three major areas, which are 1) to develop sensitive joint imaging modalities to detect early changes and diagnose the etiology of acute and chronic pain, 2) to explore diagnostic and predictive biomarkers, and 3) to understand the underlying pathobiology of HA. This review summarizes the current progress in all those areas, and aims to bring this information into clinical context.
Novel Imaging Modalities
The desire to explore structural changes and disease burden of hemophilic joints dates back almost one century, when x-ray technology was developed and first applied for the diagnosis and staging of medical conditions.16 From the 1960s to the 1980s, efforts focused on devising radiographic grading systems to document progression of joint disease.17 Two main classification systems were introduced, the Arnold-Hilgartner scale,18 and the Pettersson score during that time.19 These systems differ in several respects. Arnold-Hilgartner scoring includes soft tissue changes and is progressive, whereby the worst imaging finding dictates the stage of arthropathy. Pettersson scoring excludes soft tissue changes and is additive, whereby each abnormality is assigned points until a maximum score is reached. Based on recommendations by the World Federation of Hemophilia, Pettersson scoring has become the most widely used radiographic staging system for several reasons that include ease of administration, exclusion of soft tissue changes that cannot be reliably assessed by x-rays, and a lesser ceiling effect as encountered with the progressive scale.17 At the time, systems such as the Pettersson score were invaluable to document beneficial long-term effects of prophylactic clotting factor treatment on joint outcomes in observational cohorts.3, 20
Since radiography captures only irreversible late stage changes, several new scoring systems using magnetic resonance imaging (MRI) were proposed in the early 2000s.21 MRI permits direct visualization of cartilage, soft tissue, hemosiderin deposits, joint effusions, bony cysts, osteopenia, and marrow edema, and therefore detection of early, possibly reversible, pathology. The increasing adoption of prophylactic rather than episodic clotting factor treatment in children in industrialized countries,8 and the need to detect early, subtle changes to evaluate treatment efficacy in young patients anticipated to have near-normal life spans,11 spurred investigations as to which MRI scale would be most suitable. As with radiography, several progressive and additive scales were developed, and excellent reviews of their advantages, disadvantages and accuracy to quantify pathological tissue changes are provided elsewhere.21–23 In 2012, the International Prophylaxis Study Group merged systems into one, attempting to enable easier comparisons across studies and populations, separating soft tissue and osteochondral changes for more detailed information.24
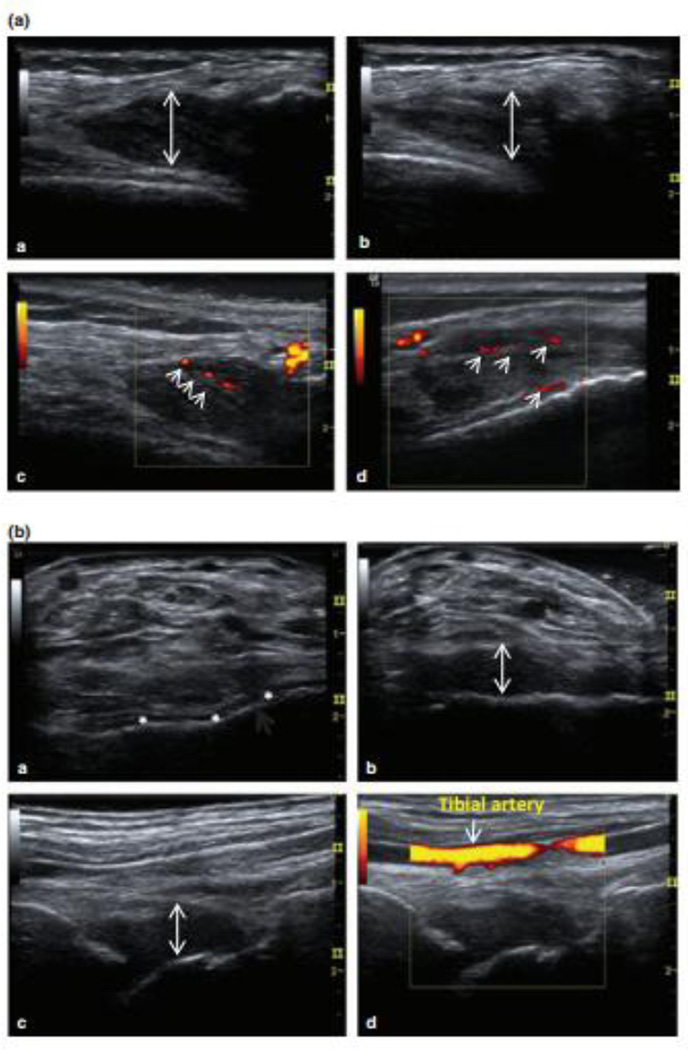
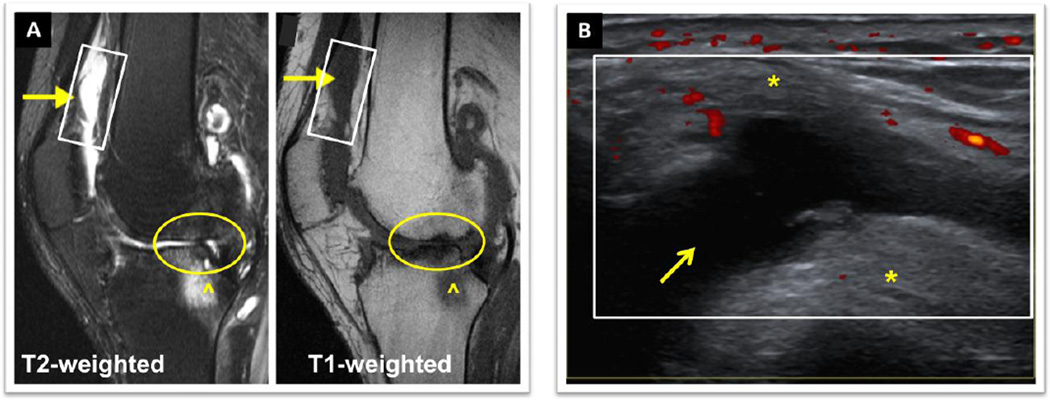
However, problems with MRI include the necessity to sedate children, cost, availability and inability to frequently scan multiple joints. This prompted investigation into alternative new imaging modalities, which led to the adoption of musculoskeletal ultrasound (MSKUS) for the imaging of hemophilic joints and its recent introduction into clinical practice.25–28 Continued innovative development and wide-spread utilization for HA care are expected over the next few years. Advances in ultrasound technology permit high resolution imaging for detailed visualization of anatomy and pathology of the musculoskeletal system, including tendons, muscles, ligaments and fluid. With high-frequency transducers, spatial compounding and power Doppler, soft tissue resolution approaches, or is sometimes greater than, the sensitivity of routine MRI.29–31 Comparison of MSKUS to MRI in hemophilia has demonstrated reliable detection of soft tissue and osteochondral abnormalities, cartilage destruction and effusions.32–34 MSKUS has been demonstrated to be a critical tool to differentiate if bleeding is associated with acute and/or chronic pain and to determine if sprains, tendon/ligament tears or enthesopathies contribute to pain (Figure 1). MSKUS examination with sonopalpation is able to distinguish complex bloody from simple serous effusions with high accuracy as confirmed by needle aspiration.25, 35 In addition, power Doppler permits rapid and dynamic assessment of synovial blood flow, thereby demarcating synovitis from fluid. In contrast, conventional MRI cannot easily differentiate joint fluid from synovitis without contrast administration,36 nor can it easily distinguish bloody from non-bloody effusions (Figure 2). The MRI signals of acute/subacute blood products in the joint have not been studied, and the diagnosis of hemarthrosis with MRI depends heavily on the clinical context.
Figure 1. Two case studies illustrating musculoskeletal ultrasound (MSKUS) in the diagnosis of bleeding and non-bleeding episodes during acute painful episodes.
(A) 37-year-old man with severe haemophilia A presented with perceived joint bleeding. a: Axial view of the lateral gutter of the left knee showed heterogeneous hypoechoic intra-articular material (arrow). b: The intra-articular (i/a) material was hardly compressible and not displaceable (arrow) suggestive of thickened synovial tissue. c/d: power Doppler (PD) revealed vascularization of thickened hypervascular synovial tissue without evidence of sonographic effusion. This episode was reclassified as synovitis.
(B) 23-year-old man with severe haemophilia B presented with ankle pain and perceived “arthritis pain”. a: Baseline axial view of the ankle showed thin anechoic joint space in the tibiotalar joint (*). b: Interval examination during pain episode showed marked volume increased tibiotalar synovial space (arrow). Complex echogenic pattern and compressibility of the synovial space were consistent with complex effusion//intra-articular bleed. c/d: Orthogonal view without and with power Doppler demonstrated absence of tissue vascularization. This episode was reclassified as acute haemarthrosis
Ceponis, A. et al. Haemophilia 2013. 19: pp 790–798. Modified. With permission form the journal.
Figure 2. Comparison of MRI and MSKUS of the knee of a 54 year old man with moderate Hemophilia A. Both imaging studies were performed on the same day.
The patient presented with persistent knee swelling and pain 2 weeks after a fall. A) MRI (sagittal view): ^ Marrow edema, O cartilage and meniscus destruction. The arrows (→) indicate areas in the suprapatellar bursa and patellar recesses where diagnostic distinctions of synovial hypertrophy/synovitis from effusion, and of bloody from non-bloody effusions were difficult by conventional MRI. The rectangle depicts the same area scanned with B) MSKUS. MSKUS demonstrated a simple non-bloody effusion (anechoic, compressible area (→)) surrounded by *fat pad and *synovium. Power Doppler (red dots) revealed areas of synovial perfusion. MRI, Magnet Resonance Imaging. MSKUS, Musculoskeletal Ultrasound. University of California San Diego Case Series, Images provided by Annette von Drygalski, MD. Patient provided written informed consent.
The ability to diagnose inflammatory soft tissue changes and synovitis with power Doppler25, 26,33, 35 is important in HA. As in rheumatoid arthritis (RA), synovial hypertrophy is often present and can be accompanied by synovitis characterized by increased synovial blood flow. Power Doppler is sensitive to slow blood flow in the microcirculation,37 and therefore able to quantify synovial perfusion abnormalities. In rheumatic joints, the quantification of power Doppler signals has been validated to evaluate the response to anti-inflammatory agents by comparisons with histological specimens and contrast-enhanced MRI.37 Based on new evidence in hemophilia, positive power Doppler signals in HA are associated with vascular remodeling and joint bleeding,38 whereby bleeding risk increases significantly with increasing power Doppler signals. This observation adds a new dimension and may become an important consideration when monitoring hemophilic joints with power Doppler.
Compared with MRI, MSKUS is rapid, less costly, does not require contrast administration or sedation and is therefore an ideal point-of-care imaging modality.36, 39 Imaging with MSKUS has been validated for a wide spectrum of musculoskeletal pathology in rheumatology, orthopedics and sports medicine, and has been recommended by the American College of Rheumatology as the point-of care imaging modality to assess disease activity.40 For hemophilia, this is of utmost importance. Rather than assuming that a painful joint represents bleeding, MSKUS can diagnose musculoskeletal conditions for which specific treatments other than clotting factor are available. MSKUS is also mobile, with a potential for use in patients’ homes or work place, and it can monitor resolution of hemarthrosis or inflammation.27, 28
However, among the potential difficulties with ultrasound assessment of the joints are the nonvisualization of internal bone structure, bone edema, and the inability to penetrate deeper structures such as cruciate ligaments in the knee. There is no doubt that ultrasound is operator dependent with a learning curve for the inexperienced operator.36 However, diagnostic ultrasound is now emerging rapidly in many medical disciplines including medical education.41 It is expected that appropriate training will result in the prerequisite understanding of anatomy, pathology, tissue discrimination and planes. This will enable non-radiologists to use point-of-care ultrasound to improve diagnosis and inform treatment decisions for a variety of medical conditions in the future.
In addition to the utilization of MSKUS for point-of care imaging of painful hemophilic joints, MSKUS has been proposed for scoring joint health in analogy to radiographic or MRI scales.35, 42, 43 The most recent published scoring system (HEAD-US) uses several defined transducer positions to semi-quantitatively evaluate synovium, cartilage and subchondral bone on low cost portable machines.43 However, this scoring algorithm has not yet been validated, and while good to excellent inter- and intra-operator reliability were reported for experienced radiologists, the same has not yet been established for non-radiologists. Long-term monitoring of joint health with ultrasound holds great promise since the ease of scanning permits fast and frequent monitoring of all joints. In general, MSKUS is evolving rapidly into a promising tool to guide treatment decisions, although the impact of MSKUS-guided treatment decisions on outcomes remains to be documented in future studies.
Biomarkers
As defined by the Food and Drug Administration, a biomarker can be “objectively measured and evaluated as an indicator of normal biologic process, pathogenic process, or pharmacologic response to a therapeutic intervention”.44 Compared to imaging, biomarkers have the advantage of rapidly capturing processes of tissue turnover that indicate structural and/or early changes at a molecular level. In hemophilia, where joint disease is currently treated with multiple self-infusions of costly clotting factor concentrates without knowing whether it is the most appropriate treatment for every patient, availability of biomarkers for diagnosis, prognosis and determination of treatment efficacy is therefore highly desirable. Biomarker analyses could identify patients for targeted treatment to improve outcomes and reduce cost, which has become an important paradigm in the era of precision medicine45.
The ideal properties of a biomarker will vary according to the underlying condition and clinical question being asked. However, to be clinically useful, biomarkers must have high disease specificity, high correlation with disease burden, and be predictive of outcomes if used to gauge treatment success. Biomarker research in hemophilia is complicated by the largely unknown mechanisms that underlie the pathogenesis of HA, including processes associated with repeated joint bleeding. Studies in hemophilia published to date exclusively mirror efforts in osteorarthritis (OA) and RA that have traditionally focused on biomarkers of cartilage and bone turnover46, 47 as a final common denominator of joint destruction. Generally speaking, OA is a degenerative disease of aging, RA an systemic inflammatory disease with a predilection for joints, and HA is caused by joint bleeding. Thus, although OA, RA and HA all result in progressive cartilage destruction, their pathogenesis appears distinct and this needs to be considered when developing markers of early disease activity. Since biomarker research in HA is currently in its infancy, it is relevant to provide a brief background of the studies of biomarkers in OA and RA.
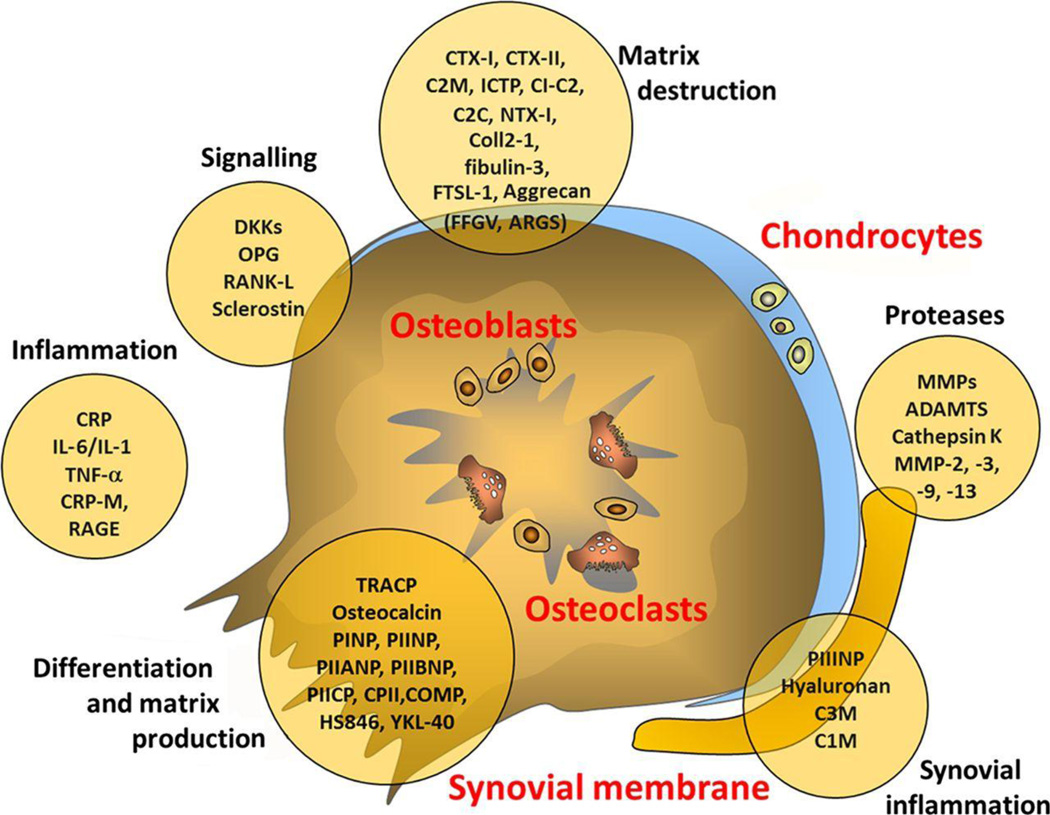
The desire to determine effects of new disease modifying agents and to guide treatment decisions in OA and RA spurred efforts to identify biomarkers over the past decade. Both OA and RA show a patient-specific onset and time course of chronic progressive joint destruction, involving cartilage and bone degradation. Therefore, candidate biomarkers to determine disease activity and predict progression originated mostly at the level of tissue degradation where different pathways converge. Prototypic candidates are neoepitopes of cleaved cartilage matrix components or markers of bone or collagen turnover. It is felt that their levels in urine, serum or synovial fluid may reveal early molecular changes not yet detectable by imaging methods.48 Selected commercially available candidates include C-terminal telopeptides of type I or II collagen (CTX-I, CTX-II), cartilage oligomeric matrix protein (COMP), hyaluronan, various cartilage cleavage products, procollagen type II N-terminal propeptide, chondroitin sulfate (CS)-846 or MMP-3.44 Figure 3 depicts sources of candidate biomarkers explored in OA. Despite intense effort, no single biomarker or composite algorithms involving multiple biomarkers have been validated to be clinically useful.48–51
Figure 3. Sources of possible biomarkers in osteoarthritis.
10–20 C2C, cleavage of type II collagen; C2M, collagen type II-specific neoepitope; C3M, collagen type III-specific neoepitope; Coll 2-1, 9-amino acid peptide of type II collagen (nitrated form Coll 2-1 NO2); COMP, cartilage oligomeric protein; CPII, type II collagen propeptide; CRP, C reactive protein; CTX, C-terminal telopeptide of collagen; DKK, wnt inhibitor; FSTL-1, follistatin-like protein 1; ICTP, type I collagen-derived cross-linked carboxy-terminal telopeptide; IL, interleukin; MMP, matrix metalloproteinase; NTX, N-terminal telopeptide of collagen OPG, osteoprotegerin; PIIANP, N-propeptide IIA of type II collagen; PIIBNP, N-propeptide IIB of type II collagen; PIICP, C-propeptide of collagen type II; PINP, N-propeptide of type I collagen; PIINP, N-propeptide of type II collagen; RANK-L, receptor activator of nuclear factor κB ligand; RAGE, receptor for advanced glycation endproducts; TNF, tumour necrosis factor.
Lotz M, et al. Postgrad Med J 2014; 90: pp 171–178. With permission from the Journal.
The identification of markers of synovitis and inflammation has been pursued more in RA than OA, since synovial inflammation is a hallmark of RA and associated with progressive joint deterioration. However, the fact that RA is a systemic inflammatory disease with immunologic abnormalities not only in joints but also other organs52 has complicated the identification of useful biomarkers. Inflammatory markers such as C-reactive protein (CRP) may be elevated in RA, but reflect overall systemic inflammation and are not specific for synovitis or joint tissue status. This is underscored by the fact that systemic and joint tissue levels of inflammatory cytokines such as TNF-α and IL-1β do not always correlate in RA, making it difficult to distinguish synovial contributions from overall inflammation. Consequently, it is difficult to use inflammatory biomarkers for treatment decisions at the joint level.53, 54 Therefore, biomarkers (for example CRP) are embedded into clinical algorithms to determine disease activity,55 whereby the abnormal vascular perfusion associated with synovitis is often quantified by MSKUS.56
The anticipated availability of many new clotting factor products and treatments for hemophilia has spurred new efforts to identify tailored treatment approaches to individual patients, similar to efforts in OA and RA. The identification of specific biomarkers that could be applied to customize the choice of treatment according to inter-individual bleeding propensity, personal clotting factor pharmacokinetics, subclinical bleeding and other variables, would be a major step forward. Biomarkers that could diagnose bleeding or joint destruction, or could assess response to treatment, would considerably advance hemophilia care. It has been shown that diagnosing joint bleeding by patient and/or physician perception is imprecise25 and that current imaging techniques for cartilage damage, bone and soft tissue changes lack sensitivity for early changes following treatment intervention.57, 58,19, 59 Hence, the identification of biomarkers, that are also predictive of long-term joint health, would permit point-of-care diagnosis and decisions for individual treatment, thereby avoiding delays in the detection of joint destruction.
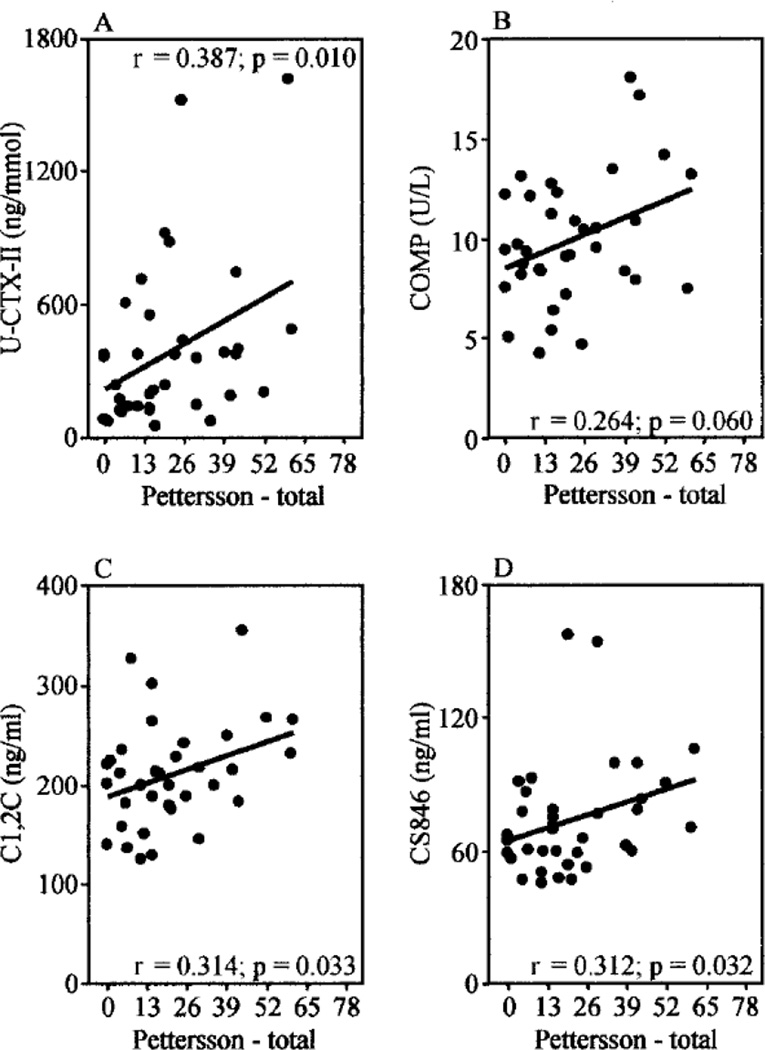
Analysis of biomarkers in HA is currently limited to a few studies. A small cross-sectional analysis of markers of bone and cartilage degradation demonstrated that a composite score of levels of CTX-II, COMP and CS-846 correlated best with radiographic joint changes during bleed-free intervals, while CTX-I and cartilage cleavage products (C1, 2C and C2C) did not (Figure 4). CTX-II, COMP and CS-846 are all indicative of cartilage degradation, originating from different matrix components.46 Interestingly, a significant rise of CTX-II and CS-846, but not COMP, was demonstrated within 5 days after self-reported joint bleeding in 10 adult hemophilia patients.47 The individual percentage increase for CTX-II and CS-846 in response to bleeding ranged from ~25 to 500% and from ~80 to 120%, returning gradually to baseline during the following 10 days. The wide inter-individual initial rise of CTX-II may have been related to imprecision of self-reported bleeding as etiology of painful episodes,25, 26 bleeding volume or individual joint responses to the blood. For CTX-II, similar findings were noted in a canine model when blood was injected into the joint.47 In summary, these pilot studies suggest that some biomarkers of cartilage degradation correlate to some extent with radiographic joint destruction in hemophilia, and that joint bleeding can cause their acute, but reversible, rise. However, the predictive value of such biomarker levels for progression of HA is undetermined. VEGF as a proangiogenic mediator may be another interesting direction for synovial changes. VEGF is elevated in hemophilia patients with arthropathy compared to patients with other bleeding disorders without joint involvement.60 VEGF is also elevated in RA and OA61, 62 and levels appear to be related to disease activity and synovitis.60, 62, 63 VEGF is thought to contribute to the pathogenesis of arthritis by various mechanisms, although its correlation with extent of synovitis, dynamic neoangiogenesis and long-term joint outcomes requires more study.64 In general, the detection of early tissue changes and their meaning for progression of arthropathy require the discovery of new biomarkers specific for HA. Towards that end, defining the pathobiology of HA will become paramount and will pave the way for innovative biomarker research including proteomics, mass spectrometry and molecular signatures.
Figure 4.
The correlation of radiographic joint damage with biomarker levels of cartilage turn-over with radiographic Pettersson scores in hemophilic arthropathy. Jansen N.W.D., et al. Arthritis & Rheumatism 2009; 60: pp 290–298. With permission from the Journal.
The Pathobiology of Hemophilic Arthropathy
The pathobiology of HA is unique in that it is influenced by repeated release of hemoglobin and iron depositions in the joint. Although HA may resemble OA or RA, and some molecular changes may have common denominators such as inflammation or cartilage destruction,65 HA does not fit either category and remains poorly understood. Whereas the clinical findings of advanced HA are well documented, the pathobiology is not. In particular, there remains a large knowledge gap as to why bleeding occurs into joints, and what facilitates re-bleeding and the development of target joints sometimes despite clotting factor administration. Also, the heterogeneity of bleeding phenotypes and severity of arthropathy is not understood; especially genetic modifiers such as the presence of genetic risk factors for thrombosis in the general population (e.g. Factor VLeiden mutation) remain to be fully appreciated.66, 67
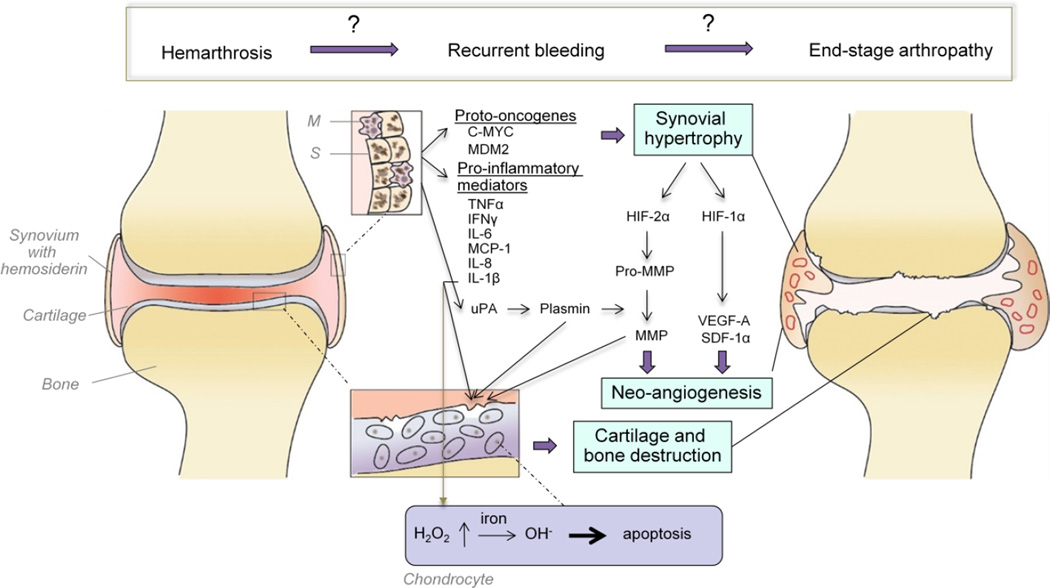
The current paradigm for HA proposes that recurrent bleeding induces hyper-reactive synovial changes including the formation of villi protruding into the joint space. Hypertrophic synovium is often associated with increased vascular perfusion, which is a hallmark of synovial inflammation, termed synovitis. Metabolic changes in cartilage and subchondral bone ensue and result in their destruction.65, 68, 69 This current model of HA is based on studies of synovial and cartilage changes in response to joint bleeding using in vitro assays, ex vivo histology and animal models of experimental HA. However, the relative contributions of various molecular pathways involved and the proper sequence of events clearly need further definition. A view of our current understanding of HA is shown in Figure 5.
Figure 5. Pathobiology of hemophilic arthropathy. A provisional scheme with many open questions.
Erythrocytes in the joint space are taken up by synoviocytes (S) and tissue macrophages (M), that store iron released from erythrocytes as hemosiderin. The expression of the oncoproteins C-MYC and MDM2 in response to iron results in synovial hypertrophy. Synovial hypertrophy creates a hypoxic environment causing a rise in hypoxia inducible factor (HIF)-1α and HIF-2α, which induce increased expression of the pro-angiogenic mediators vascular endothelial growth factor-A (VEGF-A) and stromal-cell derived factor 1α (SDF-1α), as well as pro-matrix metalloproteinases (pro-MMP). VEGF-A stimulates synovial neoangiogenesis and plasmin-mediated conversion to MMPs results in glycosaminoglycan release from the cartilage matrix, and cartilage and subchondral bone destruction. Synoviocytes and tissue macrophages also release pro-inflammatory mediators, such as tumor necrosis factor alpha (TNFα), interferon-γ, interleukin (IL)-1β, IL-6, monocyte chemoattractant protein-1 (MCP-1) fueling these processes. Secreted IL-1β induces increased production of hydrogen peroxide (H2O2) in the chondrocytes, which upon reaction with iron (Fe2+) forms cytotoxic hydroxyl radicals (OH−) and prompts chondrocyte apoptosis. Neoangiogenesis and vascular remodeling are assumed to maintain a vicious cycle of re-bleeding that results in progressive hemophilic arthropathy, characterized by synovial hypertrophy, cartilage and bone destruction and joint deformities.
Synovial hypertrophy
Erythrocytes in the joint are removed through their uptake by macrophages and synoviocytes. Iron released from erythrocytes is bound as hemosiderin and cleared.70–72 However, with excessive and/or repeated joint bleeding the iron burden may exceed iron clearance capacity despite increased expression of iron regulators.73, 74 Synovial hypertrophy is facilitated by the exposure to iron that triggers the upregulation of the oncogenes MDM2 and c-MYC, thereby promoting proliferation.75, 76 Additionally, local synovial upregulation of inflammatory cytokines and chemokines such as interleukin(IL)-1β, IL-6, tumor necrosis factor alpha (TNFα), interferon-γ, monocyte chemoattractant protein-1 and IL-8, enhance the catabolic activity of chondrocytes to promote cartilage destruction.77–79 The hypoxic environment ensuing synovial hypertrophy results in the expression of hypoxia-inducible factor (HIF)-1α, HIF-2-α, and subsequent upregulation of vascular endothelial growth factor (VEGF)-A, stromal cell-derived factor 1α, pro-matrix metalloproteinase (proMMP)3 and proMMP13, These processes fuel a vicious cycle of neoangiogenesis and enzymatic tissue destruction.60, 78
A worrisome and clinically relevant observation is that synovial neoangiogenesis may be perpetuated systemically in HA, a conclusion supported by significantly increased levels of VEGF-A and bone marrow-derived endothelial progenitor cells expressing VEGF-receptor 2 (VEGFR2/KDR) in the peripheral blood of patients with hemophilia.60 Further support for systemic activation is provided by the fact that peripheral blood mononuclear cells from patients with hemophilia can induce VEGF-dependent synovial cell proliferation in vitro.60 There is also new evidence in mice and humans that systemically-mediated angiogenesis and continued vascular remodeling may perpetuate bleeding and facilitate progression of HA.38 Vascular abnormalities consisted of vascular architecture changes and highly abnormal joint tissue perfusion patterns, as well as pronounced expression of α-Smooth Muscle Actin, a marker of mesenchymal-type progenitor cells involved in vascular remodeling. These changes appeared unique to hemophilia, as they are not present in rheumatoid arthritis or osteoarthritis.38 The mediators of these neoangiopoietic pathways in hemophilia remain to be determined, but may involve inflammatory cytokines because modulation of cytokine levels, whether by inhibiting pro-inflammatory cytokines (e.g. IL-6 receptor antagonist80) or by administering protective cytokines (e.g. IL-1081 and/or IL-482–84), alleviated the sequelae of experimental hemarthrosis.
The fibrinolytic system in the joint provides another important aspect in relation to hemarthosis.85–87 Extravascular fibrin deposition in the inflamed synovium is known to be deleterious in RA, OA and with joint trauma and is induced by the complex interplay between inflammation and hemostasis.88–91 For instance, one interaction between inflammation and hemostasis is the direct engagement of fibrin(ogen) with the integrin receptor alpha(M)beta(2) on leukocytes, thereby regulating leukocyte function in the joint.92 Modulation of fibrinolysis in the joint is clinically relevant since antifibrinolytics are often used in hemophilic bleeding93, 94 without an understanding of the effect of such treatment on joint health. Studies in RA and OA have shown ongoing fibrin degradation that correlates with the degree of inflammation, involving increased expression of tissue-type plasminogen activator (t-PA) in OA89, 95 and urokinase (uPA) in RA87.89 In this context, plasmin generated from plasminogen is a central player. Plasmin promotes fibrin degradation, tissue disintegration and remodeling with direct detrimental effects on cartilage.96 Plasmin facilitates proteoglycan release, potentially by plasmin-mediated activation of pro-MMPs as demonstrated on healthy human cartilage explants.97 Consistent with this, systemic or intra-articular injection of α2-antiplasmin during experimental hemarthrosis led to improvement of histological scores of synovial inflammation and cartilage destruction in hemophilia mice.98 Plasmin levels in the synovial fluid and the number of uPA-expressing synovial cells were shown to be elevated in hemarthrosis, although fibrinolysis inhibition was induced simultaneously as evidenced by elevated plasminogen activator inhibitor 1 (PAI-1) levels.97 Enhanced PAI-1 expression, for example mediated by TNFα in RA, may on the other hand facilitate fibrin depositions,99 which all together suggests that the de-regulation of fibrinolysis contributes to joint damage in various, not yet fully understood ways. This is further underlined by the fact that opposing effects of plasmin modulation were observed in a model of rheumatoid arthritis in the plasminogen-deficient mouse and depended on the type of joint.100 In this model mice developed severe arthritis in the distal joints despite decreased MMP activity, whereas proximal joints were protected.100 The extent to which fibrinolysis dysregulation comprising fibrin depositions, hyperfibrinolysis or reduced fibrinolysis may play a role for HA therefore remains an important open question. In hemophilia, increased fibrinolysis is known to contribute to bleeding due to defective thrombin-mediated activation of thrombin-activatable fibrinolysis inhibitor (TAFI).101–103 Hence, it is conceivable that impaired TAFI activation may contribute to an imbalanced regulation of fibrinolysis in hemophilic joints. Interestingly, activated TAFI (TAFIa) not only impairs fibrinolysis, but also exerts anti-inflammatory effects104 that are protective in RA and OA.105–107 The anti-inflammatory properties result from inhibition of thrombin-cleaved osteopontin, bradykinin and the anaphylatoxins (complement C3a and C5a) by TAFIa.104 In animal studies, synovial inflammation and arthritis were significantly increased in TAFI(−/−) mice subjected to experimental RA due to decreased TAFI-dependent inactivation of C5a.108 TAFI’s important role in modulating inflammatory joint disease in humans is further supported by the fact that TAFI is expressed in synovium and in synovial fluid,90 and that patients with RA with the enzymatically more stable TAFI polymorphism (rs1926447, Thr325Ile) are better protected against joint disease.108 The extent to which TAFI may provide an important molecular link between joint inflammation, joint degeneration and hemostasis in HA is interesting and remains to be studied.
Cartilage degradation
Cartilage is composed of chondrocytes and cartilage matrix, mainly containing collagen type II and proteoglycan (aggrecan) which are required for the tensile strength and resilience of the cartilage tissue. Joint hemorrhage may lead to cartilage deterioration in a number of ways. First, cartilage damage may be inflicted by hemophilic synovitis through the production of catabolic cytokines and tissue-destructive enzymes as mentioned above.77 However, studies in the dog have verified early signs of cartilage erosion prior to or independent of synovitis after experimental hemarthrosis, and those findings closely resembled the pathology of OA.109–111 Therefore, and second, cartilage damage may also be induced by direct exposure to blood, which simultaneously decreases synthesis and increases release of cartilage proteoglycan-glycosaminoglycan (GAG). Hemoglobin-derived iron from erythrocytes and cytokines released from mononuclear cells were shown to be responsible for these effects.112 Additionally, IL-1β-induced hydrogen peroxide release from activated monocytes/macrophages appears to affect GAG metabolism. Hydrogen peroxide reacts with hemoglobin-derived iron to form hydroxyl radicals that induce chondrocyte apoptosis and destruction of the cartilage matrix; this was partially prevented by the oxygen radical scavenger, N-acetylcysteine.112–114 Taken together, cartilage destruction may be induced directly by contact with blood, but also indirectly through catabolic downstream effects during soft tissue inflammation or alteration.
Summary
HA is emerging as a major morbidity of hemophilia as the average age of the patient with hemophilia increases. HA is distinct from other arthropathies in that it is propelled by repeated bleeding, rather than systemic inflammation as in RA, or age-related degeneration in OA. The pathobiological mechanisms that result in hypertrophic synovium, hypervascularity and cartilage destruction are incompletely characterized, but newest evidence suggests involvement of systemic neoangiogenesis in response to joint bleeding that is associated with vascular remodeling and perpetuated bleeding.38, 60 To better address care of the hemophilic joint, novel strategies involving diagnostic biomarkers and point-of-care imaging techniques are being explored. These advancements will permit a shift from empiric diagnosis and treatment to precision medicine and targeted treatment to preserve or improve joint function especially for the aging generation of hemophilia patients.
Acknowledgments
This manuscript was supported, in part, by a National Hemophilia Foundation/Novo Nordisk Career Development Award and a Bayer Early Career Development Award (A.v.D.), and a National Institutes of Health (NHLBI) grant HL104165 (L.O.M.).
Disclosures
A.v.D. has received honoraria for participating on scientific advisory boards for industry, consulting and speaking engagements for Baxter Biosciences, Pfizer, Biogen, CSL-Behring, Novo Nordisk and Grifols and also has received research support from Biogen, Baxter, Pfizer and Novo Nordisk.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berntorp E, Shapiro AD. Modern haemophilia care. Lancet. 2012;379:1447–1456. doi: 10.1016/S0140-6736(11)61139-2. [DOI] [PubMed] [Google Scholar]
- 2.Soucie JM, Cianfrini C, Janco RL, et al. Joint range-of-motion limitations among young males with hemophilia: prevalence and risk factors. Blood. 2004;103:2467–2473. doi: 10.1182/blood-2003-05-1457. [DOI] [PubMed] [Google Scholar]
- 3.Aledort LM, Haschmeyer RH, Pettersson H. A longitudinal study of orthopaedic outcomes for severe factor-VIII-deficient haemophiliacs. The Orthopaedic Outcome Study Group. J Intern Med. 1994;236:391–399. doi: 10.1111/j.1365-2796.1994.tb00815.x. [DOI] [PubMed] [Google Scholar]
- 4.Oldenburg J, Zimmermann R, Katsarou O, et al. Controlled, cross-sectional MRI evaluation of joint status in severe haemophilia A patients treated with prophylaxis vs. on demand. Haemophilia. 2015;21:171–179. doi: 10.1111/hae.12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fischer K, Steen Carlsson K, Petrini P, et al. Intermediate-dose versus high-dose prophylaxis for severe hemophilia: comparing outcome and costs since the 1970s. Blood. 2013;122:1129–1136. doi: 10.1182/blood-2012-12-470898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson SC, Yang M, Minuk L, et al. Prophylaxis in older Canadian adults with hemophilia A: lessons and more questions. BMC Hematol. 2015;15:4. doi: 10.1186/s12878-015-0022-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aznar JA, Lucia F, Abad-Franch L, et al. Haemophilia in Spain. Haemophilia. 2009;15:665–675. doi: 10.1111/j.1365-2516.2009.02001.x. [DOI] [PubMed] [Google Scholar]
- 8.Schramm W, Gringeri A, Ljung R, et al. Haemophilia care in Europe: the ESCHQoL study. Haemophilia. 2012;18:729–737. doi: 10.1111/j.1365-2516.2012.02847.x. [DOI] [PubMed] [Google Scholar]
- 9.Baumgardner J, Elon L, Antun A, et al. Physical activity and functional abilities in adult males with haemophilia: a cross-sectional survey from a single US haemophilia treatment centre. Haemophilia. 2013;19:551–557. doi: 10.1111/hae.12134. [DOI] [PubMed] [Google Scholar]
- 10.You CW. The impact of haemarthropathy on the QoL of Korean severe haemophilia A patients: the critical level of haemarthropathy for the QoL. Haemophilia. 2013;19:637–641. doi: 10.1111/hae.12131. [DOI] [PubMed] [Google Scholar]
- 11.Darby SC, Kan SW, Spooner RJ, et al. Mortality rates, life expectancy, and causes of death in people with hemophilia A or B in the United Kingdom who were not infected with HIV. Blood. 2007;110:815–825. doi: 10.1182/blood-2006-10-050435. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez-Merchan EC. Haemophilic synovitis of the elbow: radiosynovectomy, open synovectomy or arthroscopic synovectomy? Thromb Res. 2013;132:15–18. doi: 10.1016/j.thromres.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 13.Galli E, Baques A, Moretti N, Candela M, Caviglia H. Hemophilic chronic synovitis: therapy of hemarthrosis using endovascular embolization of knee and elbow arteries. Cardiovasc Intervent Radiol. 2013;36:964–969. doi: 10.1007/s00270-012-0480-3. [DOI] [PubMed] [Google Scholar]
- 14.Vanderhave KL, Caird MS, Hake M, et al. Musculoskeletal care of the hemophiliac patient. J Am Acad Orthop Surg. 2012;20:553–563. doi: 10.5435/JAAOS-20-09-553. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez-Merchan EC. Aspects of current management: orthopaedic surgery in haemophilia. Haemophilia. 2012;18:8–16. doi: 10.1111/j.1365-2516.2011.02544.x. [DOI] [PubMed] [Google Scholar]
- 16.Key JA. Hemophilic Arthritis: Bleeder's Joints. Ann Surg. 1932;95:198–225. doi: 10.1097/00000658-193202000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kilcoyne RF, Lundin B, Pettersson H. Evolution of the imaging tests in hemophilia with emphasis on radiography and magnetic resonance imaging. Acta Radiol. 2006;47:287–296. doi: 10.1080/02841850600550708. [DOI] [PubMed] [Google Scholar]
- 18.Arnold WD, Hilgartner MW. Hemophilic arthropathy. Current concepts of pathogenesis and management. J Bone Joint Surg Am. 1977;59:287–305. [PubMed] [Google Scholar]
- 19.Pettersson H, Ahlberg A, Nilsson IM. A radiologic classification of hemophilic arthropathy. Clin Orthop Relat Res. 1980:153–159. [PubMed] [Google Scholar]
- 20.Nilsson IM, Berntorp E, Lofqvist T, Pettersson H. Twenty-five years' experience of prophylactic treatment in severe haemophilia A and B. J Intern Med. 1992;232:25–32. doi: 10.1111/j.1365-2796.1992.tb00546.x. [DOI] [PubMed] [Google Scholar]
- 21.Feldman BM, Funk S, Lundin B, Doria AS, Ljung R, Blanchette V. Musculoskeletal measurement tools from the International Prophylaxis Study Group (IPSG) Haemophilia. 2008;14(Suppl 3):162–169. doi: 10.1111/j.1365-2516.2008.01750.x. [DOI] [PubMed] [Google Scholar]
- 22.Chan MW, Leckie A, Xavier F, et al. A systematic review of MR imaging as a tool for evaluating haemophilic arthropathy in children. Haemophilia. 2013;19:e324–e334. doi: 10.1111/hae.12248. [DOI] [PubMed] [Google Scholar]
- 23.Jelbert A, Vaidya S, Fotiadis N. Imaging and staging of haemophilic arthropathy. Clin Radiol. 2009;64:1119–1128. doi: 10.1016/j.crad.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Lundin B, Manco-Johnson ML, Ignas DM, et al. An MRI scale for assessment of haemophilic arthropathy from the International Prophylaxis Study Group. Haemophilia. 2012;18:962–970. doi: 10.1111/j.1365-2516.2012.02883.x. [DOI] [PubMed] [Google Scholar]
- 25.Ceponis A, Wong-Sefidan I, Glass CS, von Drygalski A. Rapid musculoskeletal ultrasound for painful episodes in adult haemophilia patients. Haemophilia. 2013;19:790–798. doi: 10.1111/hae.12175. [DOI] [PubMed] [Google Scholar]
- 26.Kidder W, Nguyen S, Larios J, Bergstrom J, Ceponis A, von Drygalski A. Point-of-care musculoskeletal ultrasound is critical for the diagnosis of hemarthroses, inflammation and soft tissue abnormalities in adult patients with painful haemophilic arthropathy. Haemophilia. 2015 doi: 10.1111/hae.12637. [DOI] [PubMed] [Google Scholar]
- 27.Aznar JA, Abad-Franch L, Perez-Alenda S, Haya S, Cid AR, Querol F. Ultrasonography in the monitoring of management of haemarthrosis. Haemophilia. 2011;17:826–828. doi: 10.1111/j.1365-2516.2011.02538.x. [DOI] [PubMed] [Google Scholar]
- 28.Aznar JA, Perez-Alenda S, Jaca M, et al. Home-delivered ultrasound monitoring for home treatment of haemarthrosis in haemophilia A. Haemophilia. 2015;21:e147–e150. doi: 10.1111/hae.12622. [DOI] [PubMed] [Google Scholar]
- 29.Jacobson JA. Musculoskeletal ultrasound: focused impact on MRI. AJR Am J Roentgenol. 2009;193:619–627. doi: 10.2214/AJR.09.2841. [DOI] [PubMed] [Google Scholar]
- 30.Wittoek R, Jans L, Lambrecht V, Carron P, Verstraete K, Verbruggen G. Reliability and construct validity of ultrasonography of soft tissue and destructive changes in erosive osteoarthritis of the interphalangeal finger joints: a comparison with MRI. Ann Rheum Dis. 2011;70:278–283. doi: 10.1136/ard.2010.134932. [DOI] [PubMed] [Google Scholar]
- 31.Shanbhogue AK, Sandhu MS, Singh P, Ojili V, Khandelwal N, Sen R. Real time spatial compound ultrasound in the evaluation of meniscal injuries: a comparison study with conventional ultrasound and MRI. Knee. 2009;16:191–195. doi: 10.1016/j.knee.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 32.Doria AS, Keshava SN, Mohanta A, et al. Diagnostic accuracy of ultrasound for assessment of hemophilic arthropathy: MRI correlation. AJR Am J Roentgenol. 2015;204:W336–W347. doi: 10.2214/AJR.14.12501. [DOI] [PubMed] [Google Scholar]
- 33.Acharya SS, Schloss R, Dyke JP, et al. Power Doppler sonography in the diagnosis of hemophilic synovitis--a promising tool. J Thromb Haemost. 2008;6:2055–2061. doi: 10.1111/j.1538-7836.2008.03160.x. [DOI] [PubMed] [Google Scholar]
- 34.Sierra Aisa C, Lucia Cuesta JF, Rubio Martinez A, et al. Comparison of ultrasound and magnetic resonance imaging for diagnosis and follow-up of joint lesions in patients with haemophilia. Haemophilia. 2014;20:e51–e57. doi: 10.1111/hae.12268. [DOI] [PubMed] [Google Scholar]
- 35.Melchiorre D, Linari S, Innocenti M, et al. Ultrasound detects joint damage and bleeding in haemophilic arthropathy: a proposal of a score. Haemophilia. 2011;17:112–117. doi: 10.1111/j.1365-2516.2010.02380.x. [DOI] [PubMed] [Google Scholar]
- 36.Rowbotham EL, Grainger AJ. Rheumatoid arthritis: ultrasound versus MRI. AJR Am J Roentgenol. 2011;197:541–546. doi: 10.2214/ajr.11.6798. [DOI] [PubMed] [Google Scholar]
- 37.Porta F, Radunovic G, Vlad V, et al. The role of Doppler ultrasound in rheumatic diseases. Rheumatology. 2012;51:976–982. doi: 10.1093/rheumatology/ker433. [DOI] [PubMed] [Google Scholar]
- 38.Bhat V, Olmer M, Joshi S, et al. Vascular Remodeling Underlies Re-bleeding In Hemophilic Arthropathy. Am J Hematol. 2015 Aug 7; doi: 10.1002/ajh.24133. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moore CL, Copel JA. Point-of-care ultrasonography. N Engl J Med. 2011;364:749–757. doi: 10.1056/NEJMra0909487. [DOI] [PubMed] [Google Scholar]
- 40.McAlindon T, Kissin E, Nazarian L, et al. American College of Rheumatology report on reasonable use of musculoskeletal ultrasonography in rheumatology clinical practice. Arthritis Care Res. 2012;64:1625–1640. doi: 10.1002/acr.21836. [DOI] [PubMed] [Google Scholar]
- 41.Solomon SD, Saldana F. Point-of-care ultrasound in medical education--stop listening and look. N Engl J Med. 2014;370:1083–1085. doi: 10.1056/NEJMp1311944. [DOI] [PubMed] [Google Scholar]
- 42.Muca-Perja M, Riva S, Grochowska B, Mangiafico L, Mago D, Gringeri A. Ultrasonography of haemophilic arthropathy. Haemophilia. 2012;18:364–368. doi: 10.1111/j.1365-2516.2011.02672.x. [DOI] [PubMed] [Google Scholar]
- 43.Martinoli C, Della Casa Alberighi O, Di Minno G, et al. Development and definition of a simplified scanning procedure and scoring method for Haemophilia Early Arthropathy Detection with Ultrasound (HEAD-US) Thromb Haemost. 2013;109:1170–1179. doi: 10.1160/TH12-11-0874. [DOI] [PubMed] [Google Scholar]
- 44.Attur M, Krasnokutsky-Samuels S, Samuels J, Abramson SB. Prognostic biomarkers in osteoarthritis. Curr Opin Rheumatol. 2013;25:136–144. doi: 10.1097/BOR.0b013e32835a9381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jameson JL, Longo DL. Precision medicine--personalized, problematic, and promising. N Engl J Med. 2015;372:2229–2234. doi: 10.1056/NEJMsb1503104. [DOI] [PubMed] [Google Scholar]
- 46.Jansen NW, Roosendaal G, Lundin B, et al. The combination of the biomarkers urinary C-terminal telopeptide of type II collagen, serum cartilage oligomeric matrix protein, and serum chondroitin sulfate 846 reflects cartilage damage in hemophilic arthropathy. Arthritis Rheum. 2009;60:290–298. doi: 10.1002/art.24184. [DOI] [PubMed] [Google Scholar]
- 47.van Vulpen LF, van Meegeren ME, Roosendaal G, et al. Biochemical markers of joint tissue damage increase shortly after a joint bleed; an explorative human and canine in vivo study. Osteoarthritis Cartilage. 2015;23:63–69. doi: 10.1016/j.joca.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 48.Kraus VB. Osteoarthritis year 2010 in review: biochemical markers. Osteoarthritis Cartilage. 2011;19:346–353. doi: 10.1016/j.joca.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lotz M, Martel-Pelletier J, Christiansen C, et al. Value of biomarkers in osteoarthritis: current status and perspectives. Ann Rheum Dis. 2013;72:1756–1763. doi: 10.1136/annrheumdis-2013-203726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bay-Jensen AC, Sondergaard BC, Christiansen C, Karsdal MA, Madsen SH, Qvist P. Biochemical markers of joint tissue turnover. Assay Drug Dev Technol. 2010;8:118–124. doi: 10.1089/adt.2009.0199. [DOI] [PubMed] [Google Scholar]
- 51.Karsdal MA, Henriksen K, Leeming DJ, et al. Biochemical markers and the FDA Critical Path: how biomarkers may contribute to the understanding of pathophysiology and provide unique and necessary tools for drug development. Biomarkers. 2009;14:181–202. doi: 10.1080/13547500902777608. [DOI] [PubMed] [Google Scholar]
- 52.Manzo A, Bombardieri M, Humby F, Pitzalis C. Secondary and ectopic lymphoid tissue responses in rheumatoid arthritis: from inflammation to autoimmunity and tissue damage/remodeling. Immunol Rev. 2010;233:267–285. doi: 10.1111/j.0105-2896.2009.00861.x. [DOI] [PubMed] [Google Scholar]
- 53.Rooney T, Roux-Lombard P, Veale DJ, FitzGerald O, Dayer JM, Bresnihan B. Synovial tissue and serum biomarkers of disease activity, therapeutic response and radiographic progression: analysis of a proof-of-concept randomised clinical trial of cytokine blockade. Ann Rheum Dis. 2010;69:706–714. doi: 10.1136/ard.2009.108324. [DOI] [PubMed] [Google Scholar]
- 54.Bugatti S, Manzo A, Bombardieri M, et al. Synovial tissue heterogeneity and peripheral blood biomarkers. Curr Rheumatol Rep. 2011;13:440–448. doi: 10.1007/s11926-011-0201-y. [DOI] [PubMed] [Google Scholar]
- 55.van Riel PL. The development of the disease activity score (DAS) and the disease activity score using 28 joint counts (DAS28) Clin Exp Rheumatol. 2014;32:S-65–S-74. [PubMed] [Google Scholar]
- 56.Senolt L, Grassi W, Szodoray P. Laboratory biomarkers or imaging in the diagnostics of rheumatoid arthritis? BMC Med. 2014;12:49. doi: 10.1186/1741-7015-12-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zukotynski K, Jarrin J, Babyn PS, et al. Sonography for assessment of haemophilic arthropathy in children: a systematic protocol. Haemophilia. 2007;13:293–304. doi: 10.1111/j.1365-2516.2006.01414.x. [DOI] [PubMed] [Google Scholar]
- 58.Doria AS, Babyn PS, Lundin B, et al. Reliability and construct validity of the compatible MRI scoring system for evaluation of haemophilic knees and ankles of haemophilic children. Expert MRI working group of the international prophylaxis study group. Haemophilia. 2006;12:503–513. doi: 10.1111/j.1365-2516.2006.01310.x. [DOI] [PubMed] [Google Scholar]
- 59.Nuss R, Kilcoyne RF, Rivard GE, Murphy J. Late clinical, plain X-ray and magnetic resonance imaging findings in haemophilic joints treated with radiosynoviorthesis. Haemophilia. 2000;6:658–663. doi: 10.1046/j.1365-2516.2000.00433.x. [DOI] [PubMed] [Google Scholar]
- 60.Acharya SS, Kaplan RN, Macdonald D, Fabiyi OT, DiMichele D, Lyden D. Neoangiogenesis contributes to the development of hemophilic synovitis. Blood. 2011;117:2484–2493. doi: 10.1182/blood-2010-05-284653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yuan Q, Sun L, Li JJ, An CH. Elevated VEGF levels contribute to the pathogenesis of osteoarthritis. BMC Musculoskelet Disord. 2014;15:437. doi: 10.1186/1471-2474-15-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Azizi G, Boghozian R, Mirshafiey A. The potential role of angiogenic factors in rheumatoid arthritis. Int J Rheum Dis. 2014;17:369–383. doi: 10.1111/1756-185X.12280. [DOI] [PubMed] [Google Scholar]
- 63.Ballara S, Taylor PC, Reusch P, et al. Raised serum vascular endothelial growth factor levels are associated with destructive change in inflammatory arthritis. Arthritis Rheum. 2001;44:2055–2064. doi: 10.1002/1529-0131(200109)44:9<2055::AID-ART355>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 64.Mapp PI, Walsh DA. Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nat Rev Rheumatol. 2012;8:390–398. doi: 10.1038/nrrheum.2012.80. [DOI] [PubMed] [Google Scholar]
- 65.Valentino LA. Blood-induced joint disease: the pathophysiology of hemophilic arthropathy. J Thromb Haemost. 2010;8:1895–1902. doi: 10.1111/j.1538-7836.2010.03962.x. [DOI] [PubMed] [Google Scholar]
- 66.Franchini M, Lippi G. Thromb Res. Vol. 125. United States: 2009 Elsevier Ltd; 2010. Factor V Leiden and hemophilia; pp. 119–123. [DOI] [PubMed] [Google Scholar]
- 67.Valentino LA, Hakobyan N, Enockson C, et al. Exploring the biological basis of haemophilic joint disease: experimental studies. Haemophilia. 2012;18:310–318. doi: 10.1111/j.1365-2516.2011.02669.x. [DOI] [PubMed] [Google Scholar]
- 68.Rodriguez-Merchan EC. Pathogenesis, early diagnosis, and prophylaxis for chronic hemophilic synovitis. Clin Orthop Relat Res. 1997:6–11. [PubMed] [Google Scholar]
- 69.Hakobyan N, Enockson C, Cole AA, Sumner DR, Valentino LA. Experimental haemophilic arthropathy in a mouse model of a massive haemarthrosis: gross, radiological and histological changes. Haemophilia. 2008;14:804–809. doi: 10.1111/j.1365-2516.2008.01689.x. [DOI] [PubMed] [Google Scholar]
- 70.Fabry G. Ultrastructural changes in synovium and cartilage in experimental hemarthrosis in dogs. Arch Orthop Trauma Surg. 1990;109:21–29. doi: 10.1007/BF00441905. [DOI] [PubMed] [Google Scholar]
- 71.Roy S, Ghadially FN. Pathology of experimental haemarthrosis. Ann Rheum Dis. 1966;25:402–415. doi: 10.1136/ard.25.5.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Roosendaal G, Mauser-Bunschoten EP, De Kleijn P, et al. Synovium in haemophilic arthropathy. Haemophilia. 1998;4:502–505. doi: 10.1046/j.1365-2516.1998.440502.x. [DOI] [PubMed] [Google Scholar]
- 73.Nieuwenhuizen L, Schutgens RE, van Asbeck BS, et al. Identification and expression of iron regulators in human synovium: evidence for upregulation in haemophilic arthropathy compared to rheumatoid arthritis, osteoarthritis, and healthy controls. Haemophilia. 2013;19:e218–e227. doi: 10.1111/hae.12208. [DOI] [PubMed] [Google Scholar]
- 74.Nieuwenhuizen L, Schutgens RE, Coeleveld K, et al. Hemarthrosis in hemophilic mice results in alterations in M1-M2 monocyte/macrophage polarization. Thromb Res. 2014;133:390–395. doi: 10.1016/j.thromres.2013.10.039. [DOI] [PubMed] [Google Scholar]
- 75.Hakobyan N, Kazarian T, Jabbar AA, Jabbar KJ, Valentino LA. Pathobiology of hemophilic synovitis I: overexpression of mdm2 oncogene. Blood. 2004;104:2060–2064. doi: 10.1182/blood-2003-12-4231. [DOI] [PubMed] [Google Scholar]
- 76.Wen FQ, Jabbar AA, Chen YX, Kazarian T, Patel DA, Valentino LA. c-myc proto-oncogene expression in hemophilic synovitis: in vitro studies of the effects of iron and ceramide. Blood. 2002;100:912–916. doi: 10.1182/blood-2002-02-0390. [DOI] [PubMed] [Google Scholar]
- 77.Roosendaal G, Vianen ME, Wenting MJ, et al. Iron deposits and catabolic properties of synovial tissue from patients with haemophilia. J Bone Joint Surg Br. 1998;80:540–545. doi: 10.1302/0301-620x.80b3.7807. [DOI] [PubMed] [Google Scholar]
- 78.Sen D, Chapla A, Walter N, Daniel V, Srivastava A, Jayandharan GR. Nuclear factor (NF)-kappaB and its associated pathways are major molecular regulators of blood-induced joint damage in a murine model of hemophilia. J Thromb Haemost. 2013;11:293–306. doi: 10.1111/jth.12101. [DOI] [PubMed] [Google Scholar]
- 79.Ovlisen K, Kristensen AT, Jensen AL, Tranholm M. IL-1 beta, IL-6, KC and MCP-1 are elevated in synovial fluid from haemophilic mice with experimentally induced haemarthrosis. Haemophilia. 2009;15:802–810. doi: 10.1111/j.1365-2516.2008.01973.x. [DOI] [PubMed] [Google Scholar]
- 80.Narkbunnam N, Sun J, Hu G, et al. IL-6 receptor antagonist as adjunctive therapy with clotting factor replacement to protect against bleeding-induced arthropathy in hemophilia. J Thromb Haemost. 2013;11:881–893. doi: 10.1111/jth.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jansen NW, Roosendaal G, Hooiveld MJ, et al. Interleukin-10 protects against bloodinduced joint damage. Br J Haematol. 2008;142:953–961. doi: 10.1111/j.1365-2141.2008.07278.x. [DOI] [PubMed] [Google Scholar]
- 82.van Meegeren ME, Roosendaal G, Coeleveld K, Nieuwenhuizen L, Mastbergen SC, Lafeber FP. A single intra-articular injection with IL-4 plus IL-10 ameliorates blood-induced cartilage degeneration in haemophilic mice. Br J Haematol. 2013;160:515–520. doi: 10.1111/bjh.12148. [DOI] [PubMed] [Google Scholar]
- 83.van Meegeren ME, Roosendaal G, Jansen NW, et al. IL-4 alone and in combination with IL-10 protects against blood-induced cartilage damage. Osteoarthritis Cartilage. 2012;20:764–772. doi: 10.1016/j.joca.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 84.van Meegeren ME, Roosendaal G, van Veghel K, Mastbergen SC, Lafeber FP. A short time window to profit from protection of blood-induced cartilage damage by IL-4 plus IL-10. Rheumatology. 2013;52:1563–1571. doi: 10.1093/rheumatology/ket005. [DOI] [PubMed] [Google Scholar]
- 85.Storti E, Magrini U, Ascari E. Synovial fibrinolysis and haemophilic haemarthrosis. Br Med J. 1971;4:812. doi: 10.1136/bmj.4.5790.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pandolfi M, Ahlberg A, Traldi A, Nilsson IM. Fibrinolytic activity of human synovial membranes in health and in haemophilia. Scand J Haematol. 1972;9:572–576. doi: 10.1111/j.1600-0609.1972.tb00988.x. [DOI] [PubMed] [Google Scholar]
- 87.Busso N, Peclat V, So A, Sappino AP. Plasminogen activation in synovial tissues: differences between normal, osteoarthritis, and rheumatoid arthritis joints. Ann Rheum Dis. 1997;56:550–557. doi: 10.1136/ard.56.9.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cole HA, Ohba T, Nyman JS, et al. Fibrin accumulation secondary to loss of plasmin-mediated fibrinolysis drives inflammatory osteoporosis in mice. Arthritis Rheumatol. 2014;66:2222–2233. doi: 10.1002/art.38639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Weinberg JB, Pippen AM, Greenberg CS. Extravascular fibrin formation and dissolution in synovial tissue of patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum. 1991;34:996–1005. doi: 10.1002/art.1780340809. [DOI] [PubMed] [Google Scholar]
- 90.So AK, Varisco PA, Kemkes-Matthes B, et al. Arthritis is linked to local and systemic activation of coagulation and fibrinolysis pathways. J Thromb Haemost. 2003;1:2510–2515. doi: 10.1111/j.1538-7836.2003.00462.x. [DOI] [PubMed] [Google Scholar]
- 91.Raghu H, Flick MJ. Targeting the coagulation factor fibrinogen for arthritis therapy. Curr Pharm Biotechnol. 2011;12:1497–1506. doi: 10.2174/138920111798281144. [DOI] [PubMed] [Google Scholar]
- 92.Flick MJ, LaJeunesse CM, Talmage KE, et al. Fibrin(ogen) exacerbates inflammatory joint disease through a mechanism linked to the integrin alphaMbeta2 binding motif. J Clin Invest. 2007;117:3224–3235. doi: 10.1172/JCI30134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hvas AM, Sorensen HT, Norengaard L, Christiansen K, Ingerslev J, Sorensen B. Tranexamic acid combined with recombinant factor VIII increases clot resistance to accelerated fibrinolysis in severe hemophilia A. J Thromb Haemost. 2007;5:2408–2414. doi: 10.1111/j.1538-7836.2007.02755.x. [DOI] [PubMed] [Google Scholar]
- 94.Rainsford SG, Jouhar AJ, Hall A. Tranexamic acid in the control of spontaneous bleeding in severe haemophilia. Thromb Diath Haemorrh. 1973;30:272–279. [PubMed] [Google Scholar]
- 95.Ronday HK, Smits HH, Van Muijen GN, et al. Difference in expression of the plasminogen activation system in synovial tissue of patients with rheumatoid arthritis and osteoarthritis. Br J Rheumatol. 1996;35:416–423. doi: 10.1093/rheumatology/35.5.416. [DOI] [PubMed] [Google Scholar]
- 96.Judex MO, Mueller BM. Plasminogen activation/plasmin in rheumatoid arthritis: matrix degradation and more. Am J Pathol. 2005;166:645–647. doi: 10.1016/S0002-9440(10)62285-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nieuwenhuizen L, Roosendaal G, Coeleveld K, et al. Haemarthrosis stimulates the synovial fibrinolytic system in haemophilic mice. Thromb Haemost. 2013;110:173–183. doi: 10.1160/TH13-01-0080. [DOI] [PubMed] [Google Scholar]
- 98.Nieuwenhuizen L, Roosendaal G, Mastbergen SC, et al. Antiplasmin, but not amiloride, prevents synovitis and cartilage damage following hemarthrosis in hemophilic mice. J Thromb Haemost. 2014;12:237–245. doi: 10.1111/jth.12467. [DOI] [PubMed] [Google Scholar]
- 99.Kamper EF, Kopeikina LT, Trontzas P, Potamianou A, Tsiroglou E, Stavridis JC. The effect of disease activity related cytokines on the fibrinolytic potential and cICAM-1 expression in rheumatoid arthritis. J Rheumatol. 2000;27:2545–2550. [PubMed] [Google Scholar]
- 100.Raghu H, Jone A, Cruz C, et al. Plasminogen is a joint-specific positive or negative determinant of arthritis pathogenesis in mice. Arthritis Rheumatol. 2014;66:1504–1516. doi: 10.1002/art.38402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Broze GJ, Jr, Higuchi DA. Coagulation-dependent inhibition of fibrinolysis: role of carboxypeptidase-U and the premature lysis of clots from hemophilic plasma. Blood. 1996;88:3815–3823. [PubMed] [Google Scholar]
- 102.Foley JH, Nesheim ME. Soluble thrombomodulin partially corrects the premature lysis defect in FVIII-deficient plasma by stimulating the activation of thrombin activatable fibrinolysis inhibitor. J Thromb Haemost. 2009;7:453–459. doi: 10.1111/j.1538-7836.2008.03261.x. [DOI] [PubMed] [Google Scholar]
- 103.Mosnier LO, Lisman T, van den Berg HM, Nieuwenhuis HK, Meijers JC, Bouma BN. The defective down regulation of fibrinolysis in haemophilia A can be restored by increasing the TAFI plasma concentration. Thromb Haemost. 2001;86:1035–1039. [PubMed] [Google Scholar]
- 104.Myles T, Nishimura T, Yun TH, et al. Thrombin activatable fibrinolysis inhibitor, a potential regulator of vascular inflammation. J Biol Chem. 2003;278:51059–51067. doi: 10.1074/jbc.M306977200. [DOI] [PubMed] [Google Scholar]
- 105.Leung LL, Nishimura T, Myles T. Regulation of tissue inflammation by thrombin-activatable carboxypeptidase B (or TAFI) Adv Exp Med Biol. 2008;632:61–69. [PubMed] [Google Scholar]
- 106.Lepus CM, Song JJ, Wang Q, et al. Brief report: carboxypeptidase B serves as a protective mediator in osteoarthritis. Arthritis Rheumatol. 2014;66:101–106. doi: 10.1002/art.38213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sharif SA, Du X, Myles T, et al. Thrombin-activatable carboxypeptidase B cleavage of osteopontin regulates neutrophil survival and synoviocyte binding in rheumatoid arthritis. Arthritis Rheum. 2009;60:2902–2912. doi: 10.1002/art.24814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Song JJ, Hwang I, Cho KH, et al. Plasma carboxypeptidase B downregulates inflammatory responses in autoimmune arthritis. J Clin Invest. 2011;121:3517–3527. doi: 10.1172/JCI46387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fabry G. Early changes in the ground substance of articular cartilage in experimental hemarthrosis in dogs, measured by the fixed-charge density method. Arch Orthop Trauma Surg. 1989;108:76–91. doi: 10.1007/BF00932161. [DOI] [PubMed] [Google Scholar]
- 110.Hoaglund FT. Experimental hemarthrosis. The response of canine knees to injections of autologous blood. J Bone Joint Surg Am. 1967;49:285–298. [PubMed] [Google Scholar]
- 111.Roosendaal G, TeKoppele JM, Vianen ME, van den Berg HM, Lafeber FP, Bijlsma JW. Blood-induced joint damage: a canine in vivo study. Arthritis Rheum. 1999;42:1033–1039. doi: 10.1002/1529-0131(199905)42:5<1033::AID-ANR24>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 112.Roosendaal G, Vianen ME, van den Berg HM, Lafeber FP, Bijlsma JW. Cartilage damage as a result of hemarthrosis in a human in vitro model. J Rheumatol. 1997;24:1350–1354. [PubMed] [Google Scholar]
- 113.Hooiveld MJ, Roosendaal G, van den Berg HM, Bijlsma JW, Lafeber FP. Haemoglobin-derived iron-dependent hydroxyl radical formation in blood-induced joint damage: an in vitro study. Rheumatology. 2003;42:784–790. doi: 10.1093/rheumatology/keg220. [DOI] [PubMed] [Google Scholar]
- 114.Roosendaal G, Vianen ME, Marx JJ, van den Berg HM, Lafeber FP, Bijlsma JW. Blood-induced joint damage: a human in vitro study. Arthritis Rheum. 1999;42:1025–1032. doi: 10.1002/1529-0131(199905)42:5<1025::AID-ANR23>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]