Abstract
Background
Hyperhomocysteinemia (HHcy) is a well-known risk factor for ischemic stroke. However, whether HHcy can influence the treatment outcome of acute ischemic stroke (AIS) patients has yet to be fully determined. In this study, we investigated the relationship between serum homocysteine (Hcy) level and prognosis in AIS patients who received tissue plasminogen activator (tPA) treatment.
Material/Methods
Patients were recruited according to the research criteria and grouped by their serum Hcy levels. Neurological outcome was evaluated by National Institute of Health Stroke Scale (NIHSS) score system before and 1 week after treatment, and functional outcome was evaluated by modified Rankin Scale (MRS) score system after 3 months. All patients took CT/MRI examination to detect cerebral hemorrhage in 24 hours after tPA treatment. Receiver operating characteristic curve (ROC) was employed to assess if serum homocysteine level can be used as an index to predict the outcome after tPA treatment.
Results
The mean (±SD) serum Hcy level of 194 patients was 22.62±21.23 μmol/L. After 1-week tPA treatment, the NIHSS scores of high Hcy level group were significantly higher than those of low level group (p<0.05), meantime the high Hcy group showed obvious symptomatic intracerebral hemorrhage risk after 24 hours (p<0.05). Poor outcome was presented in mRS score results after 3 months in high Hcy level group, which compared with low Hcy level group (p<0.01). The ROC showed that Hcy level was a moderately sensitive and specific index to predict the prognosis with an optimal cut-off value at 19.95 μmol/L (sensitivity [58.2%], specificity [80.3%]).
Conclusions
High serum homocysteine level could potentially predict poor prognosis in acute ischemic stroke patients after tPA treatment.
MeSH Keywords: Homocysteine, Patient Outcome Assessment, Stroke, Thrombolytic Therapy
Background
Acute ischemic stroke (AIS) is a leading cause of death and permanent disability in China, and is also a cause of enormous financial burden for patients and society [1]. Currently, intravenous thrombolysis (IVT) with alteplase is the only approved therapy in patients with AIS presenting within 4.5 hours after the onset [2–4], but extra attention must be given to its most likely complication, which is symptomatic intracerebral hemorrhage (sICH). To the best of our knowledge, there is a lack of reliable factors to predict neurological function deterioration, sICH, and prognosis. There is an urgent need to identify pretreatment predictors of sICH to help select patients to receive t-PA treatment.
Recently, mounting evidence shows that hyperhomocysteinemia (HHcy) is associated with increased risk of ischemic stroke, which has attracted the attention of clinicians. The possible mechanism may be the increasing homocysteine level caused neurotoxicity, endothelial dysfunction, and other associated prothrombotic factors, which is reported to be an independent predictor of poor outcome in patients with ischemic stroke [5–7]. However, whether it is also can be used as an independent predictor of poor outcome after routine tPA treatment has not been fully addressed. Therefore, this study was designed to retrospectively investigate the possible relationship between elevated Hcy levels and prognosis following acute thrombolytic treatment.
Material and Methods
This study protocol was approved by the Medical Ethics Review Committee of Shihezi University. Data from consecutive acute ischemic stroke patients were collected from January 2011 to January 2015 and retrospectively analyzed in the First Affiliated Hospital, School of Medicine, Shihezi University, Xinjiang Uygur Autonomous Region, China. All patients provided written informed consent.
All patients had been admitted to the Neurological Department. Inclusion criteria were: (1) Age ≥18; (2) Diagnosis of ischemic stroke causing measurable neurological deficit, confirmed by computed tomography (CT) of the head; and (3) Onset of symptoms ≤4.5 h before treatment began. Exclusion criteria were: (1) Significant head trauma or prior stroke in the previous 3 months; (2) Symptoms suggesting SAH; (3) History of previous intracranial hemorrhage; (4) Recent intracranial or intraspinal surgery; (5) Active internal bleeding; (6) Elevated blood pressure (systolic >185 mmHg or diastolic >110 mmHg); (7) Acute bleeding diathesis; (8) Blood glucose concentration < 50 mg/dl (2.7 mmol/l); (9) CT demonstrating multilobar infarction (hypodensity >1/3 cerebral hemisphere); and (10) Unwilling to participate.
All patients underwent a CT exam as a baseline check before they were recruited. The following variables were recorded at admission: age, sex, history of alcohol consumption and smoking, hypertension, diabetes mellitus, coronary atherosclerotic cardiovascular disease, atrial fibrillation, previous stroke, time of thrombolysis, systolic and diastolic blood pressure and stroke subtype including total anterior circulation infarcts (TACI), partial anterior circulation infarcts (PACI), posterior circulation infarcts (POCI), and lacunar infarcts (LACI). The National Institutes of Health Stroke Scale (NIHSS) score was used to evaluate the severity of initial stroke on admission, which served as the study baseline. One week after thrombolysis treatment, all recruited patients repeated the NIHSS score evaluation. Related pretreatment predictors of AIS were performed on the first day: fasting blood glucose level, hemoglobin level, platelet count, fibrinogen level, uric acid level, serum lipid profile, and serum homocysteine level. Serum samples for homocysteine level measuring were separated from the whole blood by centrifuging at 3500 rpm for 15 min, and then a chemiluminescent microparticle immunoassay was performed [6].
Treatment
All patients received intravenous alteplase (0.9 mg/kg, maximum 90 mg, Boehringer Ingelheim) within 4.5 h of onset (10% as a bolus within 1 min, and the remainder as a continuous intravenous infusion over a period of 60 min) [2–4].
Outcome
Initial stroke severity and progression were identified using the NIHSS score on admission and 1 week after thrombolysis treatment. All patients underwent non-contrast brain computed tomography (CT) or magnetic resonance imaging (MRI) scan within 24 h after treatment. Symptomatic intracerebral hemorrhage (sICH) was assessed as the secondary end-point. We defined sICH as having detectable hemorrhage and parenchymal hematoma (visible on cerebral CT/MRI scan within 24 h following tPA treatment), and a neurological deterioration (NIHSS score ≥4) [8]. The neurological status of patients was assessed daily by trained neurologists. The follow-up was conducted either during a clinic visit or by telephone interview. All patients were assessed at 3 months after stroke onset for clinical outcome by modified Rankin Scale (mRS) score. Poor functional outcome was defined with a mRS score of 3 or more after 3 months.
Statistical analysis
Total homocysteine concentration was examined by quartiles of increasing levels to evaluate for possible threshold effects. Baseline characteristics of continuous variables were compared in 2 groups using the t test. Categorical variables are expressed as percentage and compared between groups using the Pearson chi-square test or Fisher exact test. A multiple logistic regression analysis for variables with P values <0.05 (2-tailed) in univariate analysis was performed to evaluate possible contributors to poor functional outcome. The area under the receiver operating characteristic curve (AUC) was developed to investigate the ability to predict patient prognosis based on homocysteine level, and cut-off points values were calculated. All statistical analyses were performed with SPSS version 19.0.
Results
This study cohort consisted of 194 patients (118 males and 76 females, mean age 62.2±12.3 years). The mean homocysteine (Hcy) level was 22.62±21.23 μmol/L. Hcy concentration was divided into quartiles grouped as 1, 2, 3, and 4, the Hcy concentration range of the groups were 2.26 to 11.50 μmol/L, 11.50 to 15.50 μmol/L, 15.50 to 23.85 μmol/L, and 23.85 to 138.90 μmol/L, respectively. The value of 15.50 μmol/L was the median of all patients’ Hcy concentration and we classified those patients with Hcy concentrations less than 15.50 μmol/L as the low concentration group, and the remaining patients with Hcy concentration equal to or higher than 15.50 μmol/L were classified as the high concentration group.
Epidemiological and clinical characteristics baselines of low and high Hcy concentration groups are listed in Table 1. As expected, we found more male patients in the high concentration group (P<0.01). Diabetes mellitus, current smoking status, alcohol consumption, hemoglobin level, and uric acid level were associated with high Hcy by univariate analysis (P<0.05), but Hcy levels were not associated with age, hypertension, coronary artery disease, atrial fibrillation, previous stroke, time of thrombolysis, systolic and diastolic blood pressure, stroke subtype blood glucose level, platelet count, fibrinogen level, or serum lipid profile.
Table 1.
Comparison of baseline characteristics and clinical outcomes between low concentration homocysteine and high concentration homocysteine level groups.
| Homocysteine level | P-value | ||
|---|---|---|---|
| Low concentration <15.5 μmol/L (n=96) | High concentration ≥15.5 μmol/L (n=98) | ||
| Demographics | |||
| Age, y | 62.60±12.01 | 61.99±12.56 | 0.728 |
| Sex, male (%) | 45 (46.90) | 73 (74.50) | <0.001* |
| Risk factors | |||
| Alcohol consumption (%) | 11 (11.50) | 24 (24.50) | 0.018* |
| Current smoking status (%) | 28 (29.20) | 54 (55.10) | <0.001* |
| Hypertension (%) | 59 (61.50) | 64 (65.30) | 0.578 |
| Diabetes mellitus (%) | 29 (30.20) | 15 (15.30) | 0.013* |
| Coronary atherosclerotic cardiovascular disease (%) | 30 (31.20) | 34 (34.70) | 0.61 |
| Atrial fibrillation (%) | 9 (9.40) | 16 (16.30) | 0.149 |
| Previous stroke (%) | 26 (27.10) | 19 (19.40) | 0.204 |
| Parameters on admission | |||
| Time of thrombolysis (SD), hour | 3.21±0.98 | 3.32±0.89 | 0.421 |
| NIHSS on admission (SD) | 7.94±3.84 | 8.39±3.92 | 0.42 |
| Initial systolic blood pressure (SD), mmHg | 148.58±24.07 | 146.70±21.27 | 0.565 |
| Initial diastolic blood pressure (SD), mmHg | 86.50±11.43 | 88.06±12.50 | 0.365 |
| Stroke subtype | 0.232 | ||
| TACI (%) | 13 (13.50) | 21 (21.40) | |
| PACI (%) | 56 (58.30) | 60 (61.20) | |
| POCI (%) | 9 (9.40) | 6 (6.10) | |
| LACI (%) | 18 (18.80) | 11 (11.20) | |
| Blood test on admission | |||
| Hemoglobin level (SD), g/l | 134.78±16.24 | 140.55±19.41 | 0.03* |
| Platelets count (SD), ×109/l | 210.26±60.95 | 222.97±83.63 | 0.24 |
| Fibrinogen level (SD), g/l | 3.00±0.72 | 3.00±0.94 | 0.995 |
| Uric acid level (SD), μmol/l | 258.21±81.59 | 301.67±95.80 | <0.001* |
| Blood glucose level (SD), mmol/l | 6.36±2.35 | 6.09±2.78 | 0.485 |
| Triglyceride (SD), mmol/l | 1.57±1.00 | 1.71±1.11 | 0.377 |
| Low density lipoprotein (SD), mmol/l | 2.73±0.80 | 2.81±1.10 | 0.558 |
| High density lipoprotein(SD), mmol/l | 1.26±0.34 | 1.20±0.34 | 0.301 |
| Total cholesterol (SD), mmol/l | 4.59±1.02 | 4.78±1.32 | 0.274 |
| Outcome | |||
| sICH (%) | 2 (22.20) | 10 (76.90) | 0.027** |
| NIHSS 1 week after stroke (SD) | 4.59±5.56 | 6.72±7.70 | 0.029* |
| mRS (SD) | 1.80±1.46 | 2.41±1.71 | 0.009* |
SD – standard deviation; NIHSS – National Institutes of Health Stroke Scale; TACI – total anterior circulation infarcts; PACI – partial anterior circulation infarcts; POCI – posterior circulation infarcts; LACI – lacunar infarcts; sICH – symptomatic intracerebral hemorrhage; mRS – modified Rankin Score. An asterisk (*) indicates a significant difference between two groups at P<0.05;
Fisher exact test.
NIHSS score obtained on admission showed no difference between the 2 groups, but the scores in the low Hcy concentration group were significantly improved after 7-day treatment compared with those in the high concentration group (P<0.05). Intracerebral hemorrhage (ICH) was detected in 21 patients (10.8%) within 24 h after administering thrombolysis treatment. We found symptomatic ICH (sICH) in 57.1% of patients. Elevated Hcy level was significantly associated with sICH within 24 h (P<0.05), and modified Rankin Scale (mRS) scores indicated poor outcome after 3 months (P<0.01).
Univariate analysis showed initial NIHSS score, diabetes mellitus, uric acid level, and Hcy level were associated with poor outcome. Multiple logistic regression analysis showed that initial NIHSS and homocysteine levels were independent predictors of poor outcome (P<0.001). Calculated OR representing the functional outcome increased along with increasing quartiles of homocysteine level, and the first quartile served as the reference value, classified as an OR greater than 1, indicating that Hcy concentration is a risk factor for poor outcome (P<0.05) (Table 2). The fourth quartile (>23.86 μmol/L) of Hcy level was identified as an independent predictor of poor functional outcome (fourth quartile odds ratio, 13.65; 95%CI: 3.58–51.97; P<0.001). As shown in the Figure 1, the risk of poor outcome increased with increasing level of homocysteine. ROC analysis of Hcy level showed an AUC value of 0.685 (95%CI: 0.603–0.768) and cut-off at 19.95 μmol/L (sensitivity 58.2%, specificity 80.3%).
Table 2.
Modified rankin scale and odds ratio according to homocysteine quartiles.
| OR | 95%CI | P-value | |
|---|---|---|---|
| Homocysteine quartile, μmol/L | |||
| Q1 (2.26~11.50, reference) | |||
| Q2 (11.50~15.50) | 1.83 | 0.49–6.81 | 0.370 |
| Q3 (15.50~23.85) | 2.29 | 0.62–8.43 | 0.241 |
| Q4 (23.85~138.90) | 13.65 | 3.58–51.97 | <0.001* |
| Sex | 0.711 | 0.181–2.80 | 0.626 |
| Age | 1.00 | 0.966–1.04 | 0.965 |
| Alcohol consumption | 1.65 | 0.495–5.48 | 0.415 |
| Current smoking status | 0.468 | 0.138–1.59 | 0.223 |
| Diabetes mellitus | 0.981 | 0.324–2.97 | 0.974 |
| Hemoglobin level | 1.00 | 0.978–1.03 | 0.771 |
| Uric acid level | 0.999 | 0.994–1.00 | 0.598 |
| NIHSS on admission | 1.61 | 1.38–1.86 | <0.001* |
CI – confidence interval; NIHSS – National Institutes of Health Stroke Scale; OR – odds ratio. ORs were calculated using a logistic regression model after adjusting for sex, age, alcohol consumption, current smoking status, diabetes mellitus, hemoglobin level, and uric acid level.
P<0.05.
Figure 1.
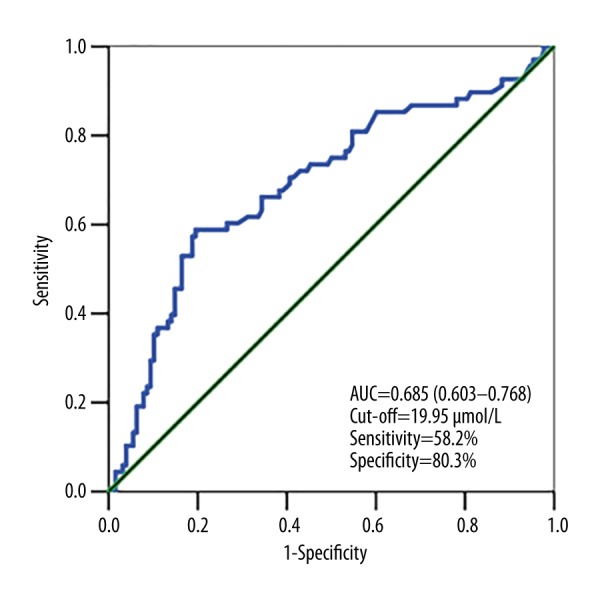
ROC analysis was conducted to assess the ability of circulating homocysteine (Hcy) level to predict stroke outcome.
Discussion
Currently, intravenous thrombolysis (IVT) with alteplase is the only approved therapy in patients with AIS presenting within 4.5 h after onset [2–4]. However, symptomatic intracranial hemorrhage (sICH) is a fatal complication after thrombolytic therapy (IV tPA), which limits its application in clinical practice. The European Cooperative Acute Stroke Study III (ECASS III) noted that subjects treated with intravenous rtPA commonly have early neurological deterioration and poor prognosis caused by sICH [3]. Therefore, it will be clinical meaningful if we can find an index to predict poor outcome after tPA treatment.
The possibility of using Hcy level to predict AIS has been reported previously [5–7], but to date there has been no study on the controversial relationship between elevated Hcy level and sICH. Some groups [9] have found that elevated serum HCY levels were associated with larger hematoma volume. The underlying mechanism may impairment of vessel wall integrity and disruption of cerebrovascular permeability caused by elevated Hcy levels, which would result in endothelial dysfunction, elastic structure damage, and basal lamina injury of cerebral arteriole and microvessels [10,11]., but other studies have found no differences according to hemorrhagic transformation after tPA treatment or clinical outcome [12]. Therefore, in our study we tried to determine if there is a positive relationship between Hcy level and sICH and if Hcy level can be used to predict poor prognosis after tPA treatment.
Our study showed that a concentration of 15.5 μmol/L was a useful cut-off level to judge if the patient’s serum Hcy level was higher than normal. Compared with the low concentration patients, the patients with higher Hcy level showed worse outcome after tPA treatment, which was further supported by NIHSS score after 7 days and mRS score after 3 months. These results indicate that higher Hcy levels can predict poor outcome after tPA treatment.
Over the last decade, a large body of epidemiological evidence has supported the association between high Hcy level and increased risk of ischemic stroke. However, the prognostic value of Hcy level in patients after AIS remains controversial. Our results are consistent with those of some studies that have already identified increased Hcy level is an independent risk factor for early neurological deterioration and short- or long-term poor outcome following AIS [5–7,13,14]. Several epidemiological studies have indicated that Hcy is a modifiable risk factor. Toole et al. [15] showed that a decrease of 3 μmol/L in total Hcy level was associated with a 10% lower risk of stroke. Based on the ROC model, we determined that out the cut-off value for Hcy was 19.95 μmol/L, and a similar cut-off value for Hcy (17.64 μmol/L) was reported by Yan et al. [16]. A cut-off value of 19.95 μmol/L of Hcy can be used as an index to predict poor prognosis in patients with AIS after thrombolysis treatment. To the best of our knowledge, the present retrospective study is the first to report a positive relationship between Hcy level and prognosis following acute thrombolytic treatment.
Our study also has several limitations. First, this was a retrospective analysis with limited sample size and suffers from selection bias because we did not include a control group consisting of patients not receiving thrombolytic treatment. Therefore, the results should be interpreted with caution and confirmed in another multicenter cohort study. Second, Hcy level is affected by genetic factors, poor general health, poor nutrition, and various diseases (e.g., diabetes mellitus and nephropathy) [17], and it was difficult to adjust for confounding and risk factors in a consistent way during the follow-up.
Conclusions
In conclusion, our results suggest that elevated Hcy level independently predicts sICH and poor functional prognosis in patients with AIS receiving thrombolytic treatment. The optimal cut-off point of Hcy also provides a reference point for future studies in Chinese patients.
Footnotes
Conflict of interest
The authors declare that they have no conflicts of interest relevant to this work.
Source of support: This work was supported by grants from the Natural Science Foundation of China (81471200 to X. Luo) and the National Basic Research Program (2011CB504403 to W. Wang)
References
- 1.Sun H, Zou X, Liu L. Epidemiological factors of stroke: a survey of the current status in china. J Stroke. 2013;15:109–14. doi: 10.5853/jos.2013.15.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–87. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 3.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–29. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 4.Lees KR, Bluhmki E, von Kummer R, et al. Time to treatment with intravenous alteplase and outcome in stroke: An updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375:1695–703. doi: 10.1016/S0140-6736(10)60491-6. [DOI] [PubMed] [Google Scholar]
- 5.Zhong C, Lv L, Liu C, et al. High homocysteine and blood pressure related to poor outcome of acute ischemia stroke in Chinese population. PLoS One. 2014;9:e107498. doi: 10.1371/journal.pone.0107498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwon HM, Lee YS, Bae HJ, Kang DW. Homocysteine as a predictor of early neurological deterioration in acute ischemic stroke. Stroke. 2014;45:871–73. doi: 10.1161/STROKEAHA.113.004099. [DOI] [PubMed] [Google Scholar]
- 7.Shi Z, Guan Y, Huo YR, et al. Elevated total homocysteine levels in acute ischemic stroke are associated with long-term mortality. Stroke. 2015;46:2419–25. doi: 10.1161/STROKEAHA.115.009136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adeoye O, Hornung R, Khatri P, Kleindorfer D. Recombinant tissue-type plasminogen activator use for ischemic stroke in the United States: A doubling of treatment rates over the course of 5 years. Stroke. 2011;42:1952–55. doi: 10.1161/STROKEAHA.110.612358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou F, Chen B, Chen C, et al. Elevated homocysteine levels contribute to larger hematoma volume in patients with intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2015;24:784–88. doi: 10.1016/j.jstrokecerebrovasdis.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Mach F, Schönbeck U, Bonnefoy JY, et al. Activation of monocyte/macrophage functions related to acute atheroma complication by ligation of CD40 induction of collagenase, stromelysin, and tissue factor. Circulation. 1997;96:396–99. doi: 10.1161/01.cir.96.2.396. [DOI] [PubMed] [Google Scholar]
- 11.Rosell A, Ortega-Aznar A, Alvarez-Sabín J, et al. Increased brain expression of matrix metalloproteinase-9 after ischemic and hemorrhagic human stroke. Stroke. 2006;37:1399–406. doi: 10.1161/01.STR.0000223001.06264.af. [DOI] [PubMed] [Google Scholar]
- 12.Ribo M, Montaner J, Molina CA, et al. Admission fibrinolytic profile is associated with symptomatic hemorrhagic transformation in stroke patients treated with tissue plasminogen activator. Stroke. 2004;35:2123–27. doi: 10.1161/01.STR.0000137608.73660.4c. [DOI] [PubMed] [Google Scholar]
- 13.Tu WJ, Zhao SJ, Liu TG, et al. Combination of high-sensitivity C-reactive protein and homocysteine predicts the short-term outcomes of Chinese patients with acute ischemic stroke. Neurol Res. 2013;35:912–21. doi: 10.1179/1743132813Y.0000000228. [DOI] [PubMed] [Google Scholar]
- 14.Gong X, Zou X, Liu L, et al. Prognostic value of inflammatory mediators in 1-year outcome of acute ischemic stroke with middle cerebral artery stenosis. Mediators Inflamm. 2013;2013:850714. doi: 10.1155/2013/850714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toole JF, Malinow MR, Chambless LE, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA. 2004;291:565–75. doi: 10.1001/jama.291.5.565. [DOI] [PubMed] [Google Scholar]
- 16.Ji Y, Song B, Xu Y, et al. Prognostic Significance of Homocysteine Levels in Acute Ischemic Stroke: A Prospective Cohort Study. Curr Neurovasc Res. 2015;12:334–40. doi: 10.2174/1567202612666150807112205. [DOI] [PubMed] [Google Scholar]
- 17.He Y, Li Y, Chen Y, Feng L, Nie Z. Homocysteine level and risk of different stroke types: A meta-analysis of prospective observational studies. Nutr Metab Cardiovasc Dis. 2014;24:1158–65. doi: 10.1016/j.numecd.2014.05.011. [DOI] [PubMed] [Google Scholar]


