Abstract
Bovine papillomavirus types 1 and 2 (BPV-1 and BPV-2) are known to induce common equine skin tumours, termed sarcoids. Recently, it was demonstrated that vaccination with BPV-1 virus-like particles (VLPs) is safe and highly immunogenic in horses. To establish a BPV-1 challenge model for evaluation of the protective potential of BPV-1 VLPs, four foals were injected intradermally with infectious BPV-1 virions and with viral genome-based and control inocula, and monitored daily for tumour development. Blood was taken before inoculation and at weekly intervals. BPV-1-specific serum antibodies were detected by a pseudo-virion neutralization assay. Total nucleic acids extracted from tumours, intact skin and PBMCs were tested for the presence of BPV-1 DNA and mRNA using PCR and RT-PCR, respectively. Intralesional E5 oncoprotein expression was determined by immunofluorescence. Pseudo-sarcoids developed exclusively at sites inoculated with virions. Tumours became palpable 11–32 days after virion challenge, reached a size of ≤20 mm in diameter and then resolved in ≤6 months. No neutralizing anti-BPV-1 serum antibodies were detectable pre- or post-challenge. BPV-1 DNA was present in lesions but not in intact skin. In PBMCs, viral DNA was already detectable before lesions were first palpable, in concentrations correlating directly with tumour growth kinetics. PBMCs from two of two foals also harboured E5 mRNA. Immunofluorescence revealed the presence of the E5 protein in tumour fibroblasts, but not in the apparently normal epidermis overlying the lesions. Together with previous findings obtained in horses and cows, these data suggest that papillomavirus infection may include a viraemic phase.
Introduction
Papillomaviruses (PVs) are small, non-enveloped viruses consisting of an icosahedral capsid harbouring a circular dsDNA genome. The latter comprises early and late coding regions, as well as a non-coding long control region that provides cis elements required for virus replication and transcription (Campo, 2006a). PVs are usually host specific and are described as having a stringent tropism for cutaneous or mucosal epithelia (Chow & Broker, 2006). Epithelial abrasion is one of the prerequisites for PV infection. Virions enter the host via basal epidermal cells, which provide the appropriate surface and secondary receptor molecules for virion attachment and uptake. There is evidence for surface heparan sulphate proteoglycans representing primary PV attachment sites (Joyce et al., 1999; Giroglou et al., 2001; Shafti-Keramat et al., 2003; Schiller et al., 2010). Subsequent PV endocytosis possibly involves clathrin- and caveolin-mediated mechanisms (Day et al., 2003; Smith et al., 2007), or may necessitate the presence of tetraspanin-enriched microdomains (Spoden et al., 2008). Interestingly, experimental data support the hypothesis that PVs can bind to receptor(s) expressed by a wide range of mammalian cells (Roden et al., 1994). This may also apply to lymphocytes.
In cattle, infection with bovine papillomavirus types 1 and 2 (BPV-1 and BPV-2) results mainly in the development of transient cutaneous and mucosal tumours. In some cases, however, and especially following ingestion of natural immunosuppressants, lesions may persist and progress to squamous cell carcinoma. BPV-induced transformation is predominantly mediated by its major oncoprotein, E5 (Campo, 2006b).
In addition to disease induced in bovines, BPV-1 and BPV-2 also infect equids and contribute chiefly to the development of common skin tumours termed sarcoids (Chambers et al., 2003). Sarcoid disease hence represents one of the rare examples of a cross-species infection by a PV (Campo, 2006a). Although the mechanisms of disease propagation within equid populations are still unclear, there is increasing evidence towards a virion-mediated transmission from equid to equid (Nasir & Reid, 1999; Bogaert et al., 2005, 2010; Marais et al., 2007; Brandt et al., 2008a, 2011c; Nasir & Campo, 2008).
Sarcoids are non-metastasizing tumours. However, due to their locally aggressive behaviour and their tendency to recur in more severe forms, sarcoids can seriously compromise the welfare of affected equids and cause substantial economic losses (Nasir & Reid, 2006). To date, no prophylactic vaccine is available to prevent this common, therapy-resistant disease.
In several animal species and humans, immunization with virus-like particles (VLPs) of papillomaviruses has been shown to provide efficient protection from PV infection (Breitburd et al., 1995; Suzich et al., 1995; Kirnbauer, 1996; Kirnbauer et al., 1996; Zinkernagel, 2003; Villa et al., 2005; Schiller, 2007). In a clinical phase I trial conducted in 15 horses, we recently demonstrated that BPV-1 L1 protein VLPs constitute a safe and highly immunogenic vaccine candidate (Shafti-Keramat et al., 2009; Hainisch et al., 2011). We next wanted to establish a robust virus challenge model to study the protective potential of BPV-1 L1 VLP vaccination in equids. Based on tumour induction experiments conducted in horses more than 50 years ago (Olson & Cook, 1951; Ragland & Spencer, 1969; Voss, 1969), four weanling foals were inoculated with BPV-containing and virus-free inocula and monitored for experimental infection and resulting pseudo-tumour formation using clinical, histological and molecular biological methods.
Results
Only inoculation with BPV-1 virions leads to pseudo-sarcoid formation
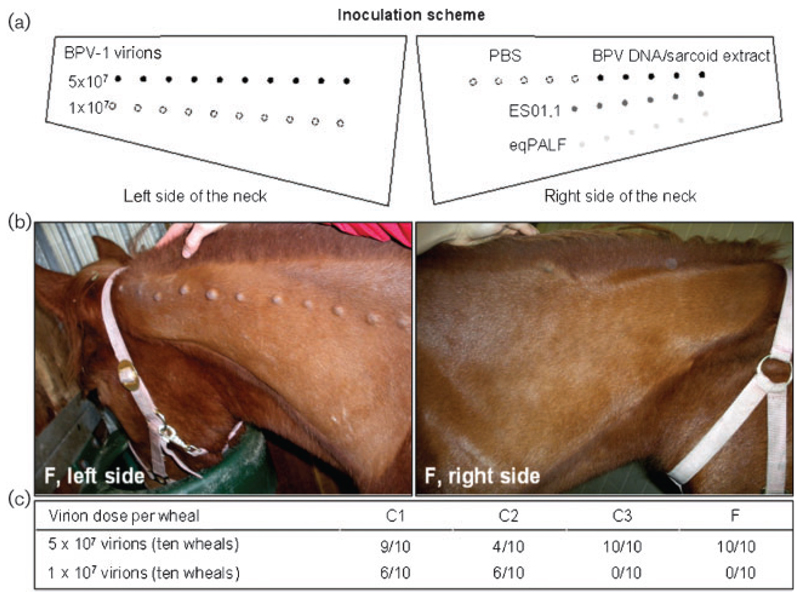
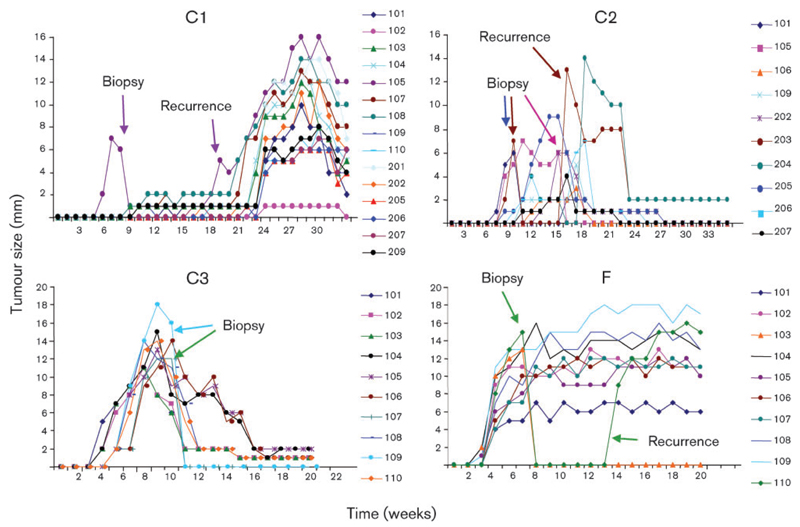
The inoculation scheme used is outlined in Fig 1(a). Tumours developed in all four horses at cutaneous sites inoculated intradermally with BPV-1 virions (Fig. 1b, c). Lesions became palpable after 11–32 days, reached sizes of up to 20 mm within 8–30 weeks and then resolved spontaneously (Fig. 2). Lesions mainly developed at sites injected with the higher virion dose, i.e. 5 × 107 particles per wheal (Fig. 1b, c). Tumour numbers varied between ten (colts C2 and C3 and filly F) and 15 (colt C1) per animal (Fig. 1c). Remarkably, no lesions developed following intradermal injection of either primary sarcoid fibroblasts containing viral episomes (one copy per cell) or naked full-length BPV-1 genomes. Injection of virus-free equine fibroblasts or PBS used as control inocula had no apparent effect, as expected (Fig. 1b). Interestingly, surgical removal of one to three lesions per horse (seven lesions in total) resulted in transient recurrence of three lesions in three out of four animals (Fig. 2). Spontaneous tumour regression was complete in all horses after 36 weeks (June/August 2009). No events of recurrence had been observed by June 2011.
Fig. 1.
Intradermal injection with BPV-1 virions results in the development of transient pseudosarcoids in foals. (a) Inoculation scheme (see Methods). (b) Pseudo-sarcoids induced by intradermal injection with virion (left side) in filly F. Inoculation with the viral genome or sarcoid cells containing viral episomes had no apparent effect (right side). (c) Summary of results in the four foals, C1–C3 and F.
Fig. 2.
Pseudo-sarcoid excision results in tumour recurrence. In horses C1, C2 and F, surgical excision of pseudo-sarcoids led to the transient recurrence of three lesions in a more aggressive form. Numbers (101–110 and 201–210) represent the different tumours.
Intradermal inoculation with BPV-1 virions fails to induce a neutralizing antibody response
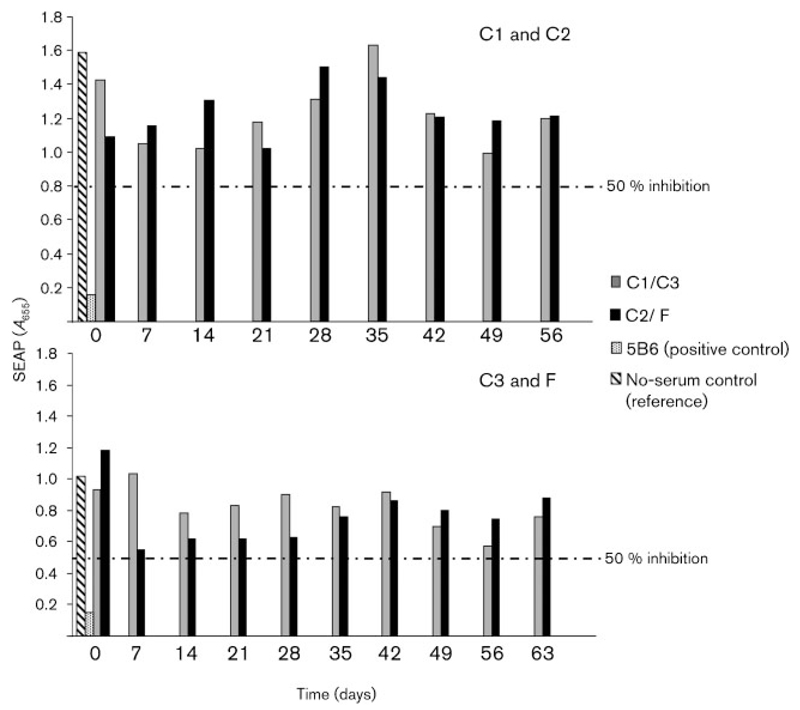
Sera collected immediately before experimental BPV-1 inoculation and then at weekly intervals were assessed for BPV-1 neutralizing antibodies by a pseudo-virion (PsV) neutralization assay. None of the sera from any point in time scored positively in this experiment, showing that multiple intradermal inoculations with the indicated amounts of BPV-1 virions in the absence of adjuvant were insufficient to induce a detectable amount of neutralizing anti-BPV-1 antibodies. As expected, robust neutralization was achieved by BPV-1-specific neutralizing mAb 5B6 in the assay (positive control; Fig. 3), whereas an anti-HPV-16 mAb was negative (negative control; not shown).
Fig. 3.
Intradermal inoculation with BPV-1 virions fails to induce specific neutralizing antibodies. BPV-1 PsVs containing a plasmid vector coding for secreted placental alkaline phosphatase (SEAP) as a reporter gene were incubated in duplicate in diluted horse serum and plated on to 293TT cells. After 48 h, the inhibition of SEAP by BPV-1 neutralizing antibody was measured as mean absorbance. Sera were deemed to be neutralizing when SEAP was inhibited by >50%. BPV-1-specific neutralizing mAb 5B6 was used as a positive control, and a no-serum control was used as a reference for maximum SEAP, which was set at 100%.
Experimental lesions harbour the BPV-1 genome and transcripts and E5 oncoprotein
DNA extracted from one to three pseudo-sarcoids and one intact skin biopsy per individual was screened for the presence of BPV-1 DNA by qualitative and quantitative PCR. All pseudo-sarcoids scored positively by these assays, revealing viral DNA loads of between 11 and 60 copies per cell. In contrast, no BPV-1 DNA was detected in intact skin taken from the chest. Subsequently, one lesion per individual was assessed for the presence of BPV-1 E5 transcripts. RT-PCR revealed E5 mRNA for all four tumours (data not shown), whereas samples for which reverse transcriptase was omitted (no-enzyme control) were consistently negative.
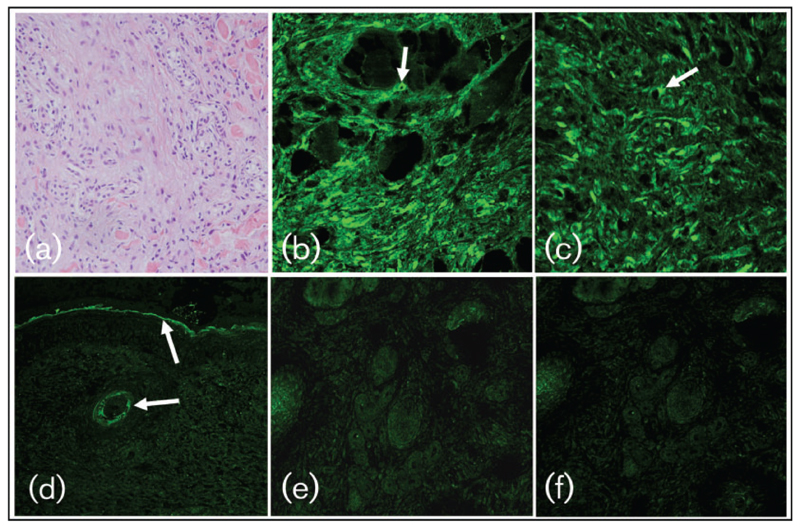
Routine haematoxylin and eosin staining (H&E) of tumour sections revealed collagen fibres in the dermis and neoplastic fibroblasts in a whorled pattern, as described for natural sarcoids (Scott & Miller, 2003; Fig. 4a) but no epidermal changes (data not shown). Cytoplasmic (Fig. 4b, arrow) or a typical juxtanuclear localization of E5 protein (Fig. 4c, arrow) was seen by immunofluorescence staining. In contrast, no E5 expression was observed for the apparently normal epidermal layer overlying the lesion (Fig. 4d). When the anti-E5 antibody was substituted with non-specific sheep IgG (Fig. 4e) or the former was omitted (Fig. 4f), no staining was observed.
Fig. 4.
E5 protein is expressed in pseudo-sarcoid fibroblasts but not in apparently intact epidermis overlying the tumour. (a) H&E staining of pseudo-sarcoid sections. Neoplastic fibroblasts are arranged in whorls as is typically seen for naturally acquired sarcoids. (b–f) Immunofluorescent staining of pseudo-sarcoid sections. (b) Neoplastic fibroblasts showing cytoplasmic E5 staining (arrow). (c) Neoplastic fibroblast with typical juxtanuclear localization of the E5 protein (arrow). (d) Absence of E5 expression in the apparently intact epidermis overlying the tumour. Arrows indicate non-specific keratin staining. (e, f) When irrelevant sheep IgG (e) or no IgG (f) were applied to pseudo-sarcoids, no specific staining was recorded.
Infection of PBMCs precedes pseudo-sarcoid formation
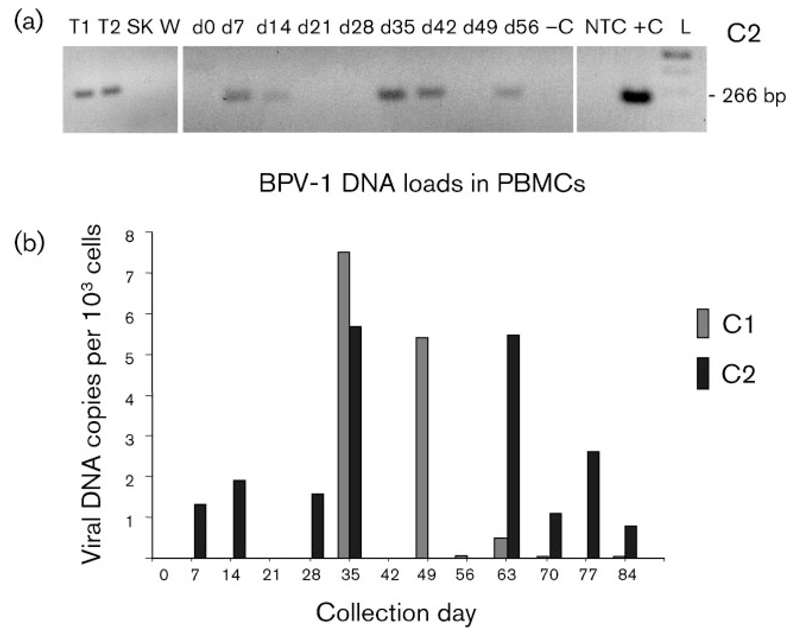
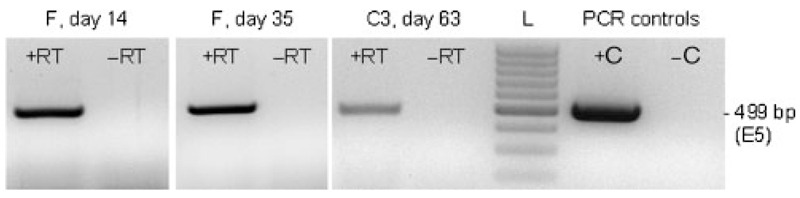
Remarkably, viral DNA was demonstrated in PBMCs of all four horses by 2–4 weeks before lesions were first palpable, whereas PBMCs obtained prior to inoculation (day 0) were negative, as expected. In the case of individual C2, viral DNA was first detectable in PBMCs from day 7 post-inoculation (Fig. 5a). Due to the lower sensitivity of quantitative PCR, viral DNA concentrations in PBMCs could not be measured for all PBMC DNA isolates that were positive by qualitative PCR. Nevertheless, the values obtained seemed to correlate directly with tumour growth kinetics. Viral DNA loads ranged between 0.2 and 7.5 copies per 103 PBMCs, with loads consecutively increasing during tumour growth and then decreasing towards zero in the course of spontaneous tumour regression (Fig. 5b). No more viral DNA was detected in PBMCs after lesions had resolved. Subsequently, all BPV PCR-positive PBMC isolates from individuals C3 and F were assessed for the presence of E5 mRNA. Following β-actin RT-PCR, which confirmed the accuracy of RNA isolation and DNase digestion (data not shown), E5 PCR from the validated cDNA was positive for PBMCs from days 14 and 35 (F) and day 63 (C3) (Fig. 6).
Fig. 5.
BPV-1 DNA loads correlate with tumour growth kinetics. (a) Qualitative detection of a 266 bp region of the BPV-1 gene L1 from pseudo-sarcoids (T1 and T2), an intact skin biopsy (SK), a juvenile wart (W) and PBMCs from day 0 (d0; before inoculation) and days 14–56 (d14–d56) after inoculation of horse C2. Note that viral DNA was detectable in PBMCs from day 7. –C, Virus-free equine DNA (negative control); NTC, no template control (sterile water); +C, sarcoid DNA (positive control); L, 100 bp DNA ladder (Fermentas). (b) BPV-1 DNA load in PBMCs of individuals C1 and C2, determined by quantitative PCR.
Fig. 6.
Detection of E5 mRNA in PBMCs by RT-PCR. A 499 bp fragment of the E5 gene was detected by RT-PCR, as described in Methods. +RT, Reverse transcription of mRNA with enzyme; –RT, no-enzyme control; L, 100 bp DNA ladder (Fermentas); +C, sarcoid DNA (positive control); –C, virus-free equine skin DNA (negative control).
Discussion
The main purpose of this study was to investigate whether pseudo-sarcoid induction studies conducted more than 50 years ago (Olson & Cook, 1951; Ragland & Spencer, 1969; Voss, 1969) would be reproducible in our hands, thus providing us with an experimental challenge model for the evaluation of BPV-1 VLPs as a prophylactic vaccine in horses. Intradermal inoculation of foals with infectious, cow wart-derived BPV-1 virions resulted in the robust formation of transient sarcoid-like lesions of the nodular type. In contrast, injection with fibroblasts harbouring viral episomes or with naked BPV-1 genome did not induce overt tumours. These findings strengthen the concept of intact virions being required for BPV infection of equids resulting in skin tumours (Brandt et al., 2011c) and that vaccination with BPV-1 VLPs may become an efficient strategy to protect equids from BPV infection and related skin malignancies (Chambers et al., 2003; Yuan et al., 2007; Hainisch et al., 2011).
Interestingly, surgical excision of pseudo-sarcoids resulted in transient recurrence of some of the lesions. Whereas primary tumours were of the nodular type (i.e. firm nodules covered by apparently intact skin), recrudescing lesions reached almost double size and resembled fibroblastic sarcoids, i.e. ulcerated nodules with destroyed epidermis (Knottenbelt, 2005). This behaviour is consistent with the observation of trauma acting as a co-factor in the development of natural sarcoids and their recurrence in a more aggressive form (Chambers et al., 2003), thus further supporting the experimental inoculation model as a surrogate for natural infection.
We have shown recently that intramuscular injection of horses with BPV-1 VLPs is well tolerated and induces a long-lasting humoral immune response (Villa et al., 2005; Hainisch et al., 2011). The fact that intradermal injection of virions failed to induce BPV-1 capsid-specific antibodies in foals is in agreement with the finding of anti-BPV antibodies being detected only rarely in naturally infected cows (Campo, 2006b). Spontaneous regression of PV-induced human and bovine papillomas is accompanied by a pronounced intralesional accumulation of activated CD4+, γδ and CD8+ lymphocytes (Coleman et al., 1994; Knowles et al., 1996). Similarly, the observed pseudosarcoid regression in foals may be achieved by a cell-mediated immune response.
Histologically, experimental lesions consisted mainly of neoplastic fibroblasts containing E5 oncoprotein, whereas no E5 expression was noted for the apparently normal epidermis overlying the lesions. Early in situ hybridization experiments failed to detect viral DNA in the epidermis of natural sarcoids so that BPV-1 infection was thought to be abortive in equids, with virus residing solely in fibroblasts in an episomal form (Lancaster et al., 1977; Amtmann et al., 1980; Lancaster, 1981). However, there is recent evidence of BPV-1 infection also involving the epidermis and being productive in horses (Brandt et al., 2008a, 2011c; Bogaert et al., 2010). In particular, epidermal E5 expression in naturally acquired sarcoids seems to distinguish the latter from experimentally induced lesions. In sarcoids, E5 protein is thought to compromise cell-mediated tumour clearance via downregulation of MHC class I (Marchetti et al., 2009), and by this may account for the persistence of the tumours.
The presence of viral DNA was demonstrated for most PBMC samples collected in the course of tumour growth, and respective concentrations correlated with tumour size. During spontaneous tumour regression, viral DNA loads decreased to zero, an indication for the elimination of infected blood cells in the course of cell-mediated tumour clearance. Remarkably, viral DNA was already detectable 2–4 weeks before lesions were first palpable. This unexpected finding is particularly interesting as it may shed new light on the mechanisms of BPV infection. In naturally infected horses and bovines, the presence of viral DNA (Stocco dos Santos et al., 1998; de Freitas et al., 2003; Brandt et al., 2008b, Roperto et al., 2008) and mRNA (Borzacchiello et al., 2010; Brandt et al., 2011b) has been demonstrated consistently. In BPV-2-infected bovines, the viral genome has been shown to reside predominantly in T lymphocytes, where it is also transcribed (Borzacchiello et al., 2010). Based on these findings, it seems reasonable to assume that viral infection in horses may also involve this cell type. The reported confinement of BPV infection to specific PBMC subsets may explain our finding of relatively low viral DNA concentrations in PBMCs and the rare detection of viral transcripts in these cells. Remarkably, there is recent evidence for early BPV-2 E5 and E7 and late L1 capsid protein also being expressed in bovine T and B lymphocytes (Roperto et al., 2011), suggesting that these cell subsets are permissive for a productive BPV infection. In concert with the findings reported here of early PBMC infection after experimental inoculation of horses with BPV-1 virions, it is conceivable that infection by BPV-1, and possibly other PVs, comprises a viraemic phase that may even constitute a prerequisite for disease onset and progression. Further investigations are warranted to elucidate this novel aspect of PV infection.
Methods
Animals
Four 8-month-old warmblood foals (colts C1–C3 and filly F) in good general condition and showing no clinical signs of BPV-induced skin malignancies were purchased and stabled in pairs (C1 and C2; C3 and F) at the Equine Clinic of the Veterinary University Vienna, Vienna, Austria. The absence of BPV-1/-2 infection was confirmed as follows. Horse sera were analysed for the presence of anti-BPV-1 antibody by PsV neutralization assay, as described elsewhere (Hainisch et al., 2011). Skin biopsies were taken from the neck of each individual using a 4 mm biopsy punch. DNA was extracted from these samples using a DNeasy Blood and Tissue kit according to instructions of the manufacturer (Qiagen) and tested for PCR compatibility by standard equine β-actin PCR (Brandt et al., 2011b). Subsequently, DNA isolates were screened for the presence of BPV-1/-2 DNA using a previously described PCR protocol (Brandt et al., 2011b; Hainisch et al., 2011).
Preparation of inocula
BPV-1 virions were obtained by homogenization of cow wart tissue in PBS and subsequent virion purification, as described previously (Shafti-Keramat et al., 2003). The concentration of infectious virions was determined by in vitro focal formation assay (Roden et al., 1996). Prior to inoculation, 5 × 107 and 1 × 107 BPV-1 virions were suspended in 50 μl PBS. A virus-free equine palatal fibroblast cell line (eqPALF; negative control) and a sarcoid-derived fibroblast cell line containing episomal BPV-1 DNA (ES01.1; positive control) were kindly provided by Lubna Nasir (University of Glasgow, Glasgow, UK) and expanded in DMEM with GlutaMax containing 10% FCS and 1% penicillin/streptomycin. Immediately before inoculation, cells were harvested and adjusted to 2 × 105 cells in 50 μl PBS. Full-length genomic BPV-1 DNA was obtained by HindIII (Roche) digestion of plasmid pML-BPV1 (Brandt et al., 2008a), subsequent gel purification (QiaEX II gel extraction kit; Qiagen) and final adjustment to a concentration of 0.36 μg viral genome in 50 μl PBS. PBS aliquots (50 μl) were prepared as additional control inocula to determine possible side effects that might be induced by buffer alone.
Inoculation and monitoring of horses
Following a 4-week period of adaptation of the foals to their new environment, inoculation of individuals C1 and C2 was performed on day 0. The left side of the neck was injected intradermally with cow wart-derived BPV-1 virions (ten wheals with 5 × 107 virions per wheal and ten wheals with 1 × 107 virions per wheal). The right side was injected intradermally with sarcoid-derived ES01.1 fibroblasts containing episomal BPV-1 DNA (six wheals with 2 × 105 ES01.1 cells per wheal), virus-free eqPALF cells (six wheals with 2 × 105 eqPALF cells per wheal), BPV-1 genome (five wheals with 360 ng BPV-1 DNA per wheal) or PBS alone (five wheals). The injection volume per wheal was 50 µl (Fig. 1).
Inoculation of individuals C3 and F was carried out 4 months later with the only difference being that genomic DNA was substituted by sarcoid-extract supernatant (five wheals with 50 µl per wheal) containing capsid protein-complexed viral genome (37 000 complexes in 50 µl) as inoculum (Brandt et al., 2008a). Following inoculation, the horses were clinically monitored, the injection sites palpated and all observations were recorded on a daily basis.
Sample collection and processing
On day 41 after each inoculation, one to three lesions per horse were excised and intact skin was biopsied from the chest of each individual. Tissue aliquots were stored in DNA and RNA extraction buffers ATL and RLT, respectively (Qiagen), and embedded in paraffin for immunohistological analyses. Whole blood (8 ml per horse) and heparinized blood (16 ml per horse) were collected immediately before inoculation and then at weekly intervals until the induced lesions had resolved completely. Heparinized blood was subjected to PBMC isolation using Ficoll-Paque PLUS (GE Healthcare Bio-Sciences) gradient centrifugation. PBMC aliquots were stored at −80 °C as pellets or in TriPure Isolation Reagent (Roche) for subsequent DNA and RNA isolation. The PBMCs in TriPure of individuals C1 and C2 were unfortunately lost due to storage problems. Whole blood was incubated at 4 °C overnight. The sera were then decanted and stored at −20 °C until use.
PsV neutralization assay
Neutralizing serum antibody titres were determined by a BPV-1 PsV neutralization assay as described previously (Hainisch et al., 2011). BPV-1 PsVs containing a plasmid vector coding for secreted placental alkaline phosphatase (SEAP) as a reporter gene were incubated in duplicate in 1 : 50 dilutions of horse serum and plated on to 293TT cells. After 48 h, SEAP in the cell culture medium was detected and measured as mean absorbance at 655 nm (A655) as described by Pastrana et al. (2004). The following controls were included in the test: a background control (293TT cells in growth medium), a no-serum control yielding maximum SEAP set at 100 % (reference; PsV-infected 293TT cells without antiserum), a positive control (293TT cells treated with PsVs that had been pre-incubated with neutralizing anti-BPV-1 L1 mAb 5B6; a gift from R. Roden, John Hopkins University, Baltimore, MD, USA) and a negative control (cells treated with PsVs that had been pre-incubated with non-specific anti-HPV-16 mAb V5; a gift from R. Roden). Serum dilutions causing at least a 50% reduction in SEAP in comparison with the no-serum control were considered neutralizing.
PCR- and RT-PCR-based analyses
The presence of BPV-1 DNA was investigated in tissue samples and PBMCs of the four horses using PCR. Purified PBMCs as well as collected tissue samples were subjected to DNA extraction using a DNeasy Blood and Tissue kit. Following β-actin PCR (Brandt et al., 2008b, 2011a) to test for the accuracy of DNA extraction, the DNA was subjected to BPV-1 PCR and quantitative PCR (Brandt et al., 2011b). DNA from virus-free equine skin and sarcoids, and sterile water were used as PCR negative, positive and no-template controls, respectively.
PBMC isolates from individuals C3 and F, which were positive for BPV-1 PCR, were subsequently subjected to RNA extraction followed by DNase digestion and reverse transcription, as described previously (Kohrgruber et al., 2004). A no-enzyme control was included for every sample. Following β-actin PCR (Brandt et al., 2008b, 2011b), which confirmed the accuracy of RNA extraction and successful DNase digestion, cDNA samples as well as the above-mentioned controls were subjected to BPV-1 PCR (Brandt et al., 2011b). Amplification products (16 μl) from all qualitative reactions were visualized on 1.5% Tris/acetate agarose gels by ethidium bromide staining.
Immunofluorescence and confocal laser-scanning microscopy
Paraffin-embedded pseudo-sarcoid sections (5 μm) were dewaxed, rehydrated and subjected to routine H&E staining or immunofluoresence. Antigen enhancement was achieved by heating in a microwave (2 × 5 min at 750 W). The sections were then blocked with donkey serum for 30 min and incubated with primary sheep anti-E5 antibody (diluted 1 : 50; kindly provided by Professor M. S. Campo, University of Glasgow, UK) in a humidified chamber at 4 °C overnight. The slides were washed three times with PBS and incubated with Alexa Fluor 488-labelled donkey anti-sheep antibody (diluted 1 : 100; Molecular Probes) for 1 h at room temperature. After washing with PBS, the slides were finally mounted in PBS : glycerol (1 : 1) and evaluated using a confocal laser-scanning microscope (LSM-510; Zeiss). Alexa Fluor 488 was irradiated at 488 nm and fluorescence emission was revealed with a 505–530 nm bandpass filter. The negative controls were obtained by either omitting the primary antibody or using non-specific sheep IgG as a substitute.
Acknowledgements
This study was funded by the University of Veterinary Medicine, Vienna, Austria (University start-up programme ‘Profillinie’) and in part by a grant to R. K. from the Austrian Science Foundation (FWF) (P18990P13). The animal trial has been approved by the institutional ethics committee, the Advisory Committee for Animal Experiments (§12 of Law for Animal Experiments, Tierversuchsgesetz – TVG) and the Federal Ministry for Science and Research, Approval No. GZ 68.205/0054-II/10b/2008.
References
- Amtmann E, Müller H, Sauer G. Equine connective tissue tumors contain unintegrated bovine papilloma virus DNA. J Virol. 1980;35:962–964. doi: 10.1128/jvi.35.3.962-964.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogaert L, Martens A, De Baere C, Gasthuys F. Detection of bovine papillomavirus DNA on the normal skin and in the habitual surroundings of horses with and without equine sarcoids. Res Vet Sci. 2005;79:253–258. doi: 10.1016/j.rvsc.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Bogaert L, Martens A, Kast WM, Van Marck E, De Cock H. Bovine papillomavirus DNA can be detected in keratinocytes of equine sarcoid tumors. Vet Microbiol. 2010;146:269–275. doi: 10.1016/j.vetmic.2010.05.032. [DOI] [PubMed] [Google Scholar]
- Borzacchiello G, Roperto F, Campo MS, Venuti A. 1st International Workshop on Papillomavirus E5 Oncogene – a report. Virology. 2010;408:135–137. doi: 10.1016/j.virol.2010.09.015. [DOI] [PubMed] [Google Scholar]
- Brandt S, Haralambus R, Shafti-Keramat S, Steinborn R, Stanek C, Kirnbauer R. A subset of equine sarcoids harbours BPV-1 DNA in a complex with L1 major capsid protein. Virology. 2008a;375:433–441. doi: 10.1016/j.virol.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Brandt S, Haralambus R, Schoster A, Kirnbauer R, Stanek C. Peripheral blood mononuclear cells represent a reservoir of bovine papillomavirus DNA in sarcoid-affected equines. J Gen Virol. 2008b;89:1390–1395. doi: 10.1099/vir.0.83568-0. [DOI] [PubMed] [Google Scholar]
- Brandt S, Apprich V, Hackl V, Tober R, Danzer M, Kainzbauer C, Gabriel C, Stanek C, Kofler J. Prevalence of bovine papillomavirus and Treponema DNA in bovine digital dermatitis lesions. Vet Microbiol. 2011a;148:161–167. doi: 10.1016/j.vetmic.2010.08.031. [DOI] [PubMed] [Google Scholar]
- Brandt S, Schoster A, Tober R, Kainzbauer C, Burgstaller JP, Haralambus R, Steinborn R, Hinterhofer C, Stanek C. Consistent detection of bovine papillomavirus in lesions, intact skin and peripheral blood mononuclear cells of horses affected by hoof canker. Equine Vet J. 2011b;43:202–209. doi: 10.1111/j.2042-3306.2010.00147.x. [DOI] [PubMed] [Google Scholar]
- Brandt S, Tober R, Corteggio A, Burger S, Sabitzer S, Walter I, Kainzbauer C, Steinborn R, Nasir L, Borzacchiello G. BPV-1 infection is not confined to the dermis but also involves the epidermis of equine sarcoids. Vet Microbiol. 2011c;150:35–40. doi: 10.1016/j.vetmic.2010.12.021. [DOI] [PubMed] [Google Scholar]
- Breitburd F, Kirnbauer R, Hubbert NL, Nonnenmacher B, Trin-Dinh-Desmarquet C, Orth G, Schiller JT, Lowy DR. Immunization with viruslike particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J Virol. 1995;69:3959–3963. doi: 10.1128/jvi.69.6.3959-3963.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campo MS. Introduction. In: Campo MS, editor. Papillomavirus Research: From Natural History to Vaccines and Beyond. Norfolk, UK: Caister Academic Press; 2006a. pp. 1–2. [Google Scholar]
- Campo MS. Bovine papillomavirus: old system, new lessons? In: Campo MS, editor. Papillomavirus Research: From Natural History to Vaccines and Beyond. Norfolk, UK: Caister Academic Press; 2006b. pp. 373–387. [Google Scholar]
- Chambers G, Ellsmore VA, O’Brien PM, Reid SWJ, Love S, Campo MS, Nasir L. Association of bovine papillomavirus with the equine sarcoid. J Gen Virol. 2003;84:1055–1062. doi: 10.1099/vir.0.18947-0. [DOI] [PubMed] [Google Scholar]
- Chow LT, Broker TR. Mechanisms and regulation of papillomavirus DNA replication. In: Campo MS, editor. Papillomavirus Research: From Natural History to Vaccines and Beyond. Norfolk, UK: Caister Academic Press; 2006. pp. 53–71. [Google Scholar]
- Coleman N, Birley HD, Renton AM, Hanna NF, Ryait BK, Byrne M, Taylor-Robinson D, Stanley MA. Immunological events in regressing genital warts. Am J Clin Pathol. 1994;102:768–774. doi: 10.1093/ajcp/102.6.768. [DOI] [PubMed] [Google Scholar]
- Day PM, Lowy DR, Schiller JT. Papillomaviruses infect cells via a clathrin-dependent pathway. Virology. 2003;307:1–11. doi: 10.1016/s0042-6822(02)00143-5. [DOI] [PubMed] [Google Scholar]
- de Freitas AC, de Carvalho C, Brunner O, Birgel EH, Jr, Melville Paiva Dellalibera AM, Benesi FJ, Gregory L, Beçak W, de Cassia Stocco dos Santos R. Viral DNA sequences in peripheral blood and vertical transmission of the virus: a discussion about BPV-1. Braz J Microbiol. 2003;34:76–78. [Google Scholar]
- Giroglou T, Florin L, Schäfer F, Streeck RE, Sapp M. Human papillomavirus infection requires cell surface heparan sulfate. J Virol. 2001;75:1565–1570. doi: 10.1128/JVI.75.3.1565-1570.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainisch EK, Brandt S, Shafti-Keramat S, van den Hoven R, Kirnbauer R. Safety and immunogenicity of BPV-1 L1 virus-like particles in a dose-escalation vaccination trial in horses. Equine Vet J. 2011 doi: 10.1111/j.2042-3306.2011.00390.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce JG, Tung JS, Przysiecki CT, Cook JC, Lehman ED, Sands JA, Jansen KU, Keller PM. The L1 major capsid protein of human papillomavirus type 11 recombinant virus-like particles interacts with heparin and cell-surface glycosaminoglycans on human keratinocytes. J Biol Chem. 1999;274:5810–5822. doi: 10.1074/jbc.274.9.5810. [DOI] [PubMed] [Google Scholar]
- Kirnbauer R. Papillomavirus-like particles for serology and vaccine development. Intervirology. 1996;39:54–61. doi: 10.1159/000150475. [DOI] [PubMed] [Google Scholar]
- Kirnbauer R, Chandrachud LM, O’Neil BW, Wagner ER, Grindlay GJ, Armstrong A, McGarvie GM, Schiller JT, Lowy DR, Campo MS. Virus-like particles of bovine papillomavirus type 4 in prophylactic and therapeutic immunization. Virology. 1996;219:37–44. doi: 10.1006/viro.1996.0220. [DOI] [PubMed] [Google Scholar]
- Knottenbelt DC. A suggested clinical classification for the equine sarcoid. Clin Tech Equine Pract. 2005;4:278–295. [Google Scholar]
- Knowles G, O’Neil BW, Campo MS. Phenotypical characterization of lymphocytes infiltrating regressing papillomas. J Virol. 1996;70:8451–8458. doi: 10.1128/jvi.70.12.8451-8458.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohrgruber N, Gröger M, Meraner P, Kriehuber E, Petzelbauer P, Brandt S, Stingl G, Rot A, Maurer D. Plasmacytoid dendritic cell recruitment by immobilized CXCR3 ligands. J Immunol. 2004;173:6592–6602. doi: 10.4049/jimmunol.173.11.6592. [DOI] [PubMed] [Google Scholar]
- Lancaster WD. Apparent lack of integration of bovine papillomavirus DNA in virus-induced equine and bovine tumor cells and virus-transformed mouse cells. Virology. 1981;108:251–255. doi: 10.1016/0042-6822(81)90433-5. [DOI] [PubMed] [Google Scholar]
- Lancaster WD, Olson C, Meinke W. Bovine papillomavirus: presence of virus-specific DNA sequences in naturally occurring equine tumors. Proc Natl Acad Sci U S A. 1977;74:524–528. doi: 10.1073/pnas.74.2.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marais HJ, Nel P, Bertschinger HJ, Schoeman JP, Zimmerman D. Prevalence and body distribution of sarcoids in South African Cape mountain zebra (Equus zebra zebra) J S Afr Vet Assoc. 2007;78:145–148. doi: 10.4102/jsava.v78i3.306. [DOI] [PubMed] [Google Scholar]
- Marchetti B, Gault EA, Cortese MS, Yuan Z, Ellis SA, Nasir L, Campo MS. Bovine papillomavirus type 1 oncoprotein E5 inhibits equine MHC class I and interacts with equine MHC I heavy chain. J Gen Virol. 2009;90:2865–2870. doi: 10.1099/vir.0.014746-0. [DOI] [PubMed] [Google Scholar]
- Nasir L, Campo MS. Bovine papillomaviruses: their role in the aetiology of cutaneous tumours of bovids and equids. Vet Dermatol. 2008;19:243–254. doi: 10.1111/j.1365-3164.2008.00683.x. [DOI] [PubMed] [Google Scholar]
- Nasir L, Reid SWJ. Bovine papillomaviral gene expression in equine sarcoid tumours. Virus Res. 1999;61:171–175. doi: 10.1016/s0168-1702(99)00022-2. [DOI] [PubMed] [Google Scholar]
- Nasir L, Reid SWJ. Bovine papillomaviruses and equine sarcoids. In: Campo MS, editor. Papillomavirus Research: From Natural History to Vaccines and Beyond. Norfolk, UK: Caister Academic Press; 2006. pp. 389–397. [Google Scholar]
- Olson C, Jr, Cook RH. Cutaneous sarcoma-like lesions of the horse caused by the agent of bovine papilloma. Proc Soc Exp Biol Med. 1951;77:281–284. doi: 10.3181/00379727-77-18750. [DOI] [PubMed] [Google Scholar]
- Pastrana DV, Buck CB, Pang YY, Thompson CD, Castle PE, FitzGerald PC, Krüger Kjaer S, Lowy DR, Schiller JT. Reactivity of human sera in a sensitive, high-throughput pseudovirus-based papillomavirus neutralization assay for HPV16 and HPV18. Virology. 2004;321:205–216. doi: 10.1016/j.virol.2003.12.027. [DOI] [PubMed] [Google Scholar]
- Ragland WL, Spencer GR. Attempts to relate bovine papilloma virus to the cause of equine sarcoid: equidae inoculated intradermally with bovine papilloma virus. Am J Vet Res. 1969;30:743–752. [PubMed] [Google Scholar]
- Roden RB, Kirnbauer R, Jenson AB, Lowy DR, Schiller JT. Interaction of papillomaviruses with the cell surface. J Virol. 1994;68:7260–7266. doi: 10.1128/jvi.68.11.7260-7266.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roden RB, Greenstone HL, Kirnbauer R, Booy FP, Jessie J, Lowy DR, Schiller JT. In vitro generation and type-specific neutralization of a human papillomavirus type 16 virion pseudotype. J Virol. 1996;70:5875–5883. doi: 10.1128/jvi.70.9.5875-5883.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roperto S, Brun R, Paolini F, Urraro C, Russo V, Borzacchiello G, Pagnini U, Raso C, Rizzo C, et al. Detection of bovine papillomavirus type 2 in the peripheral blood of cattle with urinary bladder tumours: possible biological role. J Gen Virol. 2008;89:3027–3033. doi: 10.1099/vir.0.2008/004457-0. [DOI] [PubMed] [Google Scholar]
- Roperto S, Comazzi S, Ciusani E, Paolini F, Borzacchiello G, Esposito I, Lucá R, Russo V, Urraro C, et al. PBMCs are additional sites of productive infection of bovine papillomavirus type 2. J Gen Virol. 2011;92:1787–1794. doi: 10.1099/vir.0.031740-0. [DOI] [PubMed] [Google Scholar]
- Schiller JT. Papillomavirus vaccines. In: Garcia R, DiMaio D, editors. The Papillomaviruses. New York: Springer; 2007. pp. 337–369. [Google Scholar]
- Schiller JT, Day PM, Kines RC. Current understanding of the mechanism of HPV infection. Gynecol Oncol. 2010;118(Suppl):S12–S17. doi: 10.1016/j.ygyno.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DW, Miller WH., Jr . Equine Dermatology. St Louis, Missouri, USA: Elsevier Science; 2003. Sarcoid; pp. 719–731. [Google Scholar]
- Shafti-Keramat S, Handisurya A, Kriehuber E, Meneguzzi G, Slupetzky K, Kirnbauer R. Different heparan sulfate proteoglycans serve as cellular receptors for human papillomaviruses. J Virol. 2003;77:13125–13135. doi: 10.1128/JVI.77.24.13125-13135.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafti-Keramat S, Schellenbacher C, Handisurya A, Christensen N, Reininger B, Brandt S, Kirnbauer R. Bovine papillomavirus type 1 (BPV1) and BPV2 are closely related serotypes. Virology. 2009;393:1–6. doi: 10.1016/j.virol.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JL, Campos SK, Ozbun MA. Human papillomavirus type 31 uses a caveolin 1- and dynamin 2-mediated entry pathway for infection of human keratinocytes. J Virol. 2007;81:9922–9931. doi: 10.1128/JVI.00988-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoden G, Freitag K, Husmann M, Boller K, Sapp M, Lambert C, Florin L. Clathrin- and caveolin-independent entry of human papillomavirus type 16 – involvement of tetraspanin-enriched microdomains (TEMs) PLoS ONE. 2008;3:e3313. doi: 10.1371/journal.pone.0003313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocco dos Santos RC, Lindsey CJ, Ferraz OP, Pinto JR, Mirandola RS, Benesi FJ, Birgel EH, Pereira CA, Beçak W. Bovine papillomavirus transmission and chromosomal aberrations: an experimental model. J Gen Virol. 1998;79:2127–2135. doi: 10.1099/0022-1317-79-9-2127. [DOI] [PubMed] [Google Scholar]
- Suzich JA, Ghim SJ, Palmer-Hill FJ, White WI, Tamura JK, Bell JA, Newsome JA, Jenson AB, Schlegel R. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc Natl Acad Sci U S A. 1995;92:11553–11557. doi: 10.1073/pnas.92.25.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa LL, Costa RL, Petta CA, Andrade RP, Ault KA, Giuliano AR, Wheeler CM, Koutsky LA, Malm C, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 2005;6:271–278. doi: 10.1016/S1470-2045(05)70101-7. [DOI] [PubMed] [Google Scholar]
- Voss JL. Transmission of equine sarcoid. Am J Vet Res. 1969;30:183–191. [PubMed] [Google Scholar]
- Yuan Z, Philbey AW, Gault EA, Campo MS, Nasir L. Detection of bovine papillomavirus type 1 genomes and viral gene expression in equine inflammatory skin conditions. Virus Res. 2007;124:245–249. doi: 10.1016/j.virusres.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Zinkernagel RM. On natural and artificial vaccinations. Annu Rev Immunol. 2003;21:515–546. doi: 10.1146/annurev.immunol.21.120601.141045. [DOI] [PubMed] [Google Scholar]