Abstract
Due to their unique properties, in recent years, ionic liquids (ILs) have been largely investigated in the field of analytical chemistry. Particularly during the last sixteen years, they have been successfully applied in the chromatographic and electrophoretic analysis of value-added compounds extracted from biomass. Considering the growing interest in the use of ILs in this field, this critical review provides a comprehensive overview on the improvements achieved using ILs as constituents of mobile or stationary phases in analytical techniques, namely in capillary electrophoresis and its different modes, in high performance liquid chromatography, and in gas chromatography, for the separation and analysis of natural compounds. The impact of the IL chemical structure and the influence of secondary parameters, such as the IL concentration, temperature, pH, voltage and analysis time (when applied), are also critically addressed regarding the achieved separation improvements. Major conclusions on the role of ILs in the separation mechanisms and the performance of these techniques in terms of efficiency, resolution and selectivity are provided. Based on a critical analysis of all published results, some target-oriented ILs are suggested. Finally, current drawbacks and future challenges in the field are highlighted. In particular, the design and use of more benign and effective ILs as well as the development of integrated (and thus more sustainable) extraction–separation processes using IL aqueous solutions are suggested within a green chemistry perspective.
Introduction
Interest in the extraction of value-added compounds or fine-chemicals from biomass (extractives) has largely increased in recent years. This growing interest is a result of public concern regarding the adverse effects of synthetic compounds on human health, combined with the increasing number of scientific reports demonstrating the enhanced performance of bio-based compounds in nutraceutical, cosmeceutical and pharmaceutical applications.1–5 Furthermore, the attention given to these compounds has also been stimulated, in an integrated perspective, by the search for sustainable approaches to produce fuels, energy, chemicals and materials from biomass,6 driven by the decline in oil reserves, along with the serious environmental damage caused by the over-exploitation of non-renewable resources and the constant need for industries to remain competitive.
The identification, extraction and isolation of new valuable compounds from complex biomass sources, which are mainly composed of cellulose, hemicelluloses and lignin, in addition to extractives and inorganic compounds, require the use of efficient extraction procedures, as well as powerful separation methods, which often are chromatographic techniques. Most methods for the extraction/fractionation and analysis of value-added components from biomass require the use of organic, volatile, and often toxic solvents. Nevertheless, these processes usually present several drawbacks, such as the requirement of long extraction times, a high energy input, and the possible degradation of value-added compounds when high operating temperatures are employed, among others.3,7 Therefore, the search for alternative solvents and technologies, while fulfilling sustainability requirements, has been a crucial challenge in the past few decades.8–10 Although a large array of extraction and separation techniques are currently available, some advances have been made in recent years, in which ionic liquids (ILs) have demonstrated a large positive impact in the development of more cost-effective and environmentally-friendly extraction and separation processes.
ILs are salts typically composed of large and asymmetric organic cations and organic or inorganic anions, being liquid at temperatures below 100 °C.11 Due to their ionic nature, they present two outstanding properties: negligible volatility and non-flammability. Mainly because of these two characteristics, ILs have been receiving a “green” connotation, although other properties, such as biodegradability and toxicity, while not always successfully met, should be additionally considered. Furthermore, one of the main advantages concerning the applicability of ILs, particularly in the extraction, separation and analysis of value-added compounds from biomass, is the ability to tailor their polarities and affinities by a proper manipulation of the cation/anion chemical structure.12,13 Based on these features, ILs have been largely investigated as alternative solvents either for the extraction of value-added compounds from biomass14 or for the enhancement of their analysis (comprising both the identification and quantification aspects),15 although no studies comprising an integrated process of extraction and further analysis using the same IL solution have been reported so far.
After the pioneering study by Du et al.16 in which aqueous solutions of ILs were proposed as alternative and effective solvents for the extraction of trans-resveratrol from a Chinese medicine herb, a large number of research studies have been reported in the literature regarding the application of ILs, either pure or as mixtures with molecular solvents (mainly water and alcohols), for the extraction of value-added compounds from natural sources.14 In addition to their use in extractions,14 ILs have also been used in sample pre-treatment approaches to improve the fractionation and isolation of target compounds from biomass extracts, by applying solid-phase extraction (SPE) and solid-phase micro-extraction (SPME) techniques.17,18 In these, the incorporation of ILs in stationary phases allows specific interactions with the target compounds, which has resulted in high extraction efficiencies and selectivity while reducing the amount of hazardous organic solvents typically used.
Apart from efficient extraction/fractionation processes, high resolution chromatographic analytical techniques are fundamental for the characterization of value-added extracts obtained from biomass, either for quality control or for the isolation of pure target compounds. It is indeed possible to identify a number of important contributions and advances in the separation and analysis of natural value-added compounds resulting from the use of ILs, either as constituents of liquid mobile phases or running electrolytes in high performance liquid chromatography (HPLC) and capillary electrophoresis (CE) or as stationary phases of HPLC or gas chromatography (GC) techniques. Based on an extended compilation of the data hitherto reported comprising the use of ILs in chromatographic and electrophoretic techniques for the identification and separation of natural compounds, this work provides a comprehensive overview on the improvements achieved using ILs as components of mobile or stationary phases in CE, HPLC and GC and the main challenges that still need to be addressed, namely the search for more target-oriented and benign ILs and the development of integrated and more sustainable extraction–separation processes.
Improvement of chromatographic and electrophoretic methods using ILs
ILs have been used in several analytical techniques, namely in CE and some of its different modes (capillary zone electrophoresis – CZE, micellar electrokinetic capillary chromatography – MEKC and non-aqueous capillary electrophoresis – NACE),19,20 in HPLC21 and in GC,22 aiming at enhancing the separation and further analysis of target compounds.
Several advances in the performance of analytical techniques by the use of ILs as mobile and stationary phase additives have been reported.23–26 The high thermal stability and tailored polarity of IL-based stationary phases, when compared to conventional highly polar columns, as well as the application of ILs as buffer additives or electroosmotic flow modifiers, are the major features behind the obtained chromatographic and electrophoretic improved separations.23–26 Furthermore, within the framework of a green analytical chemistry perspective, these studies demonstrated that the use of ILs in separation techniques, particularly in CE and HPLC as additives in mobile phases, can lead to a decrease in the amount of organic solvents and additives used, as well as to a decrease in the energy consumption by increasing the speed of analysis without compromising the analytical performance or even improving it.27,28 Most of these accomplishments have been demonstrated using mixtures of synthetic-derived analytes, for which significant reviews have already been published.25,26,29 Nonetheless, the number of published studies regarding the application of ILs in the separation and analysis of value-added compounds directly extracted from biomass is much more limited, with a total of 25 manuscripts reported in the literature in the past sixteen years.30–54 Within these studies, CE was the most studied technique, followed by HPLC and GC applications. On the other hand, phenolic compounds and alkaloids represent the major classes of compounds investigated, followed by carbohydrates, essential oils and lipids.
Within the analytical techniques considered, a large number of ILs were investigated. The name and acronym of each IL (divided by the cation and anion) employed in the analysis of natural value-added compounds are presented in Table 1, while their chemical structures are depicted in Fig. 1. The following discussion is divided into different sections regarding the advances brought about by the use of ILs in the separation and analysis (identification and quantification) of natural compounds using CE, HPLC and GC. The families of compounds studied, as well as the optimization of their separation, including the selection of ILs and their concentration and a range of different experimental conditions, are also presented and discussed. Whenever possible, the overall separation/analysis performance is outlined and discussed in terms of the IL chemical structure and IL–analyte interactions, allowing us to suggest some target-oriented ILs. Finally, the design and use of more benign and effective ILs, in addition to the development of integrated (and thus more sustainable) extraction–separation processes using IL aqueous solutions, not attempted hitherto, are suggested as the steps to follow.
Table 1.
Name and respective acronym of IL cation–anion combinations employed in the separation and analysis of natural value-added compounds
| Cations |
Anions |
||||
|---|---|---|---|---|---|
| Name | Acronym | Name | Acronym | ||
| i | Imidazolium | [Im]+ | i | Bromide | Br– |
| ii | 1-Alkylimidazolium | [CnIm]+ | ii | Chloride | Cl– |
| iii | 1-Alkyl-3-methylimidazolium | [CnC1Im]+ | iii | Iodide | I– |
| iv | 1-Alkylpyridinium | [Cnpy]+ | iv | Hydroxide | [OH]– |
| v | 1-Alkyl-1-methylpyrrolidinium | [CnC1pyr]+ | v | Acesulfamate | [Ace]– |
| vi | 1-Butyl-2,3-dimethylimidazolium | [C4C1C1Im]+ | vi | Tetrafluoroborate | [BF4]– |
| vii | 1-Propylamine-3-methylimidazolium | [(NH2)C3C1Im]+ | vii | Nitrate | [NO3]– |
| viii | 1-(4-sulfonylbutyl)-3-methylimidazolium | [(HSO3)C4C1Im]+ | viii | Bis(trifluoromethylsulfonyl)imide | [NTf2]– |
| ix | 1-Cyclohexyl-3-methylimidazolium | [C6H11C1Im]+ | ix | Hexafluorophosphate | [PF6]– |
| x | 1-Benzyl-3-methylimidazolium | [PhC1Im]+ | x | Saccharinate | [Sac]– |
| xi | N,N-Dimethyl(cyanoethyl)ammonium | [N113N0]+ | xi | Dimethylcarbamate | [N(C1)2CO2]– |
| xii | 2-(Dodecyloxy)-N,N,N-trimethyl-2-oxoethanaminium | [N111C2O(O)C12]+ | xii | Hydrogenosulfate | [HSO4]– |
| xiii | N,N-Dimethylammonium | [N1100]+ | xiii | Dihydrogenophosphate | [H2PO4]– |
| xiv | Tetraalkylammonium | [Nnnnn]+ | xiv | Alkylsulfate | [CnSO4]– |
| xv | Cholinium | [N1112OH]+ | xv | Trifluoromethanesulfonate | [CF3SO3]– |
| xvi | N,N-Dimethyl-N-(2-hydroxyethoxyethyl)ammonium | [N112(O)2OH0]+ | |||
| xvii | N,N-Dimethyl(2-methoxyethyl)ammonium | [N112(O)10]+ | |||
| xviii | 1,12-Di-(2,3-dimethylimidazolium)dodecane | [C12(C1C1Im)2]+ | |||
| xix | 1-Nonyl-3-vinylimidazolium | [C9vIm]+ | |||
| xx | 1,9-Di-(3-vinylimidazolium)nonane | [C9(vIm)2]+ | |||
| xxi | 1,12-Di-(tripropylphosphonium)dodecane | [C12(P333)2]+ | |||
Fig. 1.
Chemical structures of IL cations and anions employed in the separation and analysis of natural value-added compounds. The nomenclature of each ion is presented in Table 1.
Capillary electrophoresis
CE belongs to electrokinetic separation methods, i.e., the separation of molecules occurs through the use of high voltages, which generate electroosmotic and electrophoretic flows of buffer solutions and ionic species, respectively. Fused-silica capillaries with negatively charged silanol groups on the inner surface are usually applied, thus resulting in the formation of an electroosmotic flow (EOF). CE allows the separation of a large variety of compounds, ranging from charged ions to a complex array of large and small neutral molecules, with excellent efficiency and selectivity, along with low eluent and sample consumptions. For these reasons, CE is also recognized as a greener separation method.55 Furthermore, CE usually provides better peak shape and faster analysis when compared, for instance, with HPLC.30
Different modes of CE can be used, for example, capillary zone electrophoresis (CZE), micellar electrokinetic capillary chromatography (MEKC) and non-aqueous capillary electrophoresis (NACE). CZE analysis is considered the simplest form of CE, where the separation mechanism is based on differences in the charge-to-mass ratio. As an extension of CZE, MEKC comprises the addition of a surfactant to the running buffer leading to the formation of micelles as a pseudo-phase, while allowing the separation of both neutral and charged analytes. In some cases, additional modifiers can be used in order to improve the efficiency, selectivity and reproducibility.23 The most commonly used surfactant is sodium dodecyl sulphate (SDS). However, due to the strongly hydrophobic nucleus of SDS micelles in aqueous media, particularly when dealing with highly hydrophobic compounds, the SDS-based MEKC technique is often insufficiently selective because all compounds tend to be incorporated into the micelles.56 On the other hand, NACE has received considerable attention due to its additional advantages, such as the ability to separate water-insoluble compounds that cannot be separated with traditional aqueous CE. Furthermore, faster separations can be obtained with NACE due to the higher EOF created, while the use of organic solvents turns feasible a direct online mass spectroscopy detection.23
The major drawback of CE-based analyses is the separation reproducibility, which can be affected by interactions between the inner capillary surface and analytes.23 In order to increase the CE separation performance, and based on their high conductivity and tunable miscibility with water, ILs have been used as supported electrolytes (by covalent bonding or dynamic coating) to modify the properties of the capillary wall and/or additives of running buffers and as remarkable alternatives to the most commonly used salts, namely sodium tetrafluoroborate (NaBF4) and sodium tetraborate.25 Contrarily to conventional salts, the low-charge density of IL cations allows a wider variety of interactions to occur between analytes and ILs, employed either as additives or as supported electrolytes, thus resulting in improved separations as a result of a proper selection of their chemical structures. Indeed, the ability of ILs to establish dispersive-type, hydrogen-bonding, electrostatic and ion–dipole/ion-induced dipole interactions with analytes is the main advantage arising from their use in separation processes. It has already been demonstrated that ILs can act as salting-in or salting-out agents in aqueous media,57 as well as hydrotropes.58 Both salting-in and hydrotropic effects are beneficial to increase the solubility of natural value-added compounds in aqueous media, allowing enhanced extractions and separations to occur. Moreover, it should be stressed that it is possible to design the IL chemical structure for a target-oriented purpose, e.g. salting-in versus salting-out, and that ILs possess a broader range of hydrophobicity–hydrophilicity behaviours than conventional salts – a valuable feature to increase the extraction yields and the separation performance of analytical techniques.
Particularly regarding the application of ILs to enhance the separation and analysis of value-added compounds extracted from biomass, several studies have been published reporting their use in CZE,30–39 MEKC,40,41 and NACE.42 In this context, the following discussion is divided into two different sections that correspond to the ILs’ application in several modes of CE. The optimization of the separation and analysis conditions as well as the selection of ILs and their concentration, running electrolyte, pH and applied voltage are presented and discussed. Moreover, the overall separation performance of CZE, MEKC and NACE using ILs is outlined and discussed, and whenever possible target-oriented ILs are suggested.
Capillary zone electrophoresis
In the field of CZE using ILs as running electrolytes or additives, phenolic compounds,30–37 alkaloids38 and carbohydrates39 were the most studied compounds, as summarized in Table 2. Table 2 also reports the optimum separation conditions and detection limits attained.
Table 2.
Value-added bioactive compounds extracted from natural sources and analysed by CZE using ILs as running electrolytes or additives: the optimum separation conditions (OSC) and detection limits (DL) in µg mL−1
| Value-added compound | Natural source | ILs and application | OSC | DL | |
|---|---|---|---|---|---|
| Phenolic compounds | |||||
| i | (–)-Catechin | Grape seeds | [N1111][BF4], [N2222][BF4] and [N3333][BF4] as running electrolytes | IL: [N2222][BF4]; [IL]: 150 mM; pH: 4; voltage: 20 kV; anal. time: 38 min.30 | (No inf.) |
| ii | (–)-Epicatechin | ||||
| iii | (–)-Epicatechin gallate | ||||
| iv | (–)-Gallocatechin gallate | ||||
| v | (–)-Catechin gallate | ||||
| vi | trans-Resveratrol | [C2C1Im][BF4], [C2C1Im][PF6], [C2C1Im][NO3], [C2C1Im][CF3SO3], [C4C1Im][BF4] and [C4C1Im][PF6] as running electrolytes | IL: [C4C1Im][BF4]; [IL]: 150 mM; pH: 4; voltage: 20 kV; anal. time: 27 min.31 | (No inf.) | |
| vii | Gallic acid | ||||
| viii | Arteanoflavone | Seriphidium santolinum (Schrenk) Poljak | [C2C1Im][BF4], [C4C1Im][BF4] and [C4C1Im][PF6] as running electrolytes | IL: [C4C1Im][BF4]; [IL]: 30 mM; [β-CD]: 5 mM; solvent: water; pH: 11.2; voltage: 20 kV.32 | 0.64–0.14 |
| ix | Eupatilin | ||||
| x | Hispidulin | ||||
| xi | 5,7,4′-Trihydroxy-6,3′,5′-trimethoxyflavone | ||||
| xii | Kaempferol | Hippophae rhamnoides L. | [C2C1Im][BF4], [C3C1Im][BF4], [C4C1Im][BF4], [C5C1Im][BF4], [C4C1Im][PF6], [C4C1Im]Br, [C4C1Im] I and [C4C1C1Im][BF4] as additives into running electrolytes | IL: [C4C1Im][BF4]; [IL]: 4 mM; [borate buffer]: 20 mM; solvent: water; pH: 10; voltage: 20 kV.33 | 1–5 |
| xiii | Quercetin | ||||
| xiv | isorhamnetin | ||||
| xv | Asebotin | Saussurea mongolica (Franch.) Franch. | C2C1Im][BF4], [C3C1Im][BF4], [C4C1Im][BF4], [C5C1Im][BF4], [C4C1Im][PF6], [C4C1Im]Br, [C4C1Im] I and [C4C1C1Im][BF4] as additives into running electrolytes | IL: [C4C1Im][BF4]; [IL]: 5 mM; [borate buffer]: 20 mM; solvent: water; pH: 9; voltage: 20 kV.34 | 0.5–5 |
| xvi | Kaempferol-3-O-β-d-glucopyranoside | ||||
| xvii | Kaempferol-3-O-α-l-rhamnopyranoside | ||||
| xviii | Kaempferol-7-methoxy-3-O-α-l-rhamnopyranoside | ||||
| xix | Quercetin-3-O-β-d-glucopyranoside | ||||
| xx | Quercetin-3-O-α-l-rhamnopyranoside | ||||
| xxi | Ononin | Dried root of Pueraria lobate (Willd.) Ohwi and Pueraria thomsonii Benth. | [N1111][BF4], [C4C1Im][BF4], [C4C1pyr]Br and [C8C1Im]Cl as additives into running electrolytes | IL: [C4C1Im][BF4]; [IL]: 50 mM; [sodium tetraborate]: 30 mM; solvent: water; pH: 9.5; voltage: 18 kV.35 | 1.72–4.92 |
| xxii | Genistin | ||||
| xxiii | Biochanin A | ||||
| xxiv | Formononetin | ||||
| xxvi | Genistein | ||||
| xxvii | Daidzein | ||||
| xxviii | Puerarin | ||||
| xxix | l,3-Dihydroxy-2-hydroxymethyl-9,10-anthraquinone-3-O-β-d-xylosyl(1–6)-β-d-glucoside | Paedicalyx attopevensis Pierre ex Pit. | [C4C1Im][BF4] as running electrolyte | [IL]: 60 mM; [β-CD]: 4 mM; pH: 10; voltage: 20 kV.36 | 0.19–3.75 |
| xxx | 1-Hydroxy-2-methoxy-3-hydroxymethyl-9,10-anthraquinone-1-O-β-d-glucoside | ||||
| xxxi | 1-Methoxy-2-methyl-3-hydroxy-9,10-anthraquinones(rubiadin-l-methylether) | ||||
| xxxii | 1-Methoxy-2-formyl-3-hydroxy-9,10-anthraquinone | ||||
| xxxiii | Aloe-emodin |
Rheum palmatum L.
and Rheum hotaoense C. Y. Cheng & T. C. Kao |
[C2C1Im][BF4], [C4C1Im][BF4] as running electrolyte | IL: [C4C1Im][BF4]; [IL]: 90 mM; pH: 10; voltage: 20 kV.37 | 0.33–0.62 |
| xxxiv | Emodin | ||||
| xxxv | Chrysophanol | ||||
| xxxvi | Physcion | ||||
| xxxvii | Rhein | ||||
| Alkaloids | |||||
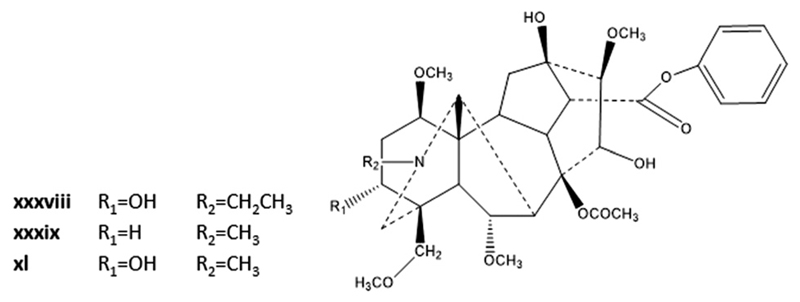
| xxxviii | Aconitine | Aconitum kusnezoffii Rchb. and Aconitum carmichaeli Debx. | [C4C1Im][BF4] as running electrolyte | IL: [C4C1Im][BF4]; [IL]: 35 mM; solvent: water; pH: 8.5; voltage: 15 kV.38 | 2.94–3.20 |
| xxxix | Hypaconitine | ||||
| xl | Meaconitine | ||||
| Carbohydrates | |||||
| xli | Sucrose | Carrot, cabbage, cucumber, onion, potato, beet and tomato juices | [C2C1Im]Cl, [C4C1Im]Cl, [C12C1Im] Cl, [C2C1C1Im][C2SO4] and [C2C1Im][C8SO4] as additives into running electrolytes | IL: [C2C1Im]Cl; [IL]: 20 mM; [NaOH]: 30 mM; solvent: water; voltage: 20 kV.39 | 0.06–0.08 |
| xlii | d-Fructose | ||||
| xliii | d-Ribose | ||||
| xliv | d-Galactose | ||||
| xlv | d-Glucose | ||||
Yanes et al.30 were the first group to report the application of [Nnnnn][BF4] as a running electrolyte to successfully separate and analyse five phenolic compounds, namely (−)-epicatechin, (+)-catechin, (−)-catechin gallate, (−)-gallocatechin gallate, (−)-epicatechin gallate, gallic acid and trans-resveratrol (Fig. 2) extracted from grape seeds (Table 2). In a first approach, Na [BF4] and citrate solutions containing [N2222][BF4] at 150 mM were evaluated as running buffers. When using Na[BF4] as the main buffer, only two phenolic compounds (epicatechin and catechin) were identified; however, when ILs were applied, it was possible to achieve an effective separation of the whole mixture. The best separation of the five phenolic compounds was achieved with the [N2222]-based IL.30 Based on the results obtained, it was concluded that the magnitude of the EOF as well as the separation performance of phenolic compounds are strongly affected by the size of the aliphatic moiety of the IL (the magnitude of EOF increases with increasing the IL cation alkyl side chain length). The authors30 concluded that the size of the uncharged phenolic compounds and their different degrees of association with the tetraalkylammonium cation seem to provide effective electrophoretic mobility differences, allowing to reach the successful separation of the entire mixture of natural compounds. Although most studies employing ILs in analytical techniques were confined to imidazolium-based fluids, as will be shown in the following sections, this work30 is an example of improved resolutions achieved by using tetraalkylammonium-based ILs. The same group31 reported the application of [CnC1Im]-based ILs as running electrolytes to separate the same phenolic compounds, also from grape seed extracts – cf. Table 2. The influence of different IL cations ([C2C1Im]+ and [C4C1Im]+) and anions ([BF4]−, [PF6]−, [NO3]− and [CF3SO3]−) as running electrolytes was evaluated. As with tetraalkylammonium-based ILs,30 also imidazolium ions coat the capillary walls, thus producing anodic EOF. The authors31 further suggested that the delay observed in the migration time of the studied phenolic compounds can be a result of their association with the positively charged imidazolium groups coating the capillary wall or with free imidazolium ions in the bulk solution. This association could be partially driven by hydrogen-bonding and ion–dipole/ion-induced-dipole interactions. Yanes et al.31 concluded that the IL cation not only acts as an EOF modifier but also plays an active role through its association with phenolic compounds. On the other hand, the role of the IL anion was also investigated. The authors31 observed that, for the same cation, e.g. [C2C1Im]+, no separations were obtained with ILs composed of [NO3]− and [CF3SO3]− anions. In contrast, [BF4]− and [PF6]− anions allow an improved separation, with similar profiles for both anions. The authors31 suggested that the high cation–anion interaction energies observed for these oxygen containing anions and the imidazolium cation can be the main reason behind these results. Nevertheless, in our opinion, this conclusion casts some doubts since [BF4]- and [PF6]-based ILs display stronger cation–anion interactions or higher cohesive energies than [CF3SO3]-based fluids (with a common imidazolium cation), as previously demonstrated.59 Overall, the low solubility of [C2C1Im][PF6] in water60 conditioned the reproducibility of the results and thus the authors31 selected [C2C1Im][BF4] as the best additive (since it is completely water-miscible at temperatures close to room temperature). Fig. 3 displays a schematic representation of the proposed mechanism involved in the process proposed by the authors.31
Fig. 2.
Chemical structures of phenolic compounds extracted from grape seeds30,31 and analysed by CZE using ILs as running electrolytes or additives. The nomenclature of each compound is presented in Table 2.
Fig. 3.
Schematic representation of the CE inherent mechanism using ILs as running electrolytes: EOF – electroosmotic flow, µep – electrophoretic mobility. Adapted from Yanes et al.31
Comparing the two classes of ILs used in different studies, i.e. [N2222][BF4]30 versus [C4C1Im][BF4],31 both to improve the separation of phenolic compounds from grape seed extracts, it is evident that both ILs allow a successful separation and identification of phenolic compounds, in the same sequence, and thus the same separation mechanism is playing a role. However, a faster analysis and better resolution were observed with [C4C1Im][BF4] (27 min instead of 38 min) at the same IL concentration (150 mM) and applied voltage (20 kV). In order to better understand the role of the cation in these results (with a fixed anion), it is relevant to stress the two main forces which dominate the separation performance: (i) the capacity of the IL cation to interact with the capillary wall (suppressing therefore interactions of the silanol groups with the analyte and by reducing or reversing the EOF) and (ii) the ability of the IL to interact with analytes in the bulk solution. Even though ammonium cations display a stronger affinity to the capillary wall than the imidazolium counterparts, given the higher EOF reversal power at low concentrations as demonstrated by Mendes et al.61 and Laamanen et al.,62 the interactions established between ILs and phenolic compounds in aqueous media also play an important role. In this context, the better performance showed by imidazolium-based ILs seems to be related with its aromatic character and ability to establish hydrogen-bonding and π⋯π interactions with phenolic compounds.
The use of ILs for the separation of natural flavonoids has also been studied using a series of [CnC1Im]-based ILs as running electrolytes in CZE (Table 2 and Fig. 4).32–34 In particular, the effect of different IL cations ([C2C1Im]+ versus [C4C1Im]+) with the common [BF4]− anion, and the effect of β-cyclodextrin (β-CD) as an EOF modifier32 and [CnC1Im]-based ILs combined with the [BF4]−, [PF6]−, Br− and I− anions were investigated.33,34 As previously observed by Yanes et al.31 for the separation of diverse phenolic compounds from grape seeds, these different studies32–34 proved that imidazolium cations play a pivotal role in the separation of flavonoids (Fig. 5 and 6). In fact, not only a better separation in a shorter analysis time was observed, but also a steadier baseline was obtained due to strong interactions occurring between the imidazolium cations and the capillary wall and between the imidazolium cations and the analytes. As previously stated, the aromatic character of imidazolium-based cations and their ability to establish hydrogen-bonding and π⋯π interactions with flavonoids seem to be the major reasons for the high separation performance observed in CZE. Moreover, both with ammonium- and with imidazolium-based ILs, it was found that ILs bearing cations with longer alkyl side chains are better candidates to improve the separation of flavonoids (Fig. 5) and phenolic compounds. This feature is certainly related with weaker cation–anion interactions that occur in long-chain ILs, thus leaving the IL cation “freer” to interact with the capillary wall and analytes. On the other hand, when the IL anion effect is evaluated (Fig. 6), an increase in the retention time was observed with [C4C1Im][PF6] when compared to the studied halides. This behaviour seems to be also related to the IL cation–anion interaction strength. Amongst the studied ILs, [C4C1Im][PF6] displays the weakest cation–anion interactions, as previously observed by us through the determination of cation–anion interaction energies for a series of [C4C1Im]-based ILs, which correlate with the IL anion radius.59 This weakest attraction leaves the IL imidazolium cation more available to interact with analytes and with the capillary wall, resulting thus in longer retention times.
Fig. 4.
Chemical structures of phenolic compounds extracted from S. santolinum,32 H. rhamnoides,33 S. mongolica,34 P. lobate and P. thomsonii35 and P. attopevensis36 and analysed by CZE using ILs as running electrolytes or additives. The nomenclature of each compound is presented in Table 2.
Fig. 5.
Separation of flavonoids present in S. santolinum samples using CZE running electrolytes composed of (A) 25 mM of borate, pH 10.20 and 6 mM of β-CD; (B) 30 mM of [C2C1Im][BF4], pH 11.20 and 5 mM of β-CD; (C) 30 mM of [C4C1Im][BF4], pH 10.10 and 7 mM of β-CD. (1) Arteanoflavone; (2) eupatilin; (3) hispidulin; (4) 5,7,4-trihydroxy-6,3,5-trimethoxyflavone. Reproduced and adapted with permission from Qi et al.32
Fig. 6.
Effect of the IL (A) anion and (B) cation on the separation of flavonoid-O-glycosides by CZE. Analytical conditions: 20 mM borate buffer with 5 mg mL−1 IL at pH 9.00; voltage, 15 kV; temperature, 25 °C; UV detection at 280 nm. (1) Asebotin; (2) kaempferol-3-O-β-d-glucopyranoside; (3) kaempferol-3-O-α-l-rhamnopyranoside; (4) kaempferol-7-methoxy-3-O-α-l-rhamnopyranoside; (5) quercetin-3-O-β-d-glucopyranoside; (6) quercetin-3-O-α-l-rhamnopyranoside. Reproduced and adapted with permission from Yue et al.34
It is well known that the selectivity and resolution provided by CE can be enhanced by the addition of cyclodextrins (CDs) that act as chiral selectors due to their ability to include a wide variety of water-insoluble molecules into their hydrophobic cavity. The same enhancement was observed by the addition of β-CD as a modifier to the IL-based running electrolyte, providing a better separation of analytes.32 In general, the separation efficiency of flavonoids using ILs as running electrolytes (30 mM of [C4C1Im][BF4], at a pH of 11.2, with 5 mM of β-CD and with 20 kV of applied voltage) was better than that achieved using the traditional CZE technique with borate (detection limits ranging from 0.64 to 0.14 µg mL−1 versus 0.94 to 1.04 µg mL−1).32
More recently, the use of ILs as additives in a sodium tetraborate solution for the analysis of eight isoflavones (ononin, daidzin, genistin, biochanin A, formononetin, genistein, daidzein and puerarin – cf. Fig. 4) from dry roots of P. lobate and P. thomsonii was reported.35 Different operational conditions, among them different ILs ([N1111][BF4], [C4C1Im][BF4], [C4C1pyr]Br and [C8C1Im]Cl), were investigated to improve the resolution and separation of isoflavones (Table 2). It was demonstrated that the migration of most analytes is affected by the concentration of sodium tetraborate and pH (due to the degree of solute ionization and EOF velocity). Indeed, the migration time increases with the concentration and pH of the running buffer. The authors35 further observed that [C4C1Im][BF4] improves the separation of isoflavones, mainly biochanin A and formononetin, when compared with the sodium tetraborate electrolyte. However, direct evidence on the resolution attained with different ILs has not been reported,35 thus making difficult a more comprehensive interpretation of the IL chemical structure effect. On the other hand, using [C4C1Im][BF4] as an additive and studying its concentration over the migration time, peurarin was the only compound that presented a good separation in terms of migration time. For instance, at 50 mM of [C4C1Im][BF4], the pairs daidzin/genistin, biochanin A/formononetin and genistein/daidzein present similar migration times (5.0/5.2 min, 6.0/6.2 min and 8.0/8.2 min, respectively). Therefore, although the isoflavone separation was enhanced by the addition of IL to the sodium tetraborate running buffer, an overall poor resolution was obtained in this study.35 The negative effect of ammonium- and pyrrolidinium-based ILs through the flavonoid separation resolution might be related to the poor interactions occurring between these non-aromatic ILs and analytes, as previously discussed. Furthermore, the self-aggregation of long alkyl side chain ILs in aqueous media63 (such as in [C8C1Im]Cl) cannot be discarded since these will make more difficult the establishment of H-bonds and π⋯π interactions between the cations and the analytes.
Improvements achieved with [CnC1Im]-based ILs as running electrolytes in CZE for the separation and analysis of anthraquinones have also been reported36,37 (data are summarized in Table 2). Qi et al.36 evaluated the performance of [C4C1Im][BF4] and the effect of β-CD as a modifier on the analysis of four anthraquinones (Fig. 7) extracted from the Chinese herb P. attopevensis, while Tian et al.37 investigated the effect of [C2C1Im][BF4] and [C4C1Im][BF4] as electrolytes for the analysis of aloe-emodin, emodin, chrysophanol, physcion and rhein (Fig. 7) extracted from R. palmatum and R. hotaoense. The overall results obtained demonstrate that the reproducibility and detection limits are improved and the analysis time is reduced when using ILs (detention limit from 0.19 to 3.75 µg mL−1 and analysis time of 4.5 min) when compared with the traditional CE technique using the borax buffer (detection limit from 1.76 to 4.56 µg mL−1 and an analysis time of 39 min).36 An increase in resolution with the increase of the IL concentration and its alkyl chain length, as shown in Fig. 8, was also demonstrated.37 In summary, and as previously reported for other phenolic compounds,31–35 [C4C1Im][BF4] seems to be one of the best IL candidates for application as an electrolyte in CZE. Again, these results are a consequence of the strong interactions established between the target analytes and ILs. In particular, ion association constants between anthraquinones and IL anions and cations were determined in order to clarify the mobility change in the presence of ILs, confirming that [C4C1Im][BF4] leads to higher ion association constants.37
Fig. 7.
Chemical structures of phenolic compounds extracted from P. attopevensis,36 R. palmatum and R. hotaoense37 and analysed by CZE using ILs as running electrolytes or additives. The nomenclature of each compound is presented in Table 2.
Fig. 8.
Effect of IL concentration on the resolution of chrysophanol and aloe-emodin. Analytical conditions: 30–105 mM [C2C1Im][BF4] (●) or [C4C1Im][BF4] (▲) at pH 11.0; voltage, 20 kV; temperature, 25.0 °C; UV-detection at 254 nm. Reproduced and adapted with permission from Tian et al.37
Alkaloids were also analysed by CZE and advances provided by ILs as running electrolytes were investigated in a single work38 – cf. Table 2 and Fig. 9. Again, and as discussed before for other natural compounds, the improvements observed in the alkaloid separation are related to the favourable interactions between alkaloids and the imidazolium groups coating the capillary wall or with free imidazolium ions in solution. It was also concluded that the use of ILs as running electrolytes decreases the Joule heating and improves resolution, as previously observed by the same researchers.38 Finally, the best resolution was obtained using [C4C1Im][BF4] in aqueous solution; the three analytes were well separated within 5 min and with high detection limits (2.94 to 3.20 µg mL−1) when compared with the analysis time of 22 min obtained by CZE using 70 mM of tri-borate at pH 8.5 with methanol (60/40, v/v) (detection limits of 1.60 to 2.30 µg mL−1).38
Fig. 9.
Chemical structures of alkaloids extracted from A. kusnezoffii and A. carmichaeli38 and analysed by CZE using ILs as running electrolytes or additives. The nomenclature of each compound is presented in Table 2.
In addition to the natural compounds described before, the analysis of neutral carbohydrates (sucrose, d-galactose, d-glucose, d-fructose and d-ribose – cf. Fig. 10) present in plant juices, by CZE using ILs as additives in a sodium hydroxide electrolyte solution, was investigated.39 Different imidazolium-based ILs ([C2C1Im]Cl, [C4C1Im]Cl, [C12C1Im]Cl, [C2C1C1Im][C2SO4] and [C2C1Im][C8SO4]) were used taking into account two objectives: (i) to act as chromophores, thus enabling indirect UV detection to overcome the carbohydrates’ low sensitivity to absorb UV light; and (ii) to interact selectively with the analytes to improve their separation. Vaher et al.39 initially demonstrated that a sodium hydroxide electrolyte solution without the addition of ILs does not enable the detection of carbohydrates. Since indirect UV absorption–detection consists of the quantification of a negative peak generated by the non-absorbing analyte, only in the presence of a chromophore, in this case the IL, it is possible to analyse carbohydrates by UV spectroscopy detection. This effect, as well as the IL cation and anion influence on separation and resolution, can be observed in the electropherograms presented in Fig. 11. In general, it was found that: (i) higher concentrations of ILs are required to improve sensitivity; (ii) both the IL cation and anion influence the detection sensitivity; (iii) the migration time of analytes depends linearly on the sodium hydroxide concentration in the electrolyte solution containing the IL; (iv) the migration order (sucrose, d-galactose, d-glucose, d-fructose and d-ribose, Fig. 11) primarily depends on the degree of ionization of the analytes and also on their charge/-size ratio; and (v) the migration order of sugars is similar for Different ILs; however an increase in mobility was observed in the following order: [C2C1Im]Cl < [C4C1Im]Cl < [C2C1C1Im][C2SO4].
Fig. 10.
Chemical structures of carbohydrates extracted from fruit juices39 and analysed by CZE using ILs as running electrolytes or additives. The nomenclature of each compound is presented in Table 2.
Fig. 11.
Effect of ILs as background electrolytes in the separation of carbohydrates by CZE. Analytical conditions: 20 mM of IL, 30 mM of NaOH; voltage, 20 kV; temperature, 171 °C; UV detection at 207 nm. (1) Sucrose; (2) galactose; (3) glucose; (4) fructose; (5) ribose. Reproduced and adapted with permission from Vaher et al.39
Overall, considering the ILs application as additives of running electrolytes in CZE, all results reported to date30–39 reveal their effect in the improvement of the separation and analysis of the studied natural compounds as a consequence of favourable interactions established between the analytes and the IL ions either coating the capillary wall or existing as free ions in solution. Contrary to conventional salts, ILs are composed of organic cations which allow other types of interactions to occur, and which are favourable to improve separation and resolution in CZE. Furthermore, recognizing that the Joule heating is a critical factor for an efficient separation in CZE, it was also found that ILs lead to a larger decrease of this parameter when compared to conventional salts.
In general, [CnC1Im]-based ILs are the best candidates to be used as additives of running electrolytes in CZE due to their high capacity to establish a variety of interactions with target compounds when compared with the sodium cation commonly used in this technique. Their ability to establish stronger hydrogen-bonding and π⋯π interactions seems to play a pivotal role, which is further supported by the poorer results achieved with non-aromatic ammonium- and pyrrolidinium-based ILs. Moreover, long-alkyl side chain or surface-active ILs are not promising candidates for use as additives of running electrolytes in CZE. [C4C1Im][BF4] was the most widely investigated IL and appears to be the best choice to improve the analysis of phenolics, flavonoids and alkaloids by CZE. In fact, high-melting temperature salts composed of [BF4]−, such as Na[BF4],30,34 are used as conventional additives in the separation of natural compounds by this technique, which could justify why most authors choose this counter ion without exploring the effect of other anions in the separation process. Nevertheless, it should be highlighted that ILs composed of [BF4]− are water unstable and undergo hydrolysis producing HF even at room temperature.64 Based on this possibility, it is important to consider the analysis time and the kinetics of [BF4]− hydrolysis in order to avoid the damage of the silica support and other parts of the system. Since it was found that weaker cation–anion interactions are favourable to increase the performance of CZE, other IL anions appear to be promising, although not studied to date. For instance, [NTf2]−, perfluoroalkylsulfonate and perfluoroalkylsulphate anions display weaker cation–anion interaction energies, in addition to being water-stable, when compared with the well-studied [BF4]-based fluids. On the other hand, it is clear from all the results reported in the literature that aromatic ILs are the best candidates to improve the separation of aromatic natural compounds. Yet, this assumption is only supported by results attained for the imidazolium-based family against non-aromatic ammonium- and pyrrolidinium-based ILs. In this context, other aromatic ILs, such as pyridinium-based as well as quaternary ammonium- or phosphonium-based with aromatic functionalized groups, should be investigated. In the same line, the use of aromatic anions has not been investigated in CZE to date, although there is today a plethora of novel ILs composed of aromatic anions, such as tosylate, salicylate, etc.
Even though low amounts of additives are used in analytical techniques, it is important to explore other options and the application of more stable and less toxic ILs. In particular, more benign cations and anions should be explored in future studies. One promising option could be the use of cholinium-based ILs, even with benzyl functionalized groups, combined with aromatic and natural-derived anions.65
Micellar electrokinetic capillary chromatography and non-aqueous capillary electrophoresis
The optimum separation conditions and the detection limits of several phenolic compounds analysed by MEKC or NACE with ILs as additives or running electrolytes are summarized in Table 3. The chemical structures of these compounds are shown in Fig. 12.
Table 3.
Value-added bioactive compounds extracted from natural sources and analysed by MEKC or NACE using ILs as running electrolytes or additives: the optimum separation conditions (OSC) and detection limits (DL) in μg mL−1
| Value-added compound | Natural source | ILs and application | OSC | DL | |
|---|---|---|---|---|---|
| Phenolic compounds | |||||
| xlvi | Schisandrin | Schisandra chinensis (Turcz.) Baill. and Schisandra henryi C. B. Clarke | [C4C1Im][BF4] as additive into running electrolyte (MECK) | [IL]: 10 mM; 5 mM borate + 5 mM phosphate + 20 mM SDS; solvent: water; pH: 9.2; voltage: 25 kV.40 | 0.4–0.7 |
| xlvii | Schisantherin A | ||||
| xlviii | Deoxyschisandrin | ||||
| xlix | γ-Schisandrin | ||||
| l | Baicalin | Roots of Scutellariae genus | [C4C1Im][BF4] as additive into running electrolyte (MECK) | Micro-emulsion: 0.88% (m/v) of SDS + 0.8% (v/v) of ethyl acetate + 0.2% (v/v) of butanol + 92.5% water; running buffer: 25% (v/v) of acetonitrile + 7.5 mM of [C4C1Im][BF4] + 10 mM NaH2PO4; pH: 8.2; voltage: 17.5 kV.41 | 0.39–1.05 |
| li | Wogonin | ||||
| lii | Baicalein | ||||
| liii | Kaempferol | Dry seeds of Plantago depressa Willd. or Plantago asiatica L. | [C2C1Im]Cl, [C2C1Im][HSO4] and [C2C1Im][BF4] as running electrolytes (NACE) | IL: [C2C1Im][HSO4]; IL concentration: 5 mM; solvent: acetonitrile; voltage: 20 kV.42 | 0.3–0.5 |
| liv | Quercetin | ||||
| lv | Luteolin | ||||
Fig. 12.
Chemical structures of lignans and flavonoids extracted from S. chinensis and S. henryi,40 Scutellariae genus41 and P. depressa and P. asiatica42 analysed by MEKC or NACE using ILs as running electrolytes or additives. The nomenclature of each compound is presented in Table 3.
In order to expand the MEKC performance when dealing with more hydrophobic solutes, Tian et al.40 studied the separation of lignans (schisandrin, deoxyschisandrin, γ-schisandrin and schisantherin A) from S. chinensis and S. henryi, using [C4C1Im][BF4] as a modifier of the running electrolyte (Table 3). The authors40 investigated the effects of the IL concentration, applied voltage, background electrolyte and pH on the resolution and retention times of lignans. By comparing the separation of lignans by MEKC using [C4C1Im][BF4] or β-CD as modifiers it was concluded that all analytes have no electrophoretic mobility and migrate according to the electroosmotic velocity. Moreover, the separation of lignans using SDS as an additive to the borate–phosphate (1 : 1) background electrolyte led to a non-successful separation of the studied compounds. On the other hand, it was demonstrated that the addition of [C4C1Im][BF4] to anionic surfactant systems, in this case SDS, significantly improves the lignan separations and MEKC resolution.40 These advances might be a result of electrostatic attractions occurring between the positively charged imidazolium cations and the negatively charged SDS surface micelles, which are thus able to neutralize the effective head group charge while reducing electrostatic repulsion.
[C4C1Im][BF4] was also studied as an additive in MEKC for the separation of flavones (baicalein, baicalin and wogonin, shown in Fig. 12) from extracts of Scutellariae genus.41 A running buffer prepared by mixing a micro-emulsion (prepared with ethyl acetate (3.2%, v/v), SDS (3.5%, w/v), butanol (0.8%, v/v) and water (92.5%, v/v)), acetonitrile, IL and 20 mM of NaH2PO4 was used. The impact of ILs and running buffer concentration, pH, acetonitrile and micro-emulsion contents and applied voltage was further investigated (Table 3). The authors41 suggested that the interactions between imidazolium cations and the micro-emulsion droplets change the character of the micro-emulsion itself and may thereby change the distribution of the analytes while increasing their separation ability, as previously discussed with lignans.40 The enhanced separation of flavones with the addition of ILs seems to derive from the association of flavones with imidazolium ions or their distribution into the micro-emulsion phase, which are driven by hydrogen-bonding, electrostatic and dispersive-type interactions.
The separation and analysis of the flavonoids kaempferol, quercetin and luteolin (Fig. 12), present in the dry roots of P. depressa or P. asiatica, were attempted by NACE using ILs as background electrolytes and acetonitrile and acetonitrile–methanol mixtures as the main solvents.42 Acetonitrile is a well-suited medium for NACE and enables a wider range of CE applications with more hydrophobic species. In fact, the large miscibility of ILs with acetonitrile, contrary to conventional salts, allows them to be used in the adjustment of the analytes’ mobility and separation in CE. In general, it was found that the EOF decreases with the increase of the IL concentration – independently of the IL nature.42 The EOF is a consequence of electrostatic forces established between the electrolyte ions and the inner capillary surface (acidic silanol groups) forming a double layer; when applying a high potential along the capillary wall, some cations of the diffuse layer migrate towards the cathode (the surface remains negative), and this migration drags water osmotically in the same direction, creating a relevant flow. When ILs are used as additives or running electrolytes, strong interactions are established between the IL ions and the inner capillary surface (dynamically coating the inner wall), thus changing the surface charge. By increasing the IL concentration, more IL ions will be present in the diffusive layer, reducing the zeta potential and consequently decreasing the EOF. As a result, flavonoids can be separated under a positive voltage with a low IL concentration, and under a negative voltage with a high IL concentration. With [C2C1Im]Cl and [C2C1Im][HSO4] as the main electrolytes, flavonoids display negative charge and are well separated.42 Moreover, the authors42 demonstrated that 5 mM of [C2C1Im][HSO4] leads to better peak shapes and reduced analysis time than those obtained with 5 mM of [C2C1Im]Cl (analysis time from 30 to 7 min, respectively). On the other hand, when methanol was added to the ILs as background electrolytes, changes in mobility and EOF were observed.42 An increase in the methanol content results in a reduction of the EOF, further leading to a decreased mobility of flavonoids. These results were explained based on the disruption of the IL–solute heteroconjugation, a proton sensitive complex, by the addition of methanol (amphiprotic species). In summary, the authors42 stated that ILs increase the solubility of flavonoids in acetonitrile, thus allowing their improved analysis by NACE (the solubility of quercetin, kaempferol and luteolin in acetonitrile is 0.6 mg mL−1, 0.4 mg mL−1 and 0.3 mg mL−1, respectively, which could be increased to 1.0 mg mL−1, 1.0 mg mL−1 and 0.6 mg mL−1 with 8 mM of [C2C1Im]Cl or [C2C1Im][HSO4]).
All authors40–42 demonstrated relevant advances in the separation of phenolic compounds by MEKC and NACE techniques through the use of imidazolium-based ILs as additives or running electrolytes. [C4C1Im][BF4] and [C2C1Im][HSO4] were identified as the best candidates to improve the separation of the most hydrophobic analytes. Higher resolution and better detection limits were obtained when compared with conventional additives or running electrolytes. Both ILs lead to acidic solutions, although, at this point, no major conclusions can be drawn about the relationship between enhanced analysis and pH since a few studies are available in the literature on the use of ILs in MEKC and NACE. Therefore, additional ILs should be investigated in future studies so that the impact of their chemical structure and of the target compounds speciation could be evaluated and better understood.
In summary, the CE separation efficiency is often compromised by the strength of the interactions occurring between the analytes and the silanol groups on the inner capillary surface and by the EOF which further depends on the electrolyte pH. ILs have been successfully applied as additives or running electrolytes in aqueous and non-aqueous CE. The results obtained reveal that both the IL cation and anion exert an influence through the separation mechanism and performance of the analytical technique. However, to date, the resolution of CE was mainly addressed by studying the effect of the IL cation. Several imidazolium-, pyrrolidinium- and ammonium-based ILs have been studied, among which [C4C1Im][BF4] was identified as the best cation–anion pair to be used as a running electrolyte or additive. However, taking into consideration that favourable interactions established between IL ions and analytes increase the CE performance, with aromatic imidazolium-based fluids appearing as the most promising class due to the possibility of establishing additional H-bonding and π⋯π interactions, it is clear that other ILs with aromatic character and with high hydrogen-bond basicity and/or acidity should be investigated in the near future. Although outside the topic of this review, considering the effect of ILs on the separation performance of analytical techniques for mixtures of standard and synthetic derived compounds, the following rank of IL cations can be established according to their ability to interact with the capillary wall: phosphonium > ammonium > sulfonium > pyrrolidinium > piperidinium > pyridinium > imidazolium, while the inverse is observed for the interactions with analytes.61,62 This trend provides important clues to the choice of adequate ILs to be applied in the CE separation of natural compounds. On the other hand, the possibility of using surface-active ILs as potential alternatives to common ionic surfactants in MEK can also be foreseen, although not attempted to date in the separation of natural compounds.
High performance liquid chromatography
HPLC is the improved form of liquid chromatography and allows the separation of compounds based on their partition between the liquid mobile phase and the stationary phase at high pressure. Depending on the relative polarity of the mobile and stationary phases, two HPLC variants can be used: normal phase (NP) chromatography and reverse phase (RP) chromatography. In the NP-HPLC technique, organic solvents (or their mixtures) are used as the mobile phase, while stationary phases are silica-based. On the other hand, aqueous solutions or mixtures with organic solvents as mobile phases and silica-modified stationary phases (the most common one is silica surface bonded with C18) are used in RP-HPLC.66
To improve the separation and analysis of value-added compounds extracted from biomass, ILs have been used in multiple roles in HPLC. They have been mainly employed as mobile phase additives (instead of amines or divalent cation compounds) with the purpose of surpassing the negative effect of free silanol groups on the long retention time of analytes, improving therefore the chromatographic resolution. Yet, ILs can also be used as stationary phases, by modifying silica through the formation of IL-based stationary phases to prepare HPLC columns with improved efficiency and stability.21,25,67,68 The following discussion is divided in two sections, covering first the use of ILs as mobile phase additives, followed by their use in stationary phase design, in both cases addressing the HPLC analysis of natural compounds, namely alkaloids, phenolic compounds and carbohydrates.
Ionic liquids as mobile phase additives
The outcome of ILs as mobile phase additives in HPLC separations seems to involve multiple interactions established between IL ions and silanol groups, producing therefore a weak bilayer electronic structure which leads to the repulsion of analytes or to a stronger attraction with the stationary phase. Furthermore, the addition of ILs can change the mobile phase polarity and thus the affinity of analytes to this phase. Some studies considering these effects were found on the separation and analysis of alkaloids43–46 and phenolic47 compounds extracted from Different species followed by HPLC analysis (Table 4).
Table 4.
Value-added compounds extracted from natural sources and analysed by HPLC with ILs as mobile phase additives under the optimum separation conditions (OSC)
| Value-added compound | Natural source | ILs and application | OSC | |
|---|---|---|---|---|
| Alkaloids | ||||
| lvi | Octopamine | Citrus reticulata Blanco (mature and immature tangerine peels) and Citrus aurantium L. | [C2py]Br, [C4py][BF4], [C2C1Im]Br, [C2C1Im][BF4], [C4C1Im]Cl and [C4C1Im][BF4] as water mobile phase additives | IL: [C2C1Im][BF4]; [IL]: 2 mM; pH: 4; temperature: 30 °C.43 |
| lvii | Synephrine | |||
| lviii | Tyramine | |||
| lix | Oxymatrine | Sophora flavescens Aiton | [C4C1Im][BF4], [C6C1Im][BF4] and [C8C1Im][BF4] as methanol/water (45/55, v/v) mobile phase additives | IL: [C6C1Im][BF4]; [IL]: 0.1 mM; pH: 11.3.44 |
| lx | Matrine | |||
| lxi | Sophoridine | |||
| lxii | Sophocarpine | |||
| lxiii | Fangchinoline | Stephania tetrandra S. Moore | [C2C1Im][BF4], [C4C1Im][BF4], [C6C1Im][BF4] and [C6C1Im]Cl as methanol/water (80/20, v/v) mobile phase additives | IL: [C6C1Im][BF4]; [IL]: 50 mM; pH: 3.0.45 |
| lxiv | Tetrandrine | |||
| lxv | Palmatine | Bark of Phellodendron chinense C. K. Schneid. | [C2C1Im][BF4], [C4C1Im][BF4], [C6C1Im][BF4] and [C6C1Im]Cl as acetonitrile/water (25/75, v/v) mobile phase additives | IL: [C6C1Im][BF4]; [IL]: 16 mM; pH: 3.0.46 |
| lxvi | Jatrorrhizine | |||
| lxvii | Berberine | |||
| Phenolic compounds | ||||
| lxviii | Scoparone (coumarin) | Artemisia capillaris Thunb. | [C4C1Im][BF4], [C6C1Im][BF4], [C8C1Im][BF4], [C2C1Im][C1SO4] and [C4C1Im]Cl as acetonitrile/water (40/60, v/v) mobile phase additives | IL: [C4C1Im][BF4]; [IL]: 10 mM; pH: 11.3.47 |
| lxix | Chlorogenic acid (cinnamic acid) | |||
| lxx | Caffeic acid (cinnamic acid) | |||
| lxxi | Rutin (flavonoid) | |||
Tang et al.43 reported the application of IL aqueous solutions ([C2py]Br, [C4py][BF4], [C2C1Im]Br, [C2C1Im][BF4], [C4C1Im]Cl and [C4C1Im][BF4]) for the HPLC separation of alkaloids (Fig. 13) present in tangerine peels (C. reticulata and C. aurantium). It was observed that the addition of ILs to the mobile phase has a significant influence on the analytes retention times; for instance, when [C2C1Im][BF4] was added to the mobile phase the following retention times were obtained: octopamine 3.2 min, synephrine 4.2 min and tyramine 7.0 min against the original 3.2, 3.8 and 8.9 min, respectively. For the same anion ([BF4]− or Br−), imidazolium-based ILs led to an improved resolution and to a decrease in the retention time of octopamine/synephrine and synephrine/tyramine when compared with pyridinium-based ILs. On the other hand, for the same cation ([C2C1Im]+ or [C4C1Im]+), the authors43 suggested that the lyotropic character of the anions is also responsible for improving the resolution and the peak shape (due to ion-pair interactions with the cationic analyte). Anions with a lower lyotropic character, namely [BF4]−, are more favourable for enhanced separations than Br− and Cl−. Nevertheless, and as discussed before with the CZE technique, the strength of the IL cation–anion interaction may play the pivotal role. In fact, [BF4]-based fluids display weaker cohesive energies than halogen-based ILs.59 In addition to the well-studied imidazolium-based ILs, it is important to highlight the use of a pyridinium-based IL as an additive of the mobile phase in this work.43 In general, the addition of ILs to the polar mobile phase reduces its polarity, thereby increasing the affinity of analytes to this phase. Therefore, the lower retention times were achieved for all analytes when [C4C1Im]-based ILs were employed. Imidazolium-based ILs with a butyl chain instead of an ethyl moiety interact more efficiently with the stationary phase surpassing in a more effective way the negative effect of free silanol groups. There is thus a decrease in the interactions between analytes and the stationary phase which leads to a decrease in their retention times. Nevertheless, it was observed that the addition of [C4C1Im][BF4] further resulted in a poorer resolution of all studied analytes, showing that although longer alkyl side chain length ILs reduce the retention times they can have a negative impact in the resolution. It is however important to mention that this behaviour is the opposite to that observed in other studies discussed below. Therefore, and based on the overall results,43 [C2C1Im][BF4] was selected as the best IL additive to the mobile phase for the analysis of moderately polar alkaloids by HPLC.
Fig. 13.
Chemical structures of alkaloids extracted from C. reticulata and C. aurantium,43 S. flavescens,44 S. tetrandra45 and P. chinense46 and analysed by HPLC using ILs as mobile phase or additives. The nomenclature of each compound is presented in Table 4.
It was also suggested that the pH of the mobile phase in the retention times was only marginal, which is in agreement with the fact that in the pH range evaluated (2 to 7), the studied molecules are always positively charged. In contrast a more significant and positive effect of temperature on the analytes retention time was observed.43
The effect of Different ILs, such as [CnC1Im][BF4] and [C6C1Im]Cl, as additives in methanol/water mobile phases was also investigated in order to improve the HPLC resolution of alkaloids from extracts of S. flavescens44 and S. tetrandra45 (Fig. 13 and Table 4). The chromatograms obtained by the authors44 with Different mobile phases (Fig. 14), namely without modifier, with diethylamine, and with diethylamine and IL, clearly demonstrate that the IL addition to the mobile phase allows significant improvements of resolution, peak shape and retention time.44 Moreover, ILs composed of cations with longer alkyl side chains and anions with a more chaotropic character improve the HPLC analysis in terms of resolution and retention time, as stated above.43 Even though the authors43,44 make use of chaotropic and lyotropic definitions to explain the results obtained, these anions are also those that display weaker cation–anion interactions strength (leaving thus both the IL cation and anion freer to interact with analytes and the stationary phase). More recently, and following the same approach, Ding et al.46 investigated the effect of the same ILs in the separation of jatrorrhizine, palmatine and berberine (Fig. 13), from extracts of the bark of P. Chinense. The reported results are in good agreement with those discussed above.44,45 The application of imidazolium-based ILs as additives improves the separation of alkaloids while the increase of the IL cation alkyl chain length and more “chaotropic” anions reduce the retention time.
Fig. 14.
Chromatograms of standard alkaloids in methanol/water (45/55, v/v) as the mobile phase (A) without modifier, (B) with diethylamine and (C) with diethylamine and IL. (1) Oxymatrine; (2) sophoridine; (3) sophocarpine; (4) matrine. Reproduced and adapted from Tian et al.44
The separation of phenolic compounds by RP-HPLC using ILs as mobile phase additives was also investigated47 (Table 4). Different additives, namely acetic acid, triethylamine, inorganic salts (NaH2PO4 and Na2HPO4) and several ILs ([C4C2Im][BF4], [C6C1Im][BF4], [C8C1Im][BF4], [C2C1Im][C1SO4] and [C4C1Im]Cl) were appraised in a methanol/water (40/60, v/v) mobile phase in the separation of chlorogenic and caffeic acids, scoparone and rutin (Fig. 15) present in the extract of A. capillaris.47 The authors47 observed that slightly acidic mobile phases are more suitable for the separation of the studied phenolic compounds, also supported by the favourable results obtained with the addition of NaH2PO4 against Na2HPO4. Concerning the application of ILs as additives, a significant effect of the IL cation alkyl side chain length and IL anion nature on the separation performance was observed, as previously reported for alkaloids.44–46 For chlorogenic acid an increase in the retention time was observed when using ILs with longer alkyl side chains, which seems to be related to the increase of hydrophobicity of the IL. On the other hand, no significant changes in the retention time of scoparone, rutin and caffeic acid were observed.
Fig. 15.
Chemical structures of phenolic compounds extracted from A. capillaris47 and analysed by HPLC using ILs as mobile phase additives. The nomenclature of each compound is presented in Table 4.
Considering that it was previously reported69 that the addition of [C2C1Im][C1SO4] to the mobile phase increases its acidic character, the authors47 also evaluated its effect on the separation of phenolic compounds. In fact, better results were attained with [C2C1Im][C1SO4] compared to the addition of conventional additives, namely acetic acid and NaH2PO4. However, when the authors47 evaluated the anion effect on the separation (with Cl− and BF4− anions combined with the common [C4C1Im]+ cation), it was not possible to separate the two less polar organic compounds (chlorogenic and caffeic acids) – again a result of the higher cation–anion interaction energies established in halogen-based ILs. In summary, ILs display a promising potential as additives for the separation of phenolic compounds when compared with acetic acid and NaH2PO4 (e.g., the retention time of rutin is 14.9 min, 12.6 min and 9.4 min using 10 mM of acetic acid, 20 mM of NaH2PO4 and 10 mM of [C4C1Im][BF4], respectively).47
All the previously highlighted studies demonstrate the potential of ILs as new and alternative additives for RP-HPLC mobile phases. In general, the IL chemical structure plays a pivotal role in the separation performance. The IL ions not only compete with the analyte for the free silanol groups on the surface of the stationary phase but also play an active part in changing the mobile phase polarity and by establishing specific interactions with analytes, further affecting the retention time and resolution. IL cations with long alkyl side chains and anions with high capability to interact with analytes appear as the best candidates to improve the separation of less polar compounds. Nevertheless, and particularly in HPLC, the selection of the best IL strongly depends on the analyte polarity and their speciation degree. Still, the number of studies concerning the application of ILs as additives in HPLC mobile phases to separate natural compounds is scarce since only imidazolium-based ILs combined with a limited number of anions were studied hitherto. More research work is still missing in this arena, and other IL families (such as pyridinium-, pyrrolidinium-, ammonium- and phosphonium-based) should be investigated in order to better understand the molecular-level separation mechanisms responsible for enhanced resolution and short retention times.
Ionic-liquid-based stationary phases
The development of new HPLC stationary phases based on ILs has received a large attention after the pioneering work of Liu et al.,70 in which an imidazolium-based IL was used to modify the silica surface and was then applied in the separation of standard alkaloids. IL-based stationary phases combine the chemical functionalities provided by ILs with the advantages of conventional C18-based-silica columns and can indeed lead to significant improvement on the separation of bioactive compounds extracted from natural sources. The main advantage of IL-based stationary phases is the ILs’ tunability character that can be designed to separate complex mixtures containing both polar and nonpolar compounds.
HPLC separations of three families of natural compounds (alkaloids, phenolic compounds and carbohydrates) have been studied (Table 5). For instance, Bi et al.48 studied the separation of caffeine, theophylline and theobromine (Fig. 16), obtained from green tea extracts, using water as the mobile phase and a silica-IL-based stationary phase RP-HPLC column. Three Different imidazole-based compounds, such as imidazole, 1-methylimidazole and 2-ethyl-4-methylimidazole, were used to prepare the supported IL-phases (ILs covalently bonded to silica using an alkyl-most often propyl-spacer, Silpr-[IL]), namely Silpr[Im]Cl, Silpr[C1Im]Cl and Silpr[2-C2-4-C1Im]Cl (Fig. 17). The authors48 demonstrated that the Silpr[2-C2-4-C1Im]Cl stationary phase provides a better retention ability when compared with Silpr[Im]Cl and Silpr[C1Im]Cl (90 min of total retention time instead of 20 or 35 min, respectively). While with normal reverse phase columns the separation of theobromine from caffeine and other catechins was not possible, as reported in previous studies,71 silica-IL-based stationary phases allowed that separation, reinforcing thus the benefits afforded by ILs.48 The positive effects of silica-IL-based stationary phases on the retention time of alkaloids are related to the specific interactions established between the IL ions and analytes.
Table 5.
Value-added compounds extracted from natural sources and analysed by HPLC using IL-based stationary phases under the optimum separation conditions (OSC)
| Value-added compound | Natural source | Stationary phases | OSC | |
|---|---|---|---|---|
| Alkaloids | ||||
| lxxii | Caffeine | Green tea | Silpr[Im]Cl, Silpr[C1Im]Cl and Silpr[2-C2-4-C1Im]Cl (optimal) | Mobile phase: deionized water; temperature: 25 °C.48 |
| lxxiii | Theophylline | |||
| lxxiv | Theobromine | IL-modified monolithic column | Mobile phase: 60 mM NaH2PO4 aqueous solution; temperature: 25 °C.49 | |
| Carbohydrates | ||||
| xlv | Glucose | Enzymatically hydrolyzed Water Hyacinth | Silpr[Im]Cl, Silpr[C1Im]Cl (optimal), Silpr [2-C2-4-C1Im]Cl, Silpr[Im][BF4] and Silpr [Im][NTf2] | Mobile phase: acetonitrile/water (90/10, v/v); temperature: 25 °C.50 |
| lxxv | Xylose | |||
| Phenolic compounds | ||||
| lxviii | Scoparone (coumarin) | Artemisia capillaris Thunb. | SilprCl, Silpr[Im]Br and Silpr[(NH2)C3Im] Br (optimal) | Mobile phase: acetonitrile/water (50/50, v/v) with the addition of modifier; temperature: 25 °C.51 |
| lxix | Chlorogenic acid (cinnamic acid) | |||
| lxx | Caffeic acid (cinnamic acid) | |||
| lxxi | Rutin (flavonoid) | |||
Fig. 16.
Chemical structure of alkaloids and carbohydrates extracted from green tea extracts48,49 and enzymatically hydrolysed Water Hyacinth,50 analysed by HPLC using IL-based stationary phases. The nomenclature of each compound is presented in Table 5.
Fig. 17.
Schematic representation of the Silpr[C1Im]Cl stationary phase preparation. Adapted from Qiu et al.72
In addition to silica-modified columns, a new monolith-IL-based stationary phase for the separation of caffeine and theophylline (standard compounds) was recently suggested.49 Monolithic stationary phases are made up of highly interconnected channel networks with high porosity and low column backpressure. Depending on the nature of the monolithic material, it can be divided into two major types: organicpolymer-based and silica-based monolithic materials which can be chemically modified for specific applications. Their unique morphology confers them physico-mechanical properties that allow a faster separation and higher separation efficiency.49 Five monolith-IL-based columns were prepared by polymerization of methacrylic acid/glycidyl methacrylate in different monomer ratios, followed by grafting of [C3Im]Cl (Fig. 18). In general, it was observed that the methacrylic acid/-glycidyl methacrylate ratio rules the properties of the monolithic column – when this ratio increases, the porosity of the monolithic column decreases, as well as the analyte resolution. This pattern is most probably related to the availability of epoxy groups present in the glycidyl methacrylate structure to react with ILs. When monolithic IL-based columns are compared with conventional ones,49 and although no significant differences on pore structures and physical properties of the materials were observed, a better resolution was achieved with the IL-based ones (retention time variation between caffeine and theophylline of 10 min compared with 4 min obtained with conventional monolithic columns), as shown in Fig. 19. In summary, both silica- and monolith-based columns modified with imidazolium-based ILs lead to improve separations of alkaloids. Nevertheless, at this point, it can only be concluded that aromatic ILs appear as promising candidates due to H-bonding and π⋯π interactions that they can establish with aromatic compounds. Further investigations with non-aromatic ILs are still required to better understand the relevant molecular features of ILs, when acting as stationary phases, and which lead to enhanced separations by HPLC.
Fig. 18.
Schematic representation of monolith-IL-based stationary phase synthesis. Adapted from Zhu et al.49
Fig. 19.
Chromatograms of alkaloids using (A) a non-modified-IL monolithic column and (B) a monolith-IL-based column. Mobile phase composition: 0.06 mol L−1 Na2HPO4, pH 9.0. (1) Caffeine; (2) theophylline. Reproduced and adapted with permission from Zhu et al.49
The separation of phenolic compounds (Fig. 15) by HPLC using silica-IL-based stationary phases, namely SilprCl, Silpr[Im]Br and Silpr[(NH2)C3Im]Br, was also investigated (Table 5).51 In general Silpr[(NH2)C3Im]Br was identified as the most suitable stationary phase for the separation of phenolic compounds, justified by the column higher hydrophobic character and possible ion-exchange interactions that can occur between the stationary phase and the analytes.51 Even so, the claimed higher performance of IL-based columns was not supported by a direct comparison with conventional ones. However, previous studies reported by the same authors73 using conventional C18 columns for the separation of the same phenolic compounds confirm the better separation, not only in terms of retention time but also in resolution, when incorporating ILs in the stationary phase.
Based on the results obtained for the separation of alkaloids,48 recently, the same researchers50 studied the separation of xylose and glucose (Fig. 10 and 16) using silica-IL-based stationary phases (Silpr[Im]Cl, Silpr[C1Im]Cl, Silpr[2-C2-4-C1Im]Cl, Silpr[Im][BF4] and Silpr[Im][NTf2] – Fig. 17), for which a summary of the conditions and results obtained is presented in Table 5. The effects of the IL chemical structure, the mobile phase composition (methanol or acetonitrile aqueous solutions) and temperature were investigated. Comparing the performance of conventional SilprNH2 with Silpr [Im]Cl, an increase in the retention times and in resolution were obtained with silica-IL-based columns. For instance, using acetonitrile/water (90/10, v/v) as the mobile phase, the resolution of xylose and glucose increased from 0.78 to 4.03 with Silpr[Im]Cl. Contrary to the results previously discussed for alkaloids,48 ILs composed of cations with longer aliphatic moieties lead to lower retention times and resolution. This behaviour is probably related to the decrease of the stationary phase polarity which decreases the carbohydrates affinity to this phase. Considering the anion effect in the separation, the authors further observed that Silpr[Im]Cl showed the best retention time for xylose and glucose when compared with Silpr[Im][BF4] and Silpr[Im][NTf2]. It seems that the high ability of Cl− anions to interact with water allows a better retention of sugars while the hydrophobic character of [NTf2]− anions results in an opposite effect.
Overall, it was demonstrated48–51 that imidazolium-based ILs allow to decrease the polar character of HPLC stationary phases. In addition, imidazolium cations establish favourable interactions with aromatic compounds, such as those previously discussed, resulting therefore in high selectivity and resolution. Indeed, the major factors that seem to contribute to the retention of analytes on these types of columns appear to be dispersive-type, hydrogen bonding and π⋯π interactions, although ion-exchange cannot be discarded if dealing with charged analytes. Unfortunately, and as observed in the application of ILs as additives of HPLC mobile phases, only imidazolium-based ILs were studied so far. Considering the previously discussed positive effect of the IL cation [C4C1Im]+ used as an additive in mobile phases of HPLC for the separation of phenolic compounds, and taking into account that more hydrophobic ILs composed of longer alkyl side chains seem to improve the resolution, at this point it can be suggested the synthesis of new silica-IL-based stationary phase using other longer alkyl side chain imidazolium- as well as pyridinium-based ILs. It should be remarked that quinolinium- and glucaminium-based ILs have been recently applied in HPLC as silica-IL-based stationary phases for the separation of several standard compounds, including phenols and flavonoids, although not directly applied in the separation of natural extracts.74 Additionally, multi-cation ILs have been recently reported as new supported IL-phases.74 These exhibit a higher thermal stability and higher selectivity (due to a broad number of interaction sites) when compared with single-cation ILs. However, to the best of our knowledge, the application of multiple-cation ILs in stationary phases for the separation of natural compounds by HPLC was not investigated so far. In the same line, only one research group50 studied the effect of the IL anion, and particularly on the separation of carbohydrates. It was observed that the IL anion also plays an important role in the separation of natural compounds. However, a larger number of anions needs to be considered and used to separate other types of compounds in order to better understand the separation mechanisms and to be able to design target-oriented ILs in separation approaches.
Gas chromatography
Over the past few decades, GC has become the most used technique for the separation of volatile and semi-volatile analytes, including those extracted from biomass.25 This technique allows the separation of volatile compounds (using a carrier gas as the mobile phase at a high temperature) based on their Different affinities for the stationary phase. Due to their low volatility, high thermal stability and tunable selectivity, ILs have attracted considerable attention as components of GC stationary phases. ILs are capable of undergoing multiple interactions (Coulombic, dispersive and hydrogen-bonding), thereby providing unique selectivity towards a wide range of analytes when compared with conventional stationary phases.25 Due to remarkable results attained with IL-based stationary phases, some of these, namely SLB®-IL59 (fused silica non-bonded phase, [C12(P333)2][NTf2], with a temperature limit of 300 °C), SLB®-IL82 (fused silica non-bonded phase, [C12(C1C1Im)2][NTf2], with a temperature limit of 270 °C) and SLB®-IL100 (fused silica non-bonded phase, [C9(vIm)2][NTf2], with a temperature limit of 230 °C), are already commercially available.75
Most publications involving IL-based stationary phases for GC are limited to studies of standard compounds or their mixtures.25 Only three studies52–54 reported the application of ILs as GC stationary phases components aiming at improving the separation and analysis of natural compounds, namely essential oils52,53 and lipids54 (Table 6).
Table 6.
Essential oils and lipids extracted from natural sources and analysed by GC using IL-based stationary phases under the optimum separation conditions (OSC)
| Value-added compounds | Natural sources | Stationary phases | OSC |
|---|---|---|---|
| Essential oil | |||
| Hydrocarbons, oxygenated compounds and others | Dried seeds of Foeniculum vulgare Mill, Myristica fragrans Houtt., and from the bark of Cinnamomum zeylanicum J. Presl | [C9(vIm)2][NTf2]; [C9vIm][NTf2] + [C9(vIm)2][NTf2] + polysiloxane (optimal); HP-5MS; HP-INNOWax | Mobile phase: helium; temperature programme: 60 °C (5 min), 8 °C min−1, 180 °C (10 min).52 |
| Flavour and fragrance compounds | Lemon essential oil | SLB®-IL59 ([C12(P333)2][NTf2]) | SLB®-IL59; mobile phase: helium; temperature programme: 50–300 °C; at 3 °C min−1.53 |
| Lipids | |||
| Fatty acids | Cylindrotheca closterium (Ehrenberg) Reimann & J. C. Lewin and Seminavis robusta D. B. Danielidis & D. G. Mann | SLB®-IL82 ([C9(vIm)2][NTf2]) (optimal) SLB®-IL100 ([C12(C1C1Im)2][NTf2]) | GC × GC using DB-1MS for the first dimension and SLB®-IL82 for the second dimension; mobile phase: helium; temperature programme: 60–175 °C at 15 °C min−1, and at 2 °C min−1 to 240 °C (10 min).54 |
Qi et al.52 were the first evaluating the capability of two polymer-IL-based stationary phases on the separation of essential oils from dried seeds of F. vulgare, M. fragrans, and from the bark of C. zeylanicum, by GC-MS. The authors52 also compared the performance of the modified stationary phases with conventional columns, namely HP-5MS and HP-INNOWax. The polymer-IL-based stationary phases were prepared by coating fused-silica capillary columns by radical polymerization of highly thermally-stable ILs, such as dicationic crosslinkers and monocationic monomers ([C9(vIm)2][NTf2] and [C9vIm][NTf2], respectively) or their mixtures with PDMS.76 Results obtained in the analysis of the three essential oil samples demonstrate that the mixed dicationic stationary phase exhibits better selectivity than single dicationic and conventional stationary phases, namely HP-5MS and HP-INNOWax columns (Fig. 20). Furthermore, 74 compounds were identified in the C. zeylanicum essential oil using the mixed dicationic stationary phase column, which accounts for 99.45% of the entire sample, against 65 compounds identified by a conventional column (87.35% of the entire sample).52
Fig. 20.
GC-MS total ion chromatograms for the analysis of fennel essential oils from Foeniculum vulgare Mill using four fused-silica capillary columns: (A) mixed dicationic IL stationary phase; (B) single dicationic IL stationary phase; (C) HP-5 MS; and (D) HP-INNOWax. Reproduced and adapted with permission from Qi et al.52
Ragonese et al.53 studied the SLB®-IL59 column75 in the GC-FID analysis of flavour and fragrance compounds from lemon essential oil. According to the McReynolds classification, the SLB®-IL59 column showed a degree of polarity (polarity number of 59) comparable with the commercial SUPELCOWAX® 10, and significantly higher than the nonpolar column SLB®-5 commonly used in the analysis of flavour and fragrance compounds.53 Furthermore, SLB®-IL59 displays a high thermal stability (300 °C versus 280 °C for the conventional SUPELCOWAX® 10 column). A lower bleeding was also obtained with SLB®-IL59 resulting in a higher sensitivity (better signal/noise ratio) and resolution (Fig. 21).53
Fig. 21.
GC-MS chromatograms of a lemon essential oil sample on (A) an SLB®-5 ms column and (B) an SLB®-IL59 column. Adapted with permission from Ragonese et al.53 Copyright 2011, American Chemical Society.
The analysis of fatty acid methyl esters (FAMES, present in C. closterium and S. robusta) by GC-MS using conventional non-polar columns could result in a low separation efficiency,54 particularly in complex mixtures of isomers of unsaturated FAMES leading to chromatograms with overlapped peaks. In order to overcome these limitations, Gu et al.54 proposed a two dimensional (GC × GC) methodology, using a commercial non-polar DB-1MS column for the first dimension and a commercial polar IL phase, namely SLB®-IL 82 or SLB®-IL 100,75 for the second. The performance of this two dimensional GC analysis was compared with the conventional HP-88 column (Table 6). The authors54 showed that the application of polymer-IL-based stationary phases (SLB®-IL 82 or SLB®-IL 100) in the second dimension improves the resolution, notably in the separation of some specific and unusual unsaturated FAMES isomers that could not be detected in one dimensional GC (Fig. 22).
Fig. 22.
GC × GC of FAMEs present in C. closterium in three configurations with 0.10 mm ID DB-1MS as the first dimensional column, and the second dimensional column: (a) SLB-IL 100; (b) SLB-IL 82. Reproduced and adapted with permission from Gu et al.54
In summary, the potential application of ILs as new stationary phase components (single-cation or multi-cation ILs) in conventional and multidimensional GC to improve the separation and analysis of natural value-added compounds was demonstrated by some authors.52–54 Overall, an improvement of the resolution and selectivity of volatile compounds from essential oils and fatty acids was observed while using more thermally stable and lower bleeding columns. Although few studies still exist concerning the application of ILs in GC stationary phases for the separation and analysis of natural compounds, some IL-based stationary phases are already commercially available, e.g. SLB®-IL59, SLB®-IL82 and SLB®-IL100. This recent commercial interest in IL-based GC columns is a result of the improvements in analytical performance achieved by the use of ILs. They allow the tailoring of the stationary phases’ polarities and their chemical properties, creating thus a new range of supported materials that can be designed for the separation and analysis of specific and/or complex samples. Concerning the application of this modified GC columns to the analysis of value-added compounds derived from biomass other types of IL-based GC columns should be evaluated, namely phosphonium-, pyridinium- and pyrrolidinium-based stationary phases, which present a high thermal stability when compared with imidazolium-based columns, allowing the study of a large number of compounds.
Green assessment of IL-based analytical techniques and future perspectives
The demand for more environmentally safe and cost-effective analytical methods has been one of the driving forces behind the recent interest in ILs, either as constituents of mobile or stationary phases. The most remarkable advantage is the ILs tailoring ability which leads to enhanced chromatographic resolution when considering the separation of compounds with similar chemical structures or features. In most of the reported studies discussed before, and particularly when ILs are used as additives of mobile phases in CE and HPLC, it was demonstrated that the use of ILs allows a decrease in the analysis time which leads to lower solvent and energy consumptions, and therefore to a lower amount of generated waste – identified as major accomplishments within a green analytical chemistry perspective.77
According to Gałuszka et al.,78 the evaluation of analytical techniques in the context of green chemistry is highly complex due to the large number and diversity of analytes and analytical methods, and analytical criteria required in each case (e.g., resolution, selectivity, accuracy and limit of detection). In the same line, Koel27 recently proposed that the environmental impact of analytical methods should be carried out through a life cycle analysis of the components used in the overall process (chemicals, solvents, instrumentation, data processing equipment, etc.). However, and according to the author,27 in many cases this is a laborious and not easy task due to a lack of essential information on the production of instruments and chemicals. Still taking into account this difficulty, an analytical “ecological scale” was proposed by Gałuszka et al.78 to evaluate the greenness of analytical methods in a more quantitative way than NEMI (National Environmental Methods Index)79 labelling and GCAW (Green Chemical Alternatives Wizard)80 databases that consider only four criteria: persistent/bioaccumulative/toxic, hazardous, corrosive and waste chemicals. In the same direction, the HPLC-environmental assessment tool (HPLC-EAT) was proposed by Gaber et al.,81 being the first tool dedicated to the identification of hazards related to the use of liquid chromatography mobile phases. In summary, and although ILs have been successfully applied in separation processes, further characterization of their physical and chemical properties as well as of their biodegradability and toxicity features still need to be accomplished to allow the evaluation of the greenness of analytical IL-based methods through the tools discussed before. Nevertheless, and as highlighted before, ILs are non-volatile solvents, are used in small quantities as additives of mobile phases and improve the resolution and time analysis of analytical techniques, which certainly contribute to a decrease of the overall carbon footprint of IL-based analytical techniques. Even so, we would like to encourage the academic community to consider the use of these tools (ecological scale and HPLC-EAT) in future studies in order to assess the real environmental impact and the economic viability of the proposed IL-based processes.
Although not attempted to date, the integration of extraction and chromatographic separation processes using IL aqueous solutions can be seen as a promising strategy to develop more sustainable processes within a green chemistry perspective. It is important to stress that in all studies considered in this review, volatile organic solvents, such as ethanol, methanol, and acetonitrile, among others, were used for the extraction of compounds from biomass. Then, the extracts were recovered and ILs were added as part of the mobile or stationary phases in chromatographic and electrophoretic analysis. Although ILs improve the separation performance of analytical techniques, the “green” nature of the overall process is reduced by the use of volatile, often toxic, solvents in the extraction step. However, ILs (either pure or as aqueous solutions) have already shown to be remarkable solvents for the extraction of value-added compounds from biomass.14 Therefore, at this stage, the link between the two approaches is missing. In fact, it seems possible to use IL solutions for the extraction of value-added compounds from biomass and then to use the same IL solutions to improve the separation performance of analytical methods, in a fully integrated route. Several studies regarding the use of ILs as extraction solvents of natural compounds reported the direct injection of the IL-extract sample82–89 or after a sample pretreatment step90 in HPLC, either for the analysis of the sample composition or for the quantification of target compounds. In most of these studies,84,88,89 the authors stated that there are no significant variations in the peaks resolution due to the presence of ILs. Still, in these studies, the authors were mainly interested in demonstrating the ability of ILs to act as alternative solvents to extract natural compounds, and thus, the evaluation of the IL effect and its concentration and the optimization of the operational conditions on the efficiency of the chromatographic process, compared with the analysis of samples in which ILs were not used, were not taken into consideration. One isolated work regarding the use of ILs for the extraction85 and another one using the same IL as an HPLC mobile phase additive47 were found in the literature, and both deal with the same natural compound – rutin. Therefore, the integrated extraction–separation route here proposed seems to be a viable strategy, albeit not addressed in these studies nor in any of the studies discussed in this review.
It should be pointed out that the use of ILs as additives in chromatographic analysis has been studied in concentrations ranging from 0.1 to 50 mM, considerably lower ranges than those in which ILs are used as extraction media (0.5 to 4 M);14 even so, a dilution step (often part of most analytical processes) could simply overcome this gap. Furthermore, some pre-treatment strategies can be employed, e.g. with IL-based aqueous biphasic systems (ABS),12 to “clean” and concentrate samples before chromatographic analyses, and still within an integrated perspective as far as the same ILs are used. Additional studies91,92 can be found on the use of ILs for the extraction of value-added compounds from biomass at concentrations similar to those applied in chromatographic studies, mainly with imidazolium-based ILs with long alkyl side chains, creating therefore the required conditions for the development of an integrated extraction–separation process. Yet, in order to successfully integrate both processes, additional studies concerning the application of ILs at higher concentrations as constituents of mobile phases in chromatographic and electrophoretic techniques, as well as further investigations regarding the use of IL solutions at lower concentrations for the extraction of value-added compounds from biomass, need to be carried out.
Remarkable studies that open new perspectives into the integration of extraction and separation processes were recently reported. Fukaya et al.93 and Kuroda et al.94 demonstrated the potential of pure ILs to extract and to dissolve polysaccharides and lignin from biomass and to be further used as eluents in HPLC analysis. In the field of GC, Mokhtar et al.95 proposed a new approach that allows the direct injection of Eucalyptus leaf essential oil compounds dissolved in [C4C1Im][NTf2]. To this end, a programmable temperature vaporisation (PTV) injector was used to retain the IL, avoiding therefore damages in the analytical column and changes in the performance of the analytical GC column. Finally, the authors95 demonstrated the viability of ILs in single drop microextraction experiments or as a bulk extraction material. Although the first two studies93,94 deal with the extraction and analyses of polysaccharides and lignin and the last one95 deals with standard mixtures, these studies provide clear evidence on the possibility of developing integrated and more sustainable extraction–separation processes.
Conclusions
The use of ILs in mobile and stationary phases in CE, HPLC and GC leads to significant improvement on the separation and resolution of these techniques when considering the analysis of value-added compounds extracted from biomass – a main result of the ILs outstanding properties and structural diversity.
Considering the ILs application as additives or running electrolytes in CE and its Different modes, all authors conveyed the perspective that the ILs’ effect in the improvement of separation derives from the possibility of establishing favourable interactions between the analytes and the IL ions either coating the capillary wall or existing as free ions in solution. ILs were also successfully applied in HPLC as mobile phase additives, namely in RP-HPLC, and allowed to surpass the negative effect of free silanol groups responsible for the commonly increased retention times of analytes. Moreover, it was demonstrated that ILs lead to improved resolution, efficiency, permeability and stability when employed as IL-silica-based HPLC stationary phases. The successful application of ILs for the development of new GC stationary phases was also established. IL-stationary phases usually exhibit a higher thermal stability and a higher polarity number compared to conventional GC columns. Due to their remarkable performance in separations, some IL-based GC stationary phases are already commercially available. In summary, as components of mobile and stationary phases in analytical techniques, ILs provide superior selectivity for analytes and an exceptional analytical performance.
In both chromatographic and electrophoretic analyses of value-added compounds extracted from biomass, imidazolium-based ILs have been the preferred choice, justified by their favourable and specific interactions with target compounds (most often aromatic compounds, allowing thus strong hydrogen-bonding and π⋯π interactions). On the other hand, ILs composed of fluorinated anions, and mainly [BF4]-based fluids, were the most studied in the literature, where their enhanced resolution in analytical techniques seems to result from weak IL cation–anion interactions (allowing the IL cation to better interact with analytes). At this stage, it is safe to admit that ILs with aromatic character and with weak electrostatic interactions appear to be the most promising candidates to be used as additives or running electrolytes of mobile phases. In this context, other ILs composed of aromatic cations, such as pyridinium-based or tetraalkylammonium or tetraalkylphosphonium ILs with aromatic functionalized groups at the aliphatic moieties, are worthy of investigation. In the same line, and although not studied to date, there is the possibility of introducing an extra aromatic character using ILs composed of anions such as tosylate, salicylate, etc. Furthermore, although ILs are used in small amounts in analytical techniques, the application of more bio-compatible and biodegradable ILs in chromatographic analysis, particularly when employed as constituents of mobile phases that are continuously discharged, is a crucial demand for the development of more sustainable processes. The research on task-specific and more benign ILs can ultimately broaden the usefulness of these compounds within the analytical chemistry arena.
Several tools for addressing the green character of IL-based analytical techniques were highlighted in this review. However, further characterization of the ILs’ physical and chemical properties as well as their biodegradability and toxicity features still needs to be accomplished to completely evaluate the greenness of IL-based analytical methods.
The development of integrated extraction–separation processes using IL aqueous solutions was identified as the main lacuna in the literature, while all evidence seems to support the viability of integrated and more sustainable extraction–separation processes. Overall, the improvements brought about by the use of ILs in both the extraction/purification of value-added compounds from biomass and in their chromatographic and electrophoretic analysis clearly support the potential of these solvents towards the development of a more bio-based economy.
Acknowledgements
This work was developed within the scope of the project CICECO-Aveiro Institute of Materials, POCI-01-0145-FEDER-007679 (FCT Ref. UID/CTM/50011/2013), financed by national funds through the FCT/MEC and, when appropriate, co-financed by the FEDER under the PT2020 Partnership Agreement. The authors also acknowledge FCT for the doctoral grants SRH/BD/85248/2012 and SRH/BDE/103257/2014 of H. Passos and B. Soares, respectively. C. S. R. Freire acknowledges the FCT/MCTES (Portugal) for a contract under Investigador FCT 2012 contract number IF/01407/2012. M. G. Freire acknowledges the European Research Council under the European Union’s Seventh Framework Programme (FP7/2007-2013)/ERC grant agreement no. 337753.
Notes and references
- 1.Da Silva V, Rodrigues C. Chem Biol Technol Agric. 2014;1–6:14. [Google Scholar]
- 2.Herrero M, Castro-Puyana M, Mendiola JA, Ibañez E. TrAC, Trends Anal Chem. 2013;43:67–83. [Google Scholar]
- 3.Joana Gil-Chávez G, Villa JA, Fernando Ayala-Zavala J, Basilio Heredia J, Sepulveda D, Yahia EM, González-Aguilar GA. Compr Rev Food Sci Food Saf. 2013;12:5–23. [Google Scholar]
- 4.Ren Q, Xing H, Bao Z, Su B, Yang Q, Yang Y, Zhang Z. Chin J Chem Eng. 2013;21:937–952. [Google Scholar]
- 5.Zhang L, Kujawinski DM, Federherr E, Schmidt TC, Jochmann MA. Anal Chem. 2012;84:2805–2810. doi: 10.1021/ac203197d. [DOI] [PubMed] [Google Scholar]
- 6.FAO. Food Agriculture Organization Statistical Yearbook 2013 - World food and agriculture. 2013 Report 9788578110796. [Google Scholar]
- 7.Wang L, Weller CL. Trends Food Sci Technol. 2006;17:300–312. [Google Scholar]
- 8.Capello C, Fischer U, Hungerbühler K. Green Chem. 2007;9:927–934. [Google Scholar]
- 9.Gałuszka A, Migaszewski Z, Namieśnik J. Trends Anal Chem. 2013;50:78–84. [Google Scholar]
- 10.Koel M, Kaljurand M. Pure Appl Chem. 2006;78:1993–2002. [Google Scholar]
- 11.Seddon KR. J Chem Technol Biotechnol. 1997;68:351–356. [Google Scholar]
- 12.Freire MG, Cláudio AFM, Araújo JMM, Coutinho JAP, Marrucho IM, Lopes JNC, Rebelo LPN. Chem Soc Rev. 2012;41:4966–4995. doi: 10.1039/c2cs35151j. [DOI] [PubMed] [Google Scholar]
- 13.Freire MG, Teles ARR, Rocha MAA, Schröder B, Neves CMSS, Carvalho PJ, Evtuguin DV, Santos LMNBF, Coutinho JAP. J Chem Eng Data. 2011;56:4813–4822. [Google Scholar]
- 14.Passos H, Freire MG, Coutinho JA. Green Chem. 2014;16:4786–4815. doi: 10.1039/C4GC00236A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaz S., Jr Anal Methods. 2014;6:8094–8105. [Google Scholar]
- 16.Du F-Y, Xiao X-H, Li G-K. J Chromatogr A. 2007;1140:56–62. doi: 10.1016/j.chroma.2006.11.049. [DOI] [PubMed] [Google Scholar]
- 17.Pereira MM, Coutinho JAP, Freire MG. Ionic Liquids in the Biorefinery Concept: Challenges and Perspectives. The Royal Society of Chemistry; 2016. pp. 227–257. [DOI] [Google Scholar]
- 18.Vidal L, Riekkola M-L, Canals A. Anal Chim Acta. 2012;715:19–41. doi: 10.1016/j.aca.2011.11.050. [DOI] [PubMed] [Google Scholar]
- 19.Tang S, Liu S, Guo Y, Liu X, Jiang S. J Chromatogr A. 2014;1357:147–157. doi: 10.1016/j.chroma.2014.04.037. [DOI] [PubMed] [Google Scholar]
- 20.Vaher M, Koel M, Kaljurand M. Chim Oggi. 2007;25:86–88. [Google Scholar]
- 21.Pino V, Afonso AM. Anal Chim Acta. 2012;714:20–37. doi: 10.1016/j.aca.2011.11.045. [DOI] [PubMed] [Google Scholar]
- 22.Ragonese C, Sciarrone D, Tranchida PQ, Dugo P, Mondello L. J Chromatogr A. 2012;1255:130–144. doi: 10.1016/j.chroma.2012.04.069. [DOI] [PubMed] [Google Scholar]
- 23.Anderson JL, Armstrong DW, Wei G-T. Anal Chem. 2006;78:2892–2902. doi: 10.1021/ac069394o. [DOI] [PubMed] [Google Scholar]
- 24.Han D, Row KH. Molecules. 2010;15:2405–2426. doi: 10.3390/molecules15042405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ho TD, Zhang C, Hantao LW, Anderson JL. Anal Chem. 2014;86:262–285. doi: 10.1021/ac4035554. [DOI] [PubMed] [Google Scholar]
- 26.Koel M. Crit Rev Anal Chem. 2005;35:177–192. [Google Scholar]
- 27.Koel M. Green Chem. 2016;18:923–931. [Google Scholar]
- 28.Kaljurand M, Koel M. Crit Rev Anal Chem. 2011;41:2–20. [Google Scholar]
- 29.Sun P, Armstrong DW. Anal Chim Acta. 2010;661:1–61. doi: 10.1016/j.aca.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 30.Yanes EG, Gratz SR, Stalcup AM. Analyst. 2000;125:1919–1923. doi: 10.1039/b004530f. [DOI] [PubMed] [Google Scholar]
- 31.Yanes EG, Gratz SR, Baldwin MJ, Robison SE, Stalcup AM. Anal Chem. 2001;73:3838–3844. doi: 10.1021/ac010263r. [DOI] [PubMed] [Google Scholar]
- 32.Qi S, Li Y, Deng Y, Cheng Y, Chen X, Hu Z. J Chromatogr A. 2006;1109:300–306. doi: 10.1016/j.chroma.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 33.Yue M-E, Shi Y-P. J Sep Sci. 2006;29:272–276. doi: 10.1002/jssc.200500292. [DOI] [PubMed] [Google Scholar]
- 34.Yue M-E, Xu J, Li Q, HOU W. J Food Drug Anal. 2011;19:146–150. [Google Scholar]
- 35.Xiao W, Wang F-Q, Li C-H, Zhang Q, Xia Z-N, Yang F-Q. Anal Methods. 2015;7:1098–1103. [Google Scholar]
- 36.Qi S, Cui S, Chen X, Hu Z. J Chromatogr A. 2004;1059:191–198. doi: 10.1016/j.chroma.2004.09.090. [DOI] [PubMed] [Google Scholar]
- 37.Tian K, Wang Y, Chen Y, Chen X, Hu Z. Talanta. 2007;72:587–593. doi: 10.1016/j.talanta.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 38.Qi S, Cui S, Cheng Y, Chen X, Hu Z. Biomed Chromatogr. 2006;20:294–300. doi: 10.1002/bmc.562. [DOI] [PubMed] [Google Scholar]
- 39.Vaher M, Koel M, Kazarjan J, Kaljurand M. Electrophoresis. 2011;32:1068–1073. doi: 10.1002/elps.201000575. [DOI] [PubMed] [Google Scholar]
- 40.Tian K, Qi S, Cheng Y, Chen X, Hu Z. J Chromatogr A. 2005;1078:181–187. doi: 10.1016/j.chroma.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 41.Zhang H, Tian K, Tang J, Qi S, Chen H, Chen X, Hu Z. J Chromatogr A. 2006;1129:304–307. doi: 10.1016/j.chroma.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 42.Lu Y, Jia C, Yao Q, Zhong H, Breadmore MC. J Chromatogr A. 2013;1319:160–165. doi: 10.1016/j.chroma.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 43.Tang F, Tao L, Luo X, Ding L, Guo M, Nie L, Yao S. J Chromatogr A. 2006;1125:182–188. doi: 10.1016/j.chroma.2006.05.049. [DOI] [PubMed] [Google Scholar]
- 44.Tian M, Liu J, Row KH. Molecules. 2009;14:2127–2134. doi: 10.3390/molecules14062127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang Y, Sun A, Liu R, Zhang Y. Anal Chim Acta. 2013;767:148–154. doi: 10.1016/j.aca.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 46.Ding X, Tang Y, Sun A, Liu R. J Sep Sci. 2015;38:374–380. doi: 10.1002/jssc.201400649. [DOI] [PubMed] [Google Scholar]
- 47.Li S, Tian M, Row KH. Int J Mol Sci. 2010;11:2229–2240. doi: 10.3390/ijms11052229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bi W, Row KH. Chromatographia. 2010;71:25–30. [Google Scholar]
- 49.Zhu T, Bi W, Row KH. J Appl Polym Sci. 2010;118:3425–3430. [Google Scholar]
- 50.Bi W, Zhou J, Row KH. Anal Chim Acta. 2010;677:162–168. doi: 10.1016/j.aca.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 51.Tian M, Row KH. J Anal Chem. 2011;66:580–585. [Google Scholar]
- 52.Qi M, Armstrong DW. Anal Bioanal Chem. 2007;388:889–899. doi: 10.1007/s00216-007-1290-3. [DOI] [PubMed] [Google Scholar]
- 53.Ragonese C, Sciarrone D, Tranchida PQ, Dugo P, Dugo G, Mondello L. Anal Chem. 2011;83:7947–7954. doi: 10.1021/ac202012u. [DOI] [PubMed] [Google Scholar]
- 54.Gu Q, David F, Lynen F, Vanormelingen P, Vyverman W, Rumpel K, Xu G, Sandra P. J Chromatogr A. 2011;1218:3056–3063. doi: 10.1016/j.chroma.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 55.Koel M, Kaljurand M. Pure Appl Chem. 2006;78:1993–2002. [Google Scholar]
- 56.Yashima T, Tsuchiya A, Morita O, Terabe S. Anal Chem. 1992;64:2981–2984. doi: 10.1021/ac00047a018. [DOI] [PubMed] [Google Scholar]
- 57.Visak PZ, Canongia Lopes NJ, Rebelo NLP. Monatsh Chem. 2007;138:1153–1157. [Google Scholar]
- 58.Cláudio AFM, Neves MC, Shimizu K, Canongia Lopes JN, Freire MG, Coutinho JAP. Green Chem. 2015;17:3948–3963. doi: 10.1039/C5GC00712G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fernandes AM, Rocha MAA, Freire MG, Marrucho IM, Coutinho JAP, Santos LMNBF. J Phys Chem B. 2011;115:4033–4041. doi: 10.1021/jp201084x. [DOI] [PubMed] [Google Scholar]
- 60.Neves CMSS, Kurnia KA, Shimizu K, Marrucho IM, Rebelo LPN, Coutinho JAP, Freire MG, Canongia Lopes JN. Phys Chem Chem Phys. 2014;16:21340–21348. doi: 10.1039/c4cp02008a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mendes A, Branco LC, Morais C, Simplício AL. Electrophoresis. 2012;33:1182–1190. doi: 10.1002/elps.201100486. [DOI] [PubMed] [Google Scholar]
- 62.Laamanen P-L, Busi S, Lahtinen M, Matilainen R. J Chromatogr A. 2005;1095:164–171. doi: 10.1016/j.chroma.2005.07.111. [DOI] [PubMed] [Google Scholar]
- 63.Blesic M, Marques MH, Plechkova NV, Seddon KR, Rebelo LPN, Lopes A. Green Chem. 2007;9:481–490. [Google Scholar]
- 64.Freire MG, Neves CMSS, Marrucho IM, Coutinho JAP, Fernandes AM. J Phys Chem A. 2010;114:3744–3749. doi: 10.1021/jp903292n. [DOI] [PubMed] [Google Scholar]
- 65.Sintra TE, Luís A, Rocha SN, Lobo Ferreira AIMC, Gonçalves F, Santos LMNBF, Neves BM, Freire MG, Ventura SPM, Coutinho JAP. ACS Sustainable Chem Eng. 2015;3:2558–2565. doi: 10.1021/acssuschemeng.5b00751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lough WJ, Wainer IW. High Performance Liquid Chromatography: Fundamental Principles and Practice. Taylor & Francis; 1995. [Google Scholar]
- 67.Polyakova Y, Koo Y, Row K. Biotechnol Bioprocess Eng. 2006;11:1–6. [Google Scholar]
- 68.Wang Y, Tian M, Bi W, Kyung HR. Int J Mol Sci. 2009;10:2591–2610. doi: 10.3390/ijms10062591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zheng J, Polyakova Y, Row KH. J Chromatogr Sci. 2007;45:256–262. doi: 10.1093/chromsci/45.5.256. [DOI] [PubMed] [Google Scholar]
- 70.Liu SJ, Zhou F, Xiao XH. Chin Chem Lett. 2004;15:1060–1062. [Google Scholar]
- 71.Nakakuki H, Horie H, Yamauchi Y, Kohata K. J Chromatogr A. 1999;848:523–527. doi: 10.1016/s0021-9673(99)00418-5. [DOI] [PubMed] [Google Scholar]
- 72.Qiu H, Jiang S, Liu X. J Chromatogr A. 2006;1103:265–270. doi: 10.1016/j.chroma.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 73.Han D, Tian M, Row KH. J Liq Chromatogr Relat Technol. 2009;32:2407–2416. [Google Scholar]
- 74.Shi X, Qiao L, Xu G. J Chromatogr A. 2015;1420:1–15. doi: 10.1016/j.chroma.2015.09.090. [DOI] [PubMed] [Google Scholar]
- 75.Sigma-Aldrich. GC Column Selection Guide - Achieve Optimal Method Performance. 2013 [Google Scholar]
- 76.Anderson JL, Armstrong DW. Anal Chem. 2005;77:6453–6462. doi: 10.1021/ac051006f. [DOI] [PubMed] [Google Scholar]
- 77.Koel M, Kaljurand M. ch. 2. In: Koel M, Kaljurand M, editors. Green Analytical Chemistry. Royal Society of Chemistry; 2010. [Google Scholar]
- 78.Gałuszka A, Migaszewski ZM, Konieczka P, Namieśnik J. TrAC Trends Anal Chem. 2012;37:61–72. [Google Scholar]
- 79.NEMI - National Environmental Methods Index. [accessed June 2016]; http://www.nemi.gov.
- 80.Massachusetts Institute of Technology. GCAW - Green Chemical Alternatives Wizard. [accessed June 2016]; http://ehs.mit.edu/greenchem/
- 81.Gaber Y, Tornvall U, Kumar MA, Ali Amin M, Hatti-Kaul R. Green Chem. 2011;13:2021–2025. [Google Scholar]
- 82.Du FY, Xiao XH, Li GK. J Chromatogr A. 2007;1140:56–62. doi: 10.1016/j.chroma.2006.11.049. [DOI] [PubMed] [Google Scholar]
- 83.Ma W, Lu Y, Hu R, Chen J, Zhang Z, Pan Y. Talanta. 2010;80:1292–1297. doi: 10.1016/j.talanta.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 84.Yang L, Liu Y, Zu Y-G, Zhao C-J, Zhang L, Chen X-Q, Zhang Z-H. Chem Eng J. 2011;175:539–547. [Google Scholar]
- 85.Zeng H, Wang Y, Kong J, Nie C, Yuan Y. Talanta. 2010;83:582–590. doi: 10.1016/j.talanta.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 86.Zhang L, Geng Y, Duan W, Wang D, Fu M, Wang X. J Sep Sci. 2009;32:3550–3554. doi: 10.1002/jssc.200900413. [DOI] [PubMed] [Google Scholar]
- 87.Liu T, Sui X, Zhang R, Yang L, Zu Y, Zhang L, Zhang Y, Zhang Z. J Chromatogr A. 2011;1218:8480–8489. doi: 10.1016/j.chroma.2011.09.073. [DOI] [PubMed] [Google Scholar]
- 88.Yang L, Wang H, Zu Y-G, Zhao C, Zhang L, Chen X, Zhang Z. Chem Eng J. 2011;172:705–712. [Google Scholar]
- 89.Bogdanov MG, Svinyarov I. Sep Purif Technol. 2013;103:279–288. [Google Scholar]
- 90.Bi W, Tian M, Zhou J, Row KH. J Chromatogr B Biomed Appl. 2010;878:2243–2248. doi: 10.1016/j.jchromb.2010.06.034. [DOI] [PubMed] [Google Scholar]
- 91.Ressmann AK, Zirbs R, Pressler M, Gaetner P, Bica K. Z Naturforsch B: J Chem Sci. 2013;68:1129–1137. [Google Scholar]
- 92.Wu K, Zhang Q, Liu Q, Tang F, Long Y, Yao S. J Sep Sci. 2009;32:4220–4226. doi: 10.1002/jssc.200900398. [DOI] [PubMed] [Google Scholar]
- 93.Fukaya Y, Tsukamoto A, Kuroda K, Ohno H. Chem Commun. 2011;47:1994–1996. doi: 10.1039/c0cc05307d. [DOI] [PubMed] [Google Scholar]
- 94.Kuroda K, Fukaya Y, Yamada T, Ohno H. Anal Methods. 2015;7:1719–1726. [Google Scholar]
- 95.Mokhtar SU, Chin ST, Vijayaraghavan R, MacFarlane DR, Drummer OH, Marriott PJ. Green Chem. 2015;17:573–581. [Google Scholar]