Abstract
Background
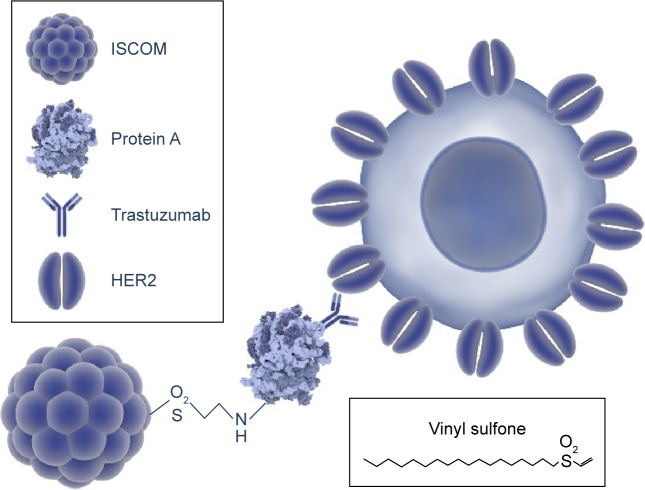
Around 20%–30% of breast cancers overexpress the proto-oncogene human epidermal growth receptor 2 (HER2), and they are characterized by being very invasive. Therefore, many current studies are focused on testing new therapies against tumors that overexpress this receptor. In particular, there exists major interest in new strategies to fight breast cancer resistant to trastuzumab (Tmab), a humanized antibody that binds specifically to HER2 interfering with its mitogenic signaling. Our team has previously developed immunostimulating complexes (ISCOMs) as nanocapsules functionalized with lipid vinyl sulfones, which can incorporate protein A and bind to G immunoglobulins that makes them very flexible nanocarriers.
Methods and results
The aim of this in vitro study was to synthesize and evaluate a drug delivery system based on protein A-functionalized ISCOMs to target HER2-overexpressing cells. We describe the preparation of ISCOMs, the loading with the drugs doxorubicin and paclitaxel, the binding of ISCOMs to alkyl vinyl sulfone-protein A, the coupling of Tmab, and the evaluation in both HER2-overexpressing breast cancer cells (HCC1954) and non-overexpressing cells (MCF-7) by flow cytometry and fluorescence microscopy. Results show that the uptake is dependent on the level of overexpression of HER2, and the analysis of the cell viability reveals that targeted drugs are selective toward HCC1954, whereas MCF-7 cells remain unaffected.
Conclusion
Protein A-functionalized ISCOMs are versatile carriers that can be coupled to antibodies that act as targeting agents to deliver drugs. When coupling to Tmab and loading with paclitaxel or doxorubicin, they become efficient vehicles for the selective delivery of the drug to Tmab-resistant HER2-overexpressing breast cancer cells. These nanoparticles may pave the way for the development of novel therapies for poor prognosis resistant patients.
Keywords: targeted drug delivery, doxorubicin, HER2, nanoparticle, paclitaxel, protein A, trastuzumab
Introduction
Nanomedicine is an expanding field of research that allows the development of nanoparticles (NPs) capable of improving the stability, transport, bioavailability, and specificity during drug delivery to tumor cells.1 Additionally, NPs may also be useful to increase the sensitivity of different diagnostic techniques, improving the early detection of the disease and the success of the treatment.2 The first NPs used as vehicles of antitumor drugs were liposomes conjugated with doxorubicin (Dox) or with albumin and paclitaxel (Pac). Currently, there exist a wide diversity of NPs, including some of inorganic nature such as iron oxide and gold NPs.2
Breast cancer remains the leading cause of cancer death in women, with a frequency that increases with age until menopause.3 Among the types of breast cancer, ~20%–30% overexpress the proto-oncogene human epidermal growth factor receptor 2 (HER2), and they are characterized as being very invasive. Similarly, tumors in other locations such as colon, stomach, pancreas, bladder, lung, and testicle may also overexpress HER2.4,5 At present, much research is focused on the study of the prognostic value of HER2 and the development of new therapies specifically directed against tumors that overexpress this receptor.6
Depending on the type of breast cancer and its stage, treatment can include surgical resection, radiotherapy, chemotherapy, and hormone therapy. However, there is still a high rate of recurrence and metastasis, due in part to the resistance that tumor cells develop to some drugs. In addition, it is necessary to find more specific drugs that reduce or avoid side effects from conventional chemotherapy, such as, for example, the cardiac dysfunction associated with anthracycline-based therapy.5,7
The humanized antibody trastuzumab (Tmab) represented a great advance in the treatment of breast tumors that overexpress HER2. This antibody specifically binds to HER2 and is widely used to treat early, advanced, and metastatic stages of cancer.2,8 Tmab interferes with mitogenic signaling induced by the receptor, and its action is highly specific against HER2-overexpressing cells.9 However, a large proportion of patients develop primary or secondary resistance in the course of the treatment.10,11
It has been found that the combined treatment of Tmab and doxorubicin produces a synergistic antitumor effect against HER2-overexpressing breast tumor cells.12 Unfortunately, there exists evidence that Dox may cause cardiac toxicity, which limits its use. However, Tmab-conjugated NPs loaded with doxorubicin fortify the antitumor effect of the drug, reducing its cardiotoxicity.13
In this regard, the conjugation of drugs with antibodies that recognize specific antigens of target cells allows the improvement of the specificity of the treatment and, in many cases, reduces the undesired side effects. This strategy has tremendous promise as a new targeted cancer therapy, and a relatively large number of antibody–drug conjugates are already in clinical development.14 In particular, Tmab conjugated to the cytotoxic agent emtansine was approved by the US Food and Drug Administration in 2013 for the treatment of patients with HER2-positive breast cancer, and it is currently in the market.15
However, the linkage of the drug to the antibody is far from trivial, and in this context our group has already reported the construction of immunostimulating complexes (ISCOM) functionalized with vinyl sulfone lipid nanocapsules. The reactivity of the vinyl sulfone group toward amine and thiol groups was exploited to obtain ISCOMs functionalized with protein A, the affinity of which for IgG allows the anchoring of an antibody that targets the ISCOMs to specific cells.16 In this work, we go one step further: ISCOMs functionalized with protein A and loaded with Pac or Dox are conjugated to Tmab and assayed against Tmab-resistant HER2-overexpressing breast cancer cells (HCC1954) and non-overexpressing cells (MCF-7).
Materials and methods
Drugs
Paclitaxel (Pac) and doxorubicin hydrochloride (Dox) were purchased from Sigma-Aldrich Co. (St Louis, MO, USA). Pac and Dox were solubilized at 1 mg/mL in methanol and water, respectively. Tmab (Herceptin) was purchased from F. Hoffmann-La Roche Ltd (Basel, Switzerland) and was solubilized according to the manufacturer’s procedure.
Preparation of the ISCOM matrix
The method used to prepare the ISCOMs was adapted from the technique of Rönnberg et al17 that was previously described by Cruz-Bustos et al.16 Briefly, to prepare the ISCOM matrix, 30 mg of cholesterol and 30 mg of phosphatidylcholine were mixed and stirred in 2 mL of Milli-Q quality water and 400 mg of detergent MEGA-10. Then, 2 mL of phosphate-buffered saline (PBS, pH 7.4) was mixed with 0.8 mL of this stock solution and 0.6 mL of Quil A (100 mg/mL) and incubated at room temperature under magnetic stirring for 1 hour. The final volume was adjusted to 12 mL by the addition of PBS.
The resulting mixture was dialyzed through Dialysis Membrane-50 (molecular weight cutoff 5,000–10,000) against water for 48 hours at 4°C with at least five changes. ISCOMs were purified by centrifugation in a sucrose gradient (10%, 25%, and 50% sucrose in PBS) at 50,000× g (Beckman Ultracentrifuge, JA 30.50 Ti. Rotor; Beckman Coulter, Brea, CA, USA) at 4°C for 18 hours following Papadopoulou et al18 and Myschik et al.19 The pellets containing the ISCOMs were resuspended in 10 mL PBS, dialyzed against water at 4°C for 48 hours, and then lyophilized and stored until used.
Preparation of ISCOMs containing paclitaxel or doxorubicin
The drug, 10 mg/mL Pac or Dox, was incorporated into the NP during the preparation of the ISCOMs following the protocol described earlier for the preparation of the ISCOM matrix. The amount of drug incorporated by the ISCOMs was determined spectrophotometrically. An amount of 10 mg aliquot of the final lyophilized product was placed in a solution of chloroform–methanol 50% water and sonicated over five cycles of 2 minutes in order to break the NPs and release the drug. The aqueous phase was measured spectrophotometrically at 230 nm for Pac and at 420 nm for the Dox, and the concentration was estimated from calibration curves.20,21 The concentration of drug (DC) inside the ISCOMs and the encapsulation efficiency (EE%) were calculated using the following formulas:
where Wt is the total amount of drug in the NP suspension and Wi is the drug initially added for the preparation of the ISCOMs.
Preparation of ISCOMs labeled with nile red
We used nile red (Sigma-Aldrich Co.) as a fluorochrome. The nile red (5 mg/mL) was incorporated into the nanocapsules as described earlier for the drugs.
Functionalization of the ISCOMs
ISCOMs were functionalized as reported by Cruz-Bustos et al,16 and lipid vinyl sulfone (LVS) was synthesized as described by Cruz-Bustos et al and Morales-Sanfrutos et al.16,22 In order to link the LVS to the NP, 2 mg/mL of the lipid sulfone was solubilized in methanol and mixed (1:1) with 0.125 mM carbonate buffer (pH 8.3). The solution was then mixed in an orbital shaker with 1 mL of a 2 mg/mL solution of protein A in carbonate buffer at a proportion of 1:1, and it was allowed to react at 4°C for 12 hours. Then, the unreacted vinyl sulfone groups were blocked with a solution of 1 M glycine in carbonate buffer for 4 hours to yield protein A-functionalized ISCOMs that were purified by dialysis and centrifugation as described earlier.
The different types of NPs, ISCOM matrix, ISCOM-paclitaxel (I-Pac), ISCOM-doxorubicin (I-Dox), and ISCOM-nile red (I-NR), were treated as described earlier and then functionalized with Tmab by incubation with a 2 mg/mL antibody solution in carbonate buffer (0.125 mM carbonate, pH 8.3) at a proportion of 1:1 for 12 hours at 4°C and orbital shaking. Unbound Tmab was eliminated by centrifugation (50,000× g, three times).
Electron microscopy characterization
An aliquot of each ISCOM preparation was analyzed by transmission electron microscopy (C. Zeiss EM 902; Carl Zeiss Meditec AG, Jena, Germany) by depositing the suspension on carbon-covered meshes stained with 1% phosphotungstic acid.
Cell lines and culture conditions
Human breast cancer cell lines MCF-7 and HCC1954 were supplied by the Department of Cell Cultures of the Granada University Scientific Instrumentation Center (Spain). Cells were cultured with Dulbecco’s Modified Eagle’s Medium (MCF-7) or RPMI-1640 medium (HCC1954) supplemented with 10% heat-inactivated fetal bovine serum, 10 mL/L penicillin–streptomycin 10×, and 2 mM l-glutamine, in a humidified atmosphere of 5% CO2 at 37°C. Culture media and respective supplements were supplied by Sigma-Aldrich Co. The authors advise that the Ethics Committee of the University of Granada did not require ethics approval for the use of the human cell line in this study as the cell line used is a commercialized ATCC cell line.
Analysis of HER2 expression by flow cytometry
MCF-7 and HCC1954 cells were harvested with PBS–ethylenediaminetetraacetic acid (EDTA), washed with PBS, and resuspended at 1×106 cells/mL in a blocking buffer (2% bovine serum albumin and 0.07% EDTA in PBS) for 30 minutes at 4°C. Cells were then centrifuged, washed with PBS, and resuspended in 100 µL of primary antibody against HER2 (Santa Cruz Biotechnology, Inc., Dallas, TX, USA, sc-74241) at the recommended dilution and incubated at 4°C for 30 minutes. Then cells were washed with PBS and incubated at 4°C for 30 minutes with the secondary antibody (Santa Cruz Biotechnology, Inc., sc-2010) at the recommended dilution. Finally, cells were analyzed in a FACScan flow cytometer (BD Biosciences, San Jose, CA, USA), and the results were processed using FlowJo software (v 7.6.5, Tree Star, Inc., Ashland, Oregon, USA). Normal isotype antibody was used for negative controls.
Uptake studies of nile red-loaded NPs
MCF-7 and HCC1954 cells were seeded at 3×104 cells/cm2 under the culture conditions detailed earlier. After 24 hours, cells were treated for 30 minutes with I-NR, conjugated (I-NR-Tmab) or not with Tmab. Samples of cultures were analyzed by fluorescence microscopy (Leica DM IL LED Fluo; Leica Microsystems, Wetzlar, Germany). Other samples were processed as follows: cells were washed with PBS, detached with PBS-EDTA, washed twice with cold PBS, and collected by centrifugation at 500× g for 10 minutes. Subsequently, cells were fixed with 4% formalin for 20 minutes and then pelleted, resuspended in PBS, and analyzed for red fluorescence in a FACScan flow cytometer (Becton Dickinson). Results were processed using FlowJo software (v 7.6.5, Tree Star, Inc.).
In vitro cytotoxicity assay
MCF-7 and HCC1954 cells (1×104) were plated into 96-well trays by sextuplicate under the culture conditions detailed earlier. After 24 hours, cells were maintained with normal medium (control), free drug (Pac or Dox), Tmab-conjugated ISCOMs (I-Tmab), and I-Pac-Tmab or I-Dox-Tmab for 24 hours. Free or encapsulated drugs were used at 0.1 µM Pac or 3 µM Dox. Subsequently, cells were fixed with 10% cold trichloroacetic acid and stained with 0.4% sulforhodamine in 1% acetic acid. The colorant was solubilized in 10 mM Tris-base pH 10.5, and optical density values at 492 nm were determined (Multiskan EX; Thermo Fisher Scientific, Waltham, MA, USA).
Statistical analysis
SPSS 14 for Windows (SPSS Inc., Chicago, IL, USA) was used for the statistical analysis. The results were compared with Student’s t-test with P<0.05 considered significant. Data were graphically represented using Microsoft Excel 2013 software (Microsoft Corporation, Redmond, WA, USA).
Results
Characterization of the NPs
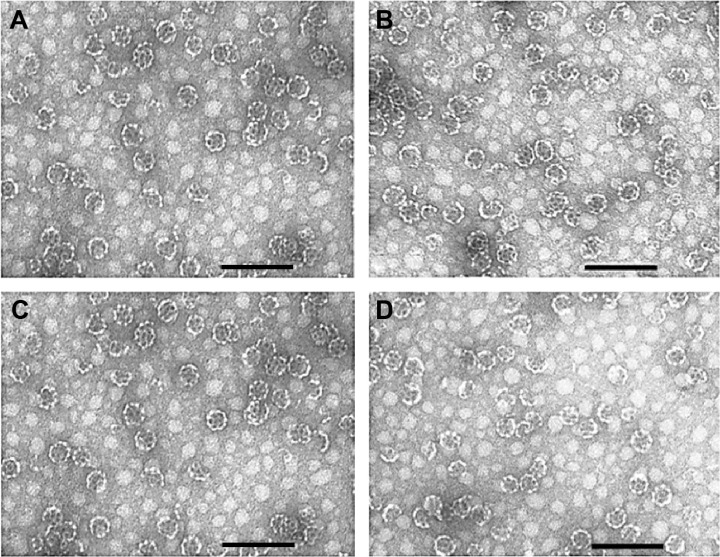
The structure of the resulting Tmab-conjugated ISCOMs is depicted in Figure 1. The morphology and the size of the different NPs were checked by transmission electron micro scopy. ISCOM matrix (Figure 2A) and Tmab-conjugated ISCOMs loaded with Pac (Figure 2B), Dox (Figure 2C), or nile red (Figure 2D) particles showed a homogeneous typical shape of the ISCOM with an average size of 45.42±1.62 nm. The amount of drug carried in delivery systems was 0.17 mg and 0.18 mg per mg of I-Pac-Tmab and I-Dox-Tmab, respectively, and encapsulation efficiency was 21.5% for Pac and 3.6% for Dox.
Figure 1.
Building-up of the NPs.
Notes: The union of the nanoparticle to the protein A and the antibody (Trastuzumab) are modified from Cruz-Bustos T, González-González G, Morales-Sanfrutos J, Megía-Fernández A, Santoyo-González F, Osuna A. Functionalization of immunostimulating complexes (ISCOMs) with lipid vinyl sulfones and their application in immunological techniques and therapy. Int J Nanomedicine. 2012;7:5941–5956.16 This figure was made using graphic components obtained from the website: www.somersault1824.com.
Abbreviations: NPs, nanoparticles; ISCOM, immunostimulating complex; HER2, human epidermal growth factor receptor 2.
Figure 2.
Transmission electron microscope images.
Notes: ISCOM matrix (A), I-Pac-Tmab (B), I-Dox-Tmab (C), and I-NR-Tmab (D) NPs. Bar =150 nm.
Abbreviations: ISCOM, immunostimulating complex; NPs, nanoparticles; I, ISCOM; Pac, paclitaxel; Tmab, trastuzumab; Dox, doxorubicin; NR, nile red.
Analysis of HER2 expression by flow cytometry
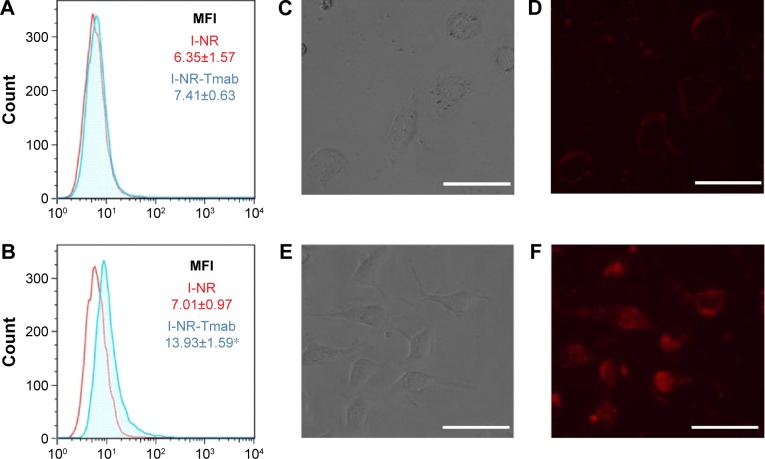
The expression levels of the HER2 receptor were analyzed by flow cytometry. Results show that the expression was much lower in MCF-7 cells than in HCC1954 cells, achieving a mean fluorescence intensity of 7.69 (Figure 3A) and 92.73 (Figure 3B), respectively.
Figure 3.
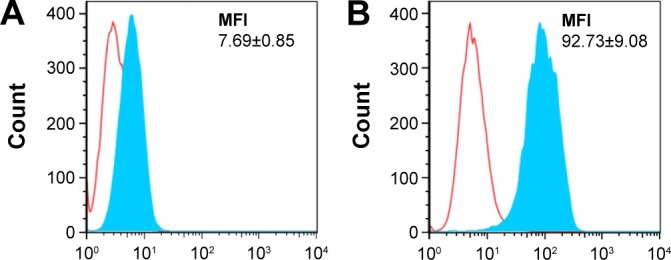
HER2 expression in MCF-7 (A) and HCC1954 (B) cells measured by flow cytometry. Empty peaks denote cells stained with isotype control antibody, and filled peaks represent cells stained with anti-HER2-specific antibody.
Notes: MFI of the filled area is listed in each graph. MFI values indicate higher HER2 expression in HCC1954 than in MCF-7.
Abbreviations: HER2, human epidermal growth factor receptor 2; MFI, mean fluorescence intensity.
Uptake studies of nile red-loaded ISCOM NPs
The ability of HCC1954 and MCF-7 cells to capture ISCOMs after 30 minutes of incubation was evaluated by flow cytometry and fluorescence microscopy (Figure 4). I-NR produced a similar level of fluorescence in both cell lines (Figure 4A and B; mean fluorescence intensity being 6.35 in MCF-7 and 7.01 in HCC1954). However, the uptake of ISCOMs conjugated with Tmab was dependent on the level of overexpression of HER2, being significantly higher for HCC1954 cells the mean fluorescence intensity of which increases from 7.01 to 13.93 (Figure 4B). This result was further confirmed by fluorescence microscopy, since cells that do not overexpress HER2 (Figure 4C) accumulated lews fluorescence (Figure 4D) than those that do it (Figure 4E and F).
Figure 4.
Cellular uptake efficiency of Tmab-conjugated ISCOM-based NPs.
Notes: MCF-7 (A) and HCC1954 (B) cells were exposed for 30 minutes to nile red-loaded ISCOM NPs, conjugated (I-NR-Tmab; blue peak) or not (I-NR; red peak) with Tmab. The MFI values measured by flow cytometry are listed in each graph. *P<0.05 Student’s t-test between both experimental groups of HCC1954 cells. MCF-7 (C and E) and HCC1954 (D and F) cells exposed for 30 minutes to I-NR-Tmab NPs were visualized by bright field microscopy (C and D) and by fluorescence microscopy (E and F). Bars (C–F): 50 µm.
Abbreviations: Tmab, trastuzumab; ISCOM, immunostimulating complex; NPs, nanoparticles; NR, nile red; MFI, mean fluorescence intensity.
In vitro cytotoxicity assay
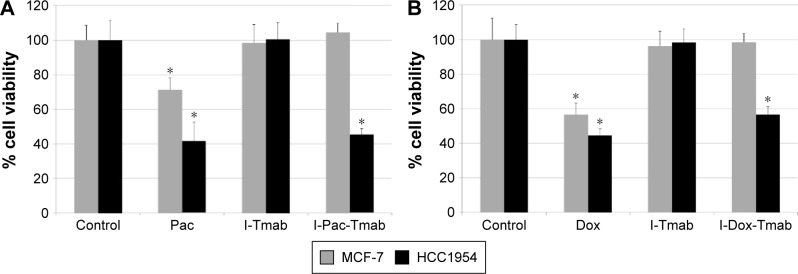
The effect of ISCOMs loaded with Pac or Dox on MCF-7 and HCC1954 cell viability was studied. The following experimental groups were analyzed: drugs alone (Pac or Dox), Tmab-conjugated ISCOM without drugs (I-Tmab), and Tmab-conjugated ISCOMs loaded with Pac or Dox (I-Pac-Tmab, I-Dox-Tmab). Free or encapsulated drugs were used at 0.1 µM (Pac) or 3 µM (Dox). Results are summarized in Figure 5 that show that HCC1954 cells are more sensitive than MCF-7 cells to the drug delivered by Tmab-conjugated ISCOMs.
Figure 5.
Effect of ISCOM-based NPs loaded with Pac (A) or Dox (B) on cell viability.
Notes: MCF-7 and HCC1954 cells were incubated for 24 hours with normal medium (control), nontargeted drug (Pac or Dox), Tmab-conjugated ISCOM NPs (I-Tmab), or I-Tmab loaded with Pac (I-Pac-Tmab) or Dox (I-Dox-Tmab). Histogram depicts the mean ± SD of six determinations of the percentage of cell viability with respect to controls, set as 100%. *P<0.05. Student’s t-test, compared with respective control.
Abbreviations: ISCOM, immunostimulating complex; NPs, nanoparticles; Pac, paclitaxel; Dox, doxorubicin; Tmab, trastuzumab.
Cells treated with untargeted Pac (Figure 5A) underwent a significant viability reduction, reaching values of 71% in MCF-7 and 41% in HCC1954, compared to the control group. As expected, I-Tmab did not reduce the viability of either cell line, but when the NPs were loaded with paclitaxel (ie, I-Pac-Tmab) only reduced the viability of HCC1954 cells (45%). Similar results were found for Dox (Figure 5B). Untargeted Dox reduced the viability of both cell lines (56% in MCF-7 and 44% in HCC1954), I-Tmab did not reduce the viability of either cell line, and targeted Dox (ie, I-Dox-Tmab) affected only HCC1954 cells (56% cell viability).
Discussion
Currently, Tmab therapy is indicated for patients with advanced breast cancer whose tumors overexpress the HER2 receptor. In addition, there are some trials with short-term monitoring showing that the addition of Tmab to chemotherapy halves the risk of relapse.9,23,24 Because of the good results achieved in metastatic patients, this agent has been tested in patients with early HER2+ breast cancer, and, in fact, it is common to include a year of Tmab as part of the adjuvant treatment.25,26 More recently, in 2013, Tmab conjugated to the cytotoxic agent emtansine was approved by the US Food and Drug Administration for the treatment of patients with HER2-positive metastatic breast cancer, who previously received Tmab and a taxane, separately or in combination.15
However, despite this apparent success, there are still issues such as the mechanism of action in vivo, the selection of patients based on drug resistance, the prevention of the resistance to Tmab, and the definition of the best therapy for patients whose tumors progress during treatment that demand further research.25,27,28 In particular, resistance is a major issue since it has been reported that ~70% of the patients who initially responded to Tmab experience progression to metastatic disease within a year.29 Additionally, both in vitro and in vivo studies have found that the combined treatment of Tmab and Dox produces a synergistic antitumor effect against HER2-overexpressing breast tumor cells, but the cardiotoxicity caused by this drug is an important disadvantage.12,24 Nevertheless, some studies have shown that NPs conjugated with Tmab can reduce the adverse effects that traditional chemotherapy involves.13
In this context, the synthesis of a drug delivery system by coupling Tmab and ISCOM is especially appealing since it combines three synergic effects: 1) the therapeutic effect of the drug and, eventually, of Tmab, 2) the targeting effect of Tmab over breast cancer cells overexpressing HER2, and 3) the high load capacity of ISCOMs. The election of the strategy to couple Tmab to ISCOMs is critical to preserve the functionality of the former, and the good reactivity of vinyl sulfone group toward proteins was envisaged as the tool to obtain the bioconjugate.30,31 From a chemical point of view, the coupling relies on the reactivity of the vinyl sulfone group toward Cys, His, and Lys residues, and the fact that according to UniProtKB/Swiss-Prot release 2016_16, Lys is the eighth most frequent amino acid virtually present in any natural protein makes this approach a general coupling strategy. As already reported by our group, the reaction of protein A with LVS promotes the incorporation of protein A to ISCOMs, and the specific interaction between IgGs and protein A can be exploited to further derivatize the ISCOMs with IgGs that act as targeting agents.16
It is important to highlight that ISCOMs functionalized with protein A are a good example of a flexible drug carrier system that can be loaded with a large number of drugs and coupled to any IgG (Figure 1).32 For the purpose of this study, ISCOMs were loaded with Pac (taxane) or Dox (anthracycline), whose efficiency against breast cancer is well established,12,33,34 and have been coupled to Tmab to yield regular vesicles with an average diameter of 45.42 nm (Figure 2).
The resulting drug delivery systems were assayed on two breast cancer cell lines, MCF-7 and HCC1954. The rationale behind the election of these two cell lines is the different level of expression of HER2, MCF-7 showing a low expression profile whereas HCC1954 overexpressing HER2 and displaying primary resistance to Tmab. Flow cytometry confirmed the functionality of the delivery system and the different level of expression of HER2 by the two cell lines assayed. As expected, the uptake of ISCOMs conjugated with Tmab was dependent on the level of overexpression of HER2, being significantly higher for HCC1954 (Figure 4), and as a consequence, the drug delivery system was more efficient on HCC1954 cells (Figure 5). These results are in full agreement with those reported by Hendriks et al,35 who found that the cytotoxic effect of liposomal NPs conjugated with Tmab and loaded with Dox was higher as the cells attain higher levels of the receptor.
In terms of cell viability, the comparison between targeted and nontargeted drug shows that untargeted drugs are more efficient, discouraging the use of these delivery systems in therapy. However, a closer analysis reveals that the targeted drugs are selective toward HCC1954 cells, whereas MCF-7 cells seem to remain unaffected, although the former are resistant to Tmab. Interestingly, disulfide-linked Tmab-emtansine also showed high selective activity toward the resistant cells.36 A possible justification of this result may be that HCC1954 cells overexpress a nonfunctional HER, being resistant to the therapeutic action of Tmab,37 but that maintains the antigenic properties of recognition, being targetable by Tmab.
Although in the context of drug delivery, the use of LVSs for the functionalization of ISCOMs with antibodies is a feasible strategy and our in vitro studies are promising, in vivo studies are needed to confirm whether ISCOM NPs are therapeutically effective and reduce the side effects associated to Dox and Pac and to study how they are affected by biological and pathological factors. In particular, the structure, physiology, growth, and environment of tumor as well as permeability and renal filtration are important factors that may influence the effectiveness of NPs for drug delivery.38 Our approach can be implemented to develop novel NPs, and it would be interesting to assay NPs coupled to other targeting antibodies such as bevacizumab (targets vascular endothelial growth factor)39 and cetuximab40 or panitumumab41 (target epidermal growth factor receptor).
Conclusion
We have demonstrated that the combination of ISCOMs and protein A previously functionalized with an LVS yields a versatile nanocarrier that can exploit the reactivity of the vinyl sulfone group toward Cys, His, and Lys to couple to any antibody to deliver drugs to the site of action. In particular, ISCOMs functionalized with Tmab and loaded with Pac or Dox are selective in vitro toward cell lines overexpressing HER2 regardless of the resistance to the antibody.
Although there exist few articles reporting the development of NPs with the same purpose as our study,35,42,43 our results are relevant because they may set the groundwork to develop treatments for patients with resistance to the antibody. Although promising in in vitro assays, in vivo studies are necessary to confirm whether they are therapeutically effective and able to reduce the adverse effects associated with Dox and Pac. If successful, these in vivo studies may open a range of possibilities to solve the high cost of health care involving the indiscriminate administration of Tmab, as they could ensure the action of this drug, regardless of whether it is administered to a resistant patient.
Acknowledgments
This study was supported by the Ministry of Economy and Competitiveness (Projects AGL2011-26098, CTQ2014-55474-C2-1-R, and CTQ2014-56611-R) and by the Regional Government of Andalusia (Project P11-CTS-7651). The authors thank http://www.somersault1824.com/resources/ for allowing the use of its platform in the design of the figures. This paper is related to the PhD thesis of Nuria Mut-Salud.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Boulaiz H, Alvarez PJ, Ramirez A, et al. Nanomedicine: application areas and development prospects. Int J Mol Sci. 2011;12(5):3303–3321. doi: 10.3390/ijms12053303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang M, Thanou M. Targeting nanoparticles to cancer. Pharmacol Res. 2010;62(2):90–99. doi: 10.1016/j.phrs.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Carrasco E, Álvarez PJ, Melguizo C, et al. Novel merosesquiterpene exerts a potent antitumor activity against breast cancer cells in vitro and in vivo. Eur J MedChem. 2014;79:1–12. doi: 10.1016/j.ejmech.2014.03.071. [DOI] [PubMed] [Google Scholar]
- 4.Caba O, Rodríguez-Serrano F, Díaz-Gavilán M, et al. The selective cytotoxic activity in breast cancer cells by an anthranilic alcohol-derived acyclic 5-fluorouracil O,N-acetal is mediated by endoplasmic reticulum stress-induced apoptosis. Eur J Med Chem. 2012;50:376–382. doi: 10.1016/j.ejmech.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 5.Weigelt B, Geyer FC, Reis-Filho JS. Histological types of breast cancer: how special are they? Mol Oncol. 2010;4(3):192–208. doi: 10.1016/j.molonc.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pohlmann PR, Mayer IA, Mernaugh R. Resistance to trastuzumab in breast cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2009;15(24):7479–7491. doi: 10.1158/1078-0432.CCR-09-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romond EH, Jeong J-H, Rastogi P, et al. Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node-positive, human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2012;30(31):3792–3799. doi: 10.1200/JCO.2011.40.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou Z, Badkas A, Stevenson M, Lee J-Y, Leung Y-K. Herceptin conjugated PLGA-PHis-PEG pH sensitive nanoparticles for targeted and controlled drug delivery. Int J Pharm. 2015;487(1–2):81–90. doi: 10.1016/j.ijpharm.2015.03.081. [DOI] [PubMed] [Google Scholar]
- 9.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 10.Ahmad S, Gupta S, Kumar R, Varshney GC, Raghava GPS. Herceptin resistance database for understanding mechanism of resistance in breast cancer patients. Sci Rep. 2014;4:4483. doi: 10.1038/srep04483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilken JA, Maihle NJ. Primary trastuzumab resistance: new tricks for an old drug. Ann N Y Acad Sci. 2010;1210:53–65. doi: 10.1111/j.1749-6632.2010.05782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slamon D, Eiermann W, Robert N, et al. Phase III randomized trial comparing doxorubicin and cyclophosphamide followed by docetaxel (AC→T) with doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab (AC→TH) with docetaxel, carboplatin and trastuzumab (TCH) in Her2neu positive early breast cancer patients: BCIRG 006 study. Cancer Res. 2009;69(24 suppl):62. [Google Scholar]
- 13.Chiang C-S, Hu S-H, Liao B-J, Chang Y-C, Chen S-Y. Enhancement of cancer therapy efficacy by trastuzumab-conjugated and pH-sensitive nanocapsules with the simultaneous encapsulation of hydrophilic and hydrophobic compounds. Nanomed Nanotechnol Biol Med. 2014;10(1):99–107. doi: 10.1016/j.nano.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 14.Hamilton GS. Antibody-drug conjugates for cancer therapy: the technological and regulatory challenges of developing drug-biologic hybrids. Biologicals. 2015;43(5):318–332. doi: 10.1016/j.biologicals.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Niculescu-Duvaz I. Trastuzumab emtansine, an antibody-drug conjugate for the treatment of HER2+ metastatic breast cancer. Curr Opin Mol Ther. 2010;12(3):350–360. [PubMed] [Google Scholar]
- 16.Cruz-Bustos T, González-González G, Morales-Sanfrutos J, Megía-Fernández A, Santoyo-González F, Osuna A. Functionalization of immunostimulating complexes (ISCOMs) with lipid vinyl sulfones and their application in immunological techniques and therapy. Int J Nanomedicine. 2012;7:5941–5956. doi: 10.2147/IJN.S35556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rönnberg B, Fekadu M, Morein B. Adjuvant activity of non-toxic Quillaja saponaria Molina components for use in ISCOM matrix. Vaccine. 1995;13(14):1375–1382. doi: 10.1016/0264-410x(95)00105-a. [DOI] [PubMed] [Google Scholar]
- 18.Papadopoulou G, Karagouni E, Dotsika E. ISCOMs vaccine against experimental leishmaniasis. Vaccine. 1998;16(9–10):885–892. doi: 10.1016/s0264-410x(97)00308-3. [DOI] [PubMed] [Google Scholar]
- 19.Myschik J, Lendemans DG, McBurney WT, Demana PH, Hook S, Rades T. On the preparation, microscopic investigation and application of ISCOMs. Micron Oxf Engl 1993. 2006;37(8):724–734. doi: 10.1016/j.micron.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 20.Manasa E, Vanitha Prakash K, Ravi Pratap P, Susena S. Method development and validation of doxorubicin HCL in API and its formulation by spectrophotometry. Int J Pharm Chem Biol Sci. 2013;3(4):1006–1009. [Google Scholar]
- 21.Yang T, Cui F-D, Choi M-K, et al. Enhanced solubility and stability of PEGylated liposomal paclitaxel: in vitro and in vivo evaluation. Int J Pharm. 2007;338(1–2):317–326. doi: 10.1016/j.ijpharm.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 22.Morales-Sanfrutos J, Megia-Fernandez A, Hernandez-Mateo F, Giron-Gonzalez MD, Salto-Gonzalez R, Santoyo-Gonzalez F. Alkyl sulfonyl derivatized PAMAM-G2 dendrimers as nonviral gene delivery vectors with improved transfection efficiencies. Org Biomol Chem. 2011;9(3):851–864. doi: 10.1039/c0ob00355g. [DOI] [PubMed] [Google Scholar]
- 23.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after Adjuvant Chemotherapy in HER2-Positive Breast Cancer. N Engl J Med. 2005;353(16):1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 24.Mandler R, Kobayashi H, Hinson ER, Brechbiel MW, Waldmann TA. Herceptin-geldanamycin immunoconjugates: pharmacokinetics, biodistribution, and enhanced antitumor activity. Cancer Res. 2004;64(4):1460–1467. doi: 10.1158/0008-5472.can-03-2485. [DOI] [PubMed] [Google Scholar]
- 25.Wong ALA, Lee S-C. Mechanisms of Resistance to Trastuzumab and Novel Therapeutic Strategies in HER2-Positive Breast Cancer. Int J Breast Cancer. 2012;2012:415170. doi: 10.1155/2012/415170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365(14):1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kataoka Y, Mukohara T, Shimada H, Saijo N, Hirai M, Minami H. Association between gain-of-function mutations in PIK3CA and resistance to HER2-targeted agents in HER2-amplified breast cancer cell lines. Ann Oncol. 2010;21(2):255–262. doi: 10.1093/annonc/mdp304. [DOI] [PubMed] [Google Scholar]
- 28.Valabrega G, Montemurro F, Aglietta M. Trastuzumab: mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancer. Ann Oncol. 2007;18(6):977–984. doi: 10.1093/annonc/mdl475. [DOI] [PubMed] [Google Scholar]
- 29.Gajria D, Chandarlapaty S. HER2-amplified breast cancer: mechanisms of trastuzumab resistance and novel targeted therapies. Expert Rev Anticancer Ther. 2011;11(2):263–275. doi: 10.1586/era.10.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lopez-Jaramillo FJ, Hernandez-Mateo F, Santoyo-Gonzalez F. Vinyl sulfone: a multi-purpose function in proteomics. In: Eastwood Leung H-C, editor. Integrative Proteomics. Croatia: InTech; 2012. pp. 301–326. [Google Scholar]
- 31.Ortega-Muñoz M, Morales-Sanfrutos J, Megia-Fernandez A, Lopez-Jaramillo FJ, Hernandez-Mateo F, Santoyo-Gonzalez F. Vinyl sulfone functionalized silica: a “ready to use” pre-activated material for immobilization of biomolecules. J Mater Chem. 2010;20(34):7189–7196. [Google Scholar]
- 32.del Castillo T, Morales-Sanfrutos J, Santoyo-Gonzalez F, Magez S, Lopez-Jaramillo FJ, Garcia-Salcedo JA. Monovinyl sulfone β-cyclodextrin. A flexible drug carrier system. ChemMedChem. 2014;9(2):383–389. doi: 10.1002/cmdc.201300385. [DOI] [PubMed] [Google Scholar]
- 33.Tolaney SM, Barry WT, Dang CT, et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med. 2015;372(2):134–141. doi: 10.1056/NEJMoa1406281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Untch M, Rezai M, Loibl S, et al. Neoadjuvant treatment with trastuzumab in HER2-positive breast cancer: results from the GeparQuattro study. J Clin Oncol. 2010;28(12):2024–2031. doi: 10.1200/JCO.2009.23.8451. [DOI] [PubMed] [Google Scholar]
- 35.Hendriks BS, Klinz SG, Reynolds JG, Espelin CW, Gaddy DF, Wickham TJ. Impact of tumor HER2/ERBB2 expression level on HER2-targeted liposomal doxorubicin-mediated drug delivery: multiple low-affinity interactions lead to a threshold effect. Mol Cancer Ther. 2013;12(9):1816–1828. doi: 10.1158/1535-7163.MCT-13-0180. [DOI] [PubMed] [Google Scholar]
- 36.Lewis Phillips GD, Li G, Dugger DL, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008;68(22):9280–9290. doi: 10.1158/0008-5472.CAN-08-1776. [DOI] [PubMed] [Google Scholar]
- 37.Vu T, Claret FX. Trastuzumab: updated mechanisms of action and resistance in breast cancer. Front Oncol. 2012;2:62. doi: 10.3389/fonc.2012.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steichen SD, Caldorera-Moore M, Peppas NA. A review of current nanoparticle and targeting moieties for the delivery of cancer therapeutics. Eur J Pharm Sci. 2013;48(3):416–427. doi: 10.1016/j.ejps.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pham E, Yin M, Peters CG, et al. Preclinical efficacy of bevacizumab with CRLX101, an investigational nanoparticle-drug conjugate, in treatment of metastatic triple-negative breast cancer. Cancer Res. 2016;76(15):4493–4503. doi: 10.1158/0008-5472.CAN-15-3435. [DOI] [PubMed] [Google Scholar]
- 40.Kaluzova M, Bouras A, Machaidze R, Hadjipanayis CG. Targeted therapy of glioblastoma stem-like cells and tumor non-stem cells using cetuximab-conjugated iron-oxide nanoparticles. Oncotarget. 2015;6:8788–8806. doi: 10.18632/oncotarget.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yook S, Cai Z, Lu Y, Winnik MA, Pignol JP, Reilly RM. Radiation nanomedicine for EGFR-positive breast cancer: panitumumab-modified gold nanoparticles complexed to the β-particle-emitter, (177)Lu. Mol Pharm. 2015;12(11):3963–3972. doi: 10.1021/acs.molpharmaceut.5b00425. [DOI] [PubMed] [Google Scholar]
- 42.Sun B, Ranganathan B, Feng S-S. Multifunctional poly(D,L-lactide-co-glycolide)/montmorillonite (PLGA/MMT) nanoparticles decorated by trastuzumab for targeted chemotherapy of breast cancer. Biomaterials. 2008;29(4):475–486. doi: 10.1016/j.biomaterials.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 43.Anhorn MG, Wagner S, Kreuter J, Langer K, von Briesen H. Specific targeting of HER2 overexpressing breast cancer cells with doxorubicin-loaded trastuzumab-modified human serum albumin nanoparticles. Bioconjug Chem. 2008;19(12):2321–2331. doi: 10.1021/bc8002452. [DOI] [PubMed] [Google Scholar]