Abstract
Purpose
Studies suggest that COPD prevalence may vary between countries. We conducted an ecological study of data from COPD prevalence articles to assess the influence of differences in country-level risk factors on COPD prevalence.
Patients and methods
Our study covered English language articles published during 2003–2014. Qualified articles used spirometry to assess COPD prevalence and used representative samples from national or subnational populations. Stepwise binomial regression was used to analyze associations between study- and country-level factors and COPD prevalence.
Results
Eighty articles provided 1,583 measures of COPD prevalence for subjects in different sex, age, and smoking categories for 112 districts in 41 countries. Adjusted prevalence rates for COPD were significantly lower for Australia/New Zealand and the Mediterranean and significantly higher for Latin America, compared to North America, Southeast Asia, and Northern Europe. Country-level socioeconomic development variables had an uneven and mixed association with COPD prevalence. High elevation above sea level was shown to be a protective factor for COPD. Study-level variables for the established risk factors of sex, age, and smoking explained 64% of variability in COPD prevalence. Country-level risk factors raised the explanatory power to 72%. Approximately 28% of worldwide variability in COPD prevalence remained unexplained.
Conclusion
Our study suggests that COPD prevalence varies across world regions, even after adjustment for established risk factors. Major country-level risk factors contributing to the worldwide epidemic of COPD remain to be investigated.
Keywords: country effects, ecological study, epidemiology, lung disease, risk factors, spirometry
Introduction
Historically, studies of COPD prevalence have used diverse survey designs, varied protocols, and a wide range of diagnostic criteria. This diversity has impeded efforts to undertake a comprehensive statistical analysis of global COPD prevalence because of between-study heterogeneity. In the past decade, however, research reports on COPD prevalence have become more consistent, rigorous, and comparable. Recent improvements in the quality of studies are likely attributable to the Burden of Obstructive Lung Disease (BOLD) initiative, which developed and published instructions for a standard protocol and methodology for COPD prevalence studies.1 The quality of COPD prevalence studies has similarly improved because the Global initiative for chronic Obstructive Lung Disease (GOLD) provided investigators with a standard framework for diagnosing COPD.2
Numerous COPD prevalence studies across many countries have shown that being male, older, and a former or current smoker are consistent risk factors associated with COPD.3,4 We subsequently refer to age, sex, smoking status, and smoking amount collectively as the established risk factors for COPD because they have been authoritatively implicated in the disease.4 The literature has also shown that occupational and domestic exposures to smoke and fumes, as well as other risk factors, contribute significantly to COPD risk.5 Systematic reviews of COPD prevalence studies have summarized these prevalence patterns internationally, confirming these general tendencies, but these reviews have also noted a wide variability in COPD prevalence rates across national borders.6 We hypothesized that some of the worldwide variability in COPD prevalence may be explained by differences in country-level risk factors that describe the living conditions of populations from which study participants are selected.
The objective of this ecological study was to conduct a statistical analysis of a large pool of recently published articles (2003–2014) to examine the influence of country-level demographic, socioeconomic, environmental, and geographic factors on the prevalence of COPD within populations. Our choice of an ecological approach was motivated in part by the success of a similar approach taken by Blanc et al7 in their study of the relationship between occupational exposures and COPD prevalence in three international cohorts covering 45 study sites.
Patients and methods
Diagnostic criteria for airflow obstruction
GOLD diagnostic guidelines for COPD were the accepted standard throughout the decade covered by our study and, hence, were adopted for our global analysis of COPD prevalence. We classified airflow obstruction into: 1) mild to severe disease (GOLD stages I–IV; forced expiratory volume in 1 second [FEV1]/forced vital capacity [FVC] <0.70) or 2) moderate to severe disease (GOLD stages II–IV; FEV1/FVC <0.70 and FEV1%pred <80%). FEV1%pred is the ratio of FEV1 for a subject to the average FEV1 for healthy subjects of the same sex, age, height and race. The GOLD criteria call for postbronchodilator spirometric measurement of FEV1 and FVC.2 Some studies that were otherwise well qualified only used bronchodilators for participants who had a degree of airflow obstruction.8,9 These studies were accepted for the analysis, but we incorporated an indicator variable to adjust for this departure from the GOLD protocol. The subsequent analysis shows its impact.
Qualified studies
To qualify for inclusion in our analysis, studies had to
Appear in a peer-reviewed English language publication dated 2003–2014.
Report COPD prevalence rates based on GOLD I–IV or GOLD II–IV criteria.
Cover a representative sample of a national population or a subnational population within a well-delineated city or district.
Report prevalence rates for categories defined by one or more of the established risk factors of sex, age, smoking status, and/or smoking amount, considered singly or jointly.
The literature search for qualified studies used the following steps:
An initial search of the medical literature was conducted on PubMed using nonoverlapping combinations of the following search terms: COPD, prevalence, spirometry, GOLD, population, for articles published in the English language during the interval 2003–2014. Supplementary material lists the search combinations.
Cited references in all qualified articles and systematic reviews found in step 1 were examined for additional qualified articles and systematic reviews. Cited references in these additional articles were searched until no new qualified articles were uncovered.
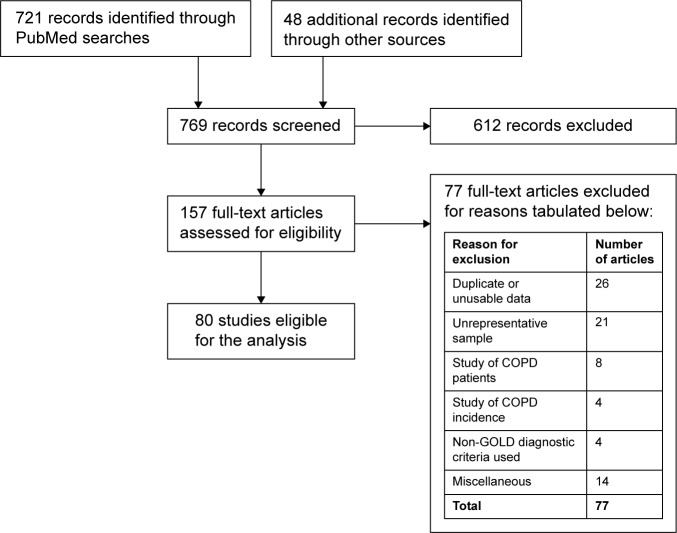
Screening for qualification involved an initial review of titles and abstracts, followed by detailed reading of articles that might be admissible. Reasons for disqualification included inadequate data, duplicate data from another study already in the database, focus on a limited, highly specialized, or unrepresentative subpopulation (eg, an occupational group), or inadequate documentation to establish qualification or precision of prevalence results. Figure 1 shows a PRISMA flow chart of the articles that were identified, screened, and found eligible in the literature search.10
Figure 1.
PRISMA flow chart for articles that are identified, screened, and found eligible in the literature search.
Data sources
Prevalence rates and other study-level data were extracted from tables, figures, and texts of the qualified articles. Public databases for country-level statistics were searched for statistics on demographic, socioeconomic, environmental, and geographic factors that might be associated with COPD. Details concerning data extraction and sources, as well as summary statistics for the study- and country-level data, are presented in the Supplementary material.
Statistical procedures
The data set consists of records with counts of COPD cases among samples of respondents in different risk categories. As this is a binomial sampling situation, binomial regression analysis was used. The analysis assumed simple random sampling. The regression function links the probability of COPD to covariates through a logistic function. Analyses were undertaken using Stata 12 software (College Station, TX, USA). Supplementary material gives a detailed explanation of the computational procedures.
Stepwise binomial regression was used. Groups of variables were stepped into the regression function in the following sequence: COPD definition variables, study-level established risks, country-level established risks, socioeconomic variables, environmental variables, physical geography variables, and finally, world region indicators. The COPD definition variables (GOLD severity level and postbronchodilation spirometry) were included in the first step because they adjust for the definition of the outcome measure. The study-level-established risks (sex, age, and smoking) were added in the next step because they are characteristics of actual participants in the studies. The remaining groups of country-level variables were added in an order that, in our judgment, proceeded from the most direct to the least direct country-level factors that could influence COPD prevalence.
Results
Results of the literature search
As shown in Figure 1, the literature search led to review of 157 full-text articles and retention of 80 qualified articles. Literature citations for the qualified articles are presented in the Supplementary material. Reasons for exclusion of the 77 full-text articles that were reviewed but not included in the analysis are tabulated in Figure 1. The 80 qualified articles yielded 1,583 measures of COPD prevalence for subjects in different sex, age, and smoking categories. These articles covered 112 districts in 41 countries. There were 950 prevalence readings on mild or more severe COPD (GOLD I–IV) and 633 readings on moderate or more severe COPD (GOLD II–IV). Postbronchodilator measurements of lung function were collected in 69% of readings.
Table 1 shows the number of countries and qualified articles for each world region. Research attention to COPD prevalence was geographically uneven in the English language literature during this decade. Very few publications were found for several large regions of the world including Africa, the Arabian Peninsula, Central Asia, Eastern Europe, and Russia. The category “other” contains single articles from Saudi Arabia, Cape Verde (Africa), and South Africa.
Table 1.
Number of countries and qualified articles for each world region
| World region | Number of countries | Number of articles |
|---|---|---|
| Australasia | 2 | 6 |
| Latin America | 5 | 3 |
| Mediterranean | 10 | 18 |
| North America | 2 | 11 |
| Northern Europe | 14 | 30 |
| Southeast Asia | 5 | 17 |
| Other | 3 | 3 |
| Total | 41 | 88 |
Notes: Some countries were covered by several articles, so the number of articles exceeds the number of countries. The total number of articles exceeds 80 because some qualified articles studied multiple countries.
Influence of outcome measure for COPD prevalence
The first step of the stepwise binomial regression analysis takes account of the prevalence outcome measure. Table 2 shows the results. The regression function includes indicator variables for whether the outcome was GOLD I–IV and whether postbronchodilation spirometry was used. The function also includes an interaction term that is a product of these two variables. The interaction captures any differential effect of postbronchodilation spirometry on the prevalence of GOLD I–IV and GOLD II–IV severity. All covariates have significant effects (P<0.001). Table 3 shows the fitted prevalence rate (in percent), as well as the number of records, stratified by the COPD severity class and whether a bronchodilator was used or not. The results show that use of a bronchodilator reduces the prevalence of GOLD I–IV COPD by one-third, from 15.0% to 10.0% on average. The prevalence of GOLD II–IV COPD (ie, moderate or severe COPD) is slightly reduced if a bronchodilator is used (7.9% vs 7.3% on average).
Table 2.
Stepwise binomial regression results for the prevalence outcome measure
| Variable | Adjusted odds ratio (95% CI) | P-value |
|---|---|---|
| GOLD I–IV (indicator) | 2.067 (2.035–2.100) | <0.001 |
| Postbronchodilation (indicator) | 0.926 (0.904–0.948) | <0.001 |
| GOLD I–IV × postbronchodilation interaction (indicator) | 0.683 (0.665–0.702) | <0.001 |
| Intercept | 0.0854 (0.0842–0.0865) |
Abbreviation: GOLD, Global Initiative for Chronic Obstructive Lung Disease.
Table 3.
Fitted binomial regression estimates of mean COPD prevalence rates (%) for the two GOLD levels according to whether postbronchodilation was used or not
| Fitted mean prevalence rate (%) | No bronchodilation | Bronchodilation |
|---|---|---|
| GOLD II–IV | 7.9% (n=199) | 7.3% (n=434) |
| GOLD I–IV | 15.0% (n=294) | 10.0% (n=656) |
Note: Sample sizes shown are numbers of records (total =1,583).
Abbreviation: GOLD, Global Initiative for Chronic Obstructive Lung Disease.
Influence of study-level established risk factors on COPD prevalence
The second step of the stepwise binomial regression takes account of the study-level risk factors for sex, age, smoking status (current, ever, and never), and smoking amount (in total pack-years). Studies differed in their reporting formats for these risk factors. Frequently one or more of the four risk factors were omitted. Where risk factors were reported, they might appear alone or jointly (that is, cross-classified) in various combinations across the 80 studies. There are 16 possible combinations or profiles of these four risk factors. The frequencies of the different profiles are tabulated in the Supplementary material. The most common reporting profile for prevalence was a cross-classification of sex and age group (370 among the 1583 records). Two of the 16 possible profiles did not occur in our data set. A separate binomial regression was run for each of the other 14 profiles with the reported established risk factors for that profile included in the regression function. Each of the 14 regression functions also included the fitted regression function for the COPD outcome measure from step 1 as a fixed offset.
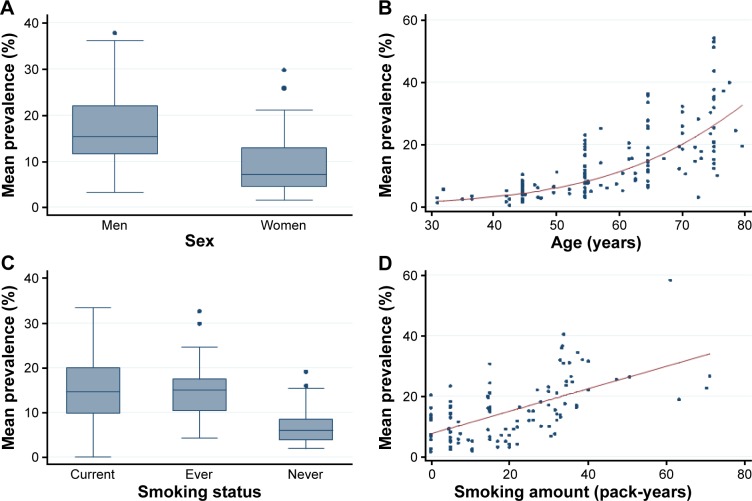
Details for these 14 regression analyses are too bulky to be reported here. Figure 2 presents an overview of the results. The figure displays plots of COPD GOLD I–IV prevalence rates for all studies that used postbronchodilator spirometry. The prevalence rates were generally higher for men than for women and rates tended to rise with age at an accelerating rate. Smokers had higher prevalence rates than those who had never smoked, and prevalence rates rose steadily with amount smoked in pack-years. A striking feature of the graphs in Figure 2 is the great amount of variability in COPD prevalence rates that is not explained by the individual established risk factors of sex, age, and smoking.
Figure 2.
Postbronchodilator COPD prevalence rates for GOLD I–IV, plotted as a function of established risk factors.
Notes: Prevalence numbers for each risk factor are averaged over all other established risk factors without weighting. Panels (A) and (C) are box plots. Panels (B) and (D) are scatter plots that also show least-squares fitted logistic and linear regression curves, respectively.
Abbreviation: GOLD, Global Initiative for Chronic Obstructive Lung Disease.
The fitted values from each of these study-level regressions were carried forward to the third regression step as fixed offsets. Carrying forward these offsets assures that the influences of COPD severity and use of postbronchodilator spirometry, and the study-level risk factors (sex, age, smoking status, and smoking amount), are taken into account before proceeding to consider country-level effects in the next regression step.
Influence of country-level risk factors on COPD prevalence
In the remaining regression steps, we consider the incremental influence of groups of country-level risk factors on prevalence rates (Table 4).
Table 4.
Results for stepwise regression of COPD prevalence on successive groups of country-level variables
| Variable | Adjusted odds ratio (95% CI) | P-value |
|---|---|---|
| Country-level established risk factors | ||
| Smoking prevalence (men, %) | 0.994 (0.993–0.995) | <0.001 |
| Smoking prevalence (women, %) | 1.012 (1.011–1.013) | <0.001 |
| Working-age men (15–64, %) | 0.922 (0.913–0.931) | <0.001 |
| Working-age women (15–64, %) | 1.061 (1.047–1.075) | <0.001 |
| Seniors (men, >64, %) | 0.954 (0.935–0.973) | <0.001 |
| Seniors (women, >64, %) | 0.968 (0.954–0.981) | <0.001 |
| Socioeconomic risk factors | ||
| Literacy (standardized) | 1.013 (1.002–1.025) | 0.024 |
| Gross national income per capita (standardized) | 1.059 (1.049–1.069) | <0.001 |
| Health system performance (WHO ranking, standardized) | 0.933 (0.922–0.944) | <0.001 |
| Environmental risk factors | ||
| CO2 emissions per capita (standardized) | 0.985 (0.977–0.993) | <0.001 |
| Vehicles per capita (standardized) | 1.009 (1.001–1.018) | 0.030 |
| Physical geography risk factors | ||
| Latitude (north or south, truncated to nearest degree) | 0.996 (0.996–0.997) | <0.001 |
| High elevation (log-elevation above 470 m) | 0.566 (0.541–0.593) | <0.001 |
| Effects of world region | ||
| Australia/New Zealand | 0.761 (0.725–0.798) | <0.001 |
| Latin America | 1.102 (1.066–1.138) | <0.001 |
| Mediterranean | 0.952 (0.928–0.976) | <0.001 |
| North America | 1.020 (1.003–1.036) | 0.018 |
| North Europe | Reference | |
| Southeast Asia | 0.983 (0.968–0.998) | 0.023 |
| Other | 1.492 (1.384–1.608) | <0.001 |
Notes: The regression analysis has already taken into account the effects of the COPD prevalence outcome variables and the study-level established risk factors in the first two regression steps before stepping in the groups of country-level variables. At each step, the regression function includes an offset covariate calculated in the preceding step. The offset therefore captures the regression effects of all risk factors that have entered in earlier steps.
a) Country-level established risk factors
In this step, we consider the six country-level smoking and age–sex variables in Table 4. These are picking up residual established risk effects for countries that are not captured by the study-level effects of sex, age, and smoking. As pointed out by Blanc et al,7 population-level variables have the ability to capture influences on COPD prevalence that might be missed by study-level variables considered alone. The regression effects for country-level smoking rates of men and women shown in Table 4 suggest that these variables influence COPD prevalence even after taking account of study-level smoking rates. For example, countrywide smoking rates in women have a small adverse residual impact on COPD prevalence not picked up by the study-level smoking variables for participants (odds ratio [OR] =1.012, 95% CI 1.011–1.013).
b) Socioeconomic risk factors
Table 4 shows that COPD prevalence was modestly elevated in countries with greater literacy (OR =1.013, 95% CI 1.002–1.025), with a lower confidence limit barely exceeding 1. COPD prevalence was higher in countries with higher per capita gross national income (OR =1.059, 95% CI 1.049–1.069) and lower in countries with more highly ranked health system performance (OR =0.933, 95% CI 0.922–0.944).
c) Environmental risk factors
CO2 emissions per capita and the number of vehicles per capita were used to estimate country-level environmental risks. Table 4 shows the adjusted effects of CO2 emissions and vehicle numbers on prevalence of COPD. We can only speculate on the behavior of these environmental variables since they represent only two facets of a complicated environmental reality. Countries with higher CO2 emissions, for example, may also be countries that have more protections in place to mitigate the impact of emission exposures on COPD prevalence, thereby giving the variable a counter-intuitive protective aspect. As for vehicles per capita, it is intuitively reasonable that having a greater density of vehicles would contribute to COPD prevalence, although the lower limit of the confidence interval barely exceeds 1.
d) Physical geography risk factors
Table 4 shows the regression contributions from two variables related to physical geography. The latitude variable indicates that countries and districts farther from the equator, whether north or south, tended to have lower COPD prevalence rates (OR =0.996, 95% CI 0.996–0.997). High elevation had a strong significant negative association with COPD prevalence (OR =0.566, 95% CI 0.541–0.593). The high elevation variable is a piecewise linear variable on a logarithmic scale. The variable is set to zero below 470 m and then allowed to vary linearly above this threshold. Figure 3 shows the change in the fitted COPD prevalence rate as a function of elevation. The change in fit is measured after all other risk factors have entered the regression function except world region. The protective effect of elevation above the 470 m threshold is evident in the figure.
Figure 3.
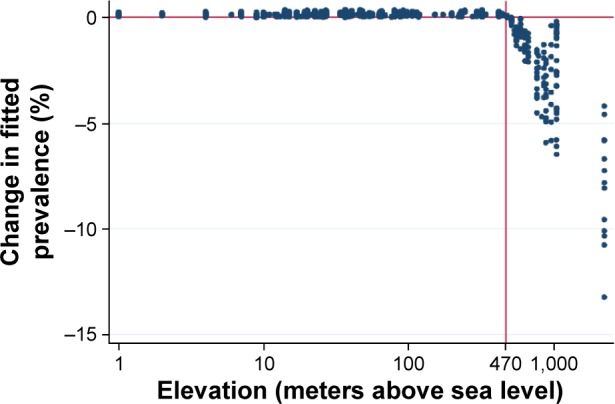
Change in fitted prevalence (in percent) with the addition of the high elevation risk factor.
Notes: Elevation (in meters above sea level) is displayed on a logarithmic scale. A vertical line is shown at the 470 m threshold where prevalence begins to decline. The prevalence change is measured after all other risk factors are incorporated in the regression model except world region.
e) Effects of world region
Table 4 shows the effect on COPD prevalence when the world region for each country is taken into account in the final regression step. Northern Europe serves as the reference category because it is the most heavily represented region in our data set. After adjustment for study-level and country-level established risk factors and country-level socioeconomic, environmental, and geographical risk factors, the adjusted ORs indicate that Australia and New Zealand (OR =0.761, 95% CI 0.725–0.798) and Mediterranean countries (OR =0.952, 95% CI 0.928–0.976) have significantly lower COPD prevalence than countries in Northern Europe. Countries in Latin America have significantly higher COPD prevalence than those in Northern Europe (OR =1.102, 95% CI 1.066–1.138). Prevalence rates of COPD in countries from North America and Southeast Asia were roughly similar to those from Northern Europe. Prevalence of COPD in countries in the other region is significantly elevated relative to those of Northern Europe (OR =1.492, 95% CI 1.384–1.608), but this category only contains two studies from Africa and a single study from Saudi Arabia, so the estimate is imprecise.
Analysis of deviance
The contribution made by each group of variables to explaining the observed variability of our 1,583 COPD prevalence rates is summarized in Table 5. A pseudo R2 value represents the fraction of the total deviance that is explained by incorporating each additional set of explanatory risk factors in the stepwise procedure. Study-level established risk factors in the 80 studies, considered alone, accounted for 64.2% of the total variation in COPD prevalence rates. This percentage increased to 71.6% with the addition of country-level established risk factors, socioeconomic variables, environmental variables, physical geography variables, and world region indicators. Thus, 7.4% points of additional explanation of variability in COPD prevalence came from the country-level risk factors in Table 4. Approximately 28% of the variability remains unexplained by any of our explanatory variables.
Table 5.
Stepwise contributions of groups of risk factors to explaining variability in COPD prevalence rates
| Risk factor groups | Variables added to the regression model | Total deviance | Pseudo R2 |
|---|---|---|---|
| Prevalence outcome measures (null regression model) | Prevalence severity, post-bronchodilation | 64,671 | 0.000 |
| Study-level established risks | Sex, age, smoking status, smoking amount | 23,170 | 0.642 |
| Country-level established risks | Sex, age, smoking status | 19,671 | 0.696 |
| Socioeconomic risks | Literacy, gross national income, health system performance | 19,409 | 0.700 |
| Environmental risks | CO2 emissions, vehicles | 19,395 | 0.700 |
| Physical geography risks | Elevation, latitude | 18,706 | 0.711 |
| World regions (full regression model) | World region | 18,397 | 0.716 |
Notes: Pseudo R2 is the proportion of total deviance explained. Deviance would be 0 if the regression model provided an exact fit to 1,583 prevalence rates from the 80 study articles.
Discussion
Our analysis of 80 COPD prevalence studies confirms that sex, age, and smoking are major determinants of COPD; indeed, about two-thirds of the variability in COPD is explained by these established risk factors for study participants. Importantly, just over 7% of variation in COPD prevalence is explained by our country-level variables, over and above participant-level risk factors. Furthermore, even after adjustment for participant risk factors and several groups of country-level risk factors, our study shows that countries in different world regions have varying residual risk levels for COPD. Importantly, 28% of the variability in COPD prevalence remains unexplained, suggesting that there are additional personal-, regional-, and country-level factors driving disease prevalence that are unaccounted for by the study-level and country-level risks we have considered.
Our study showed, as expected, that use of postbronchodilator spirometry, as specified in GOLD guidelines, lowers the estimate of COPD GOLD I–IV prevalence compared to studies that used only prebronchodilator spirometry. We incorporated this variable in the first step of our binomial stepwise regression procedure to adjust for the effect of this design feature on prevalence rates. Our findings suggest that GOLD I–IV COPD prevalence may be lowered by ~5% points by use of a bronchodilator.
We expected to find clear signals for the correlation of country-level variables with COPD prevalence. Variables describing the socioeconomic development of a country, however, had an uneven and mixed relationship with COPD prevalence. We assessed socioeconomic development using three country-level indices and found that COPD prevalence tended to be slightly higher in countries with higher literacy rates and in countries with higher gross national income per capita. COPD prevalence was lower in countries whose health systems had better performance rankings in WHO’s World Health Report 2000. A supplemental analysis (not shown here) showed that COPD prevalence was higher in countries with larger per capita health expenditures. The WHO ranking formula suggests that expenditure by itself is not an adequate measure of health system performance.
Our analysis revealed significant gaps in global coverage of COPD prevalence assessment as few or no eligible publications were found for several large regions of the world including North and Central Africa, the Arabian Peninsula, Central Asia, Eastern Europe and Russia, and South Asia. The fact that our analysis only includes studies published in English may account for some of this poor coverage. The published literature on COPD prevalence also has a potential bias caused by the fact that research funding, infrastructure, opportunities, and incentives are not uniformly distributed across regions of the globe. For example, few African countries have the financial capability to mount a prevalence study without external assistance.
Earlier studies have considered the association of elevation with COPD prevalence. Menezes et al11 observed a perfect inverse rank correlation between prevalence and altitude in the five Latin American cities in the PLATINO study, and Laniado-Laborin et al12 detected a significant inverse relationship in their study of 27 Mexican cities with altitudes ranging from 1 m to 2,680 m. Our results support an inverse relationship of COPD prevalence to elevation above a threshold of ~500 m. Whether this correlation is a physiological response to altitude or a consequence of overlaid patterns of human settlement and topography is unclear. It is possibly a combination of these influences. A study of Himalayan Sherpas, who live and work at very high elevations, shows that genetic selection, chronic hypoxia, and high levels of habitual exercise give them larger FEV1 and FVC values and, hence, possibly greater protection from developing COPD.13 The EPAS1 gene locus has been implicated in this adaptation.14
Even after adjustment for the established risk factors of sex, age, and smoking, socioeconomic development risk factors, and differences in latitude and elevation, our study shows systematic residual differences in COPD prevalence across world regions. Figure 4 shows ORs for COPD prevalence by world region, before and after adjustment for study-and country-level risks. After adjustments, the countries in the Australasia region (Australia and New Zealand) and the Mediterranean have COPD prevalence rates that are markedly lower than those in Northern Europe, while countries in Latin America have higher prevalence. The highest regional effect on prevalence is found among countries in the other category, which is dominated by one study for Capetown, South Africa. This finding raises the possibility that worrying COPD prevalence levels may be found in other African countries, but confirmation awaits further studies.
Figure 4.
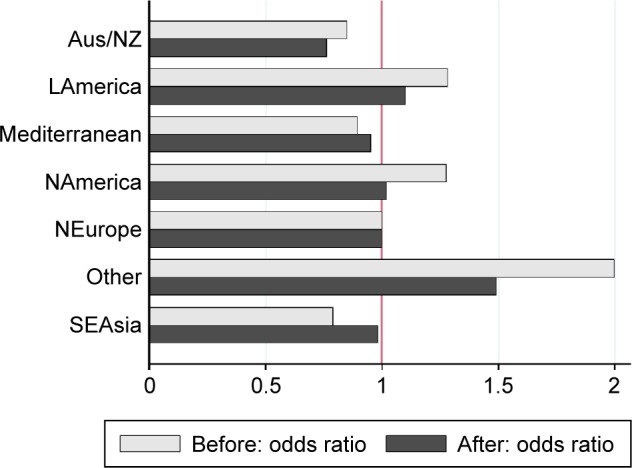
Odds ratios for COPD prevalence by world region, before and after adjustment for study- and country-level risks.
Note: Northern Europe serves as the reference region.
Abbreviations: Aus/NZ, Australia/New Zealand; LAmerica, Latin America; NAmerica, North America; NEurope, North Europe; SEAsia, Southeast Asia.
Our analysis has shown that 36% of variability in COPD prevalence rates is not explained by the established risk factors of sex, age, and smoking risk factors captured at the level of individual studies. Our limited set of country-level risk factors explain just >7% of the variability, which is a useful contribution, but much remains to be done to understand country-to-country variability in COPD prevalence rates. This persistent challenge of understanding and measuring the influence of country-level risk factors on COPD prevalence will require comprehensive international research initiatives.
Our study has some limitations. Our study aim was not to estimate a global COPD prevalence level. Rather we wanted to look at how differences in COPD prevalence in populations and subpopulations are influenced, first, by study-level variables and, then, by country-level variables. Thus, our methodology and approach are more consistent with an “ecological study” and do not constitute a meta-analysis. As such, our literature search followed predetermined inclusion criteria as described earlier, but the protocol did not provide for multiple independent searches of the literature by multiple investigators. Thus, our study does not have the benefit of an extra check on whether all potential qualified literature studies were included.
Many study-level risk factors for COPD, such as domestic and occupational exposures to smoke and fumes, could not be accommodated in our analysis because they were not covered consistently across studies. Similarly, many relevant country-level variables could not be included because of incomplete or no data, such as airborne particulate matter. Finally, some country-level variables are difficult to construct or characterize, such as cultural and ethnic factors and country climate.
Any regression analysis is a study of correlations and does not establish causes and effects. We acknowledge that observed associations between COPD prevalence and country-level variables may reflect influences of omitted variables. Thus, some confounding of attribution is present. We also caution that effects of country-level characteristics (such as literacy rates) do not necessarily apply to individuals within populations of these countries (the so-called ecological fallacy).
In matching COPD prevalence studies for different countries to country-level variables, it would be ideal to match the time period of the country-level measures with the time period of exposures that produce the COPD being discovered in the prevalence studies. This ideal match is difficult to achieve because data reporting is intermittent and data sources are incomplete. Also, the matching time periods differ for study participants in different age groups. We have selected country-level variables that are sufficiently stable historically so this timing influence is moderated. Country-level variables such as latitude, elevation, and world region, of course, are unchanging through time. Also, data sources were too coarse to allow us to match district-level prevalence data to district-level risk factors for sex, age, and smoking rates or social, economic, and environmental variables. Finally, diagnostic criteria for COPD were limited to GOLD I–IV and GOLD II–IV. Competing criteria such as the lower limit of normal were not included because they were not systematically reported.
The increments in the pseudo R2 value reported in Table 5 produced by each group of added country-level variables would be somewhat different if the stepwise procedure proceeded in a different order. For example, if world regions were added before country-level established risk factors, they would be credited with a different explanatory effect. As mentioned in the main report, we chose an order for our groups of variables that we felt proceeded from the most direct to the least direct expected effects on COPD prevalence.
Conclusion
The strong dependence of COPD prevalence on study-level risk factors of sex, age, and smoking is well established but the explanatory effects of country-level risk factors are not well known. Our study of data from 80 COPD prevalence articles covering 41 countries aimed to assess the influence of country-level risk factors on COPD prevalence. Variables representing demography, social and economic conditions, environment, physical geography, and world region were included. The analysis revealed significant contributions from these country-level risk factors, including major differences by world region. Yet, a large portion of variability in COPD prevalence remains unexplained.
Acknowledgments
The study investigators would like to thank the study participants, clinicians, and researchers worldwide who have provided the great quantity of data summarized in the articles we have selected for this analysis. This study was supported by The Canadian Respiratory Research Network (CRRN). The CRRN is an academic research network established by The Canadian Institutes of Health Research. The abstract of this paper was presented at the American Thoracic Society 2016 International Conference, May 13–18, 2016, San Francisco, CA, USA, and the abstract has been published in American Journal of Respiratory and Critical Care Medicine, Vol 193, Meeting Abstracts, 2016, A58 Epidemiology of COPD and Lung Cancer.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Buist AS, Vollmer WM, Sullivan SD, et al. The burden of obstructive lung disease initiative (BOLD): rationale and design. COPD. 2005;2(2):227–283. [PubMed] [Google Scholar]
- 2.Global Initiative for Chronic Obstructive Lung Disease (GOLD) [web-page on the Internet] Global Strategy for the Diagnosis, Management and Prevention of COPD. 2016. [Accessed July 23, 2016]. Available from: http://goldcopd.org/global-strategy-diagnosis-management-prevention-copd-2016/
- 3.Buist AS, McBurnie MA, Vollmer WM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet. 2007;370(9589):741–750. doi: 10.1016/S0140-6736(07)61377-4. [DOI] [PubMed] [Google Scholar]
- 4.Lindberg A, Jonsson AC, Rönmark E, et al. Prevalence of chronic obstructive pulmonary disease according to BTS, ERS, GOLD and ATS criteria in relation to doctor diagnosis, symptoms, age, gender, and smoking habits. Respiration. 2005;72(5):471–479. doi: 10.1159/000087670. [DOI] [PubMed] [Google Scholar]
- 5.Fullerton DG, Bruce N, Gordon SB. Indoor air pollution from biomass fuel smoke is a major health concern in the developing world. Trans R Soc Trop Med Hyg. 2008;102(9):843–851. doi: 10.1016/j.trstmh.2008.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halbert RJ, Natoli JL, Gano A, Badamgarav E, Buist AS, Mannino DM. Global burden of COPD: systematic review and meta-analysis. Eur Respir J. 2006;28(3):523–532. doi: 10.1183/09031936.06.00124605. [DOI] [PubMed] [Google Scholar]
- 7.Blanc PD, Menezes AM, Plana E, et al. Occupational exposures and COPD: an ecological analysis of international data. Eur Respir J. 2009;33(2):298–304. doi: 10.1183/09031936.00118808. [DOI] [PubMed] [Google Scholar]
- 8.Eriksson B, Lindberg A, Müllerova H, et al. Association of heart diseases with COPD and restrictive lung function – results from a population survey. Respir Med. 2013;107(1):98–106. doi: 10.1016/j.rmed.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 9.Fukuchi Y, Nishimura M, Ichinose M, et al. COPD in Japan: the Nippon COPD Epidemiology study. Respirology. 2004;9(4):458–465. doi: 10.1111/j.1440-1843.2004.00637.x. [DOI] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 11.Menezes AM, Perez-Padilla R, Jardim JRB, et al. Chronic obstructive pulmonary disease in five Latin American cities (the PLATINO study): a prevalence study. Lancet. 2005;366(9500):1875–1881. doi: 10.1016/S0140-6736(05)67632-5. [DOI] [PubMed] [Google Scholar]
- 12.Laniado-Laborin R, Rendón A, Batiz F, Alcantar-Schramm JM, Bauerle O. High altitude and chronic obstructive pulmonary disease prevalence: a casual or causal correlation? Arch Bronconeumol. 2012;48(5):156–160. doi: 10.1016/j.arbres.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Havryk AP, Gilbert M, Burgess KR. Spirometry values in Himalayan high altitude residents (Sherpas) Respir Physiol Neurobiol. 2002;132(2):223–232. doi: 10.1016/s1569-9048(02)00072-1. [DOI] [PubMed] [Google Scholar]
- 14.Beall CM, Cavalleri GL, Deng L, et al. Natural selection on EPAS1 (HIF2alpha) associated with low hemoglobin concentration in Tibetan highlanders. Proc Natl Acad Sci U S A. 2010;107(25):11459–11464. doi: 10.1073/pnas.1002443107. [DOI] [PMC free article] [PubMed] [Google Scholar]