Abstract
Background
A trans-generational influence of prenatal tobacco smoke exposure on asthma development has been proposed but the evidence remains sparse.
Methods
We examined the grandmother’s smoking when pregnant with the mother in relation to asthma outcomes in the grandchild [current asthma at 36 months (N=53,169,cases=3,013), current asthma at 7 years (N=25,394,cases=1,265) and dispensed asthma medications at 7 years in the Norwegian Prescription Database (N=45,607,cases=1,787)] within the Norwegian Mother and Child Cohort Study(MoBa). We calculated adjusted relative risks (adj. RR) and 95% confidence intervals (CI) using log binomial regression.
Results
A total of 23.5% of mothers reported that their mother smoked when pregnant with them. The grandmother’s smoking when pregnant with the mother was positively associated with asthma at 36 months [adj.RR 1.15 (95% CI: 1.06, 1.24)], asthma at 7 years [adj.RR 1.21 (95% CI: 1.07, 1.37)] and dispensed asthma medications at 7 years [adj.RR 1.15 (95% CI: 1.04, 1.26)]. This positive association did not differ significantly by the mother’s smoking status when pregnant with the child (P-values for multiplicative interaction >0.1).
Conclusion
The grandmother’s smoking when pregnant with the mother increased the risk of asthma in the grandchild independent of the mother’s smoking status. However, given limited information on the grandmother’s socio-economic status, asthma status and other factors, unmeasured confounding may be present.
Keywords: Asthma, grandmother, smoking, pregnancy
INTRODUCTION
Observational studies indicate that parental smoking is positively associated with asthma development in the child. Meta-analyses summarizing the evidence find that the most consistent positive association is seen for maternal smoking during pregnancy [1–3]. In concordance with these findings, exposure to maternal smoking during pregnancy is associated with decreased lung function both in childhood and adulthood [4–7].
Whether there are trans-generational effects of tobacco smoke exposure is an interesting question. Trans-generational inheritance of an asthma phenotype from in utero nicotine exposure has been reported in a rat model [8]. Of the two human studies that have examined whether the grandmother’s smoking when pregnant with the mother influenced asthma in the grandchild, one found a positive association while the other reported no association [9 10]. A biological plausibility exists for a trans-generational influence of prenatal tobacco smoke exposure on asthma development. Three previous studies reported that the grandmother’s smoking when pregnant with the mother might increase the grandchild’s birth weight [11–13]. Birth weight is also a predictor for asthma [14 15]. Differential methylation, an epigenetic alternation, has also been reproducibly observed in newborns whose mothers smoked during pregnancy [16]. Epigenetic mechanisms therefore constitute one underlying mechanisms which might explain a trans-generational effect of tobacco smoke exposure.
Given the limited human data on potential trans-generational effects of prenatal tobacco smoke exposure with asthma development, we examined the association between the grandmother’s smoking when pregnant with the mother and asthma development in the grandchild using the Norwegian Mother and Child Cohort Study (MoBa).
METHODS
Study population
MoBa is a prospective population-based pregnancy cohort [17 18]. MoBa recruited pregnant women between 1999 and 2008, at approximately 18 weeks gestation. The participation rate was 40.6% of all invited pregnant women. Mothers could participate with more than one pregnancy, resulting in approximately 95,200 mothers and 114,500 children. All participants gave a written informed consent. In May 2014, the 114,761 children participating in MoBa were linked to the Medical Birth Registry of Norway (henceforth referred to as “birth registry”) and the Norwegian Prescription Database (henceforth referred to as “prescription registry”) using unique personal identification numbers. Children not linked to the birth registry (n=499) and children from multiple births (n=3,971) were not eligible for the current study. We used information from questionnaires completed at 18 and 30 gestational weeks, and when the child was 6 months, 36 months and 7 years [19]. The Norwegian Data Inspectorate and the Regional Ethics Committee for South/East Norway approved this study. The study is reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) recommendations.
Exposure
Mothers in MoBa were asked “did your mother smoke when she was pregnant with you?” at approximately 18 gestational weeks. The response alternatives were “yes”, “no” and “don’t know”. This information identified the grandmother’s smoking when she was pregnant with the mother. Mothers further reported their own smoking habits during pregnancy at 18 gestational weeks, 30 gestational weeks and retrospectively when the child was 6 months. To evaluate whether the grandmother’s smoking had an independent association with asthma development in the grandchild from maternal smoking during pregnancy, we classified the children into four mutually exclusive exposure groups: not exposed to smoking, only exposed to the grandmother’s smoking, only exposed to the mother’s smoking during pregnancy and both the grandmother and the mother smoked.
Outcome
We examined two asthma outcomes based on the MoBa questionnaires and one outcome based on asthma medications as registered in the prescription registry. (1) We defined current asthma at 36 months as a “yes” by the mother to a question about current asthma in combination with reported names of inhaled glucocorticoids and/or beta-2 agonists in an open ended question of which medications the child had used the past year. (2) Current asthma at 7 years was defined based on the mother’s report that the child had experienced asthma symptoms in the past year in combination with “yes” to a closed ended question of whether the child had used any medications for asthma in the past year. (3) We classified a child as having redeemed asthma medications based on the prescription registry if there was at least one dispensed prescription for asthma medications in the past year at 7 years in addition to a second dispensed prescription within one year after the first. The prescription registry classifies medications according to the Anatomical Therapeutic Chemical (ATC) classification system. Medications classified as asthma medications included inhaled short- and long-acting beta (2)-agonists (R03AC), inhaled corticosteroids (R03BA), fixed-dose combinations of inhaled beta(2)-agonists and corticosteroids (R03AK), and leukotriene antagonists (R03DC).
Covariates/confounders
Characteristics plausibly associated with the grandmother’s smoking and child asthma development were included as potential confounders. Since we did not have information concerning socio-economic or health characteristics of the grandmother, we relied on maternal characteristics as indirect measures. Maternal age at delivery, parity (primiparous, 1, 2, and 3 or more), education (less than high school, high school, up to 4 years of college and more than 4 years of college), yearly salary (<200,000, 200,000–400,000, and >400,000 NOK), and pre-pregnancy BMI (underweight, normal weight, overweight, and obese; categorized according to WHO guidelines) were included as indicators of socio-economic status/health status. Maternal asthma was included as an indicator of genetic predisposition. The child’s birth weight was considered as a potential intermediate factor.
Statistical Analysis
We used log binomial regression to estimate relative risks (RR) and 95% confidence intervals (CI) for asthma in the grandchild related to the grandmother’s smoking when pregnant with the mother. In the first multivariable regression model we adjusted for all the potential confounding factors specified (Model 1), reporting adjusted RR (adj.RR) and 95% CI. We used robust cluster variance estimations to account for siblings. The second multivariable model adjusted for all factors included in Model 1 in addition to maternal smoking during pregnancy (Model 2). We tested for multiplicative interaction of the mother’s smoking during pregnancy by including product terms in the multivariable regression analysis. Furthermore, the grandmother’s and the mother’s smoking was evaluated in combination, classified into four mutually exclusive exposure categories. Finally, we examined the influence of adjustment for the potential intermediate factor: the child’s birth weight. In the multivariable analysis, up to 10% of observations had missing information on one or more of the covariates. To evaluate the potential influence of missing covariate information we conducted multiple imputation by chained equations, imputing a total of 20 datasets. As the results using complete case and multiple imputation were similar, we only present the measures of association from the multiple imputation analysis. The results from the complete case analysis are included in Supplement Table 2 and 3. We used inverse probability weighting to evaluate selection bias [20]. The weights reflected the probability of having the necessary follow-up information to be included in the respective analysis among all eligible children. The statistical significance level was 5%. All analyses were conducted in STATA version 13 (Statacorp, Texas).
RESULTS
Of the 110,291 eligible children, 53,169 had the follow-up information to be included in the analysis of current asthma at 36 months (Figure 1). Among these 53,169 children, 41,209 had reached 7 years by July 1st 2014, out of which 25,394 children had information from the 7 year questionnaire (Figure 1). These children were included in the analysis of current asthma at 7 years. A total of 45,607 children were included when evaluating children who had dispensed asthma medications at 7 years registered in the prescription registry (Figure 1). Supplement Table 1 indicates the distribution of characteristics within these three study populations.
Figure 1.
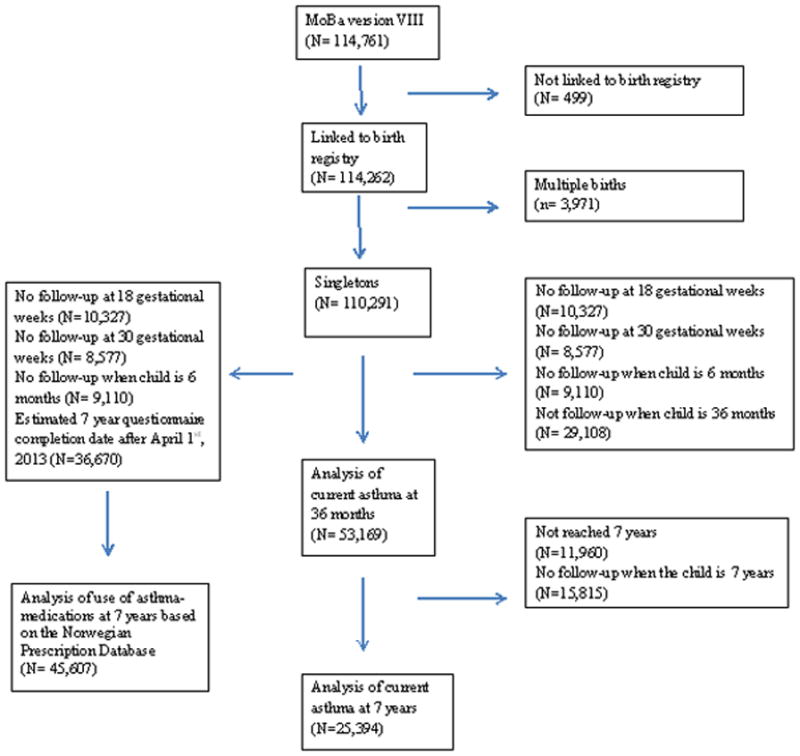
Illustration of sample selection
The grandmother’s smoking when pregnant with the mother was reported for 23.5% of children, while mothers of 66.9% of children reported that their mother did not smoke when pregnant with them, and for 9.6% of children the mother reported “don’t know”. Mothers who reported prenatal tobacco smoke exposure were younger, had a higher parity, lower education, lower salary, were more likely to be overweight/obese and more likely to have a history of asthma. The birth weight of the grandchild was also slightly higher if the grandmother had smoked when she was pregnant with the mother.
Current asthma at 36 months was reported for 5.7% of children, current asthma at 7 years for 5.1% while 4.8% had dispensed asthma medications at 7 years in the prescription registry. The grandmother’s smoking when pregnant with the mother was modestly associated with asthma at 36 months, adj.RR 1.15 (95% CI: 1.06, 1.24), asthma at 7 years, adj.RR 1.21 (95% CI: 1.07, 1.37) and dispensed asthma medications in the prescription registry, adj.RR 1.15 (95% CI: 1.04, 1.26) (Model 1, Table 2). Maternal age, pre-pregnancy BMI and asthma explained the majority of the change from the crude associations. Further adjustment for maternal smoking during pregnancy did not change the association between the grandmother’s smoking and asthma in the grandchild (Model 2, Table 2). Children of mothers who responded “don’t know” to the question regarding whether their mother smoked when pregnant with them had risk estimates that were positive but smaller than the exposed category for all outcomes (Table 2). There was no indication of multiplicative interaction of maternal smoking during pregnancy (all p-values >0.1).
Table 2.
Association between the grandmother’s smoking when pregnant with the mother and asthma development in the grandchild
| Outcome | Exposure | n, total | n, cases | Crude RR(95% CI) | Model 1 adj.RR(95% CI) | Model 2 adj. RR(95% CI) |
|---|---|---|---|---|---|---|
| Asthma at 36 months (N=53,169) | Grandmother smoked during pregnancy | |||||
| No | 34,460 | 1,843 | 1 | 1 | 1 | |
| Yes | 12,104 | 803 | 1.24 (1.15, 1.35) | 1.15 (1.06, 1.24) | 1.15 (1.06, 1.25) | |
| Don’t know | 4,933 | 307 | 1.16 (1.03, 1.31) | 1.11 (0.99, 1.24) | 1.11 (0.99, 1.24) | |
| Asthma at 7 years (N=25,394) | Grandmother smoked during pregnancy | |||||
| No | 16,301 | 759 | 1 | 1 | 1 | |
| Yes | 5,756 | 348 | 1.31 (1.15, 1.48) | 1.21 (1.07, 1.37) | 1.21 (1.07, 1.37) | |
| Don’t know | 2,276 | 129 | 1.21 (1.01, 1.46) | 1.15 (0.96, 1.39) | 1.15 (0.96, 1.39) | |
| Use of asthma medications at 7 years based on prescription registry (N=45,607) | Grandmother smoked during pregnancy | |||||
| No | 28,821 | 1,281 | 1 | 1 | 1 | |
| Yes | 11,367 | 627 | 1.24 (1.13, 1.37) | 1.15 (1.04, 1.26) | 1.13 (1.03, 1.25) | |
| Don’t know | 4,448 | 226 | 1.14 (1.00, 1.31) | 1.07 (0.93, 1.23) | 1.07 (0.93, 1.23) |
Model 1 adjusted for maternal age, maternal parity, maternal education, maternal salary, maternal pre-pregnancy BMI, and maternal asthma.
Model 2 adjusted for maternal age, maternal parity, maternal education, maternal salary, maternal pre-pregnancy BMI, maternal asthma and maternal smoking during pregnancy.
Multiple imputation of missing covariate information by chained equations conducted for all measures of association presented. There were a total of 1,069 children in the analysis of asthma at 36 months, 535 children in the analysis of asthma at 7 years and 971 children in the analysis of use of asthma medication for whom we imputed missing information on the grandmother’s smoking status. Furthermore, missing information on asthma status was imputed for 610 children with regard to current asthma at 36 months and 540 children with regard to current asthma at 7 years. For all other covariates, the amount of missing information that was imputed was less than 1.5%.
We also evaluated the grandmother’s smoking when pregnant with the mother and maternal smoking when pregnant with the child in combination (Table 3). For all three asthma outcomes, exposure to the grandmother’s smoking alone, or exposure to both the grandmother’s smoking and the mother’s smoking showed a tendency for a positive association (Table 3). Notably, there was no independent statistically significant association of exposure to maternal smoking during pregnancy alone and asthma development.
Table 3.
Association of the grandmother’s and the mother’s smoking during pregnancy with asthma development in the grandchild
| Outcome | Exposure | n, total | n, cases | Crude RR(95% CI) | Adj RR(95% CI)a |
|---|---|---|---|---|---|
| Asthma at 36 months (N=53,169) | Mother and grand maternal smoking during pregnancy | ||||
| Neither | 32,701 | 1,735 | 1 | 1 | |
| Only grandmother | 10,411 | 686 | 1.24 (1.14, 1.35) | 1.15 (1.06, 1.26) | |
| Only mother | 1,728 | 106 | 1.15 (0.94, 1.41) | 1.06 (0.87, 1.30) | |
| Both grandmother and mother | 1,685 | 117 | 1.32 (1.10, 1.59) | 1.15 (0.95, 1.40) | |
| Asthma at 7 years (N=25,394) | Mother and grand maternal smoking during pregnancy | ||||
| Neither | 15,426 | 708 | 1 | 1 | |
| Only grandmother | 4,973 | 299 | 1.32 (1.16, 1.50) | 1.23 (1.08, 1.40) | |
| Only mother | 858 | 49 | 1.30 (1.00, 1.70) | 1.17 (0.89, 1.53) | |
| Both grandmother and mother | 775 | 49 | 1.42 (1.09, 1.87) | 1.22 (0.92, 1.60) | |
| Use of asthma medications at 7 years based on prescription registry (N=45,607) | Mother and grand maternal smoking during pregnancy | ||||
| Neither | 26,600 | 1,157 | 1 | 1 | |
| Only grandmother | 9,172 | 483 | 1.22 (1.10, 1.35) | 1.14 (1.03, 1.26) | |
| Only mother | 2,180 | 122 | 1.27 (1.06, 1.53) | 1.11 (0.92, 1.34) | |
| Both grandmother and mother | 2,181 | 143 | 1.49 (1.26, 1.76) | 1.25 (1.05, 1.49) |
Adjusted for maternal age, maternal parity, maternal education, maternal salary, maternal pre-pregnancy BMI, and maternal asthma.
Multiple imputation of missing covariate information by chained equations conducted for all measures of association presented.
Values for the respondents reporting “don’t know” to the grandmother’s smoking when pregnant with the mother were imputed using multiple imputation together with all other missing covariate information. There were a total of 6,097 children in the analysis of asthma at 36 months, 2,884 children in the analysis of asthma at 7 years and 5,474 children in the analysis of use of asthma medication in the prescription database for whom we imputed missing information on the combined exposure of both grandmother and mother smoking during pregnancy.
The birth weight of the grandchild, which has been associated with the grandmother’s smoking during pregnancy, could be on the causal pathway to asthma. Further adjustment for the grandchild’s birth weight did not change the association between the grandmother’s smoking when pregnant with the mother and asthma in the grandchild.
We conducted a number of sensitivity analyses. The mother’s asthma status might be considered an intermediate factor. We therefore explored the multivariable analyses with and without adjustment for the mother’s asthma status which indicated similar associations (Supplement table 4). Further multivariable adjustment for the mother’s smoking status the first 6 months of the child’s life did not change the association between the grandmother’s smoking and asthma in the grandchild (Supplement table 4, Model 3). Likewise, multivariable adjustment for the father’s asthma status and the father’s smoking status both during pregnancy and the first 6 months of the child’s life also left the association unchanged (Supplement table 4, Model 4). Furthermore, there was no difference in the associations by the child’s gender (all p-values for interaction >0.1). Finally, the results of the analysis using inverse probability weighting also yielded similar results.
DISCUSSION
In this large-scale population based study, the grandmother’s smoking when pregnant with the mother was positively associated with asthma in the grandchild. This positive association was independent of the mother’s smoking status when pregnant with the child.
Our findings support those from the Children’s Health Study in California indicating a positive association between the grandmother’s smoking when pregnant with the mother and asthma in the grandchild by 5 years of age, adj.OR 2.1 (95% CI: 1.4–3.2) [9]. In contrast, the ALSPAC study showed no association between the grandmother’s smoking when pregnant with the mother and asthma in the grandchild by 7 years of age, adj.OR 1.01 (95% CI: 0.84–1.22) [10]. Interestingly, the ALSPAC study found an association between the grandmother’s smoking when pregnant with the father and asthma in the grandchild [10].
One potential explanation for the positive association between the grandmother’s smoking and asthma in the grandchild are trans-generational epigenetic changes. Evidence from one human study support a link between prenatal smoke exposure, DNA methylation changes and asthma related lung function [21]. Furthermore, there is some animal data that epigenetic changes due to prenatal smoke exposure might be inherited by second generation offspring with potential consequences for an asthma related phenotype [22]. In MoBa, we have previously shown that maternal smoking during pregnancy influences cord blood DNA methylation [16]. However, we did not find that the grandmother’s smoking when pregnant with the mother was associated with cord blood DNA methylation in the grandchild at loci associated with maternal smoking during pregnancy [23]. This does not exclude the possibility that the grandmother’s smoking is associated with DNA methylation in the grandchild in other areas of the genome.
Another explanation for the association between the grandmother’s smoking and asthma in the grandchild is through the lower birth weight in mothers born to smokers [24 25] which is positively associated with BMI during adulthood [26 27]. Maternal pre-pregnancy BMI is positively associated with asthma in the offspring [28–30]. In the current study, an association between the grandmother’s smoking and asthma in the grandchild remained after adjustment for the mother’s pre-pregnancy BMI. Three previous studies reported that the grandmother’s smoking when pregnant with the mother might increase the grandchild’s birth weight [11–13]. Low birth weight increases the risk of asthma [14 15]. Whether there might exist a nonlinear association between birth weight and asthma development, with higher birth weight also constituting a risk factor, is less clear. Adjustment for the grandchild’s birth weight did not change the observed associations. The association between the grandmother’s smoking and the grandchild’s birth weight might be differential by the grandchild’s gender [11]. This would have rendered our evaluation of mediation by the grandchild’s birth weight invalid. However, we found no indication of a differential association between the grandmother’s smoking and the grandchild’s birth weight by gender in MoBa.
The observed association between the grandmother’s smoking and asthma in the grandchild could reflect unmeasured confounding by the grandmother’s socio-economic status, lifestyle factors or asthma status. The ALSPAC study, which adjusted for direct measures of the grandmother’s socio-economic status, reported no association between the grandmother’s smoking when pregnant with the mother and asthma in the grandchild. The modest positive association between maternal smoking during pregnancy and asthma in the offspring observed in the crude analyses in the current study was attenuated and became non-significant after multivariable adjustment. This might support the notion that the positive association between the grandmother’s smoking and asthma in the offspring is influenced by unmeasured confounding.
The current study has additional limitations. Relying on maternal report of her own in utero tobacco smoke exposure might have resulted in misclassification. If mothers are underreporting their exposure, this could have attenuated the observed associations towards the null. In contrast, if mothers who smoke themselves are more likely to report their own prenatal tobacco smoke exposure, such a differential misclassification could have biased the associations either towards or away from the null. A large validation study using the U.S. Nurses’ Health Study indicated that the adult daughter’s report of her mother’s smoking both prenatally and during childhood had a high validity [31]. Furthermore, the reproducibility of the mother’s report that her mother’s smoked when pregnant with her has been evaluated among mothers who participated with more than one pregnancy in MoBa, yielding a kappa statistic of 0.80 [32]. Maternal self-report of her own smoking during pregnancy in MoBa compared with cotinine levels measured around 18 gestational weeks shows a high degree of validity [33]. Using maternal report of the child’s asthma status might also have resulted in misclassification. However, in MoBa maternal report that the child used asthma medications the past year on the 7 years questionnaire compared well to a dispensed asthma medication in the prescription registry [34]. The prevalence of current asthma at 36 months and 7 years in MoBa was relatively low compared to estimates from the Environment and Childhood Asthma Study in Oslo [35]. This could be due to the fairly stringent asthma definitios used in the current study and/or due to the overall characteristics of the MoBa cohort which includes relatively high educated and health conscious mothers.
A selection bias might have occurred due to the initial participation rate. Comparing MoBa participants to all pregnant women who gave birth during the MoBa inclusion period as registered in the birth registry indicated that MoBa participants were older, less likely to be single, less likely to have more than two previous deliveries and less likely to smoke [18]. However, these differences were not found to influence a number of exposure outcome associations [18]. Overall, we do not think that the initial participation rate is a strong source of selection bias. However, this could influence the generalizability of our results. The current study further required information from a large number of follow-up questionnaires. We were able to evaluate selection bias to some degree at age seven by using the prescription registry as an additional outcome. Reassuringly, we saw similar association of the grandmother’s smoking with asthma at 7 years in the grandchild classified based on questionnaire data and classified based on the prescription registry (Table 2). Furthermore, the analysis using inverse probability weighting to make the study sample more representatives of all eligible MoBa participants gave similar results.
In contrast to the Children’s Health Study, we did not find an independent positive association of maternal smoking during pregnancy with asthma development [9]. There are some potential explanations for this finding. The Norwegian government banned smoking from all public buildings in 2004. Furthermore, the Norwegian Health Directorate has conducted multiple health awareness campaigns during the MoBa enrollment period targeted at getting pregnant women to stop smoking. The proportion of women who smoke during pregnancy has gone down during the MoBa enrollment period. It is possible that the low proportion of smokers in MoBa compared to earlier studies (13–38% of mothers smoked during pregnancy in a pooled analysis of 8 European birth cohorts), attenuated the power to detect an association between maternal smoking during pregnancy and asthma seen in other studies [2].
In conclusion, the grandmother’s smoking when pregnant with the mother increased the risk of asthma in the grandchild independent of the mother’s smoking status. However, given limited information on the grandmother’s socio-economic status, asthma status and other factors, unmeasured confounding may be present.
Supplementary Material
Table 1.
Maternal characteristics and child birth weight by the grandmother’s smoking when pregnant with the mother
| Characteristic | No (n=34,859) | Yes (n=12,253) | Don’t know (n=4,988) |
|---|---|---|---|
|
| |||
| Maternal age at delivery (Mean (sd)) | 30.5 (4.4) | 30.1 (4.5) | 30.6 (4.5) |
|
| |||
| Maternal parity (%) | |||
| Primiparous | 49.1 | 45.0 | 46.7 |
| 1 | 33.4 | 36.0 | 35.1 |
| 2 | 13.7 | 15.0 | 14.6 |
| 3 or more | 3.8 | 4.1 | 3.6 |
|
| |||
| Maternal education (%) | |||
| Less than high school | 4.1 | 8.7 | 6.3 |
| High school | 23.9 | 32.9 | 30.2 |
| Up to 4 years of college | 44.4 | 40.4 | 41.4 |
| More than 4 years of college | 27.5 | 18.0 | 22.1 |
|
| |||
| Maternal yearly salary (%) | |||
| <200,000 NOK | 10.0 | 10.1 | 9.4 |
| 200,000–400,000 NOK | 54.7 | 57.5 | 57.3 |
| > 400,000 NOK | 35.3 | 32.4 | 33.3 |
|
| |||
| Maternal pre-pregnancy BMI (%) | |||
| Underweight (<18.5) | 3.1 | 2.5 | 2.9 |
| Normal weight (18.5–24.9) | 69.2 | 60.3 | 64.0 |
| Overweight (25–29.9) | 20.2 | 25.0 | 23.2 |
| Obese (>=30) | 7.4 | 12.3 | 10.0 |
|
| |||
| Maternal asthma (%) | |||
| No | 93.4 | 91.5 | 91.9 |
| Yes | 6.6 | 8.5 | 8.1 |
|
| |||
| Maternal smoking during pregnancy (%) | |||
| No | 95.0 | 86.1 | 91.2 |
| Yes | 5.0 | 13.9 | 8.8 |
|
| |||
| Child birth weight, grams (Mean (sd)) | 3599 (534) | 3626 (555) | 3605 (540) |
Frequencies based on sample used for current asthma at 36 months classified based on follow-up MoBa questionnaire.
Missing covariate information <2% for each individual covariate.
Given the large sample size, all P-values for chi-square tests (categorical covariates) and ANOVA (continuous covariates) <0.01.
What is the key question?
Does the grandmother’s smoking when pregnant with the mother influence asthma development in the grandchild?
What is the bottom line?
The grandmother’s smoking when pregnant with the mother increased the risk of asthma in the grandchild independent of the mother’s smoking status.
Why read on?
Our findings support the idea that prenatal tobacco smoke exposure might have a trans-generational influence on asthma development.
Acknowledgments
The Norwegian Mother and Child Cohort Study is supported by NIH (NIH/NIEHS contract number N01-ES-75558, NIH/NINDS grant no.1 UO1 NS 047537-01 and grant no.2 UO1 NS 047537-06A1) and the Norwegian Research Council/FUGE (grant number 151918/S10). Supported in part by the Intramural Research Program of the NIH, NIEHS (ZIA ES049019). Ms. Magnus is also supported by the Norwegian Extra-Foundation for Health and Rehabilitation (grant number 2011.2.0218). We are grateful to all families participating in the Norwegian Mother and Child Cohort Study.
References
- 1.Burke H, Leonardi-Bee J, Hashim A, et al. Prenatal and passive smoke exposure and incidence of asthma and wheeze: systematic review and meta-analysis. Pediatrics. 2012;129(4):735–44. doi: 10.1542/peds.2011-2196. [DOI] [PubMed] [Google Scholar]
- 2.Neuman A, Hohmann C, Orsini N, et al. Maternal smoking in pregnancy and asthma in preschool children: a pooled analysis of eight birth cohorts. Am J Respir Crit Care Med. 2012;186(10):1037–43. doi: 10.1164/rccm.201203-0501OC. [DOI] [PubMed] [Google Scholar]
- 3.Vork KL, Broadwin RL, Blaisdell RJ. Developing asthma in childhood from exposure to secondhand tobacco smoke: insights from a meta-regression. Environ Health Perspect. 2007;115(10):1394–400. doi: 10.1289/ehp.10155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilliland FD, Berhane K, Li YF, et al. Effects of early onset asthma and in utero exposure to maternal smoking on childhood lung function. Am J Respir Crit Care Med. 2003;167(6):917–24. doi: 10.1164/rccm.200206-616OC. [DOI] [PubMed] [Google Scholar]
- 5.Hayatbakhsh MR, Sadasivam S, Mamun AA, et al. Maternal smoking during and after pregnancy and lung function in early adulthood: a prospective study. Thorax. 2009;64(9):810–14. doi: 10.1136/thx.2009.116301. [DOI] [PubMed] [Google Scholar]
- 6.Li YF, Gilliland FD, Berhane K, et al. Effects of in utero and environmental tobacco smoke exposure on lung function in boys and girls with and without asthma. Am J Respir Crit Care Med. 2000;162(6):2097–104. doi: 10.1164/ajrccm.162.6.2004178. [DOI] [PubMed] [Google Scholar]
- 7.Moshammer H, Hoek G, Luttmann-Gibson H, et al. Parental smoking and lung function in children: an international study. Am J Respir Crit Care Med. 2006;173(11):1255–63. doi: 10.1164/rccm.200510-1552OC. [DOI] [PubMed] [Google Scholar]
- 8.Rehan VK, Liu J, Sakurai R, et al. Perinatal nicotine-induced transgenerational asthma. American journal of physiology Lung cellular and molecular physiology. 2013;305(7):L501–7. doi: 10.1152/ajplung.00078.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li YF, Langholz B, Salam MT, et al. Maternal and grandmaternal smoking patterns are associated with early childhood asthma. Chest. 2005;127(4):1232–41. doi: 10.1378/chest.127.4.1232. [DOI] [PubMed] [Google Scholar]
- 10.Miller LL, Henderson J, Northstone K, et al. Do grandmaternal smoking patterns influence the aetiology of childhood asthma? Chest. 2013;145(6):1213–8. doi: 10.1378/chest.13-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller LL, Pembrey M, Davey SG, et al. Is the growth of the fetus of a non-smoking mother influenced by the smoking of either grandmother while pregnant? PLoS One. 2014;9(2):e86781. doi: 10.1371/journal.pone.0086781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Misra DP, Astone N, Lynch CD. Maternal smoking and birth weight: interaction with parity and mother’s own in utero exposure to smoking. Epidemiology. 2005;16(3):288–93. doi: 10.1097/01.ede.0000158198.59544.cf. [DOI] [PubMed] [Google Scholar]
- 13.Rillamas-Sun E, Harlow SD, Randolph JF., Jr Grandmothers’ Smoking in Pregnancy and Grandchildren’s Birth Weight: Comparisons by Grandmother Birth Cohort. Matern Child Health J. 2013;18(7):1691–8. doi: 10.1007/s10995-013-1411-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bjerg A, Hedman L, Perzanowski M, et al. A strong synergism of low birth weight and prenatal smoking on asthma in schoolchildren. Pediatrics. 2011;127(4):e905–e12. doi: 10.1542/peds.2010-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kindlund K, Thomsen SF, Stensballe LG, et al. Birth weight and risk of asthma in 3–9-year-old twins: exploring the fetal origins hypothesis. Thorax. 2010;65(2):146–49. doi: 10.1136/thx.2009.117101. [DOI] [PubMed] [Google Scholar]
- 16.Joubert BR, Haberg SE, Nilsen RM, et al. 450K epigenome-wide scan identifies differential DNA methylation in newborns related to maternal smoking during pregnancy. Environ Health Perspect. 2012;120(10):1425–31. doi: 10.1289/ehp.1205412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magnus P, Irgens LM, Haug K, et al. Cohort profile: the Norwegian Mother and Child Cohort Study (MoBa) Int J Epidemiol. 2006;35(5):1146–50. doi: 10.1093/ije/dyl170. [DOI] [PubMed] [Google Scholar]
- 18.Nilsen RM, Vollset SE, Gjessing HK, et al. Self-selection and bias in a large prospective pregnancy cohort in Norway. Paediatr Perinat Epidemiol. 2009;23(6):597–608. doi: 10.1111/j.1365-3016.2009.01062.x. [DOI] [PubMed] [Google Scholar]
- 19.Norwegian Institute of Public Health. The Norwegian Mother and Child Cohort Study Questionnaires. Secondary The Norwegian Mother and Child Cohort Study Questionnaires. 2014 [cited 01.09.14]. http://www.fhi.no/eway/default.aspx?pid=238&trg=MainArea_5811&MainArea_5811=5903:0:15,3138:1:0:0:::0:0.
- 20.Sheikh K. Investigation of selection bias using inverse probability weighting. Eur J Epidemiol. 2007;22(5):349–50. doi: 10.1007/s10654-007-9131-4. [DOI] [PubMed] [Google Scholar]
- 21.Patil VK, Holloway JW, Zhang H, et al. Interaction of prenatal maternal smoking, interleukin 13 genetic variants and DNA methylation influencing airflow and airway reactivity. Clin Epigenetics. 2013;5(1):22. doi: 10.1186/1868-7083-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rehan VK, Liu J, Naeem E, et al. Perinatal nicotine exposure induces asthma in second generation offspring. BMC Med. 2012;10:129. doi: 10.1186/1741-7015-10-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joubert BR, Haberg SE, Bell DA, et al. Maternal smoking and DNA methylation in newborns: In utero effect or epigenetic inheritance? Cancer Epidemiol Biomarkers Prev. 2014;23(6):1007–17. doi: 10.1158/1055-9965.EPI-13-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walsh RA. Effects of maternal smoking on adverse pregnancy outcomes: examination of the criteria of causation. Hum Biol. 1994;66(6):1059–92. [PubMed] [Google Scholar]
- 25.Wang X, Tager IB, Van VH, et al. Maternal smoking during pregnancy, urine cotinine concentrations, and birth outcomes. A prospective cohort study. Int J Epidemiol. 1997;26(5):978–88. doi: 10.1093/ije/26.5.978. [DOI] [PubMed] [Google Scholar]
- 26.Gluckman PD, Hanson MA, Morton SM, et al. Life-long echoes--a critical analysis of the developmental origins of adult disease model. Biol Neonate. 2005;87(2):127–39. doi: 10.1159/000082311. [DOI] [PubMed] [Google Scholar]
- 27.Harding JE. The nutritional basis of the fetal origins of adult disease. Int J Epidemiol. 2001;30(1):15–23. doi: 10.1093/ije/30.1.15. [DOI] [PubMed] [Google Scholar]
- 28.Haberg SE, Stigum H, London SJ, et al. Maternal obesity in pregnancy and respiratory health in early childhood. Paediatr Perinat Epidemiol. 2009;23(4):352–62. doi: 10.1111/j.1365-3016.2009.01034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harpsoe MC, Basit S, Bager P, et al. Maternal obesity, gestational weight gain, and risk of asthma and atopic disease in offspring: a study within the Danish National Birth Cohort. J Allergy Clin Immunol. 2013;131(4):1033–40. doi: 10.1016/j.jaci.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 30.Pike KC, Inskip HM, Robinson SM, et al. The relationship between maternal adiposity and infant weight gain, and childhood wheeze and atopy. Thorax. 2013;68(4):372–79. doi: 10.1136/thoraxjnl-2012-202556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simard JF, Rosner BA, Michels KB. Exposure to cigarette smoke in utero: comparison of reports from mother and daughter. Epidemiology. 2008;19(4):628–33. doi: 10.1097/EDE.0b013e3181761cbd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cupul-Uicab LA, Ye X, Skjaerven R, et al. Reproducibility of reported in utero exposure to tobacco smoke. Annals of Epidemiology. 2011;21(1):48–52. doi: 10.1016/j.annepidem.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kvalvik LG, Nilsen RM, Skjaerven R, et al. Self-reported smoking status and plasma cotinine concentrations among pregnant women in the Norwegian Mother and Child Cohort Study. Pediatr Res. 2012;72(1):101–07. doi: 10.1038/pr.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Furu K, Karlstad O, Skurtveit S, et al. High validity of mother-reported use of antiasthmatics among children: a comparison with a population-based prescription database. J Clin Epidemiol. 2011;64(8):878–84. doi: 10.1016/j.jclinepi.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lodrup Carlsen KC, Haland G, Devulapalli CS, et al. Asthma in every fifth child in Oslo, Norway: a 10-year follow up of a birth cohort study. Allergy. 2006;61(4):454–60. doi: 10.1111/j.1398-9995.2005.00938.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


