Abstract
The human Solute Carrier (SLC) transporters are important targets for drug development. Structure-based drug discovery for SLC transporters requires the description of their structure, dynamics, and mechanism of interaction with small molecule ligands and ions. The recent determination of atomic structures of human SLC transporters and their homologs, combined with improved computational power and prediction methods have led to an increased applicability of structure-based drug design methods for human SLC members. In this review, we provide an overview of the SLC transporters’ structures and transport mechanisms. We then describe computational techniques, such as homology modeling and virtual screening that are emerging as key tools to discover chemical probes for human SLC members. We illustrate the utility of these methods by presenting case studies in which rational integration of computation and experiment was used to characterize SLC members that transport key nutrients and metabolites, including the amino acid transporters LAT-1 and ASCT2, the SLC13 family of citric acid cycle intermediate transporters, and the glucose transporter GLUT1. We conclude with a brief discussion about future directions in structure-based drug discovery for the human SLC superfamily, one of the most structurally and functionally diverse protein families in human.
SLC Structure
In recent years, the SLC transporters have been increasingly acknowledged as key biopharmaceutical targets.1-3 Due to limited number of high resolution structures of the human SLC members, most efforts for developing chemical probes or drugs against these proteins have focused on ligand-based approaches such as pharmacophore modeling and quantitative structure-activity relationship (QSAR).2, 4-6 These approaches have been used to develop ligands for transporters such as the Apical Sodium Dependent Bile Acid Transporter (ASBT/SLC10A2)7 and Multidrug and Toxin Extrusion Transporter 1 (MATE1, SLC47A1),8 as well as to guide the discovery of tofogliflozin, an SGLT2 (SLC5A2) ligand currently prescribed to treat type-2 diabetes in Japan.9 However, ligand-based methods are limited in their ability to capture molecules that are chemically distinct from known ligands and to describe the molecular interactions between the transporter and its ligand. Conversely, computational approaches that rely on three-dimensional structures of proteins can be useful for identifying novel chemical scaffolds with virtual screening of large compound libraries,10 or optimizing lead compounds or known drugs with methods such as Molecular Dynamics (MD) simulations and free energy calculations.11 Moreover, structural characterization of transporters in complex with small molecule ligands and drugs can contribute to the understanding of the effect of nonsynonymous polymorphyisms (nsSNPs) on transporter function including drug binding or transport.12 For example, a homology model of MATE2-K, a H+/organic cation antiporter primarily found in the kidney, was used to rationalize the effects of four nsSNPs on its ability to transport cationic substrates and protons.13
Productive application of structure-based drug design methods includes the characterization of SLC structures in different conformational states and the description of their mode of interaction with small molecules and ions. Recent advancements in experimental techniques such as X-ray crystallography, electron microscopy, and NMR,14 as well as improved computational methods and computer power,15, 16 have facilitated the characterization of human SLC transporters with structure-based approaches, as demonstrated by the growing number of such published studies.1-3, 12, 17, 18 Currently there are experimentally determined high-resolution structures of only four human SLC members representing three human SLC families. They include the Glucose transporters GLUT119 and GLUT320 (SLC2A1 and SLC2A3, respectively), the Anion exchanger 121 (AE1; SLC4A1), and the ammonium channel or Rh protein (RhCG; SLC42A3).22 However, in recent years there has also been a surge in the number of atomic structures of SLC homologs from a variety of eukaryotes (e.g. the Drosophila dopamine transporter DAT (SLC6A4))23 and prokaryotes (e.g., the Shewanella oneidensis di-/tri- peptide transporter PEPTso (an SLC15 homolog))24. Many of these structures share sequence identity of ~30% or more and conserved binding site with their human homologs, thereby providing useful templates for elucidating the substrate specificity of the human SLC members. For example, the aspartate transporter GltPh, from the Achaean organism Pyrococcus horikoshii,25 shares sequence identity of 24%-35% with the human SLC1 family of glutamate or neutral amino acid transporters. Models of human SLC1 members including the glutamate transporter EAAT3 (SLC1A1)26 and the neutral amino acid transporters ASCT1 (SLC1A4)27, 28 and ASCT2 (SLC1A5)29 based on GltPh structures have revealed mechanisms of ion coordination26, 27 and differential amino acid selectivity28 in this family; ASCT2 models were also subjected to successful ligand discovery30 and optimization29 campaigns.
Unlike other functionally defined “Superfamilies” such as ABC transporters and GPCRs, the SLC members are highly diverse in structure, consisting of a variety of folds, many of which are unlikely to be evolutionarily related to each other. For example, the two structural folds predicted to be the most common in the human SLCs are the structurally dissimilar Major Facilitator Superfamily (MFS) and the Leucine transporter (LeuT)-like folds (Fig. 1A,C). In brief, the MFS fold usually contains 12 transmembrane helices (TM) arranged into two inverted structural pseudo-repeats of six TMs. The MFS fold is one of the most common membrane protein folds in nature and includes various human families such as the glucose facilitative transporters GLUTs/SLC2 and the proton oligopeptide cotransporter family SLC15. The core of a LeuT-like fold possesses 10 TMs, with two five-TMs inverted pseudo-repeats, where the first two TMs in each of the two repeats (i.e., the ‘bundle domain’) are tilted relative to the three remaining TMs of the repeat (i.e., ‘scaffold domain’). This fold is adopted by multiple biomedically important families such as the SLC5 family of Na+/glucose transporters and the SLC6 family of Na+/Cl−-dependent neurotransmitter transporters
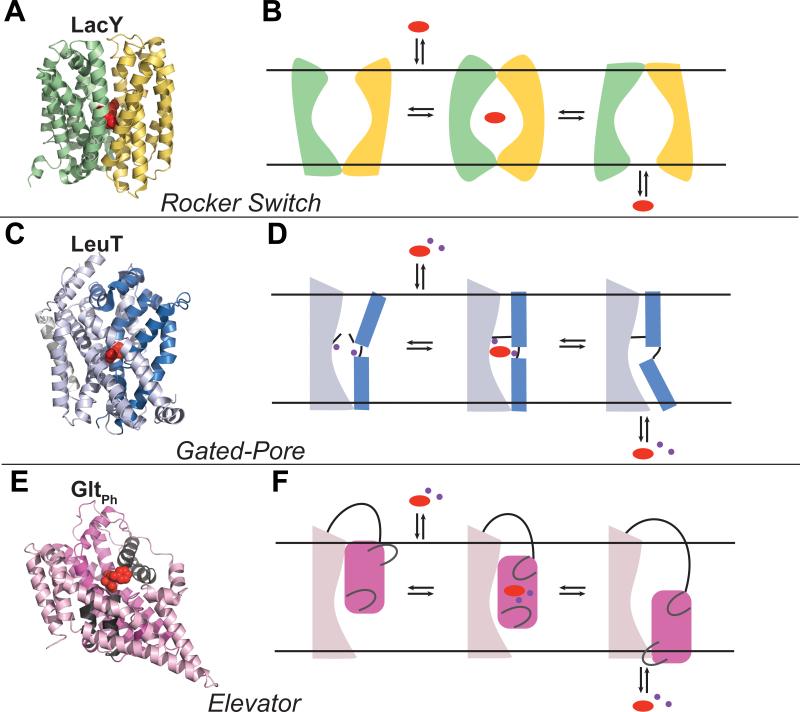
Figure 1. Transport mechanism models.
In each case, a representative structure of a transporter that uses the mechanism described is shown in (A, C, E) and three states of the transport cycle of the associated transporter are depicted in (B, D, F): outward-open, occluded and inward-open. Rocker Switch. (A) LacY is shown in an outward-open bound conformation (PDB: 4OAA137) with the N- and C-terminal halves in green and yellow cartoons respectively, and the substrate in red spheres. (B) The substrate binds to a V-shape conformation facing the extracellular side of the membrane, triggering an intermediate occluded state. The substrate is then released from an inverted V-shape inward-open conformation. Gated-pore. (C) LeuT is shown an outward-open bound conformation (PDB: 4FXZ138), with the scaffold and bundle domains represented in light and dark blue cartoons respectively, the substrate and ions in red and purple spheres. (D) The scaffold domain remains static, whereas the bundle domain experiences conformational changes to bind and release the substrate. The binding site is enclosed by a thin gate (i.e., a salt bridge) on the extracellular side, and a thick gate (TM1) on the intracellular side. Elevator. (E) GltPh is shown in an outward-open bound conformation (PDB: 2NWW25), with the oligomerization and transport domain represented in light pink and magenta cartoons, respectively, and the two gates (HP1 and HP2) in gray cartoons. The inhibitor is shown in red spheres, and sodium ions in purple spheres. (F) The oligomerization domain remains invariant while the transport domain moves in a piston-like movement to transport the substrate from the extracellular side to the intracellular side of the membrane.
SLC Function
Human SLC transporters belonging to structurally dissimilar families or folds can have chemically similar substrates. For example, cellular concentration of glucose in human is mediated by three SLC families: the SLC2, facilitative transporters with an MFS fold; the SLC5, secondary active sodium-dependent transporters with a LeuT-like fold; and SLC50A1/SWEET,31 a glucose uniporter with a unique fold of 7 TMs. Conversely, SLC transporters belonging to the same fold, or even family, do not always share substrate specificity, ion stoichiometry, or energy coupling mechanism (e.g. antiport vs. contransport/symport). For example, the SLC22 family consists of organic cation transporters (OCTs), organic zwitterion/cation transporters (OCTNs), and organic anion transporters (OATs);32 members of this family can be uniporter facilitative transporters, anion exchangers (OATs), and Na+/zwitterion cotransporters (OCTNs). Therefore, annotating the function of a transporter including its potential small molecule ligands, based on its homolog requires detailed structural characterization. For instance, the human SLC6 family members such as the serotonin, norepinephrine, and dopamine transporters (SERT, NET, and DAT, respectively) transport monoamines and share a conserved binding site with LeuT, a transporter of amino acids such as alanine and leucine that are chemically distinct from monoamines. Docking of NET ligands and decoys against the template structure LeuT identified NET's ligands as accurately as random indicating that the human NET model is required for functional annotation of its ligands and structure based ligand discovery.33
The SLC members mediate movement across the membrane using a variety of energy coupling mechanisms; they include secondary active transporters, ion channels (the ammonium channel SLC42), and other membrane proteins such as SLC51B and members from the SLC3 family that do not have transport capability on their own and interact with other SLC members (SLC51A and SLC7 members, respectively) to form functional heterodimers. Here we focus on SLC transporters that transport via secondary active transport (e.g., antiporters and symporters) and facilitative transport that utilize the electrochemical gradient of ions and substrates across the cell membrane. Transport by SLC transporters is a dynamic process generally described by the ‘alternating access’ model. In the alternating access mechanism the transporter alternates between outward and inward facing conformations, with multiple intermediate states, such as an occluded state, where the binding site is not accessible from either side of the membrane. A description of the transport function by transporters includes the identifications of domain movement and interaction, as well as transporter interactions with ions and ligands. Notably, structures of distinct states of the transport cycle can guide the design of conformation-specific ligands that can stabilize a particular conformation.
Three types of alternating access mechanisms have been described for SLC transporters (Fig. 1): i) Rocker-switch. The N- and C- terminal halves of the transporter oscillate back and forth from an outward facing state to an inward facing state along the symmetry axis perpendicular to the membrane. Transporters with an MFS fold have been proposed to transport substrates via the rocker-switch mechanism, where the majority of the studies characterizing this mechanism have been performed on the E. coli Lactose Permease LacY (Fig. 1A,B).34, 35 ii) Gated-pore. The binding site of the transporter is enclosed by two gates.36 The opening of the outward-facing gate allows the binding of the substrate, which is then released into the cell by the opening of the second gate that faces the cytosol. Proteins of the LeuT-like fold transport solutes via the gated-pore mechanism, as was demonstrated for LeuT with multiple X-ray structures37, 38 as well as with other techniques such as FRET39 and EPR40 (Fig. 1C,D). iii) Elevator. The domain containing the binding site, which is also referred as transport domain, moves along the axis perpendicular to the membrane, whereas an oligomerization domain remains static (Fig. 1 E,F). The elevator mechanism has been proposed based on the unique structure of GltPh, which was determined as a trimer in various conformations.41-44 Recent structures of the Vibrio cholerae INDY (VcINDY) suggest that this protein also utilizes a similar elevator mechanism.45, 46 Interestingly, GltPh includes two gates facilitating the binding and releasing of the substrate, similarly to the gated-pore mechanism, but it is not clear whether the presence of dynamic gates is a general theme among elevator transporters.
Homology modeling and virtual screening
Structure-based drug design approaches are particularly useful for identifying novel chemical scaffolds (e.g., with virtual screening) and for optimizing lead molecules or known drugs. Moreover, characterizing distinct conformational states of target proteins and protein complexes enables us to identify conformation- and interface-specific modulators, which are likely to be more selective toward the target relative to homologs as well as modulate specific function of the protein. Once initial hits are identified, more computationally expensive methods such as MD simulations and free energy calculations can be applied to evaluate binding affinity for ligand optimization.47-51 For a protein target without an experimentally determined structure, structure-based drug discovery can be performed with homology models that are based on experimentally determined structures of related proteins.1, 46, 47 Homology modeling typically includes four steps: template selection (or fold assignment), template–target alignment, model building, and model evaluation.52 More recently, homology modeling has been combined with molecular docking to optimize models for protein-ligand complementarity and structure-based drug design.53 This approach has been applied to discover novel ligands for a variety of challenging membrane protein targets including GPCRs54, 55 and SLC transporters.30, 33, 56-59
In brief, in template selection, a related known structure is identified through sequence search. Threading algorithms that use observed or predicted secondary structure elements as well as methods that rely on multiple sequences (e.g., profiles) are usually more sensitive than methods that use sequence information alone (e.g., BLAST60). Interestingly, although a reliably assigned template typically shares sequence identity of at least 40% with the target, many SLC transporters share the same fold, despite a lower sequence identity (~10%).12, 58
Fold assignment programs typically provide target-template alignment, however, the alignment may be suboptimal, especially for those targets that share sequence identity of 30% or lower with their template structures.52 Thus, additional alignment tools are often used to improve the initial alignment. For example, Promals3D61 combines structure and sequence information as well as user-defined constraints to align multiple structure and/or sequences; AlignMe62 is a membrane protein alignment program that relies on membrane proteins substitution matrices, hydrophobicity scales and secondary structure predictions. For challenging targets such as many SLC members, it is recommended to use multiple methods, visually inspect the results, and manually adjust the alignment, which can help minimize gaps in the transmembrane helices or align functionally important residues and motifs.52
Once a template-target alignment is constructed, a homology model of the protein target is built. MODELLER,63 for example, relies on satisfaction of spatial restraints derived from the template structure guided by the template-target alignment, as well as stereochemical restraints. Initial homology models often contain a variety of errors resulted from the sequence divergence between the target and the template, such as erroneous side chain packing, distortion of correctly aligned regions, and errors in regions without a template, such as nonconserved loop regions.52 Thus, the top-ranked models are visually inspected and may be refined. For instance, the sidechains of residues constituting the target binding site can be reoriented on a fixed backbone to face the binding pocket (e.g., with the sidechain modeling program SCWRL464).
Evaluation of the model's accuracy is essential for correct interpretation of the model. For example, accurate modeling of the sidechains conformation of the binding site can be critical for generating meaningful results in virtual screening.65 There are multiple approaches and servers that assess homology models. Some methods evaluate stereochemical properties such as chirality, bond lengths, and torsion angles (e.g., PROCHECK66). Other, more common approaches include knowledge-based statistical potentials that evaluate the environment of each residue in the model with respect to the environment found in experimentally determined structures. For example, Z-DOPE67 is a normalized atomic distance-dependent statistical potential implemented in MODELLER. ProQM68 is a knowledge-based method developed for membrane proteins, by taking into account specific features, such as the topology and the absolute Z-coordinate (i.e., the distance from the membrane center).
Furthermore, a model can be evaluated for its utility in virtual screening and structure-based drug design. Docking can be performed against the model to evaluate the ability of the model and docking program to discriminate the known ligands among decoy molecules (i.e., compounds with physical properties similar to those of known ligands, but are different topologically). For example, enrichment plots that show the percentage of known ligands docked on the y-axis as a function of the top-ranked subset of the entire database on the x-axis, are generated, and the Area Under the Curve (AUC) and LogAUC are calculated.69 Decoys can be generated using the Directory of Useful Decoys Enhanced70 (DUD-E), and the set of known ligands and decoys is docked against the binding site of the model. Such enrichment calculations can estimate whether a model is sufficiently accurate for virtual screening as well as a guide to optimize the model for drug design; by iteratively generating large number of binding site conformers and calculating the enrichment, the models are optimized for protein-ligand complementarity. If a desirable enrichment is observed, the model is subjected to virtual screening in which a virtual library of small organic molecules, such as those in ZINC,71 is docked against the protein target and ranked based on their complementarity to the target binding site.
Docking programs differ mainly by their sampling algorithm and scoring functions. For example, in OpenEye FRED72 (Fast Rigid Exhaustive Docking), the flexibility of the ligands is explored by generating the conformers prior to the docking, which are then rigidly docked into the receptor during the screening. Various scoring functions are implemented in FRED. For instance, the default Chemgauss4 uses smoothed Gaussian potentials to measure the shape and chemical complementarities of the ligand to the binding site.72 In DOCK,73, 74 the shape of the binding site is described by a set of spheres and the ligands are then matched to these spheres using a graphical algorithm. The scoring function is derived by a sum of van der Waals, Poisson-Boltzmann electrostatics and ligand desolvation penalty terms.74, 75 Finally, compounds are often selected for experimental testing based on visual inspection of the top-ranked prediction, to discard molecules with incorrect ionization states, tautomers, or strained conformations.
Notably, SLC transporters are dynamic molecules that populate different conformational states during transport. Because the majority of the current docking methods do not take into account the flexibility of the transporter (i.e., ‘rigid docking’), they are often limited in their applicability to human SLC members. Therefore, for rigid docking applications, the conformational space of the binding site can be explored prior to docking, using approaches such as normal mode analysis,53 sidechain modeling,64 or MD simulations, which can be performed with implicit or explicit membrane.76, 77 For example, when using MD simulations, the snapshots of the resulting trajectories can be clustered to generate representative conformers that are subjected to virtual screening which are then processed. Alternatively, some docking programs (e.g. GOLD78) incorporate sidechain flexibility in the binding site residues during docking. For example, Jurik et al used a combination of MD simulations and flexible docking to explore the mode of interaction of the GABA transporter (GAT1/SLC6A1) with the anticonvulsant drug Tiagabine.76 The snapshots resulting from the MD simulations, combined with ligand docking, were initially used to build a pharmacophore model for Tiagabine and its analogs. They then performed a pharmacophore-based virtual screening, which was followed by experimental testing, to identify thyroid hormones as novel GAT1 ligands.76 Recently, induced fit docking (IFD) has been implemented in the widely-used Schrödinger suite.79 IFD is constituted of three steps. First, the ligand is docked, recording multiple binding poses. Second, the sidechains of the binding site and the ligand are minimized for each of these poses. Third, the ligand is re-docked in the minimized conformations and the poses are ranked according to a scoring function. This method has been employed in combination with mutagenesis experiments, to propose a unique binding mode of the anti-depressant drug Vortioxetine in homology models of the human serotonin transporter (SLC6A4/SERT).80
In addition, one relevant limitation of ligand docking is that it cannot accurately predict binding affinity, which can be critical for generating useful structure activity relationship (SAR) models. Methods such as linear interaction energy81 (LIE), Free Energy Perturbation82 (FEP), and thermodynamic integration83 (TI) can directly estimate binding affinity with varying precision and computing time. Although these methods are more accurate than docking, due to their expensive computing time, it is impractical to screen large libraries such as ZINC against a target protein. Moreover, the estimated energy of these methods depends on the precise configuration of the binding site, which may not be optimal in a low resolution homology model of a human SLC transporter. Recently, Samsudin et al have combined calculations with LIE and TI, with uptake experiments, to rationalize the substrate specificity of the di- and tri- peptide transporter PepTSt from Streptococcus thermophiles, prokaryotic homolog of the human PepT1 (SLC15A1).84 For example, they found that the N-terminal residue of a di-peptide substrate determines its selectivity, and proposed that di-peptide substrates with basic amino acids have similar binding modes to those of tri-peptides. Interestingly, the predicted affinity for the human PepT1 based on a homology model that was built using the PepTSt structure as template did not correlate with experimentally determined cellular uptake of substrates, suggesting that the quality of the protein structure or model determines the accuracy of the predicted affinities. Notably, no known structure-based computational method can distinguish between substrates that get transported by the transporter and high-affinity inhibitors that bind the target without getting transported.85 Taken together, these studies suggest that using computational techniques and analyzing their output should be done with careful understanding of their limitations. We now discuss case studies in which integration of computational methods and experimental testing were used to characterize a variety of nutrient and metabolite SLC transporters.
Neutral amino acid exchangers: ASCT2 and LAT-1
Amino acids are essential molecules that are involved in numerous biological processes such as protein synthesis, neurotransmitter synthesis, or cell signaling. Multiple human SLC transporter families mediate the transport of amino acids, including members of the SLC1, SLC6, SLC7, SLC17, SLC32, SLC38, and SLC43 families.86, 87 The amino acid transporters have been previously grouped into ‘systems’ based on the amino acid type that they transport;27, 28 however, this classification scheme may include evolutionarily unrelated members which can be confusing in the fold assignment step during modeling. For example, System L amino acid transporters (LAT) transport Leucine and other large neutral amino acids, and include members of the SLC7 (LAT-1 and LAT-2) and SLC43 families (LAT-3 and LAT-4), which are predicted to have LeuT-like and MFS folds, respectively.
Notably, malfunctions in amino acid transporters have been associated with a variety of diseases and disorders.2 In cancer, cells can undergo a metabolic shift toward anaerobic glycolysis, resulting in decreased energy yield per glucose molecule.88-90 The cancer cell relies on increasing the uptake and metabolism of glucose and amino acids such as glutamine and leucine, to support the increased energy consumption and nutrition. Therefore, the dependence of cancer cells on sugar and glutamine metabolic pathways provides appealing new therapeutic targets such as GLUT1 and the neutral amino acid transporters ASCT2 (SLC1A5) and LAT-1 (SLC7A5), which function cooperatively in such reprogrammed metabolic networks.91 Specifically, LAT-1 exports glutamine that has been imported to the cell by ASCT2 in exchange for importing additional amino-acid nutrients (e.g., leucine), which control cell proliferation via the mammalian target of rapamycin (mTOR) pathway.92 These two exchangers are up-regulated in various types of cancers91 such as triple-negative breast cancer,93, 94 prostate cancer,95, 96 or glioblastoma multiforme (GBM),91 and have become well-pursued targets for cancer therapy. A potential drug or chemical probe targeting ASCT2 and LAT-1 can be a potent inhibitor blocking nutrient uptake, or a substrate interacting with a different target within the cell (e.g., the metabolic enzyme glutaminase (GLS)).97 Here, we discuss recent structure-based drug discovery studies of LAT-1 and ASCT2.
ASCT2
The Alanine Serine Cysteine Transporter 2 (ASCT2) is an exchanger of neutral amino acids such as serine, threonine and glutamine, typically found in various peripheral tissues such as the lung and kidney.98 It belongs to the SLC1 family, which includes the neutral amino acid ASCT1 (SLC1A4) and five glutamate transporters (Excitatory Amino Acid Transporters, EAATs). We recently modeled ASCT230 based on the structures of the glutamate transporter homolog GltPh from P. horikoshii in occluded and outward-open conformations.25 The new models reveal a conserved binding site between GltPh and ASCT2, in agreement with previous studies.29 In brief, the binding site consists of two hairpin loops, HP1 on the intracellular side and HP2 on the extracellular side, which likely act as gates that allow the release and binding of the substrate, similarly to the corresponding loops in GltPh. In the occluded conformation, the binding site is buried by the closed HP2, whereas the opening of this hairpin reveals an additional a pocket, “pocket A”, specific to the outward-open conformation (Fig. 2).
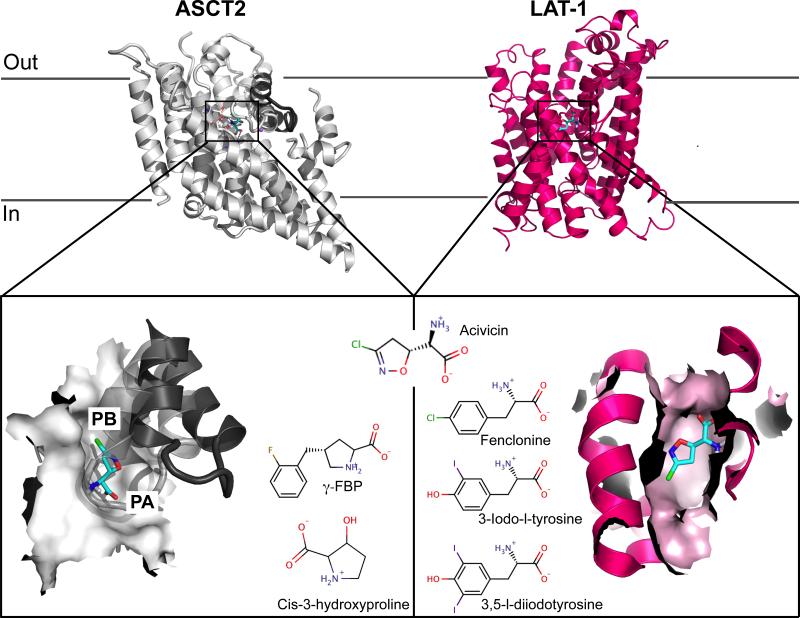
Figure 2. Homology models of the amino acid transporters ASCT2 and LAT-1 bound to acivicin.
The top panel shows both transporters viewed from the membrane bilayer plane. ASCT2 is represented in gray cartoons, with the HP2 gate in the outward-open conformation shown in dark gray cartoons. LAT-1 is represented in pink cartoons. The bottom panels show the binding site of each transporter, represented in surface, with acivicin docked. Pockets A and B revealed by the models of ASCT2 are labeled PA and PB, respectively. The newly discovered ligands are shown next to their respective targets.
In addition, a key amino acid substitution in the binding site between GltPh and ASCT2 contributes to the different substrate specificities among these transporters (acidic amino acid ligands in GltPh, vs. neutral amino acid ligands in ASCT2, respectively): Arg397 in GltPh is replaced with Cys467 in ASCT2, thereby (i) creating an additional pocket (“pocket B”) that increases the size of the binding site and changes its shape (Fig. 2); and (ii) changing the electrostatic potential of the binding site from basic in GltPh to neutral/hydrophobic in ASCT2. Importantly, the newly discovered pockets A and B can be targeted via conformation-specific small molecule probes for ASCT2.29
Virtual screening using the ASCT2 models, followed by experimental testing with electrophysiological methods, revealed five activators and two inhibitors for this protein.30 For example, aminooxetane-3-carboxylate is a more efficient activator than any known natural or unnatural ASCT2 substrate. Moreover, three of these compounds were chemically different from known ASCT2 ligands (e.g., acivicin; Fig. 2) or chemically similar to experimentally verified non-ligands. For example, the activator cis-3-hydroxyproline (Km of 190 μM) and inhibitor γ-FBP (Km of 87 μM) are both proline derivatives, yet proline is a confirmed non-ligand of ASCT2. These results enable us to hypothesize about the chemical basis for discriminating ASCT2 inhibitors from activators. For the activator cis-3-hydroxyproline, the hydroxyl group at Cβ of the proline interacts with residue Asn487 that also interacts with all other activators, which are all relatively small molecules. Conversely, for the inhibitor γ-FBP, the bulky fluorobenzyl group at the Cγ of the proline likely enhances the affinity of the molecule through π-π and fluorine-peptide bond interactions, thereby stabilizing an inactive conformation.
LAT-1
LAT-1 is a sodium-independent transporter that forms heterodimers with the glycoprotein 4F2hc (SLC3A2) via a disulfide bridge to import large neutral amino acids (e.g., leucine and phenylalanine) across the cell membrane in exchange of intracellular amino acids (e.g., glutamine).99, 100 It was suggested that the affinity of LAT-1 for its intracellular substrates is higher than the extracellular amino acids, indicating that the transport rate is mediated by intracellular substrates.101 LAT-1 is mainly located in the blood-brain-barrier (BBB)102 where it mediates the transport of nutrients and drugs into the central nervous system (CNS). Thus, LAT-1 is a relevant target for the design of drugs with optimal BBB permeability against CNS targets. Furthermore, the expression of LAT-1 is increased in variety of cancers, particularly in the early stages of cancer development, where high leucine concentration induces cell proliferation via the mTOR pathway.95, 103 LAT-1 is also upregulated together with LAT-3 in prostate cancer, suggesting that targeting multiple transporters simultaneously via polypharmacology might be a useful strategy for cancer therapy.95, 104
LAT-1 belongs to the acid/polyamine/organo-cation transporter (APC) family and is predicted to adopt a LeuT-like fold with 12 TMs. Recently, we built a homology model of LAT-157 based on the arginine/agmatine transporter AdiC from E. coli in an outward-occluded substrate-bound conformation (Fig. 2).105 The majority of the transporter-ligand interactions observed in the X-ray structure of AdiC are predicted to be conserved in our model. However, key substitutions in the binding site of LAT-1 result in a larger and more hydrophobic binding site than that of the template structure, rationalizing the different binding specificities of the two transporters. Virtual screening of drug and metabolite libraries from KEGG106 followed by cis-inhibition assay revealed four compounds that inhibit the transport of a radiolabeled substrate.57 Trans-stimulations experiment showed that 3,5-diiodo-L-tyrosine and 3-iodo-L-tyrosine are LAT-1 inhibitors whereas the other two ligands, acivicin and fenclonine are substrates (Fig. 2). This suggests that these substrates may cross the BBB via LAT-1, to exert their positive and negative therapeutic effects, respectively. Interestingly, two compounds were shown to decrease proliferation of a GBM cell line via distinct mechanisms. They include the inhibitor 3-iodo-L-tyrosine, which likely obtains its effect via nutrient deprivation, and the substrate acivicin, which achieves its cytotoxic effect by targeting multiple intracellular targets such as aldehyde deshydrogenase ALDH4A1,107 an enzyme involved in cell viability.108
Facilitative glucose transporter: GLUT1
D-glucose is an essential energy source that powers biological functions. The cellular concentration of glucose is regulated in part by the facilitative glucose transporters (GLUTs) of the SLC2 family, which includes 14 members.109 Although the primary substrates and physiological functions of over half of the GLUTs are not well characterized, GLUT1 (or SLC2A1) has been studied extensively as the prototype transporter of this family.110 GLUT1 is responsible for maintaining the basal glucose level in most cell types. It is highly expressed in erythrocytes111 and in barrier tissues such as the epithelial cells of blood-brain barrier.112 Abnormal expression and genetic variations in GLUT1 have been associated with a variety of metabolic disorders113, 114 as well an in altered metabolism in cancer.115 Thus, GLUT1 has emerged as a key transporter of biomarkers for cancer detection (e.g., 18F-DG116), and an attractive target for cancer therapy via nutrient deprivation.117-119
The atomic structure of the human GLUT1 confirmed that it adopts an MFS fold with 12 transmembrane helices.19 This landmark structure was solved in an inward-open ligand-free conformation with the sugar-binding site exposed to the cytoplasm (Fig. 3A). It shares similar fold and conformation to its homolog, the xylose transporter XylE.120, 121 Importantly, XylE structures have also been determined with glucose in multiple other conformations including the partially occluded outward-open conformation (the ‘occluded’ conformation, Fig. 3B), which has a compact, solvent excluded sugar-binding site.122 The recent structures of the human GLUT3,20 and rat and bovine GLUT5,123 in various conformations ranging from outward-open, outward-occluded, and inward-open in different ligand-bound states, show similar MFS fold and conformation repertoire. These structures also provide useful templates for modeling GLUT1 in various states of the transport cycle, and support the postulation that GLUTs transport solutes via a “rocker-switch” alternating-access mechanism.35, 124, 125
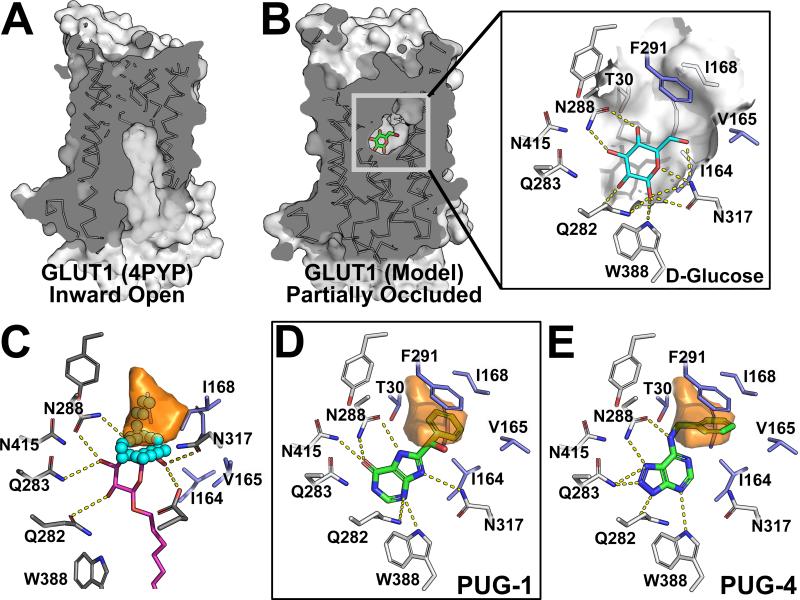
Figure 3. Structure-based drug discovery targeting the occluded conformation of the human GLUT1.
Comparison of GLUT1 in (A) crystal structure in an inward-open conformation (PDBID: 4PYP) and (B) homology model in an occluded conformation with the glucose-binding site highlighted in surface representation. (C) The H-pocket, lined by hydrophobic residues (blue sticks), observed in the occluded model (orange volume) is larger than in the inward-open structure (cyan spheres), e.g. 4PYP (grey sticks and magenta alkyl sugar analog). (D and E) Ligands (green sticks) discovered through virtual screening against the occluded GLUT1 model (white sticks) occupy the glucose-binding site and the H-pocket (orange volume).
Our recent study of the human GLUT1 using an integrated computational and experimental approach revealed a potentially druggable pocket adjacent to the sugar-binding site and previously unknown chemical probes for this transporter.126 We analyzed atomic structures of GLUT1 and its bacterial homolog XylE, and generated models of GLUT1 based on the ligand-bound, occluded XylE structures.122 Structural analysis of the GLUTs and XylE structures, and the newly constructed GLUT1 models, revealed a hydrophobic pocket, termed the H-pocket (Fig. 3C). Mutations in key residues that make up the H-pocket have resulted in reduced transport activity by GLUT1. In brief, this pocket constitutes part of the sugar-binding site and is lined by conserved, hydrophobic residues Gly27, Thr30, Ile164, Val165, Ile168, and Phe291. The size and shape of this pocket vary according to the conformational state: the occluded conformation has larger H-pocket (4GC0: 37.5Å3) than the inward-open conformation (4PYP: 10.0 Å3).
Virtual screening against the occluded GLUT1 models followed by experimental testing with cis-inhibition assays yielded novel and potent ligands for this transporter. In particular, of the 19 molecules that were tested for their ability to inhibit [3H]-2-DG uptake in CHO cells overexpressing GLUT1, eight compounds exhibited significant level of specific inhibition close to the known GLUT1 inhibitor phloretin at 50 μM. These newly identified GLUT1 ligands are predicted to form critical hydrogen bonds similar to those made by glucose, the primary substrate of GLUT1, and may utilize the H-pocket for binding (Fig. 3B-E).
The newly discovered ligands can be grouped into the following four groups based on their chemical structures: (i) xanthine-derivative compounds (Fig. 3D) that are related to caffeine, a known noncompetitive inhibitor of GLUT1 with Ki ~ 1.5 mM;127, 128 (ii) N-substituted adenine-derivative compounds, which have a purine core similar to adenine and a hydrophobic substitution at the 1-amino position (Fig. 3E); (iii) glycosylated polyphenolic compounds that are likely to form hydrogen bonds similar to those formed with the natural GLUT1 substrates; (iv) pyridazinone-like compounds, small fragments with high ratio of carbon-to-heteroatom that bear chemical features different from those described above. Notably, one compound belonging to group (i) (Fig. 3D), exhibits IC50 of 450 nM, while all other ligands inhibit GLUT1 with IC50 values in the low μM range (12-60 μM). Furthermore, six of the confirmed inhibitors are chemically different from published GLUT1 inhibitors. This study expands the chemical space of GLUT1 inhibitors, as well as describes a novel druggable pocket in GLUT1 that can potentially be targeted with structure-based drug design.
The Na+/di-tri carboxylates transporters of the SLC13 family
Intermediates from the citric acid cycle (CAC) such as citrate and succinate, regulate multiple metabolic processes, including fatty acid synthesis, glucose metabolism, and renal function.129-131 In humans, CAC intermediates are transported into cells by three Na+-dependent transporters from the SLC13 family,132 including NaDC1 (SLC13A2), NaDC3 (SLC13A3), and NaCT (SLC13A5), which have different tissue distributions. Genetic variations in these transporters have been associated with multiple metabolic disorders, aging, type-2 diabetes, and epilepsy, and they have been proposed to be drug targets for a variety of metabolic disorders.133 Despite the high sequence similarity among the SLC13 members, especially in their ligand binding region, they exhibit differences in substrate specificity and substrate:Na+ stoichiometry. For instance, NaDC1 transports citrate2− and four-carbon dicarboxylates, whereas NaDC3 has a broader substrate specificity and can transport dicarboxylates with larger chemical groups. NaCT is specific for citrate3− in which all three carboxy groups are deprotonated and charged.134
The recently solved X-ray structure of a SLC13 homolog VcINDY in a citrate- and Na+-bound inward-open conformation45 facilitated characterization of the human family with computational methods.56, 59 We used homology modeling and virtual screening combined with a variety of cell-based assays, including site-directed mutagenesis, cis-inhibition, and transport specificity ratio experiments, to improve our understanding of the structural components that are important for substrate specificity in the SLC13 family.56, 59 In brief, similarly to the VcINDY structure, the human SLC13 models consist of a highly conserved binding site with two hairpin loops HPin and HPout, each containing a Serine-Asparagine-Threonine (SNT) motif that interacts with the ligand and at least one Na+ (Fig. 4). For example, Thr485 of the C-terminal SNT motif in NaDC3 is critical for substrate binding and transport, as well as Na+ coordination.59 This position is variable within the SLC13 family, corresponding to a valine in NaDC1 and NaCT and may play a role in substrate specificity. Our models revealed other key residues in the binding site, such as Thr253 in NaDC3, which is essential for substrate transport. Interestingly, in NaCT, the mutation of the corresponding residue (Thr227) to a methionine as well as other mutations including T142M of the N-terminal SNT motif, have been identified in patients with neonatal epileptic encephalopathy.135 In fact, it has been shown that these mutations result in a loss of citrate transport,135 in agreement with the role of these residues in substate binding proposed by modeling and mutagenesis.59 Specifically, the sidechains of these threonine residues are predicted to make hydrogen bonds with the subsrate and ion which are expected to be lost in the variants (Fig. 4B). Overall, these results highlight the utility of modeling in rationalizing mutations’ effects on function and disease.
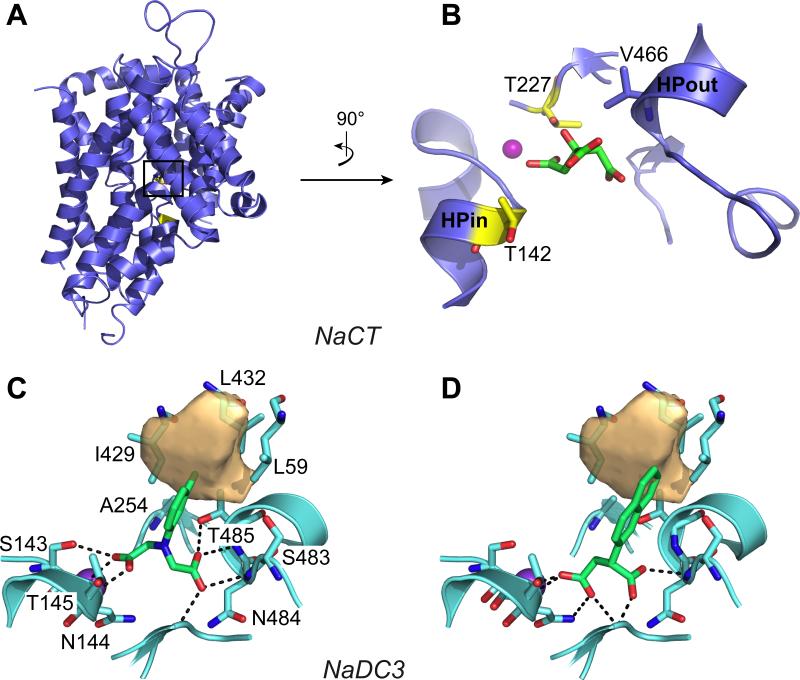
Figure 4. Homology models of the human transporter NaCT (SLC13A5) and NaDC3 (SLC13A3).
(A) The NaCT model in an inward-open conformation is shown in blue cartoon within the membrane plane. The NaCT binding site, with citrate (green sticks) and Na+ (purple sphere) is shown in (B). The position of the Na+ is derived from the X-ray structure of the template structure VcINDY; the coordinates of citrate are predicted with docking. The two binding site residues (yellow sticks) whose mutations are associated with epileptic encephalopathy are shown. The predicted binding pose of two of the newly discovered SLC13 ligands (green sticks) in NaDC3 are shown in (C) and (D). The NaDC3 binding site is represented in cyan cartoons, with the residues interacting with the ligands in sticks. The hydrophobic pocket specific to this transporter is shown in light orange volume.
In addition, our group has recently performed structure/function characterization of five human and mouse SLC13 transporters.56 Virtual screening was conducted against NaDC1 and NaDC3, and top predicted compounds were tested experimentally in five SLC13 members from human and mouse. Three key findings emerged from this study. First, two variable binding site residues among the SLC13 members likely contribute to define substrate specificity. The first is Ala254 in NaDC3, which is in close proximity to the C-terminal SNT motif and is a part of a hydrophobic pocket unique to this protein (Fig. 4 C,D); and the second residue is the third residue of the C-terminal SNT motif (Thr485 in NaDC3, Val477 in NaDC1 and Val466 in NaCT).
Second, we identified nine inhibitors for SLC13 members with IC50 values in the μM range and varying specificities. All new ligands contain dicarboxylate and aromatic rings with carbon chain in different lengths, similarly to new NaCT inhibitors found in a recent study.136 These ligands interact with a hydrophobic subpocket in NaDC3 (e.g., with Leu59, Leu429, Leu432, Fig. 4C,D), suggesting a previously unknown mode of interaction between SLC13A3 and its ligands. The size and carbon chain lengths of the newly discovered dicarboxylate ligands correlated with their preference to a particular transporter. It is likely that the combined subtle differences affect the overall hydrophobicity and shape of their corresponding binding sites, which is reflected in the binding specificities of their ligands. Notably, some differences in inhibitor specificity between the five SLC13 members from human and mouse could not be predicted by the models, highlighting the need for new structures in additional conformations.
Conclusions and future directions
In human, there are 418 SLC transporters important for a variety of functions such as ion and nutrient uptake into the cell, or drug absorption, distribution, metabolism and excertion (ADME). Therefore, SLCs have been the subjects of increasing number of studies to develop drugs targeting these transporters directly or drugs interacting with SLC transporters for optimal ADME properties.
We first provided an overview of the SLC transporters structure and function (Fig. 1), as well as a description of the computational techniques commonly used to characterize these transporters. We then presented examples of how these computational methods were used in ligand discovery for SLC targets, primarily focusing on the identification of new low-affinity hits that interact with the substrate binding site of the transporter (Figs. 2-4). High quality structures of new SLC targets are solved at a rapid pace, and combined with improved computational methods, these advances enable us to develop structure-activity relationship (SAR) of compounds and targets and optimize useful lead molecules. The iterative process of evaluating binding of new analogs using modern computational methods such as free energy estimation and experimental testing, progressively refines homology models and advances our understanding of ligands activities. For example, models of ASCT2 (Fig. 2), GLUT1 (Fig. 3) and NaDC3 (Fig. 4) revealed unique hydrophobic pockets that can provide a framework for developing chemical probes with improved affinity and specificity.
In addition, in polypharmacology a drug or chemical probe interacts with multiple targets to obtain an optimal therapeutic effect. For example, many CNS drugs such as Selective Serotonin Reuptake Inhibitors (SSRIs) interact with numerous GPCRs and transporter targets. While the majority of these polypharmacological probes have been optimized using ligand-based approaches, due to the improvement of computational resources and our understanding of transporter structure and function, such polypharmacological probes can now potentially be optimized with structure-based drug design. For example, high resolution structures of DAT and homologs in complex with a variety of CNS drugs can be combined with computational modeling for optimization of compounds with improved therapeutic index.
Finally, characterization of the distinct conformations of SLC transporters during the transport cycle as well as determination of higher order oligomeric structures that some SLC members adopt, can provide novel approaches for modulating SLC activities and unique chemical tools to further characterize their physiological roles. For instance, potential interface-specific modulators that stabilize or disrupt SLC heterodimers (e.g., LAT-1 and SLC3A2) are likely to be chemically different from the natural substrates (amino acids) and are thus expected to exhibit improved specificity. Allosteric compounds can also be used as chemical tools to elucidate the dynamics of the transporters. For example, an allosteric modulator targeting the interface between the transport and oligomerization domains in elevator transporters (e.g., the SLC1 family) can potentially inhibit transport by stabilizing a specific state.
ACKNOWLEDGMENTS
We are thankful to OpenEye Scientific Software Inc. for granting us access to its high-performance molecular modeling applications through its academic license program. This study was supported by a grant from the National Institutes of Health (R01 GM108911) and a grant from the Department of Defense (W81XWH-15-1-0539).
Footnotes
The authors declare no competing interests.
REFERENCES
- 1.Schlessinger A, Khuri N, Giacomini KM, Sali A. Curr Top Med Chem. 2013;13:843–856. doi: 10.2174/1568026611313070007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin L, Yee SW, Kim RB, Giacomini KM. Nature reviews. Drug discovery. 2015;14:543–560. doi: 10.1038/nrd4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cesar-Razquin A, Snijder B, Frappier-Brinton T, Isserlin R, Gyimesi G, Bai X, Reithmeier RA, Hepworth D, Hediger MA, Edwards AM, Superti-Furga G. Cell. 2015;162:478–487. doi: 10.1016/j.cell.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 4.Bareford LM, Swaan PW. Advanced drug delivery reviews. 2007;59:748–758. doi: 10.1016/j.addr.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang C, Ekins S, Bahadduri P, Swaan PW. Adv Drug Deliv Rev. 2006;58:1431–1450. doi: 10.1016/j.addr.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, Dahlin A, Evers R, Fischer V, Hillgren KM, Hoffmaster KA, Ishikawa T, Keppler D, Kim RB, Lee CA, Niemi M, Polli JW, Sugiyama Y, Swaan PW, Ware JA, Wright SH, Yee SW, Zamek-Gliszczynski MJ, Zhang L. Nature Reviews Drug Discovery. 2010;9:215–236. doi: 10.1038/nrd3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng X, Pan Y, Acharya C, Swaan PW, Polli JE. Bioconjugate chemistry. 2010;21:2038–2048. doi: 10.1021/bc100273w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wittwer MB, Zur AA, Khuri N, Kido Y, Kosaka A, Zhang X, Morrissey KM, Sali A, Huang Y, Giacomini KM. Journal of medicinal chemistry. 2013 doi: 10.1021/jm301302s. DOI: 10.1021/jm301302s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohtake Y, Sato T, Kobayashi T, Nishimoto M, Taka N, Takano K, Yamamoto K, Ohmori M, Yamaguchi M, Takami K, Yeu SY, Ahn KH, Matsuoka H, Morikawa K, Suzuki M, Hagita H, Ozawa K, Yamaguchi K, Kato M, Ikeda S. Journal of medicinal chemistry. 2012;55:7828–7840. doi: 10.1021/jm300884k. [DOI] [PubMed] [Google Scholar]
- 10.Shoichet BK. Nature. 2004;432:862–865. doi: 10.1038/nature03197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jorgensen WL. Science. 2004;303:1813–1818. doi: 10.1126/science.1096361. [DOI] [PubMed] [Google Scholar]
- 12.Schlessinger A, Yee SW, Sali A, Giacomini KM. Clin Pharmacol Ther. 2013;94:19–23. doi: 10.1038/clpt.2013.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi JH, Yee SW, Ramirez AH, Morrissey KM, Jang GH, Joski PJ, Mefford JA, Hesselson SE, Schlessinger A, Jenkins G, Castro RA, Johns SJ, Stryke D, Sali A, Ferrin TE, Witte JS, Kwok PY, Roden DM, Wilke RA, McCarty CA, Davis RL, Giacomini KM. Clinical Pharmacology and Therapeutics. 2011;90:674–684. doi: 10.1038/clpt.2011.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bill RM, Henderson PJ, Iwata S, Kunji ER, Michel H, Neutze R, Newstead S, Poolman B, Tate CG, Vogel H. Nature biotechnology. 2011;29:335–340. doi: 10.1038/nbt.1833. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Wen PC, Moradi M, Tajkhorshid E. Current opinion in structural biology. 2015;31:96–105. doi: 10.1016/j.sbi.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Enkavi G, Li J, Mahinthichaichan P, Wen PC, Huang Z, Shaikh SA, Tajkhorshid E. Methods in molecular biology. 2013;924:361–405. doi: 10.1007/978-1-62703-017-5_14. [DOI] [PubMed] [Google Scholar]
- 17.Venkatesan S, Saha K, Sohail A, Sandtner W, Freissmuth M, Ecker GF, Sitte HH, Stockner T. PLoS computational biology. 2015;11:e1004551. doi: 10.1371/journal.pcbi.1004551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stockner T, Montgomery TR, Kudlacek O, Weissensteiner R, Ecker GF, Freissmuth M, Sitte HH. PLoS computational biology. 2013;9:e1002909. doi: 10.1371/journal.pcbi.1002909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng D, Xu C, Sun P, Wu J, Yan C, Hu M, Yan N. Nature. 2014;510:121–125. doi: 10.1038/nature13306. [DOI] [PubMed] [Google Scholar]
- 20.Deng D, Sun P, Yan C, Ke M, Jiang X, Xiong L, Ren W, Hirata K, Yamamoto M, Fan S, Yan N. Nature. 2015;526:391–396. doi: 10.1038/nature14655. [DOI] [PubMed] [Google Scholar]
- 21.Arakawa T, Kobayashi-Yurugi T, Alguel Y, Iwanari H, Hatae H, Iwata M, Abe Y, Hino T, Ikeda-Suno C, Kuma H, Kang D, Murata T, Hamakubo T, Cameron AD, Kobayashi T, Hamasaki N, Iwata S. Science. 2015;350:680–684. doi: 10.1126/science.aaa4335. [DOI] [PubMed] [Google Scholar]
- 22.Gruswitz F, Chaudhary S, Ho JD, Schlessinger A, Pezeshki B, Ho CM, Sali A, Westhoff CM, Stroud RM. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:9638–9643. doi: 10.1073/pnas.1003587107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Penmatsa A, Wang KH, Gouaux E. Nature. 2013;503:85–90. doi: 10.1038/nature12533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newstead S, Drew D, Cameron AD, Postis VL, Xia X, Fowler PW, Ingram JC, Carpenter EP, Sansom MS, McPherson MJ, Baldwin SA, Iwata S. EMBO Journal. 2011;30:417–426. doi: 10.1038/emboj.2010.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boudker O, Ryan RM, Yernool D, Shimamoto K, Gouaux E. Nature. 2007;445:387–393. doi: 10.1038/nature05455. [DOI] [PubMed] [Google Scholar]
- 26.Tao Z, Rosental N, Kanner BI, Gameiro A, Mwaura J, Grewer C. The Journal of biological chemistry. 2010;285:17725–17733. doi: 10.1074/jbc.M110.121798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scopelliti AJ, Heinzelmann G, Kuyucak S, Ryan RM, Vandenberg RJ. The Journal of biological chemistry. 2014;289:17468–17479. doi: 10.1074/jbc.M114.565242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scopelliti AJ, Ryan RM, Vandenberg RJ. The Journal of biological chemistry. 2013;288:8250–8257. doi: 10.1074/jbc.M112.441022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Albers T, Marsiglia W, Thomas T, Gameiro A, Grewer C. Molecular pharmacology. 2012;81:356–365. doi: 10.1124/mol.111.075648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Colas C, Grewer C, Otte NJ, Gameiro A, Albers T, Singh K, Shere H, Bonomi M, Holst J, Schlessinger A. PLoS computational biology. 2015;11:e1004477. doi: 10.1371/journal.pcbi.1004477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wright EM. Molecular aspects of medicine. 2013;34:183–196. doi: 10.1016/j.mam.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 32.Koepsell H, Endou H. Pflugers Archiv : European journal of physiology. 2004;447:666–676. doi: 10.1007/s00424-003-1089-9. [DOI] [PubMed] [Google Scholar]
- 33.Schlessinger A, Geier E, Fan H, Irwin JJ, Shoichet BK, Giacomini KM, Sali A. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:15810–15815. doi: 10.1073/pnas.1106030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Radestock S, Forrest LR. Journal of molecular biology. 2011;407:698–715. doi: 10.1016/j.jmb.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 35.Kaback HR, Smirnova I, Kasho V, Nie Y, Zhou Y. The Journal of membrane biology. 2011;239:85–93. doi: 10.1007/s00232-010-9327-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Forrest LR, Rudnick G. Physiology (Bethesda) 2009;24:377–386. doi: 10.1152/physiol.00030.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh SK, Piscitelli CL, Yamashita A, Gouaux E. Science. 2008;322:1655–1661. doi: 10.1126/science.1166777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E. Nature. 2005;437:215–223. doi: 10.1038/nature03978. [DOI] [PubMed] [Google Scholar]
- 39.Singh SK, Pal A. Methods in enzymology. 2015;557:167–198. doi: 10.1016/bs.mie.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Penmatsa A, Gouaux E. The Journal of physiology. 2014;592:863–869. doi: 10.1113/jphysiol.2013.259051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reyes N, Ginter C, Boudker O. Nature. 2009;462:880–885. doi: 10.1038/nature08616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verdon G, Boudker O. Nat Struct Mol Biol. 2012;19:355–357. doi: 10.1038/nsmb.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verdon G, Oh S, Serio RN, Boudker O. eLife. 2014;3:e02283. doi: 10.7554/eLife.02283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yernool D, Boudker O, Jin Y, Gouaux E. Nature. 2004;431:811–818. doi: 10.1038/nature03018. [DOI] [PubMed] [Google Scholar]
- 45.Mancusso R, Gregorio GG, Liu Q, Wang DN. Nature. 2012;491:622–626. doi: 10.1038/nature11542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mulligan C, Fitzgerald GA, Wang DN, Mindell JA. The Journal of general physiology. 2014;143:745–759. doi: 10.1085/jgp.201311141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuhn B, Gerber P, Schulz-Gasch T, Stahl M. Journal of medicinal chemistry. 2005;48:4040–4048. doi: 10.1021/jm049081q. [DOI] [PubMed] [Google Scholar]
- 48.Heinzelmann G, Bastug T, Kuyucak S. The journal of physical chemistry. B. 2013;117:5486–5496. doi: 10.1021/jp4010423. [DOI] [PubMed] [Google Scholar]
- 49.Grazioso G, Limongelli V, Branduardi D, Novellino E, De Micheli C, Cavalli A, Parrinello M. Journal of the American Chemical Society. 2012;134:453–463. doi: 10.1021/ja208485w. [DOI] [PubMed] [Google Scholar]
- 50.Gu Y, Shrivastava IH, Amara SG, Bahar I. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:2589–2594. doi: 10.1073/pnas.0812299106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shrivastava IH, Jiang J, Amara SG, Bahar I. The Journal of biological chemistry. 2008;283:28680–28690. doi: 10.1074/jbc.M800889200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marti-Renom MA, Stuart AC, Fiser A, Sanchez R, Melo F, Sali A. Annual review of biophysics and biomolecular structure. 2000;29:291–325. doi: 10.1146/annurev.biophys.29.1.291. [DOI] [PubMed] [Google Scholar]
- 53.Carlsson J, Coleman RG, Setola V, Irwin JJ, Fan H, Schlessinger A, Sali A, Roth BL, Shoichet BK. Nature Chemical Biology. 2011;7:769–778. doi: 10.1038/nchembio.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cooke RM, Brown AJ, Marshall FH, Mason JS. Drug discovery today. 2015;20:1355–1364. doi: 10.1016/j.drudis.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 55.Congreve M, Langmead C, Marshall FH. Advances in pharmacology. 2011;62:1–36. doi: 10.1016/B978-0-12-385952-5.00011-7. [DOI] [PubMed] [Google Scholar]
- 56.Colas C, Pajor AM, Schlessinger A. Biochemistry. 2015;54:4900–4908. doi: 10.1021/acs.biochem.5b00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Geier EG, Schlessinger A, Fan H, Gable JE, Irwin JJ, Sali A, Giacomini KM. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:5480–5485. doi: 10.1073/pnas.1218165110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schlessinger A, Matsson P, Shima JE, Pieper U, Yee SW, Kelly L, Apeltsin L, Stroud RM, Ferrin TE, Giacomini KM, Sali A. Protein Science. 2010;19:412–428. doi: 10.1002/pro.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schlessinger A, Sun NN, Colas C, Pajor AM. The Journal of biological chemistry. 2014;289:16998–17008. doi: 10.1074/jbc.M114.554790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 61.Pei J, Kim BH, Grishin NV. Nucleic acids research. 2008;36:2295–2300. doi: 10.1093/nar/gkn072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stamm M, Staritzbichler R, Khafizov K, Forrest LR. Nucleic acids research. 2014;42:W246–251. doi: 10.1093/nar/gku291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sali A, Blundell TL. J Mol Biol. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- 64.Krivov GG, Shapovalov MV, Dunbrack RL., Jr. Proteins. 2009;77:778–795. doi: 10.1002/prot.22488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schlessinger A, Geier E, Fan H, Irwin JJ, Shoichet BK, Giacomini KM, Sali A. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:15810–15815. doi: 10.1073/pnas.1106030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Laskowski RA, MacArthur MW, Moss DS, Thornton JM. Journal of Applied Crystallography. 1993;26:283–291. [Google Scholar]
- 67.Shen MY, Sali A. Protein Sci. 2006;15:2507–2524. doi: 10.1110/ps.062416606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ray A, Lindahl E, Wallner B. Bioinformatics. 2010;26:3067–3074. doi: 10.1093/bioinformatics/btq581. [DOI] [PubMed] [Google Scholar]
- 69.Fan H, Irwin JJ, Webb BM, Klebe G, Shoichet BK, Sali A. Journal of chemical information and modeling. 2009;49:2512–2527. doi: 10.1021/ci9003706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mysinger MM, Carchia M, Irwin JJ, Shoichet BK. Journal of medicinal chemistry. 2012;55:6582–6594. doi: 10.1021/jm300687e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Irwin JJ, Shoichet BK. Journal of chemical information and modeling. 2005;45:177–182. doi: 10.1021/ci049714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McGann M. J. Chem. Inf. Model. 2011;51:578–596. doi: 10.1021/ci100436p. [DOI] [PubMed] [Google Scholar]
- 73.Mysinger MM, Shoichet BK. Journal of chemical information and modeling. 2010;50:1561–1573. doi: 10.1021/ci100214a. [DOI] [PubMed] [Google Scholar]
- 74.Shoichet BK, Stroud RM, Santi DV, Kuntz ID, Perry KM. Science. 1993;259:1445–1450. doi: 10.1126/science.8451640. [DOI] [PubMed] [Google Scholar]
- 75.Mysinger MM, Weiss DR, Ziarek JJ, Gravel S, Doak AK, Karpiak J, Heveker N, Shoichet BK, Volkman BF. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:5517–5522. doi: 10.1073/pnas.1120431109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jurik A, Zdrazil B, Holy M, Stockner T, Sitte HH, Ecker GF. Journal of medicinal chemistry. 2015;58:2149–2158. doi: 10.1021/jm5015428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schlessinger A, Wittwer MB, Dahlin A, Khuri N, Bonomi M, Fan H, Giacomini KM, Sali A. Journal of Biological Chemistry. 2012;287:37745–37756. doi: 10.1074/jbc.M112.388157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Verdonk ML, Cole JC, Hartshorn MJ, Murray CW, Taylor RD. Proteins. 2003;52:609–623. doi: 10.1002/prot.10465. [DOI] [PubMed] [Google Scholar]
- 79.Sherman W, Beard HS, Farid R. Chemical biology & drug design. 2006;67:83–84. doi: 10.1111/j.1747-0285.2005.00327.x. [DOI] [PubMed] [Google Scholar]
- 80.Andersen J, Ladefoged LK, Wang D, Kristensen TN, Bang-Andersen B, Kristensen AS, Schiott B, Stromgaard K. ACS chemical neuroscience. 2015;6:1892–1900. doi: 10.1021/acschemneuro.5b00225. [DOI] [PubMed] [Google Scholar]
- 81.Aqvist J, Medina C, Samuelsson JE. Protein engineering. 1994;7:385–391. doi: 10.1093/protein/7.3.385. [DOI] [PubMed] [Google Scholar]
- 82.Zwanzig RW. The Journal of Chemical Physics. 1954;22:1420–1426. [Google Scholar]
- 83.Kirkwood JG. The Journal of Chemical Physics. 1935;3:300–313. [Google Scholar]
- 84.Samsudin F, Parker Joanne L., Mark SP, Sansom S, Newstead, Fowler Philip W. Cell Chemical Biology. 23:299–309. doi: 10.1016/j.chembiol.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Colas C, Smith DE, Schlessinger A. Cell Chem Biol. 2016;23:211–213. doi: 10.1016/j.chembiol.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hediger MA, Romero MF, Peng JB, Rolfs A, Takanaga H, Bruford EA. Pflugers Arch. 2004;447:465–468. doi: 10.1007/s00424-003-1192-y. [DOI] [PubMed] [Google Scholar]
- 87.Schweikhard ES, Ziegler CM. Current topics in membranes. 2012;70:1–28. doi: 10.1016/B978-0-12-394316-3.00001-6. [DOI] [PubMed] [Google Scholar]
- 88.Hanahan D, Weinberg RA. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 89.Warburg O, Wind F, Negelein E. The Journal of general physiology. 1927;8:519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vander Heiden MG, Cantley LC, Thompson CB. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fuchs BC, Bode BP. Semin Cancer Biol. 2005;15:254–266. doi: 10.1016/j.semcancer.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 92.Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B, Yang H, Hild M, Kung C, Wilson C, Myer VE, MacKeigan JP, Porter JA, Wang YK, Cantley LC, Finan PM, Murphy LO. Cell. 2009;136:521–534. doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.van Geldermalsen M, Wang Q, Nagarajah R, Marshall AD, Thoeng A, Gao D, Ritchie W, Feng Y, Bailey CG, Deng N, Harvey K, Beith JM, Selinger CI, O'Toole SA, Rasko JE, Holst J. Oncogene. 2015 doi: 10.1038/onc.2015.381. DOI: 101038/onc.2015.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Furuya M, Horiguchi J, Nakajima H, Kanai Y, Oyama T. Cancer science. 2012;103:382–389. doi: 10.1111/j.1349-7006.2011.02151.x. [DOI] [PubMed] [Google Scholar]
- 95.Sakata T, Ferdous G, Tsuruta T, Satoh T, Baba S, Muto T, Ueno A, Kanai Y, Endou H, Okayasu I. Pathology international. 2009;59:7–18. doi: 10.1111/j.1440-1827.2008.02319.x. [DOI] [PubMed] [Google Scholar]
- 96.Wang Q, Hardie RA, Hoy AJ, van Geldermalsen M, Gao D, Fazli L, Sadowski MC, Balaban S, Schreuder M, Nagarajah R, Wong JJ, Metierre C, Pinello N, Otte NJ, Lehman ML, Gleave M, Nelson CC, Bailey CG, Ritchie W, Rasko JE, Holst J. The Journal of pathology. 2015;236:278–289. doi: 10.1002/path.4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen L, Cui H. International journal of molecular sciences. 2015;16:22830–22855. doi: 10.3390/ijms160922830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pochini L, Scalise M, Galluccio M, Indiveri C. Frontiers in chemistry. 2014;2:61. doi: 10.3389/fchem.2014.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Verrey F, Closs EI, Wagner CA, Palacin M, Endou H, Kanai Y. Pflugers Archiv : European journal of physiology. 2004;447:532–542. doi: 10.1007/s00424-003-1086-z. [DOI] [PubMed] [Google Scholar]
- 100.Fotiadis D, Kanai Y, Palacin M. Molecular aspects of medicine. 2013;34:139–158. doi: 10.1016/j.mam.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 101.Meier C, Ristic Z, Klauser S, Verrey F. EMBO Journal. 2002;21:580–589. doi: 10.1093/emboj/21.4.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Boado RJ, Li JY, Nagaya M, Zhang C, Pardridge WM. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:12079–12084. doi: 10.1073/pnas.96.21.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang Q, Bailey CG, Ng C, Tiffen J, Thoeng A, Minhas V, Lehman ML, Hendy SC, Buchanan G, Nelson CC, Rasko JE, Holst J. Cancer Res. 2011;71:7525–7536. doi: 10.1158/0008-5472.CAN-11-1821. [DOI] [PubMed] [Google Scholar]
- 104.Wang Q, Tiffen J, Bailey CG, Lehman ML, Ritchie W, Fazli L, Metierre C, Feng YJ, Li E, Gleave M, Buchanan G, Nelson CC, Rasko JE, Holst J. Journal of the National Cancer Institute. 2013;105:1463–1473. doi: 10.1093/jnci/djt241. [DOI] [PubMed] [Google Scholar]
- 105.Gao X, Zhou L, Jiao X, Lu F, Yan C, Zeng X, Wang J, Shi Y. Nature. 2010;463:828–832. doi: 10.1038/nature08741. [DOI] [PubMed] [Google Scholar]
- 106.Okuda S, Yamada T, Hamajima M, Itoh M, Katayama T, Bork P, Goto S, Kanehisa M. Nucleic acids research. 2008;36:W423–426. doi: 10.1093/nar/gkn282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kreuzer J, Bach NC, Forler D, Sieber SA. Chemical science. 2014;6:237–245. doi: 10.1039/c4sc02339k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Muzio G, Maggiora M, Paiuzzi E, Oraldi M, Canuto RA. Free radical biology & medicine. 2012;52:735–746. doi: 10.1016/j.freeradbiomed.2011.11.033. [DOI] [PubMed] [Google Scholar]
- 109.Thorens B, Mueckler M. American journal of physiology. Endocrinology and metabolism. 2010;298:E141–145. doi: 10.1152/ajpendo.00712.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Carruthers A, DeZutter J, Ganguly A, Devaskar SU. American journal of physiology. Endocrinology and metabolism. 2009;297:E836–848. doi: 10.1152/ajpendo.00496.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mueckler M, Caruso C, Baldwin SA, Panico M, Blench I, Morris HR, Allard WJ, Lienhard GE, Lodish HF. Science. 1985;229:941–945. doi: 10.1126/science.3839598. [DOI] [PubMed] [Google Scholar]
- 112.Farrell CL, Yang J, Pardridge WM. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 1992;40:193–199. doi: 10.1177/40.2.1552163. [DOI] [PubMed] [Google Scholar]
- 113.Seidner G, Alvarez MG, Yeh JI, O'Driscoll KR, Klepper J, Stump TS, Wang D, Spinner NB, Birnbaum MJ, De Vivo DC. Nature genetics. 1998;18:188–191. doi: 10.1038/ng0298-188. [DOI] [PubMed] [Google Scholar]
- 114.Weber YG, Storch A, Wuttke TV, Brockmann K, Kempfle J, Maljevic S, Margari L, Kamm C, Schneider SA, Huber SM, Pekrun A, Roebling R, Seebohm G, Koka S, Lang C, Kraft E, Blazevic D, Salvo-Vargas A, Fauler M, Mottaghy FM, Munchau A, Edwards MJ, Presicci A, Margari F, Gasser T, Lang F, Bhatia KP, Lehmann-Horn F, Lerche H. The Journal of clinical investigation. 2008;118:2157–2168. doi: 10.1172/JCI34438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Calvo MB, Figueroa A, Pulido EG, Campelo RG, Aparicio LA. International journal of endocrinology. 2010:2010. doi: 10.1155/2010/205357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Larson SM, Schwartz LH. J. Nucl. Med. 2006;47:901–903. [PubMed] [Google Scholar]
- 117.Rastogi S, Banerjee S, Chellappan S, Simon GR. Cancer Lett. 2007;257:244–251. doi: 10.1016/j.canlet.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 118.Chan DA, Sutphin PD, Nguyen P, Turcotte S, Lai EW, Banh A, Reynolds GE, Chi JT, Wu J, Solow-Cordero DE, Bonnet M, Flanagan JU, Bouley DM, Graves EE, Denny WA, Hay MP, Giaccia AJ. Sci. Transl. Med. 2011;3:94ra70. doi: 10.1126/scitranslmed.3002394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lopez-Serra P, Marcilla M, Villanueva A, Ramos-Fernandez A, Palau A, Leal L, Wahi JE, Setien-Baranda F, Szczesna K, Moutinho C, Martinez-Cardus A, Heyn H, Sandoval J, Puertas S, Vidal A, Sanjuan X, Martinez-Balibrea E, Vinals F, Perales JC, Bramsem JB, Orntoft TF, Andersen CL, Tabernero J, McDermott U, Boxer MB, Vander Heiden MG, Albar JP, Esteller M. Nat. Commun. 2014;5:3608. doi: 10.1038/ncomms4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Quistgaard EM, Low C, Moberg P, Tresaugues L, Nordlund P. Nat. Struct. Mol. Biol. 2013;20:766–768. doi: 10.1038/nsmb.2569. [DOI] [PubMed] [Google Scholar]
- 121.Wisedchaisri G, Park MS, Iadanza MG, Zheng H, Gonen T. Nat. Commun. 2014;5:4521. doi: 10.1038/ncomms5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sun L, Zeng X, Yan C, Sun X, Gong X, Rao Y, Yan N. Nature. 2012;490:361–366. doi: 10.1038/nature11524. [DOI] [PubMed] [Google Scholar]
- 123.Nomura N, Verdon G, Kang HJ, Shimamura T, Nomura Y, Sonoda Y, Hussien SA, Qureshi AA, Coincon M, Sato Y, Abe H, Nakada-Nakura Y, Hino T, Arakawa T, Kusano-Arai O, Iwanari H, Murata T, Kobayashi T, Hamakubo T, Kasahara M, Iwata S, Drew D. Nature. 2015;526:397–401. doi: 10.1038/nature14909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wisedchaisri G, Park MS, Iadanza MG, Zheng H, Gonen T. Nature communications. 2014;5:4521. doi: 10.1038/ncomms5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Smirnova I, Kasho V, Kaback HR. Biochemistry. 2011;50:9684–9693. doi: 10.1021/bi2014294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ung PM, Song W, Cheng L, Zhao X, Hu H, Chen L, Schlessinger A. Joural of Physiiology. 2016 In Revision. [Google Scholar]
- 127.Sage JM, Cura AJ, Lloyd KP, Carruthers A. Am. J. Physiol. Cell Physiol. 2015;308:C827–834. doi: 10.1152/ajpcell.00001.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ojeda P, Perez A, Ojeda L, Vargas-Uribe M, Rivas CI, Salas M, Vera JC, Reyes AM. Am. J. Physiol. Cell Physiol. 2012;303:C530–539. doi: 10.1152/ajpcell.00145.2012. [DOI] [PubMed] [Google Scholar]
- 129.Gullans SR, Kone BC, Avison MJ, Giebisch G. The American journal of physiology. 1988;255:F1170–1177. doi: 10.1152/ajprenal.1988.255.6.F1170. [DOI] [PubMed] [Google Scholar]
- 130.Ruderman NB, Saha AK, Vavvas D, Witters LA. The American journal of physiology. 1999;276:E1–E18. doi: 10.1152/ajpendo.1999.276.1.E1. [DOI] [PubMed] [Google Scholar]
- 131.Stoppa GR, Cesquini M, Roman EA, Prada PO, Torsoni AS, Romanatto T, Saad MJ, Velloso LA, Torsoni MA. The Journal of endocrinology. 2008;198:157–168. doi: 10.1677/JOE-07-0428. [DOI] [PubMed] [Google Scholar]
- 132.Birkenfeld AL, Lee HY, Guebre-Egziabher F, Alves TC, Jurczak MJ, Jornayvaz FR, Zhang D, Hsiao JJ, Martin-Montalvo A, Fischer-Rosinsky A, Spranger J, Pfeiffer AF, Jordan J, Fromm MF, Konig J, Lieske S, Carmean CM, Frederick DW, Weismann D, Knauf F, Irusta PM, De Cabo R, Helfand SL, Samuel VT, Shulman GI. Cell metabolism. 2011;14:184–195. doi: 10.1016/j.cmet.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pajor AM. Pflugers Arch. 2014;466:119–130. doi: 10.1007/s00424-013-1369-y. [DOI] [PubMed] [Google Scholar]
- 134.Inoue K, Zhuang L, Ganapathy V. Biochemical and biophysical research communications. 2002;299:465–471. doi: 10.1016/s0006-291x(02)02669-4. [DOI] [PubMed] [Google Scholar]
- 135.Hardies K, de Kovel CG, Weckhuysen S, Asselbergh B, Geuens T, Deconinck T, Azmi A, May P, Brilstra E, Becker F, Barisic N, Craiu D, Braun KP, Lal D, Thiele H, Schubert J, Weber Y, van 't Slot R, Nurnberg P, Balling R, Timmerman V, Lerche H, Maudsley S, Helbig I, Suls A, Koeleman BP, De Jonghe P, E. R. E. S. C. autosomal recessive working group of the Euro Brain : a journal of neurology. 2015;138:3238–3250. doi: 10.1093/brain/awv263. [DOI] [PubMed] [Google Scholar]
- 136.Huard K, Brown J, Jones JC, Cabral S, Futatsugi K, Gorgoglione M, Lanba A, Vera NB, Zhu Y, Yan Q, Zhou Y, Vernochet C, Riccardi K, Wolford A, Pirman D, Niosi M, Aspnes G, Herr M, Genung NE, Magee TV, Uccello DP, Loria P, Di L, Gosset JR, Hepworth D, Rolph T, Pfefferkorn JA, Erion DM. Scientific reports. 2015;5:17391. doi: 10.1038/srep17391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kumar H, Kasho V, Smirnova I, Finer-Moore JS, Kaback HR, Stroud RM. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:1784–1788. doi: 10.1073/pnas.1324141111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang H, Gouaux E. EMBO reports. 2012;13:861–866. doi: 10.1038/embor.2012.110. [DOI] [PMC free article] [PubMed] [Google Scholar]