Abstract
Background. While the association between renal impairment and cardiovascular disease (CVD) is well established in the general population, the association remains poorly understood in human immunodeficiency virus (HIV)–positive individuals.
Methods. Individuals with ≥2 estimated glomerular filtration rate (eGFR) measurements after 1 February 2004 were followed until CVD, death, last visit plus 6 months, or 1 February 2015. CVD was defined as the occurrence of centrally validated myocardial infarction, stroke, invasive cardiovascular procedures, or sudden cardiac death.
Results. During a median follow-up duration of 8.0 years (interquartile range, 5.4–8.9 years) 1357 of 35 357 individuals developed CVD (incidence rate, 5.2 cases/1000 person-years [95% confidence interval {CI}, 5.0–5.5]). Confirmed baseline eGFR and CVD were closely related with 1.8% of individuals (95% CI, 1.6%–2.0%) with an eGFR > 90 mL/minute/1.73 m2 estimated to develop CVD at 5 years, increasing to 21.1% (95% CI, 6.6%–35.6%) among those with an eGFR ≤ 30 mL/minute/1.73 m2. The strong univariate relationship between low current eGFR and CVD was primarily explained by increasing age in adjusted analyses, although all eGFRs ≤ 80 mL/minute/1.73 m2 remained associated with 30%–40% increased CVD rates, and particularly high CVD rates among individuals with an eGFR ≤ 30 mL/minute/1.73 m2 (incidence rate ratio, 3.08 [95% CI, 2.04–4.65]).
Conclusions. Among HIV-positive individuals in a large contemporary cohort, a strong relation between confirmed impaired eGFR and CVD was observed. This finding highlights the need for renal preventive measures and intensified monitoring for emerging CVD, particularly in older individuals with continuously low eGFRs.
Keywords: eGFR, renal impairment, kidney disease, cardiovascular disease, myocardial infarction, stroke, invasive cardiovascular procedures, sudden cardiac death, HIV
The association between impaired renal function and cardiovascular disease (CVD) is well established in the general population, particularly for severe levels of renal impairment [1–6]. As such, >50% of all deaths among individuals with end-stage renal disease are related to a CVD event [7]. In contrast, most prior studies that have investigated the relation between renal impairment and CVD in human immunodeficiency virus (HIV)–positive individuals have been small, have used relatively broad definitions of CVD, or have focused on single measures of renal function, which are subjected to random variation and the transient effects of acute illness [8–13]. The influence of a more sustained impairment of the estimated glomerular filtration rate (eGFR) on well-defined CVD events in HIV-positive individuals is less clear.
Renal impairment is projected to become more prevalent among HIV-positive individuals in future years owing to aging and an accumulating burden of comorbidities and lifestyle-related risk factors. CVD is furthermore now one of the leading causes of non–AIDS-defining death in HIV-positive individuals [14]. A better understanding of the rates of CVD among HIV-positive individuals with renal impairment is therefore warranted to assist identification of those at highest risk with a need for intensified monitoring and initiation of preventive measures [15].
The relationship between renal impairment and CVD is complex and may be mediated through a variety of different pathways [3, 6, 14]. These include accelerated coronary and cerebrovascular atherosclerosis (which may be mediated in part by increased inflammation and oxidative stress) atrial fibrillation, and ventricular hypertrophy, which are common at severe levels of renal impairment and may, similar to electrolyte abnormalities, promote dysrhythmias, resulting in stroke or sudden cardiac death [3, 15–20]. Finally, renal impairment and CVD are known to share a common underlying risk factor profile, which includes hypertension, diabetes, dyslipidemia, smoking, injection drug use, obesity, ongoing inflammation, and black African origin [20, 21]. CVD, renal impairment, and many of the underlying shared individual risk factors are more prevalent among HIV-positive individuals than in the general population, and, hence, the association between renal impairment and CVD may be stronger in HIV-positive individuals [22, 23]. The aim of this analysis is to investigate the nature and relationship of various levels of sustained eGFR impairment with centrally adjudicated CVD endpoints in a large heterogeneous and contemporary cohort of primarily white HIV-positive individuals.
METHODS
Study Population
The Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study is a large, prospective cohort collaboration established in 1999 and following >49 000 HIV–positive persons from 11 cohorts in Europe, the United States, and Australia; details have been published previously [17]. Data on centrally validated clinical events, including myocardial infarction, sudden cardiac death, stroke, invasive cardiovascular procedures, end-stage renal disease, and death, is collected in real time during routine clinical care. Information on sociodemographic factors, antiretroviral treatment, HIV viral load, CD4+ T-cell counts, AIDS-defining events, viral hepatitis, levels of creatinine and other laboratory biomarkers, and cardiovascular risk factors is collected electronically at enrollment and every 6 months thereafter.
End Point Definition
CVD events are reported using designated event forms (more information is available at: http://www.chip.dk/Studies/DAD/Study-Documents) and are defined as centrally validated fatal and nonfatal myocardial infarction, stroke, coronary angioplasty, coronary bypass, carotid endarterectomy, and sudden cardiac death. A fatal CVD event is defined as one of the above events leading to death within 28 days. Adjudication of CVD events is made in accordance with predefined algorithms, and only confirmed events are included in analysis. Sudden cardiac death is defined as a sudden death in which the underlying cause could not be established as a myocardial infarction, owing to the lack of data on symptoms, electrocardiogram findings, and changes in cardiac biomarkers, but in which cardiovascular risks were present at the time of death, according to the World Health Organization MONICA Dundee score [24], and without evidence of other nonatherosclerotic or noncardiovascular causes of death. All sudden cardiac deaths in the D:A:D study are reviewed by an external cardiologist.
Statistical Methods
D:A:D study participants with ≥2 eGFR measurements after 1 February 2004 (baseline for initiation of systematic creatinine collection) were included and followed until the earliest of the following: first CVD event, death, 6 months after the last visit, or 1 February 2015. Persons with <3 months of follow-up from the first to the last eGFR measurement were excluded. The Cockcroft-Gault equation [25], standardized for body surface area [26], was used to estimate creatinine clearance, a surrogate for eGFR in this analysis [27, 28]. As several cohorts participating in D:A:D are prohibited from collecting ethnicity information, the Cockcroft-Gault equation was used, rather than an equation including ethnicity. Where eGFR measurements were performed more frequently than every 28 days, the median value was used and assigned to the median date. Confirmed baseline and time-updated (current) eGFRs were defined using 2 consecutive eGFR measurements, regardless of the time between measurements (per the definition minimum of 28 days). The confirmed baseline and current eGFRs were subsequently allocated to the following eGFR strata: >90, >60 to ≤90, >30 to ≤60, and ≤30 mL/minute/1.73 m2. Where 2 consecutive eGFRs (<15% of all values) did not fall within the same eGFR strata, the mean of 2 eGFRs carried forward was used to assign an eGFR category.
Individuals with a prior CVD event were included, but only the first CVD event experienced during prospective follow-up after baseline was included as an event. Individuals could, however, experience ≥2 different types of CVD events on the same date.
Incidence rates were calculated per 1000 person-years of follow-up. Kaplan–Meier estimation was used to investigate time to CVD, stratified according to confirmed baseline eGFRs ( > 90, ≤90 to >60, ≤60 to >30, and ≤30 mL/minute/1.73 m2).
Poisson regression models stratified according to the confirmed current eGFR were used to model the CVD incidence rate ratios (IRRs), overall and stratified by individual CVD events. Potential confounders included in multivariate models were age (per 10 years older), sex, ethnicity, D:A:D enrollment cohort, nadir CD4+ T-cell count, HIV acquisition group, and family history of CVD. All remaining variables were adjusted for as time updated, including hepatitis B virus (HBV)/hepatitis C virus (HCV) coinfection, HIV RNA level (per log10), CD4+ T-cell count, prior AIDS, hypertension (>150/>100 mm Hg or receipt of antihypertensive treatment), diabetes (confirmed diagnosis of diabetes mellitus or receipt of antidiabetic treatment), confirmed eGFR strata, smoking status (current, previous, never), dyslipidemia (total cholesterol level >6.2 mmol/L, high-density lipoprotein cholesterol level <0.9 mmol/L, triglyceride level >2.3 mmol/L, or receipt of lipid-level-lowering treatment), and prior CVD (confirmed diagnosis). Antiretroviral drug use was fitted as time-updated cumulative use (per 5 years; zidovudine, didanosine, zalcitabine, stavudine, lamivudine, emtricitabine, tenofovir disoproxil fumarate, abacavir, efavirenz, nevirapine, indinavir, saquinavir, ritonavir, nelfinavir, (fos)ampreavir, atazanavir, and darunavir) and current use (currently receipt and use within the last 6 months; zidovudine, didanosine, zalcitabine, stavudine, lamivudine, emtricitabine, tenofovir disoproxil fumarate, and abacavir).
A number of sensitivity analyses were performed to test the robustness of the results. One analysis investigated death as a potential competing risk of CVD. Another analysis excluded all with a prior CVD event. Other analyses adjusted for the D:A:D chronic kidney disease (CKD) risk score [29] and the predicted CVD risk based on the Framingham CVD prediction model [30] to estimate how much of the CVD risk is explained through common renal and CVD risk factors. The D:A:D CKD risk score is a 9-variable prediction score estimating the 5-year risk of developing CKD in HIV-positive individuals. Individuals in the low CKD risk group (score, <0) have a 1 in 393 (0.3%) 5-year CKD risk, rising to 1 in 47 (2.1%) in the medium-risk group (score, 0–4) and 1 in 6 (16.7%) in the high-risk group (score ≥5) [29]. A final analysis investigated the association between current nadir eGFR and the percentage of follow-up time spent with an eGFR of ≤ 60 mL/minute/1.73 m2 and CVD.
RESULTS
Study Population
A total of 35 357 persons with follow-up after 2004 and at least 2 eGFR measurement were included in analysis, Supplementary Figure 1. Included individuals were predominantly white (48.1%) and male (73.9%), and the median age was 41 years (interquartile range [IQR], 35–48 years; Table 1). While 41.6% were smokers, 4.0% had diabetes, 8.9% had hypertension, and 0.7% had experienced a prior CVD event. At baseline, the median estimated 5-year risk of CKD was low overall (−1 [IQR −3 to 4]; corresponding to 0.3%) and medium in those developing a CVD event (4 [IQR, −1 to 9], corresponding to 2.1%; Table 1). A total of 558 persons were excluded from analysis because of missing CD4+ T-cell counts or viral load at baseline or because of insufficient follow-up. Excluded persons were more likely to be young, to be white, to be naive to combination antiretroviral therapy, to be positive for HCV, to have no family history of CVD, and to have experienced a prior AIDS event.
Table 1.
Baseline Characteristics
| Characteristic | All Persons (n = 35 357) | Persons Developing CVD (n = 1357) |
|---|---|---|
| Male sex | 26 124 (73.9) | 1181 (87.3) |
| Ethnicity | ||
| White | 17 016 (48.1) | 697 (51.4) |
| Black | 2450 (6.9) | 40 (3.0) |
| Other | 716 (2.0) | 12 (0.9) |
| Unknown | 15 175 (42.9) | 608 (44.8) |
| HIV acquisition group | ||
| MSM | 16 234 (45.9) | 728 (53.7) |
| IDU | 4529 (12.8) | 154 (11.4) |
| Heterosexual | 12 436 (35.2) | 386 (28.4) |
| Other | 2158 (6.1) | 89 (6.6) |
| HBVa | ||
| Positive | 1597 (4.5) | 46 (3.4) |
| Negative | 31 169 (88.2) | 1 213 (89.4) |
| Unknown | 2591 (7.3) | 98 (7.2) |
| HCVb | ||
| Positive | 6479 (18.3) | 236 (17.4) |
| Negative | 25 535 (72.2) | 973 (71.7) |
| Unknown | 3343 (9.5) | 148 (10.9) |
| cART use | 26 425 (74.7) | 1 197 (88.2) |
| Prior AIDS event | 8768 (24.8) | 462 (34.1) |
| HIV RNA load < 400 copies/mL | 20 828 (58.9) | 956 (70.4) |
| Current smoker | 14 715 (41.6) | 688 (50.7) |
| BMI >30c | 1830 (5.2) | 78 (5.7) |
| Family history of CVD | 2712 (7.7) | 179 (13.2) |
| Prior CVDd | 240 (0.7) | 72 (5.3) |
| Hypertensione | 3133 (8.9) | 264 (19.5) |
| Diabetesf | 1425 (4.0) | 163 (12.0) |
| eGFR, mL/min/1.73 m2g | ||
| >90 | 24 937 (70.5) | 656 (48.3) |
| >60 to ≤90 | 9378 (26.5) | 559 (41.2) |
| >30 to ≤60 | 999 (2.8) | 13.5 (10.0) |
| ≤30 | 43 (0.1) | 7 (0.5) |
| Framingham risk score (%) | ||
| Low (0–5) | 24 111 (68.2) | 275 (18.9) |
| Moderate (5–10) | 5821 (16.5) | 290 (21.4) |
| High (>10) | 5425 (15.3) | 810 (59.7) |
| D:A:D study CKD risk score median (IQR)h | −1 (−3 to 4) | 4 (−1 to 9) |
| Age, median (IQR) | 41 (35–48) | 50 (44–59) |
| CD4+ T-cell count, cells/mm3 median (IQR) | 44 (290–625) | 441 (289–640) |
Data are no. (%) of subjects or median value (interquartile range). Baseline was defined as 1 February 2004.
Abbreviations: cART, combination antiretroviral therapy; CKD, chronic kidney disease; CVD, cardiovascular disease; D:A:D, Data Collection on Adverse Events of Anti-HIV Drugs; eGFR, estimated glomerular filtration rate; HIV, human immunodeficiency virus; IDU, injection drug use; IQR, interquartile range; MSM, men who have sex with men.
a Positivity was defined as detection of hepatitis B virus (HBV) surface antigen, HBV e antigen, or HBV DNA.
b Positivity was defined as detection of anti–hepatitis C virus (HCV) antibody and either detection or unknown status of HCV RNA.
c Body mass index (BMI) was calculated as the weight in kilograms divided by the height in meters squared.
d As diagnosed on a D:A:D study CVD event form.
e Defined as a blood pressure of >150/>100 mm Hg or receipt of antihypertensive treatment.
f Defined as the recording of a diagnosis of diabetes on a D:A:D study event form or receipt of antidiabetic treatment.
g Calculated using the Cockcroft-Gault equation.
h A score of <0 indicated a low 5-year CKD risk (0.3%); 0–4, a medium risk (2.1%); and ≥5, a high risk (16.7%).
Age and eGFR
Among individuals younger than 40 years, 87.0% (13 660) had normal baseline renal function (confirmed eGFR, > 90 mL/minute/1.73 m2), and only 0.04% (7) had advanced renal impairment (confirmed eGFR, ≤ 30 mL/minute/1.73 m2). In contrast, among individuals older than 60 years, only 15.8% (321) had a confirmed baseline eGFR > 90 mL/minute/1.73 m2, and 0.8% (17) had a confirmed baseline eGFR ≤ 30 mL/minute/1.73 m2.
CVD Events
Over a median follow-up time of 8.0 years (IQR, 5.4–8.9 years; total person-years of follow-up, 258 480), 1357 persons developed 1646 CVD events (incidence rate, 5.2 events per 1000 person-years of follow-up [95% confidence interval, CI, 5.0–5.5]). The CVD events included 586 myocardial infarctions (11.1% fatal), 430 strokes (8.6% fatal), 510 coronary angioplasties (1.6% fatal), 96 coronary bypasses (2.1% fatal), 19 carotid endarterectomies (0% fatal), and 5 sudden cardiac deaths. A total of 284 persons (21.0%) experienced >1 CVD event on the same date, most commonly a myocardial infarction and coronary angioplasty (n = 259).
Median eGFRs and Incident CVD
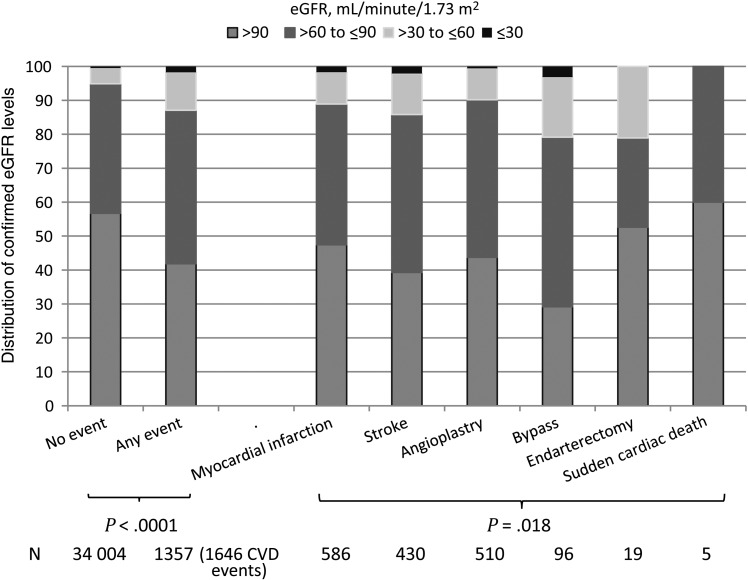
The median eGFR measured in individuals prior to their CVD event was significantly lower (85 mL/minute/1.73 m2 [IQR, 69–102 mL/minute/1.73 m2]) than the median eGFR measured during follow-up in individuals not experiencing a CVD event (94 mL/minute/1.73 m2 [IQR, 79–110 mL/minute/1.73 m2]; P < .0001). Likewise, a greater proportion of individuals experiencing a CVD event had some level of confirmed reduced eGFR, compared with individuals not experiencing an event (Figure 1). When comparing the individual types of CVD events, those experiencing a coronary bypass event had significantly lower confirmed eGFRs as compared to those with all other CVD event types (P = .018). When excluding coronary bypass events, there were no statistically significant differences in confirmed eGFR levels prior to a CVD event (P = .068). Likewise, when comparing those with an invasive cardiovascular procedures (coronary angioplasty, carotid endarterectomy, or coronary bypass) to those with a myocardial infarction and/or stroke, there was no statistically significant difference (P = .55; Figure 1).
Figure 1.
Confirmed current estimated glomerular filtration rate (eGFR) levels prior to cardiovascular disease (CVD) event. Confirmed current eGFR for those with a CVD event is the last measured median eGFR prior the event. For those without a CVD event, confirmed current eGFR is the last measured median eGFR during follow-up.
Confirmed Baseline eGFRs and Incident CVD
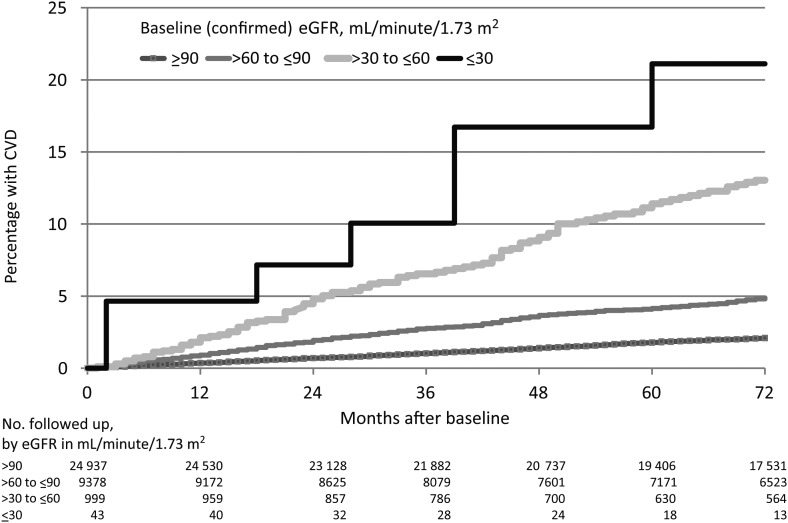
We observed a clear inverse relationship between confirmed eGFRs at baseline and incident CVD, with 1.8% (95% CI, 1.6%–2.0%) estimated to have progressed to CVD at 5 years among those with a confirmed baseline eGFR > 90 mL/minute/1.73 m2, increasing to 4.1% (95% CI, 3.5%–4.6%) for those with a baseline eGFR of 60–90 mL/minute/1.73 m2, 10.8% (95% CI, 8.7%–12.9%) for those with a baseline eGFR of 30–60 mL/minute/1.73 m2, and 21.1% (95% CI, 6.6%–35.6%) for those with confirmed baseline eGFR ≤ 30 mL/minute/1.73 m2 (Figure 2).
Figure 2.
Kaplan–Meier progression to cardiovascular disease (CVD), by confirmed baseline estimated glomerular filtration rate (eGFR).
Among individuals with moderately impaired baseline eGFR (confirmed eGFR ≤ 60 mL/minute/1.73 m2) who developed a CVD event, we did not observe a statistically significant differences (P = .63) in the time to different CVD events, with a median time to CVD event of 45 months (IQR, 21–76 months).
Confirmed Current eGFR and Incident CKD
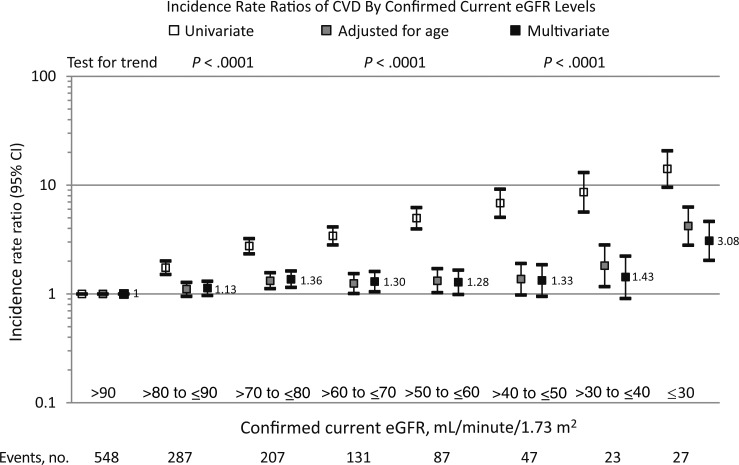
There was a strong and inverse linear relationship between confirmed current eGFR and CVD in univariate analysis: IRRs increased from 1.00 at eGFR > 90 mL/minute/1.73 m2 to 14.09 (95% CI, 9.58–20.74) at eGFR ≤ 30 mL/minute/1.73 m2 (Figure 3). Adjustment for increasing age explained most of the relationship between eGFR and CVD at eGFRs >30 mL/minute/1.73 m2, although all eGFRs <80 mL/minute/1.73 m2 were associated with an increased incidence of CVD of approximately 30%–40%. At a confirmed current eGFR ≤ 30 mL/minute/1.73 m2, a significantly increased incidence of CVD remained independent of age (IRR, 4.21 [95% CI, 2.81–6.30]; Figure 3). Further adjustment for other potential confounders, including individual antiretroviral drugs, had relatively limited impact on the overall association (IRR, 3.08 [95% CI, 2.04–4.65] at confirmed eGFR ≤ 30 mL/minute/1.73 m2 as compared to confirmed eGFR ≥ 90 mL/minute/1.73 m2; Figure 3). The exclusion of the 240 individuals with a CVD event prior to baseline led to entirely consistent results (data not shown).
Figure 3.
Cardiovascular disease (CVD) incidence rate ratios by confirmed current estimated glomerular filtration rate (eGFR). Multivariate analysis adjusted for age, sex, ethnicity, Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study enrollment cohort, nadir CD4+ T-cell count, mode of human immunodeficiency virus (HIV) acquisition group, and family history of CVD at baseline. Time-updated variables include hepatitis B virus/hepatitis C virus coinfection, HIV RNA level, CD4+ T-cell count, prior AIDS, hypertension, diabetes, confirmed eGFR strata, smoking status, dyslipidemia, prior CVD, exposure to antiretroviral drugs fitted as cumulative use (to zidovudine, didanosine, zalcitabine, stavudine, lamivudine, emtricitabine, tenofovir disoproxil fumarate, abacavir, efavirenz, nevirapine, indinavir, saquinavir, ritonavir, nelfinavir, (fos)ampreavir, atazanavir, and darunavir) and current use (zidovudine, didanosine, zalcitabine, lamivudine, stavudine, emtricitabine, tenofovir disoproxil fumarate, and abacavir). Abbreviation: CI, confidence interval.
In a bivariate analysis, adjustment for the Framingham score (as a continuous variable) explained some of the association between confirmed current eGFR and CVD, but not to the same extent as age alone (data not shown). In another analysis, which adjusted for the estimated 5-year D:A:D CKD risk score, individuals with a medium CKD risk (ie, a score of 0–4) had a 2.56-fold increased incidence of CVD (IRR, 2.56 [95% CI, 2.22–2.95]), and individuals with a high CKD risk (ie, a score of ≥5) had an almost 5-fold increased incidence of CVD (IRR, 4.98 [95% CI, 4.37–5.68]) as compared to persons with a low estimated CKD risk (ie, a score of <0). After adjustment for other potential confounders (as shown in Figure 3) not included in the D:A:D CKD risk score (with the exception of age), those with a medium or high CKD risk score continued to have a significantly higher risk of CVD (IRR, 1.29 [95% CI, 1.10–1.50] and 1.43 [95% CI, 1.19–1.71], respectively).
There was no strong evidence suggesting that the observed association between confirmed current eGFRs and CVD differed among the individual types of CVD events. When restricting the analysis to fatal CVD events only, all observed associations were further strengthened (data not shown). Our findings were furthermore consistent in different age groups (test for interaction, P = .88) and after accounting for death as a possible competing risk for CVD (data not shown). The association between CVD and confirmed eGFR seen in the primary analyses was largely unchanged by fitting renal function as the current nadir eGFR and as the percentage of follow-up spent with a moderately impaired eGFR (eGFR ≤ 60 mL/minute/1.73 m2; data not shown).
Confirmed Current eGFRs and Number of CVD Events
Individuals with higher confirmed current eGFRs experienced ≥2 CVD events (at the same date) more frequently than those with lower eGFR levels (24.7% at eGFRs > 90 mL/minute/1.73 m2 vs 4.2% at eGFRs ≤ 30 mL/minute/1.73 m2; P = .0034), most commonly myocardial infarction and coronary angioplasty. Furthermore, the proportion of individuals experiencing a fatal CVD event (with death occurring ≤28 days following the event) was strongly related to the confirmed current eGFR, increasing from 4.4% in individuals with a confirmed current eGFR > 90 mL/minute/1.73 m2 to 25.0% in individuals with a confirmed current eGFR ≤ 30 mL/minute/1.73 m2 (P < .0001).
DISCUSSION
In this large heterogeneous cohort of HIV-positive individuals, we found a strong association between centrally adjudicated CVD events and advanced levels of renal impairment (confirmed eGFR ≤ 30 mL/minute/1.73 m2). Almost 60% of all individuals experiencing a CVD event had an eGFR ≤ 90 mL/minute/1.73 m2, based on the latest median eGFR before the event, compared with <40% of those without an event. We further showed that development of a CVD event was considerately faster among those with a severely impaired eGFR at baseline. Among HIV-positive individuals with a confirmed baseline eGFR ≤ 30 mL/minute/1.73 m2, >20% were estimated to have developed CVD after 5 years.
In previous studies from D:A:D, we investigated the inverse relation between CVD events and eGFR, focusing on CVD as a risk factor for various levels of chronic renal impairment [28, 29, 31]. Interestingly, these previous data also supported a strong association between CVD and renal function, which significantly diminished after accounting for other risk factors, suggesting an underlying biological mechanism at least partly mediated by other factors. We have also previously showed an association between the use of certain antiretroviral drugs and CVD and renal impairment [28, 30, 32]. The results of this analysis are entirely consistent with these prior findings, and adjustment for the use of individual antiretroviral drugs did not have any major impact on the association between impaired eGFR and CVD. Data from this analysis point toward increasing age as the main underlying driver of the inverse relationship between eGFR and CVD, in particular at mild to moderately impaired eGFR levels [14]. At more advanced levels of renal impairment (eGFR ≤ 30 mL/minute/1.73 m2), there are additional pathways between renal impairment and CVD that are not immediately related to any of the known common risk factors on the shared causal pathway, such as diabetes, hypertension, and immunosuppression. Regardless of the underlying pathology, the high rates of CVD observed in older individuals with mild to moderate renal impairment highlight the need for intensified monitoring and a search for effective prophylactic measures for impaired renal function and CVD in the aging HIV-population.
In other studies of HIV-positive individuals, a smaller cross-sectional analysis in the FRAM study did not confirm an association between carotid intima-media thickness and eGFR after accounting for older age, sex, and ethnicity [13]. Likewise, a British study did not find an association between eGFR as a continuous variable and coronary heart disease, although those with an eGFR < 75 mL/minute/1.73 m2 already had a >4-fold increased incidence [9]. In a recent EuroSIDA study, both the follow-up time with a low eGFR and an eGFR ≤ 30 mL/minute/1.73 m2 were predictive of non–AIDS-defining events, including CVD, but power was limited [12]. An older large cohort study among HIV-positive US veterans showed an almost 6-fold higher association between an eGFR ≤ 30 mL/minute/1.73 m2, albuminuria, and CVD, although this study also included peripheral artery disease and heart failure [10].
Our findings do not suggest that the association between declining renal function and CVD is stronger or starts at higher eGFR levels in HIV-positive persons than in the general population, as was hypothesized on the basis of the higher occurrence of common renal and CVD risk factors and increased immune activation [1, 4, 33, 34]. There is, however, ongoing ambiguity in the general population with regard to the strength of the association between impaired renal function and CVD. Some studies report only an association with CVD at advanced levels of renal impairment (eGFRs ≤ 30 mL/minute/1.73 m2), while others report associations already at higher eGFR levels [1, 4, 5, 9, 10, 14, 33, 34]. However, the definitions of CVD differ considerately in these studies, ranging from subclinical imaging-verified diagnoses of atherosclerosis to various clinical events ascertained with different levels of certainty. The differences in the incidence of common risk factors and of CVD and renal impairment may also partly explain the conflicting results. Importantly, the D:A:D study focuses on hard clinical CVD events exclusively, and information on nonfatal heart failure or milder forms of ischemic CVD such as angina pectoris is not collected. This methodology may explain why more severe levels of renal impairment are necessary to establish an association with CVD. Interestingly, none of the widely accepted CVD risk prediction models currently include renal impairment in the estimates [30, 32], but the proportion of individuals with advanced renal impairment may be too limited to date.
We also found that fatal outcomes of a CVD event were more common at lower as compared to higher eGFR levels, which may be related to a more severe clinical event or to the fact that those with advanced levels of renal impairment provide a more fragile phenotype with less ability to cope with CVD complications. Likewise, fewer multiple CVD events occurred on the same date among those with lower eGFRs. This finding may be related to the increased fatality rate at lower eGFRs or to the possibility that those with lower eGFRs are less likely to undergo invasive cardiovascular procedures as secondary prophylaxis, owing to concerns about radiocontrast-induced nephrotoxicity. Interestingly, there was no evidence of a relation between the eGFR level and type of CVD outcome (ie, a myocardial infarction did not seem to occur at different eGFRs levels as compared to other CVD events), with the exception of coronary bypass. Coronary bypass was more commonly performed at lower eGFRs, compared with other CVD events, which may suggest more-advanced atherosclerosis with multiple vessel disease in this population.
The potential limitations of the analysis should be acknowledged. We may have underestimated the proportion of individuals with an impaired eGFR, as those excluded from analysis were more likely to have common renal risk factors; hence, the provided relation between eGFR and CVD is of a conservative nature. Proteinuria is a potential source of unmeasured confounding because it not measured systematically in the D:A:D study and may also have moderating effects, as it is a strong independent risk factor for both CVD and CKD [35]. Furthermore, renal impairment may have developed secondary to a CVD event as part of a cardiorenal syndrome, with potential for reverse causality. However, in this analysis, eGFR impairment preceded all prospectively investigated CVD events [36]. Finally, nonischemic events such as cardiac arrhythmias and ventricular hypertrophy were not directly included in the CVD definition but may have contributed more indirectly via stroke and sudden cardiac death events.
In summary, in a large, contemporary cohort of HIV-positive individuals, we observed a strong relationship between confirmed impaired renal function and incident CVD. More than 1 in 5 of those with advanced levels of renal impairment were estimated to have developed CVD by 5 years, with an increasing 28-day CVD fatality rate as the eGFR declined. Our findings highlight the need for intensified monitoring for emerging CVD, in particular in older individuals with continuously low eGFRs levels. Our findings also call for an increased focus on applying different renal and cardiovascular preventive measures in HIV-positive individuals.
STUDY GROUP COHORTS AND MEMBERS
D:A:D participating cohorts (locations): the Australian HIV Observational Database (AHOD; Australia), Aquitaine (France), Athena (the Netherlands), the Barcelona Antiretroviral Surveillance Study (BASS; Spain), the Terry Beirn Community Programs for Clinical Research on AIDS (CPCRA; United States), EuroSIDA (multinational), HivBivus (Sweden), the Italian Cohort Naive to Antiretrovirals (ICONA; Italy), Nice (France), the Swis HIV Cohort Study (SHCS; Switzerland), and St. Pierre (Belgium). D:A:D steering committee: W. El-Sadr, G. Calvo, F. Dabis, O. Kirk, M. Law, A. d′Arminio Monforte, L. Morfeldt, C. Pradier, P. Reiss, R. Weber, S. De Wit, B. Powderly, N. Shortman, C. Moecklinghoff, G. Reilly, X. Franquet, C. A. Sabin, A. Phillips, A. Mocroft, L. Ryom, and J. D. Lundgren (chair).
Cohort principal investigators (cohort): W. El-Sadr (CPCRA), G. Calvo (BASS), F. Dabis (Aquitaine), O. Kirk (EuroSIDA), M. Law (AHOD), A. d'Arminio Monforte (ICONA), L. Morfeldt (HIVBivus), C. Pradier (Nice), P. Reiss (ATHENA), R. Weber (SHCS), and S. De Wit (Brussels). Members of the D:A:D steering committee from the oversight committee: B. Powderly, N. Shortman, C. Moecklinghoff, G. Reilly, and X. Franquet. D:A:D central coordination: C. I. Hatleberg, L. Ryom, C. A. Sabin, D. Kamara, C. J. Smith, A. Phillips, A. Mocroft, A. Bojesen, A. L. Grevsen, C. Matthews, D. Raben, and J. D. Lundgren. D:A:D cohort coordinators and data managers (role or cohort): A. Lind-Thomsen (data manager coordinator), R. Salbøl Brandt; M. Hillebreght, S. Zaheri, and F. W. N. M. Wit (ATHENA); F. Schöni-Affolter (SHCS); A. Travellia and I. Fanti (ICONA); O. Leleux (Aquitaine); E. Thulin and A. Sundström (HivBivus); G. Bartsch and G. Thompsen (CPCRA); M. Delforge (Brussels); E. Fontas, C. Caissotti, and K. Dollet (Nice); S. Mateu and F. Torres (BASS); R. Puhr (AHOD); and D. Kristensen (EuroSIDA)
Verification of end points: A. Sjøl (CVD), P. Meidahl (oncology), J. Helweg-Larsen (hematology), and J. Schmidt Iversen (nephrology). Kidney working group: L. Ryom, A. Mocroft, O. Kirk, P. Reiss, C. Smit, M. Ross, C. A. Fux, P. Morlat, E. Fontas, D. A. Kamara, C.J. Smith, and J. D. Lundgren. Mortality working group: C. J. Smith, L. Ryom, C. I. Hatleberg, A. Phillips, R. Weber, P. Morlat, C. Pradier, P. Reiss, F. W. N. M. Wit, N. Friis-Møller, J. Kowalska, and J. D. Lundgren. Cancer working group: C. Sabin, M. Law, A. d'Arminio Monforte, F. Dabis, F. Bonnet, P. Reiss, F. W. N. M. Wit, C. J. Smith, D. A. Kamara, J. Bohlius, M. Bower, G. Fätkenheuer, A. Grulich, L. Ryom, C. I. Hatleberg, and J. D. Lundgren. For a complete list of acknowledgements for the members of the 11 cohorts in the D:A:D study, please see Supplementary Document 2.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Disclaimer. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of any of the institutions mentioned above.
Financial support. The Data collection on Adverse events of Anti-HIV Drugs [D:A:D] study was supported by the Highly Active Antiretroviral Therapy Oversight Committee, a collaborative committee with representation from academic institutions, the European Agency for the Evaluation of Medicinal Products, the Food and Drug Administration, the patient community, and the following pharmaceutical companies with licensed anti-human immunodeficiency virus (HIV) drugs in the European Union: AbbVie, Bristol-Myers Squibb, Gilead Sciences, ViiV Healthcare, Merck, and Janssen Pharmaceuticals (to the D:A:D: study); the Danish National Research Foundation (grant DNRF126 to CHIP and PERSIMUNE); the Dutch Ministry of Health, Welfare, and Sport, through the Center for Infectious Disease Control, National Institute for Public Health and the Environment, to Stichting HIV Monitoring (via a grant to ATHENA); the Agence nationale de recherches sur le sida et les hépatites virales (Action Coordonnée no. 7, Cohortes; grant to the Aquitaine cohort); the Asia Pacific HIV Observational Database, a program of the Foundation for AIDS Research, amfAR (to the Australian HIV Observational Database [AHOD]); the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH) (grant U01-AI069907 to the AHOD and grants 5U01AI042170-10 and 5U01AI046362-03 to the Terry Beirn Community Programs for Clinical Research on AIDS); Merck Sharp and Dohme (unconditional grant to the AHOD); Gilead Sciences (unconditional grant to the AHOD); Bristol-Myers Squibb (unconditional grant to the AHOD; Boehringer Ingelheim (unconditional grant to the AHOD); Janssen-Cilag (unconditional grant to the AHOD); and ViiV Healthcare (unconditional grant to the AHOD); the Australian Government Department of Health and Ageing (to the Kirby Institute); the Fondo de Investigación Sanitaria (grant FIS 99/0887 to the Barcelona Antiretroviral Surveillance Study [BASS]); the Fundación para la Investigación y la Prevención del SIDA en Espanã (grant FIPSE 3171/00 to the BASS); the NIAID, NIH (grants number 5U01AI042170-10 , 5U01AI046362-03), to the Terry Beirn Community Programs for Clinical Research on AIDS, CPCRA; the European Union's Seventh Framework Programme for research, technological development, and demonstration, under EuroCoord grant 260694 (to the EuroSIDA study); Janssen R&D (unrestricted grant to the EuroSIDA study); Bristol-Myers Squibb (unrestricted grant to the EuroSIDA study); Merck (unrestricted grant to the EuroSIDA study); Pfizer (unrestricted grant to the EuroSIDA study and unrestricted educational grant to the Italian Cohort Naive to Antiretrovirals [ICONA] Foundation); GlaxoSmithKline (unrestricted grant to the EuroSIDA study); the Swiss National Science Foundation (grant 108787 to the Swiss participating centers in the EuroSIDA study); AbbVie (unrestricted educational grant to the ICONA Foundation); Gilead Sciences (unrestricted educational grant to the ICONA Foundation); Janssen Pharmaceuticals (unrestricted educational grant to the ICONA Foundation); Bristol-Myers Squibb (unrestricted educational grant to the ICONA Foundation); Pfizer (unrestricted educational grant to the ICONA Foundation); GlaxoSmithKline (unrestricted educational grant to the ICONA Foundation) and the Swiss National Science Foundation (grant 148522 to the Swiss HIV Cohort Study, SHCS). The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of any of the institutions mentioned above.
Potential conflicts of interest. O. K. had prior/present board membership at ViiV Healthcare, Gilead Sciences, and Merck; received payment for lectures and/or for development of educational presentations from Abbott, Gilead Sciences, and Tibotec; and had travel/accommodations/meeting expenses paid by Abbott, BMS, Gilead Sciences, Merck, and ViiV Healthcare. P. M. has received honoraria, speaker fees, travel support, or honoraria from AbbVie, Bristol-Myers Squibb, Gilead Sciences, ViiV Healthcare, Merck, and Janssen Pharmaceuticals. C. A. F. is an advisory board member for Gilead Sciences and MSD, has grants pending from Gilead Sciences and Abbott, and has received payment for lectures from Gilead HIV and the body. M. L. has received research grants from Boehringer Ingelheim, Bristol Myer Squibb, Gilead Sciences, GlaxoSmithKline, Janssen Pharmaceuticals, Merck, Pfizer, and Hoffman-LaRoche. C. S. received personal fees from Gilead Sciences, Bristol-Myers Squibb, Janssen Pharmaceuticals, Abbott Pharmaceuticals, and ViiV Healthcare. A. M. has received consultancy fees/honoraria/speaker fees from Bristol-Myers Squibb, Pfizer, Merck, Boehringer Ingelheim, and Gilead Sciences. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Collaborators: for the D:A:D Study Group, W. El-Sadr, G. Calvo, F. Dabis, O. Kirk, M. Law, A. d'Arminio Monforte, L. Morfeldt, C. Pradier, P. Reiss, R. Weber, S. De Wit, B. Powderly, N. Shortman, C. Moecklinghoff, G. Reilly, X. Franquet, C.I. Hatleberg, L. Ryom, C.A. Sabin, D. Kamara, CJ. Smith, A. Phillips, A. Mocroft, A. Bojesen, A.L. Grevsen, C. Matthews, D. Raben, J.D. Lundgren, A. Lind-Thomsen, R. Salbøl Brandt, M. Hillebreght, S. Zaheri, F.W.N.M. Wit, F. Schöni-Affolter, A. Travelli, I. Fanti, O. Leleux, E. Thulin, A. Sundström, G. Bartsch, G. Thompsen, M. Delforge, E. Fontas, C. Caissotti, K. Dollet, S. Mateu, F. Torres, R. Puhr, D. Kristensen, A. Sjøl, P. Meidahl, J. Helweg-Larsen, J. Schmidt Iversen, L. Ryom, A. Mocroft, O. Kirk, P. Reiss, C. Smit, M. Ross, C.A. Fux, P. Morlat, E. Fontas, D.A. Kamara, C.J. Smith, J.D. Lundgren, C.J. Smith, L. Ryom, C. I. Hatleberg, A. Phillips, R. Weber, P. Morlat, C. Pradier, P. Reiss, F.W.N.M. Wit, N. Friis-Møller, J. Kowalska, J.D. Lundgren, C. Sabin, M. Law, A. d'Arminio Monforte, F. Dabis, F. Bonnet, P. Reiss, F.W.N.M. Wit, CJ. Smith, D.A. Kamara, J. Bohlius, M. Bower, G. Fätkenheuer, A. Grulich, L. Ryom, C.I. Hatleberg, and J.D. Lundgren
References
- 1.Lee M, Saver JL, Chang KH, Liao HW, Chang SC, Ovbiagele B. Low glomerular filtration rate and risk of stroke: meta-analysis. BMJ 2010; 341:c4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shara NM, Wang H, Mete M et al. Estimated GFR and incident cardiovascular disease events in American Indians: the Strong Heart Study. Am J Kidney Dis 2012; 60:795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang AY, Lai KN. Use of cardiac biomarkers in end-stage renal disease. J Am Soc Nephrol 2008; 19:1643–52. [DOI] [PubMed] [Google Scholar]
- 4.Di Angelantonio E, Danesh J, Eiriksdottir G, Gudnason V. Renal function and risk of coronary heart disease in general populations: new prospective study and systematic review. PLoS Med 2007; 4:e270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arbel Y, Halkin A, Finkelstein A et al. Impact of Estimated Glomerular Filtration Rate on Vascular Disease Extent and Adverse Cardiovascular Events in Patients Without Chronic Kidney Disease. Can J Cardiol 2013; 29:1374–81. [DOI] [PubMed] [Google Scholar]
- 6.de Bie MK, Buiten MS, Rabelink TJ, Jukema JW. How to reduce sudden cardiac death in patients with renal failure. Heart 2012; 98:335–41. [DOI] [PubMed] [Google Scholar]
- 7.Collins AJ, Roberts TL, St Peter WL, Chen SC, Ebben J, Constantini E. United States Renal Data System assessment of the impact of the National Kidney Foundation-Dialysis Outcomes Quality Initiative guidelines. Am J Kidney Dis 2002; 39:784–95. [DOI] [PubMed] [Google Scholar]
- 8.George E, Lucas GM, Nadkarni GN, Fine DM, Moore R, Atta MG. Kidney function and the risk of cardiovascular events in HIV-1-infected patients. AIDS 2010; 24:387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell LJ, Desai M, Hegazi A et al. Renal impairment is associated with coronary heart disease in HIV-positive men. HIV Clinical Trials 2012; 13:343–9. [DOI] [PubMed] [Google Scholar]
- 10.Choi AI, Li Y, Deeks SG, Grunfeld C, Volberding PA, Shlipak MG. Association between kidney function and albuminuria with cardiovascular events in HIV-infected persons. Circulation 2010; 121:651–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serrano-Villar S, Estrada V, Gomez-Garre D et al. Incipient renal impairment as a predictor of subclinical atherosclerosis in HIV-infected patients. JAIDS 2012; 59:141–8. [DOI] [PubMed] [Google Scholar]
- 12.Mocroft A, Ryom L, Begovac J et al. Deteriorating renal function and clinical outcomes in HIV-positive persons. AIDS 2014; 28:727–37. [DOI] [PubMed] [Google Scholar]
- 13.Jotwani V, Scherzer R, Choi A et al. Reduced kidney function and preclinical atherosclerosis in HIV-infected individuals: the study of fat redistribution and metabolic change in HIV infection (FRAM). Am J Nephrol 2011; 33:453–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Natali A, Boldrini B, Baldi S et al. Impact of mild to moderate reductions of glomerular filtration rate on coronary artery disease severity. Nutr Metab Cardiovasc Dis 2014; 24:681–8. [DOI] [PubMed] [Google Scholar]
- 15.Spoto B, Mattace-Raso F, Sijbrands E et al. Association of IL-6 and a functional polymorphism in the IL-6 gene with cardiovascular events in patients with CKD. Clin J Am Soc Nephrol N 2015; 10:232–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klawitter J, Reed-Gitomer BY, McFann K et al. Endothelial dysfunction and oxidative stress in polycystic kidney disease. Am J Renal Physiol 2014; 307:F1198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsushita K, Sang Y, Ballew SH et al. Cardiac and kidney markers for cardiovascular prediction in individuals with chronic kidney disease: the Atherosclerosis Risk in Communities study. Arterioscler Thromb Vasc Biol 2014; 34:1770–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foley RN, Murray AM, Li S et al. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol 2005; 16:489–95. [DOI] [PubMed] [Google Scholar]
- 19.Baber U, Howard VJ, Halperin JL et al. Association of chronic kidney disease with atrial fibrillation among adults in the United States: REasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Circ Arrhythm Electrophysiol 2011; 4:26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta SK, Kitch D, Tierney C, Melbourne K, Ha B, McComsey GA. Markers of renal disease and function are associated with systemic inflammation in HIV infection. HIV Med 2015; 16:591–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kong X, Ma X, Cui M, Xu D. Association of clustering of major cardiovascular risk factors with chronic kidney disease in the adult population. Clin Nephrol 2014; 82:92–7. [DOI] [PubMed] [Google Scholar]
- 22.Schouten J, group oboAHs. Comorbidity and ageing with HIV. Washington, DC: IAS, 2012. [Google Scholar]
- 23.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007; 92:2506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tunstall-Pedoe H, Kuulasmaa K, Amouyel P, Arveiler D, Rajakangas AM, Pajak A. Myocardial infarction and coronary deaths in the World Health Organization MONICA Project. Registration procedures, event rates, and case-fatality rates in 38 populations from 21 countries in four continents. Circulation 1994; 90:583–612. [DOI] [PubMed] [Google Scholar]
- 25.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16:31–41. [DOI] [PubMed] [Google Scholar]
- 26.Mosteller RD. Simplified calculation of body-surface area. N Engl J Med 1987; 317:1098. [DOI] [PubMed] [Google Scholar]
- 27.Vrouenraets SM, Fux CA, Wit FW et al. A comparison of measured and estimated glomerular filtration rate in successfully treated HIV-patients with preserved renal function. Clin Nephrol 2012; 77:311–20. [DOI] [PubMed] [Google Scholar]
- 28.Ryom L, Mocroft A, Kirk O et al. Association Between Antiretroviral Exposure and Renal Impairment Among HIV-Positive Persons With Normal Baseline Renal Function: the D:A:D Study. JID 2013; 207:1359–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mocroft A, Lundgren JD, Ross M et al. Development and validation of a risk score for chronic kidney disease in HIV infection using prospective cohort data from the D:A:D study. PLoS Med 2015; 12:e1001809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D'Agostino RB Sr, Vasan RS, Pencina MJ et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008; 117:743–53. [DOI] [PubMed] [Google Scholar]
- 31.Ryom L, Mocroft A, Kirk O et al. Predictors of advanced chronic kidney disease and end-stage renal disease in HIV-positive persons. AIDS 2014; 28:187–99. [DOI] [PubMed] [Google Scholar]
- 32.Friis-Moller N, Ryom L, Smith C et al. An updated prediction model of the global risk of cardiovascular disease in HIV-positive persons: The Data-collection on Adverse Effects of Anti-HIV Drugs (D:A:D) study. Eur J Prev Cardiol 2015; 23:214–23. [DOI] [PubMed] [Google Scholar]
- 33.Matsushita K, van der Velde M, Astor BC et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010; 375:2073–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arbel Y, Halkin A, Finkelstein A et al. Impact of estimated glomerular filtration rate on vascular disease extent and adverse cardiovascular events in patients without chronic kidney disease. Can J Cardiol 2013; 29:1374–81. [DOI] [PubMed] [Google Scholar]
- 35.Hemmelgarn BR, Manns BJ, Lloyd A et al. Relation between kidney function, proteinuria, and adverse outcomes. JAMA 2010; 303:423–9. [DOI] [PubMed] [Google Scholar]
- 36.Lekawanvijit S, Krum H. Cardiorenal syndrome: acute kidney injury secondary to cardiovascular disease and role of protein-bound uraemic toxins. J Physiol 2014; 592:3969–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.