Abstract
Cross-reactive influenza virus–specific antibody-dependent cellular cytotoxicity (ADCC)–activating antibodies are readily detected in healthy adults. However, little is known about the kinetics of these ADCC responses. We used retrospective serial blood samples from healthy donors to investigate this topic. All donors had ADCC responses against 2009 pandemic influenza A(H1N1) virus (A[H1N1]pdm09) and avian influenza A(H7N9) virus hemagglutinins (HAs) despite being seronegative for these viruses in standard hemagglutination inhibition and microneutralization serological assays. A(H1N1)pdm09 exposure did not boost ADCC responses specific for H7 HA antigens. H7 HA ADCC responses were variable longitudinally within donors, suggesting that these cross-reactive antibodies are unstable. We found no correlation between ADCC responses to the H7 HA and either influenza virus–specific immunoglobulin G1 concentration or age.
Keywords: influenza, antibody, ADCC, NK cells
In 2009, a novel strain of influenza A(H1N1)pdm virus emerged that rapidly spread worldwide, causing the first influenza pandemic of this century. Older adults were partly protected from the disease because of preexisting immunity, whereas younger adults and children were more readily infected. Owing to the unique hemagglutinin (HA) head of the 2009 virus, vaccination or infection in some adults boosted antibody responses to conserved epitopes of the functionally conserved HA stem [1]. More recently, in 2013, a novel influenza A(H7N9) virus emerged in China, resulting in >781 cases and 313 deaths [2]. Unlike seasonal A(H1N1) and influenza A(H3N2) virus, the H7 subtype still cannot cause sustainable transmissions in humans, and the great majority of the population is immunologically naive to this newly emerged virus. Thus, the frequent occurrence of human cases of A(H7N9) infection continues to be a major public health emergency of pandemic concern.
Growing evidence suggest that a significant portion of healthy individuals carry nonneutralizing antibodies that are cross-reactive with influenza viruses of multiple subtypes [3, 4]. Such antibodies can be used by the innate and adaptive immune system synergistically to fight viral infection through antibody-dependent cellular cytotoxicity (ADCC). ADCC-activating antibodies cross-link virus-infected cells and Fc receptors of CD16+/CD32+/CD64+ cells, such as B cells, neutrophils, macrophages, and natural killer (NK) cells. ADCC activation of NK cells results in degranulation and the release of preformed cytotoxic granules and proinflammatory antiviral cytokines. Thus, ADCC could have a protective role against influenza virus infection.
Previously, ADCC-activating antibodies specific for H5 HA were detected in healthy individuals without prior influenza A(H5N1) virus exposure. In addition, a correlation with ADCC responses and reduced disease severity with increased age was found for adults aged >45 years prior to the 2009 pandemic due to influenza A(H1N1) virus (A[H1N1]pdm09), whereas A(H1N1)pdm09-specific ADCC responses were boosted in individuals aged 15–45 years following exposure during the course of the pandemic [5].
The presence of broadly cross-reactive influenza virus–specific antibodies has ignited an interest in universal antibody-based vaccines that could able to recruit other immune cells. However, much remains to be deciphered about influenza virus–specific ADCC responses. In this study, we report the stability of ADCC responses over the long term in serial blood donors and the high baseline magnitude of responses toward different influenza virus proteins within the same individual, before and after A(H1N1)pdm09 exposure.
Prior to the 2009 pandemic, the population was predominantly serologically naive to the novel virus, resulting in the rapid global spread of A(H1N1)pdm09. Infection with or vaccination against A(H1N1)pdm09 in some individuals elicited broadly reactive antibodies, owing to the heterologous nature of H1 HA. We aimed to investigate the relationship of A(H1N1)pdm09 and cross-reactive avian influenza virus–specific ADCC-activating antibodies. Therefore, we probed for H7 HA avian-specific ADCC-activating antibody responses in healthy individuals in Hong Kong, a community which is serologically naive to A(H7N9) in China, and for which A(H7N9) remains a public health threat. Furthermore, as H7 is a group 2 HA subtype, there is less cross-reactivity with existing group 1 HA responses for H1 and H5 viruses. The conserved nucleoprotein (NP) was included in our study as an internal control between H1 HA and H7 HA. Furthermore, mouse studies have shown a protective role of NP-specific nonneutralizing antibodies upon adoptive transfer [6, 7]. Therefore, NP-specific ADCC activity could be a potential immune correlate for influenza virus infection. Thus, we hypothesized that A(H1N1)pdm09 exposure could increase ADCC responses to divergent influenza viruses, such as avian A(H7N9), owing to boosting of antibodies to conserved epitopes, like the HA stem.
METHODS
Subjects and Samples
All serum samples were derived from samples collected from blood donors at the Hong Kong Red Cross Blood Transfusion Services from June 2009 to June 2010. Longitudinal samples from repeat blood donors were collected at multiple time points, with 2–6 months (median time, 4 months) between consecutive donations. Paired or serial samples were collected from donors with 2 (n = 10), 3 (n = 5), or 4 (n = 2) blood donations within the studied period. Exposure to A(H1N1)pdm09 (A/California/07/2009) was determined by standard hemagglutination inhibition (HAI) and microneutralization (MN) assays [8]. A(H1N1)pdm09 exposure was defined as a MN titer of ≥ 1:40 and a HAI titer of ≥ 1:40, and A(H1N1)pdm09-negative donors were defined as individuals with a MN titer of < 1:20. All serum was frozen and heat inactivated at 56°C for 60 minutes before use. Serum for HAI and MN assays was also treated with receptor-destroying enzyme.
Protein-Specific ADCC Responses
A protein plate-bound NK ADCC assay was adopted from Jegaskanda et al [9]. The assay measures degranulation (CD107a) and antiviral (interferon γ [IFN-γ]) function of human NK cells due to cross-linking of influenza virus–specific immunoglobulin G (IgG) in donor sera. Briefly, U-bottomed enzyme-linked immunosorbent assay (ELISA) plates are coated overnight with recombinant influenza virus proteins, H7 HA (H7N9; A/Anhui/01/2013), H1 HA (H1N1; A/California/04/2009), and NP (H7N9; A/Anhui/01/2013; >95% homology with H1N1-derived NP; 400 ng/well; SinoBiological) in phosphate-buffered saline (PBS). Background ADCC activity by NK cells was determined by analysis of paired serum specimens with plate-bound nonspecific protein (allantoic fluid), with positive controls including purified CD16 antibody (Biolegend) for coating and pooled human sera (n = 4 donors pooled) tested against each protein.
Fresh peripheral blood mononuclear cells (PBMCs) from a healthy donor were isolated by Ficoll-Paque density gradient centrifugation on the day of the experiment. Protein-bound plates were thoroughly washed, and heat-inactivated immune serum was allowed to bind for 2 hours at 37°C. Plates were then further washed and incubated with PBMCs (2–5 × 105 cells) in the presence of brefeldin A/monensin and anti-human CD107a-APC. After 5 hours, cells were stained with anti-human CD14-PerCPCy5.5, CD3-APCCy7, and CD56-PE (Biolegend) in fluorescence-activated cell-sorting buffer (PBS with 1% fetal bovine serum and 0.1% sodium azide). Cells were then fixed (BD Cytoperm/cytofix buffer) and stained with anti-human IFN-γ–FITC (Biolegend) in BD Perm Wash buffer and then acquired on an LSR Fortessa.
ELISA
Briefly, recombinant influenza virus proteins (H1 HA, H7 HA, and NP, as described above; 80 ng/mL; SinoBiological) were coated on ELISA plates (Nunc-Immuno MaxiSorp, NUNC) overnight. After plates were blocked with fetal bovine serum and washed, diluted serum samples (1:10) were bound for 2 hours, washed, and detected by mouse anti-human IgG1–horseradish peroxidase (BD Biosciences) as the secondary antibody. TMB/peroxide was used as substrate, the reaction was stopped by addition of sulfuric acid (R&D systems), and absorbance was read at 450 nm.
RESULTS
A(H1N1)pdm Seroconversion Does Not Correlate With Increased H7 HA ADCC Responses
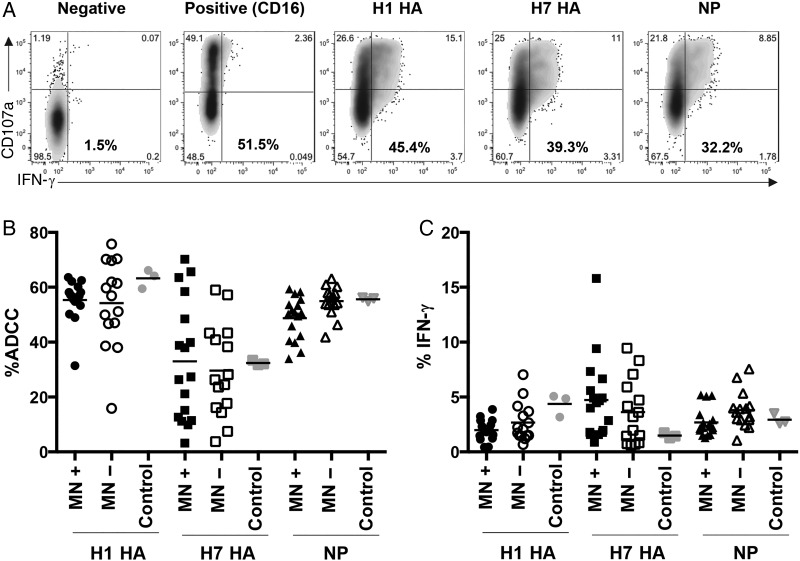
To determine whether A(H1N1)pdm09 exposure increased influenza virus–specific ADCC, serum from A(H1N1)pdm09-positive and A(H1N1)pdm09-negative donors was tested for ADCC responses to H7 HA, H1 HA, and NP (Figure 1). Responses by total ADCC (CD107a+ and/or IFN-γ+; Figure 1B) and IFN-γ+ (Figure 1C) were comparable. These observations were further confirmed by using diluted sera (1:20; data not shown). Overall, there was no significant difference in ADCC responses or different proteins between A(H1N1)pdm09-positive and A(H1N1)pdm09-negative donors. However, the response magnitude was protein dependent, with an immunodominance hierarchy of H1 HA, NP, and then H7 HA.
Figure 1.
Influenza A(H1N1)pdm09 virus seroconversion does not correlate with increased anti–influenza virus antibody-dependent cellular cytotoxicity (ADCC) responses in the community. A, Representative fluorescence-activated cell-sorting plots show the amount of ADCC (in bold) as the summed total of CD107a+ and/or interferon γ (IFN-γ)+ natural killer (NK) cells (CD14−CD3−CD56+). B and C, A(H1N1)pdm09 microneutralization (MN) assay positive (n = 17; black symbols), MN assay negative (n = 15; white symbols), and pooled serum (pooled from 4 Red Cross blood donors after the pandemic; gray symbols) were assessed for ADCC-activating antibody responses toward the H1 hemagglutinin (HA), H7 HA, and conserved nucleoprotein (NP). The amount of ADCC is the summed total of CD107a+ and/or IFN-γ+ (B) or IFN-γ+ (C) NK cells.
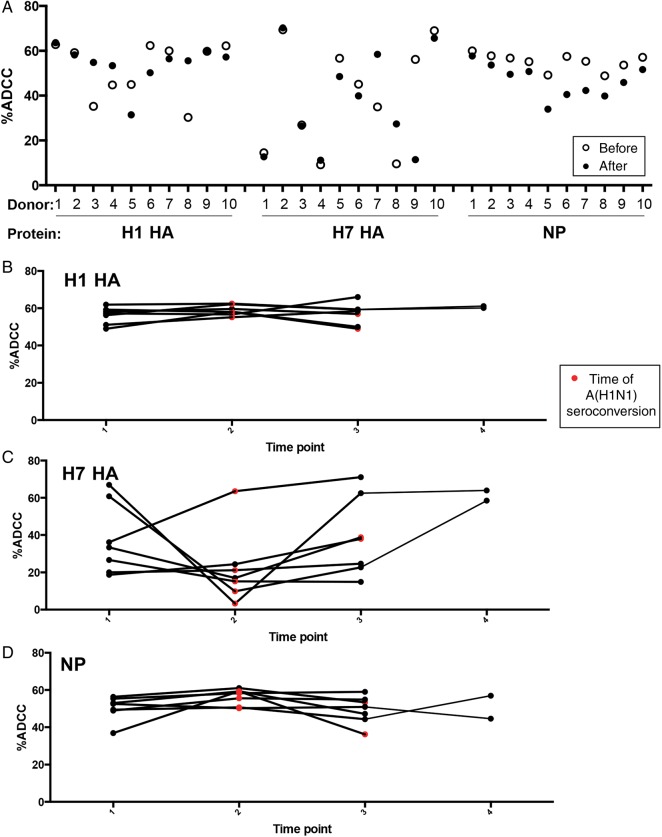
Paired serum from the same donor before and after A(H1N1)pdm09 infection was again compared across H7 HA, H1 HA, and NP (Figure 2). Again there was no consistent increase in the ADCC response magnitude after recent A(H1N1)pdm09 exposure. For H1 HA, 4 donors had reduced ADCC responses after exposure, 3 had increased responses, and responses in 3 remained unchanged (Figure 2A), whereas for H7 HA, 3 donors had reduced ADCC responses after exposure, 3 had increased responses, and responses in 5 remained unchanged. NP-specific ADCC responses were reduced in all 10 donors after A(H1N1)pdm09 exposure but were within a close range of their original baseline responses.
Figure 2.
Longitudinal stability of anti-influenza virus antibody-dependent cellular cytotoxicity (ADCC) responses is protein specific. A, Paired sera from the same donor (n = 10) before and after influenza A(H1N1)pdm virus exposure (confirmed A[H1N1]pdm positivity was defined as a microneutralization [MN] titer of > 1:40 and a hemagglutination inhibition titer of > 1:40) was assessed for ADCC toward H1 hemagglutinin (HA), H7 HA, and nucleoprotein (NP). Serial longitudinal serum of donors positive for A(H1N1)pdm09 by MN assay (before and after A[H1N1]pdm seroconversion) from 3 (n = 5) and 4 time points (n = 2) were tested for the magnitude of the ADCC response to H1 HA (B), H7 HA (C), and NP (D). The amount of ADCC is the summed total of CD107a+ and/or interferon γ+ cells. Individual responses are given. Red symbols indicate the point of A(H1N1)pdm09 seroconversion, based on MN findings. Time points are intervals between Red Cross blood donations, which are 2–6 months (median, 4 months).
Further comparison of the stability of ADCC responses longitudinally over 3–4 time points for repeated blood donors (Figure 2B–D) showed again that A(H1N1)pdm09 seroconversion did not result in increased H1 HA responses. H1 HA–specific ADCC responses were stable from 2 to 6 months between blood donations. Similarly, NP-specific ADCC responses were stable, whereas cross-reactive H7 HA ADCC responses showed higher levels of variation within donors longitudinally (Figure 2C). Interestingly, a substantial decrease in the ADCC response against H7 HA was detected in these individuals at the time when they were found to have seroconverted to A(H1N1)pdm09.
ADCC Responses Do Not Correlate With Increased Age or IgG Antibody Concentration
H1 HA–, H7 HA–, and NP-specific antibodies were further assessed by quantification of their concentration, using IgG1 ELISA (Supplementary Figure 1). Levels of H1 HA IgG1 antibodies were increased in A(H1N1)pdm09-positive subjects, whereas levels of H7 HA– and NP-specific IgG1 were unchanged (Supplementary Figure 1A). Again there was no difference in H7 HA–specific IgG1 levels after A(H1N1)pdm09 exposure in either prepandemic and postpandemic community samples (Supplementary Figure 1A) or in donor paired samples (Supplementary Figure 1B). Furthermore, longitudinal serum samples did not show a rise in H7 HA antibodies after A(H1N1)pdm09 exposure. NP IgG levels had similar results. Overall, there was no increase in H7 HA IgG antibody levels or ADCC responses among individuals with A(H1N1)pdm09 seroconversion.
A(H1N1)pdm09-positive and A(H1N1)pdm09-negative donors (Figure 1B) were assessed for correlation between age and the magnitude of the ADCC response to multiple influenza virus proteins (Supplementary Figures 2A and 2B). No significant correlation was found between increased age and the magnitude of the ADCC responses. In addition, there was no correlation with NP-specific IgG1 and NP-specific ADCC responses in A(H1N1)pdm09-positive donors (R2 = 0.23; Supplementary Figure 2C). These results are in agreement with our lack of correlation for increased ADCC response and recent A(H1N1)pdm09 infection.
DISCUSSION
High-level baseline responses were found in our Hong Kong study cohort for H1 HA and H7 HA in healthy unexposed subjects. Recently, high levels of A(H7N9) and A(H5N1) ADCC-activating and complement-fixing antibodies were found in all US infants and adults aged ≥8 years [10]. Conversely, in an Australian study, when individual donor serum was tested, only 1 of 69 adult donors had A(H7N9)-specific ADCC-activating antibodies, with antibody levels increasing after the 2009 pandemic [11]. Furthermore, Australian intravenous immunoglobulin preparations (antibody preparations pooled from thousands of donors) contained ADCC-activating antibodies for H1, H2, H3, H4, H5, and H7 [11]. However, the H7N9-Anhui/01/2013 ADCC responses were very low in IVIG preparations, compared with other HA subtypes, and ADCC responses against other A(H7N9) variants (A/Shanghai/01/2013 and A/Hangzhou/01/2013) were at even lower background levels. Our results are consistent with those of the recent US study in that all adults had cross-reactive avian-specific ADCC-activating antibodies.
In our study, A(H1N1)pdm09-exposed donors showed no increase in H1 HA ADCC–activating antibodies following exposure to A(H1N1)pdm09. This is most likely attributed to the high-level baseline H1 HA ADCC responses that cross-react with previous seasonal A(H1N1) exposure. Previously, an age-related increase was found for H1 HA ADCC response prior to the 2009 pandemic [5], whereby adults aged <45 years had reduced ADCC activity, which normalized after the pandemic. We did not find a significant correlation with age and H1 HA–, H7 HA–, or NP-specific ADCC-activating antibodies, whereas the recent US ADCC study found a correlation (R2 = 0.46) between age (1–18 years) and A(H7N9)-specific ADCC activity [10]. Importantly, their donors were children, whereas our cohorts comprise adults aged 17–55 years. Previous data reported by Jegaskanda et al [3, 12] from studies of macaque models show that vaccination with inactivated influenza virus vaccines does not elicit detectable ADCC responses but that A(H1N1)pdm09 challenge of vaccinated macaques recalls a homologous H1 HA–specific ADCC response [3, 12]. Macaque influenza virus–specific ADCC responses are mounted early after infection, wane in magnitude, and remain stable to memory time points. Naive animal models and infants (age, <2 years) demonstrate an induction of influenza virus–specific ADCC responses after influenza exposure. Therefore, it is possible that ADCC activity reaches a ceiling threshold in adults and that ADCC responses are accumulated after seasonal influenza exposure up to an immune ceiling. This would explain why we found a high baseline ADCC responses and no boosting effect after recent exposure in adults, as ADCC responses had already reached a threshold level.
In our study, the magnitude of ADCC responses toward H1 HA and NP was relatively stable before, immediately after, during the 6 months following A(H1N1)pdm09 seroconversion in our 7 donors tested longitudinally. However, H7 HA ADCC responses were more variable with time and between donors, making the kinetics and stability of cross-reactive H7N9 ADCC responses unclear. The same donor could show high-level responses and, at the next time point, have a response that had decreased to lower levels. Future vaccine strategies should aim to establish and stabilize avian influenza virus–specific ADCC responses to ensure this emerging immune correlate is used.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank Dr Sinth Jegaskanda for his expert advice about the antibody-dependent cellular cytotoxicity assay.
Flow cytometry data were acquired using equipment maintained by the University of Hong Kong Li Ka Shing Faculty of Medicine Faculty Core Facility.
Financial support. This work was supported by the Health and Medical Research Fund (project 15141052), the National Institutes of Allergy and Infectious Diseases (contract HHSN272201400006C), Hong Kong University small project seed funding (project 201409176181), the Harvard Center for Communicable Disease Dynamics from the National Institute of General Medical Sciences (project U54 GM088558 to J. T. W., K. L., and Y. Z.), and the Health and Medical Research Fund from the Government of the Hong Kong Special Administrative Region (commissioned grant to J. T. W., K. L., and Y. Z.).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Corti D, Lanzavecchia A. Broadly neutralizing antiviral antibodies. Annu Rev Immunol 2013; 31:705–42. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Influenza at the human-animal interface: Summary and assessment, 9 May to 13 June 2016. http://www.who.int/influenza/human_animal_interface/Influenza_Summary_IRA_HA_interface_06_13_2016.pdf?ua=1. Accessed 22 July 2016.
- 3.Jegaskanda S, Weinfurter JT, Friedrich TC, Kent SJ. Antibody-dependent cellular cytotoxicity is associated with control of pandemic H1N1 influenza virus infection of macaques. J Virol 2013; 87:5512–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laidlaw BJ, Decman V, Ali MA et al. . Cooperativity between CD8+ T cells, non-neutralizing antibodies, and alveolar macrophages is important for heterosubtypic influenza virus immunity. PLoS Pathog 2013; 9:e1003207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jegaskanda S, Laurie KL, Amarasena TH et al. . Age-associated cross-reactive antibody-dependent cellular cytotoxicity toward 2009 pandemic influenza A virus subtype H1N1. J Infect Dis 2013; 208:1051–61. [DOI] [PubMed] [Google Scholar]
- 6.Carragher DM, Kaminski DA, Moquin A, Hartson L, Randall TD. A novel role for non-neutralizing antibodies against nucleoprotein in facilitating resistance to influenza virus. J Immunol 2008; 181:4168–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LaMere MW, Lam HT, Moquin A et al. . Contributions of antinucleoprotein IgG to heterosubtypic immunity against influenza virus. J Immunol 2011; 186:4331–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. WHO global influenza surveillance network: manual for the laboratory diagnosis and virological surveillance of influenza Geneva: World Health Organization, 2011. [Google Scholar]
- 9.Jegaskanda S, Job ER, Kramski M et al. . Cross-reactive influenza-specific antibody-dependent cellular cytotoxicity antibodies in the absence of neutralizing antibodies. J Immunol 2013; 190:1837–48. [DOI] [PubMed] [Google Scholar]
- 10.Terajima M, Co MD, Cruz J, Ennis FA. High antibody-dependent cellular cytotoxicity antibody titers to H5N1 and H7N9 avian influenza A viruses in healthy US adults and older children. J Infect Dis 2015; 212:1052–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jegaskanda S, Vandenberg K, Laurie KL et al. . Cross-reactive influenza-specific antibody-dependent cellular cytotoxicity in intravenous immunoglobulin as a potential therapeutic against emerging influenza viruses. J Infect Dis 2014; 210:1811–22. [DOI] [PubMed] [Google Scholar]
- 12.Jegaskanda S, Amarasena TH, Laurie KL et al. . Standard trivalent influenza virus protein vaccination does not prime antibody-dependent cellular cytotoxicity in macaques. J Virol 2013; 87:13706–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.